Abstract
PURPOSE:
Pulmonary arterial hypertension (PAH) is a rare, progressive vasculopathy with significant cardiopulmonary morbidity and mortality. Genetic testing is currently recommended for adults diagnosed with heritable, idiopathic, anorexigen-, hereditary hemorrhagic telangiectasia-, and congenital heart disease-associated PAH, PAH with overt features of venous/capillary involvement, and all children diagnosed with PAH. Variants in at least 27 genes have putative evidence for PAH causality. Rigorous assessment of the evidence is needed to inform genetic testing.
METHODS:
An international panel of experts in PAH applied a semi-quantitative scoring system developed by the NIH Clinical Genome Resource to classify the relative strength of evidence supporting PAH gene-disease relationships based on genetic and experimental evidence.
RESULTS:
Twelve genes (BMPR2, ACVRL1, ATP13A3, CAV1, EIF2AK4, ENG, GDF2, KCNK3, KDR, SMAD9, SOX17, and TBX4) were classified as having definitive evidence and three genes (ABCC8, GGCX, and TET2) with moderate evidence. Six genes (AQP1, BMP10, FBLN2, KLF2, KLK1, and PDGFD) were classified as having limited evidence for causal effects of variants. TOPBP1 was classified as having no known PAH relationship. Five genes (BMPR1A, BMPR1B, NOTCH3, SMAD1, and SMAD4) were disputed due to a paucity of genetic evidence over time.
CONCLUSIONS:
We recommend that genetic testing includes all genes with definitive evidence and that caution be taken in the interpretation of variants identified in genes with moderate or limited evidence. Genes with no known evidence for PAH or disputed genes should not be included in genetic testing.
Keywords: pulmonary arterial hypertension, genetics, molecular diagnosis, genomic medicine
INTRODUCTION
Pulmonary arterial hypertension (PAH) (OMIM #178600) is a rare, often lethal, disease with pulmonary artery remodeling leading to increased pulmonary vascular resistance, right ventricular (RV) hypertrophy, and right heart failure [1–3]. PAH can occur in a heritable manner (HPAH), idiopathically (IPAH) or associated with other diseases including congenital heart disease, autoimmune connective tissue diseases or risk-factor exposure (APAH). PAH may be caused by genetic, epigenetic, and environmental factors, as well as gene-environment interactions which may modify genetic risk. Pathogenic BMPR2 (bone morphogenetic protein receptor 2) variants are the major cause of HPAH, yet twenty-six additional genes have been implicated in IPAH and some HPAH cases primarily driven by the advent of massively-parallel sequencing technologies. Independent validation of PAH gene-disease relationships is critical to avoid over-interpretation of genetic findings. Moreover, reporting of large numbers of variants of uncertain significance (VUS) often negatively influences patient well-being. Variable levels of evidence for gene-disease relationships complicate the clinical interpretation of genetic testing results and the prioritization of research strategies in the field. Currently, patient education about the option of genetic testing is recommended for adult H/IPAH, anorexigen-, hereditary hemorrhagic telangiectasia-, and congenital heart disease-associated PAH, PAH with overt features of venous/capillary involvement (PVOD/PCH), and all pediatric PAH patients [4–7]. Thus, systematic review of the strength of evidence for PAH gene-disease relationships is needed.
An international panel of scientists, with extensive research experience in PAH gene discovery and characterization, was assembled to systematically assess evidence for PAH gene-disease relationships. We applied the National Institutes of Health Clinical Genome Resource (ClinGen) [8] framework for semiquantitative classification [9], as used for six other cardiovascular diseases [10–15]. Here, we report the results of evidence-based gene curation for twenty-seven genes implicated in PAH.
METHODS
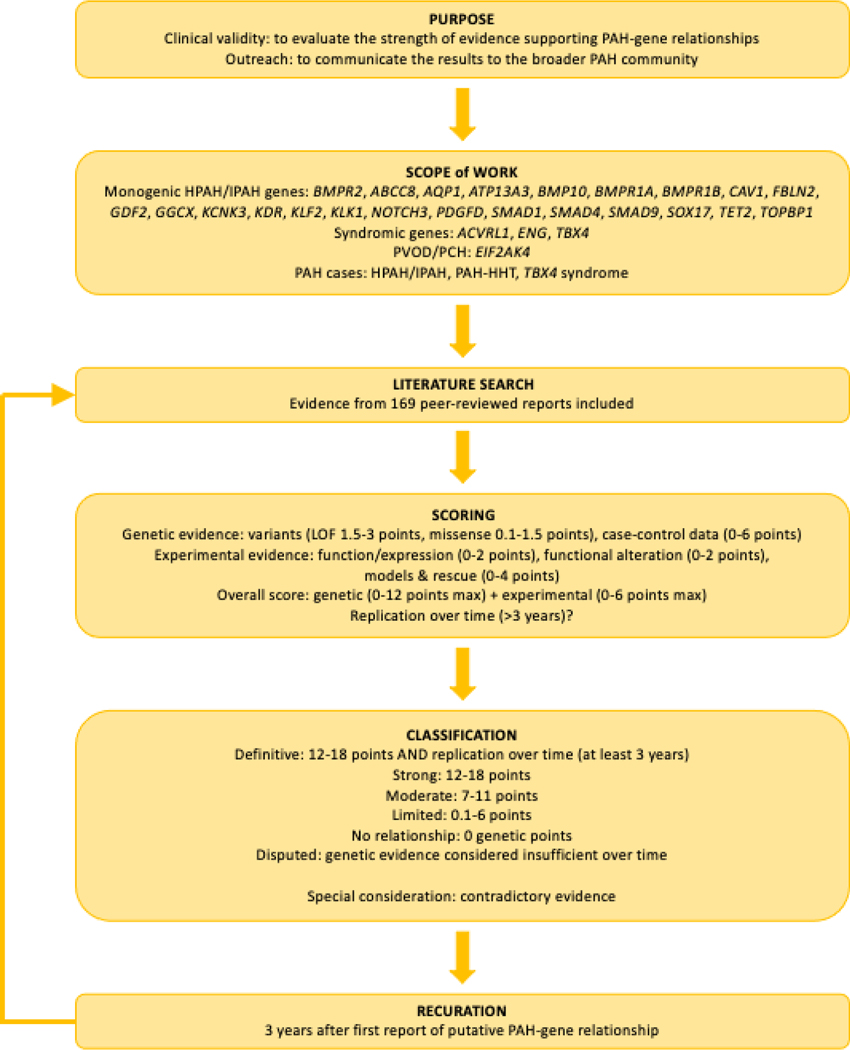
Study design and criteria
We assembled a ClinGen pulmonary hypertension gene curation expert panel (PH GCEP) (https://clinicalgenome.org/affiliation/40071/) consisting of fifteen members from eight institutions and representing six countries. An overview of the gene classification process and scoring criteria is provided in Figure 1. The scope of work included genes implicated in isolated H/IPAH: BMPR2, ABCC8, AQP1, ATP13A3, BMP10, BMPR1A, BMPR1B, CAV1, FBLN2, GDF2, GGCX, KCNK3, KDR, KLF2, KLK1, NOTCH3, PDGFD, SMAD1, SMAD4, SMAD9, SOX17, TET2, and TOPBP1. We curated three syndromic PAH genes: ACVRL1 (PAH associated with hereditary hemorrhagic telangiectasia, PAH-HHT), ENG (PAH-HHT), and TBX4 (small patella or TBX4 syndrome) [16]. EIF2AK4 was curated based on diagnoses of PVOD/PCH, which can be misdiagnosed as IPAH. APAH and persistent pulmonary hypertension of the newborn cases were excluded. Genes were assigned to expert panel members without conflicts of interest. A total of 168 peer-reviewed reports were evaluated for relevant genetic and experimental evidence for the 27 genes. Genome-wide sequencing data from several moderate- to large-sized cohorts were utilized in multiple gene curations. The UK NIHR Bioresource – Rare Diseases Study for PAH (NBR PAH cohort) comprises whole-genome sequencing of 1,038 unrelated, predominantly European adult IPAH patients [17–19]. The US National Biological Sample and Data Repository for PAH case-control study (PAH Biobank) included 2,572 exome-sequenced unrelated pediatric and adult PAH cases (43% IPAH, 48% APAH, 4% HPAH and 5% other PAH) of mixed ancestry [18–21]. Wang et al comprises 331 Han Chinese IPAH cases (Han Chinese IPAH cohort) with exome or genome sequencing [22]. The Spanish Registry includes 300 cases with H/IPAH and APAH analyzed by panel or exome sequencing [23–26].
Figure 1.
Flowchart.
Genetic and functional evaluations of gene-disease relationships
Lead curators scored genetic and experimental evidence according to the updated ClinGen framework (Standard Operating Protocol v9.0). The predominant type of genetic evidence scored was case-level variant data as family segregation and case-control association analyses rarely reached ClinGen criteria for inclusion. The threshold for inclusion was allele frequency less than 1/10,000 (gnomAD all, v2.1.1 controls, or relevant genetic ancestry) and variant type predicted loss of function (pLOF; nonsense, frameshift, canonical splice variant, and whole exon deletions) or missense with in silico predictions of deleteriousness ((CADD score ≥20 [27] or REVEL score with gene-specific thresholds [20]). Case-level evidence scores were weighted based on variant type, available functional data, and de novo inheritance. Experimental evidence included: a) expression in PAH relevant tissues/cells; b) known function including intracellular pathways, cell proliferation, apoptosis; c) functional alteration in variant-positive patient cells; d) PAH-relevant animal models and rescue. Expression and functional studies referenced in the curations specified whether cell/tissue samples were from affected PAH patients or healthy controls. Where noted, expression data were taken from the Genotype-Expression Project, GTEx. Comparative studies utilized age-matched controls but genetic ancestry was not always available. As defined by SOP v9.0 guidelines individual PAH gene-disease relationships were classified as strong, moderate, limited, no known relationship, or disputed due to a lack of genetic evidence over time. Definitive classifications were assigned to genes with strong evidence for causality plus independent evaluation over ≥3 years post-discovery without contradictory evidence. Provisional gene curations were discussed by the full PH GCEP monthly. Genes curated before the 3-year post-discovery timepoint underwent recuration at the 3-year timepoint. Once a consensus decision was made, final classifications were published to the ClinGen website and made publicly available via our PH GCEP (https://search.clinicalgenome.org/kb/affiliate/10071) or the Hemostasis/Thrombosis GCEP (https://search.clinicalgenome.org/kb/affiliate/10028) for ACVRL1 and ENG.
Curation and classification of the twenty-seven PAH genes occurred over a two-year period, mid 2019–2021. Group meetings were held on a monthly or bi-monthly basis and genes were assigned to distribute individual curator burden from month to month. Approximately 1–4 genes were curated per month, with re-evaluations when additional information was needed (i.e. relatedness of probands or functional experiment details), curation inconsistencies were identified (i.e. case inclusion criteria or variable use of in silico predictors of variant deleteriousness), or at the 3-year recuration timepoint.
