Abstract
Poultry coccidiosis is an intestinal infection caused by an intracellular parasitic protozoan of the genus Eimeria. Coccidia-induced gastrointestinal inflammation results in large economic losses, hence finding methods to decrease its prevalence is critical for industry participants and academic researchers. It has been demonstrated that coccidiosis can be effectively controlled and managed by employing anticoccidial chemical compounds. However, as a result of their extensive use, anticoccidial drug resistance in Eimeria species has raised concerns. Phytochemical/herbal medicines (Artemisia annua, Bidens pilosa, and garlic) seem to be a promising strategy for preventing coccidiosis, in accordance with the “anticoccidial chemical-free” standards. The impact of herbal supplements on poultry coccidiosis is based on the reduction of oocyst output by preventing the proliferation and growth of Eimeria species in chicken gastrointestinal tissues and lowering intestinal permeability via increased epithelial turnover. This review provides a thorough up-to-date assessment of the state of the art and technologies in the prevention and treatment of coccidiosis in chickens, including the most used phytochemical medications, their mode of action, and the applicable legal framework in the European Union.
Keywords: Eimeria Species, Immunity, Intestinal Health, Microbiota, Poultry Production, Probiotics
INTRODUCTION
Poultry are the primary source of animal protein around the world [1,2]. According to the USDA, 102.9 million tons of chicken meat were produced in January 2020, reflecting a 3.9%-increase over the previous year [3]. This growth is crucial, since by 2050 the world population is expected to reach approximately nine billion people, making the production of sustainable and safe protein a top priority [4]. In intensive systems, stress levels and disease prevalence are higher, mainly due to birds being kept together in large numbers and at high stocking densities [5]. Therefore, any disease that decreases the effectiveness of the aforementioned production system may pose a risk to the global food chain [6].
The poultry sector is affected by different parasitic diseases (“hidden foes”) that lead to potential chronic losses without causing apparent clinical symptoms [7]. In the United States, coccidiosis-related annual costs are estimated to be over USD 127 million [8], whereas those in China exceed USD 73 million. Coccidiosis alone accounts for 30% of the overall spending on the pharmacological control of all potential poultry diseases [9]. There are seven Eimeria species that have been associated with coccidiosis in chickens, with the most important being E. tenella in broilers. The remaining ones include E. acervuline, E. brunitti, E. maxima, E. necatrix, E. praecox, and E. mitis. They are all unique in terms of pathogenicity and each of them affects different sections of the intestines [10]. Eimeria infection destroys host mucosal cells, increasing cell permeability, and allowing nutrients and proteins to seep out. This, in turn, impairs digestion and absorption of proteins as well as other nutrients both of which directly contribute to the subclinical and clinical symptoms of coccidiosis [11].
Coccidiosis prophylaxis is of utmost importance to pro mote substantial growth in chicken industry and to protect the sources of financial income [12]. In Europe, it will not be possible to maintain the current level of chicken production without a comprehensive anticoccidial management program. Therefore, almost all poultry farms use antiparasitic drugs as feed additives for pullets and broiler breeders for a period of 12 to 16 weeks, as well as for broiler chickens during nearly their whole lives. This approach substantially facilitates the maintenance of the high standards of poultry safety and welfare set forth by the European Union (EU) [13]. Since 1930, a multitude of ionophore and chemical anticoccidial agents has been extensively utilized to combat these possibly hazardous parasites [14]. However, drug residues in chicken products have adverse effects on consumers’ health and lead to the emergence of drug resistance [15]. This challenge is addressed, on the other hand, by the application of coccidiostats, which are synthetic chemicals or microbial products suppressing protozoan growth. Since 1940, by Directive 70/524/EEC of November 23, they have been used in numerous farm animals to prevent, inhibit and control parasitic protozoa of the genus Eimeria (the most common one), Isospora, Neospora, and Cryptosporidium, all of which belong to the phylum Apicomplexa and cause virulent infectious diseases of the intestinal system in chickens [16]. Eleven coccidiostats are approved as feed additives in the EU and divided into two groups: synthetics (decoquinate, diclazuril, halofuginone, nicarbazin, and robenidine) and bacterial polyether ionophores (lasalocid, monensin, maduramicin, narasin, salinomycin, and semduramicin) [17]. They are usually administered from the first day of the birds’ life up to seven days before slaughter to guard against contracting the disease due to the omnipresence of oocysts. This review provides a thorough up-to-date assessment of the state of the art and technologies in the prevention and treatment of coccidiosis in chickens, including the most used phytochemical medications, their mode of action, and the applicable legal framework in the European Union.
COCCIDIOSIS AND EIMERIA
The Eimeria seven-day life cycle in poultry normally occurs both outside (sporogony) and inside the host, where both sexual (schizogony and gametogenic) and asexual reproductive stages take place [18]. Fresh oocysts (capsules with protective walls shielding the parasite eggs), shed in faces at an undifferentiated (unsporulated) stage, are not infectious until they sporulate outside the host (in the environment) [19]. For the majority of Eimeria species, this process requires warmth and oxygen and takes 24 to 48 hours, depending on the environmental factors. Four sporocysts, each with two sporozoites, are contained in each sporulated oocyst. This is a direct cycle commencing after oral infection with active oocysts, during the infectious transmission stage. Sporocysts are released upon the mechanical action of the gizzard and sporozoites are released by the action of the bile and protein degrading enzymes in the small intestine [20].
These eukaryotic, host-specific, unicellular protozoa infiltrate the host intestinal tissue, rapidly reproducing and damaging gastrointestinal cells, thus impairing food absorption, and leading to the development of diarrhea and hemorrhage in the absence of treatment. Even in mild cases, gastrointestinal lesions induced by parasite proliferation in epithelial cells frequently facilitate other infections deteriorating animal health [21]. At least seven Eimeria species have been identified to parasitize intestinal epithelial cells in chickens. The pathophysiology of Eimeria spp. infection varies, however Eimeria tenella, Eimeria necatrix, and Eimeria brunetti are more hazardous and cause serious disease outbreaks in poultry. Three economically significant Eimeria species, Eimeria acervulina, Eimeria maxima, and Eimeria tenella, are the most important for broiler chickens. Following the first exposure of young animals to the infectious agent, immunity quickly develops, shielding birds against subsequent infections. Unfortunately, there is no cross-immunity among various Eimeria species, and recurrent occurrence of the disease may happen [22].
METHODS USED TO CONTROL AVIAN COCCIDIOSIS
The cornerstones for the prevention and control of coccidiosis are the application of vaccines, natural feed additives, preventive anticoccidial medications, and optimal handling measures on farms [23]. Appropriate handling measures are essential at poultry farms to safeguard the health and welfare of chickens and maintain the maximum production of high-quality chicken products. Some vital measures to consider for the best management at a chicken farm are, all farm employees should receive comprehensive guidance in proper chicken handling techniques. This comprises recognizing poultry behaviours, using safe lifting and carrying techniques, and emphasizing gentle handling to reduce stress [24].
A well-designed chicken farm will enable effective and secure handling. This includes the proper lighting system, adequate ventilation, temperature management and properly designed equipment and walkways to reduce the risk of possible injury to chickens. Assure that chickens have access to clean and fresh water and sufficient diet during handling and transportation. Provision of ample electrolytes supplementation to poultry birds during stressful conditions should be considered to properly maintain their health and well-being [25]. Additionally, implement stringent biosecurity measures to avoid disease introduction and spread in the poultry farm. This specifically entails limiting access to the poultry farm, outfitting employees in proper clothing and footwear, and putting in hygienic procedures including hand washing and disinfecting tools and transportation [26]. Facility cleaning and disinfection, appropriate ventilation, and the use of clean water are some effective means of maintaining litter conditions that reduce oocyst sporulation. The application of anticoccidials to prevent disease outbreaks (prophylaxis) has been a key element in broiler chicken production [27].
Coccidiostats
Effective anticoccidial feed additives have been conventionally used in broiler chickens and turkeys since the 1950s. According to Agri Stats Inc., during the late 1990s, 99% of broiler birds received anticoccidial treatment in one or more phases of their growth and this practice is still common around the world [28]. Meanwhile, customer preferences have evolved in different countries and currently some of the major global chicken producers, including the USA, produce as much as 60% of their broilers without anticoccidials [3]. Based on their mode of action, anticoccidial agents are classified as coccidiostats or coccidiocides. The former hinders parasite development by restricting reproduction and growth, but their effect can be significantly reduced after their elimination from the diet, which results in infection resurgence and potential disease. The latter, on the other hand, act by destroying or imposing irreparable damage to the parasites [15].
