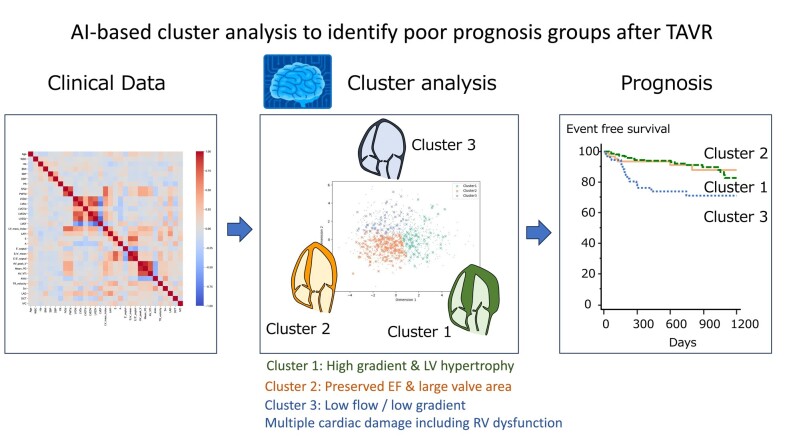
Abstract
Aims
The aim of this study was to identify phenotypes with potential prognostic significance in aortic stenosis (AS) patients after transcatheter aortic valve replacement (TAVR) through a clustering approach.
Methods and results
This multi-centre retrospective study included 1365 patients with severe AS who underwent TAVR between January 2015 and March 2019. Among demographics, laboratory, and echocardiography parameters, 20 variables were selected through dimension reduction and used for unsupervised clustering. Phenotypes and outcomes were compared between clusters. Patients were randomly divided into a derivation cohort (n = 1092: 80%) and a validation cohort (n = 273: 20%). Three clusters with markedly different features were identified. Cluster 1 was associated predominantly with elderly age, a high aortic valve gradient, and left ventricular (LV) hypertrophy; Cluster 2 consisted of preserved LV ejection fraction, larger aortic valve area, and high blood pressure; and Cluster 3 demonstrated tachycardia and low flow/low gradient AS. Adverse outcomes differed significantly among clusters during a median of 2.2 years of follow-up (P < 0.001). After adjustment for clinical and echocardiographic data in a Cox proportional hazards model, Cluster 3 (hazard ratio, 4.18; 95% confidence interval, 1.76–9.94; P = 0.001) was associated with increased risk of adverse outcomes. In sequential Cox models, a model based on clinical data and echocardiographic variables (χ2 = 18.4) was improved by Cluster 3 (χ2 = 31.5; P = 0.001) in the validation cohort.
Conclusion
Unsupervised cluster analysis of patients after TAVR revealed three different groups for assessment of prognosis. This provides a new perspective in the categorization of patients after TAVR that considers comorbidities and extravalvular cardiac dysfunction.
Keywords: Artificial intelligence, Machine learning, Aortic stenosis, Echocardiography
Graphical Abstract
Graphical Abstract.
Introduction
The incidence of aortic stenosis (AS) continues to rise with the rapid aging of society, and severe AS is a fatal condition without intervention. Current guidelines categorize AS into severity stages based on echocardiographic evaluations of the aortic valve and symptoms.1–3 Recently, the advancement of transcatheter aortic valve replacement (TAVR) has greatly improved the outcomes of elderly patients. However, the prognosis of patients who have undergone TAVR is variable, with complex interactions between patient characteristics and associated cardiac and non-cardiac factors.4 This suggests that the current classification focused on valve severity may not fully capture the diversity in patients with AS who have undergone TAVR. Due to the rising number of comorbidities, the phenotypes and outcomes of patients with AS who have undergone TAVR are more diverse than anticipated for varying outcomes from treatments.5 Identifying subpopulations (phenotypes) relevant to patient prognosis profiles based on several clinical parameters may be important for providing optimal patient care.6
Single-factor analysis (such as the use of only aortic valve peak velocity) may oversimplify the complexity of patients who have undergone TAVR. Recently, machine learning techniques, particularly unsupervised clustering, have been used to identify relationships among many factors.7 The unsupervised approach offers the potential to uncover novel phenotypes that may not be immediately obvious from clinical prognoses. While similar cluster analyses have been conducted in the context of heart failure and atrial fibrillation,8–10 we can incorporate machine learning to evaluate the meaning of clusters beyond traditional statistical methods in patients with TAVR. We aim to explore whether unsupervised cluster analysis could yield novel insights in discerning phenotypes with significant prognostic value after TAVR, rather than concentrating only on native AS phenotypes.
Methods
Ethics statements
The study protocol received approval from each participating institution's local ethics committee and was conducted in accordance with the guidelines set forth in the Declaration of Helsinki. To maintain the anonymity of the participants, the need for informed consent was waived, and the data were de-identified.
Study population
This was a retrospective analysis of a multi-centre registry of TAVR11 that involved 17 cardiovascular centres across Japan, including St. Marianna Medical University Hospital, National, Cerebral and Cardiovascular Center, Dokkyo Medical University Hospital, University of Tsukuba Hospital, Osaka University Hospital, Sakakibara Heart Institute, Iwate Medical University Hospital, Oita University Hospital, Kobe City Medical Center General Hospital, Hokkaido University Hospital, Nihonkai General Hospital, Asahikawa Medical University Hospital, Jichi Medical University Hospital, Hiroshima City Hiroshima Citizens Hospital, Tokushima University Hospital, Shimane University Hospital, and Japanese Red Cross Ise Hospital. Patients who underwent TAVR for severe symptomatic AS between January 2015 and 31 March 2019, with pre-procedural echocardiographic evaluation and follow-up after TAVR, were included in the study. Those who lacked complete baseline data for echocardiographic parameters and follow-up information after TAVR or who had poor image quality were excluded from the analysis. For the unsupervised cluster analysis, patient data were divided into a derivation cohort (80%) and a validation cohort (20%) by stratified sampling for the year of TAVR.
