Abstract
Most of the adenine residues in GATC sequences in the Escherichia coli chromosome are methylated by the enzyme deoxyadenosine methyltransferase (Dam). However, at least 20 GATC sequences remain nonmethylated throughout the cell cycle. Here we examined how the DNA methylation patterns of GATC sequences within the regulatory regions of the pyelonephritis-associated pilus (pap) operon and the glucitol utilization (gut) operon were formed. The results obtained with an in vitro methylation protection assay showed that the addition of the leucine-responsive regulatory protein (Lrp) to pap DNA was sufficient to protect the two GATC sequences in the pap regulatory region, GATC-I and GATC-II, from methylation by Dam. This finding was consistent with previously published data showing that Lrp was essential for methylation protection of these DNA sites in vivo. Methylation protection also occurred at a GATC site (GATC-44.5) centered 44.5 bp upstream of the transcription start site of the gutABD operon. Two proteins, GutR and the catabolite gene activator protein (CAP), bound to DNA sites overlapping the GATC-44.5-containing region of the gutABD operon. GutR, an operon-specific repressor, was essential for methylation protection in vivo, and binding of GutR protected GATC-44.5 from methylation in vitro. In contrast, binding of CAP at a site overlapping GATC-44.5 did not protect this site from methylation. Mutational analyses indicated that gutABD gene regulation was not controlled by methylation of GATC-44.5, in contrast to regulation of Pap pilus expression, which is directly controlled by methylation of the pap GATC-I and GATC-II sites.
In Escherichia coli, the enzyme deoxyadenosine methyltransferase (Dam) methylates the adenine residue of DNA GATC sequences. This methylation is important in various cellular processes, including methyl-directed mismatch repair and the timing of the initiation of chromosome replication (16). These processes are regulated by the hemimethylation of the DNA that occurs following the synthesis of a new DNA strand after passage of the replication fork. Hemimethylated GATC sequences are substrates for Dam, and the nonmethylated strand becomes methylated during the remainder of the cell cycle. However, chromosome analysis has shown that at least 20 GATC sequences are maintained in a nonmethylated state on both DNA strands throughout the cell cycle, establishing methylation patterns on the chromosome (4, 8, 10, 21, 30). The methylation patterns on the chromosome can be modulated by growth media, growth phase, and the presence of the global regulator leucine-responsive regulatory protein (Lrp) (10, 21). DNA methylation patterns are thought to be formed by the binding of regulatory proteins, such as Lrp, at GATC sites or by natural or protein-induced DNA conformational changes near GATC sites (5, 8, 9, 13).
Some of the nonmethylated GATC sequences in the E. coli chromosome are present in the regulatory regions of operons involved in metabolic processes (10, 30). Nonmethylated GATC sites are also present in the regulatory regions of the fimbrial operons pap, daa, and sfa of pathogenic E. coli. The pap operon has been examined in greatest detail (27), but the sfa and daa operons appear to be regulated by a similar Dam-dependent mechanism (29). Pap phase variation, which is a reversible switch between pilus expression and nonexpression states, requires differential methylation of two GATC sequences, denoted GATC-I and GATC-II, in the pap upstream regulatory region (6). In cells expressing pili (phase-on transcription state), GATC-I is nonmethylated, whereas in cells not expressing pili (phase-off transcription state), GATC-II is nonmethylated. Both the GATC-I and the GATC-II sequences are fully methylated in an lrp null mutant at 37°C (5), showing that Lrp is required to block methylation of these GATC sequences. Moreover, the pap GATC-I and GATC-II sequences are present within two different sets of binding sites for Lrp, designated sites 4 and 5 and sites 1, 2, and 3, respectively (18). Analysis of Lrp binding in vitro has indicated that Lrp has the highest affinity for sites 1, 2, and 3, which overlap the papBAp promoter. We hypothesized that binding of Lrp to pap sites 1, 2, and 3 in vivo blocks methylation of the GATC-II site and forms the phase-off DNA methylation pattern (18, 27).
The switch from the phase-off to the phase-on Pap pilus expression state requires the pap-encoded coregulatory protein PapI (6, 14). In addition, PapI is required for methylation protection of the GATC-I site (19), consistent with genetic evidence indicating that binding of Lrp to pap sites 4 and 5 near GATC-I is essential for transcription activation (18). PapI binds to the Lrp moiety of Lrp-pap DNA complexes and increases the affinity of Lrp for pap sites 4 and 5 (14). These findings are consistent with the hypothesis that binding of Lrp-PapI to pap DNA sites 4 and 5 blocks methylation of GATC-I and forms the phase-on DNA methylation pattern.
Our understanding of the role of DNA methylation in Pap phase variation comes from the analysis of mutant pap DNA sequences containing altered GATC sites. Mutation of GATC-I to GCTC resulted in a uniform phase-on phenotype (locked-on), whereas mutation of GATC-II to GCTC resulted in a uniform phase-off phenotype (locked-off) (6). The effects of these mutations appeared to be due to inhibition of GATC methylation, since the affinities of Lrp and Lrp-PapI for pap mutant DNA were not significantly altered (6). These results indicated that methylation of GATC-I blocks pap transcription, whereas methylation of GATC-II is essential for pap transcription. In vitro DNA binding studies showed that the affinity of Lrp-PapI for pap sites 4 and 5 was reduced by methylation of GATC-I (19). Based on these data, we hypothesized that methylation of GATC-I locks cells in the phase-off state by preventing binding of Lrp-PapI to sites 4 and 5 (27). The switch from phase off to phase on therefore would require DNA replication to generate a hemimethylated GATC-I site, allowing binding of Lrp-PapI (6, 19). The mechanism by which methylation of GATC-II stimulates pap transcription is not known but may involve a reduction in the affinity of Lrp for pap sites 1, 2, and 3. Since these Lrp binding sites overlap the papBAp promoter, binding of RNA polymerase could occur.