Mode of action of coccidiostats
Coccidiostats are classified into two groups. The first one contains polyether ionophores, which are natural compounds produced by bacteria of the family Streptomycetaceae [29]. They consist of multiple tetrahydrofuran rings conjugated into spiroketal moieties. The second group comprises synthetic coccidiostats (also known as chemicals) such as guanidines, triazines, quinolones, pyridines, alkaloids, or thiamine analogs [30]. Two modes of action (MoA) of ionophore antibiotics lead to changes in the ion concentration ratios on both sides of the cell membrane. Two antibiotic molecules dimerize under the first MoA to build a channel for cation transport through lipid bilayer [31]. The second MoA consists in the binding of cations by ionophores and their efficient transport across the cell membrane. The cations are subsequently released into the cytoplasm [32]. The cells effectively protect themselves against the aforementioned effect under physiological conditions. Ion concentrations on both sides of the cell membrane are regulated by the enzymes Ca2+/Mg2+-ATPase and Na+/K+-ATPase. As previously stated, ionophore antibiotics alter these concentrations. For example, lasalocid causes efflux of K+ ions while elevating the concentration of Na+ and Ca2+ within the cell [17]. The high concentration of Ca2+ in turn impairs mitochondrial activity, resulting in decreased energy production and cytotoxicity. Elevated Ca2+ levels also contribute to apoptosis due to the induction of specific endonucleases. Additionally, an inappropriate ratio of Na+ influx to efflux modifies the physiological ion concentrations on both sides of the cell membrane. A higher supply of energy for ATPases is essential to restore the proper functioning of the system [33]. However, the appropriate ion gradient cannot be maintained by the cell after exceeding a certain threshold and apoptosis occurs. Finally, higher levels of Na+ and Ca2+ enhance osmosis, causing cell expansion and rupture. Different chemical coccidiostats have distinct mechanisms of action. Decoquinate prevents parasite multiplication by interfering with mitochondrial electron transport [34]. Robenidine and nicarbazin are presumed to block mitochondrial energy production, but their exact mechanism of action is unknown. Halofuginone and diclazuril MoA is seldom mentioned in the literature [17].
Chemical coccidiostats
Currently, eleven coccidiostats, mainly synthetic chemicals and ionophores, are approved in the EU to limit disease spread, reduce parasite multiplication, and boost the immune system [21]. Ionophores eliminate parasites by preventing ions from passing through the cell membrane and altering the osmotic equilibrium. At present, they are the cornerstone of infection control. Synthetic chemicals influence parasite metabolism by blocking their physiological processes [35]. Halofuginone, robenidine, diclazuril, decoquinate, nicarbazin, toltrazuril, clopidol, ethopabate, amprolium, sulfadimethoxine, and sulfaquinoxaline are some examples of synthetic compounds [36]. Each of them has a specific mechanism of action against coccidia. Compounds of this type are referred to as “chemicals” due to their chemical composition and operate by interfering with one or more stages of the parasite life cycle [37]. They have an effect against its intestinal phases as soon as it attacks the host gastrointestinal system, being potentially more effective in the case of serious infections, but over time, resistance may appear [13].
Ionophores coccidiostats
Ionophores have a more complex and convoluted mode of action as compared to synthetic anticoccidials and do not exert so much selective pressure on the parasite. Furthermore, this mode of action is oriented towards sporozoites (the developmental stage of the parasite in the intestinal epithelium before host cell penetration), and does not result in the complete elimination of the parasite [38]. Instead, they allow low numbers of the parasite to survive and cause the host to acquire immunity. Currently, six ionophore anticoccidials are widely utilized in poultry production: lasalocid, maduramicin, salinomycin, monensin, narasin, and semduramicin [16].
Vaccines
Vaccination is a crucial element of coccidiosis control strategies. It stimulates the immune response that provides protection against future Eimeria challenges [39,40]. This response can be immediately triggered by the production of B and T lymphocytes and lymphoid cells [23]. Vaccines are an important alternative to medications in the combat against coccidiosis. Vaccination must be carried out appropriately to be efficient and to ensure adequate protection of birds. Vaccines containing oocysts obtained from Eimeria strains (E. acervulina, E. maxima, E. tenella, E. necatrix, and E. brunetti). E. maxima generate the maximum immune response in the host, i.e. a single sporocyst can provoke comprehensive immune defense, and five E. maxima oocysts can initiate a complete immune response against infection [41]. Vaccines constitute an important component of coccidiosis prevention since they induce adaptive immune responses within 3–4 weeks, depending on host genotype, duration and frequency of infection, and parasite concentration [13]. Present day vaccination procedure is quite challenging, since there is no certainty that all poultry birds in the flocks are subjected to the same concentration of the of the coccidia. Several factors like unhygienic or insufficient administration, potentially results in suboptimal performance as compared to the preventive care given to poultry birds. The most recent advance is “in ovo” immunization that is applied to 18-day embryonated chicken eggs. This technique involves the precise and consistent administration of the vaccine to the embryo amniotic cavity [42]. In ovo inoculation is a special approach that allows us to introduce substances directly to the chicken embryo throughout the incubation period. The administration of an extensive variety of additional substances was abruptly investigated using the in ovo method. In 2003, the idea of in ovo feeding was presented with the injection of the nutrients and other natural substances that may regulate the hatchling’s gastrointestinal growth into the embryonic amnion [43].
A non-metallic vital micronutrient called selenium has the capability to modulate the immunological response in broilers exposed to C. perfringens and Eimeria maxima at 14 and 18 days after hatching, respectively. In comparison to the non-treated group, the treated group received 10 to 20 μg of selenium/egg produced and had both fewer intestinal lesions and oocysts and exhibited higher serum antibody levels against C. perfringens and NetB toxin. This suggests that the immune response was improved in the post-hatched period [44]. In ovo injection of the raffinose family oligosaccharides from the Lupinus lutes seeds after 12 days of incubation resulted in a 2.5 log reduction in C. perfringens number and an 89% decrease in the shedding of oocysts from Eimeria spp [45]. In ovo treatment of probiotics on day 18 of the incubation results in a considerable reduction in the degree of severity of macroscopic lesions induced by Eimeria spp. in all gastrointestinal segments as well as an improvement in the zootechnical capabilities in the broilers [46]. Sokale et al [47] employed a commercial multi-egg injector to inject 50 μL volume of commercial coccidiosis vaccine comprising E. acervuline, E. maxima, and E. tenella oocysts (Inovocox EMI) to Ross 708 broiler hatching eggs after 18.5 days of incubation. According to the study’s findings, administering the EMI vaccination between 18.0 and 18.8 days of incubation may safely and efficiently stimulate the broilers immune system early enough to provide protection from further coccidial assaults. Live Eimeria parasites administered in ovo can be potentially effective in preventing coccidiosis in chicken production. The in ovo route of administration of a live vaccine (Inovocox, Pfizer) containing oocysts of the coccidian parasites species of Eimeria tenella, Eimeria acervuline, and Eimeria maxima has been proven to confer protective immunity in chickens [48]. It has been documented that the DNA vaccine for Eimeria tenella that uses the miconeme recombinant gene (EtMIC2) stimulates protective immunity in the gastrointestinal tract against coccidiosis [49]. In ovo immunization with a recombinant protein subunit vaccine has additionally been shown to be successful in safeguarding chickens against coccidiosis [50]. Furthermore, according to Hamid et al [12], immunization combined with in-feed ionophores produces the best outcomes in terms of commercial broiler performance. Vaccines can be administered directly (fed as gels or added to water), topically (sprayed into the eye), or in the hatchery where chickens are raised. However, their successful implementation may be limited by their cost associated with unskilled labor and high prices [51].
The first live coccidiosis vaccine was produced more than seven decades ago. In the EU, laying pullets, commercial broilers, and replacement breeders were vaccinated for the first time in 1992, and a vaccine for commercial layers was introduced in 2000 [27]. Currently, three vaccine types are widely used under field conditions: recombinant, attenuated, and non-attenuated. Each of them contains a wide spectrum of attenuated and non-attenuated parasites [52]. Non-attenuated vaccines are effective in preventing parasite spread and have been extensively administered for about 50 years. They are used as an alternative to in-feed anticoccidials, which may be inefficient in some cases [53].
The initial choice of the Eimeria strain isolates can decrease the efficacy of the attenuated anticoccidial vaccines as compared to the Eimeria with normal life cycle. Although attenuated anticoccidial vaccines are still often employed today however, the lower level of the immune response can be increased by the potential application of the adjuvants, composed of cytokines [54]. An analysis of surface and intracellular antigens of Eimeria at various phases of its life cycle is necessary for producing recombinant vaccines and stimulating an efficient immune response. Problems with the identification of appropriate antigens hinders the development of synthetic vaccines [55]. However, coccidiosis in chickens can be prevented over time by rotation strategies that use both medicines and vaccines in succeeding flocks [56]. As genetic technology progresses, vaccines containing genes encoding immunomodulatory polypeptides will be developed [57].
Natural alternatives (Plants and their components)
Alternative coccidiosis control strategies have been developed to reduce the use of veterinary medicines in the food supply chain. Natural treatments such as prebiotics and probiotics, plant and fungal extracts, and essential oils are examples of alternative therapeutic options. Typically, natural compounds influence gastrointestinal flora and the immune system rather than directly combating parasites [58].
Garlic
Garlic (Allium sativum L.) has been regarded as a medicinal plant for centuries. Allicin constitutes the most important organosulfur compound, accounting for over 70% of all thiosulphates and being responsible for the garlic aroma [59]. In general, allicin interacts with sulfhydryl groups of cysteine residues in thiol-containing enzymes produced by pathogenic bacteria [60].