Data collection and outcome assessment
Clinical and demographic data were collected from patient electronic medical records through manual extraction. Follow-up was conducted through chart review, and the date of the last follow-up or death was recorded (last reviewed on 31 December 2019). Mortality data and hospitalization due to cardiovascular events, such as heart failure, arrhythmia, coronary artery disease, stroke, prosthetic valve-related issues, and device implantation, were obtained from patient medical records or available electronic databases. The primary outcome was a composite of cardiovascular death and re-hospitalization due to cardiovascular causes. Secondary outcomes included the components of the primary outcome and all-cause mortality. Follow-up was completed in all included patients, and an outcome review committee reviewed all events to avoid ascertainment bias.
Echocardiographic assessment
All patients underwent echocardiographic evaluation with commercially available ultrasound systems. Readers performed the assessments in accordance with current guidelines and reviewed all echocardiographic measurements.12,13 These readers are highly trained and experienced, ensuring a high level of consistency and reproducibility in the measurements. To measure baseline left ventricular (LV) global longitudinal strain (GLS), two-dimensional speckle-tracking echocardiography was performed offline with vendor-independent software (Image Arena 4.6; TOMTEC Imaging Systems, Munich, Germany). The apical four-chamber, two-chamber, and long-axis views were captured for analysis, and the average of the estimated peak systolic strain values from each view was used to calculate GLS, reported as an absolute value according to the American Society of Echocardiography/European Association of Cardiovascular Imaging recommendations. A single observer, blinded to the clinical and other echocardiographic data and outcomes, performed all strain measurements.
Variable preparation for cluster analysis
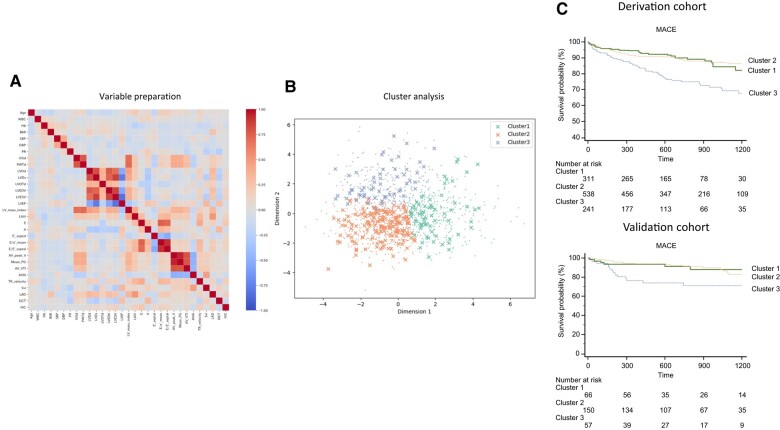
All variables used throughout the analysis are from pre-TAVR echocardiography and clinical characteristics. Variables used in the study were selected from clinical and echocardiographic measures used to evaluate the severity of AS, to stratify risk, or that have prognostic value for AS. Most were standard echocardiographic parameters, including aortic valve measurements [atrioventricular (AV) peak velocity, mean pressure gradient (PG), aortic velocity time interval, and aortic valve area index (AVAi)], LV systolic function [LV ejection fraction (LVEF) and stroke volume index (SVi)], cardiac geometry (LV end-diastolic diameter, LV end-systolic diameter, LV end-diastolic volume, LV end-systolic volume, LV mass index, and left atrial volume index), LV diastolic function (E wave, A wave, e′, and E/e′), and tricuspid regurgitation (TR) velocity. Non-echocardiographic parameters, such as age, body mass index, blood pressure, heart rate, and laboratory results, were also included. Patients with missing values for any variables were removed by listwise deletion. Pearson's coefficient matrix was calculated for each variable pair, and if the two were strongly correlated, the variable with greater clinical importance was kept and the other was discarded (Figure 1A). The distribution patterns of each variable were evaluated, and log-transformation was applied to two variables with substantial right skewness. All variables were standardized by transforming them to have a mean of 0 and a standard deviation (SD) of 1.
Figure 1.
Overall scheme of the study design. (A) Data from patients with aortic stenosis were prepared, and pivotal variables for clustering were selected. The correlation matrix among 27 candidate variables is shown. With dimensionality reduction, 20 variables were selected. (B) K-means clustering was performed. For simple visualization, the classification plot was projected onto the subspace where the 20 variables were reduced to two dimensions by principal component analysis. (C) Each cluster’s phenotype was interpreted. Outcomes were compared among the clusters, and different associations sought. MACE, major adverse cardiovascular event.
