Abstract
A strain designated Acinetobacter indicus WMB-7 with the ability to hydrolyze phthalate esters (PAEs) was isolated from the fermented grains of Baijiu. The genome of the strain was sequenced with a length of 3,256,420 bp and annotated with 3183 genes, of which 36 hydrolases encoding genes were identified. The hydrolases were analyzed by protein structure modeling and molecular docking, and 14 enzymes were docked to the ligand di-butyl phthalate with the catalytic active regions, and showed binding affinity. The 14 enzymes were expressed in E. coli and 5 of them showed the ability for PAEs hydrolysis. Enzyme GK020_RS15665 showed high efficiency for PAEs hydrolysis and could efficiently hydrolyze di-butyl phthalate under an initial concentration of 1000 mg/L with a half-life of 4.24 h. This work combined a series of methods for identifying PAEs hydrolases and offered a molecular basis for PAEs degradation of A. indicus strains from Baijiu.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01334-w.
Keywords: Baijiu, Acinetobacter indicus, Molecular docking, Phthalate esters, Ester bond hydrolysis
Introduction
Traditional fermented Baijiu is a distilled beverage with a history dating back thousands of years, and is loved by consumers in China (Xu et al., 2022a; Xu et al., 2023a). Baijiu production mainly continues with the traditional methods, and the manufacturing process is relatively backward (Xu et al., 2017; Xu et al., 2023b; Zhao et al., 2023). There is still no scientific analysis of the formation, migration or degradation of harmful substances in the products, thus leading to the lack of useful control methods during manufacturing process, and generating serious safety issues (Dong et al., 2019). Ensuring the safety of products is an important aspect of Baijiu, while a series of compounds with negative effects on safety have been detected, such as phthalate esters, ethyl carbamates, heavy metals and pesticides (Dong et al., 2019). Among them, phthalate esters (PAEs) show serious negative effects on the quality of Baijiu as they could cause health problems after oral intake (Lai et al., 2023; Zhang et al., 2018).
PAEs are a class of endocrine disruptors, and show reproductive toxicity, cause abnormal lipid metabolism, induce childhood obesity, interfere with immune response, and cause neuropsychological diseases (Huang et al., 2019; Tian et al., 2019). Previous studies recognize that the manufacturing process of Baijiu does not produce PAEs, and those substances in products are caused by migration from plastic contact materials during processing, transport and storage (Fan et al., 2014). Recent studies indicate other sources of PAEs in Baijiu. That are, PAEs in raw materials is an important source of such substances in Baijiu products (Dong et al., 2019; Sun et al., 2018). Previous studies report that crops such as sorghum, wheat, and rice can enrich PAEs and easily accumulate them in seeds (Ma et al., 2013; Sun et al., 2015). Therefore, a certain amount of PAEs will inevitably exist in grains for manufacturing of Baijiu, and finally migrates into the products (Xu et al., 2020).
Natural degradation of PAEs takes a long time, thus developing effective treatment method is a necessary way to solve PAEs contamination (Huang et al., 2019; Tan et al., 2016). Nowadays, the main methods for solving PAEs contamination are physical and chemical methods, such as UV irradiation combined with catalytic degradation using chemical catalyst (Yuan et al., 2008), activated carbon adsorption (Mohan et al., 2007) or activated carbon as carrier combined with chemical catalytic degradation (Li et al., 2009). Although these methods are effective, there are limitations when applying these methods in food industry, such as incomplete degradation or secondary pollution (Xu et al., 2020). It has been found that the concentrations of PAEs in Baijiu raw materials are significantly higher than those in the products, indicating that the fermentation process is conducive to the degradation of PAEs (Dong et al., 2019). Microorganisms play an important role for the conversion of materials in the fermentation process of Baijiu (Xu et al., 2023b). Meanwhile, studies indicate that microorganisms from Baijiu show high PAEs degradation ability (Xu et al., 2021). Microbial degradation has the characteristics of mild reaction conditions, few by-products, and high efficiency (Xu et al., 2020). Analyzing the PAEs degradation characteristics of microorganisms from Baijiu, and moderately strengthening the fermentation process will help to reduce the concentrations of PAEs in the final products (Xu et al., 2022b).
In this study, Acinetobacter indicus WMB-7 isolated from Baijiu and capable of degrading PAEs was investigated. The genome was sequenced by high-throughput sequencing and annotated thereafter. Combined with protein homology modeling, molecular docking and enzyme property analysis, five PAEs hydrolases were identified. Although previous studies reported PAEs degrading Acinetobacter sp. (Fang et al., 2017; Li et al., 2022; Liang et al., 2010; Sharma et al., 2021; Wang et al., 2021b; Wu et al., 2013), to the best of our knowledge, this work first reported the genomic information of a PAEs degrading A. indicus strain from Baijiu and systematically investigated the related PAEs hydrolases. These investigations will provide direct molecular references for comprehensive understanding of PAE-degrading properties of A. indicus. Identifying PAEs degrading microorganisms from Baijiu and analyzing the genetic information will promote the application of microorganisms in the fermentation process and ensure the quality and safety of products in the future.
Materials and methods
Strains, vectors and materials
Escherichia coli BL21(DE3) competent cell was provided by TIANGEN (Beijing, China). The vector pET-28a(+) was purchased from Novagen Inc. (Darmstadt, Germany). Di-methyl phthalate (DMP) (purity 99.0%), di-ethyl phthalate (DEP) (99.5%), di-butyl phthalate (DBP) (99.0%), di-isobutyl phthalate (DIBP) (99.0%) and di-(2-ethylhexyl) phthalate (DEHP) (99.0%) were all purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Luria-Bertan (LB) medium contained tryptone (10 g/L), yeast extract (5 g/L), sodium chloride (10 g/L), and the pH was adjusted to 7.0. Solid LB medium was added by 2% agar powder.