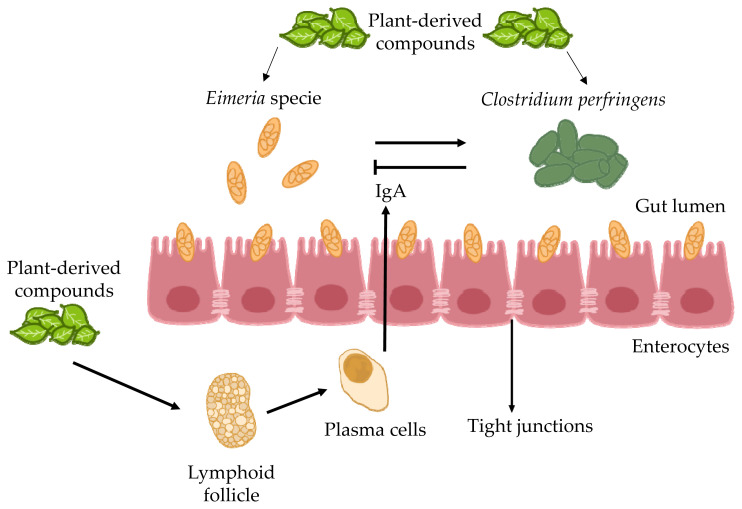
The antioxidant and anti-inflammatory properties of garlic result from significant amounts of organosulfur compounds such as allicin, diallyl sulphide, and diallyl trisulfide. According to Kim et al [61], the potential anticoccidial effect of garlic is attributed to its immunomodulatory activity as shown in Figure 1. Aqueous garlic extract contains a high concentration of phenols, flavonoids, and other sulfur compounds [62]. Phenolic complex alters cytoplasmic membrane permeability to various cations, which affects physiological activities at the molecular level, leading to lower membrane potential, the loss of vital cellular constituents to the surrounding environment, decreased synthesis of proteins and ATP, and ultimately cellular death [63–65].
Figure 1.
The schematic process illustrates the coordinated efforts of Gut-associated T cells and macrophages to orchestrate the immune response to anticoccidian herbal compounds in chickens.
Different forms of garlic contain various active compounds with specific functions. Antioxidant activity is exhibited by powdered garlic and its aqueous extract (containing diallyl disulphide as well as phenol and flavonoids, respectively) [66], whereas the anti-inflammatory function is characteristic of powder and essential oils [67]. Moreover, diallyl trisulfide contained in the latter has antiviral properties, while the water extract also alters the permeability of the cytoplasmic membrane [68]. Finally, garlic tincture, rich in sulphuric acid, shows immunostimulatory activity and all of the above-mentioned forms are capable of inhibiting oocyst sporulation to some extent, either in vivo or in vitro [69].
Artemisia annua
Artemisia annua, a perennial plant from the Asteraceae family, is a common component of the native flora of many regions, including China, Argentina, France, Bulgaria, Hungary, Romania, Spain, Italy, and the United States [70]. The chemical constituent (artemisinin), derived from the leaves of A. annua, has been associated with the efficient treatment of malaria, which is a considerable problem worldwide [71]. It has been documented that the synergistic effect between plant ingredients does not decrease the incidence rate of malaria. On the other hand, A. annua leaves can yield 40 times higher levels of artemisinin in the bloodstream as compared to pure artemisinin [72,73]. Several recent studies on artemisinin and its derivatives have revealed that this plant may be of potential therapeutic interest for the treatment of several other diseases, such as chicken coccidiosis [74].
The number of oocysts per gram of feces in chickens ad ministered A. annua decreased, and the overall lesion score was 80% lower as compared to the control group. Dietary supplementation of A. annua in chickens resulted in reduced body weight gains but also in improved feed conversion in comparison with the control. Artemisia annua can be regarded as a promising candidate for the prevention of poultry coccidiosis. Furthermore, A. annua-supplemented broiler feeds have beneficial zootechnical and health-related properties in terms of parasitic diseases and gastrointestinal microbiota [70]. According to Almeida [75], supplementation of Eimeria sp. infected feed with A. annua dried leaves significantly reduced the number of excreted oocysts in chickens. Fatemi et al [76] also reported a decrease in the number of oocysts per gram by adding alcoholic extract of A. annua to poultry feed, which indicates that the potential anticoccidial effect is associated with the influence of artemisinin on oocysts. Fatemi et al [77] found that A. annua alcoholic and petroleum ether preparations impeded oocyst sporulation by morphological modification of the oocyst wall, whereas Del Cacho et al [78] showed that artemisinin inhibited the expression of SERCA (sarco/endoplasmic reticulum Ca2+ -ATPase having a vital function in transportation of calcium from the cytosol into the sarcoplasmic reticulum) in macrogametes, which had a significant impact on oocyst wall development. Furthermore, artemisinin and A. annua leaves promote host cell death and inhibit the inflammatory reaction. In addition, it was demonstrated that A. annua leaves alleviated clinical manifestations by apoptosis induction and inflammatory reaction decrease, especially in comparison with artemisinin. A. annua also has a substantial concentration of tannins, saponins, and flavonoids that serve as antioxidants and inhibit cellular antioxidants mediated by reactive oxygen compounds, a phenomenon observed in coccidiosis [79].
Biden pilosa
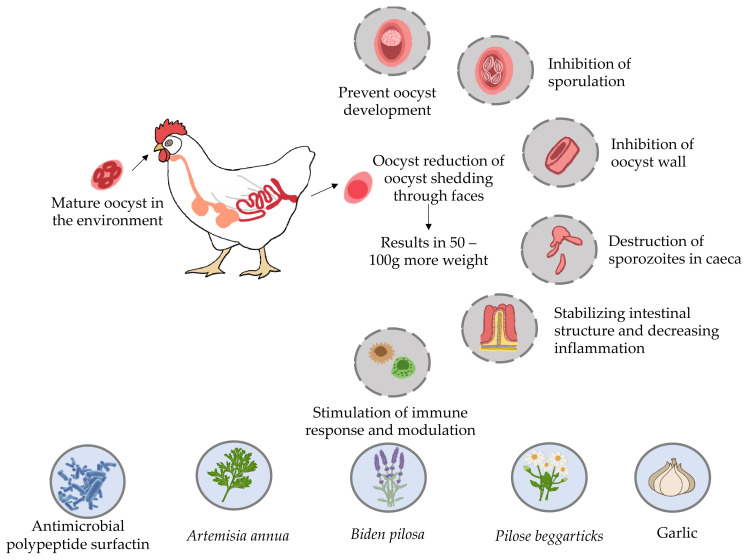
B. pilosa (BP), belonging to the family Asteraceae, occurs all over the world. It has been marketed as a medicinal and culinary herb [80]. This plant has been successfully used to treat more than 41 types of infection, including protozoan and bacterial ones as well as different sorts of disorders (gastrointestinal, immunological and other) [81]. Around 200 phytochemicals such as aliphatics, flavonoids, terpenoids, phenylpropanoids, aromatics, and porphyrins, have been documented in BP. Its chemical constituents are responsible for potential therapeutic effects. In small-scale research, 68 plants, including BP, were found to have antiprotozoal activity. BP was first used as a herbal plant to treat malaria, i.e. human coccidial disease [82]. According to in vitro assays, BP and its active components may be successful therapeutic interventions for malaria. Polyacetylenes and flavonoids derived from BP are the key molecules responsible for its antimalarial effectiveness [83]. However, the anticoccidial properties of BP and its active ingredients require further investigation. Therefore, BP impact on Eimeria, the avian coccidial parasite, was explored. More recently it has been demonstrated that BP decreased the severity of Eimeria infection and drug tolerance in poultry. Additionally, BP positively affected symbiotic bacteria and reduced the number of pathogenic microorganisms in the gastrointestinal tract, which is the main mechanism responsible for its anticoccidial action as shown in Figure 2 [84]. Chang et al [82] found that supplementation of feed with BP at a concentration of 0.025% or higher significantly decreased the probability of Eimeria infection. This addition enhanced growth performance by increasing body weight gains and lowering feed conversion ratio. It also improved the anticoccidial index and reduced the number of oocysts per gram of feces, thus playing an important role in gastrointestinal pathophysiology, and decreasing mortality rate. In general, the above-described results indicate that BP can minimize the occurrence of eimeriosis in chicken production. Hence, BP can be used to effectively control this disease.
Figure 2.
The life cycle of Eimeria, alongside an exploration of the intellectual prospects for employing natural coccidiostats.
In a study comparing control with positive untreated challenged birds, feed containing probiotics (Bacillus subtilis, Clostridium butyricum and Lactobacillus at 5×108 CFU per g), BP, and probiotic+BP increased antioxidant enzyme activity, the level of tight junction proteins and pro-inflammatory cytokines. Consequently, feeding probiotics and BP (either separately or together) to poultry seems beneficial for disease prevention and reduced intensity of Eimeria infection [85].
Oregano essential oil
The labiate family, including thyme, sage, lavender, and oregano, in particular (in the form of extract and steam-distilled oil), are the most intensively investigated plants for preventing parasitic infections in poultry. One of their most important components (polyphenols) has anticoccidial properties against chicken coccidiosis [86]. The two main phenols (carvacrol and thymol) accounting for approximately 70% to 80% of oregano essential oil, possess anticoccidial activity [87]. During Eimeria infection, oregano essential oil increased gastrointestinal absorption and enhanced antioxidative defense system. According to Tsinas et al [88], broilers challenged with Eimeria acervulina and E. maxima and supplemented with 300 or 600 ppm of an oregano product demonstrated decreased lesion score and without compromising growth performance. In broilers experimentally vaccinated with 50× doses of E. acervulina, E. maxima, and E. tenella, oregano oil supplementation at a concentration of 500 ppm decreased coccidiosis infection severity [89].
