Abstract
Purpose:
With increasing utilization of genetic testing, sharing genetic information can become part of general family health communication while providing biological relatives with important information about their own genetic risk. Importantly, little is known about motivations for and barriers to family communication of genetic information in historically underserved populations.
Methods:
Using mixed methods, we explored patient experiences with family communication in a study population of English- and Spanish-speaking adults aged 18-49 years enriched for participants from historically underserved backgrounds. Risk screening for hereditary cancer guided genetic testing for cancer risk genes and other medically actionable findings.
Results:
Most participants overall (91%), including most with normal findings (89%), shared or planned to share their results with relatives. Common motivations for sharing results were to give relatives information about their genetic risk and because the participant thought the results were interesting. Reasons for not sharing were limited contact with relatives, perceptions of limited clinical utility for relatives, and concern that discussion of genetic information was stigmatized or taboo.
Conclusion:
Results demonstrate high rates of sharing genetic information, indicate motivations for sharing go beyond facilitating genetic testing for relatives, and suggest general willingness to share genetic information as part of family health communication.
Keywords: hereditary cancer, genetic counseling, family communication, cascade testing, historically underserved
Introduction
With both clinical and direct-to-consumer genetic testing on the rise1, genetic information can become a more common factor in family health communication. Some people may share their genetic findings with biological relatives because it is relevant to their health as well2 and relatives can make decisions about seeking genetic counseling and testing (i.e., cascade testing)3. Studies of individuals with a pathogenic or likely pathogenic (P/LP) variant for dominant, medically actionable conditions (e.g., BRCA1/2 and risk for breast and ovarian cancer) indicate high rates of family communication, with up to 99% of participants sharing genetic information with at least one first degree relative2,4–6. Other individuals may want to share their personal reactions to or feelings about genetic test results7. Indeed, prior studies have reported rates of family communication of normal test results (i.e., no P/LP variant or variant of uncertain significance [VUS] reported) of up to 99%4,8. Thus, motivations for sharing genetic information with relatives go beyond facilitating cascade testing. Overall, family history and genetic risk for health outcomes are important aspects of clinical health risk assessment; thus, regardless of motivation, communication about genetic risk information among families provides relatives with valuable health-relevant information.
Most studies of family communication of genetic information have been limited to study populations primarily of European descent and middle to high socioeconomic status. Two studies assessed populations with economic and/or racial diversity; both reported high rates of sharing genetic test results with relatives. One surveyed 1103 individuals heterozygous for BRCA1/2 P/LP variants who were predominantly White, served by both public and private hospitals, and had a range of economic backgrounds; 97% shared their result with at least one relative4. A second interviewed 73 individuals heterozygous for BRCA1/2 P/LP variants, a majority of whom were African American, Asian/Pacific Islander, Hispanic, or identified as more than one race, and many of whom had public insurance or were served by a public hospital9. Participants had shared genetic test results with 73% of their first- and second-degree relatives. These findings suggest high rates of sharing genetic information in diverse study populations; however, they focused only on individuals with BRCA1/2 variants and did not evaluate sharing genetic information for other medically actionable conditions, sharing normal genetic results, or motivations for and barriers to communication.
Using mixed-methods, we explored rates of sharing genetic test results with biological relatives, motivations for and barriers to sharing, and uptake of cascade testing in relatives among adults primarily from historically underserved populations who underwent genetic testing for hereditary cancer and other medically actionable conditions.
Methods
Study Population
Participants were healthy adults ages 18 to 49 who spoke English or Spanish recruited as part of the Cancer Health Assessments Reaching Many (CHARM) study10, a Clinical Sequencing Evidence-generating Research (CSER) Consortium project11. Participants were recruited from two healthcare systems: Kaiser Permanente Northwest, an integrated healthcare system in Oregon and southwest Washington, and Denver Health, an integrated safety-net health system in Colorado. All study procedures were reviewed and approved by the Kaiser Permanente Northwest Institutional Review Board (IRB). This study was conducted in accordance with the ethical standards outlined in the U.S. Federal Policy for the Protection of Human Subjects (also known as the Common Rule). Informed consent was obtained from all study participants prior to enrollment in the study.
Individuals were eligible for CHARM if they screened positive on a hereditary cancer risk assessment12 or if they had limited knowledge of their family cancer history due to factors such as adoption or small family size. Genetic findings returned to participants included P/LP variants and VUS from 39 genes associated with increased cancer risk. Participants could also opt in for P/LP variants from 77 genes associated with non-cancer medically actionable conditions. A subset of participants could opt in for carrier screening for pathogenic variants in 14 genes. More detail about genes and conditions tested can be found elsewhere10.
During result disclosure, study genetic counselors reviewed any clinical implications and options for genetic testing for biological relatives. However, some participants with a negative finding received their results by letter with the option to speak with a genetic counselor. Participants with P/LP findings in cancer or medically actionable genes were informed their first- and second-degree relatives could undergo genetic testing for the familial variant, at no cost through the study or through their healthcare provider. These participants were given a letter and a copy of their genetic test results to share; relatives interested in cascade testing through the study could contact the study team to provide consent and a saliva sample. Outreach and uptake of cascade testing by eligible relatives was tracked by the study team.
In some instances, irrespective of the participant’s genetic result, the genetic counselor would discuss how some relatives could benefit from comprehensive genetic testing, such as relatives with a personal cancer history. When a VUS was detected, testing other relatives for the VUS was discussed by the genetic counselor if offered by the testing laboratory for aiding VUS interpretation, though not common; VUS family testing to inform variant pathogenicity was not in the CHARM study protocol.