An essential element of the Pap phase variation model is that the DNA methylation patterns in pap are a result of competition between Lrp and Dam for GATC sequences. This idea implies not only that methylation affects Lrp binding but also that Lrp binding at a GATC-containing region blocks Dam-dependent methylation of GATC sequences. Here we use an in vitro methylation protection assay to test this latter hypothesis. We show that Lrp binding at pap is indeed sufficient for the formation of the observed methylation patterns in the pap regulatory region.
Another nonmethylated GATC sequence in the E. coli chromosome is located 44.5 bp upstream of the transcription start site of the gutABD (glucitol utilization) operon (designated here as GATC-44.5) (3, 10, 30). Regulation of gutABD transcription is complex, involving repression by GutR, an operon-specific repressor, and induction by the activators GutM and cyclic AMP (cAMP)-catabolite gene activator protein (CAP) (33, 34). The DNA region containing GATC-44.5 has homology to the CAP consensus binding site (15, 30), a fact which led to the hypothesis that CAP binding contributes to methylation protection of gut GATC-44.5 (30). Here we show that CAP binds at the GATC-44.5-containing region but that CAP binding does not protect this GATC sequence from methylation by Dam. Instead, binding of GutR at the GATC-44.5-containing region establishes the observed methylation patterns both in vitro and in vivo.
MATERIALS AND METHODS
Strains and plasmids.
Luria-Bertani (LB) broth, LB agar, M9 minimal broth, and M9 minimal agar were prepared as described previously with glycerol, glucose, or d-glucitol (sorbitol) at 0.2% (wt/vol) as a carbon source for minimal media (22, 24). Antibiotics, when used, were added at the following final concentrations: ampicillin, 100 μg/ml; kanamycin, 25 μg/ml; tetracycline, 15 μg/ml; and chloramphenicol, 34 μg/ml.
All strains, plasmids, and phages are listed in Table 1. Transductions and transformations were performed as described previously (11, 17). The numbering for the gutABD operon sites was that used by Yamada and Saier (32). All gutA′-lacZ fusions were placed in a single copy on the chromosome of MC4100 as a lambda prophage as described previously (25). DL1882 contains a gutA′-lacZ operon fusion extending from bp −487 to +115 relative to the gutABD promoter. DL2453 contains the same gutA′-lacZ operon fusion as DL1882, but the GATC-44.5 site was mutated to GATT by a C-to-T mutation. This point mutation was made by sequential PCR as described previously (2). The gutABD regulatory region was sequenced to confirm that no other mutations were introduced by this procedure.
TABLE 1.
Strains, plasmids, and phages used in this study
| Strain, phage, or plasmid | Description | Reference or source |
|---|---|---|
| E. coli strain | ||
| MC4100 | recA+ F−araD ΔlacU169 strA relA mot | 7 |
| DL1882 | MC4100 λRS402 lysogen | This study |
| DL2453 | MC4100 λRS459 lysogen | This study |
| DL2583 | MC4100 Δlrp(pYZCRP) | This study; 28, 35 |
| DL2803 | DL1882 gutR::ΩCm | This study |
| DL2884 | GI1724(pDAL496) | Invitrogen; this study |
| JC7623 | recB21 recC22 sbcB15 sbcC201 | 20 |
| Phages | ||
| λRS45 | amp-lacZYA imm21 | 25 |
| λRS402 | λRS45 pDAL402 recombinant phage | This study |
| λRS459 | λRS45 pDAL459 recombinant phage | This study |
| Plasmids | ||
| pRS550 | bla-kan-lacZYA pMB1 replicon | 25 |
| pDAL402 | pRS550 with gutABD regulatory region (bp −487 to +115) containing GATC-44.5 | This study |
| pDAL459 | pRS550 with gutABD regulatory region (bp −487 to +115) containing GATT-44.5 | This study |
| pDAL483 | pT7-6 containing a 288-bp fragment of gutR | This study |
| pDAL484 | pDAL483 (gutR::ΩCm) | This study |
| pLex | Vector with tryptophan-inducible promoter; ColE1 replicon; Ampr | Invitrogen |
| pDAL496 | pLex containing a 781-bp gutR coding fragment | This study |
| pYZCRP | Plasmid containing a 633-bp crp coding fragment; Ampr | 35 |
To obtain the gutR isolate DL2803, a 288-bp BamHI-EcoRI DNA fragment containing the 5′ end of gutR was cloned into plasmid pT7-6, yielding plasmid pDAL483. The gutR fragment was obtained by PCR with chromosomal DNA from MC4100 as a template and with the primers 5′-CGGGATCCAAACCTAAATCAGGTAATCACG-3′ and 5′-GGAATTCAAGACTGCAAACTACGTTGG-3′. A DNA fragment encoding chloramphenicol resistance from plasmid pBSL119 with BamHI ends was cloned into the BglII site of the gutR gene in pDAL483 (1). The resulting plasmid, pDAL484, was electroporated into E. coli JC7623 (20). A colony that was ampicillin sensitive and chloramphenicol resistant was chosen, and chloramphenicol resistance was transduced into DL1882 by use of phage P1L4 to construct DL2803. In this isolate, the insertion of the fragment in gutR was verified by Southern blot analysis (data not shown).
Overproduction and purification of proteins.
Highly purified Lrp was a gift from Felix Vajdos and Chris Hill (University of Utah, Salt Lake City).
CAP was purified from E. coli DL2583 by affinity chromatography on a CNBr-activated Sepharose 4B-cAMP column in accordance with a protocol used by M. G. Fried. CAP was further purified by cation exchange on Bio-Rex 70 (Bio-Rad). CAP was at least 95% pure, as judged by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and staining with SyPro Orange (Molecular Probes).