In a similar way, “functional oil” consisting of castor and cashew nut shell liquid oils added to broiler chicken feed following E. maxima challenge improved body weight gains and feed conversion efficiency [90]. In addition, extract from the medicinal herb Tulbaghia violacea, inhibited oocyst proliferation in Eimeria-infected poultry, whereas the antioxidant contained in the extract has been shown to attenuate lipid peroxidation caused by coccidial infection. It should finally be stated that herbal medicinal plants and chemicals use several metabolic pathways, including linoleic acid, estrogen, and lipoid metabolism [91].
Bio-active compounds
Prebiotics
Prebiotics such as fructooligosaccharides, mannan oligosaccharides (MOS), xylooligosaccharides, and inulin, are commonly used in poultry production. They are an innovative approach to coccidiosis management and their mechanism of action is mainly based on the multiplication and activation of specialized probiotic bacteria [92]. Fructooligosaccharides and MOS have been demonstrated to modify a gut-associated inflammatory response and macrophages, which suppresses Eimeria infection [93]. Prebiotics exert their effect mainly through the regulation of intestinal flora by providing nutrients to favorable bacteria and stimulating their growth. They also reduce penetration of microbial pathogens into the gastrointestinal tract [94,95]. Shorter intestines of birds result in a larger amount of ingested glucose accumulating in the ceca, which, after subsequent fermentation, leads to lower pH and consequently affects E. tenella proliferation [96]. In the anticoccidial experiment, MOS supplementation (0.8 g/kg feed) potentially reduced the severity of cecal lesions in birds with approximately 20,000 to 30,000 sporulated oocysts of E. tenella [97]. In a series of studies, incorporation of MOS at a rate of 10 g/kg of feed in broiler diets resulted in lower oocyst excretion and severity of E. acervulina lesions. Nevertheless, other studies have not shown any beneficial effects of prebiotics on coccidiosis prevention. McCann et al [98] found that feeding 0.5 g/kg of MOS to birds did not affect severity of infection with E. maxima, E. tenella, or E. acervuline. Differences in the doses of Eimeria and MOS concentration in chicken feed were considered responsible for the discrepancies in MOS effectiveness [96].
Probiotics
Probiotics are defined as feed supplemented with live beneficial microorganisms (including Bifidobacterium, Lactobacillus, and Streptococci), yeast cultures (Candida and Saccharomyces strains), and fungi (Aspergillus awamori, A. niger, and A. Oryza). They have been demonstrated to decrease the susceptibility to coccidiosis by improving poultry performance, intestinal flora balance, and the immune response [5]. Feed supplementation with Pediococcus-based probiotics in birds infected with E. tenella conferred additional protection against growth delay. Ritzi et al [99] reported that supplementing broiler feed with probiotics containing Lactobacillus salivarius, Enterococcus faecium, and Bacillus animalis attenuated infections with E. maxima, E. tenella, and E. acervuline by decreasing oocyst shedding and lesion scores in the duodenum, jejunum, and ceca. When compared to the Eimeria-challenged positive control birds, the combination of the three aforementioned probiotic bacteria (E. faecium, B. animalis, and L. salivarius) at a proportion of 6:3:1 ameliorated growth parameters and improved gastrointestinal health (increasing ileal villus height and crypt depth ratio) [100]. Probiotics comprising Pediococcus and Saccharomyces strains synergistically affected the immune system reaction and decreased the shedding of E. acervulina and E. tenella oocysts [96]. Bacillus is another important strain that has been commonly used to treat coccidiosis in poultry. Oral administration of Bacillus subtilis significantly reduced E. tenella lesions in the ceca in comparison with the control group [101]. Similarly, eight B. subtilis strains contained in a direct-feed microbial product were administered to broiler chickens exposed to E. maxima challenge and fed the mash diet. The clinical manifestations associated with coccidiosis were reduced and the immune level was decreased by boosting cell-mediated immune responses against Eimeria [102]. The obtained results indicate that probiotic bacteria may occupy common receptors in the epithelium because of competing with Eimeria for attachment sites in the gastrointestinal tract. This competition probably prevents Eimeria from proliferating and shedding oocysts. However, the effectiveness of probiotics or prebiotics can be reduced by sever coccidiosis and further alternatives need to be explored [96].
Amino acids
Important roles of amino acids in broilers under Eimeria challenge
In broilers exposed to Eimeria spp., amino acids enhance intestinal growth, immunity, gut integrity, and antioxidant defense. Among other important amino acids, methionine, threonine, glutamine, and arginine, have received attention for their potential to mitigate the adverse effects of Eimeria infections in broilers [103]. Methionine is the first limiting amino acid in poultry diets produced from corn and soybeans. It is essential to produce cysteine, glutathione, taurine, carnitine, and polyamines as well as methyl group donors and sulfur donors. Because an Eimeria infection makes methionine harder to digest and causes more oxidative stress and inflammation inside the body, supplementing with methionine may lessen oxidative stress and enhance antioxidant defense [104]. Jankowski et al [105] revealed that broilers and turkeys supplemented with methionine had higher serum superoxide dismutase activity and total antioxidant potential. According to Castro et al [103] broilers infected with Eimeria spp., produced more glutathione, reduced oxidative stress, and boosted intestinal mucin synthesis as diet was supplemented with methionine.
Threonine is a crucial amino acid and is an essential con stituent of mucin for maintaining intestinal integrity and promoting spontaneous recovery during and after Eimeria infections as the infection damages the gastrointestinal tract [106]. According to Zhang et al [107] threonine shortage considerably enhanced the number of Eimeria oocysts shedding and amount of the gut leakage in the broilers receiving 25× Eimeria vaccine, however enhanced threonine supplementation from 0.48% to 0.96% considerably ameliorates gastrointestinal integrity and decreased oocysts shedding. Teng et al [104] documented that supplementing 0.75% threonine to low-protein diet enhanced villus height and a tight junction protein in broilers exposed to mixed Eimeria species (E. acervuline, E. maxima, and E. tenella). These findings suggest that supplementing broilers diet with threonine can help to improve gastrointestinal heath under coccidiosis challenge. Additionally, threonine is a vital amino acid maintaining inflammatory mechanism and regulates immunoglobulin synthesis. Dietary threonine supplementation improves IgA secretion as well as decreases pro-inflammatory cytokines including INF-γ and IL-1β [108].
Alkaloids
Sanguinarine (C20H14NO4) is a particular type of plant alkaloid. Alkaloids are nitrogen compounds particularly found in plants as secondary metabolites or natural products. Isoquinoline phenanthridine alkaloid is mostly present throughout the entire Macleaya cordata plant. It holds multiple potential properties including insecticidal, antibacterial, ant-inflammatory, anticancer, and immune booster [109]. As a plant derived medication, sanguinarine also has the benefits of low toxicity, little residues, and no harmful effects on the environment. As a feed supplementation, sanguinarine has been demonstrated to improve the performance of poultry. It can enhance the digestibility of nutrients and productive efficiency of laying hens fed low crude protein diet, as well as considerably safeguards laying hens under Campylobacter hepaticus challenges [110]. In vitro sanguinarine supplementation at dose rate of 1, 5, and 10 mg/L potentially inhibited coccidiosis invasion. In addition to inducing apoptosis, sanguinarine can raise sporozoites reactive oxygen species levels, lower mitochondrial membrane potential, and increase intracellular calcium ions concentrations [111].
The isoquinoline alkaloid berberine has been identified in a number of medicinal plants including Coptis chinensis Franch, Cortex phellodendri, and Berberis asiatica. Berberine has been linked to a magnitude of pharmacological activities such as anti-inflammatory, anti-diabetic, anti-atherosclerotic and cardioprotective properties [112]. The alteration of the intestinal microbiota composition and functionality by berberine in poultry may be correlated with its effects on the growth performance. Although, berberine increased the abundance of the phylum Bacteroidetes and the genus Bacteroides as well as decreased the Firmicutes, and Clostridiales in the gut of broilers [113]. Additionally, broilers can be treated with berberine to prevent coccidial infection and necrotic enteritis [114].
Nguyen et al [115] documented the anticoccidial advan tages associated with berberine-based supplemented diets in broilers chickens following oral infection with five Eimeria spp. (E. acervuline, E. maximma, E. tenella, E. mitis, and E. praecox). Broilers treated 0.5% berberine following E. maxima infection have significantly decreased fecal oocysts production.
Tannins
There are several forms of tannins with different molecular weights, which are classified as polyphenolic chemical compounds with the capability for precipitating proteins. The two different types of plants tannins are hydrolysable tannins with tannin derivatives (gallic acid and ellagic acid), and condensed tannins (CT) [116].
The bioavailability of tannins varies based on a number of parameters, such as the chemical derivatives of each tannin (such as gallic acid and ellagic acid), their affinity for proteins, molecular structure as well as their molecular weight. Tannin bioavailability is a crucial characteristic for their functionality and should be taken into account while addressing various challenges in poultry production [117]. Tonda et al [118] observed that supplementation of 500 mg/kg of gallnut tannic acid extract decreased total oocyst number in the excrete and lowered gastrointestinal lesions scores in broilers under Eimeria spp. challenges.
According to a research finding, providing chickens in fected with Eimeria tenella grapes seed proanthcyanidin extract, particularly with a high concentration in CT, as a supplement improved growth performance and reduced clinical symptoms, possibly through increasing antioxidant capacity [119]. Eimeria maxima infection drastically decreased broiler growth and development performance and compromised the gastrointestinal ecosystem. In broilers under E. maxima challenge, supplementation of tannic acid at dose of 500 to 2,750 mg/kg potentially contributed to decreased oocysts shedding, an active immune response, increased gut barrier integrity, and improved gastrointestinal impact and digestibility of nutrients. Consequently, the supplementation of tannic acid at dosage ranging from 500 to 2,750 mg/kg has the potential to act as an anti-coccidial agent and improve the gut health in broilers [120].