Genome sequence, assembly and annotation
A. indicus WMB-7 was separated from the fermented grains of Baijiu and showed the ability for degrading PAEs (Wang et al., 2021a). The genomic DNA was extracted and prepared for high-throughput sequencing according to the standard protocol of the Beijing genomics institute (BGI) Co. (Beijing, China). The whole genome of strain WMB-7 was sequenced using PacBio RSII and Illumina Hiseq™ 2000 sequencing platform, respectively, and the sequenced data was assembled after quality control. De novo assembly was performed with the custom software Rabbit developed by BGI (Beijing, China) based on PacBio sequencing results. The Illumina HiSeq™ 2000 (Illumina, San Diego, CA, USA) sequencing platform generated reads for single nucleotide correction. Genes were predicted based on the genome sequence, and annotated by Clusters of Orthologous Groups of proteins (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG), respectively.
Protein structure modeling
The sequences of annotated hydrolases were aligned with the reference proteins using ESPript 3.0 (http://espript.ibcp.fr/ESPript/ESPript/). The protein with high sequence identity to the template (> 30%) was calculated of the structure using SWISS-MODEL (https://swissmodel.expasy.org/) (Bordoli et al., 2009). The protein with low identity to the template (< 30%) was analyzed of the structure by RoseTTAFold (https://robetta.bakerlab.org/) (Baek et al., 2021). PROCHECK (https://www.ebi.ac.uk/thornton-srv/software/PROCHECK/) was used to evaluate the reliability of the protein structure (Berman et al., 2003).
Molecular docking
The three-dimensional structure of DBP was obtained from ZINC database (http://zinc15.docking.org/). The ligand was prepared by AutoDock Tools 4.0. The receptor protein was modified by hydrogenation and charge balancing, and the pocket center of the receptor protein was set as the center of the binding site. The files of receptor protein and ligand molecule were converted to the AutoDock Vina acceptable format (.pdbqt). The binding pocket of the protein was selected and docked with the ligand. The affinity of the receptor-ligand complex was evaluated by calculating the score based on the spatial effect, repulsion effect, hydrogen bond, hydrophobic interaction and molecular flexibility. Results were visualized through PyMOL software.
Gene cloning and expression
Genes were amplified through polymerase chain reaction (PCR) using the genomic DNA of A. indicus WMB-7 as the template. The primers were designed with the 18 or 20 bp homologous recombination sequence regions (Table 1), and the amplified gene was generated with the recombinant region and ligated with the linearized vector pET-28a(+) generated by PCR using the primer pair pET28a.f/pET28a.r. Genes numbered GK020_RS03995, GK020_RS05280, GK020_RS05500, GK020_RS06805, GK020_RS07540, GK020_RS08850, GK020_RS08875, GK020_RS09115, GK020_RS10440, GK020_RS11185, GK020_RS11230, GK020_RS15155, GK020_RS15275, and GK020_RS15665 were amplified using the respective primer pairs (Table 1). A Vazyme ClonExpress II One Step Cloning Kit (Nanjing, China) was used to ligate the genes with the vector to produce the expression vectors, and transferred to the host cell E. coli BL21(DE3) to generate the respective transformants.
Table 1.
Strains, vectors and primers in this work
| Name | Characteristic | Reference |
|---|---|---|
| Strain | ||
| Escherichia coli BL21(DE3) | Used as host strain | Invitrogen |
| E. coli BL21/pET-28a(+) | E. coli BL21(DE3) harboring pET-28a(+) | This work |
| E. coli BL21/pET-GK020_RS03995 | E. coli BL21(DE3) harboring pET-GK020_RS03995 | This work |
| E. coli BL21/pET-GK020_RS05280 | E. coli BL21(DE3) harboring pET-GK020_RS05280 | This work |
| E. coli BL21/pET-GK020_RS05500 | E. coli BL21(DE3) harboring pET-GK020_RS05500 | This work |
| E. coli BL21/pET-GK020_RS06805 | E. coli BL21(DE3) harboring pET-GK020_RS06805 | This work |
| E. coli BL21/pET-GK020_RS07540 | E. coli BL21(DE3) harboring pET-GK020_RS07540 | This work |
| E. coli BL21/pET-GK020_RS08850 | E. coli BL21(DE3) harboring pET-GK020_RS08850 | This work |
| E. coli BL21/pET-GK020_RS08875 | E. coli BL21(DE3) harboring pET-GK020_RS08875 | This work |
| E. coli BL21/pET-GK020_RS09115 | E. coli BL21(DE3) harboring pET-GK020_RS09115 | This work |
| E. coli BL21/pET-GK020_RS10440 | E. coli BL21(DE3) harboring pET-GK020_RS10440 | This work |
| E. coli BL21/pET-GK020_RS11185 | E. coli BL21(DE3) harboring pET-GK020_RS11185 | This work |
| E. coli BL21/pET-GK020_RS11230 | E. coli BL21(DE3) harboring pET-GK020_RS11230 | This work |
| E. coli BL21/pET-GK020_RS15155 | E. coli BL21(DE3) harboring pET-GK020_RS15155 | This work |
| E. coli BL21/pET-GK020_RS15275 | E. coli BL21(DE3) harboring pET-GK020_RS15275 | This work |
| E. coli BL21/pET-GK020_RS15665 | E. coli BL21(DE3) harboring pET-GK020_RS15665 | This work |
| Vector | ||
| pET-28a(+) | pMB1 replicon, Kanr, carried PT7 promoter | Novagen |