GutR-containing extracts were prepared from DL2884 containing the GutR-expressing plasmid pDAL496. pDAL496 was derived by cloning a 781-bp NdeI-EcoRI PCR fragment containing gutR into pLex (Invitrogen) with the forward primer 5′-GGAATTCCATATGAAACCTCGTCAGCGT-3′ and the reverse primer 5′-GGAATTCTCACTCATTGCTCTCTCCGGT-3′. GutR was partially purified from DL2884 by heparin-agarose affinity chromatography as described previously (12). Fractions were analyzed for GutR activity by a gel mobility shift assay with the gutABD regulatory region as a probe. The fraction containing the highest activity was stored at −70°C in 20% glycerol–0.1 mg of bovine serum albumin per ml. This fraction was judged to be 85% pure by SDS-PAGE.
EMSA and DNase I footprint analysis.
Electrophoretic mobility shift assays (EMSA) and DNase I footprinting were performed as described previously (6, 19). EMSA and DNase I footprinting with CAP were performed with CAP binding buffer (40 mM Tris-HCl [pH 7.5], 0.1 mM EDTA, 1 mM dithiothreitol, 60 mM KCl, 100 mM NaCl, 3 μg of herring sperm DNA, 1 μg of acetylated bovine serum albumin, 0.2 mM cAMP). DNA binding reactions for GutR or Lrp were carried out with Lrp binding buffer (60 mM Tris-HCl [pH 7.5], 40 mM KCl, 100 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol) (19). The DNA probe for analysis of gut DNA consisted of a 331-bp fragment extending from bp −206 to +115 relative to the gutABD transcription start site.
In vitro DNA methylation protection assay.
DNA binding assays were set up as described above with a 20-μl volume of Lrp binding buffer for Lrp and GutR and CAP binding buffer for CAP. 32P-labeled DNA fragments containing the gutABD (bp −206 to +115) or pap (−278 to +49) regulatory regions were used for analysis. Purified Lrp, CAP, or GutR was added to binding reaction mixtures and incubated for 20 min at 37°C. Dam (4 U; New England BioLabs) and S-adenosylmethionine (0.1 mM) were added, followed by 1 h of incubation at 37°C. To determine the levels of protein-bound and free DNAs, 10 μl of the reaction mixtures was used in an EMSA (19). The remainder of the reaction mixtures was heated at 65°C for 10 min to denature and release the bound proteins and then was digested with MboI. DNA restriction fragments were analyzed on a 4% acrylamide gel to determine the methylation states of the GATC sequences in the gut and pap DNA probes.
Analysis of DNA methylation patterns.
The methylation state of GATC-44.5 in the gutABD regulatory region was analyzed by Southern blotting as described previously (29). Chromosomal DNA was cut with the enzymes BamHI, Acc65I, and MboI as indicated in the legend to Fig. 2. Southern blots were probed with a DNA fragment that contained the gutABD regulatory region from bp −206 to +115 and that was labeled with a random primed DNA labeling kit (Boehringer Mannheim Biochemicals). Blots were quantified with the Phosphoanalyst program and a GS-250 Molecular Imager (Bio-Rad).
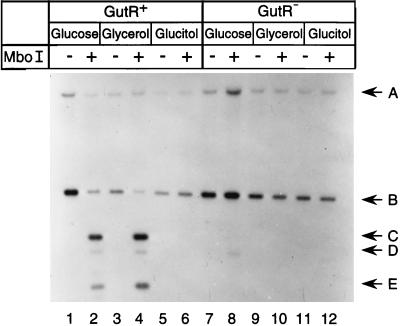
FIG. 2.
DNA methylation pattern analyses. The DNA methylation states of GATC-44.5 of the gutABD operon in isolate DL1882 (lanes 1 to 6) and isogenic gutR isolate DL2803 (lanes 7 to 12) were examined as described in Materials and Methods. Odd-numbered lanes show DNAs digested with BamHI and Acc65I, and even-numbered lanes show DNAs digested with BamHI, Acc65I, and MboI. DNA was isolated from cultures grown with glucose (lanes 1, 2, 7, and 8), glycerol (lanes 3, 4, 9, and 10), or glucitol (lanes 5, 6, 11, and 12) in the logarithmic phase of growth. DNA fragments A and E were from the gutA′-lacZ fusion, and DNA fragments B, C, and D were from the wild-type gutABD operon.
β-Galactosidase assays.
β-Galactosidase activity was measured as previously described with bacterial cultures in the logarithmic growth phase (17).
RESULTS
Methylation pattern formation in the papBA operon.
The global regulator Lrp is necessary for the in vivo formation of specific DNA methylation patterns at the pap GATC-I and GATC-II sites. Previous work showed that Lrp binds at the pap GATC sequences in vitro (5, 18, 19). To determine if Lrp binding was sufficient to protect these GATC sequences from methylation in vitro, Lrp was incubated with nonmethylated pap regulatory DNA containing GATC-I and GATC-II for 20 min, followed by the addition of Dam and the methyl group donor S-adenosyl-l-methionine to methylate accessible GATC sites (see Materials and Methods). The binding of Lrp to pap DNA was measured by a DNA mobility shift assay, and the methylation states of the pap GATC-I and GATC-II sites were analyzed by restriction enzyme analysis with MboI, which digests only nonmethylated GATC sites. The results showed a converse relationship between the fraction of methylated pap DNA and the fraction of DNA bound by Lrp (Fig. 1A). Under the conditions used here, about 70% of the pap GATC sites were methylated in the absence of Lrp. At Lrp levels at which binding saturation occurred (>40 nM), almost all of the pap GATC-I and GATC-II sites were protected from methylation (Fig. 1A). These results indicated that the binding of Lrp at its target site in the pap regulatory region directly blocked methylation of the GATC-I and GATC-II sites. This methylation protection assay was also performed with purified CAP, which binds pap DNA about 60 bp upstream of the GATC-I sequence but does not contain a GATC sequence in its target site in the pap regulatory region. Even under conditions in which 100% of pap DNA was bound by CAP, methylation of the pap GATC-I and GATC-II sites was not reduced (results not shown), indicating that the Lrp-mediated methylation protection of the GATC-I and GATC-II sites in pap was specific. Together, these results showed that Lrp binding was necessary and sufficient to protect the GATC sequences in the pap regulatory region from methylation by Dam.