RESULTS
1. Strength of evidence for genes implicated in isolated H/IPAH
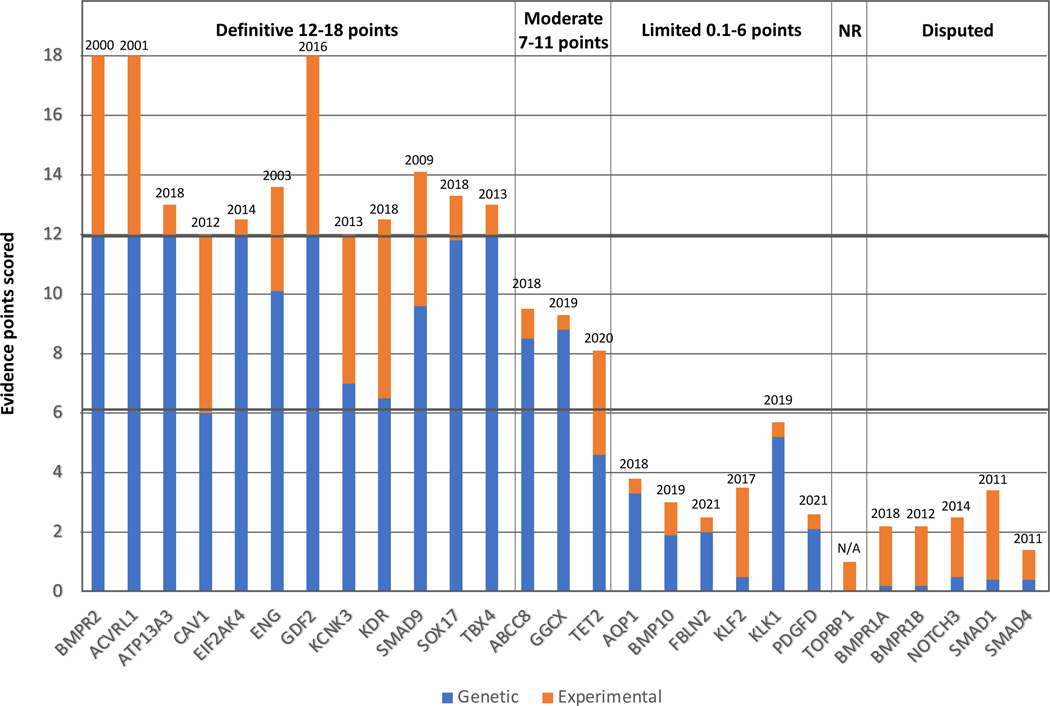
For the twenty-three genes assessed for isolated disease, gene-disease relationships were classified as definitive (n=8), moderate (n=3), limited (n=6), disputed (n=5), or no known relationship (n=1) (Table 1 and Figure 2).
Table 1.
Strength of PAH-gene relationships for genes implicated in isolated H/IPAH.
| Gene | Gene name | MOIa | Genetic evidence | Variant type scoreb | Experimental evidence | Evidence type scoredc | Total score | >3 yrs? | Classification | Tissue/cell expressiond | Molecular mechanisme |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ATP13A3 | ATPase 13A3 | AD/AR | 12 | pLOF | 1 | F/expression; FA/non-patient | 13 | Y 2018 | Definitive | PASMC, PAEC, BOEC | Unknown |
| BMPR2 | Bone morphogenetic protein receptor 2 | AD | 12 | pLOF | 6 | F/expression, biochemical, interaction; FA/patient; M/non-human; R/non-human | 18 | Y 2000 | Definitive | PASMC, PAEC | Haploinsufficiency |
| CAV1 | Caveolin 1 | AD | 6 | pLOF, missense | 6 | F/biochemical; FA/patient; M/non-human; R/non-human | Y 2012 | Definitive | Lung EC | Dominant negative | |
| GDF2 | Growth differentiation factor 2 | AD | 12 | pLOF, missense | 6 | F/expression, biochemical, interaction; FA/patient, non-patient; M/cell culture | 18 | Y 2016 | Definitive | HMVEC/PAEC | Haploinsufficiency |
| KCNK3 | Potassium two pore domain channel subfamily K member 3 | AD | 7 | missense | F/expression; FA/patient; M/non-human | 12 | Y 2013 | Definitive | Lung, PA, PASMC | LOF | |
| KDR | Kinase insert domain receptor | AD | 6.5 | 6 | F/expression; M/non-human | 12.5 | Y 2018 | Definitive | PAEC | Haploinsufficiency | |
| SMAD9 | Smad family member 9 | AD | 9.6 | pLOF, missense | 4.5 | F/biochemical, interaction; FA/patient, non-patient; R/patient cells | 14.1 | Y 2009 | Definitive | PAEC, PASMC | LOF |
| SOX17 | SRY-box transcription factor 17 | AD | 11.8 | pLOF, missense | 1.5 | F/expression; FA/non-patient | 13.3 | Y 2018 | Definitive | PAEC, PAH plexiform lesions | Haploinsufficiency |
| ABCC8 | ATP binding cassette subfamily C member 8 | AD | 9.0 | pLOF, missense | 1.0 | F/expression | 10 | Y 2018 | Moderate | Lung, PA | LOF |
| GGCX | Gamma glutamyl carboxylase | AD | 8.8 | pLOF, missense | 0.5 | F/expression | 9.3 | Y 2019 | Moderate | Lung | Unknown |
| TET2 | Tet-methylcytosine-dioxygenase-2 | AD | 4.6 | pLOF, missense | 3.5 | F/expression, biochemical; M/non-human | 8.1 | N 2020 | Moderate | Lung | LOF |
| AQP1 | Aquaporin 1 | AD | 3.3 | missense | 0.5 | F/expression | 3.8 | Y 2018 | Limited | PASMC, PAEC, BOEC | N/A |
| BMP10 | Bone morphogenetic protein 10 | AD | 1.9 | pLOF, missense | 1.1 | F/expression, biochemical, interaction | 3.0 | Y 2019 | Limited | Plasma, right atrium | Haploinsufficiency |
| FBLN2 | Fibulin 2 | AD | 2.0 | missense | 0.5 | F/expression | 2.5 | N 2021 | Limited | Heart, aorta coronaries; basement membrane | Unknown (GOF?) |
| KLF2 | Krüppel-like factor 2 | AD | 0.5 | missense | 3.0 | F/expression, interaction; FA/patient | Y 2017 | Limited | Lung, vasculature | N/A | |
| KLK1 | Tissue Kallikrein | AD | 5.2 | pLOF, missense | 0.5 | F/expression | 5.7 | Y 2019 | Limited | Lung, vasculature | Unknown (haploinsufficiency and/or LOF?) |
| PDGFD | Platelet Derived Growth Factor D | AD | 2.1 | 2.0 case-CTL data + 0.1 missense | 0.5 | 2.6 | N 2021 | Limited | Lung, vasculature, mesenchyme | Unknown (GOF?) | |
| TOPBP1 | DNA topoisomerase II binding protein 1 | N/A | 0 | none | 1.0 | F/expression; FA/non-patient | 1.0 | N/A | No known disease relationship | Lung, PAEC | N/A |
| BMPR1A | Bone morphogenetic protein receptor 1A | N/A | 0 | missense | F/expression, biochemical, interaction | 2.0 | Y 2018 | Disputed | PASMC | N/A | |
| BMPR1B | Bone morphogenetic protein receptor 1B | N/A | 0 | 2 | F/expression, biochemical, interaction | 2.0 | Y 2012 | Disputed | PASMC | N/A | |
| NOTCH3 | Notch receptor 3 | N/A | 0 | missense | 2.0 | F/expression, biochemical; FA/non-patient | 2.0 | Y 2014 | Disputed | Lung, PASMC | N/A |
| SMAD1 | Smad family member 1 | N/A | 0 | missense | 3.0 | F/biochemical; M/non-human | 3.0 | Y 2011 | Disputed | PAEC, PASMC | N/A |
| SMAD4 | Smad family member 4 | N/A | 0 | missense, other | 1.0 | F/biochemical | 1.0 | Y 2011 | Disputed | PAEC, PASMC | N/A |
MOI, mode of inheritance; AD, autosomal dominant; AR, autosomal recessive; N/A, not applicable.
pLOF, predicted loss of function, including nonsense, frameshift, and canonical splice variants.
F, function (relevant expression, biochemical function, protein interaction); FA, functional alteration (in patient or non-patient cells); M, model (human or non-human, cell culture/human or non-human); R, rescue (human or non-human, cell culture/human or non-human).
BOEC, blood outgrowth endothelial cell; HMVEC, human lung microvascular endothelial cell; PA, pulmonary artery; PAEC, pulmonary artery endothelial cell; PASMC, pulmonary artery smooth muscle cell.
LOF, loss of function; GOF, gain of function; N/A, not applicable.
Figure 2. Quantitative contributions of genetic and experimental evidence to the clinical validity classifications of genes curated for PAH.
The sums of genetic (blue) and experimental (orange) evidence scores are shown for genes classified as having definitive, moderate, or limited evidence of a monogenic relationship, no relationship (NR) or disputed relationship for H/IPAH, PVOD/PCH (EIF2AK4), or syndromic PAH (ACVRL1, ENG, TBX4). Dates above the bars indicate date of first report of a gene variant identified in a PAH case.
BMP pathway genes
Definitive
BMPR2 encodes a type II receptor of the TGF-β superfamily that in complex with type I receptors drives phosphorylation of SMAD signaling molecules and tightly regulates processes related to development, differentiation, and growth [28, 29]. Linkage analysis in autosomal dominant (AD) PAH families led to the identification of BMPR2 variants that segregated among affected family members with incomplete penetrance [30, 31]. Currently, more than 650 unique PAH-associated BMPR2 variants have been reported [17, 20, 32], of which the majority are pLOF variants. Missense variants cluster in the conserved ligand-binding and protein kinase domains. BMPR2 variants cause 70–80% of familial cases and 10–20% of sporadic cases but are rarely found in APAH cases. BMPR2 is expressed in the pulmonary vasculature with reduced expression in patient-derived cells [33, 34]. Pulmonary arterial endothelial cells (PAECs) derived from PAH patients with BMPR2 truncating variants demonstrated haploinsufficiency as a pathogenetic mechanism [35], with confirmation in mouse and rat models heterozygous for Bmpr2 null alleles [36–38] or transgenic for a dominant-negative[39]. The rodent models exhibited increased right ventricular systolic pressure (RVSP) and increased arteriole muscularization with rescue of RV function by wild-type BMPR2 [40] or BMPR2 ligand [41].
CAV1 encodes caveolin-1, the main structural and signaling protein of caveolae. CAV1 was first identified as a putative PAH gene by exome sequence analysis in a multi-generational AD PAH family with incomplete penetrance [42]. Screening of 260 unrelated H/IPAH patients identified an additional de novo frameshift variant associated with reduced CAV1 protein in small artery endothelial cells compared to a control [42]. Independent studies identified seven additional CAV1 variants among H/IPAH patients [20, 23, 43, 44]. CAV1 is expressed in lung endothelial and smooth muscle cells, expression is decreased or absent in plexiform lesions [45], and CAV1 c.474del (transcript NM_001753.5) patient fibroblasts demonstrated reduced caveolae density and caveolar protein levels [45]. Increased SMAD1/5/8 phosphorylation in CAV1 patient fibroblasts was not rescued by transduction with wild-type CAV1 [46]. Expression of the mutant protein caused reduced wild-type protein [46, 47], consistent with a dominant-negative mechanism. Cav1 knockout mice exhibited pulmonary vascular remodeling and other pathological features consistent with PAH [48, 49]. Of note, the phenotype was rescued by endothelial re-expression of CAV1 [50]. Rare variants in CAV1 cause autosomal dominant and recessive lipodystrophies but the observed variants are different from those associated with H/IPAH and the molecular mechanisms are likely different. Thus, CAV1 was curated independently by the PH and monogenic diabetes GCEPs.
GDF2 encodes BMP9, a circulating ligand member of the BMP signaling pathway. GDF2 was first identified by burden testing using the NBR PAH cohort [17]. Subsequently, 47 unrelated patients with 45 unique heterozygous variants (pLOF and missense) were identified in three independent cohorts [20, 22, 44]. David et al. identified BMP9 as a functional activator of the endothelial-specific BMPR2/ALK1 signaling pathway [51]. BMP9 plasma levels were decreased among GDF2 variant-positive patients versus controls or IPAH patients without GDF2 variants, potentially due to impaired secretion[17]. Treatment of PAECs with wild-type or mutant GDF2 supernatant resulted in mutant-specific attenuation of the anti-apoptotic response [22]. Recuration (March 16th, 2022), three years after the initial gene discovery report, identified four additional heterozygous variants, including one nonsense and three likely deleterious missense variants, based on in vitro evidence [52, 53]. Combined analysis of the UK/US PAH cohorts identified GDF2 as one of seven genes that were significantly associated with IPAH on a genome-wide basis [19]. Identification of homozygous GDF2 pLOF variants in three children with severe PAH raises the possibility of semi-dominant inheritance [54–56]; however, unaffected siblings with biallelic variants in one family suggested variable expressivity [55]. There is also an emerging picture of overlap with HHT-like phenotypes, notably pulmonary arteriovenous malformations [56–58].