Terpenoids
The Acacia tree is indigenous to Egypt and is extensively grown in tropical and subtropical regions of Asia, Australia, Africa, and America. Acacia plants produce several secondary metabolites, and these secondary metabolites possess a wide range of therapeutic applications in both the prevention and treatment measure of many poultry diseases [121]. As a result, research on the biological activities of natural compounds has been conducted and developed using medicinal plants in the most effective manner [122]. Additionally, tannins, flavonoids, and saponins are known to have anticoccidial efficacy via reducing sporulation. E. tenella oocysts sporulation was altogether inhibited by Acacia nilotica aqueous extract at a concentration of 100 mg/mL, and the morphology and size of the oocysts were markedly altered, so Acacia nilotica can be potentially employed to prevent and cope with Eimeria infections [123].
Surfactin
Surfactin is a potent biosurfactant, produced by many strains of Bacillus subtilis. The biological activities of the surfactin includes antiviral, anti-mycoplasma, and antiprotozoal actions, as well as broad-spectrum potential activities against Gram-positive and Gram-negative bacteria, and fungi. Surfactin is one of the potentially successful antibiotic substitutes. Cheng et al [124] demonstrated that supplementation of B. licheniformis fermented product 0.1% to 0.2% to the broiler’s feed could enhance weight gain and alleviate C. perfringens-induced necrosis of the gastrointestinal tract. This demonstrates that the intestinal health of the broilers appeared to be improved by surfactin. According to Lee et al [125] supplementing B. Licheniformis fermented product 0.3% to broilers diet could enhance body weight gain. When compared to the coccidial challenge group, B. licheniformis fermented products improve broiler average daily growth at 21 to 35 days of age. The B. licheniformis fermented products treated group exhibited a higher anti-coccidia index than the coccidial challenge group. The cecal digesta of the B. licheniformis fermented products treated group have more genus Lactobacillus compared to the coccidial challenge group [126]. Cheng et al [127] reported that supplementation with Bacillus licheniformis-fermented products at 1 g/kg could increase average daily body weight gains in broiler chickens subjected to coccidiosis challenge. It also improved anticoccidial efficacy and modified the composition of the gastrointestinal microflora by enhancing beneficial microorganisms and inhibiting detrimental ones.
According to Yu et al [128], adding B. licheniformis-fermented products at 1.25 and 5 g/kg to broiler feed improved their survival rate and cecal morphology after E. tenella challenge. Chickens receiving 5 or 1.25 g/kg of the above-mentioned supplements had lower oocyst-count index and cecal lesion scores, respectively. Surfactin also altered sporozoite morphology and inhibited Eimeria oocyst sporulation. These results show that surfactin, an antimicrobial lipopeptide derived from Bacillus licheniformis, has anticoccidial activity both in vitro and in vivo.
Cheng et al [129] showed that Bacillus subtilis-supplemented feed fermented for four days had the highest surfactin level and exerted the most profound antimicrobial effect on pathogenic microorganisms such as Clostridium perfringens, Staphylococcus aureus, Escherichia coli, and Salmonella typhimurium. Dietary supplementation with Bacillus subtilis-fermented products containing surfactin significantly affected gastrointestinal tract morphology and stimulated the healing of ulcerated lesions in broilers infected with Clostridium perfringens. Bacillus subtilis addition may increase broiler growth and productivity, enhance bone quality, intestinal structure, and function.
CONCLUSION
Poultry are the primary source of animal protein, contributing significantly towards meat and egg production. The demand for this type of protein rises rapidly all over the world. Each of the seven Eimeria species inhabits different sites of the gastrointestinal tract and causes poultry diseases ranging from subclinical enteritis to subacute mortality. The severity of coccidiosis depends on Eimeria species, strain, infectious dose, host genetic makeup, flock density, environmental and stress conditions, and concomitant infections. Restrictions on the use of antibiotic growth promoters and the scarcity of novel antimicrobials create an urgency to find new antibiotic alternatives. Currently, herbal products receive a great deal of attention for their potential use in the prevention and treatment of poultry coccidiosis. Research results on phytogenic substances exhibiting anti-coccidial preventive, prophylactic, or immunomodulatory activities are reviewed. However, further research is required to clarify and confirm the effectiveness of phytochemicals as promising in-feed anticoccidial agents.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
FUNDING
The authors received no financial support for this article.
REFERENCES
- 1.Govoni C, Chiarelli DD, Luciano A, et al. Global assessment of natural resources for chicken production. Adv Water Resour. 2021;154:103987. doi: 10.1016/j.advwatres.2021.103987. [DOI] [Google Scholar]
- 2.Nkukwana T. Global poultry production: Current impact and future outlook on the South African poultry industry. S Afr J Anim Sci. 2018;48:869–84. doi: 10.4314/sajas.v48i5.7. [DOI] [Google Scholar]
- 3.Mesa-Pineda C, Navarro-Ruíz JL, López-Osorio S, Chaparro-Gutiérrez JJ, Gómez-Osorio LM. Chicken coccidiosis: from the parasite lifecycle to control of the disease. Front Vet Sci. 2021;8:787653. doi: 10.3389/fvets.2021.787653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh RB, Watson RR, Takahashi T. The role of functional food security in global health. Academic Press; 2019. pp. 3–24. [DOI] [Google Scholar]
- 5.Ahmad R, Yu YH, Hsiao FSH, et al. Influence of heat stress on poultry growth performance, intestinal inflammation, and immune function and potential mitigation by probiotics. Animals. 2022;12:2297. doi: 10.3390/ani12172297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aganovic K, Hertel C, Vogel RF, et al. Aspects of high hydrostatic pressure food processing: Perspectives on technology and food safety. Compr Rev Food Sci Food Saf. 2021;20:3225–66. doi: 10.1111/1541-4337.12763. [DOI] [PubMed] [Google Scholar]
- 7.Blake DP, Knox J, Dehaeck B, et al. Re-calculating the cost of coccidiosis in chickens. Vet Res. 2020;51:115. doi: 10.1186/s13567-020-00837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahlou RA, Bounechada M, Mohammedi A, Silva LR, Alves G. Dietary use of Rosmarinus officinalis and Thymus vulgaris as anticoccidial alternatives in poultry. Anim Feed Sci Technol. 2021;273:114826. doi: 10.1016/j.anifeedsci.2021.114826. [DOI] [Google Scholar]
- 9.Geng T, Ye C, Lei Z, et al. Prevalence of Eimeria parasites in the Hubei and Henan provinces of China. Parasitol Res. 2021;120:655–63. doi: 10.1007/s00436-020-07010-w. [DOI] [PubMed] [Google Scholar]
- 10.Kers JG, Velkers FC, Fischer EA, Hermes GDA, Stegeman JA, Smidt H. Host and environmental factors affecting the intestinal microbiota in chickens. Front Microbiol. 2018;9:235. doi: 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C, Kennes YM, Lepp D, et al. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult Sci. 2020;99:936–48. doi: 10.1016/j.psj.2019.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid PH, Kristianingrum YP, Wardhana AH, Prastowo S, Silva LMR. Chicken coccidiosis in Central Java, Indonesia: A recent update. Vet Med Int. 2018;2018:8515812. doi: 10.1155/2018/8515812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins RR, Silva LJ, Pereira AM, Esteves A, Duarte SC, Pena A. Coccidiostats and poultry: A comprehensive review and current legislation. Foods. 2022;11:2738. doi: 10.3390/foods11182738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogueira VA, Franca TN, Peixoto PV. Ionophore poisoning in animals. Pesqui Vet Bras. 2009;29:191–7. doi: 10.1590/S0100-736X2009000300001. [DOI] [Google Scholar]
- 15.El-Shall NA, Abd El-Hack ME, Albaqami NM, et al. Phytochemical control of poultry coccidiosis: a review. Poult Sci. 2022;101:101542. doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noack S, Chapman HD, Selzer PM. Anticoccidial drugs of the livestock industry. Parasitol Res. 2019;118:2009–26. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rybicki MJ. Coccidiostats in treating coccidiosis. Zywnosc Nauka Technologia Jakosc. 2020;27:125–37. doi: 10.15193/zntj/2020/125/364. [DOI] [Google Scholar]