Survey Data Collection
Self-reported demographic data was collected via survey at enrollment. Six months after result disclosure, a survey queried participants about sharing their genetic test results with relatives, including if they had shared their results and, if so, with whom, and reasons for sharing or not sharing. Fixed choice and open-ended questions were used. Survey questions are publicly available (https://cser-consortium.org/resources) and are included in Supplemental Materials.
Survey Data Analysis
Descriptive statistics summarized study participant demographic characteristics and survey response patterns. Participants were divided into four groups based on type of genetic finding: (1) participants with a P/LP variant in a cancer or other medically actionable gene (“positive finding”) and were encouraged to share genetic results with relatives to facilitate cascade testing; (2) participants with a heterozygous finding, including participants with a P variant detected through carrier screening and participants with a single MUTYH P/LP variant; (3) participants with a VUS in a cancer gene; and (4) participants with normal findings. Participants with more than one genetic finding was assigned to the group with the most clinical relevance (e.g., participant with a heterozygous finding and a VUS in a cancer gene was assigned to the heterozygous group). We do not report our qualitative data using numbers given our interviews were semi-structured, and thus interviewers may not have asked all questions on the guide in each interview or in the same way in each interview.
Qualitative Interviews
Qualitative interviews were conducted in English or Spanish within one month of result disclosure and again six months after result disclosure in alignment with survey timepoints. Recruitment aimed to oversample for Spanish-preferring participants (only 13% of CHARM participants were Spanish-preferring) and participants with positive findings (only 5% of CHARM participants had a positive finding) to reach saturation in each category. One-hour, semi-structured telephone interviews asked about experiences of participating in and receiving genetic test results through the CHARM study. Interview topics included family dynamics that influence health communication, implications of genetic test results for relatives, sharing genetic test results with relatives, and cascade testing; six-month interviews additionally included family communication and cascade testing. Qualitative interview findings from other topics are presented elsewhere13.
Interviews were conducted by masters and PhD-level researchers with expertise in the social sciences and qualitative research using interview guides that explored topics such as approach to health and healthcare, familiarity and prior experience with genetics, perception of genetic counseling communication, preparation for and understanding of results, and attitudes toward recommended care and follow-up with primary care or specialist providers (see Supplementary Material for interview guides used at each timepoint). Interviews were audio recorded, transcribed, (for interviews conducted in Spanish) translated to English, then analyzed using a grounded theory approach, an iterative process involving parsing data according to themes, developing and classifying (coding) themes and concepts, and identifying associations among themes14. Our team collectively developed a preliminary codebook based on initial review of the one-month interview transcripts and further refined it with subsequent coding and review of the six-month interview transcripts. We initially coded for “family communication,” and then conducted additional analyses of coded data related to family communication to complement and inform survey results. Each transcript was coded by two researchers using Dedoose Version 8.3.43 qualitative data analysis software15; discrepancies were resolved through consensus. Emerging themes were discussed with the research team.
Results
Study Participant Characteristics
Survey data from 562 CHARM participants who received genetic test results and completed survey questions on family communication were available for analysis (Table 1). Participants in the study sample were diverse in terms of race and ethnicity, language, and income. For race and ethnicity, the most common responses were non-Hispanic white (47%), Hispanic (30%), and more than one race and ethnicity category (9%). In addition, 13% preferred speaking Spanish and 34% had a household income less than $40,000. The genetic findings of participants have been reported elsewhere16. In brief, 28 of 562 (5%) participants had a P/LP variant associated with cancer risk or other medically actionable finding, including 10 with a variant in a gene associated with hereditary breast and ovarian cancer (BRCA1 and BRCA2) and 7 with a variant in a gene associated with Lynch syndrome (MLH1, MSH2, MSH6, PMS2). Qualitative interviews were conducted with 33 participants within one month of result disclosure (Table 1). We recontacted 17 interview participants with a positive finding for a second interview six months after results disclosure; 6 passively declined and 11 were interviewed. Although we initially planned to conduct second interviews with all interviewees, we determined that additional interviews with participants who had VUS or negative results were not necessary as these participants were not recommended to take any action during that time period and thus did not have a lot to add to their initial interviews.
Table 1.