The Mothers Against Decapentaplegic Homolog 9 (SMAD9) gene encodes SMAD8, a member of the SMAD signaling protein family. Shintani et al. [59] first associated a heterozygous nonsense variant in SMAD9 with PAH using a candidate gene screen of ENG and seven SMADs in Japanese IPAH patients without BMPR2 or ACVRL1 variants (n=23). A missense [60] and a unique nonsense variant [61] were then identified in two independent candidate gene screens. Fourteen additional H/IPAH cases heterozygous for rare variants were identified in the PAH Biobank [20], NBR PAH cohort [17], and Han Chinese IPAH cohort [22]. The variants included missense (n=9, located in functional domains), nonsense (n=3) and an in-frame indel variant. SMAD9 is ubiquitously expressed, including abundant expression in lung tissue (GTEx, on May 1st, 2022) [62]. SMAD8 undergoes phosphorylation downstream of BMP type I receptors, inducing interaction with SMAD4 and transcriptional activity [63]. SMAD8 p.(Cys202*) (protein NP_001120689.1) failed to bind SMAD4 and displayed reduced transcriptional activity [59]. Transcript levels of the BMP target gene Id2 were reduced in patient pulmonary arterial smooth muscle cells (PASMCs) heterozygous for SMAD8 p.Lys43Glu (NP_001120689.1), although response to ligand was largely preserved suggesting redundancy of SMAD1/5/8 function [60]. Smad9 knockout mice are viable and show evidence of spontaneous, age-related pulmonary vascular remodeling [64]. Further, SMAD8-dependent post-transcriptional up-regulation of a subset of microRNAs exerts anti-proliferative effects in control cells that was abrogated in patient cells with SMAD8 p.Arg294* (NP_001120689.1) variant, comparable to BMPR2 exon deletion [61].
Limited
BMP10 encodes the bone morphogenetic protein 10 (BMP10) ligand, a paralogue of BMP9 with 65% amino acid homology and overlapping function [51, 65]. Eyries and colleagues conducted targeted sequencing of nine known PAH genes plus BMP10 in 263 patients. Heterozygous nonsense and missense variants in BMP10 were found in two severely affected IPAH patients [66]. Gelinas and colleagues [67] identified a missense variant in BMP10 by exome sequencing of a pediatric cohort (n=18). Two more studies independently reported heterozygous BMP10 substitutions in two IPAH patients [68, 69]. Bmp10 knockout mice die at an early embryonic stage due to retarded cardiac growth and chamber maturation [70]. In contrast, Bmp10 conditional knockout mice (induced postnatally) were reported to be viable and fertile, exhibiting no PH phenotype under normoxic or hypoxic conditions [71].
Disputed genes
BMPR1A and BMPR1B encode type I receptors integral to the canonical BMP signaling pathway [72]. A potential relationship between BMPR1B and IPAH first emerged in a candidate gene study of 74 Japanese cases wherein two missense variants were described as pathogenic [73]. However, this analysis revealed that both variants are observed at a frequency exceeding the population prevalence of PAH (8.3KJPN, https://jmorp.megabank.tohoku.ac.jp/202109/variants). Additional publications reported variants in BMPR1A or BMPR1B [20, 22, 43] but only two missense variants per gene met our minimal inclusion criteria. BMPR1A and BMPR1B are expressed at equivalent levels to BMPR2 in human PASMCs but not detected in PAECs [74]. Due to a paucity of genetic evidence over time, both genes are disputed.
SMAD1 and SMAD4 encode additional members of the SMAD signaling protein family [75]. Targeted sequencing of SMAD genes in 324 PAH cases identified SMAD4 predicted splice-site and missense variants, and a SMAD1 missense variant in three IPAH patients [60]. Overexpression of the SMAD1 variant demonstrated modestly reduced luciferase activity [60]. While additional SMAD1 and SMAD4 variants have been reported, the overall number remains small [20, 22, 23]. Both SMAD proteins are expressed in human lung (GTEx on November 24th, 2021) [62] and function as critical mediators of BMPR2 signaling [76]. SMAD1/4 protein levels were reduced in a rat PH model [77] but there are contradictory reports in animal [78] and human [79] lung tissue studies. Based on the weak human genetic data for both SMAD1 and SMAD4 over time, both gene-disease relationships are disputed.
Transporter and channel genes
Definitive
ATP13A3 encodes a transmembrane cation transporter that transports polyamines, small metabolites required for normal cell growth and proliferation [80]. Monoallelic ATP13A3 variants were identified in the NBR PAH cohort (6 pLOF, 4 missense variants) [17]. Subsequently, four missense and five pLOF/missense variants were identified in the Han Chinese IPAH cohort [22] and PAH Biobank [20], respectively. Recently, biallelic ATP13A3 variants were identified in three families with severe, early onset PAH and high mortality [81]. ATP13A3 is highly constrained for loss-of-function variants (pLoF = 1) [82] and most of the PAH-associated missense variants occur in conserved protein domains [83]. These data indicate a dose-dependent, semi-dominant mode of inheritance for ATP13A3. ATP13A3 is expressed in PASMCs, PAECs, and blood outgrowth endothelial cells (BOECs) from IPAH patients [17], with decreased proliferation and increased apoptosis of BOECs transfected with ATP13A3 siRNA [17]. Elevated ATP13A3 plasma concentrations have been reported in multiple cancers and PAH [84, 85]. The protein-truncating variants are predicted to undergo nonsense-mediated decay indicating haploinsufficiency as the likely disease mechanism. For the missense variants, the mechanism is unclear.
KCNK3 encodes a two-pore domain potassium channel, a regulator of resting membrane potential and pulmonary vascular tone [86]. Exome sequencing identified KCNK3 as the cause of AD PAH in a multi-generational family wherein a novel heterozygous missense variant co-segregated with disease, but with incomplete penetrance [87]. Targeted sequencing detected additional missense variants in 5/320 unrelated H/IPAH cases, accounting for 1.9% of the total cohort. Electrophysiological analyses indicated reduced current in mutant channels [87], independently confirmed for two variants identified in an independent Spanish cohort [88, 89], indicating a LOF disease mechanism. To date, more than 20 likely pathogenic missense variants have been reported in H/IPAH [17, 20, 22, 23, 43, 90–93]. Identification of biallelic variants in two families [88, 94], suggests potential semi-dominant inheritance for KCNK3. KCNK3 is expressed in PASMCs [86], with reduced mRNA and protein expression and increased sensitivity to selective KCNK3 channel blockade in PAH patient-derived pulmonary arteries and PASMCs compared to controls [95]. Kcnk3 mutant rats expressing a truncated channel demonstrated age-related increased RVSP and other pathological features of PAH [96].
Moderate
ATP binding cassette subfamily C member 8 (ABCC8) encodes sulfonylurea receptor-1 (SUR1), a regulatory subunit of adenosine triphosphate (ATP)-sensitive potassium channel, Kir6.2. Heterozygous ABCC8 variants were identified in 12/913 unrelated H/IPAH patients by exome sequencing [97]. Electrophysiological assays demonstrated reduced mutant channel function [97]. pLOF and missense variants were identified in twenty-one additional patients with H/IPAH [20, 24, 67], with incomplete penetrance [24, 97]. SUR1 protein expression was demonstrated in proximal pulmonary arteries and alveolar macrophages of IPAH patients [97]; however, mechanistic interpretation remains dependent on further functional data. Homozygous pathogenic variants in ABCC8 are known to cause hyperinsulinemic hypoglycemia of infancy [98]. Based on differing modes of inheritance and only a single patient exhibiting overlapping phenotypes, ABCC8 was curated separately for PAH and monogenic diabetes.
Limited
AQP1 encodes aquaporin 1, a water transport channel that also promotes endothelial cell migration and angiogenesis [99]. Increased AQP1 missense variant burden was demonstrated in the NBR PAH cohort [17]. Two missense variants were shown to co-segregate with AD PAH in three families, with incomplete penetrance, and insufficient segregation evidence to count in our scoring [17]. Three additional missense variants were identified in the Han Chinese IPAH cohort [22]. Expression of AQP1 was demonstrated in PAH lung endothelium and healthy donor PAECs [17] but no functional effects of patient variants has been reported. In mice, homozygosity for Aqp1 null alleles resulted in attenuated hypoxic PH [100]. Currently, only heterozygous missense variants are considered potentially disease relevant.
Growth and transcription/translation factor genes
Definitive
KDR encodes the kinase insert domain receptor for vascular endothelial growth factor type 2 (VEGFR2). Ligand activation of VEGFR2 promotes cell proliferation, cell survival, and migration. Protein-truncating KDR variants were reported in 4/1048 IPAH cases from the NBR PAH cohort [17]. Subsequently, 2/311 IPAH cases were identified with KDR variants associated with a low diffusion capacity for carbon monoxide (DLCO); co-segregation analysis indicated that variant heterozygotes were either affected by PAH or had decreased DLCO [101]. Four additional pLOF [18] and three missense variants, including one recurrent in three unrelated IPAH patients [19], were identified during recuration. KDR is highly expressed in PAECs [102]. Mice exposed to chronic hypoxia combined with SU5416-mediated inhibition of VEGFR resulted in vascular remodelling, PAEC proliferation and obliteration, and severe PH; biomarker analysis revealed a signature analogous to human PAH patients [103]. Endothelial-specific Kdr deletion resulted in a mild PAH phenotype under normoxia that worsened under hypoxia [104].
SOX17 is a two-exon gene encoding the SRY-box transcription factor 17. SOX17 is critical in cardiovascular morphogenesis and postnatal vascular remodeling [105]. Gene burden testing in the NBR PAH cohort revealed enrichment of SOX17 variants in IPAH cases compared to controls [17]. Subsequently, 21 pLOF and missense variants were identified in H/IPAH patients from diverse populations [20, 106–108]. Most PAH-associated nonsense and frameshift variants occur in the terminal exon, in the conserved β-catenin-binding domain, and are predicted to escape nonsense-mediated decay. Wang et al. [108] reported a terminal exon SOX17 nonsense variant in a multi-generational family associated with a 14-fold reduction of target gene NOTCH1 reporter activity and de-repression of β-catenin compared to wild-type. Of interest, endothelial-specific deletion of Notch1 results in worsened PH in mice [109]. Immunolocalization of SOX17 established endothelial specific expression in the pulmonary arterioles of wild-type cells and PAH vascular lesions [17].
Limited
KLF2 encodes Krüppel-like factor 2, a transcriptional repressor of inflammation, endothelial activation, and proliferation [110]. Eichstaedt and colleagues [111] identified a heterozygous missense variant in two affected siblings with HPAH but not an unaffected brother. Functional analyses indicated loss of KLF2 nuclear localization in patient-derived lung [112] and PAECs [113], and decreased transcriptional activity in transfected cells [112]. No KLF2 variants have been reported in other H/IPAH cases. KLF2 is highly expressed in human [114] and rodent [115] lung, and decreased in PAH lung compared to healthy lung [113]. KLF2 overexpression in pulmonary vascular cells demonstrated decreased apoptosis and cell proliferation [113]. The classification is based on limited genetic evidence to date.
Gene burden testing in a combined analysis of the PAH Biobank and the NBR PAH cohort demonstrated increased burden for five previously reported PAH genes and two new genes, PDGFD and FBLN2 (see ‘Other genes’ below) [19]. Nine IPAH cases were identified carrying seven unique and two recurrent PDGFD missense variants [19]. Gelinas et al. [67] reported an additional IPAH case with a novel missense variant. PDGFD encodes a member of the platelet-derived growth factor family, a mesenchymal mitogenic factor involved in regulation of embryonic development, cell proliferation, cell migration, survival, and chemotaxis [116–118]. PDGFD is expressed in lung (GTEx, on March 11th, 2022) [62] and arterial vasculature cells [119], but expression in the pulmonary vasculature has not been assessed. Cardiac-specific Pdgfd transgenic mice have increased SMC proliferation, vessel wall thickening, fibrosis, heart failure, and premature death [120],
No evidence
TOPBP1 encodes a topoisomerase binding protein required for DNA replication. Common variants in TOPBP1 were identified by exome sequencing in twelve IPAH patients [121] but with similar allele frequency in the control populations and without segregation with PAH [122]. Rare variants have not been reported for PAH cases. TOPBP1 expression is altered in PAH lung tissues but there is no genetic evidence for a gene-disease relationship [121].