| pET-GK020_RS03995 | pET-28a(+) carried the gene GK020_RS03995 from A. indicus WMB-7 | This work |
| pET-GK020_RS05280 | pET-28a(+) carried the gene GK020_RS05280 from A. indicus WMB-7 | This work |
| pET-GK020_RS05500 | pET-28a(+) carried the gene GK020_RS05500 from A. indicus WMB-7 | This work |
| pET-GK020_RS06805 | pET-28a(+) carried the gene GK020_RS06805 from A. indicus WMB-7 | This work |
| pET-GK020_RS07540 | pET-28a(+) carried the gene GK020_RS07540 from A. indicus WMB-7 | This work |
| pET-GK020_RS08850 | pET-28a(+) carried the gene GK020_RS08850 from A. indicus WMB-7 | This work |
| pET-GK020_RS08875 | pET-28a(+) carried the gene GK020_RS08875 from A. indicus WMB-7 | This work |
| pET-GK020_RS09115 | pET-28a(+) carried the gene GK020_RS09115 from A. indicus WMB-7 | This work |
| pET-GK020_RS10440 | pET-28a(+) carried the gene GK020_RS10440 from A. indicus WMB-7 | This work |
| pET-GK020_RS11185 | pET-28a(+) carried the gene GK020_RS11185 from A. indicus WMB-7 | This work |
| pET-GK020_RS11230 | pET-28a(+) carried the gene GK020_RS11230 from A. indicus WMB-7 | This work |
| pET-GK020_RS15155 | pET-28a(+) carried the gene GK020_RS15155 from A. indicus WMB-7 | This work |
| pET-GK020_RS15275 | pET-28a(+) carried the gene GK020_RS15275 from A. indicus WMB-7 | This work |
| pET-GK020_RS15665 | pET-28a(+) carried the gene GK020_RS15665 from A. indicus WMB-7 | This work |
| Primer name | Sequence (5′ → 3′)a | |
| pET28a.f | ATCGCACTCGAGCACCACC | |
| pET28a.r | CATGGTATATCTCCTTCTTA | |
| GK020_RS03995.f | TAAGAAGGAGATATACCATGATGGCACAAATTTATGTG | |
| GK020_RS03995.r | GGTGGTGCTCGAGTGCGATTTCCACAATAATATGAGG | |
| GK020_RS05280.f | TAAGAAGGAGATATACCATGATGAAACCATTAATCCAT | |
| GK020_RS05280.r | GGTGGTGCTCGAGTGCGATAGGTGCCTGATCCTGTAA | |
| GK020_RS05500.f | TAAGAAGGAGATATACCATGATGATTCATACCGTAATTG | |
| GK020_RS05500.r | GGTGGTGCTCGAGTGCGATAATTGCAAATTTAAATAA | |
| GK020_RS06805.f | TAAGAAGGAGATATACCATGATGGATGACGAGCACCTT | |
| GK020_RS06805.r | GGTGGTGCTCGAGTGCGATTAGCCCTTTGCGGTTTAACC | |
| GK020_RS07540.f | TAAGAAGGAGATATACCATGATGCTGCTCAATTTCCAG | |
| GK020_RS07540.r | GGTGGTGCTCGAGTGCGATTGCAGATAAAAACTGCTG | |
| GK020_RS08850.f | TAAGAAGGAGATATACCATGATGAAATTTGTGATCCAG | |
| GK020_RS08850.r | GGTGGTGCTCGAGTGCGATAGATCGTTCGCCCTGTTG | |
| GK020_RS08875.f | TAAGAAGGAGATATACCATGATGCCGTATTATGTCATG | |
| GK020_RS08875.r | GGTGGTGCTCGAGTGCGATTTGATGCTGCAATAAAAAG | |
| GK020_RS09115.f | TAAGAAGGAGATATACCATGATGAAGTATCTGATGTTA | |
| GK020_RS09115.r | GGTGGTGCTCGAGTGCGATGGGCGCGGATCTATCTGTC | |
| GK020_RS10440.f | TAAGAAGGAGATATACCATGATGAGAAAAATATGGCTT | |
| GK020_RS10440.r | GGTGGTGCTCGAGTGCGATTTGGGTAGGCATGATCGA | |
| GK020_RS11185.f | TAAGAAGGAGATATACCATGATGAATAGTTTGCGGTTA | |
| GK020_RS11185.r | GGTGGTGCTCGAGTGCGATTAATTTAAAGTGATGAGC | |
| GK020_RS11230.f | TAAGAAGGAGATATACCATGATGAAGCAGGATTCTTCG | |
| GK020_RS11230.r | GGTGGTGCTCGAGTGCGATATTTAAAGGAATCAGTTT | |
| GK020_RS15155.f | TAAGAAGGAGATATACCATGATGAAGATCAACCCGTTA | |
| GK020_RS15155.r | GGTGGTGCTCGAGTGCGATATCTTGGTCCTGCTGATG | |
| GK020_RS15275.f | TAAGAAGGAGATATACCATGATGAATTTAATTTATTTG | |
| GK020_RS15275.r | GGTGGTGCTCGAGTGCGATTTTTAGGGAATCCGATAA | |
| GK020_RS15665.f | TAAGAAGGAGATATACCATGATGAGTGCGTATCAACAG | |
| GK020_RS15665.r | GGTGGTGCTCGAGTGCGATAAAGAAGCGTGGTCGATC | |
aUnderlined region is the homologous recombinant sequence to the ends of 5′- or 3′- terminal of the linearized pET-28a(+) vector
The transformants were cultured using LB medium added with kanamycin (the final concentration 40 mg/L) and kept at 37 °C and 200 r/min. When the cells grew to a density of about 0.6–0.8 at OD600nm, isopropyl-β-d-thiogalactopyranoside was added to the medium to a final concentration of 0.5 mM. Cell cultures were then kept at 20 °C and 200 r/min for about 20 h. Cells were collected by centrifugation at 9000×g and 4 °C for 5 min, and washed twice with Tris–HCl buffer (pH 7.5, 50 mM). Cells were lysed by disrupting with a sonifier (Ningbo Xinzhi, Ningbo, China), and centrifuged at 10,000×g and 4 °C for 20 min, and the supernatant was used as the crude enzyme solution to verify the ability for PAEs hydrolysis. Enzyme expression was investigated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Identification of PAEs hydrolases
In order to identify the PAEs hydrolase, the reaction mixture contained crude enzyme solution (1 mL), PAEs mixture (DMP, DEP, DBP, DIBP and DEHP, final concentration of 200 mg/L for each substrate) and Tris–HCl buffer solution (pH 7.5, 50 mM) to the final volume of 10 mL, and kept at 37 °C and 200 r/min for 24 h. Enzyme GK020_RS15665 was added to similar reaction system with PAEs mixture replaced by different concentrations of DBP (the final concentrations of 200, 400, 600, 800 and 1000 mg/L, respectively) to investigate the ability of the enzyme for PAEs hydrolysis. n-Hexane (2 mL) was added to the reaction mixture after catalysis, vortexed for 30 s and centrifuged under 12,000×g for 10 min. The supernatant was collected and filtered by a 0.22 μm membrane and used for gas chromatography analysis.