K-means clustering and visualization of important variables
The primary cluster analysis was performed with K-means clustering, which has been widely adopted in prior studies.14–17 The optimal number of clusters was determined with the elbow method and silhouette coefficient.18,19 The sum of squared error and silhouette coefficients for changing the number of clusters in K-means clustering are plotted (Figure 1B). Clustering was done without consideration of outcome data, and after assigning individuals to each cluster, differences in phenotypes and outcomes were compared and interpreted for their clinical significance. The validity of the clustering was confirmed by inferring the trained K-means model to a validation cohort (Figure 1C). To investigate the relative importance of the explanatory variables for cluster assignment, a logistic regression model was trained with the predicted clusters obtained by K-means clustering as the ground truth, and the partial regression coefficients of the trained model were calculated. In this case, the logistic regression model was trained with a derivation cohort, and then the trained model was evaluated with a validation cohort. If the trained model had high classification accuracy, the partial regression coefficient of the trained model could be considered as the relative importance of the explanatory variables for cluster assignment. The initialization method was set to greedy k-means++ in K-means clustering.20 The regularization strength and L1 ratio in the logistic regression with elastic net regularization were optimized with Optuna.21 The K-means and the logistic regression with elastic net regularization were implemented with scikit-learn 1.0.2.22 The code has been uploaded in GitHub (https://github.com/TakumasaTsuji/TAVR-Clustering).
Statistical analysis
Continuous variables were expressed as mean ± SD for normally distributed data or median (interquartile range) for non-normal data. Normality was assessed using the Kolmogorov–Smirnov test. Differences in continuous variables were analysed with analysis of variance or the Kruskal–Wallis test, and differences in categorical variables were assessed with the chi-square or Fisher's exact tests. Kaplan–Meier curves were plotted for time to last follow-up or death and compared with the log-rank test. Cox proportional hazards analysis was used to examine the relationship between outcomes and clusters. The assumption of proportional hazards was verified by plotting the scaled Schoenfeld residuals against time, which showed no significant correlations. To determine the value of the clusters in predicting outcomes, sequential Cox models were performed to compare the global chi-square values. All statistical analyses were performed using SPSS 21.0 (SPSS, Chicago, IL, USA), MedCalc 19.5.6 (Mariakerke, Belgium), and R 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). A P-value of <0.05 was considered statistically significant.
Ethical approval
The study protocol was approved by the local ethics committee of each participating institution (Institutional Review Board of the University of Tokushima: protocol number: 3499) and followed the guidelines of the Declaration of Helsinki. Data were de-identified, so the requirement for informed consent was waived.
Results
Study population
We identified 1742 patients with severe AS who had undergone TAVR between January 2015 and March 2019. Among these, 377 patients were excluded due to missing data for any variables used for clustering data analysis. The records of the remaining 1365 patients were analysed for both deriving and validating the results (Table 1).
Table 1.
Clinical characteristics of the entire study cohort
| Number | 1365 |
|---|---|
| Clinical background | |
| Age | 84 ± 5 |
| Male | 441 (32.3) |
| BMI | 22.4 ± 3.8 |
| NYHA III/IV, n (%) | 483 (35.4) |
| CHF, n (%) | 796 (58.3) |
| CAD, n (%) | 408 (29.9) |
| Prior PCI, n (%) | 246 (18.0) |
| Hypertension, n (%) | 1084 (79.4) |
| DM, n (%) | 358 (26.2) |
| DL, n (%) | 722 (52.9) |
| Chronic lung disease | 232 (17.0) |
| Prior MI, n (%) | 74 (5.4) |
| Smoking current/quit | 342 (25.1) |
| Atrial fibrillation, n (%) | 239 (17.5) |
| STS PROM, % | 7.1 ± 5.6 |
| Laboratory data | |
| WBC, /µL | 5630 ± 1804 |
| Haemoglobin, g/dL | 11.3 ± 1.6 |
| BNP, pg/mL | 221 (103–489) |
| NT-proBNP pg/mL | 1197 (545–3098) |
| TAVR procedure | |
| Approach | |
| Trans-femoral, n (%) | 1156 (84.7) |
| Trans-apical, n (%) | 163 (11.9) |
| Trans-aorta, n (%) | 25 (1.8) |
| Others, n (%) | 21 (1.5) |
| Valve type | |
| Sapien XT, n (%) | 343 (25.1) |
| Sapien 3, n (%) | 724 (53.0) |
| CoreValve, n (%) | 76 (5.6) |
| EvoluteR, n (%) | 173 (12.7) |
| EvolutePRO, n (%) | 49 (3.6) |
| Valve size | |
| 20 mm, n (%) | 69 (5.1) |
| 23 mm, n (%) | 620 (45.4) |
| 26 mm, n (%) | 494 (36.2) |
| 29 mm, n (%) | 182 (13.3) |
| Echocardiographic variables | |
| Systolic BP, mmHg | 129 ± 21 |
| Diastolic BP, mmHg | 65 ± 13 |
| Heart rate, b.p.m. | 69 ± 13 |
| LVEDV, mL | 85 ± 32 |
| LVESV, mL | 34 ± 22 |
| LVEF, % | 62 ± 11 |
| LV mass index, g/m2 | 122 ± 39 |
| LAD, mm | 42 ± 7 |
| LAVi, mL/m2 | 56 ± 22 |
| E, cm/s | 84 ± 31 |
| E-DCT, ms | 267 ± 95 |
| E/e′ | 19.2 ± 8.9 |
| AV Vmax, m/s | 453 ± 82 |
| AV mean PG. mmHg | 50.4 ± 19.3 |
| AVAi, cm2/m2 | 0.44 ± 0.13 |
| SVi, mL/m2 | 47 ± 13 |
| TR velocity, m/s | 259 ± 45 |
| TAPSE, mm | 19.2 |
| FAC, % | 43.3 ± 8.9 |
| IVC, mm | 13.4 ± 4.1 |
| LVGLS, % | 11.9 ± 4.2 |
| More than moderate AR, n (%) | 174 (12.7) |
| More than moderate MR, n (%) | 172 (12.6) |
| More than moderate TR, n (%) | 138 (10.1) |
Data are expressed as the number of patients (percentage) and mean ± SD or median (interquartile range).