- 18.Del Cacho E, Gallego M, Lillehoj HS, et al. IL-17A regulates Eimeria tenella schizont maturation and migration in avian coccidiosis. Vet Res. 2014;45:25. doi: 10.1186/1297-9716-45-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastor-Fernández I, Collantes-Fernández E, Jiménez-Pelayo L, Ortega-Mora LM, Horcajo P. Modeling the ruminant placenta-pathogen interactions in Apicomplexan parasites: current and future perspectives. Front Vet Sci. 2021;7:634458. doi: 10.3389/fvets.2020.634458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gururajan A, Rajkumari N, Devi U, Borah P. Cryptosporidium and waterborne outbreaks – A mini review. Trop Parasitol. 2021;11:11–5. doi: 10.4103/tp.TP_68_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesse LL, Bakke AM, Eggen T, et al. The risk of development of antimicrobial resistance with the use of coccidiostats in poultry diets. Eur J Nutr Food Saf. 2019;11:40–3. doi: 10.9734/EJNFS/2019/v11i130127. ID: sea-189696. [DOI] [Google Scholar]
- 22.Mnisi CM, Mlambo V, Gila A, et al. Antioxidant and antimicrobial properties of selected phytogenics for sustainable poultry production. Appl Sci. 2023;13:99. doi: 10.3390/app13010099. [DOI] [Google Scholar]
- 23.Broom LJ. Evidence-based consideration of dietary ‘alternatives’ to anticoccidial drugs to help control poultry coccidial infections. World’s Poult Sci J. 2021;77:43–54. doi: 10.1080/00439339.2021.1873713. [DOI] [Google Scholar]
- 24.Dhaka P, Chantziaras I, Vijay D, et al. Can improved farm biosecurity reduce the need for antimicrobials in food animals? A scoping review. Antibiotics. 2023;12:893. doi: 10.3390/antibiotics12050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alagawany M, Elnesr SS, Farag MR, et al. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health – a comprehensive review. Vet Q. 2021;41:1–29. doi: 10.1080/01652176.2020.1857887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilli G, Laconi A, Galuppo F, Mughini-Gras L, Piccirillo A. Assessing biosecurity compliance in poultry farms: a survey in a densely populated poultry area in north east Italy. Animals. 2022;12:1409. doi: 10.3390/ani12111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abebe E, Gugsa G. A review on poultry coccidiosis. Abyssinia J Sci Technol. 2018;3:1–12. [Google Scholar]
- 28.Chapman HD. A landmark contribution to poultry science— Prophylactic control of coccidiosis in poultry. Poult Sci. 2009;88:813–5. doi: 10.3382/ps.2008-00316. [DOI] [PubMed] [Google Scholar]
- 29.Dembitsky VM. Natural polyether ionophores and their pharmacological profile. Mar Drugs. 2022;20:292. doi: 10.3390/md20050292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke L, Fodey TL, Crooks SRH, et al. A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci. 2014;97:358–74. doi: 10.1016/j.meatsci.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Muthamilselvan T, Kuo TF, Wu YC, Yang WC. Herbal remedies for coccidiosis control: A review of plants, compounds, and anticoccidial actions. Evid Based Complement Alternat Med. 2016;2016:2657981. doi: 10.1155/2016/2657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anthony EJ, Bolitho EM, Bridgewater HE, et al. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem Sci. 2020;11:12888–917. doi: 10.1039/D0SC04082G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller MA, Zachary JF. Mechanisms and morphology of cellular injury, adaptation, and death. Pathologic Basis of Veterinary Disease. Sixth Ed. St. Louis, MO, USA: Elsevier; 2017. [DOI] [Google Scholar]
- 34.Felici M, Tugnoli B, Piva A, Grilli E. In vitro assessment of anticoccidials: methods and molecules. Animals. 2021;11:1962. doi: 10.3390/ani11071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acharya KP, Acharya N. Alternatives to fight against coccidiosis: A review. Nepalese Vet J. 2017;34:152–67. doi: 10.3126/nvj.v34i0.22918. [DOI] [Google Scholar]
- 36.Roila R, Branciari R, Pecorelli I, et al. Occurrence and residue concentration of coccidiostats in feed and food of animal origin; human exposure assessment. Foods. 2019;8:477. doi: 10.3390/foods8100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelhady AY, El-Safty SA, Hashim M, et al. Comparative evaluation of single or combined anticoccidials on performance, antioxidant status, immune response, and intestinal architecture of broiler chickens challenged with mixed Eimeria species. Poult Sci. 2021;100:101162. doi: 10.1016/j.psj.2021.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kadykalo S, Roberts T, Thompson M, Wilson J, Lang M, Espeisse O. The value of anticoccidials for sustainable global poultry production. Int J Antimicrob Agents. 2018;51:304–10. doi: 10.1016/j.ijantimicag.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Shivaramaiah C, Barta JR, Hernandez VX, Tellez G, Hargis BM. Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these Apicomplexan parasites. Vet Med. 2014;5:23–34. doi: 10.2147/VMRR.S57839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee Y, Lu M, Lillehoj HS. Coccidiosis: Recent progress in host immunity and alternatives to antibiotic strategies. Vaccines. 2022;10:215. doi: 10.3390/vaccines10020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attree E, Sanchez AG, Jones M, et al. Controlling the causative agents of coccidiosis in domestic chickens; an eye on the past and considerations for the future. CABI Agric Biosci. 2021;2:37. doi: 10.1186/s43170-021-00056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams RB. Intercurrent coccidiosis and necrotic enteritis of chickens: rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–80. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]
- 43.Arain MA, Nabi F, Marghazani IB, et al. In ovo delivery of nutraceuticals improves health status and production performance of poultry birds: a review. World’s Poult Sci J. 2022;78:765–88. doi: 10.1080/00439339.2022.2091501. [DOI] [Google Scholar]
- 44.Lee SH, Lillehoj HS, Jang SI, et al. Effects of in ovo injection with selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickens. Poult Sci. 2014;93:1113–21. doi: 10.3382/ps.2013-03770. [DOI] [PubMed] [Google Scholar]
- 45.Stadnicka K, Bogucka J, Stanek M, et al. Injection of raffinose family oligosaccharides at 12 days of egg incubation modulates the gut development and resistance to opportunistic pathogens in broiler chickens. Animals. 2020;10:592. doi: 10.3390/ani10040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pender CM, Kim S, Potter TD, Ritzi MM, Young M, Dalloul RA. Effects of in ovo supplementation of probiotics on performance and immunocompetence of broiler chicks to an Eimeria challenge. Benef Microbes. 2016;7:699–705. doi: 10.3920/BM2016.0080. [DOI] [PubMed] [Google Scholar]
- 47.Sokale AO, Zhai W, Pote LM, Williams CJ, Peebles ED. Effects of coccidiosis vaccination administered by in ovo injection on Ross 708 broiler performance through 14 days of post-hatch age. Poult Sci. 2017;96:2546–51. doi: 10.3382/ps/pex041. [DOI] [PubMed] [Google Scholar]
- 48.Zaheer T, Abbas RZ, Imran M, et al. Vaccines against chicken coccidiosis with particular reference to previous decade: progress, challenges, and opportunities. Parasitol Res. 2022;121:2749–63. doi: 10.1007/s00436-022-07612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang H, Jiang Y, Zhou F, et al. A potential vaccine candidate towards chicken coccidiosis mediated by recombinant Lactobacillus plantarum with surface displayed EtMIC2 protein. Exp Parasitol. 2020;215:107901. doi: 10.1016/j.exppara.2020.107901. [DOI] [PubMed] [Google Scholar]
- 50.Yuan B, Sun Z, Lu M, et al. Immunization with pooled antigens for Clostridium perfringens conferred partial protection against experimental necrotic enteritis in broiler chickens. Vaccines. 2022;10:979. doi: 10.3390/vaccines10060979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blake DP, Marugan-Hernandez V, Tomley FM. Spotlight on avian pathology: Eimeria and the disease coccidiosis. Avian Pathol. 2021;50:209–13. doi: 10.1080/03079457.2021.1912288. [DOI] [PubMed] [Google Scholar]
- 52.Arczewska-Włosek A, Swiatkiewicz S, Ognik K, Jozefiak D. Effects of a dietary multi-strain probiotic and vaccination with a live anticoccidial vaccine on growth performance and haematological, biochemical and redox status indicators of broiler chickens. Animals. 2022;12:3489. doi: 10.3390/ani12243489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soutter F, Werling D, Tomley FM, Blake DP. Poultry coccidiosis: design and interpretation of vaccine studies. Front Vet Sci. 2020;7:101. doi: 10.3389/fvets.2020.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkatas J, Adeleke MA. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol Res. 2019;118:1701–10. doi: 10.1007/s00436-019-06338-2. [DOI] [PubMed] [Google Scholar]
- 55.Barbour E, Ayyash D, Iyer A, Harakeh S, Kumosani T. A review of approaches targeting the replacement of coccidiostat application in poultry production. Braz J Poult Sci. 2015;17:405–18. doi: 10.1590/1516-635x1704405-418. [DOI] [Google Scholar]
- 56.Chapman HD, Rathinam T. Focused review: the role of drug combinations for the control of coccidiosis in commercially reared chickens. Int J Parasitol Drugs Drug Resist. 2022;18:32–42. doi: 10.1016/j.ijpddr.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmad R, Yu YH, Hsiao FSH, et al. Probiotics as a friendly antibiotic alternative: assessment of their effects on the health and productive performance of poultry. Fermentation. 2022;8:672. doi: 10.3390/fermentation8120672. [DOI] [Google Scholar]