Analytical methods
Agilent 7890B gas chromatography system was used for sample analysis equipped with an Agilent 19091N-213I column (60 m × 0.32 mm × 0.50 μm). The colume temperature was kept at 80 °C for 5 min, then raised to 240 °C at a rate of 20 °C/min, and maintained at 240 °C for 25 min. The carrier gas was nitrogen with the flow rate of 1.0 mL/min, and the injection volume was 1.0 μL. Statistical analysis was analyzed using Origin 8.0 software.
Results and discussion
Genome sequencing and annotation of A. indicus WMB-7
The genome of WMB-7 was composed of a circular chromosome without plasmid. The length was 3,256,420 bp, and the DNA G + C content was 45.5%. The strain carried 3183 genes, including 81 tRNAs, 21 rRNAs, 4 sRNAs, 142 pseudogenes and 2935 protein coding genes (Fig. 1). Proteins of strain WMB-7 were sequence aligned with the COG database. A total of 2641 proteins were annotated (Fig. S1a), accounting for 83.0% of the prediction genes. Proteins of WMB-7 were also sequence aligned with the KEGG database. A total of 1887 proteins were annotated through KEGG database, accounting for 59.3% of the predicted proteins (Fig. S1b).
Fig. 1.
Circular map of A. indicus WMB-7 based on genome sequence and annotation. From outside to inside: forward strand gene, reverse strand gene, forward strand RNAs, reverse strand RNAs, repeat, GC content, GC skew
Studies found that the toxicity of PAEs correlated with ester bond linked side chains, and after hydrolyzed the ester bonds, the toxicity of PAEs were significantly reduced (Jiao et al., 2013; Tian et al., 2019). Most of the enzymes with the ability to hydrolyze the ester bonds of PAEs belonged to hydrolases (Xu et al., 2021). According to genome annotation, WMB-7 carried 36 hydrolases, including 3 serine hydrolases (Locus tag in GenBank: GK020_RS09070, GK020_RS10225, and GK020_RS01245), 27 α/β hydrolases (GK020_RS01505, GK020_RS02535, GK020_RS03995, GK020_RS04040, GK020_RS05175, GK020_RS05280, GK020_RS05500, GK020_RS06805, GK020_RS07075, GK020_RS07540, GK020_RS08640, GK020_RS08850, GK020_RS08875, GK020_RS09030, GK020_RS09115, GK020_RS09830, GK020_RS10105, GK020_RS10440, GK020_RS10775, GK020_RS10835, GK020_RS11185, GK020_RS11230, GK020_RS11310, GK020_RS13040, GK020_RS15155, GK020_RS15275, and GK020_RS15665), 5 metal ion dependent hydrolases (GK020_RS03365, GK020_RS04540, GK020_RS12300, GK020_RS13595, and GK020_RS14095), and 1 SGNH/GDSL hydrolase (GK020_RS08095).
Homology and de novo protein structure modeling
The structures of 36 hydrolases were analyzed and docked with the ligand DBP to evaluate the ability to hydrolyze PAEs. Among them, 13 hydrolases showed the sequence similarity > 30% to the respective reference templates with known protein structures by SWISS-MODEL (Table S1). Results of protein sequence alignment were shown in Fig. S2.
The left 23 enzymes showed low sequence similarities (< 30%) with the reference templates, including GK020_RS01505, GK020_RS02535, GK020_RS03365, GK020_RS04040, GK020_RS04540, GK020_RS05175, GK020_RS05280, GK020_RS06805, GK020_RS07075, GK020_RS08095, GK020_RS08640, GK020_RS08850, GK020_RS08875, GK020_RS09030, GK020_RS09830, GK020_RS10105, GK020_RS10775, GK020_RS10835, GK020_RS11310, GK020_RS12300, GK020_RS13040, GK020_RS13595, and GK020_RS14095. RoseTTAFold was used for de novo protein structure modeling.
In order to test the reliability of the predicted protein structures, the quality of protein structure was evaluated using the Ramachandran plot, which can confirm whether the amino acids of a protein structure is reliable (Sheik et al., 2002). Results of the 36 hydrolases showed that the proportion of amino acid residues in the unreliable region was all less than 5% (Table S2; Fig. S3), indicating the protein sturctures were reliable (Tam et al., 2020).
Molecular docking
Studies indicated that A. indicus could hydrolyze 200 mg/L DBP completely in 4 days, therefore DBP was used as the ligand for molecular docking with hydrolases from A. indicus WMB-7 (Wang et al., 2021a). Homology modeling combined with molecular docking can evaluate possible enzyme substrate interactions and provide useful information for revealing the catalytic mechanisms. For the 13 hydrolases with reference templates (sequence similarity > 30%), 10 of them showed binding affinity with the ligand DBP (Table S3, number 1–10, Fig. 2A–J), while DBP was unable to dock to the catalytic active pocket of GK020_ RS01245, GK020_ RS09070 or GK020_ RS10225.
Fig. 2.
Protein structure modeling of the potential PAEs hydrolases and molecular docking to DBP. (A) GK020_RS11185; (B) GK020_RS11230; (C) GK020_RS15275; (D) GK020_RS15155; (E) GK020_RS03995; (F) GK020_RS15665; (G) GK020_RS05500; (H) GK020_RS09115; (I) GK020_RS10440; (J) GK020_RS07540; (K) GK020_RS08875; (L) GK020_RS08850; (M) GK020_RS05280; (N) GK020_RS06805
All these hydrolases contained the conserved sequence A/GX1SX2G, which was consistent with the reported PAEs hydrolases (Duan et al., 2019; Kakugawa et al., 2007), indicating these enzymes with the potential abilities to catalyze the hydrolysis of DBP. When the hydrolase binds PAEs, the oxygen atom of the hydroxyl group of serine nucleophilically attacked the carbonyl carbon atom of PAEs to facilitate the cleavage of the ester bonds (Singh et al., 2017).