AR, aortic regurgitation; AV, aortic valve; AVAi, aortic valve area index; BMI, body mass index; BNP, B-type natriuretic peptide; CAD, coronary artery disease; CHF, congestive heart failure; DCT; deceleration time; DL, dyslipidaemia; DM, diabetes mellitus; E, early diastolic filling velocity; e′, early mitral annular velocity; FAC, fractional area change; GLS, global longitudinal strain; IVC, inferior vena cava; LAD, left atrial dimension; LAVi, left atrial volume index; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular stroke volume; MI, myocardial infarction; MR, mitral regurgitation; NT-pro BNP; N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association;
PCI, percutaneous coronary intervention; STS PROM; Society of Thoracic Surgeons predicted risk of mortality; SVi, stroke volume index; TAPSE; tricuspid annular plane systolic excursion; TR; tricuspid valve regurgitation; WBC; white blood cell.
Variable selection and optimal number of clusters
The heatmap for variable selection is presented in Figure 1A. Twenty of the 31 initial variables were selected for clustering analysis by removing variables with high correlation coefficients. During the K-means clustering process, the three clusters were determined to be the optimal solution, based on the elbow method and silhouette coefficient (see Supplementary material online, Figure S1). To investigate the relative importance of the 20 variables for cluster assignment, we trained a logistic regression with elastic net regularization using the predicted clusters obtained by K-means clustering as ground truth. The model was trained with a derivation cohort, and then the trained model was evaluated with a validation cohort. The classification results for these cohorts are shown in Supplementary material online, Figure S2 as a confusion matrix.
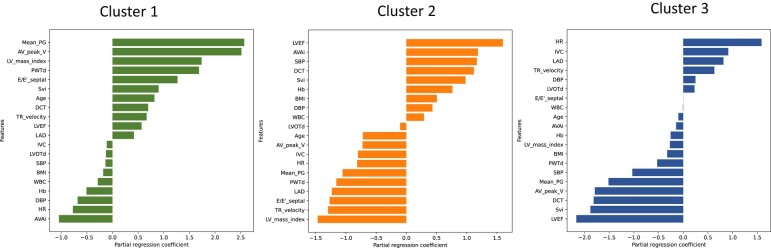
These results have high classification accuracy, suggesting that the partial regression coefficients of the logistic regression model reflect the relative importance of the 20 variables assigned to each cluster class. These partial regression coefficients for each cluster are shown in Figure 2.
Figure 2.
Significant features among clusters. These figures show the partial regression coefficients of logistic regression for each cluster. AV_peak_V, atrioventricular peak velocity; AVAi, aortic valve area index; BMI, body mass index; DBP, diastolic blood pressure; DCT, deceleration time; E/e′, the ratio of transmitral early peak velocity to mitral annulus velocity; EF, ejection fraction; Hb, haemoglobin; HR, heart rate; IVC, inferior vena cava; LAD, left atrial diameter; LV, left ventricular; LVOTd, left ventricular outflow tract diameter; Mean PG, mean pressure gradient; PWTd, posterior wall thickness diameter; SBP, systolic blood pressure; Svi, stroke volume index; TR, tricuspid valve regurgitation; WBC, white blood cell.
Cluster 1: higher mean PG, AV peak velocity, and LV mass index, lower AVAi, diastolic blood pressure, and heart rate.
Cluster 2: higher LVEF, AVA, and systolic blood pressure, lower LV mass index, TR velocity, and E/e′.
Cluster 3: higher heart rate, inferior vena cava, and left atrial dimension, lower deceleration time, SVi, and LVEF.
Comparison of clinical and echocardiography parameters among clusters
The baseline characteristics of the clusters were compared (Table 2). Cluster 1 was older; however, its patients had less coronary artery disease and less diabetes. Cluster 2 was the youngest of the three groups, with minimal symptoms, few comorbidities, and a low Society of Thoracic Surgeons (STS) short-term risk score. Cluster 3 consisted of older patients who were thin and had more comorbidities. The notable finding was the highest prevalence of atrial fibrillation in Cluster 3.
Table 2.