FIG. 1.
In vitro methylation protection. Squares represent the percentage of DNA in a complex with protein, as determined by EMSA, and circles represent the percentage of methylated DNA, as determined by an in vitro methylation protection assay (see Materials and Methods). (A) Data obtained with pap regulatory region DNA and purified Lrp. (B) Data obtained with gutABD regulatory region DNA and CAP. (C) Data obtained with gutABD regulatory region DNA and GutR.
Methylation pattern formation in the gutABD operon. (i) GutR is required for methylation protection of GATC-44.5 in vivo.
Binding of the regulatory protein Lrp at its target sites in pap resulted in methylation protection of GATC sequences located in the Lrp binding sites (see above). To determine if the previously identified DNA binding proteins involved in gutABD regulation, GutM, GutR, and cAMP-CAP, contribute to methylation protection of GATC-44.5 in gut, we examined the methylation state of this GATC sequence in vivo under growth conditions that were expected to result in transcriptional activation or repression of this operon. For this analysis, merodiploid E. coli DL1882 containing one wild-type gutABD operon as well as a single copy of the gutA′-lacZ fusion was used (Table 1). The β-galactosidase levels of this isolate grown in minimal medium cultures with glycerol, glucose, or glucitol as a carbon source (Table 2) were consistent with the regulation of gutABD transcription by catabolite repression and induction by the addition of glucitol (15, 34). The in vivo methylation states of GATC-44.5 in both copies of the regulatory region of gutABD were examined by Southern blot analyses with MboI, which digests only nonmethylated GATC sites (Fig. 2, lanes 1 to 6). Digestion of chromosomal DNA with BamHI and Acc65I yielded DNA fragments A and B, containing the regulatory regions of the gutA′-lacZ fusion and the wild-type gutABD operon, respectively (Fig. 2, lanes 1, 3, and 5). DNA fragments C, D, and E, resulting from digestion of nonmethylated GATC-44.5 sites in the gutABD regulatory regions, were present in glycerol- and glucose-grown cells (Fig. 2, lanes 2 and 4). In contrast, these DNA fragments were absent in cells grown in glucitol (Fig. 2, lane 6), indicating that under these conditions no methylation protection of GATC-44.5 occurred. Quantitation revealed 45% methylation protection of GATC-44.5 in cells grown in glucose, 63% in glycerol, and 0% in glucitol.
TABLE 2.
Transcription of chromosomal gutA′-lacZ fusions during growth with various carbon sourcesa
| Strain | Relevant genotype | Carbon source | β-Galactosidase activity (Miller units)b |
|---|---|---|---|
| DL1882 | gutA′ (GATC)-lacZ | Glucose | 89 ± 4.3 |
| Glycerol | 435 ± 27 | ||
| Glucitol | 4,159 ± 108 | ||
| DL2803 | gutA′ (GATC)-lacZ gutR | Glucose | 953 ± 127 |
| Glycerol | 4,289 ± 145 | ||
| Glucitol | 3,006 ± 197 | ||
| DL2453 | gutA′ (GATT)-lacZ | Glucose | 47 ± 3 |
| Glycerol | 202 ± 8.1 | ||
| Glucitol | 2,300 ± 80 |
The β-galactosidase level was measured in gutR+ isolate DL1882, gutR isolate DL2803, and DL2453, containing a GATT substitution at GATC-44.5. Cultures were grown with glucose, glycerol, or glucitol as the sole carbon source.
Error is expressed as the standard deviation of the mean for at least two independent cultures with three replicates each.
The results described above showed that methylation protection of GATC-44.5 occurred when gutABD transcription was repressed. We therefore examined the role of the operon-specific repressor GutR in methylation protection of GATC-44.5 (34). Compared to that in DL1882 (gutA′-lacZ gutR+), the levels of transcription of the gutA′-lacZ fusion in the isogenic gutR isolate DL2803 were about 11-fold higher in glucose-grown cells and 10-fold higher in glycerol-grown cells (Table 2). These data confirmed that GutR was a repressor of the gutABD operon (34). The levels of transcription were the same for the gutR and gutR+ isolates in glucitol-grown cultures, in agreement with the hypothesis that the induction of the gutABD operon by glucitol occurs by relief of GutR-mediated repression. The lower level of transcription of gutR isolate DL2803 in a glucose-grown culture compared to a glucitol- or glycerol-grown culture presumably reflects a lack of cAMP-CAP-dependent activation due to the lower levels of cAMP in the rich carbon source glucose.
To determine the role of GutR in methylation protection, the in vivo methylation states of GATC-44.5 in the two copies of the gutABD regulatory region in the gutR isolate DL2803 were examined (Fig. 2, lanes 7 to 12) and compared to those in the isogenic gutR+ isolate DL1882. The results showed that in glucitol-grown cultures, the GATC-44.5 sites were methylated in the gutR isolate DL2803, as they were in the gutR+ isolate DL1882 (Fig. 2, lanes 6 and 12). However, in contrast to the methylation protection observed for glucose- as well as glycerol-grown cells of DL1882 (gutR+) (Fig. 2, lanes 2 and 4), no methylation protection was observed for DL2803 (gutR) with either glucose or glycerol as the carbon source (Fig. 2, lanes 8 and 10). These data indicated that GutR was required for methylation protection of GATC-44.5 in the gutABD regulatory region in vivo.