For the 23 protein models calculated by de novo protein structure modeling, molecular docking was performed based on the suitability of the potential catalytic region and energy matching. Results showed that 4 hydrolases (GK020_RS05280, GK020_RS06805, GK020_RS08850 and GK020_RS08875) could combine with DBP with the possible catalytic region and might have the ability to hydrolyze DBP (Table S2, number 11–14, Fig. 2K–N). All these enzymes also contained the conserved catalytic sequence “A/GX1SX2G”. Due to the lack of reference template, it is necessary to verify the catalytic active sites of these proteins and investigate their catalytic properties.
Identification of PAEs hydrolases
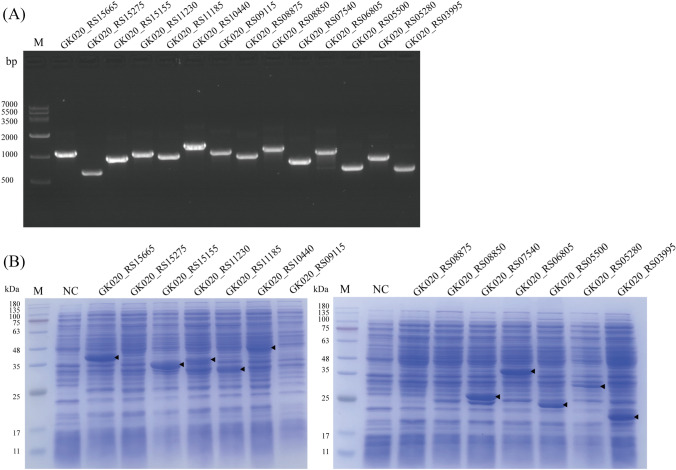
Based on molecular docking, the DNA sequences of the 14 enzymes encoding genes were amplified through PCR (Fig. 3A) and expressed in E. coli BL21(DE3). SDS-PAGE analysis showed that 10 hydrolases (GK020_RS03995, GK020_RS05280, GK020_RS05500, GK020_RS06805, GK020_RS07540, GK020_RS10440, GK020_RS11185, GK020_RS11230, GK020_RS15155 and GK020_RS15665) achieved soluble expression (Fig. 3b). The expression of enzymes GK020_ RS08850, GK020_ RS08875, GK020_ RS09115 and GK020_RS15275 could be further improved in our following work through changing the expression vector or host, optimizing the codon, and removing the N-terminal signal peptide.
Fig. 3.
PCR amplification of the genes (A) and SDS-PAGE analysis of the enzymes (B) with molecular docking interactions to DBP. M, marker; NC, negative control
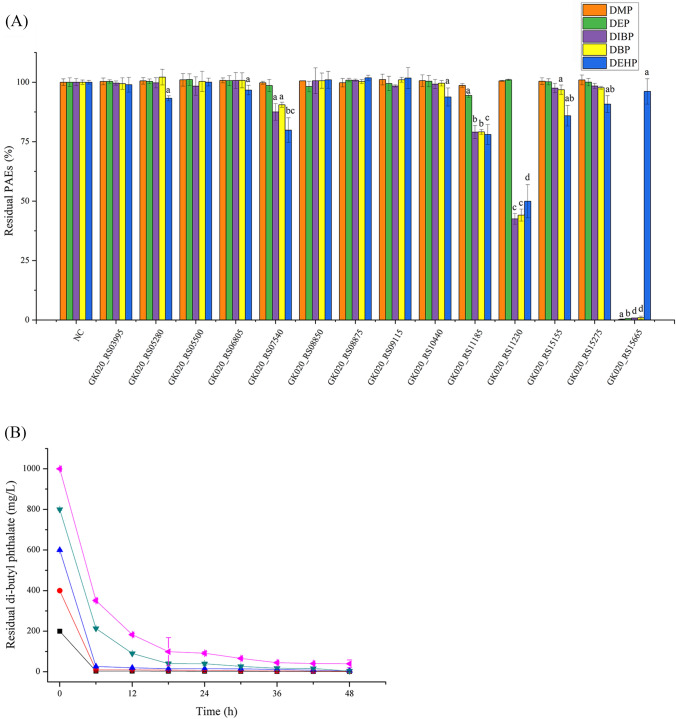
The PAEs hydrolysis abilities of the enzymes were investigated, and the results showed that GK020_ RS15665 showed the best hydrolysis performance toward PAEs among all the enzymes, which could hydrolyze 99.69%, 99.39%, 99.19% and 99.05% of DMP, DEP, DIBP, and DBP in 24 h, respectively (Fig. 4A). DMP, DEP, DIBP, DBP, and DEHP were used as the mixed substrates to verify the PAE-degrading ability of the enzymes because these 5 substrates were frequently detected in grains (Dong et al., 2019). In addition, GK020_RS11230 and GK020_RS11185 showed the hydrolysis abilities toward DBP, DIBP and DEHP compared with the control groups (p < 0.05), and the hydrolysis ratios of DBP were 55.89% and 20.91%, respectively (Fig. 4A). Enzyme GK020_RS07540 showed the ability to hydrolyze DIBP, DBP and DEHP (p < 0.05), and enzyme GK020_RS15155 showed the ability to hydrolyze DBP and DEHP (p < 0.05) (Fig. 4A). These results indicated that five enzymes showed the abilities for hydrolyzing the ester bonds of PAEs, and revealed the efficiency for identifying the target enzyme by homology modeling, molecular docking and enzymatic PAEs hydrolysis analysis.
Fig. 4.