Study Population Demographics
| Domain | Values | Survey Participants N=562 | Interview Participants N=33 |
|---|---|---|---|
|
| |||
| Age | Mean (SD) | 36.4 (8.0) | 37.5 (8.0) |
|
| |||
| Race | Asian | 29 (5.2%) | 1 (3.0%) |
| Black | 28 (5.0%) | 2 (6.1%) | |
| Hispanic | 170 (30.3%) | 14 (42.4%) | |
| Middle Eastern | 5 (0.9%) | - | |
| Native American | 12 (2.0%) | - | |
| Pacific Islander | 4 (0.7%) | - | |
| White | 262 (46.6%) | 14 (42.4%) | |
| Multiracial, non-Hispanic | 28 (5.0%) | 1 (3.0%) | |
| Multiracial, Hispanic | 23 (4.1%) | 1 (3.0%) | |
| Unknown | 1 (0.2%) | - | |
|
| |||
| Gender Identity a | Female | 403 (71.7%) | 23 (69.7%) |
| Male | 108 (19.2%) | 6 (18.2%) | |
| Transgender female | 0 (0%) | - | |
| Transgender male | 4 (0.7%) | 1 (3.0%) | |
| Non-binary/Genderqueer | 8 (1.4%) | 1 (3.0%) | |
| Not sure/questioning | 0 (0%) | - | |
| Another gender identity | 0 (0%) | - | |
| Prefer not to answer | 5 (0.9%) | - | |
|
| |||
| Level of Education b | Some high school or less | 56 (10.0%) | 4 (12.2%) |
| High school or associate collegec degree | 232 (41.3%) | 12 (36.4%) | |
| Bachelors or graduate degree | 241 (42.9%) | 15 (45.5%) | |
|
| |||
| Household Income d | <$39,999 | 191 (34.0%) | 14 (42.4%) |
| $40-79,999 | 184 (32.7%) | 12 (36.4%) | |
| ≥$80,000 | 152(27.0%) | 5 (15.2%) | |
|
| |||
| Language Chosen for Survey or Interview | Spanish | 71 (12.6%) | 11 (33.3%) |
| English | 491 (87.4%) | 22 (66.7%) | |
|
| |||
| Personal History of Cancer | No | 507 (90.2%) | 29 (87.9%) |
| Yes | 55 (9.8%) | 4 (12.1%) | |
|
| |||
| Genetic Finding e | Positive finding | 28 (5.0%) | 13 (39.4%) |
| Heterozygous Findingf | 54 (8.9%) | 6 (18.2%) | |
| VUS | 43 (7.7%) | 8 (24.2%) | |
| Normal finding | 437 (77.8%) | 6 (18.2%) | |
P/LP=pathogenic or likely pathogenic; VUS=variant of uncertain significance
Gender identity missing for 39 participants, including 2 interviewed participants;
Level of education missing for 33 participants, including 2 interviewed participants;
Includes occupational, technical, or vocational programs;
Household income missing for 35 participants, including 2 interviewed participants;
Participants who had more than 1 finding were only counted once and classified based on the more clinically significant finding (e.g., a participant with a carrier finding and a VUS was classified as having a carrier finding);
Includes participants with a single P variant from carrier screening and participants with a single MUTYH P/LP variant
Sharing Study Genetic Test Results with Biological Relatives
Survey Responses
Most participants (83%) who had biological relatives indicated they had shared their genetic test results and 8% indicated they hadn’t shared results yet but intended to in the future. This high rate of sharing was consistent across the type of result; 89% of participants with normal results either had shared or planned to share (Figure 1). Among the 28 participants with a positive result, 82% had shared and another 14% had not shared but planned to share, and only 1 (4%) did not share or plan to share this information with relatives.
Figure 1.
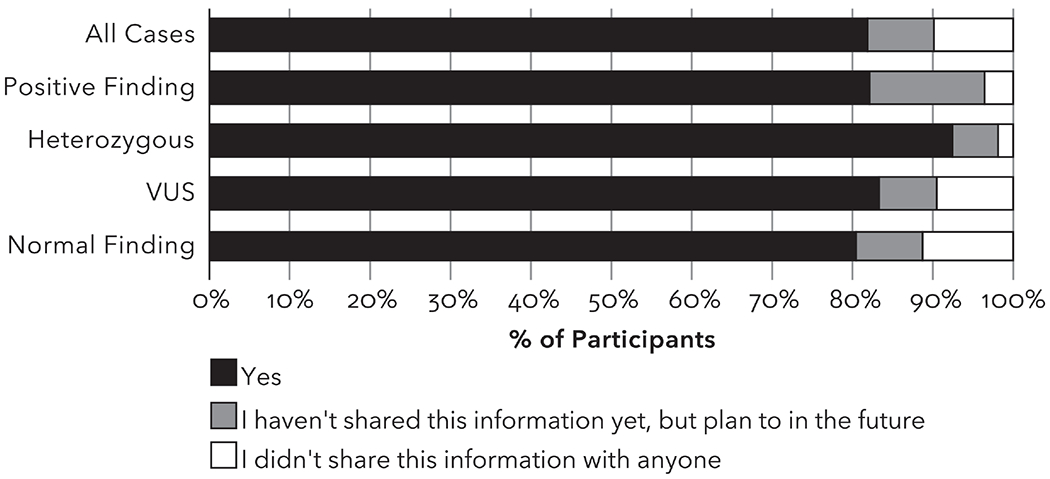
Whether study participants shared their study results with their biological relatives (n=558). Heterozygous group includes participants with a single P/LP MUTYH variant.
Participants who shared their results were asked who they talked to about their result (Figure 2). In 409 participants with ≥1 living parent, 87% had shared with a parent. In 408 participants with ≥1 living sibling, 78% had shared with a sibling. In 269 participants with ≥1 living child, 62% had shared with a child. Forty-one percent of participants indicated they had shared biological relatives beyond the first-degree relatives listed in the survey. Open text responses indicated participants had shared with aunts, uncles, cousins, grandparents, nieces, and nephews.