- 58.Abd El-Hack ME, El-Saadony MT, Salem HM, et al. Alternatives to antibiotics for organic poultry production: types, modes of action and impacts on bird’s health and production. Poult Sci. 2022;101:101696. doi: 10.1016/j.psj.2022.101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kovarovic J, Bystricka J, Vollmannova A, Toth T, Brindza J. Biologically valuable substances in garlic (Allium sativum L.) A review. J Cent Eur Agric. 2019;20:292–304. doi: 10.5513/JCEA01/20.1.2304. [DOI] [Google Scholar]
- 60.Elmahallawy EK, Fehaid A, El-Shewehy DM, et al. S-methylcysteine ameliorates the intestinal damage induced by Eimeria tenella infection via targeting oxidative stress and inflammatory modulators. Front Vet Sci. 2022;8:754991. doi: 10.3389/fvets.2021.754991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim DK, Lillehoj HS, Lee SH, Lillehoj EP, Bravo D. Improved resistance to Eimeria acervulina infection in chickens due to dietary supplementation with garlic metabolites. Br J Nutr. 2013;109:76–88. doi: 10.1017/S0007114512000530. [DOI] [PubMed] [Google Scholar]
- 62.Jang HJ, Lee HJ, Yoon DK, Ji DS, Kim JH, Lee CH. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci Biotechnol. 2018;27:219–25. doi: 10.1007/s10068-017-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhavaniramya S, Vishnupriya S, Al AMS, Vijayakumar R, Baskaran D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci Technol. 2019;2:49–55. doi: 10.1016/j.gaost.2019.03.001. [DOI] [Google Scholar]
- 64.Habibi H, Firouzi S, Nili H, Razavi M, Asadi SL, Daneshi S. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: in vitro and in vivo study. J Parasit Dis. 2016;40:401–7. doi: 10.1007/s12639-014-0517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christaki E, Florou PP, Giannenas I, Papazahariadou M, Botsoglou NA, Spais AB. Effect of a mixture of herbal extracts on broiler chickens infected with Eimeria tenella. Anim Res. 2004;53:137–44. doi: 10.1051/animres:2004006. [DOI] [Google Scholar]
- 66.Ali M, Chand N, Khan RU, Naz S, Gul S. Anticoccidial effect of garlic (Allium sativum) and ginger (Zingiber officinale) against experimentally induced coccidiosis in broiler chickens. J Appl Anim Res. 2019;47:79–84. doi: 10.1080/09712119.2019.1573731. [DOI] [Google Scholar]
- 67.Alnassan AA, Thabet A, Daugschies A, Bangoura B. In vitro efficacy of allicin on chicken Eimeria tenella sporozoites. Parasitol Res. 2015;114:3913–5. doi: 10.1007/s00436-015-4637-2. [DOI] [PubMed] [Google Scholar]
- 68.Ezeorba TPC, Chukwudozie KI, Ezema CA, Anaduaka EG, Nweze EJ, Okeke ES. Potentials for health and therapeutic benefits of garlic essential oils: Recent findings and future prospects. Pharmacol Res. 2022;3:100075. doi: 10.1016/j.prmcm.2022.100075. [DOI] [Google Scholar]
- 69.Gadelhaq SM, Arafa WM, Abolhadid SM. In vitro activity of natural and chemical products on sporulation of Eimeria species oocysts of chickens. Vet Parasitol. 2018;251:12–6. doi: 10.1016/j.vetpar.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 70.Coroian M, Pop LM, Popa V, et al. Efficacy of Artemisia annua against coccidiosis in broiler chickens: a field trial. Microorganisms. 2022;10:2277. doi: 10.3390/microorganisms10112277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong SC. Artemisia Annua, Artemisinin, ACTs, and malaria control in Africa: tradition, science, and public policy. By Dana G. Dalrymple. Washington DC, USA: Politics and Prose; 2012. J Econ Hist. 2014;74:304–6. doi: 10.1017/S0022050714000242. [DOI] [Google Scholar]
- 72.Cai TY, Zhang YR, Ji JB, Xing J. Investigation of the component in Artemisia annua L. leading to enhanced antiplasmodial potency of artemisinin via regulation of its metabolism. J Ethnopharmacol. 2017;207:86–91. doi: 10.1016/j.jep.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Zhang C, Gong M, Wang M. Combination of artemisinin-based natural compounds from Artemisia annua L. for the treatment of malaria: Pharmacodynamic and pharmacokinetic studies. Phytother Res. 2018;32:1415–20. doi: 10.1002/ptr.6077. [DOI] [PubMed] [Google Scholar]
- 74.Lang SJ, Schmiech M, Hafner S, et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine. 2019;62:152962. doi: 10.1016/j.phymed.2019.152962. [DOI] [PubMed] [Google Scholar]
- 75.De AGF, Horsted K, Thamsborg SM, Kyvsgaard NC, Ferreira JF, Hermansen JE. Use of Artemisia annua as a natural coccidiostat in free-range broilers and its effects on infection dynamics and performance. Vet Parasitol. 2012;186:178–87. doi: 10.1016/j.vetpar.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 76.Fatemi A, Asasi K, Razavi SM. Anticoccidial effects of Artemisia annua ethanolic extract: prevention, simultaneous challenge-medication, and treatment. Parasitol Res. 2017;116:2581–9. doi: 10.1007/s00436-017-5567-y. [DOI] [PubMed] [Google Scholar]
- 77.Fatemi A, Razavi SM, Asasi K, Goudarzi MT. Effects of Artemisia annua extracts on sporulation of Eimeria oocysts. Parasitol Res. 2015;114:1207–11. doi: 10.1007/s00436-014-4304-z. [DOI] [PubMed] [Google Scholar]
- 78.Del Cacho E, Gallego M, Francesch M, Quilez J, Sánchez-Acedo C. Effect of artemisinin on oocyst wall formation and sporulation during Eimeria tenella infection. Parasitol Int. 2010;59:506–11. doi: 10.1016/j.parint.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Jiao J, Yang Y, Liu M, et al. Artemisinin and Artemisia annua leaves alleviate Eimeria tenella infection by facilitating apoptosis of host cells and suppressing inflammatory response. Vet Parasitol. 2018;254:172–7. doi: 10.1016/j.vetpar.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 80.Mtenga DV, Ripanda AS. A review on the potential of underutilized Blackjack (Biden pilosa) naturally occurring in sub-Saharan Africa. Heliyon. 2022;8:e09586. doi: 10.1016/j.heliyon.2022.e09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uysal S, Ugurlu A, Zengin G, et al. Novel in vitro and in silico insights of the multi-biological activities and chemical composition of Bidens tripartita L. Food Chem Toxicol. 2018;111:525–36. doi: 10.1016/j.fct.2017.11.058. [DOI] [PubMed] [Google Scholar]
- 82.Chang CLT, Yang CY, Muthamilselvan T, Yang WC. Field trial of medicinal plant, Bidens pilosa, against eimeriosis in broilers. Sci Rep. 2016;6:24692. doi: 10.1038/srep24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang MT, Lin YX, Yang G, et al. Functional and mechanistic studies of two anti-coccidial herbs, Bidens pilosa and Artemisia indica. Planta Med. 2022;88:282–91. doi: 10.1055/a-1527-9715. [DOI] [PubMed] [Google Scholar]
- 84.Khater HF, Ziam H, Abbas A, et al. Avian coccidiosis: Recent advances in alternative control strategies and vaccine development. Agrobiol Rec. 2020;1:11–25. doi: 10.47278/journal.abr/2020.003. [DOI] [Google Scholar]
- 85.Memon FU, Yang Y, Lv F, et al. Effects of probiotic and Bidens pilosa on the performance and gut health of chicken during induced Eimeria tenella infection. J Appl Microbiol. 2021;131:425–34. doi: 10.1111/jam.14928. [DOI] [PubMed] [Google Scholar]
- 86.Bozkurt M, Ege G, Aysul N, et al. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poult Sci. 2016;95:1858–68. doi: 10.3382/ps/pew077. [DOI] [PubMed] [Google Scholar]
- 87.Abdelli N, Solà-Oriol D, Perez JF. Phytogenic feed additives in poultry: Achievements, prospective and challenges. Animals. 2021;11:3471. doi: 10.3390/ani11123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsinas A, Giannenas I, Voidarou C, Tzora A, Skoufos J. Effects of an oregano based dietary supplement on performance of broiler chickens experimentally infected with Eimeria acervulina and Eimeria maxima. J Poult Sci. 2011;48:194–200. doi: 10.2141/jpsa.010123. [DOI] [Google Scholar]
- 89.Mohiti-Asli M, Ghanaatparast-Rashti M. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev Vet Med. 2015;120:195–202. doi: 10.1016/j.prevetmed.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 90.Murakami AE, Eyng C, Torrent J. Effects of functional oils on coccidiosis and apparent metabolizable energy in broiler chickens. Asian-Australas J Anim Sci. 2014;27:981–9. doi: 10.5713/ajas.2013.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jeldi L, Taarabt KO, Mazri MA, Ouahmane L, Alfeddy MN. Chemical composition, antifungal and antioxidant activities of wild and cultivated Origanum compactum essential oils from the municipality of Chaoun, Morocco. S Afr J Bot. 2022;147:852–8. doi: 10.1016/j.sajb.2022.03.034. [DOI] [Google Scholar]
- 92.Gadde U, Kim WH, Oh ST, Lillehoj HS. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: a review. Anim Health Res Rev. 2017;18:26–45. doi: 10.1017/S1466252316000207. [DOI] [PubMed] [Google Scholar]