Verification of the PAE-degrading ability of the enzymes (A) and degradation efficiency of the enzyme GK020_RS15665 (B). All experiments were carried out in triplicate. NC, negative control, the crude enzyme solution of E. coli BL21/pET-28a(+)
GK020_RS15665 showed a relatively high catalytic efficiency toward PAEs, while other PAE-hydrolyzing enzymes like GK020_RS07540, GK020_RS11185 and GK020_RS11230 showed relatively low catalytic efficiency. This might due to the high compatibility of the enzymatic catalytic pocket of GK020_RS15665 with PAEs, including the suitable distance and angle of interactions between the key catalytic amino acids and the substrate. But this did not mean that the catalytic reaction would realize if an enzyme could docked with the ligand. The docking of an enzyme with the substrate only indicated the potential of an enzyme to catalyze the reaction, and enzymatic catalysis reaction should be performed thereafter to verify the possibility. For example, the enzyme 3h2h, with highly structure identity to GK020_RS10440, showed a good docking result with the ligand β-octyl glucoside, but no catalytic reaction was detected of the enzyme with the ligand (Aparna et al., 2009). In addition, the efficiency of an enzyme toward different substrates with similar structures were also diverged. For example, the esterase McLipB could efficiently hydrolyze DBP, but the hydrolytic efficiency toward the isomer DIBP was only half than that of DBP (Duan et al., 2019). Meanwhile, the catalytic conditions such as temperature and pH value could also affect the enzymatic catalysis. In this study, the catalytic efficiency of enzymes were investigated under the specific reaction conditions, and GK020_RS15665 showed the highest catalytic efficiency. If the reaction conditions were optimized, the catalytic efficiency of some enzymes under other reaction conditions might be improved, such as GK020_ RS07540, GK020_ RS11185 and GK020_ RS11230.
As GK020_RS15665 showed the best performance for PAEs hydrolysis, its hydrolytic ability was further investigated by reaction under different DBP concentrations (Fig. 4B). In 6 h, the degradation ratios of DBP in the reaction mixture with initial concentrations of 200, 400, 600, 800 and 1000 mg/L were 98.07%, 97.61%, 95.63%, 73.33% and 64.85%, respectively. The PAEs degrading curve fitted well with the equation C = A × exp(− t/t0) + C0, where t is the time period (h), C0 is the constant, and C is the initial substrate concentration (mg/L). The half-life (t1/2) for degrading 200, 400, 600, 800 and 1000 mg/L of DBP were 0.93, 0.76, 1.06, 3.09 and 4.24 h, respectively (Table 2). Compared with the ability to completely hydrolyze 200 mg/L of DBP in 4 d by the strain A. indicus WMB-7 (Wang et al., 2021a), the E. coli carried enzyme GK020_RS15665 hydrolyzed 64.85% of DBP with the initial concentration of 1000 mg/L in 6 h, and was an increase of about 51.88-fold in degradation rate. About 90.07% of DBP (initial concentration 1000 mg/L) was hydrolyzed by GK020_RS15665 within 18 h (Fig. 4B), reflecting the high catalytic efficiency of enzyme GK020_RS15665. As enzyme treatment was recognized as a green route for solving PAEs contamination, the results also indicated the necessity for identifying PAEs hydrolases from the PAE-degrading strains, although the wild type species might not show a good performance for PAEs degradation (Qiu et al., 2020).
Table 2.
The fitting formula and half-life (t1/2) for degrading DBP by GK020_RS15665
| DBP concentration (mg/L) | Kinetic equationa | R2 | t1/2 (h) |
|---|---|---|---|
| 200 | C = 197.93 × exp(− t/1.32) + 2.07 | 0.9967 | 0.93 |
| 400 | C = 391.76 × exp(− t/1.07) + 8.24 | 0.9956 | 0.76 |
| 600 | C = 581.58 × exp(− t/1.46) + 18.27 | 0.9832 | 1.06 |
| 800 | C = 770.10 × exp(− t/4.39) + 18.78 | 0.9914 | 3.09 |
| 1000 | C = 952.21 × exp(− t/5.70) + 47.31 | 0.9993 | 4.24 |
aC concentration, t time
In summary, the whole genome of A. indicus WMB-7 was sequenced with a length of 3,256,420 bp and annotated with 3183 genes, and 36 hydrolases were identified. The potential abilities of enzymes for hydrolyzing the ester bonds of PAEs were evaluated by protein structure modeling and docked with the ligand DBP. Thereafter, through heterologous expression and PAEs hydrolysis ability investigation, 5 enzymes were verified to hydrolyze the ester bonds of PAEs. This work combined genome sequence, gene annotation, molecular simulation and enzymatic PAEs hydrolysis analysis to efficiently identify the hydrolases capable for hydrolyzing the ester bonds of phthalates from A. indicus species, and will promote the application of the strain in actual Baijiu manufacturing process.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 32072165), Beijing Municipal Natural Science Foundation & Beijing Municipal Education Commission (No. KM202110011003).
Data availability
Genome sequence of Acinetobacter indicus WMB-7 was deposited to NCBI GenBank with accesssion number CP046392.1. The BioProject accession number was PRJNA590557, and the BioSample accession number was SAMN13333292.
Strain availability
The strain Acinetobacter indicus WMB-7 and all the transformants generated for this study are available on request to the corresponding author.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Huiqin Huang and Youqiang Xu have contributed equally to this work.
Contributor Information
Youqiang Xu, Email: xuyouqiang@btbu.edu.cn.
Xiuting Li, Email: lixt@btbu.edu.cn.