Clinical data among clusters
| ALL | Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|---|
| Number | 1092 | 311 | 538 | 243 |
| Clinical background | ||||
| Age | 84.4 ± 5 | 86 ± 5 | 83 ± 5a | 85 ± 6b |
| Male | 357 (32.7) | 90 (29) | 175 (32.5) | 92 (37.9) |
| BMI | 22.4 ± 3.8 | 21.9 ± 3.6 | 22.9 (3.9)a | 21.9 ± 3.6b |
| NYHA III/IV, n (%) | 387 (35.4) | 136 (43.7) | 132 (24.5)a | 119 (49) b |
| CHF, n (%) | 635 (58.2) | 222 (71.4) | 234 (43.5)a | 179 (73.7) b |
| CAD, n (%) | 324 (29.7) | 64 (20.6) | 164 (30.5)a | 96 (39.5) b,c |
| Prior PCI, n (%) | 193 (17.7) | 39 (12.5) | 95 (17.7)a | 59 (24.3) c |
| Hypertension, n (%) | 870 (79.7) | 242 (77.8) | 436 (81) | 192 (79) |
| DM, n (%) | 283 (25.9) | 67 (21.5) | 139 (25.8)a | 77 (31.7) c |
| DL, n (%) | 566 (51.8) | 153 (49.2) | 300 (55.8)a | 113 (46.5) b |
| Chronic lung disease | 185 (16.9) | 53 (17) | 84 (15.6) | 48 (19.8) |
| Prior MI, n (%) | 60 (5.5) | 11 (3.5) | 22 (4.1)a | 27 (11.1) b,c |
| Smoking current/quit | 279 (25.5) | 62 (20) | 139 (25.8)a | 78 (32) c |
| Atrial fibrillation, n (%) | 199 (18.2) | 46 (14.8) | 45 (8.4)a | 108 (44.4) b,c |
| STS PROM, % | 7.2 ± 5.7 | 7.5 ± 4.3 | 5.97 ± 3.6a | 9.6 ± 9.3b |
| Laboratory data | ||||
| WBC, /µL | 5626.7 ± 1834 | 5406 ± 1728 | 5764 ± 1761a | 5605 ± 2088 |
| Haemoglobin, g/dL | 11.3 ± 1.6 | 10.87 ± 1.6 | 11.58 ± 1.5a | 11.05 ± 1.7b |
| BNP, pg/mL | 105 (230–507) | 448 (240–708) | 122 (69.5–229.5)a | 415 (242–778) b |
| NT-proBNP pg/mL | 1349 (592–3172) | 2214 (1304–4798) | 649 (341–1120)a | 2968 (1450–5988) b |
| TAVR procedure | ||||
| Approach | ||||
| Trans-femoral, n (%) | 923 (84.5) | 268 (86.2) | 453 (84.2) | 202 (83.1) |
| Trans-apical, n (%) | 129 (11.8) | 35 (11.3) | 60 (11.2) | 34 (14) |
| Trans-aorta, n (%) | 21 (1.9) | 2 (0.6) | 15 (2.8) | 4 (1.6) |
| Others, n (%) | 19 (1.7) | 6 (1.9) | 10 (1.9) | 3 (1.2) |
| Valve type | ||||
| Sapien XT, n (%) | 273 (25) | 76 (24.4) | 137 (25.5) | 60 (24.7) |
| Sapien 3, n (%) | 582 (53.3) | 162 (52.1) | 284 (52.8) | 136 (56) |
| CoreValve, n (%) | 60 (5.5) | 16 (5.1) | 34 (6.3) | 10 (4.1) |
| EvoluteR, n (%) | 136 (12.5) | 45 (14.5) | 65 (12.1) | 26 (10.7) |
| EvolutePRO, n (%) | 41 (3.8) | 12 (3.9) | 18 (3.3) | 11 (4.5) |
| Valve size | ||||
| 20 mm, n (%) | 55 (5) | 16 (5.1) | 29 (5.4) | 10 (4.1) |
| 23 mm, n (%) | 505 (46.2) | 157 (50.5) | 245 (45.5) | 103 (42.4) |
| 26 mm, n (%) | 385 (35.3) | 95 (30.5) | 205 (38.1) | 85 (35) |
| 29 mm, n (%) | 147 (13.5) | 43 (13.8) | 59 (11)a | 45 (18.5) |
Other abbreviations as in Table 1. P < 0.05.
aCluster 1 vs. Cluster 2.
bCluster 2 vs. Cluster 3.
cCluster 1 vs. Cluster 3.
Regarding echocardiographic evaluation (Table 3), Cluster 2 had the most preserved cardiac function and structure. Left ventricular and left atrial (LA) size were small, and diastolic function was preserved. Cluster 1 had very severe AS with the highest AV pressure gradient among these clusters. Left ventricular and LA size were large, and diastolic dysfunction was more severe. Cluster 3 had significantly depressed LVEF, decreased GLS, lower stroke volume, and lower aortic valve mean pressure gradient. Cluster 3 also had concomitant valve disease including aortic regurgitation, mitral regurgitation, and TR. Overall, the three clusters could be characterized as follows: traditional severe AS for Cluster 1, severe AS with preserved cardiac function for Cluster 2, and cardiac dysfunction AS for Cluster 3.
Table 3.