Disputed
The Notch pathway is a highly conserved signalling cascade with important roles in human development and tissue homeostasis [123]. Two NOTCH3 missense variants were identified in IPAH patients by a targeted candidate gene screen [124]. One variant had an allele frequency (JPN8.3K, MAF: 0.002) that exceeded our threshold for inclusion and the other had functional data contradictory to a presumed gain-of-function mechanism [124]. Targeted sequencing in other cohorts [23, 92, 125–127] identified only two additional NOTCH3 missense variants in H/IPAH that met our minimal threshold. NOTCH3 is expressed in PASMCs and IPAH patient cells display overexpression of NOTCH3 [128]. However, the paucity of rare NOTCH3 variants in PAH patients indicates that the regulation of NOTCH3 signaling is independent of genetic variation.
Other genes
Moderate
Gene burden testing in the PAH Biobank [20] identified two PAH candidate genes, gamma-glutamyl carboxylase (GGCX) and kallikrein 1 (KLK1) (see ‘Limited’ below). Heterozygous GGCX variants (5 pLOF, 9 missense) were identified in 18 H/IPAH cases, with three missense variants recurrent in at least two cases each [20]. GGCX plays important roles in blood coagulation, bone formation, vascular integrity, and inflammation [129]. GGCX expression was detected in lung and liver (GTEx, on March 8th, 2021) but a potential pathogenetic mechanism cannot be established without experimental data. Biallelic variants in GGCX cause vitamin K-dependent coagulation factor deficiency (MIM #277450) [129] but the genetic variants and mode of inheritance are distinct. GGCX was curated independently by the PH and hemostasis/thrombosis GCEPs.
TET2 encodes tet-methylcytosine-dioxygenase-2, an epigenetic regulatory enzyme implicated in cancer [130–132], cardiovascular disease [133, 134] and inflammation [135]. Targeted burden analysis in the PAH Biobank demonstrated increased burden of TET2 variants in PAH cases compared to controls, largely due to heterozygous pLOF variants (9 pLOF, 3 missense) and IPAH cases (8/12 cases) [21]. Seventy-five percent were predicted germline and 25% predicted somatic. Increases in sequencing depth will likely increase TET2 variant identification among PAH cases. TET2 is expressed in lung (GTEx, on March 23rd, 2022) and decreased circulating TET2 associated with circulating proinflammatory cytokines was reported in IPAH cases compared to controls [21]. Spontaneous PH was demonstrated in hematopoietic-specific mouse models, and treatment of the mice with an IL-1beta inhibitor reversed the pro-inflammatory phenotype and PH [21].
Limited
Fibulin proteins are secreted as glycoproteins into the extracellular matrix and function in developmental processes, tissue remodeling, and maintenance of basement membrane and elastic fibers. In the PAH Biobank/NBR PAH combined analysis [19], seven unique FBLN2 variants were identified in IPAH, including a recurrent variant carried by four cases, predicted to affect splicing. FBLN2 is expressed in developing heart and smooth muscle precursor cells, amongst other tissues [117, 136, 137]. Fbln2 knockout mice are viable, fertile, and have intact elastic fiber formation. They exhibit attenuated angiotensin II-induced, TGF-β mediated, cardiac hypertrophy and myocardial fibrosis [138, 139] but have not been tested for PH.
KLK1 encodes a kininogenase contributing to the formation of the vasoactive peptide bradykinin. In the PAH Biobank [20], eight unique variants were identified (3 pLOF, 5 missense), with three of the variants recurrent in at least two cases. KLK1 is expressed in several tissues including lung and vascular tissues [140–142]. Overexpression of Klk1 resulted in hypotension in transgenic mice whereas Klk1 knockout mice were normotensive but showed blunted flow dependent vasodilatation [143–145]. While KLK1 has been implicated in pathogenic processes related to PAH development [146–148], clinical indications of PH have not been assessed.
2. Strength of evidence for genes implicated in syndromic forms of PAH
The three genes curated for syndromic forms of PAH have all been classified as having a definitive relationship with PAH (Table 2 and Figure 2).
Table 2.
Strength of PAH-gene relationships for genes implicated in syndromes including PAH.
| Gene | Gene name | Syndromea | Original ClinGen curation GCEP | Original classification | Genetic evidence for PAH | Variant type scoredb | Experimental evidence for PAH | Evidence type scoredc | Total score for PAH | >3 yrs | Classification for PAH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACVRL1 | Activin receptor like 1 | HHT | Hemostasis/ thrombosis | Definitive | 12 | pLOF, missense | 6 | F/expression, biochemical, interaction; M/non-human | 16 | Y 2001 | Definitive |
| ENG | Endoglin | HHT | Hemostasis/ thrombosis | Definitive | 10.1 | pLOF, missense, other | 3.5 | F/expression, interaction; M/nonhuman | 13.6 | Y 2003 | Definitive |
| TBX4 | T-box transcription factor 4 | TBX4 syndrome | --- | --- | 12 | pLOF | 1 | F/expression; FA/patient, non-patient | 13 | Y 2013 | Definitive |
HHT, hereditary hemorrhagic telangiectasia
pLOF, predicted loss of function, including nonsense, frameshift, and canonical splice variants..
F, function (relevant expression, biochemical function, protein interaction); FA, functional alteration (in patient or non-patient cells); M, model (human or non-human, cell culture/human or non-human); R, rescue. (human or non-human, cell culture/human or non-human)
ACVRL1 encodes activin A receptor like type 1 involved in BMP signaling [149, 150]. Variants in ACVRL1 were first identified as causal for HHT, an autosomal dominant vasculopathy characterized by abnormal blood vessel formation in multiple organs [151]. PAH is a rare complication of HHT, with most cases attributable to missense ACVRL1 variants [152–154]. The majority are harbored in the conserved protein kinase domain, especially in a nonactivating, nondown-regulating (NANDOR) box subdomain located in the terminal exon [155]. The NANDOR box is required for downstream SMAD signaling [155]; other rare HHT-PAH associated missense variants have been shown to cause subcellular mislocalization to the endoplasmic reticulum [153]. Small deletion and nonsense variants have been identified in some cases [152–154]. ACVRL1 is predominantly expressed in PAECs and in PAH plexiform lesions [152]. BMP9 and BMP10 were identified as the cognate ligands for ACVRL1 in endothelial cells [51]. Homozygous Acvrl1 knockout mice demonstrated embryonic lethality with severe vascular malformations [156]. Heterozygous Acvrl1 mice developed adult-onset spontaneous PH with increased RVSP, RV hypertrophy, and vascular remodeling [157].
ENG encodes endoglin, an accessory protein that interacts with ACVRL1 to promote TGF-β/BMP signaling [158, 159]. Like ACVRL1, heterozygous pLOF variants in ENG are predominantly associated with HHT [160]. Trembath et al. reported novel ENG nonsense variants in 2/11 HHT-PAH patients [153]. Subsequently, Harrison et al. [161] reported an HHT-PAH associated splicing variant with demonstrated exon skipping. Other studies have identified at least ten additional ENG variants (4 nonsense, 5 missense, 1 in-frame deletion) [17, 20, 32, 162]. ENG variants were rarely reported in the absence of HHT. However, in one case the diagnosis of PAH was made at three months of age, preceding the onset of HHT by eight years [161, 163]. Endoglin is expressed in healthy and PAH lung endothelial cells [164] and plays an important role in angiogenesis [165, 166]. High expression observed in some PAH vascular lesions could be considered contradictory evidence; however, the analysis did not distinguish between L-endoglin and S-endoglin isoforms, which have opposing effects on TGF-β vs BMP signaling [167]. Eng heterozygous knockout mice spontaneously develop characteristic hemodynamic features of PAH, with increased reactive oxygen species levels [168].
TBX4 encodes T-box transcription factor 4 [169], which plays a major role in lung branching morphogenesis and skeletal system development [170]. Heterozygous TBX4 variants were first reported in families with small patella syndrome (SPS, OMIM #147891), an AD skeletal dysplasia [171]. TBX4-containing microdeletions were implicated in PAH [172], followed by identification of two intragenic TBX4 frameshifts and one missense variant in SPS/pediatric-onset PAH cases [173]. Other studies have reported numerous protein-truncating and missense variants clustering in the T-box domain [17, 20, 25, 43, 174]. Luciferase reporter assays demonstrated variant-specific LOF and gain-of-function effects for the missense variants [175]. TBX4 variants are more prevalent in pediatric PAH than adult-onset cases [43], and are often associated with a syndrome involving PAH, other lung and cardiac anomalies, and SPS [176–178]. TBX4 is strongly expressed in developing lung [179], but its role in PAH pathogenesis is currently unclear. Tbx4 knockout mice showed reduced phospho-SMAD1/5 levels in fetal lung fibroblasts [180]. However, PH per se has not yet been demonstrated in the mouse model.
3. Strength of evidence for EIF2AK4 implicated in PVOD/PCH
EIF2AK4 encodes eukaryotic translation initiation factor 2 alpha kinase 4, a serine-threonine kinase inducer of nutrient-mediated changes in gene expression. Biallelic EIF2AK4 variants were identified independently in 13 PVOD families [181] and one family and two sporadic PCH cases [90], suggesting autosomal recessive inheritance. Subsequent studies reported at least ten additional probands with biallelic variants and clinical diagnoses of PVOD/PCH or early-onset IPAH with poor survival[182, 183]. EIF2AK4 was detected in lung tissue from an unaffected control and a PVOD patient without EIF2AK4 variants but was not detected in a PVOD patient with pathogenic variants in EIF2AK4 [181]. Despite the paucity of experimental data, the genetic evidence is strong and remains uncontradicted, yielding a classification of definitive.
DISCUSSION
Of twenty-four genes curated for isolated H/IPAH (or PVOD/PCH for EIF2AK4), nine were classified as definitive (ATP13A3, BMPR2, CAV1, EIF2AK4, GDF2, KCNK3, KDR, SMAD9, SOX17), three as moderate (ABCC8, GGCX, TET2), and six as limited (AQP1, BMP10, FBLN2, KLF2, KLK1, PDGFD). One gene was determined to have no known relationship (TOPBP1) and five were disputed (BMPR1A, BMPR1B, NOTCH3, SMAD1, SMAD4). Three genes curated for syndromic PAH (ACVRL1, ENG, TBX4) were classified as definitive. Four of the disputed genes are from the TGF-β/BMP pathway, originally implicated through candidate gene screens but not confirmed in larger, rigorous next generation sequencing studies. Moderate and limited genes may change classification with new evidence, and recurations can be tracked on the ClinGen website. These results offer guidance to clinicians and genetic testing laboratories.
The inclusion or classification of some genes in this report differ from the 6th World Symposium on Pulmonary Hypertension report [7] due to new genetic and experimental evidence. For example, KDR was not included in the Symposium report but is now classified as definitive, and AQP1 moved from “higher level of evidence” to “limited” in this report.
Identification of a genetic cause of PAH in individual cases can have implications for clinical management including treatment (mono- vs multimodal therapy), surgical intervention and transplantation decisions, and screening for associated conditions [184]. A genetic diagnosis can lead to early treatment of associated medical conditions, cascade genetic testing of family members to identify those at risk for developing PAH, and clarification of reproductive risks to inform family planning decisions.
Based on our analyses, we recommend a tiered genetic diagnostic testing approach. Tiered testing can simplify clinical interpretation of results and decrease the reporting of VUSs. Tier 1 should include definitive and strong genes. For cases without a genetic diagnosis from tier 1 testing, moderate (tier 2) and limited (tier 3) genes could be screened. Lack of genetic evidence over time for TOPBP1 and the five disputed genes indicates that these genes should no longer be included in routine PAH genetic testing. Given the potential reclassification of limited evidence genes and new gene discovery over time, we encourage regular review of testing panels for gene inclusion and adjustment of tiered analyses for both testing panels and exome/genome sequence data as appropriate. Thus, routine reanalysis of case-level data for undiagnosed cases by genetic testing laboratories is highly recommended.