Figure 2:
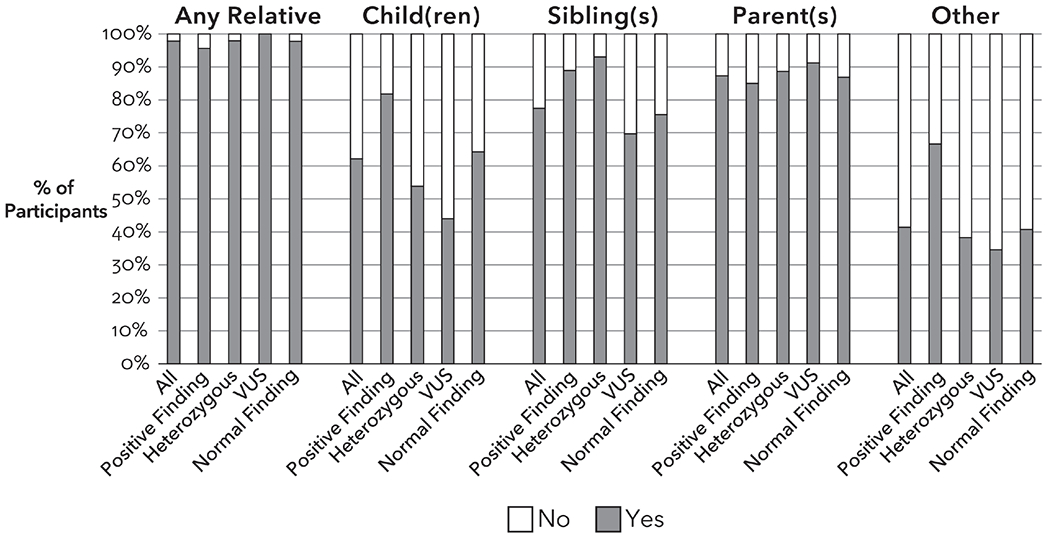
Sharing with specific biological relatives among study participants who shared with at least one biological relative (n=457). Heterozygous group includes participants with a single P/LP MUTYH variant. Results do not include “Not Applicable” responses for each relative type.
Interview Findings
Consistent with survey results, participants interviewed had shared their genetic test results with at least one biological relative by the first interview, regardless of result type. Similar to survey results, participants reported sharing their results with parents, siblings, children, aunts, uncles, nieces, and cousins. A few participants who had not shared their results as of the first interview indicated that while they planned to share, they preferred to keep the results to themselves for a while as they processed them or were waiting to share the results in person. As one participant explained,
I just told my mom but other than that I didn’t want anyone else to know…In the future yes, but not yet…I want my time and space to take it in. I don’t want people asking me about [it] or anything about what I’m going to do or ‘Did you go to the doctor?’ I don’t want any of that. (BRCA2 (Pathogenic [P]), 1st interview).
Information Shared with Relatives and How it Was Shared
Survey Responses
Among participants who shared genetic test results with relatives, most reported sharing general information about their results (92%) (Figure 3). Some (40%) talked to relatives about the relatives’ risk. Sixty-five percent of participants with a positive finding indicated they talked to their relatives about the relatives’ risk, a higher rate than participants with a heterozygous finding (55%), VUS (43%), or normal results (36%). To facilitate cascade testing, the study genetic counselors provided participants with a positive finding with a letter and a copy of their genetic test results to share with their relatives. The survey did not ask whether participants shared the letter and copy of results provided by genetic counselors specifically but did ask the method of communication to relatives. Most participants reported sharing results directly by phone (68%) and in person (58%). Other methods included email (4%), social media (3%), and text messaging (one participant; <1%).
Figure 3.
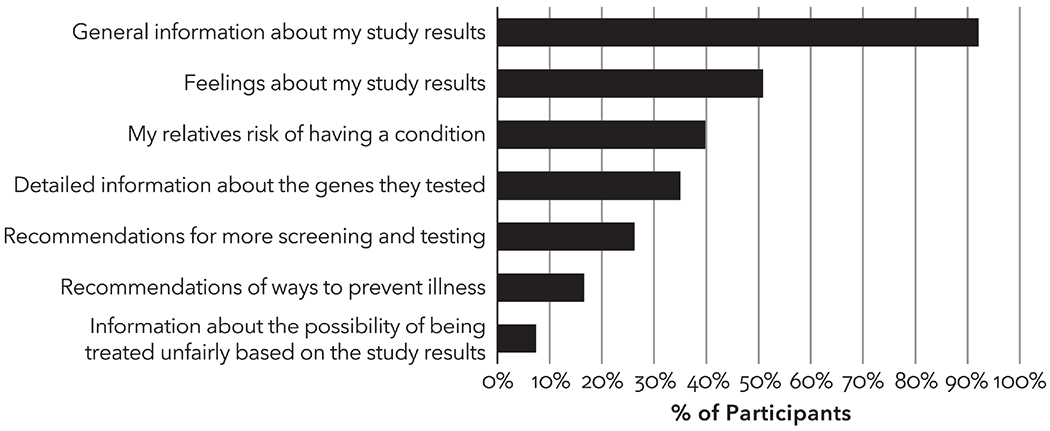
What information was shared by study participants who shared genetic test results with biological relatives (n=457). More than one response could be selected.
Interview Findings
Interview participants reported they shared their results in person, by phone, and by email. One participant whose relatives had the same variant started a group chat with her family:
Sometimes it’s just a group text message within the family. Like for this genetic result, I just texted my family that I had that CHEK2 mutation that was also found that my sister and my mom both have. I mean, it wasn’t necessarily great news but it wasn’t news that, you know, warranted a full-on family sit-down intervention or something like that. And if it’s something more severe or that needed like full-on attention, then I think you would probably just do an actual phone call, I guess. (CHEK2 (P), 1st interview).
Some interview participants reported sharing results by providing a copy of the CHARM study report or family letter. Most with a positive result relayed the risks of health conditions conferred by the genetic variant to their relatives. Many of these participants encouraged cascade testing for relatives and, in some cases, relatives’ adult children. Although study genetic counselors recommended that relatives of participants with a VUS should not receive cascade testing to inform their medical care, several participants with VUS results reported encouraging cascade testing to their family members.