(ii) CAP and GutR bind at the region containing GATC-44.5.
Our in vivo analysis of methylation protection of GATC-44.5 in the gutABD regulatory region indicated that GutR was required for methylation protection and that CAP did not contribute to methylation protection of this site. However, previous data obtained with a crp isolate suggested that CAP binding might contribute to methylation protection of GATC-44.5 (30). To address the putative role of these regulatory proteins in methylation protection, we first determined the DNA sequences within the gutABD regulatory region that were bound by CAP and GutR by using DNase I footprint analysis. The results obtained with the top (coding) DNA strand are shown in Fig. 3. CAP footprinted gutABD regulatory DNA from bp −49 to −30 (Fig. 3A). This region includes the GATC-44.5 sequence and overlaps the putative CAP binding site, which was determined by sequence homology with the CAP consensus binding site (30, 32).
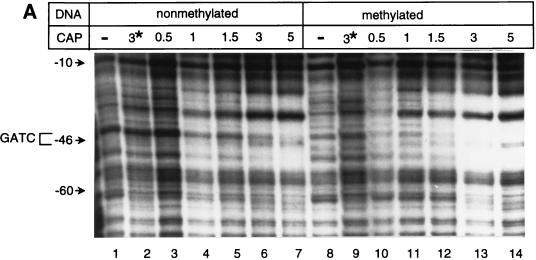
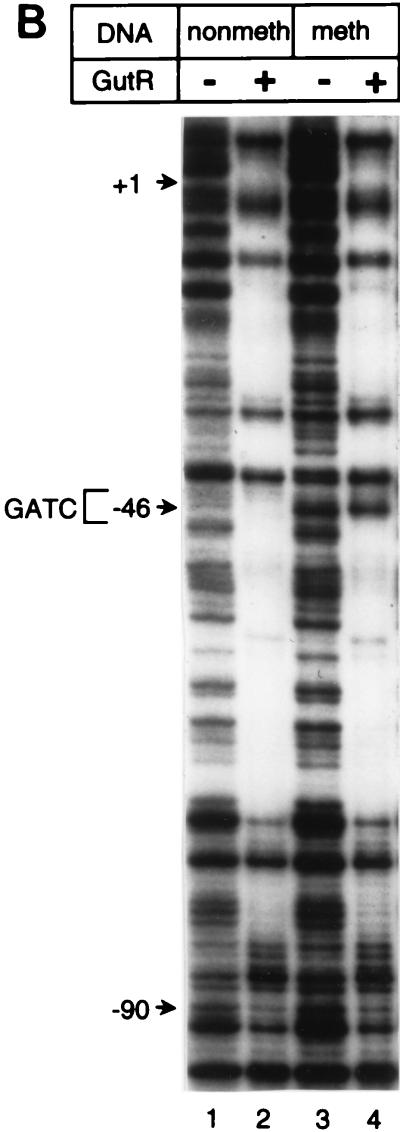
FIG. 3.
DNase I footprint analyses. (A and B) The DNase I footprint of the top strand of gutABD regulatory region DNA with CAP (A) or GutR (B) is shown. Results obtained with nonmethylated gutABD regulatory region DNA (A, lanes 1 to 7; B, lanes 1 and 2) and methylated gutABD regulatory region DNA (A, lanes 8 to 14; B, lanes 3 and 4) are shown. In panel A, the relative levels of CAP added are shown, with 1 being equal to 280 nM. In panel B, a level of GutR sufficient for binding saturation was used. In panel A, lanes 2 and 9, the asterisk indicates that the reactions took place in the presence of 840 nM CAP but in the absence of cAMP. (C) Protection by GutR and CAP of the top DNA strand. Open circles below a base designate DNase I-hypersensitive sites, and thin lines designate protection from DNase I digestion. The asterisk indicates the base (G−46) that was sensitive in methylated DNA but not in nonmethylated DNA. The GATC-44.5 sequence is boxed, and the predicted CAP binding site is underlined with a thick line (30). The numbering of the gut DNA is that of Yamada and Saier (32).
The DNase I footprint of GutR extended from 92 bp upstream of the gutABD transcription start site into the gutA coding sequence (Fig. 3B, lanes 2 and 4). Quantitative DNase I footprinting showed that GutR had the highest affinity for gutABD regulatory DNA from bp −92 to +12, a region which included GATC-44.5 (results not shown). Although GutR footprinted the same stretch of the gutABD regulatory region regardless of the methylation state of the DNA, the “G” of the GATC sequence was hypersensitive only with methylated DNA. DNase I footprinting of the bottom DNA strand yielded similar results (data not shown). In summary, both GutR and CAP bound the gutABD regulatory region DNA which includes GATC-44.5, raising the possibility that each of these proteins contributes to methylation protection of GATC-44.5.
(iii) GutR but not CAP blocks the methylation of GATC-44.5 in vitro.
The abilities of CAP and GutR to protect GATC-44.5 from Dam methylation were determined by use of the in vitro methylation protection assay. The addition of CAP to gut DNA resulted in binding saturation by 250 nM CAP and a Kd of about 70 nM. Even at saturating levels of CAP, the gut GATC-44.5 site was fully methylated (between 96 and 100% in this experiment; Fig. 1B). Thus, CAP binding at the sequence overlapping the GATC-44.5 site did not prevent Dam from methylating this site. In contrast, the addition of GutR to gut regulatory DNA resulted in a decrease in the methylation of GATC-44.5 concomitant with increased binding of GutR to gut DNA (Fig. 1C). These data indicated that binding of GutR at GATC-44.5 protected this site from methylation by Dam.
(iv) Methylation of GATC-44.5 does not control gutABD gene regulation.