- 93.Assis RCL, Luns FD, Beletti ME, et al. Histomorphometry and macroscopic intestinal lesions in broilers infected with Eimeria acervulina. Vet Parasitol. 2010;168:185–9. doi: 10.1016/j.vetpar.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 94.Santos RR, Velkers FC, Vernooij JCM, et al. Nutritional interventions to support broiler chickens during Eimeria infection. Poult Sci. 2022;101:101853. doi: 10.1016/j.psj.2022.101853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lan Y, Xun S, Tamminga S, Williams B, Verstegen M, Erdi G. Real-time PCR detection of lactic acid bacteria in cecal contents of Eimeria tenella-lnfected broilers fed soybean oligosaccharides and soluble soybean polysaccharides. Poult Sci. 2004;83:1696–702. doi: 10.1093/ps/83.10.1696. [DOI] [PubMed] [Google Scholar]
- 96.Adhikari P, Kiess A, Adhikari R, Jha R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poult Res. 2020;29:515–34. doi: 10.1016/j.japr.2019.11.005. [DOI] [Google Scholar]
- 97.Chand N, Faheem H, Khan RU, Qureshi MS, Alhidary IA, Abudabos AM. Anticoccidial effect of mananoligosacharide against experimentally induced coccidiosis in broiler. Environ Sci Pollut Res. 2016;23:14414–21. doi: 10.1007/s11356-016-6600-x. [DOI] [PubMed] [Google Scholar]
- 98.McCann M, Newell E, Preston C, Forbes K. The use of mannan-oligosaccharides and/or tannin in broiler diets. Int J Poult Sci. 2006;5:873–9. doi: 10.3923/ijps.2006.873.879. [DOI] [Google Scholar]
- 99.Ritzi MM, Abdelrahman W, Mohnl M, Dalloul RA. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult Sci. 2014;93:2772–8. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- 100.Yin G, Lin Q, Wei W, et al. Protective immunity against Eimeria tenella infection in chickens induced by immunization with a recombinant C-terminal derivative of EtIMP1. Vet Immunol Immunopathol. 2014;162:117–21. doi: 10.1016/j.vetimm.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Farnell YZ, Kiess AS, Peebles ED, Wamsley KG, Zhai W. Effects of Bacillus subtilis and coccidial vaccination on cecal microbial diversity and composition of Eimeria-challenged male broilers. Poult Sci. 2019;98:3839–49. doi: 10.3382/ps/pez096. [DOI] [PubMed] [Google Scholar]
- 102.Lee SH, Lillehoj HS, Dalloul RA, Park DW, Hong YH, Lin JJ. Influence of Pediococcus-based probiotic on coccidiosis in broiler chickens. Poult Sci. 2007;86:63–6. doi: 10.1093/ps/86.1.63. [DOI] [PubMed] [Google Scholar]
- 103.Castro FLS, Tompkins YH, Pazdro R, Kim WK. The effects of total sulfur amino acids on the intestinal health status of broilers challenged with Eimeria spp. Poult Sci. 2020;99:5027–36. doi: 10.1016/j.psj.2020.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teng PY, Choi J, Tompkins Y, Lillehoj H, Kim W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet Res. 2021;52:81. doi: 10.1186/s13567-021-00949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jankowski J, Kubinska M, Juskiewicz J, Czech A, Ognik K, Zdunczyk Z. Effect of different dietary methionine levels on the growth performance and tissue redox parameters of turkeys. Poult Sci. 2017;96:1235–43. doi: 10.3382/ps/pew383. [DOI] [PubMed] [Google Scholar]
- 106.Duangnumsawang Y, Zentek J, Boroojeni FG. Development and functional properties of intestinal mucus layer in poultry. Front Immunol. 2021;12:745849. doi: 10.3389/fimmu.2021.745849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Q, Chen X, Eicher SD, Ajuwon KM, Applegate TJ. Effect of threonine deficiency on intestinal integrity and immune response to feed withdrawal combined with coccidial vaccine challenge in broiler chicks. Br J Nutr. 2016;116:2030–43. doi: 10.1017/S0007114516003238. [DOI] [PubMed] [Google Scholar]
- 108.Chen YP, Cheng YF, Li XH, et al. Effects of threonine supplementation on the growth performance, immunity, oxidative status, intestinal integrity, and barrier function of broilers at the early age. Poult Sci. 2017;96:405–13. doi: 10.3382/ps/pew240. [DOI] [PubMed] [Google Scholar]
- 109.Sarwar MS, Zhang HJ, Tsang SW. Perspectives of plant natural products in inhibition of cancer invasion and metastasis by regulating multiple signaling pathways. Curr Med Chem. 2018;25:5057–87. doi: 10.2174/0929867324666170918123413. [DOI] [PubMed] [Google Scholar]
- 110.Quinteros JA, Scott PC, Wilson TB, et al. Isoquinoline alkaloids induce partial protection of laying hens from the impact of Campylobacter hepaticus (spotty liver disease) challenge. Poult Sci. 2021;100:101423. doi: 10.1016/j.psj.2021.101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li JY, Huang HB, Pan TX, et al. Sanguinarine induces apoptosis in Eimeria tenella sporozoites via the generation of reactive oxygen species. Poult Sci. 2022;101:101771. doi: 10.1016/j.psj.2022.101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu X, Yi H, Wu J, et al. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed Pharmacother. 2021;133:110984. doi: 10.1016/j.biopha.2020.110984. [DOI] [PubMed] [Google Scholar]
- 113.Zhu C, Huang K, Bai Y, et al. Dietary supplementation with berberine improves growth performance and modulates the composition and function of cecal microbiota in yellow-feathered broilers. Poult Sci. 2021;100:1034–48. doi: 10.1016/j.psj.2020.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuan L, Li M, Qiao Y, Wang H, Cui L, Wang M. The impact of berberine on intestinal morphology, microbes, and immune function of broilers in response to necrotic enteritis challenge. BioMed Res Int. 2021;2021:1877075. doi: 10.1155/2021/1877075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen BT, Flores RA, Cammayo PLT, Kim S, Kim WH, Min W. Anticoccidial activity of berberine against Eimeria-infected chickens. Korean J Parasitol. 2021;59:403–8. doi: 10.3347/kjp.2021.59.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang Q, Liu X, Zhao G, Hu T, Wang Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 2018;4:137–50. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mahlake SK, Mnisi CM, Kumanda C, Mthiyane DMN, Montso PK. Green tea (Camellia sinensis) products as alternatives to antibiotics in poultry nutrition: A Review. Antibiotics. 2022;11:565. doi: 10.3390/antibiotics11050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tonda RM, Rubach JK, Lumpkins BS, Mathis GF, Poss MJ. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poult Sci. 2018;97:3031–42. doi: 10.3382/ps/pey158. [DOI] [PubMed] [Google Scholar]
- 119.Wang M, Suo X, Gu J, Zhang W, Fang Q, Wang X. Influence of grape seed proanthocyanidin extract in broiler chickens: effect on chicken coccidiosis and antioxidant status. Poult Sci. 2008;87:2273–80. doi: 10.3382/ps.2008-00077. [DOI] [PubMed] [Google Scholar]
- 120.Choi J, Tompkins YH, Teng PY, Gogal RM, Jr, Kim WK. Effects of tannic acid supplementation on growth performance, oocyst shedding, and gut health of in broilers infected with Eimeria maxima. Animals. 2022;12:1378. doi: 10.3390/ani12111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lako JDW, Sube KLL, Lumori CSG, et al. Diversity and distribution of medicinal plants in the republic of South Sudan. World J Adv Res Rev. 2020;7:18–31. doi: 10.30574/wjarr.2020.7.1.0165. [DOI] [Google Scholar]
- 122.Batiha GES, Akhtar N, Alsayegh AA, et al. Bioactive compounds, pharmacological actions, and pharmacokinetics of genus Acacia. Molecules. 2022;27:7340. doi: 10.3390/molecules27217340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ahmed E, Galal M, Abdelmageed N, et al. An in vitro evaluation of the inhibitory effects of an aqueous extract of Acacia nilotica on Eimeria tenella. SVU-Int J Vet Sci. 2022;5:33–40. [Google Scholar]
- 124.Cheng YH, Horng YB, Dybus A, Yu YH. Bacillus licheniformis-Fermented Products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. J Poult Sci. 2021;58:30–9. doi: 10.2141/jpsa.0200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee TY, Lee YS, Yeh RH, Chen KH, Chen KL. Bacillus amyloliquefaciens CU33 fermented feather meal-soybean meal product improves the intestinal morphology to promote the growth performance of broilers. Poult Sci. 2022;101:102027. doi: 10.1016/j.psj.2022.102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng YH, Horng YB, Chen WJ, et al. Development and validation the efficacy of Bacillus-based fermented products as an antibiotics alternative in domestic animals. Acta Sci Pol Zootech. 2021;20:23–34. doi: 10.21005/asp.2021.20.3.03. [DOI] [Google Scholar]
- 127.Cheng YH, Horng YB, Chen WJ, Hua KF, Dybus A, Yu YH. Effect of fermented products produced by Bacillus licheniformis on the growth performance and cecal microbial community of broilers under coccidial challenge. Animals. 2021;11:1245. doi: 10.3390/ani11051245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu YH, Wu CM, Chen WJ, Hua KF, Liu JR, Cheng YH. Effectiveness of Bacillus licheniformis-fermented products and their derived antimicrobial lipopeptides in controlling coccidiosis in broilers. Animals. 2021;11:3576. doi: 10.3390/ani11123576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng YH, Zhang N, Han JC, Chang CW, Hsiao FSH, Yu YH. Optimization of surfactin production from Bacillus subtilis in fermentation and its effects on Clostridium perfringens-induced necrotic enteritis and growth performance in broilers. J Anim Physiol Anim Nutr. 2018;102:1232–44. doi: 10.1111/jpn.12937. [DOI] [PubMed] [Google Scholar]