Echocardiographic data among clusters
| ALL | Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|---|
| Echocardiographic variables | ||||
| Systolic BP, mmHg | 129 ± 21 | 123.7 ± 18.8 | 136.2 ± 20.4a | 119.9 ± 19.6b |
| Diastolic BP, mmHg | 64.7 ± 12.8 | 61.1 ± 11.5 | 66.6 ± 13.2a | 65.1 ± 12.5c |
| Heart rate, b.p.m. | 68.5 ± 12.7 | 65.7 ± 11 | 65.9 ± 10 | 78 ± 15.2b |
| LVEDV, mL | 84.6 ± 31.7 | 88.4 ± 31.9 | 77.6 ± 25.1a | 95.1 ± 39.6b,c |
| LVESV, mL | 33.4 ± 22.2 | 34.2 ± 17.8 | 25.7 ± 11.4a | 49. ± 33.93b,c |
| LVEF, % | 61.6 ± 10.7 | 62.2 ± 8.6 | 66 ± 6.7a | 51.1 ± 13.1b,c |
| LV mass index, g/m2 | 123.8 ± 39 | 155.4 ± 40.7 | 105.2 ± 26.7a | 124.4 ± 33.5b,c |
| LAD, mm | 42.1 ± 7.3 | 44 ± 7.3 | 39.6 ± 6.3a | 45.4 ± 7.4b |
| LAVi, mL/m2 | 56.5 ± 22.2 | 64.8 ± 22.5 | 47.8 ± 15.5a | 65 ± 26.6b |
| E, cm/s | 83.9 ± 31.2 | 92.8 ± 35.6 | 73.6 ± 23a | 95.5 (35.5)b |
| E-DCT, ms | 267 ± 96 | 287.9 ± 109.6 | 288.4 ± 82.2 | 192.9 ± 64.6b,c |
| E/e′ | 19.2 ± 9 | 24 ± 10.9 | 16.4 ± 6.1a | 19.9 ± 9.6b,c |
| AV Vmax, cm/s | 454.3 ± 82.5 | 538.4 ± 63.4 | 432.5 ± 56.2a | 395 ± 69.7b,c |
| AV mean PG, mmHg | 50.5 ± 19.1 | 70.6 ± 17.7 | 44.4 ± 12.1a | 38.4 ± 13.4b,c |
| AVAi, cm2/m2 | 0.44 ± 0.1 | 0.37 ± 0.1 | 0.48 ± 0.11a | 0.42 ± 0.12b,c |
| SVi, mL/m2 | 46.9 ± 13.5 | 49.7 ± 13 | 50 ± 13 | 36 ± 9b,c |
| TR velocity, cm/s | 259.5 ± 44.8 | 274.4 ± 49.4 | 244.5 ± 34.3a | 273.3 ± 48.5b,c |
| TAPSE, mm | 19.1 ± 4.4 | 19.6 ± 3.9 | 20 ± 4.2 | 17.1 ± 4.6b,c |
| FAC, % | 43.3 ± 8 | 44.7 ± 6.9 | 44.2 ± 6.4 | 40.3 ± 10.4b,c |
| IVC, mm | 13.7 ± 4.3 | 14.4 ± 3.8 | 12.8 ± 4.1a | 15.5 ± 4.7b |
| LVGLS, % | 11.9 ± 4.2 | 11.2 ± 3.8 | 13.5 ± 3.6a | 8.47 ± 3.9b,c |
| More than moderate AR, n (%) | 137 (12.5) | 54 (17.4) | 44 (8.2)a | 39 (16)b |
| More than moderate MR, n (%) | 130 (11.9) | 49 (15.8) | 24 (4.5)a | 57 (23.5)b,c |
| More than moderate TR, n (%) | 83 (7.6) | 35 (11.3) | 23 (4.3)a | 54 (22.2)b,c |
Other abbreviations as in Table 1.
P < 0.05.
aCluster 1 vs. Cluster 2.
bCluster 2 vs. Cluster 3.
cCluster 1 vs. Cluster 3.
Clinical outcomes of the clusters in the independent validation cohort
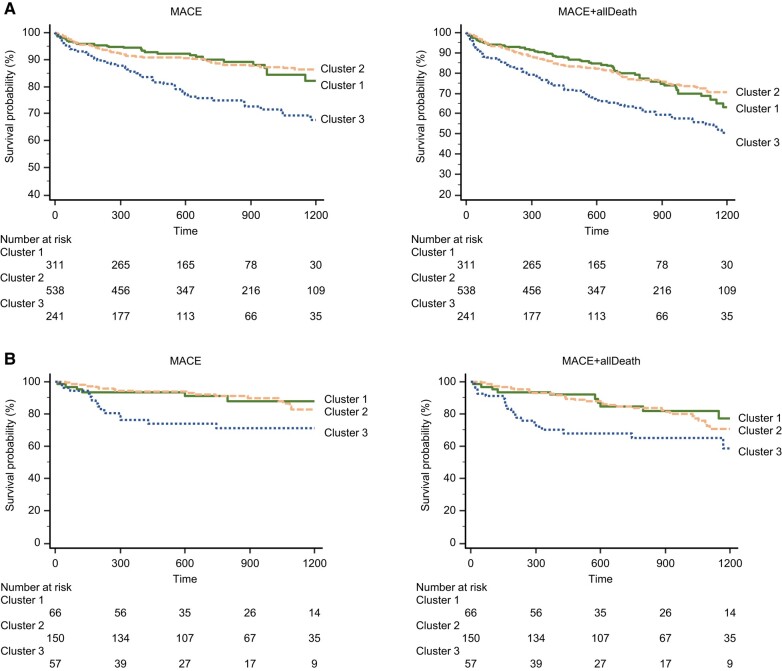
Clinical outcomes were markedly different per cluster in the derivation and validation cohorts. In the derivation cohort, during a median of 2.0 years (interquartile range, 1.0–3.0 years) of follow-up, 304 major adverse cardiovascular events (MACE) and all-cause deaths occurred. In the validation cohort, during a median of 2.2 years (interquartile range, 1.0–3.1 years) of follow-up, 69 MACE and all-cause deaths occurred. There was no difference in the event-free survival rate between the derivation and validation cohorts (see Supplementary material online, Figure S3). Cluster 3 had the highest all-cause mortality in both cohorts. Figure 3A and B shows the time to reach the primary and secondary endpoint in the derivation and validation cohorts. In both cohorts, Cluster 3 appeared to be associated with the worst outcomes.
Figure 3.
Kaplan–Meier curves by cluster. (A) Derivation cohort. (B) Validation cohort. Survival free from major adverse cardiovascular event and major adverse cardiovascular event plus all-cause death. MACE, major adverse cardiovascular event.