The use of exome (or genome) sequencing for molecular diagnosis has become more cost-effective. Benefits of exome/genome sequencing over panel testing include gene inclusion/exclusion flexibility, copy-number variant detection, and reanalysis of stored data from undiagnosed cases following evidence of new PAH gene-disease relationships.
Conclusions
Twelve genes have definitive evidence for causal effects of variants on PAH using a standardized evidence-based classification system. Our continued efforts to recurate known genes and assess evidence for newly identified genes will provide continuity of expert review.
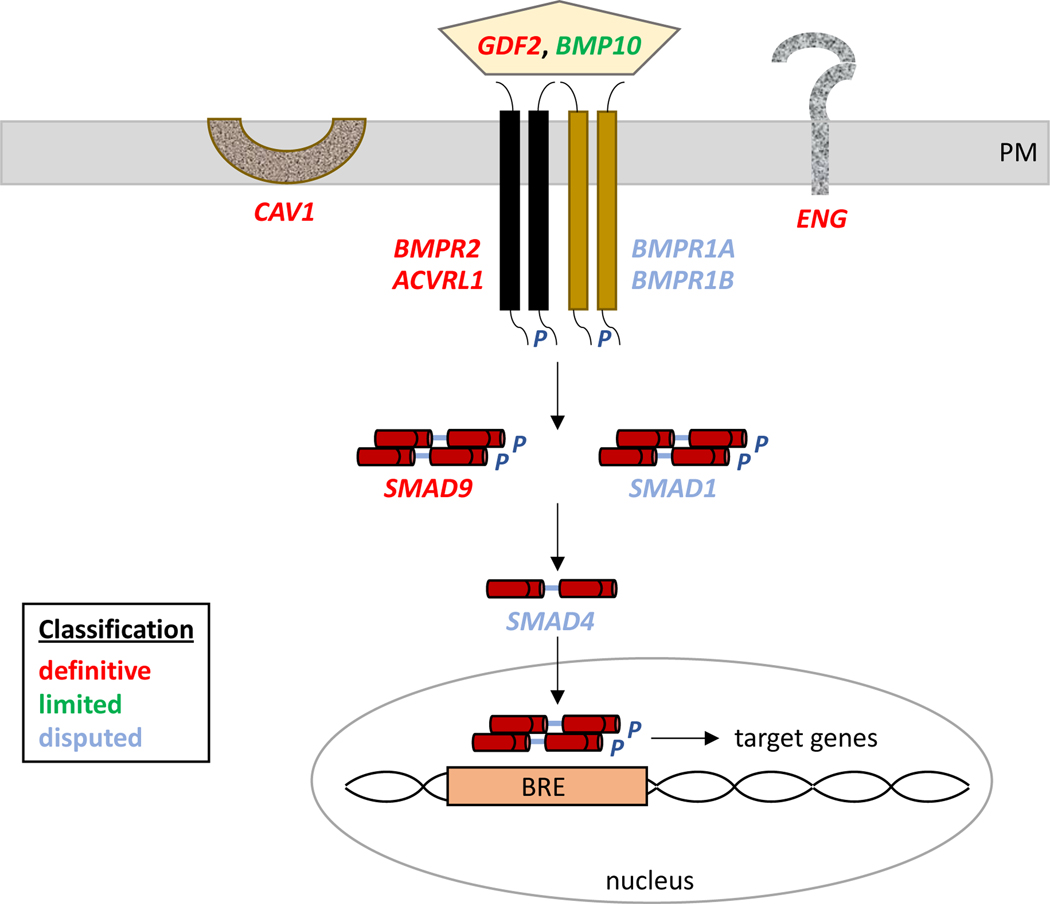
Figure 3. Updated classifications of BMPR2 pathway genes implicated in PAH.
The relative strength of evidence of curated genes is indicated by color-coded classifications. PM, plasma membrane; P, phosphate; BRE, BMPR2 response element.
Table 3.
Strength of PVOD/PCH-EIF2AK4 relationship.
| Gene | Gene name | MOIa | Genetic evidence | Variant type scoredb | Experimental evidence | Evidence type scoredc | Total score | >3 yrs? | Classification | Tissue/cell expressiond | Molecular mechanisme |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EIF2AK4 | Eukaryotic translation initiation factor 2 alpha kinase 4 | AR | 12 | pLOF, missense | 0.5 | F/expression | 12.5 | Y 2014 | Definitive | Lung, PASMCs | LOF |
PVOD/PCH, pulmonary veno-occlusive disease/pulmonary capillary hemangiomatosis.
AR, autosomal recessive
pLOF, predicted loss of function, including nonsense, frameshift, and canonical splice variants.
F, function (relevant expression).
PASMCs, pulmonary artery smooth muscle cells.
LOF, loss of function.
Take home message.
Using the semi-quantitative NIH Clinical Genome Resource model, twelve out of twenty-seven genes curated had definitive evidence, ten have emerging evidence, five were disputed, and one gene had no evidence to support causal PAH gene-disease relationships.
Points for clinical practice:
All genes with definitive evidence for a PAH gene-disease relationship are strongly recommended to be included in genetic testing.
Caution should be taken in clinical interpretation for genes with less than definitive or strong evidence, and disputed genes or genes with no known genetic evidence for PAH should not be included in genetic testing.
Four previously reported TGF-β/BMP pathway genes are disputed for a PAH gene-disease relationship.
For undiagnosed cases, genetic reanalysis is recommended over time as new evidence for PAH gene-disease relationships emerges.
Acknowledgements
Funding Statement
This work was financially supported by grants from the National Institutes of Health (NIH; U24HG009650) and NHLBI R35HL140019 (MAA). SB is supported by an AHA predoctoral fellowship #834024. LS is supported by the Springboard Scheme Funders, namely the Academy of Medical Sciences (AMS), the Wellcome Trust, the Government Department of Business, Energy and Industrial Strategy (BEIS), the British Heart Foundation and Diabetes UK [SBF005\1115]. JT is supported by Spanish FEDER-ISCIII grant PI21/01593.
Nonstandard Abbreviations and Acronyms
- APAH
pulmonary arterial hypertension associated with other diseases
- BOECs
blood outgrowth endothelial cells
- ClinGen
Clinical Genome Resource
- HPAH
heritable pulmonary arterial hypertension
- IPAH
idiopathic pulmonary arterial hypertension
- LOF
loss of function
- PAECs
pulmonary arterial endothelial cells
- PASMCs
pulmonary arterial smooth muscle cells
- PAH
pulmonary arterial hypertension
- pLOF
predicted loss of function
- RV
right ventricular
- RVSP
right ventricular systolic pressure
- SPS
small patella syndrome
- VUS
variant of uncertain significance
PH VCEP members
Emily P. Callejo1, Kristina M. Day2, Daniela Macaya3, Gabriel Maldonado-Velez2
1Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, USA. 2Indiana University School of Medicine, Indianapolis, IN, USA. 3GeneDx, Gaithersburg, MD, USA.
PAH-ICON members
Stephen L. Archer1, Kathryn Auckland2, Eric D. Austin3, Roberto Badagliacca4, Joan-Albert Barberà5, Catharina Belge6, Harm Jan Bogaard7, Sébastien Bonnet8,9, Karin A. Boomars10, Olivier Boucherat9,11, Murali M. Chakinala12, Robin Condliffe13,14, Rachel Lynn Damico15, Marion Delcroix16, Ankit A. Desai17, Anna Doboszynska18, C. Greg Elliott19, Melanie Eyries20,21, Maria Pilar Escribano Subías22,23,24,25, Henning Gall26, Stefano Ghio27, Ardeschir-Hossein Ghofrani26,28, Ekkehard Grünig29, Rizwan Hamid30, Lars Harbaum31, Paul M. Hassoun15, Anna R. Hemnes32, Katrin Hinderhofer33, Luke S. Howard31, Marc Humbert34,35,36, David G. Kiely37, David Langleben38, Allan Lawrie13, Jim E. Loyd39, Shahin Moledina40, David Montani35,41, Nichols W. Morrell42,43, William C. Nichols44, Andrea Olschewski45, Horst Olschewski45,46, Silvia Papa4, Mike W. Pauciulo44, Steve Provencher9,11, Rozenn Quarck6, Christopher J. Rhodes31, Laura Scelsi28, Werner Seeger27, Duncan J. Stewart45, Andrew Sweatt46, Emilia M. Swietlik42, Carmen Treacy2, Richard C. Trembath47, Olga Tura-Ceide48,49,50, Carmine Dario Vizza4, Anton Vonk Noordegraaf51, Martin R. Wilkins31, Roham T. Zamanian52, Dmitry Zateyshchikov53
1Department of Medicine, Queen’s University, Kingston, Ontario, Canada. 2Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK. 3Vanderbilt University Department of Pediatrics, Division of Allergy, Immunology, and Pulmonary Medicine, Nashville, TN, USA. 4Pulmonary Hypertension Unit, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Italy. 5Department of Pulmonary Medicine, Hospital Clinic-IDIBAPS, University of Barcelona, Barcelona and Biomedical Research Networking Center on Respiratory Diseases (CIBERES), Spain. 6Laboratory of Respiratory Diseases & Thoracic Surgery (BREATHE), Department of Chronic Diseases & Metabolism (CHROMETA), Clinical Department of Respiratory Diseases, University Hospitals, University of Leuven, 3000 Leuven, Belgium. 7Department of Lung Disease, Amsterdam UMC (location VUmc), Amsterdam, the Netherlands. 8Pulmonary Hypertension Research Group, Centre de Recherche de l’Institut de Cardiologie et de Pneumologie de Quebec, Quebec City, QC, Canada. 9Department of Medicine, Université Laval, Quebec City, Quebec, Canada. 10Department of Pulmonary Medicine, Erasmus MC, University Medical Center Rotterdam, the Netherlands. 11Pulmonary Hypertension and Vascular Biology Research Group, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Department of Medicine, Université Laval, Quebec City, Quebec, Canada. 12Division of Pulmonary and Critical Care Medicine, Department of Medicine, Washington University School of Medicine, St. Louis, MO, USA. 13Department of Infection, Immunity & Cardiovascular Disease, University of Sheffield, UK. 14Royal Hallamshire Hospital, Sheffield, UK. 15Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA. 16Department of Pneumology, University Hospital Leuven, Leuven, Belgium. 17Indiana University, Indianapolis, IN, USA. 18Department of Pulmonology, Faculty of Medicine, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland. 19Pulmonary and Critical Care Divisions, Intermountain Medical Center, Murray, UT, USA. 20Département de Génétique, AP-HP, Hôpital Pitié-Salpêtrière, Paris, France. 21INSERM UMRS 1166, Sorbonne Université and Institute for Cardiometabolism and Nutrition (ICAN), Paris, France. 22Department of Cardiology, Hospital Universitario 12 de Octubre, Madrid, Spain. 23Ciber-CV, Centro de investigación Biomédica en Red de Enfermedades Cardiovasculares, Madrid, Spain. 24Centro de Referencia Nacional de Hipertensión Pulmonar Compleja and ERN-Lung-Pulmonary Hypertension Referal Center, Madrid, Spain. 25Instituto de Investigación Sanitaria del Hospital Universitario 12 de Octubre (Imas12), Red SAMID, Madrid, Spain. 26Justus-Liebig University, Excellence Cluster Cardio-Pulmonary Institute (CPI) and Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany. 27Division of Cardiology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. 28Department of Medicine, Imperial College London, London, UK. 29Center for Pulmonary Hypertension, Thoraxklinik Heidelberg gGmbH at Heidelberg University Hospital, Heidelberg, Germany and Translational Lung Research Center Heidelberg (TLRC), German Center for Lung Research (DZL), Heidelberg, Germany. 30Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA. 31National Heart and Lung Institute, Imperial College London, London, UK. 32Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA. 33Laboratory of Molecular Genetic Diagnostics, Institute of Human Genetics, Heidelberg University, Heidelberg, Germany. 34Faculté de Médecine, Université Paris-Sud and Université Paris-Saclay, Le Kremlin-Bicêtre, France. 35INSERM UMR_S 999, Hôpital Marie Lannelongue, Le Plessis-Robinson, France. 36AP-HP, Service de Pneumologie, Centre de Référence de l’Hypertension Pulmonaire Sévère, Département Hospitalo-Universitaire (DHU) Thorax Innovation (TORINO), Hôpital de Bicêtre, Le Kremlin-Bicêtre, France. 37Sheffield Pulmonary Vascular Disease Unit, Royal Hallamshire Hospital, Sheffield, UK. 38Center for Pulmonary Vascular Disease, Cardiology Division, Jewish General Hospital and McGill University, Montreal, QC, Canada. 39Vanderbilt University Medical Center, Nashville, TN, USA. 40Great Ormond Street Hospital, London, UK. 41Université Paris-Saclay, AP-HP, French Referral Center for Pulmonary Hypertension, Pulmonary Department, Hôpital de Bicêtre, Le Kremlin-Bicêtre, France. 42Department of Medicine, University of Cambridge, UK. 43Department of Haematology, University of Cambridge, Cambridge, UK. 44Division of Human Genetics, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA. 45Ludwig Boltzmann Institute for Lung Vascular research, Graz, Austria. 46Medical University of Graz, Graz, Austria. 47Ottawa Hospital Research Institute, Sinclair Centre for Regenerative Medicine and the University of Ottawa, Ontario, Canada. 48Department of Pulmonary, Allergy and Critical Care Medicine, Stanford University, Stanford, CA, USA. 49Department of Medical and Molecular Genetics, King’s College London, London, UK. 50Department of Pulmonary Medicine, Hospital Clínic-Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS), University of Barcelona, Spain. 51Biomedical Research Networking center on Respiratory diseases (CIBERES), 28029 Madrid, Spain. 52Department of Pulmonary Medicine, Dr. Josep Trueta University Hospital de Girona, Santa Caterina Hospital de Salt and the Girona Biomedical Research Institute (IDIBGI), Girona, Catalonia, Spain. 53Department of Pulmonology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, VU University Medical Center, Amsterdam, the Netherlands. 54Department of Medicine, Stanford University Medical Center, Stanford, CA, USA. 55Federal Scientific Clinical Centre of Federal Medical and Biological Agency, Genetic Laboratory, Moscow, Russia.