The methylation states of the GATC-I and GATC-II sites in pap regulatory DNA directly control transcription by inhibiting the binding of the global regulator Lrp (6). We examined whether the transcription of the gutABD operon in vivo was regulated by the methylation state of GATC-44.5 by altering the GATC sequence to GATT in the gutA′-lac fusion (DL2453), rendering this site permanently nonmethylated. This C-to-T mutation did not significantly affect the binding of GutR or CAP, as determined by DNase I footprinting and EMSA (data not shown).
Induction and repression of the transcription of the gutABD operon by glycerol, glucose, and glucitol were determined by transcriptional activation of the gutA′-lacZ fusion in DL2453. The relative levels of transcription during growth with these three carbon sources were similar in the isolates with GATT-44.5 (DL2453) and with GATC-44.5 (DL1882) (Table 2), even though the absolute level of transcription was reduced twofold by the GATT mutation. These results indicated that the methylation state of GATC-44.5 did not affect the regulation of gutABD. This hypothesis was supported by the observations that methylation of the GATC-44.5 site had no observed effect on the binding affinity of CAP or GutR for gut DNA in vitro, as determined by gel mobility shift analysis or quantitative DNase I footprinting (Fig. 3 and data not shown). Interestingly, the transcription of gutA′-lacZ was affected by the level of Dam in the cells, as indicated by an increase in transcription in derivatives of DL1882 after the introduction of a dam allele or a Dam-overproducing plasmid (data not shown). However, because transcription also increased in dam and Dam-overproducing derivatives of DL2453 containing the GATT-44.5 substitution, we concluded that this Dam-dependent regulation of gutA′-lacZ was due to a nonspecific or indirect effect of the Dam level (data not shown). Together, these results indicated that the methylation state of GATC-44.5 had no effect on steady-state transcriptional regulation of the gutABD operon.
DISCUSSION
Previous results showed that stably inherited DNA methylation patterns are present in E. coli and, in the case of pap, directly regulate gene expression (6, 10, 21, 30). Here we show that DNA methylation patterns can be established in vitro by the binding of regulatory proteins to target DNA sequences containing the GATC recognition site for Dam. Binding of Lrp to pap regulatory DNA inhibited methylation of the pap GATC-I and GATC-II sites, whereas binding of GutR blocked methylation of GATC-44.5 in the gutABD operon (Fig. 1A and C). In contrast, binding of the activator CAP at the GATC sequence in the gutABD operon did not result in methylation protection of GATC-44.5 (Fig. 1B). The data obtained from our in vitro methylation protection assays were consistent with the data obtained from in vivo analyses showing that Lrp was required for pap GATC-I and GATC-II methylation protection (5, 19) and that GutR was required for methylation protection of GATC-44.5 in the gutABD operon (Fig. 2). These data confirm that methylation protection can indicate the presence of a DNA binding protein at a specific DNA site in vivo. However, these data also show that the converse is not necessarily true: the absence of methylation protection of a GATC sequence does not prove the absence of a protein binding at that site.
Lrp is required for in vivo methylation protection of the GATC-I and GATC-II sites in pap (5). The in vitro data presented here show that Lrp binding is sufficient to protect these GATC sequences from methylation (Fig. 1A). Besides Lrp, in vivo methylation protection of GATC-I requires PapI. In vitro, however, binding of Lrp can occur at the GATC-I region at high concentrations of Lrp (19). This PapI-independent binding of Lrp is what blocks the methylation of GATC-I in the methylation protection assay. The ability of Lrp to form pap DNA methylation patterns is an important element in the model for the methylation-dependent phase variation of pap. Phase variation of pap requires the translocation of Lrp between sites 4 and 5 and sites 1, 2, and 3, each containing one GATC sequence. Binding at sites 4 and 5 results in Lrp-dependent transcriptional activation, whereas binding at sites 1, 2, and 3 is required for repression of transcription (28). The DNA methylation states of the GATC-I and GATC-II sequences directly regulate Pap pilus phase variation by modulating this Lrp binding, as was shown in an analysis of mutant E. coli isolates containing site-directed mutations within GATC-I and GATC-II that abolished DNA methylation but not Lrp binding (6). Based on these data, we predict that in order to maintain an expression state, DNA methylation patterns must also be maintained. In turn, it is implied that Lrp binding must block methylation of the GATC sequences in the Lrp binding sites in pap. The data presented in this paper support this latter part of the regulatory model by showing that in vitro, Lrp binding is sufficient to block Dam-dependent methylation of the GATC sequences in the pap Lrp binding sites. It seems likely that Lrp must dissociate from pap DNA to allow methylation of the GATC sequences. It is not known if this dissociation occurs stochastically or if a cellular event such as DNA replication is required. In summary, the data presented here and previously (4, 6, 18, 19) support a model in which competition between Dam and Lrp for GATC-containing regions is an essential step in determining both the DNA methylation pattern and the transcriptional state of pap.
The role of Dam in pap phase variation as discussed above concerns the regulation of transcription at 37°C. In contrast, pap is transcriptionally inactive at 23°C, and this thermoregulation requires H-NS. At 23°C, the GATC sequences are also protected from methylation. However, in contrast to methylation protection at 37°C, this protection is not Lrp dependent but requires H-NS. Specific binding of H-NS to pap DNA in vitro also results in methylation protection of the GATC sequences (31). These data suggest that H-NS binds to the GATC-containing regions of pap at 23°C and blocks their methylation. However, in contrast to the situation with Lrp-dependent methylation protection, it is not known if methylation of GATC-I and GATC-II affects H-NS binding and thermoregulation of pap.