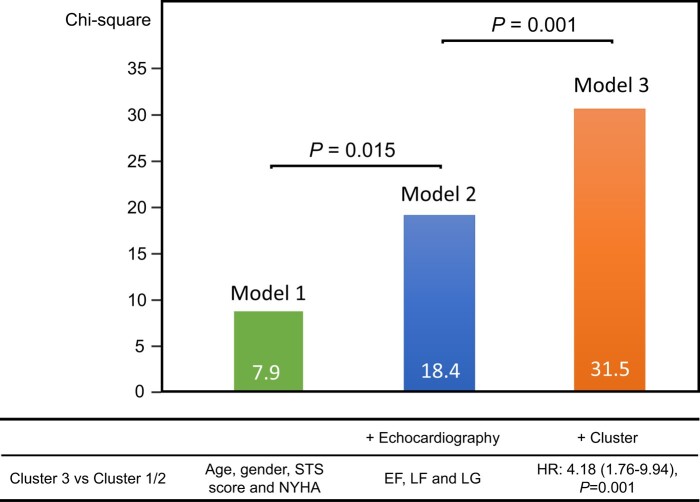
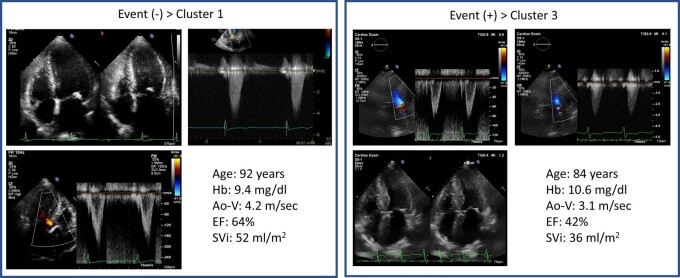
In the validation cohort, when compared with Clusters 1 and 2, Cluster 3 had higher MACE and all-cause mortality risk. Cluster 3 had the strongest risk of MACE (hazard ratio, 2.66; 95% CI 1.41–5.03, P = 0.003) and MACE + all-cause mortality (hazard ratio, 2.26; 95% CI 1.36–3.75, P = 0.004). After adjustment for clinical and echocardiographic data in a Cox proportional hazards model, Cluster 3 (hazard ratio, 4.18; 95% CI 1.76–9.94, P = 0.001) was associated with primary outcomes (Table 4). Figure 4 shows the added benefit of cluster analysis in the prediction of the primary endpoint. The addition of cluster analysis significantly improved the prognostic power of a model containing age, gender, STS score, New York Heart Association (NYHA) class, and echocardiographic data (Model 1, age, gender, STS score, and NYHA, χ2 = 7.9; Model 2, plus echocardiography, χ2 = 18.4, P = 0.015; Model 3, cluster analysis, χ2 = 31.5, P = 0.001). For the Cox model based on age, gender, STS score, NYHA, and echocardiographic data, the Harrell C concordance statistic was calculated to be 0.69 (95% CI: 0.63–0.74). When Cluster 3 was added to the model, the C-statistic significantly improved to 0.75 (95% CI: 0.69–0.80, P = 0.041 vs. without Cluster 3). Representative cases are shown in Figure 5.
Table 4.
Multivariate associations of primary outcomes
| Model 1 (χ2 = 7.9) | Model 2 (χ2 = 18.4) | Model 3 (χ2 = 31.5) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Clinical parameter | |||||||||
| Age | 1.01 | 0.95–1.07 | 0.84 | 1.01 | 0.95–1.08 | 0.68 | 1.02 | 0.96–1.08 | 0.53 |
| Gender | 1.16 | 0.58–2.31 | 0.67 | 1.18 | 0.59–2.38 | 0.64 | 1.13 | 0.56–2.28 | 0.73 |
| STS score | 0.98 | 0.91–1.04 | 0.48 | 0.99 | 0.93–1.06 | 0.81 | 0.97 | 0.91–1.04 | 0.41 |
| NYHA | 1.87 | 1.19–2.92 | 0.006 | 1.98 | 1.22–3.20 | 0.005 | 2.03 | 1.24–3.32 | 0.005 |
| Echocardiography | |||||||||
| LVEF | 1.00 | 0.97–1.03 | 0.98 | 1.02 | 0.99–1.06 | 0.19 | |||
| LF | 0.52 | 0.19–1.39 | 0.19 | 0.31 | 0.11–0.86 | 0.024 | |||
| LG | 2.73 | 1.43–5.23 | 0.002 | 1.81 | 0.89–3.68 | 0.103 | |||
| Cluster | |||||||||
| Cluster 3 vs. Cluster 1/2 | 4.18 | 1.76–9.94 | 0.001 | ||||||
HR, hazard ratio; CI, confidence interval; LF, low flow; LG, low gradient; other abbreviations as in Table 1.
Figure 4.
Incremental value of cluster data to clinical and echocardiographic data. These figures illustrate the global χ2 of sequential Cox models incorporating clinical data, echocardiographic data, and cluster group. EF, ejection fraction; HR, hazard ratio; LF, low flow; LG, low gradient; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
Figure 5.
Representative cases with and without events. Ao-V, aortic velocity; EF, ejection fraction; Hb, haemoglobin; SVi, stroke volume index.
Finally, we have performed supplementary analyses using both agglomerative clustering and model-based (Gaussian mixture) clustering. The results of these analyses have been summarized in the Supplementary material online. Both these alternative clustering methods independently identified three as the optimal number of clusters. This convergence on the number of clusters across different methods adds robustness to our original findings with K-means clustering. We have included Kaplan–Meier survival curves for each of these clusters. The survival analysis distinctly showed that the prognosis of patients was well segregated among the three clusters identified by both agglomerative and Gaussian mixture model-based clustering, which was similar with our original findings using K-means clustering.