Reasons for Sharing Genetic Test Results with Biological Relatives
Survey Responses
Among participants who shared their genetic test results with relatives or planned to do so in the future, the reasons rated as most important for sharing results were to give their relatives information about their genetic risk and because they thought it was interesting (Figure 4). In open text comments, some participants responded they had shared because the results were relevant to their family health history; they wanted to ease the relative’s mind; they wanted to encourage open communication about family health information; and they wanted to compare genetic test results with relatives who had also had genetic testing.
Figure 4.
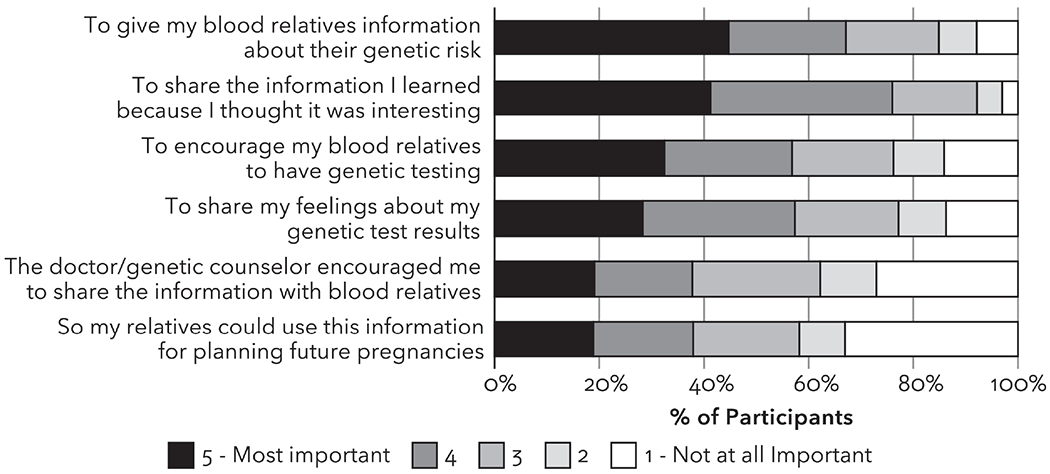
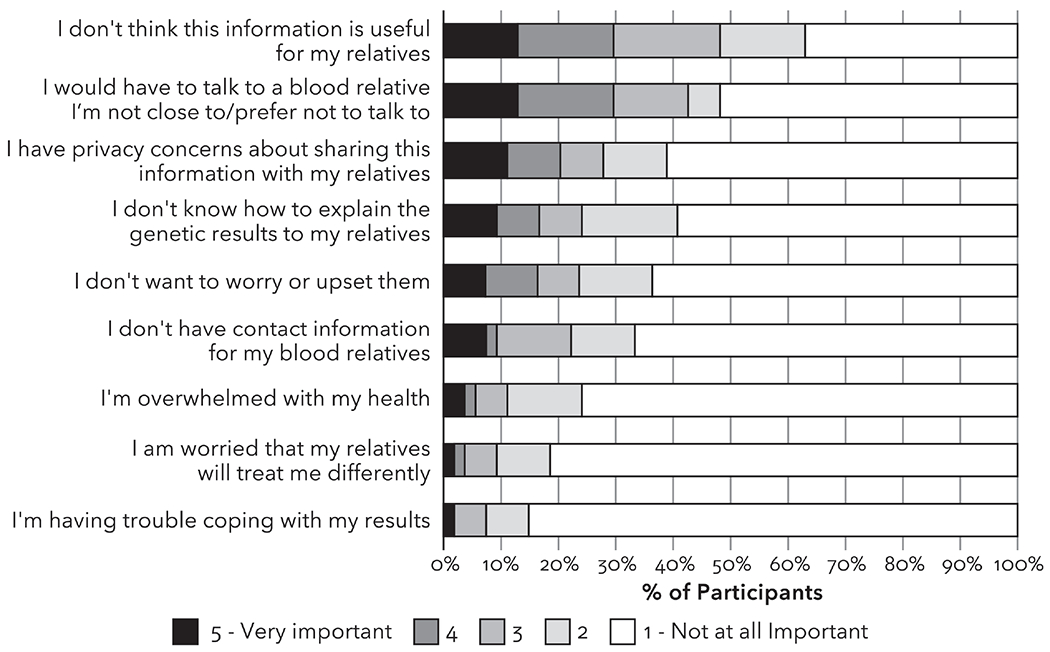
Perspectives among participants who shared their genetic test results (n=457) on the importance of various reasons for sharing their genetic test results with their relatives.
Interview Findings
Participants cited various reasons for sharing their results with relatives; primary among them was the perceived risk to their relatives and to relay the importance of cascade testing for those with a positive finding. As one participant said,
My daughter was the most important that I talked to…And my daughter has one daughter already and I talked to her about it and she said she had already talked to her doctor about it as well. And so she’s going to be looking into getting tested too ‘cause I gave her a copy of that letter. My boys, they don’t seem too concerned because they don’t have, you know, the same things as women but I know they still should get tested. So I’m trying to convince them to do it. (BRCA2 (P), 1st interview).
Even when the information was not immediately actionable for the relative, as in the case of a participant’s young child, they often felt having a note about their positive results in their child’s medical record was important and planned to share it with them when they reached screening or reproductive age. Several participants also indicated their results provided important insight into their family’s health history, and that sharing results would be informative for their relatives, especially those personally affected.