Previous data obtained by Wang and Church (30) suggested that CAP may contribute to the methylation protection of GATC-44.5. However, our in vitro methylation protection assay showed that CAP binding at the gutABD regulatory region did not result in methylation protection, despite the fact that the cAMP-CAP binding site contains the GATC-44.5 sequence (Fig. 1B and 3). This conclusion is consistent with the finding that GATC-44.5 was fully methylated in the gutR isolate DL2803 under growth conditions in which the cAMP level should have been high (glycerol as the sole carbon source), facilitating the binding of CAP at its target site in gut (Fig. 2). Also, it is consistent with our finding that in cultures grown in LB medium, the level of methylation protection of GATC-44.5 in a crp+ background was similar to the level of protection in a crp background, 58.5 and 66.5%, respectively (results not shown). Taken together, these results strongly indicate that CAP does not play a role in methylation protection of GATC-44.5.
Since genetic analyses indicated that GutR was the only methylation-blocking factor for GATC-44.5 (Fig. 2), the methylation state of GATC-44.5 provides information on the binding of the repressor GutR in vivo. The absence of methylation protection in vivo in glucitol-grown cells indicated that GutR was not bound to GATC-44.5 (Fig. 2). This finding is in agreement with data showing that glucitol or a derivative thereof is the inducer of the gut operon and acts by relieving GutR-mediated repression (15, 34). In vitro, however, we found that glucitol did not change the affinity of GutR for gutABD regulatory region DNA; thus, the physiological inducer and the mechanism of induction of gutABD remain to be determined (26a). Surprisingly, in glucose- and glycerol-grown cells, only a fraction of the GATC-44.5 sequence was fully protected from methylation, as determined by digestion of the DNA with MboI (Fig. 2). The fraction of DNA that was not digested by MboI could have been either hemimethylated or fully methylated. If this fraction represented fully methylated gut DNA, then in a part of the bacterial population, GutR was not bound under conditions in which gutABD expression was repressed. Alternatively, if the fraction of MboI-resistant DNA represented hemimethylated gut DNA, it is possible that GutR bound to and maintained a hemimethylated GATC-44.5 sequence.
Methylation protection of a Dam target site by a DNA binding protein could occur by steric hindrance or alteration of DNA conformation by DNA bending. Therefore, it is likely that the observed difference in methylation protection by GutR and CAP reflects a difference in the respective protein-DNA structures. In the gutABD sequence, the CAP half site has the sequence 5′-TGCGATCA-3′, where the underlined sequence is the nonmethylated site at −44.5. In the published crystal structure of a cAMP-CAP-DNA complex, the adenine that is in the same position as the underlined methylated adenine in the CAP half site is in the major groove on the DNA region facing away from CAP-DNA contacts (23). This finding suggests that GATC-44.5 may be accessible to Dam. Other nonmethylated GATC sequences in putative CAP binding sites have been identified (30). Based on our observations with gut, it will be of interest to determine if in these cases another protein besides CAP is required for methylation protection.
The DNA methylation states of the pap GATC-I and GATC-II sites directly regulate Pap pilus phase variation by modulating the binding of Lrp to pap DNA sites which overlap GATC sequences (6). In contrast, the data obtained here indicated that although GutR bound to the GATC-44.5 site of the gutABD operon and protected this site from Dam methylation, the methylation state of GATC-44.5 did not control gut transcription. Our results indicated that methylation of GATC-44.5 did not significantly affect binding of GutR to gut regulatory region DNA (Fig. 3B). It is possible that the adenine that was methylated was not in direct contact with GutR. Alternatively, since the GutR DNase I footprint extended over a 100-bp gut DNA region, multiple functional units of GutR might have been bound. Therefore, one GATC sequence might be insufficient to affect the binding affinity of the total complex. In the carAB operon in Salmonella typhimurium, the methylation state of a partially nonmethylated GATC sequence also does not contribute to car regulation (8). However, the GATC site in car is adjacent to and not within the binding sites of the regulatory proteins CarP and IHF; therefore, methylation is less likely to regulate carAB transcription (8). The identification of other Dam-dependent genes seems to suggest that Dam may play a role as a global regulator in E. coli and S. typhimurium (26). However, it remains to be determined if methylation patterns in the E. coli chromosome besides those in the pap family are involved in gene regulation.
ACKNOWLEDGMENTS
We thank the Protein-DNA Core Facility, Cancer Center, University of Utah, Salt Lake City, for the production of the oligonucleotides used in this study; Julie Tucker for technical assistance; Felix Vajdos for purified Lrp; and Bruce Braaten for helpful discussion. Also, we thank M. G. Fried for the CAP purification protocol and M. Saier and R. Ebright for strains and plasmids.
This work was supported by National Science Foundation grant MCB-9305166 and its supplementary REU grant.