Footnotes
Conflict of Interest
The authors have nothing to disclose.
Conflict of interest statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
All gene curations reported herein are publicly available at https://search.clinicalgenome.org/kb/affiliate/10071 or https://search.clinicalgenome.org/kb/affiliate/10028 (ACVRL1 and ENG).
References
- 1.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013: 62(25 Suppl): D22–33. [DOI] [PubMed] [Google Scholar]
- 2.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014: 115(1): 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019: 53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Rev Esp Cardiol (Engl Ed) 2016: 69(2): 177. [DOI] [PubMed] [Google Scholar]
- 5.Abman SH, Ivy DD, Archer SL, et al. Executive Summary of the American Heart Association and American Thoracic Society Joint Guidelines for Pediatric Pulmonary Hypertension. Am J Respir Crit Care Med 2016: 194(7): 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant 2019: 38(9): 879–901. [DOI] [PubMed] [Google Scholar]
- 7.Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 2019: 53(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehm HL, Berg JS, Brooks LD, et al. ClinGen--the Clinical Genome Resource. N Engl J Med 2015: 372(23): 2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strande NT, Riggs ER, Buchanan AH, et al. Evaluating the Clinical Validity of Gene-Disease Associations: An Evidence-Based Framework Developed by the Clinical Genome Resource. American journal of human genetics 2017: 100(6): 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renard M, Francis C, Ghosh R, et al. Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysm and Dissection. J Am Coll Cardiol 2018: 72(6): 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini SM, Kim R, Udupa S, et al. Reappraisal of Reported Genes for Sudden Arrhythmic Death: Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation 2018: 138(12): 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ingles J, Goldstein J, Thaxton C, et al. Evaluating the Clinical Validity of Hypertrophic Cardiomyopathy Genes. Circ Genom Precis Med 2019: 12(2): e002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler A, Novelli V, Amin AS, et al. An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation 2020: 141(6): 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James CA, Jongbloed JDH, Hershberger RE, et al. International Evidence Based Reappraisal of Genes Associated With Arrhythmogenic Right Ventricular Cardiomyopathy Using the Clinical Genome Resource Framework. Circ Genom Precis Med 2021: 14(3): e003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan E, Peterson L, Ai T, et al. Evidence-Based Assessment of Genes in Dilated Cardiomyopathy. Circulation 2021: 144(1): 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prapa M, Lago-Docampo M, Swietlik EM, et al. First Genotype-Phenotype Study in TBX4 Syndrome: Gain-of-Function Mutations Causative for Lung Disease. Am J Respir Crit Care Med 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graf S, Haimel M, Bleda M, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 2018: 9(1): 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swietlik EM, Greene D, Zhu N, et al. Bayesian Inference Associates Rare KDR Variants with Specific Phenotypes in Pulmonary Arterial Hypertension. Circ Genom Precis Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu N, Swietlik EM, Welch CL, et al. Rare variant analysis of 4241 pulmonary arterial hypertension cases from an international consortium implicates FBLN2, PDGFD, and rare de novo variants in PAH. Genome Med 2021: 13(1): 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu N, Pauciulo MW, Welch CL, et al. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med 2019: 11(1): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potus F, Pauciulo MW, Cook EK, et al. Novel Mutations and Decreased Expression of the Epigenetic Regulator TET2 in Pulmonary Arterial Hypertension. Circulation 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XJ, Lian TY, Jiang X, et al. Germline BMP9 mutation causes idiopathic pulmonary arterial hypertension. Eur Respir J 2019: 53(3). [DOI] [PubMed] [Google Scholar]
- 23.Castano JAT, Hernandez-Gonzalez I, Gallego N, et al. Customized Massive Parallel Sequencing Panel for Diagnosis of Pulmonary Arterial Hypertension. Genes (Basel) 2020: 11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lago-Docampo M, Tenorio J, Hernandez-Gonzalez I, et al. Characterization of rare ABCC8 variants identified in Spanish pulmonary arterial hypertension patients. Sci Rep 2020: 10(1): 15135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navas P, Tenorio J, Quezada CA, et al. Molecular Analysis of BMPR2, TBX4, and KCNK3 and Genotype-Phenotype Correlations in Spanish Patients and Families With Idiopathic and Hereditary Pulmonary Arterial Hypertension. Rev Esp Cardiol (Engl Ed) 2016: 69(11): 1011–1019. [DOI] [PubMed] [Google Scholar]
- 26.Pienkos S, Gallego N, Condon DF, et al. Novel TNIP2 and TRAF2 Variants Are Implicated in the Pathogenesis of Pulmonary Arterial Hypertension. Front Med (Lausanne) 2021: 8: 625763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kircher M, Witten DM, Jain P, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 2014: 46(3): 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Ventura F, Doody J, et al. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol 1995: 15(7): 3479–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrell NW, Bloch DB, ten Dijke P, et al. Targeting BMP signalling in cardiovascular disease and anaemia. Nat Rev Cardiol 2016: 13(2): 106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. American journal of human genetics 2000: 67(3): 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.International PPHC, Lane KB, Machado RD, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 2000: 26(1): 81–84. [DOI] [PubMed] [Google Scholar]
- 32.Machado RD, Southgate L, Eichstaedt CA, et al. Pulmonary Arterial Hypertension: A Current Perspective on Established and Emerging Molecular Genetic Defects. Hum Mutat 2015: 36(12): 1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atkinson C, Stewart S, Upton PD, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002: 105(14): 1672–1678. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Fantozzi I, Tigno DD, et al. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2003: 285(3): L740–754. [DOI] [PubMed] [Google Scholar]
- 35.Morrell NW, Yang X, Upton PD, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 2001: 104(7): 790–795. [DOI] [PubMed] [Google Scholar]
- 36.Song Y, Jones JE, Beppu H, et al. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 2005: 112(4): 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong KH, Lee YJ, Lee E, et al. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 2008: 118(7): 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hautefort A, Mendes-Ferreira P, Sabourin J, et al. Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension. Circulation 2019: 139(7): 932–948. [DOI] [PubMed] [Google Scholar]
- 39.West J, Fagan K, Steudel W, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 2004: 94(8): 1109–1114. [DOI] [PubMed] [Google Scholar]
- 40.Reynolds AM, Holmes MD, Danilov SM, et al. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J 2012: 39(2): 329–343. [DOI] [PubMed] [Google Scholar]
- 41.Long L, Ormiston ML, Yang X, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 2015: 21(7): 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austin ED, Ma L, LeDuc C, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 2012: 5(3): 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu N, Gonzaga-Jauregui C, Welch CL, et al. Exome Sequencing in Children With Pulmonary Arterial Hypertension Demonstrates Differences Compared With Adults. Circ Genom Precis Med 2018: 11(4): e001887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eyries M, Montani D, Nadaud S, et al. Widening the landscape of heritable pulmonary hypertension mutations in paediatric and adult cases. Eur Respir J 2019: 53(3). [DOI] [PubMed] [Google Scholar]
- 45.Achcar RO, Demura Y, Rai PR, et al. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest 2006: 129(3): 696–705. [DOI] [PubMed] [Google Scholar]
- 46.Marsboom G, Chen Z, Yuan Y, et al. Aberrant caveolin-1-mediated Smad signaling and proliferation identified by analysis of adenine 474 deletion mutation (c.474delA) in patient fibroblasts: a new perspective on the mechanism of pulmonary hypertension. Mol Biol Cell 2017: 28(9): 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copeland CA, Han B, Tiwari A, et al. A disease-associated frameshift mutation in caveolin-1 disrupts caveolae formation and function through introduction of a de novo ER retention signal. Mol Biol Cell 2017: 28(22): 3095–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao YY, Liu Y, Stan RV, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A 2002: 99(17): 11375–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maniatis NA, Shinin V, Schraufnagel DE, et al. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1−/− mice. Am J Physiol Lung Cell Mol Physiol 2008: 294(5): L865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murata T, Lin MI, Huang Y, et al. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med 2007: 204(10): 2373–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.David L, Mallet C, Mazerbourg S, et al. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 2007: 109(5): 1953–1961. [DOI] [PubMed] [Google Scholar]
- 52.Hodgson J, Swietlik EM, Salmon RM, et al. Characterization of GDF2 Mutations and Levels of BMP9 and BMP10 in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2020: 201(5): 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Heuvel LM, Jansen SMA, Alsters SIM, et al. Genetic Evaluation in a Cohort of 126 Dutch Pulmonary Arterial Hypertension Patients. Genes (Basel) 2020: 11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang G, Fan R, Ji R, et al. Novel homozygous BMP9 nonsense mutation causes pulmonary arterial hypertension: a case report. BMC Pulm Med 2016: 16: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallego N, Cruz-Utrilla A, Guillen I, et al. Expanding the Evidence of a Semi-Dominant Inheritance in GDF2 Associated with Pulmonary Arterial Hypertension. Cells 2021: 10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hodgson J, Ruiz-Llorente L, McDonald J, et al. Homozygous GDF2 nonsense mutations result in a loss of circulating BMP9 and BMP10 and are associated with either PAH or an “HHT-like” syndrome in children. Mol Genet Genomic Med 2021: 9(12): e1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Yang J, Tang X, et al. Homozygous GDF2-Related Hereditary Hemorrhagic Telangiectasia in a Chinese Family. Pediatrics 2020: 146(2). [DOI] [PubMed] [Google Scholar]
- 58.Balachandar S, Graves TJ, Shimonty A, et al. Identification and validation of a novel pathogenic variant in GDF2 (BMP9) responsible for hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. Am J Med Genet A 2022: 188(3): 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shintani M, Yagi H, Nakayama T, et al. A new nonsense mutation of SMAD8 associated with pulmonary arterial hypertension. J Med Genet 2009: 46(5): 331–337. [DOI] [PubMed] [Google Scholar]
- 60.Nasim MT, Ogo T, Ahmed M, et al. Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum Mutat 2011: 32(12): 1385–1389. [DOI] [PubMed] [Google Scholar]
- 61.Drake KM, Zygmunt D, Mavrakis L, et al. Altered MicroRNA processing in heritable pulmonary arterial hypertension: an important role for Smad-8. Am J Respir Crit Care Med 2011: 184(12): 1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013: 45(6): 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawai S, Faucheu C, Gallea S, et al. Mouse smad8 phosphorylation downstream of BMP receptors ALK-2, ALK-3, and ALK-6 induces its association with Smad4 and transcriptional activity. Biochem Biophys Res Commun 2000: 271(3): 682–687. [DOI] [PubMed] [Google Scholar]