Participants with normal results gave various reasons for sharing results with relatives, such as to put their relatives at ease; while others understood their results did not explain previous cases of cancer in their family. One participant with a strong family history of cancer noted that the normal result left her with questions about her and her son’s risk, given that she had heard that cancer can “skip a generation.” She shared her results with her only other surviving relative, who had a personal history of cancer and a fascination with genetics. Indeed, relatives’ general curiosity about genetics, or the CHARM study specifically, was a factor in many participants’ decisions to share their results. Most had told their relatives about their participation in the study and had planned on sharing the genetic findings even before they received them.
Reasons for Not Sharing Genetic Test Results with Relatives
Survey Responses
Fifty-five participants (10%) did not share their genetic test results with any relatives with reasons for not sharing most commonly endorsed as very important were, “I would have to talk to a blood relative I am not close to or prefer not to talk to” (13%), “I don’t think this information is useful for my relatives” (13%), and “I have privacy concerns about sharing this information with my relatives” (11%). Only one of the 28 participants with a positive finding indicated they had not shared their genetic test results with any relative; this participant only selected privacy concerns as a very important reason for not sharing.
Interview Findings
Interview participants who had not shared their genetic test results with any relatives or who shared with certain relatives but not others, were asked about reasons for not sharing their results. Consistent with survey results, limited family contact and a perceived lack of clinical utility or immediate actionability for the relative (in the case of normal or VUS results) were factors; additionally, some chose not to inform older relatives due to the belief that it was not worth worrying them or they had other competing health priorities. As one participant explained:
I don’t have any brothers and sisters and very little as far as cousins go. I have like one second cousin that I talk to. And my dad, he’s had a stroke so he’s kind of not 100% there, so I don’t think he’s too worried about it either.” (BRCA2 (P), 1st interview).
Family dynamics, including wariness about genetic testing, taboos around talking about health, and estranged relationships contributed to participants not sharing or planning to share their results with some relatives. One participant who had shared the results only with his mother and fiancé said,
Even when I told my family that I was doing it, they were kind of wary about it…they implied that there was like a stigma with getting genetically tested. But my family doesn’t really discuss much about how they feel…We don’t talk about [health]… Like I didn’t know my dad had colon cancer until three months afterwards. (MUTYH (P), 1st interview).
Another participant had only shared the results with a friend but planned to tell her mother and brothers explained,
I mean, we’re Latino so it’s like, you know, a lot of stuff is very taboo in my culture. But I think growing up in the States… my brothers and I, we talk about a lot of stuff, especially when it comes to health. My grandma will talk about it but, you know, she grew up in a time when you didn’t really share a lot of like your personal like health things. (CDH1 (VUS), 1st interview).
Misunderstandings about the heritability and actionability of results for relatives also played a role in participants’ decisions not to share their results, at least initially. As one participant with a BRCA1 result, who had shared the results with his mother and sister (who had already had normal genetic testing) said,
I think [my relatives] might have a risk. My sister probably doesn’t, but my aunt, I doubt she has a risk because she is the youngest and the most further down the lineage you are, the less likely you are to have the mutation. I still probably won’t bring it up. I’m not sure if I should bring it up to my brother, because I don’t know if the mutation can affect him anyway. (BRCA1 (P), 1st interview).
However, by the second interview, he had shared the results with his aunt and one of his brothers and sent a picture of the report to other relatives.
One participant decided not to share her heterozygous variant from carrier screening with her brother, saying,
There’s nothing that can be done. So I thought no, I don’t think it’s, like, to go ahead with something that I don’t know if it will happen. I mean, if there’s nothing you can do to avoid it. You know what I mean? … If she were to tell me, you know what, if you do this, the probability goes down, or it won’t happen. So there’s nothing to be done so I don’t see the point. Maybe if it comes up in a conversation, but not like to make a call specifically for that. (GJB2 (heterozygous), 1st interview).
By the second interview, she had still not shared the results with her brother, citing busy schedules and forgetfulness. Indeed, several participants noted they had simply forgotten to share the information with their relatives.
While many participants with VUS results had shared their results with relatives, a few chose not to share them because they understood that the results conveyed more uncertainty than a positive result. For example, referring to her VUS cancer results and normal secondary and normal result from carrier screening, one participant stated,
I think that regardless, my results were two no’s and one unclear, it would not give my relatives very much information anyway. (ATM (VUS), 1st interview).
Uptake of Cascade Testing Among Eligible Biological Relatives
Eight first-degree relatives of four study participants with a positive finding (3 cancer and 1 medically actionable) reached out to the study team to receive genetic testing of the familial variant. All 8 consented and 7 provided a sample for genetic testing. The CHARM study was not contacted by relatives of the 24 remaining study participants with a positive finding. It is unknown if any relatives pursued cascade testing outside of the CHARM study such as through their own provider.
Discussion
The high rate of sharing genetic test results in this study is consistent with prior studies5,8,17, including those of more diverse study populations4,9. Our results expand upon these prior studies by including individuals with actionable findings beyond cancer risk, as well as individuals with VUS and normal results. Our findings also provide important individual- and family-level context, illustrating that motivations for sharing genetic information go beyond facilitating cascade testing, and that some participants may perceive sharing of this information as similar to sharing general health information. Qualitative data indicated that some participants experienced barriers to communication related to taboos and stigma around sharing genetic information, similar to prior findings related to uptake of genetic testing18,19. Culturally sensitive approaches to family communication of genetic risk could be explored in future studies to potentially overcome these barriers.