REFERENCES
- 1.Alexeyev M F, Shokolenko I N, Croughan T P. Improved antibiotic-resistance gene cassettes and omega elements for Escherichia coli vector construction and in vitro deletion/insertion mutagenesis. Gene. 1995;160:63–67. doi: 10.1016/0378-1119(95)00108-i. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 3.Bachmann B J. Linkage map of Escherichia coli K-12, edition 7. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. pp. 807–877. [Google Scholar]
- 4.Blyn L B, Braaten B A, Low D A. Regulation of pap pilin phase variation by a mechanism involving differential Dam methylation states. EMBO J. 1990;9:4045–4054. doi: 10.1002/j.1460-2075.1990.tb07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braaten B A, Blyn L B, Skinner B S, Low D A. Evidence for a methylation-blocking factor (mbf) locus involved in pap pilus expression and phase variation in Escherichia coli. J Bacteriol. 1991;173:1789–1800. doi: 10.1128/jb.173.5.1789-1800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braaten B A, Nou X, Kaltenbach L S, Low D A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell. 1994;76:577–588. doi: 10.1016/0092-8674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 7.Casadaban M. Transposition and fusion of the lac genes to selected promoters in E. coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 8.Charlier D, Gigot D, Huysveld N, Roovers M, Piérard A, Glansdorff N. Pyrimidine regulation of the Escherichia coli and Salmonella typhimurium carAB operons: CarP and integration host factor (IHF) modulate the methylation status of a GATC site present in the control region. J Mol Biol. 1995;250:383–391. doi: 10.1006/jmbi.1995.0384. [DOI] [PubMed] [Google Scholar]
- 9.Chesnokov I, Schmid C. Specific Alu binding protein from human sperm chromatin prevents DNA methylation. J Biol Chem. 1995;270:18539–18542. doi: 10.1074/jbc.270.31.18539. [DOI] [PubMed] [Google Scholar]
- 10.Hale W B, Van der Woude M W, Low D A. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J Bacteriol. 1994;176:3438–3441. doi: 10.1128/jb.176.11.3438-3441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 12.Jackson S P. Identification and characterization of eukaryotic transcription factors. In: Hames B D, Higgins S J, editors. Gene transcription. New York, N.Y: Oxford University Press; 1993. pp. 189–243. [Google Scholar]
- 13.Jaworski A, Hsieh W-T, Blaho J A, Larson J E, Wells R D. Left-handed DNA in vivo. Science. 1987;238:773–777. doi: 10.1126/science.3313728. [DOI] [PubMed] [Google Scholar]
- 14.Kaltenbach L K, Braaten B A, Low D A. Specific binding of PapI to Lrp-pap DNA complexes. J Bacteriol. 1995;177:6449–6455. doi: 10.1128/jb.177.22.6449-6455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lengeler J, Steinberger H. Analysis of the regulatory mechanisms controlling the synthesis of the hexitol transport systems in Escherichia coli K12. Mol Gen Genet. 1978;164:163–169. doi: 10.1007/BF00267381. [DOI] [PubMed] [Google Scholar]
- 16.Marinus M G. Methylation of DNA. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 782–791. [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Nou X, Braaten B, Kaltenbach L, Low D. Differential binding of Lrp to two sets of pap DNA binding sites mediated by PapI regulates Pap phase variation in Escherichia coli. EMBO J. 1995;14:5785–5797. doi: 10.1002/j.1460-2075.1995.tb00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nou X, Skinner B, Braaten B, Blyn L, Hirsch D, Low D. Regulation of pyelonephritis-associated pili phase variation in Escherichia coli: binding of the PapI and Lrp regulatory proteins is controlled by DNA methylation. Mol Microbiol. 1993;7:545–553. doi: 10.1111/j.1365-2958.1993.tb01145.x. [DOI] [PubMed] [Google Scholar]
- 20.Oden K L, DeVeaux L C, Vibat C R T, Cronan J E, Jr, Gennis R B. Genomic replacement in Escherichia coli K-12 using covalently closed circular plasmid DNA. Gene. 1990;96:29–36. doi: 10.1016/0378-1119(90)90337-q. [DOI] [PubMed] [Google Scholar]
- 21.Rinquist S, Smith C L. The Escherichia coli chromosome contains specific, unmethylated dam and dcm sites. Proc Natl Acad Sci USA. 1992;89:4539–4543. doi: 10.1073/pnas.89.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Schultz S C, Shields G C, Steitz T A. Crystal structure of a CAP-DNA complex: the DNA is bent by 90 degrees. Science. 1991;253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 24.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 25.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 26.Torreblanca J, Casadesus J. DNA adenine methylase mutants of Salmonella typhimurium and a novel Dam-regulated locus. Genetics. 1996;144:15–26. doi: 10.1093/genetics/144.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.van der Woude, M. Unpublished results.
- 27.van der Woude M W, Braaten B, Low D. Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol. 1996;4:5–9. doi: 10.1016/0966-842x(96)81498-3. [DOI] [PubMed] [Google Scholar]
- 28.van der Woude M W, Kaltenbach L S, Low D A. Leucine-responsive regulatory protein plays dual roles as both an activator and a repressor of the Escherichia coli pap fimbrial operon. Mol Microbiol. 1995;17:303–312. doi: 10.1111/j.1365-2958.1995.mmi_17020303.x. [DOI] [PubMed] [Google Scholar]
- 29.van der Woude M W, Low D A. Leucine-responsive regulatory protein and deoxyadenosine methylase control the phase variation and expression of the sfa and daa pili operons in Escherichia coli. Mol Microbiol. 1994;11:605–618. doi: 10.1111/j.1365-2958.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang M X, Church G M. A whole genome approach to in vivo DNA-protein interactions in E. coli. Nature. 1992;360:606–610. doi: 10.1038/360606a0. [DOI] [PubMed] [Google Scholar]
- 31.White-Ziegler C A, Angus Hill M L, Braaten B A, van der Woude M W, Low D A. Thermoregulation of E. coli pap transcription: H-NS is a temperature-dependent DNA methylation blocking factor. Mol Microbiol. 1998;28:1121–1138. doi: 10.1046/j.1365-2958.1998.00872.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Saier M H., Jr Glucitol-specific enzymes of the phosphotransferase system in Escherichia coli. J Biol Chem. 1987;262:5455–5463. [PubMed] [Google Scholar]
- 33.Yamada M, Saier M H., Jr Physical and genetic characterization of the glucitol operon in Escherichia coli. J Bacteriol. 1987;169:2990–2994. doi: 10.1128/jb.169.7.2990-2994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada M, Saier M H., Jr Positive and negative regulators for glucitol (gut) operon expression in Escherichia coli. J Mol Biol. 1988;203:569–583. doi: 10.1016/0022-2836(88)90193-3. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Zhang X, Ebright R H. Random mutagenesis of gene-sized DNA molecules by use of PCR with Taq DNA polymerase. Nucleic Acids Res. 1991;19:6052. doi: 10.1093/nar/19.21.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]