- 64.Huang Z, Wang D, Ihida-Stansbury K, et al. Defective pulmonary vascular remodeling in Smad8 mutant mice. Hum Mol Genet 2009: 18(15): 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tillet E, Ouarne M, Desroches-Castan A, et al. A heterodimer formed by bone morphogenetic protein 9 (BMP9) and BMP10 provides most BMP biological activity in plasma. J Biol Chem 2018: 293(28): 10963–10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eyries M, Montani D, Nadaud S, et al. Widening the landscape of heritable pulmonary hypertension mutations in paediatric and adult cases. European Respiratory Journal 2019: 53(3). [DOI] [PubMed] [Google Scholar]
- 67.Gelinas SM, Benson CE, Khan MA, et al. Whole Exome Sequence Analysis Provides Novel Insights into the Genetic Framework of Childhood-Onset Pulmonary Arterial Hypertension. Genes (Basel) 2020: 11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hodgson J, Swietlik EM, Salmon RM, et al. Characterization of GDF2 mutations and levels of BMP9 and BMP10 in pulmonary arterial hypertension. American journal of respiratory and critical care medicine 2020: 201(5): 575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abou Hassan OK, Haidar W, Nemer G, et al. Clinical and genetic characteristics of pulmonary arterial hypertension in Lebanon. BMC medical genetics 2018: 19(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen H, Shi S, Acosta L, et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouvard C, Tu L, Rossi M, et al. Different cardiovascular and pulmonary phenotypes for single-and double-knock-out mice deficient in BMP9 and BMP10. Cardiovascular Research 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci 2005: 6(12): 945–954. [DOI] [PubMed] [Google Scholar]
- 73.Chida A, Shintani M, Nakayama T, et al. Missense mutations of the BMPR1B (ALK6) gene in childhood idiopathic pulmonary arterial hypertension. Circ J 2012: 76(6): 1501–1508. [DOI] [PubMed] [Google Scholar]
- 74.Upton PD, Long L, Trembath RC, et al. Functional characterization of bone morphogenetic protein binding sites and Smad1/5 activation in human vascular cells. Mol Pharmacol 2008: 73(2): 539–552. [DOI] [PubMed] [Google Scholar]
- 75.Waite KA, Eng C. From developmental disorder to heritable cancer: it’s all in the BMP/TGF-beta family. Nat Rev Genet 2003: 4(10): 763–773. [DOI] [PubMed] [Google Scholar]
- 76.Yang X, Long L, Southwood M, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 2005: 96(10): 1053–1063. [DOI] [PubMed] [Google Scholar]
- 77.Ramos MF, Lame MW, Segall HJ, et al. Smad signaling in the rat model of monocrotaline pulmonary hypertension. Toxicol Pathol 2008: 36(2): 311–320. [DOI] [PubMed] [Google Scholar]
- 78.Sanada TJ, Sun XQ, Happe C, et al. Altered TGFbeta/SMAD Signaling in Human and Rat Models of Pulmonary Hypertension: An Old Target Needs Attention. Cells 2021: 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geraci MW, Moore M, Gesell T, et al. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res 2001: 88(6): 555–562. [DOI] [PubMed] [Google Scholar]
- 80.Hamouda NN, Van den Haute C, Vanhoutte R, et al. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J Biol Chem 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Machado R, Welch CL, Haimel M, et al. Biallelic variants of ATP13A3 cause dose-dependent childhood-onset pulmonary arterial hypertension characterised by extreme morbidity and mortality. J Med Genet 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020: 581(7809): 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Welch CL, Chung WK. Channelopathy Genes in Pulmonary Arterial Hypertension. Biomolecules 2022: 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rhodes CJ, Ghataorhe P, Wharton J, et al. Plasma Metabolomics Implicates Modified Transfer RNAs and Altered Bioenergetics in the Outcomes of Pulmonary Arterial Hypertension. Circulation 2017: 135(5): 460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He YY, Yan Y, Jiang X, et al. Spermine promotes pulmonary vascular remodelling and its synthase is a therapeutic target for pulmonary arterial hypertension. Eur Respir J 2020: 56(5). [DOI] [PubMed] [Google Scholar]
- 86.Olschewski A, Li Y, Tang B, et al. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res 2006: 98(8): 1072–1080. [DOI] [PubMed] [Google Scholar]
- 87.Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 2013: 369(4): 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Navas Tejedor P, Tenorio Castano J, Palomino Doza J, et al. An homozygous mutation in KCNK3 is associated with an aggressive form of hereditary pulmonary arterial hypertension. Clin Genet 2017: 91(3): 453–457. [DOI] [PubMed] [Google Scholar]
- 89.Cunningham KP, Holden RG, Escribano-Subias PM, et al. Characterization and regulation of wild-type and mutant TASK-1 two pore domain potassium channels indicated in pulmonary arterial hypertension. J Physiol 2019: 597(4): 1087–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014: 145(2): 231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Higasa K, Ogawa A, Terao C, et al. A burden of rare variants in BMPR2 and KCNK3 contributes to a risk of familial pulmonary arterial hypertension. BMC Pulm Med 2017: 17(1): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang HS, Liu Q, Piao CM, et al. Genotypes and Phenotypes of Chinese Pediatric Patients With Idiopathic and Heritable Pulmonary Arterial Hypertension-A Single-Center Study. Can J Cardiol 2019: 35(12): 1851–1856. [DOI] [PubMed] [Google Scholar]
- 93.Haarman MG, Kerstjens-Frederikse WS, Vissia-Kazemier TR, et al. The Genetic Epidemiology of Pediatric Pulmonary Arterial Hypertension. J Pediatr 2020: 225: 65–73 e65. [DOI] [PubMed] [Google Scholar]
- 94.Eichstaedt CA, Sassmannshausen Z, Shaukat M, et al. Gene panel diagnostics reveals new pathogenic variants in pulmonary arterial hypertension. Respir Res 2022: 23(1): 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antigny F, Hautefort A, Meloche J, et al. Potassium Channel Subfamily K Member 3 (KCNK3) Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2016: 133(14): 1371–1385. [DOI] [PubMed] [Google Scholar]
- 96.Lambert M, Capuano V, Boet A, et al. Characterization of Kcnk3-Mutated Rat, a Novel Model of Pulmonary Hypertension. Circ Res 2019: 125(7): 678–695. [DOI] [PubMed] [Google Scholar]
- 97.Bohnen MS, Ma L, Zhu N, et al. Loss-of-Function ABCC8 Mutations in Pulmonary Arterial Hypertension. Circ Genom Precis Med 2018: 11(10): e002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Le Ribeuz H, Capuano V, Girerd B, et al. Implication of Potassium Channels in the Pathophysiology of Pulmonary Arterial Hypertension. Biomolecules 2020: 10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saadoun S, Papadopoulos MC, Hara-Chikuma M, et al. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005: 434(7034): 786–792. [DOI] [PubMed] [Google Scholar]
- 100.Liu M, Liu Q, Pei Y, et al. Aqp-1 Gene Knockout Attenuates Hypoxic Pulmonary Hypertension of Mice. Arterioscler Thromb Vasc Biol 2019: 39(1): 48–62. [DOI] [PubMed] [Google Scholar]
- 101.Eyries M, Montani D, Girerd B, et al. Familial pulmonary arterial hypertension by KDR heterozygous loss of function. Eur Respir J 2020: 55(4). [DOI] [PubMed] [Google Scholar]
- 102.Kaipainen A, Korhonen J, Pajusola K, et al. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med 1993: 178(6): 2077–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ciuclan L, Bonneau O, Hussey M, et al. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2011: 184(10): 1171–1182. [DOI] [PubMed] [Google Scholar]
- 104.Winter MP, Sharma S, Altmann J, et al. Interruption of vascular endothelial growth factor receptor 2 signaling induces a proliferative pulmonary vasculopathy and pulmonary hypertension. Basic Res Cardiol 2020: 115(6): 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corada M, Orsenigo F, Morini MF, et al. Sox17 is indispensable for acquisition and maintenance of arterial identity. Nat Commun 2013: 4: 2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hiraide T, Kataoka M, Suzuki H, et al. SOX17 Mutations in Japanese Patients with Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2018: 198(9): 1231–1233. [DOI] [PubMed] [Google Scholar]
- 107.Zhu N, Welch CL, Wang J, et al. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med 2018: 10(1): 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang TM, Wang SS, Xu YJ, et al. SOX17 Loss-of-Function Mutation Underlying Familial Pulmonary Arterial Hypertension. Int Heart J 2021: 62(3): 566–574. [DOI] [PubMed] [Google Scholar]
- 109.Miyagawa K, Shi M, Chen PI, et al. Smooth Muscle Contact Drives Endothelial Regeneration by BMPR2-Notch1-Mediated Metabolic and Epigenetic Changes. Circ Res 2019: 124(2): 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res 2007: 100(12): 1686–1695. [DOI] [PubMed] [Google Scholar]
- 111.Eichstaedt CA, Song J, Viales RR, et al. First identification of Kruppel-like factor 2 mutation in heritable pulmonary arterial hypertension. Clin Sci (Lond) 2017: 131(8): 689–698. [DOI] [PubMed] [Google Scholar]
- 112.Piva R, Deaglio S, Fama R, et al. The Kruppel-like factor 2 transcription factor gene is recurrently mutated in splenic marginal zone lymphoma. Leukemia 2015: 29(2): 503–507. [DOI] [PubMed] [Google Scholar]
- 113.Sindi HA, Russomanno G, Satta S, et al. Therapeutic potential of KLF2-induced exosomal microRNAs in pulmonary hypertension. Nat Commun 2020: 11(1): 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wani MA, Conkright MD, Jeffries S, et al. cDNA isolation, genomic structure, regulation, and chromosomal localization of human lung Kruppel-like factor. Genomics 1999: 60(1): 78–86. [DOI] [PubMed] [Google Scholar]
- 115.Anderson KP, Kern CB, Crable SC, et al. Isolation of a gene encoding a functional zinc finger protein homologous to erythroid Kruppel-like factor: identification of a new multigene family. Mol Cell Biol 1995: 15(11): 5957–5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fassler R, Sasaki T, Timpl R, et al. Differential regulation of fibulin, tenascin-C, and nidogen expression during wound healing of normal and glucocorticoid-treated mice. Exp Cell Res 1996: 222(1): 111–116. [DOI] [PubMed] [Google Scholar]
- 117.Tsuda T, Wang H, Timpl R, et al. Fibulin-2 expression marks transformed mesenchymal cells in developing cardiac valves, aortic arch vessels, and coronary vessels. Dev Dyn 2001: 222(1): 89–100. [DOI] [PubMed] [Google Scholar]
- 118.Folestad E, Kunath A, Wagsater D. PDGF-C and PDGF-D signaling in vascular diseases and animal models. Mol Aspects Med 2018: 62: 1–11. [DOI] [PubMed] [Google Scholar]
- 119.Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J 2005: 272(22): 5723–5741. [DOI] [PubMed] [Google Scholar]
- 120.Ponten A, Folestad EB, Pietras K, et al. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res 2005: 97(10): 1036–1045. [DOI] [PubMed] [Google Scholar]
- 121.de Jesus Perez VA, Yuan K, Lyuksyutova MA, et al. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2014: 189(10): 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barozzi C, Galletti M, Tomasi L, et al. A Combined Targeted and Whole Exome Sequencing Approach Identified Novel Candidate Genes Involved in Heritable Pulmonary Arterial Hypertension. Sci Rep 2019: 9(1): 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Siebel C, Lendahl U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol Rev 2017: 97(4): 1235–1294. [DOI] [PubMed] [Google Scholar]