The availability of free cascade testing of the familial variant through the study may have incentivized sharing among participants with a positive finding. Indeed, the most common motivation for sharing was to inform relatives about their own genetic risk. Interestingly, despite 82% of participants with a positive finding indicating they had shared this finding with at least one biological relative, few relatives sought cascade testing through the study. This is consistent with prior findings of low cascade testing uptake20–23. Given most study participants had no personal cancer history but were identified as high risk based on family history11, relatives may have felt less urgency about pursuing cascade testing than if their relative had a prior cancer diagnosis.
Overall, the rate of family communication of genetic risk information in a study population enriched for individuals from historically underserved backgrounds provides important insight. Future efforts could leverage these conversations and explore approaches to facilitate cascade testing to expand equitable availability of genetic risk information. For example, individuals could be provided with appropriate guidance or educational materials to share with at-risk relatives, such as printed materials or electronic tools, to provide them with information needed to make informed decisions about whether to seek genetic testing themselves.
The high rate of family communication among participants with normal test results could, at least in part, be due to discussions with the study genetic counselor during results disclosure about how relatives who had cancer could get genetic testing even if the participant had a normal finding. However, survey and interview responses indicated a common motivation for sharing with relatives was because the information was interesting. Several participants indicated in interviews they had told relatives about their study participation who had expressed interest in learning the results. Thus, sharing of test results was motivated by their relatives’ desire to know the outcome.
Higher proportions of participants shared their genetic test results with at least one parent (87%) or at least one sibling (77%) than with at least one child (62%). This was likely due to young age of the children, given participants were aged 18-49 years. Parents, siblings, and adult children are most likely to benefit from receipt of genetic risk information given the relevance to their health and opportunities to pursue cascade testing, so these patterns of sharing may be appropriate.
A key limitation of the survey component of the study is that questions did not capture detailed information on patterns of family communication, such as the number of each type of relative the participant did and did not share with and why they may have shared with some but not all relatives. However, the qualitative interviews allowed for detailed conversations about patterns of communication. Barriers included perceived lack of relevance for some family members (e.g., breast cancer risk and male relatives) feeling that discussions of genetic information was stigmatized or taboo, and being estranged with some relatives, factors also reported in prior studies 24–26. The interviews also allowed assessment of reasons why participants who planned to share their results had not shared them yet, which included the desire to process and learn more about their results before sharing, being too busy, and forgetting. Further, given genetic testing results were returned in the context of a research study and some participants received genetic counseling, these finding may not be generalizable across all contexts of genetic testing.
This study provides important insights into family communication of genetic test results in populations typically underrepresented in genetics research across a range of result types and clinical conditions. The high rate of family communication in this study is promising, particularly as genetic testing is increasingly implemented in clinical care. Additional research is needed to further explore motivations and barriers to communication of genetic test results and uptake of cascade testing among underrepresented populations to ensure equitable access to genetic risk information that can significantly impact health outcomes.
Supplementary Material
Acknowledgements
The authors would also like to thank the participants for their time and willingness to share their perspectives, stories, and experiences. The authors would also like to thank Neon Brooks for her editorial support.
Funding Statement
This work was funded as part of the Clinical Sequencing Evidence-Generating Research (CSER) consortium funded by the National Human Genome Research Institute with co-funding from the National Institute on Minority Health and Health Disparities and the National Cancer Institute. The CSER consortium represents a diverse collection of projects investigating the application of genome-scale sequencing in different clinical settings, including pediatric and adult subspecialties, germline diagnostic testing and tumor sequencing, and specialty and primary care. This work was supported by a grant from the National Human Genome Research Institute (U01HG007292; MPIs: Wilfond, Goddard, Leo), with co-funding from the National Institute on Minority Health and Health Disparities (NIMHD) and the National Cancer Institute (NCI). And with additional support from U24HG007307 (Coordinating Center, PI Jarvik). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
CHARM study team non-author contributors:
Jake Allen, Frank Angelo, Briana L. Arnold, Cecelia Bellcross, Tiffany Bendelow, Barbara B, Biesecker, Kristin D. Breslin, Kristina F. Booker, Mikaella Caruncho, James V. Davis, Sonia Deutsch, Beth Devine, Michael O. Dorschner, Devan Duenas, Donna J. Eubanks, Heather Spencer Feigelson, Amanda S. Freed, Clay Greaney, Inga Gruß, Claudia Guerra, Boya Guo, Joan Holup, Chalinya L. Ingphakorn, Paige Jackson, Gail P. Jarvik, Charisma L. Jenkins, Leah S. Karliner, Tia Kaufmann, Erin Keast, Sarah Knerr, Alyssa H. Koomas, Stephanie A. Kraft, Mi H. Lee, Robin Lee, Sandra Soo-Jin Lee, Hannah S. Lewis, Elizabeth G. Liles, Nangel M. Lindberg, Frances Lynch, Carmit K. McMullen, Elizabeth Medina, Kathleen F. Mittendorf, Kristin R. Muessig, Sonia Okuyama, C. Samuel Peterson, Angela R. Paolucci, Rosse Rodriguez Perez, Kathryn M. Porter, Chelese L. Ransom, Ana Reyes, Sperry Robinson, Bradley A. Rolf, Alan F. Rope, Emily Schield, Jennifer L. Schneider, Kelly J. Shipman, Brian H. Shirts, Elizabeth Shuster, Sapna Syngal, Britta N. Torgrimson-Ojerio, Chinedu Ukaegbu, Meredith L. Vandermeer, Alexandra M. Varga, David L. Veenstra, W. Chris Whitebirch, Larissa Lee White.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics Declaration
The Kaiser Permanente Northwest Center for Health Research IRB approved this study (Protocol #000733); Denver Health and the University of California, San Francisco relied on this review. Informed consent was obtained from all participants as required by the IRB. This trial is registered with www.clinicaltrials.gov NCT03426878.