References
- Aparna G, Chatterjee A, Sonti RV, Sankaranarayanan R. A cell wall-degrading esterase of Xanthomonas oryzae requires a unique substrate recognition module for pathogenesis on rice. Plant Cell. 2009;21:1860–1873. doi: 10.1105/tpc.109.066886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek M, DiMaio F, Anishchenko I, Dauparas J, Ovchinnikov S, Lee GR, Wang J, Cong Q, Kinch LN, Schaeffer RD, Millan C, Park H, Adams C, Glassman CR, DeGiovanni A, Pereira JH, Rodrigues AV, van Dijk AA, Ebrecht AC, Opperman DJ, Sagmeister T, Buhlheller C, Pavkov-Keller T, Rathinaswamy MK, Dalwadi U, Yip CK, Burke JE, Garcia KC, Grishin NV, Adams PD, Read RJ, Baker D. Accurate prediction of protein structures and interactions using a three-track neural network. Science. 2021;373:871–876. doi: 10.1126/science.abj8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H, Henrick K, Nakamura H. Announcing the worldwide Protein Data Bank. Nature Structural Biology. 2003;10:980–980. doi: 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
- Bordoli L, Kiefer F, Arnold K, Benkert P, Battey J, Schwede T. Protein structure homology modeling using SWISS-MODEL workspace. Nature Protocols. 2009;4:1–13. doi: 10.1038/nprot.2008.197. [DOI] [PubMed] [Google Scholar]
- Dong W, Guo RN, Sun XT, Li HH, Zhao MM, Zheng FP, Sun JY, Huang MQ, Wu JH. Assessment of phthalate ester residues and distribution patterns in Baijiu raw materials and Baijiu. Food Chemistry. 2019;283:508–516. doi: 10.1016/j.foodchem.2019.01.069. [DOI] [PubMed] [Google Scholar]
- Duan XJ, Xiang M, Wang L, Yan QJ, Yang SQ, Jiang ZQ. Biochemical characterization of a novel lipase from Malbranchea cinnamomea suitable for production of lipolyzed milkfat flavor and biodegradation of phthalate esters. Food Chemistry. 2019;297:124925. doi: 10.1016/j.foodchem.2019.05.199. [DOI] [PubMed] [Google Scholar]
- Fan YY, Liu SH, Xie QL. Rapid determination of phthalate esters in alcoholic beverages by conventional ionic liquid dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Talanta. 2014;119:291–298. doi: 10.1016/j.talanta.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Fang Y, Zhang LS, Wang J, Zhou Y, Ye BC. Biodegradation of phthalate esters by a newly isolated Acinetobacter sp strain LMB-5 and characteristics of its esterase. Pedosphere. 2017;27:606–615. doi: 10.1016/S1002-0160(17)60355-2. [DOI] [Google Scholar]
- Huang H, Zhang XY, Chen TL, Zhao YL, Xu DS, Bai YP. Biodegradation of structurally diverse phthalate esters by a newly identified esterase with catalytic activity toward di(2-ethylhexyl) phthalate. Journal of Agricultural and Food Chemistry. 2019;67:8548–8558. doi: 10.1021/acs.jafc.9b02655. [DOI] [PubMed] [Google Scholar]
- Jiao YY, Chen X, Wang X, Liao XW, Xiao L, Miao AJ, Wu J, Yang LY. Identification and characterization of a cold-active phthalate esters hydrolase by screening a metagenomic library derived from biofilms of a wastewater treatment plant. PLoS ONE. 2013;8:e75977. doi: 10.1371/journal.pone.0075977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakugawa S, Fushinobu S, Wakagi T, Shoun H. Characterization of a thermostable carboxylesterase from the hyperthermophilic bacterium Thermotoga maritima. Applied Microbiology and Biotechnology. 2007;74:585–591. doi: 10.1007/s00253-006-0687-9. [DOI] [PubMed] [Google Scholar]
- Lai JH, Huang HQ, Lin MW, Xu YQ, Li XT, Sun BG. Enzyme catalyzes ester bond synthesis and hydrolysis: the key step for sustainable usage of plastics. Frontiers in Microbiology. 2023;13:1113705. doi: 10.3389/fmicb.2022.1113705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LS, Ye WY, Zhang QY, Sun FQ, Lu P, Li XK. Catalytic ozonation of dimethyl phthalate over cerium supported on activated carbon. Journal of Hazardous Materials. 2009;170:411–416. doi: 10.1016/j.jhazmat.2009.04.081. [DOI] [PubMed] [Google Scholar]
- Li C, Liu CJ, Li RZ, Liu Y, Xie JZ, Li BW. Biodegradation of dibutyl phthalate by the new strain Acinetobacter baumannii DP-2. Toxics. 2022;10:532. doi: 10.3390/toxics10090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang RX, Wu XL, Wang XN, Dai QY, Wang YY. Aerobic biodegradation of diethyl phthalate by Acinetobacter sp JDC-16 isolated from river sludge. Journal of Central South University of Technology. 2010;17:959–966. doi: 10.1007/s11771-010-0584-3. [DOI] [Google Scholar]
- Ma TT, Christie P, Luo YM, Teng Y. Phthalate esters contamination in soil and plants on agricultural land near an electronic waste recycling site. Environmental Geochemistry and Health. 2013;35:465–476. doi: 10.1007/s10653-012-9508-5. [DOI] [PubMed] [Google Scholar]
- Mohan SV, Shailaja S, Krishna MR, Sarma PN. Adsorptive removal of phthalate ester (Di-ethyl phthalate) from aqueous phase by activated carbon: a kinetic study. Journal of Hazardous Materials. 2007;146:278–282. doi: 10.1016/j.jhazmat.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Qiu JR, Zhang YQ, Shi YN, Jiang JW, Wu SL, Li LX, Shao YT, Xin ZH. Identification and characterization of a novel phthalate-degrading hydrolase from a soil metagenomic library. Ecotoxicology and Environmental Safety. 2020;190:110148. doi: 10.1016/j.ecoenv.2019.110148. [DOI] [PubMed] [Google Scholar]
- Sharma N, Kumar V, Maitra SS, Lakkaboyana SK, Khantong S. DBP biodegradation kinetics by Acinetobacter sp33F in pristine agricultural soil. Environmental Technology & Innovation. 2021;21:101240. doi: 10.1016/j.eti.2020.101240. [DOI] [Google Scholar]
- Sheik SS, Sundararajan P, Hussain ASZ, Sekar K. Ramachandran plot on the web. Bioinformatics. 2002;18:1548–1549. doi: 10.1093/bioinformatics/18.11.1548. [DOI] [PubMed] [Google Scholar]
- Singh N, Dalal V, Mahto JK, Kumar P. Biodegradation of phthalic acid esters (PAEs) and in silico structural characterization of mono-2-ethylhexyl phthalate (MEHP) hydrolase on the basis of close structural homolog. Journal of Hazardous Materials. 2017;338:11–22. doi: 10.1016/j.jhazmat.2017.04.055. [DOI] [PubMed] [Google Scholar]