- 124.Chida A, Shintani M, Matsushita Y, et al. Mutations of NOTCH3 in childhood pulmonary arterial hypertension. Mol Genet Genomic Med 2014: 2(3): 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gomez J, Reguero JR, Junquera MR, et al. Next generation sequencing of the NOTCH3 gene in a cohort of pulmonary hypertension patients. Int J Cardiol 2016: 209: 149–150. [DOI] [PubMed] [Google Scholar]
- 126.Eichstaedt CA, Verweyen J, Halank M, et al. Myeloproliferative Diseases as Possible Risk Factor for Development of Chronic Thromboembolic Pulmonary Hypertension-A Genetic Study. Int J Mol Sci 2020: 21(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hernandez-Gonzalez I, Tenorio-Castano J, Ochoa-Parra N, et al. Novel Genetic and Molecular Pathways in Pulmonary Arterial Hypertension Associated with Connective Tissue Disease. Cells 2021: 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li X, Zhang X, Leathers R, et al. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med 2009: 15(11): 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Vilder EY, Debacker J, Vanakker OM. GGCX-Associated Phenotypes: An Overview in Search of Genotype-Phenotype Correlations. Int J Mol Sci 2017: 18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Khetarpal SA, Qamar A, Bick AG, et al. Clonal Hematopoiesis of Indeterminate Potential Reshapes Age-Related CVD: JACC Review Topic of the Week. J Am Coll Cardiol 2019: 74(4): 578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014: 371(26): 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014: 371(26): 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 2017: 377(2): 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Libby P.Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013: 368(21): 2004–2013. [DOI] [PubMed] [Google Scholar]
- 135.Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv 2018: 2(22): 3404–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang HY, Chu ML, Pan TC, et al. Extracellular matrix protein fibulin-2 is expressed in the embryonic endocardial cushion tissue and is a prominent component of valves in adult heart. Dev Biol 1995: 167(1): 18–26. [DOI] [PubMed] [Google Scholar]
- 137.Olijnyk D, Ibrahim AM, Ferrier RK, et al. Fibulin-2 is involved in early extracellular matrix development of the outgrowing mouse mammary epithelium. Cell Mol Life Sci 2014: 71(19): 3811–3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang H, Wu J, Dong H, et al. Fibulin-2 deficiency attenuates angiotensin II-induced cardiac hypertrophy by reducing transforming growth factor-beta signalling. Clin Sci (Lond) 2014: 126(4): 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khan SA, Dong H, Joyce J, et al. Fibulin-2 is essential for angiotensin II-induced myocardial fibrosis mediated by transforming growth factor (TGF)-beta. Lab Invest 2016: 96(7): 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gan L, Lee I, Smith R, et al. Sequencing and expression analysis of the serine protease gene cluster located in chromosome 19q13 region. Gene 2000: 257(1): 119–130. [DOI] [PubMed] [Google Scholar]
- 141.Shaw JL, Diamandis EP. Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem 2007: 53(8): 1423–1432. [DOI] [PubMed] [Google Scholar]
- 142.Mahabeer R, Bhoola KD. Kallikrein and kinin receptor genes. Pharmacol Ther 2000: 88(1): 77–89. [DOI] [PubMed] [Google Scholar]
- 143.Bergaya S, Meneton P, Bloch-Faure M, et al. Decreased flow-dependent dilation in carotid arteries of tissue kallikrein-knockout mice. Circ Res 2001: 88(6): 593–599. [DOI] [PubMed] [Google Scholar]
- 144.Song Q, Chao J, Chao L. High level of circulating human tissue kallikrein induces hypotension in a transgenic mouse model. Clin Exp Hypertens 1996: 18(8): 975–993. [DOI] [PubMed] [Google Scholar]
- 145.Wang J, Xiong W, Yang Z, et al. Human tissue kallikrein induces hypotension in transgenic mice. Hypertension 1994: 23(2): 236–243. [DOI] [PubMed] [Google Scholar]
- 146.Madeddu P, Emanueli C, El-Dahr S. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol 2007: 3(4): 208–221. [DOI] [PubMed] [Google Scholar]
- 147.Stone OA, Richer C, Emanueli C, et al. Critical role of tissue kallikrein in vessel formation and maturation: implications for therapeutic revascularization. Arterioscler Thromb Vasc Biol 2009: 29(5): 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xia CF, Yin H, Yao YY, et al. Kallikrein protects against ischemic stroke by inhibiting apoptosis and inflammation and promoting angiogenesis and neurogenesis. Hum Gene Ther 2006: 17(2): 206–219. [DOI] [PubMed] [Google Scholar]
- 149.Attisano L, Carcamo J, Ventura F, et al. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993: 75(4): 671–680. [DOI] [PubMed] [Google Scholar]
- 150.Yingling JM, Das P, Savage C, et al. Mammalian dwarfins are phosphorylated in response to transforming growth factor beta and are implicated in control of cell growth. Proc Natl Acad Sci U S A 1996: 93(17): 8940–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 1996: 13(2): 189–195. [DOI] [PubMed] [Google Scholar]
- 152.Trembath RC, Thomson JR, Machado RD, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001: 345(5): 325–334. [DOI] [PubMed] [Google Scholar]
- 153.Harrison RE, Flanagan JA, Sankelo M, et al. Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 2003: 40(12): 865–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Abdalla SA, Gallione CJ, Barst RJ, et al. Primary pulmonary hypertension in families with hereditary haemorrhagic telangiectasia. Eur Respir J 2004: 23(3): 373–377. [DOI] [PubMed] [Google Scholar]
- 155.Garamszegi N, Dore JJ Jr., Penheiter SG, et al. Transforming growth factor beta receptor signaling and endocytosis are linked through a COOH terminal activation motif in the type I receptor. Mol Biol Cell 2001: 12(9): 2881–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res 2003: 93(7): 682–689. [DOI] [PubMed] [Google Scholar]
- 157.Jerkic M, Kabir MG, Davies A, et al. Pulmonary hypertension in adult Alk1 heterozygous mice due to oxidative stress. Cardiovasc Res 2011: 92(3): 375–384. [DOI] [PubMed] [Google Scholar]
- 158.Gore B, Izikki M, Mercier O, et al. Key role of the endothelial TGF-beta/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS One 2014: 9(6): e100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blanco FJ, Santibanez JF, Guerrero-Esteo M, et al. Interaction and functional interplay between endoglin and ALK-1, two components of the endothelial transforming growth factor-beta receptor complex. J Cell Physiol 2005: 204(2): 574–584. [DOI] [PubMed] [Google Scholar]
- 160.McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 1994: 8(4): 345–351. [DOI] [PubMed] [Google Scholar]
- 161.Harrison RE, Berger R, Haworth SG, et al. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation 2005: 111(4): 435–441. 162. [DOI] [PubMed] [Google Scholar]
- 162.Chen YJ, Yang QH, Liu D, et al. Clinical and genetic characteristics of Chinese patients with hereditary haemorrhagic telangiectasia-associated pulmonary hypertension. Eur J Clin Invest 2013: 43(10): 1016–1024. [DOI] [PubMed] [Google Scholar]
- 163.Mache CJ, Gamillscheg A, Popper HH, et al. Early-life pulmonary arterial hypertension with subsequent development of diffuse pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia type 1. Thorax 2008: 63(1): 85–86. [DOI] [PubMed] [Google Scholar]
- 164.Malhotra R, Paskin-Flerlage S, Zamanian RT, et al. Circulating angiogenic modulatory factors predict survival and functional class in pulmonary arterial hypertension. Pulm Circ 2013: 3(2): 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Liu Z, Lebrin F, Maring JA, et al. ENDOGLIN is dispensable for vasculogenesis, but required for vascular endothelial growth factor-induced angiogenesis. PLoS One 2014: 9(1): e86273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rossi E, Bernabeu C, Smadja DM. Endoglin as an Adhesion Molecule in Mature and Progenitor Endothelial Cells: A Function Beyond TGF-beta. Front Med (Lausanne) 2019: 6: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Velasco S, Alvarez-Munoz P, Pericacho M, et al. L- and S-endoglin differentially modulate TGFbeta1 signaling mediated by ALK1 and ALK5 in L6E9 myoblasts. J Cell Sci 2008: 121(Pt 6): 913–919. [DOI] [PubMed] [Google Scholar]
- 168.Toporsian M, Jerkic M, Zhou YQ, et al. Spontaneous adult-onset pulmonary arterial hypertension attributable to increased endothelial oxidative stress in a murine model of hereditary hemorrhagic telangiectasia. Arterioscler Thromb Vasc Biol 2010: 30(3): 509–517. [DOI] [PubMed] [Google Scholar]
- 169.Yi CH, Russ A, Brook JD. Virtual cloning and physical mapping of a human T-box gene, TBX4. Genomics 2000: 67(1): 92–95. [DOI] [PubMed] [Google Scholar]
- 170.Haarman MG, Kerstjens-Frederikse WS, Berger RMF. TBX4 variants and pulmonary diseases: getting out of the ‘Box’. Curr Opin Pulm Med 2020: 26(3): 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bongers EM, Duijf PH, van Beersum SE, et al. Mutations in the human TBX4 gene cause small patella syndrome. American journal of human genetics 2004: 74(6): 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nimmakayalu M, Major H, Sheffield V, et al. Microdeletion of 17q22q23.2 encompassing TBX2 and TBX4 in a patient with congenital microcephaly, thyroid duct cyst, sensorineural hearing loss, and pulmonary hypertension. Am J Med Genet A 2011: 155A(2): 418–423. [DOI] [PubMed] [Google Scholar]
- 173.Kerstjens-Frederikse WS, Bongers EMHF, Roofthooft MTR, et al. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet 2013: 50(8): 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Levy M, Eyries M, Szezepanski I, et al. Genetic analyses in a cohort of children with pulmonary hypertension. Eur Respir J 2016: 48(4): 1118–1126. [DOI] [PubMed] [Google Scholar]
- 175.Prapa M, Lago-Docampo M, Swietlik EM, et al. First genotype-phenotype study in TBX4 syndrome: gain-of-function mutations causative for lung disease. medRxiv 2022: 2022.2002.2006.22270467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Galambos C, Mullen MP, Shieh JT, et al. Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and pediatric pulmonary hypertension. Eur Respir J 2019. [DOI] [PubMed] [Google Scholar]
- 177.Austin ED, Elliott CG. TBX4 syndrome: a systemic disease highlighted by pulmonary arterial hypertension in its most severe form. Eur Respir J 2020: 55(5). [DOI] [PubMed] [Google Scholar]
- 178.Thore P, Girerd B, Jais X, et al. Phenotype and outcome of pulmonary arterial hypertension patients carrying a TBX4 mutation. Eur Respir J 2020: 55(5). [DOI] [PubMed] [Google Scholar]
- 179.Arora R, Metzger RJ, Papaioannou VE. Multiple roles and interactions of Tbx4 and Tbx5 in development of the respiratory system. PLoS Genet 2012: 8(8): e1002866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cai Y, Yan L, Kielt MJ, et al. TBX4 Transcription Factor Is a Positive Feedback Regulator of Itself and Phospho-SMAD1/5. Am J Respir Cell Mol Biol 2021: 64(1): 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014: 46(1): 65–69. [DOI] [PubMed] [Google Scholar]
- 182.Tenorio J, Navas P, Barrios E, et al. A founder EIF2AK4 mutation causes an aggressive form of pulmonary arterial hypertension in Iberian Gypsies. Clin Genet 2015: 88(6): 579–583. [DOI] [PubMed] [Google Scholar]
- 183.Hadinnapola C, Bleda M, Haimel M, et al. Phenotypic Characterization of EIF2AK4 Mutation Carriers in a Large Cohort of Patients Diagnosed Clinically With Pulmonary Arterial Hypertension. Circulation 2017: 136(21): 2022–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Eichstaedt CA, Belge C, Chung WK, et al. Genetic counselling and testing in pulmonary arterial hypertension: a consensus statement on behalf of the International Consortium for Genetic Studies in PAH. Eur Respir J 2023: 61(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All gene curations reported herein are publicly available at https://search.clinicalgenome.org/kb/affiliate/10071 or https://search.clinicalgenome.org/kb/affiliate/10028 (ACVRL1 and ENG).