Conflicts of Interest
Laura Amendola is an employee and shareholder of Illumina. The remaining authors have no conflict of interest to disclose.
Data Availability Statement
De-identified datasets and the qualitative codebook will be made available upon request.
References
- 1.Brittain HK, Scott R, Thomas E. The rise of the genome and personalised medicine. Clinical Medicine. 2017;17(6):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn J, Milo Rasouly H, Vasquez-Loarte T, et al. Do research participants share genomic screening results with family members? J Genet Couns. 2022;31(2):447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts MC, Dotson WD, DeVore CS, et al. Delivery Of Cascade Screening For Hereditary Conditions: A Scoping Review Of The Literature. Health Aff (Millwood). 2018;37(5):801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung EL, Olson AD, Yu TM, Han PZ, Beattie MS. Communication of BRCA results and family testing in 1,103 high-risk women. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman S, Lahad A, Tomer A, et al. Familial communication and cascade testing among relatives of BRCA population screening participants. Genet Med. 2018;20(11):1446–1454. [DOI] [PubMed] [Google Scholar]
- 7.Smit AK, Bartley N, Best MC, et al. Family communication about genomic sequencing: A qualitative study with cancer patients and relatives. Patient Educ Couns. 2021;104(5):944–952 [DOI] [PubMed] [Google Scholar]
- 8.Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24(4):700–706. [DOI] [PubMed] [Google Scholar]
- 9.Fehniger J, Lin F, Beattie MS, Joseph G, Kaplan C. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns. 2013;22(5):603–612. [DOI] [PubMed] [Google Scholar]
- 10.Mittendorf KF, Kauffman TL, Amendola LM, et al. Cancer Health Assessments Reaching Many (CHARM): A clinical trial assessing a multimodal cancer genetics services delivery program and its impact on diverse populations. Contemp Clin Trials. 2021;106:106432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amendola LM, Berg JS, Horowitz CR, et al. The Clinical Sequencing Evidence-Generating Research Consortium: Integrating Genomic Sequencing in Diverse and Medically Underserved Populations. Am J Hum Genet. 2018;103(3):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittendorf KF, Lewis HS, Duenas DM, et al. Literacy-adapted, electronic family history assessment for genetics referral in primary care: patient user insights from qualitative interviews. Hered Cancer Clin Pract. 2022;20(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph G, Leo MC, Riddle L, et al. An accessible, relational, inclusive, and actionable (ARIA) model of genetic counseling compared with usual care: Results of a randomized controlled trial. Genet Med. 2022;24(11):2228–2239. [DOI] [PubMed] [Google Scholar]
- 14.Strauss A, Corbin J. Grounded theory in practice. Thousand Oaks: Sage; 1997. [Google Scholar]
- 15.Dedoose Web Application for Managing, Analyzing, and Presenting Qualitative and Mixed Method Research Data [computer program]. Sociocultural Research Consultants, LLC; 2018. [Google Scholar]
- 16.Amendola LM, Shuster E, Leo MC, et al. Laboratory-related outcomes from integrating an accessible delivery model for hereditary cancer risk assessment and genetic testing in populations with barriers to access. Genet Med. 2022;24(6):1196–1205. [DOI] [PubMed] [Google Scholar]
- 17.Stoffel EM, Ford B, Mercado RC, et al. Sharing genetic test results in Lynch syndrome: communication with close and distant relatives. Clin Gastroenterol Hepatol. 2008;6(3):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee R, Beattie M, Crawford B, et al. Recruitment, genetic counseling, and BRCA testing for underserved women at a public hospital. Genet Test. 2005;9(4):306–312. [DOI] [PubMed] [Google Scholar]
- 19.Sherman KA, Miller SM, Shaw LK, Cavanagh K, Sheinfeld Gorin S. Psychosocial approaches to participation in BRCA1/2 genetic risk assessment among African American women: a systematic review. J Community Genet. 2014;5(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landsbergen K, Verhaak C, Kraaimaat F, Hoogerbrugge N. Genetic uptake in BRCA-mutation families is related to emotional and behavioral communication characteristics of index patients. Fam Cancer. 2005;4(2):115–119. [DOI] [PubMed] [Google Scholar]
- 21.Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18(1):127–135. [DOI] [PubMed] [Google Scholar]
- 22.Miller EM, Wang Y, Ware SM. Uptake of cardiac screening and genetic testing among hypertrophic and dilated cardiomyopathy families. J Genet Couns. 2013;22(2):258–267. [DOI] [PubMed] [Google Scholar]
- 23.Sharaf RN, Myer P, Stave CD, Diamond LC, Ladabaum U. Uptake of genetic testing by relatives of lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11(9):1093–1100. [DOI] [PubMed] [Google Scholar]
- 24.Wiseman M, Dancyger C, Michie S. Communicating genetic risk information within families: a review. Fam Cancer. 2010;9(4):691–703. [DOI] [PubMed] [Google Scholar]
- 25.Daly MB, Montgomery S, Bingler R, Ruth K. Communicating genetic test results within the family: Is it lost in translation? A survey of relatives in the randomized six-step study. Fam Cancer. 2016;15(4):697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young AL, Butow PN, Rhodes P, et al. Talking across generations: Family communication about BRCA1 and BRCA2 genetic cancer risk. J Genet Couns. 2019;28(3):516–532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified datasets and the qualitative codebook will be made available upon request.