- Sun JQ, Wu XQ, Gan J. Uptake and metabolism of phthalate esters by edible plants. Environmental Science & Technology. 2015;49:8471–8478. doi: 10.1021/acs.est.5b01233. [DOI] [PubMed] [Google Scholar]
- Sun XT, Dong W, Liu M, Shen CH, Zhang YY, Sun JY, Sun BG, Li HH, Chen F. Validation of a QuEChERS-based gas chromatography-mass spectrometry (GC-MS) method for analysis of phthalate esters in grain sorghum. Journal of Food Science. 2018;83:892–901. doi: 10.1111/1750-3841.14063. [DOI] [PubMed] [Google Scholar]
- Tam B, Sinha S, Wang SM. Combining Ramachandran plot and molecular dynamics simulation for structural-based variant classification: Using TP53 variants as model. Computational and Structural Biotechnology Journal. 2020;18:4033–4039. doi: 10.1016/j.csbj.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WB, Zhang Y, He XS, Xi BD, Gao RT, Mao XH, Huang CH, Zhang H, Li D, Liang Q, Cui DY, Alshawabkeh AN. Distribution patterns of phthalic acid esters in soil particle-size fractions determine biouptake in soil-cereal crop systems. Scientific Reports. 2016;6:31987. doi: 10.1038/srep31987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian MP, Zhang X, Liu LP, Martin FL, Wang H, Zhang J, Huang QY, Wang XF, Shen HQ. Phthalate side-chain structures and hydrolysis metabolism associated with steroidogenic effects in MLTC-1 Leydig cells. Toxicology Letters. 2019;308:56–64. doi: 10.1016/j.toxlet.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Wang WH, Li XT, Xu YQ, Li WW, Sun BG. Bioinformatics analysis of esterase and degradation mechanism of PAEs came from Acinetobacter indicus. Journal of Chinese Institute of Food Science and Technology. 2021;21:31–41. [Google Scholar]
- Wang XJ, Shen S, Wu H, Wang HX, Wang LJ, Lu ZM. Acinetobacter tandoii ZM06 assists Glutamicibacter nicotianae ZM05 in resisting cadmium pressure to preserve dipropyl phthalate biodegradation. Microorganisms. 2021;9:1417. doi: 10.3390/microorganisms9071417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liao XW, Yu FB, Wei ZB, Yang LY. Cloning of a dibutyl phthalate hydrolase gene from Acinetobacter sp strain M673 and functional analysis of its expression product in Escherichia coli. Applied Microbiology and Biotechnology. 2013;97:2483–2491. doi: 10.1007/s00253-012-4232-8. [DOI] [PubMed] [Google Scholar]
- Xu YQ, Sun BG, Fan GS, Teng C, Xiong K, Zhu YP, Li JL, Li XT. The brewing process and microbial diversity of strong flavour Chinese spirits: a review. Journal of the Institute of Brewing. 2017;123:5–12. doi: 10.1002/jib.404. [DOI] [Google Scholar]
- Xu YQ, Minhazul KAHM, Wang XC, Liu X, Li XT, Meng Q, Li HH, Zhang CN, Sun XT, Sun BG. Biodegradation of phthalate esters by Paracoccus kondratievae BJQ0001 isolated from Jiuqu (Baijiu fermentation starter) and identification of the ester bond hydrolysis enzyme. Environmental Pollution. 2020;263:114506. doi: 10.1016/j.envpol.2020.114506. [DOI] [PubMed] [Google Scholar]
- Xu YQ, Liu X, Zhao JR, Huang HQ, Wu MQ, Li XT, Li WW, Sun XT, Sun BG. An efficient phthalate ester-degrading Bacillus subtilis: Degradation kinetics, metabolic pathway, and catalytic mechanism of the key enzyme. Environmental Pollution. 2021;273:116461. doi: 10.1016/j.envpol.2021.116461. [DOI] [PubMed] [Google Scholar]
- Xu YQ, Huang HQ, Lu HY, Wu MQ, Lin MW, Zhang CS, Zhao ZG, Li WW, Zhang CN, Li XT, Sun BG. Characterization of an Aspergillus niger for efficient fatty acid ethyl ester synthesis in aqueous phase and the molecular mechanism. Frontiers in Microbiology. 2022;12:820380. doi: 10.3389/fmicb.2021.820380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YQ, Zhao JR, Huang HQ, Guo XY, Li XT, Zou W, Li WW, Zhang CN, Huang MQ. Biodegradation of phthalate esters by Pantoea dispersa BJQ0007 isolated from Baijiu. Journal of Food Composition and Analysis. 2022;105:104201. doi: 10.1016/j.jfca.2021.104201. [DOI] [Google Scholar]
- Xu YQ, Wu MQ, Niu JL, Lin MW, Zhu H, Wang K, Li XT, Sun BG. Characteristics and correlation of the microbial communities and flavor compounds during the first three rounds of fermentation in Chinese sauce-flavor Baijiu. Foods. 2023;12:207. doi: 10.3390/foods12010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YQ, Zhao JR, Liu X, Zhang CS, Zhao ZJ, Li XT, Sun BG. Flavor mystery of Chinese traditional fermented baijiu: the great contribution of ester compounds. Food Chemistry. 2022;369:130920. doi: 10.1016/j.foodchem.2021.130920. [DOI] [PubMed] [Google Scholar]
- Yuan BL, Li XZ, Graham N. Reaction pathways of dimethyl phthalate degradation in TiO2-UV-O2 and TiO2-UV-Fe(VI) systems. Chemosphere. 2008;72:197–204. doi: 10.1016/j.chemosphere.2008.01.055. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Huang B, Thomsen M, Sabel CE, Hess F, Hu WY, Tian K. One overlooked source of phthalate exposure - oral intake from vegetables produced in plastic greenhouses in China. Science of the Total Environment. 2018;642:1127–1135. doi: 10.1016/j.scitotenv.2018.06.112. [DOI] [PubMed] [Google Scholar]
- Zhao JR, Xu YQ, Lu HY, Zhao D, Zheng J, Lin MW, Liang X, Ding Z, Dong WQ, Yang MC, Li WW, Zhang CN, Sun BG, Li XT. Molecular mechanism of LIP05 derived from Monascus purpureus YJX-8 for synthesizing fatty acid ethyl esters under aqueous phase. Frontiers in Microbiology. 2023;13:1107104. doi: 10.3389/fmicb.2022.1107104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genome sequence of Acinetobacter indicus WMB-7 was deposited to NCBI GenBank with accesssion number CP046392.1. The BioProject accession number was PRJNA590557, and the BioSample accession number was SAMN13333292.
The strain Acinetobacter indicus WMB-7 and all the transformants generated for this study are available on request to the corresponding author.