Discussion
The results of this study revealed the presence of three different clusters of patients with severe AS who underwent TAVR. These clusters were identified with unsupervised cluster analysis and were characterized by markedly different clinical and echocardiographic features. Cluster 1 was associated predominantly with elderly age, a high aortic valve gradient, and LV hypertrophy; Cluster 2 consisted of preserved LVEF, larger AVA, and high blood pressure; and Cluster 3 demonstrated tachycardia and low flow/low gradient AS. Adverse outcomes differed significantly among clusters during a median of 2.2 years of follow-up. After adjustment for clinical and echocardiographic data in a Cox proportional hazards model, Cluster 3 was associated with worse outcomes. The current study presents three key discoveries. First, unsupervised cluster analysis effectively identified three patient groups with TAVR with unique phenotypes. Second, each group showed significant variations in clinical data and outcomes. Finally, the findings were verified in the validation cohort.
Phenotyping after transcatheter aortic valve replacement
Cardiologists commonly concentrate on echocardiography parameters of the valves in patients with AS. Previous studies have identified different predictors of outcomes in patients undergoing TAVR, such as age, LV function, and valve characteristics.23,24 However, those studies did not consider the heterogeneity of the patient population and did not explore the existence of different subgroups. While these various elements have been investigated individually through traditional hypothesis-driven methods, a more comprehensive, data-driven cluster analysis can be a potent tool to examine heterogeneity. Our study builds upon previous research by identifying distinct subgroups of patients and highlighting the importance of considering comorbidities and extravalvular cardiac dysfunction in the prognostic assessment of patients who have undergone TAVR. Our cluster analysis found three distinct phenotypes: high gradient and cardiac hypertrophy for Cluster 1, traditional AS for Cluster 2, and comorbidities for Cluster 3. Importantly, the echocardiographic variables that were crucial for clustering were TR jet velocity, LA volume, E- and A-wave velocity, and LVEF, while none of the AS severity indices were critical. This highlights the substantial prognostic value of these cardiac imaging markers in AS beyond the diseased aortic valve.
Characteristics of clusters
Cluster 1 was characterized by elderly patients with a high aortic valve gradient and LV hypertrophy. This cluster may represent patients with more advanced stages of AS and LV remodelling, which may lead to adverse outcomes without TAVR/surgical AVR. Cluster 2 was associated predominantly with preserved LVEF, larger AVA, and high blood pressure. Notably, Cluster 2 patients, who have larger AVA and are not typically categorized as having very severe AS, also demonstrated significant benefits from TAVR. This cluster may represent patients with less cardiac remodelling, leading to better outcomes post-TAVR. A previous study suggested that the post-TAVR prognosis in patients tends to align more closely with the natural history outcomes observed in elderly populations.25 This alignment could be a contributing factor to the similarity in outcomes between Clusters 1 and 2. Despite the differences in their pre-procedural characteristics, these clusters might converge in terms of overall prognosis due to the overarching impact of TAVR, which potentially ‘normalizes’ their outcomes towards those observed in general elderly cohorts.
Cluster 3 demonstrated tachycardia and low flow/low gradient AS, which may indicate the presence of other comorbidities, such as frailty, or other cardiac dysfunctions. Cluster 3 had the worst prognosis, underscoring the observation that there are specific types of death that are more related to specific groups of patients with AS and demonstrating that non-cardiac death should not be ignored, especially in those with significant comorbidities.26 Moreover, the significant right heart dysfunction observed in Cluster 3 underscores the advanced nature of extravalvular damage.27 This underlines the necessity for thorough echocardiographic assessments including right heart examination as part of the pre-TAVR evaluation process. Such detailed evaluations are essential for devising appropriate management strategies for patients with complex clinical presentations, as indicated by the distinct phenotype of Cluster 3. These insights reinforce the clinical relevance of our findings and the need for a nuanced approach to patient care in TAVR, considering the extensive and diverse impacts of comorbidities on patient outcomes.
Clinical implications
Our study has important clinical implications for the phenotyping of patients with severe AS undergoing TAVR. By identifying these different phenotypes, clinicians can better tailor patient selection and management strategies to optimize outcomes.28 For instance, for patients in Cluster 1, which appears to represent a severe stage of AS, outcomes can be improved by a well-planned surgical procedure. Conversely, patients in Cluster 2 may require less intense monitoring post-TAVR and may have better outcomes overall. Furthermore, patients in Cluster 3 may require additional post-operative management, such as closer follow-up and optimization of medical therapy, to minimize adverse outcomes.
Additional key distinguishing features of our research is its specific focus on patients undergoing TAVR. This focus is significant as, to the best of our knowledge, there are no existing studies that have concentrated exclusively on clustering analyses within a TAVR-only patient population. By focusing solely on this patient group, our research provides insights that are directly applicable to the contemporary management of AS. The clustering approach used in our study allows for a nuanced understanding of patient subgroups within the TAVR population, potentially aiding in more personalized and effective treatment decisions.
Limitations
While this study provides important insights into the phenotypic heterogeneity of patients undergoing TAVR, there are several limitations that should be acknowledged. Determining the most suitable clustering method can be challenging. The results of the K-means clustering depend on the starting point. We varied the initial centroids in K-means clustering across 10 different scenarios. These analyses were complemented by generating Kaplan–Meier survival curves for each of the resulting cluster configurations. Despite changing the initial values, we observed minimal variation in the clustering outcomes. We conducted an additional analysis with the number of clusters set to four. This analysis aimed to explore whether a four-cluster model might provide a more accurate or distinct segmentation of our dataset. The Kaplan–Meier survival curves for the four-cluster model did not reveal a significantly different pattern compared to the three-cluster model (see Supplementary material online). A limitation in the application of K-means clustering was its exclusion of categorical variables such as sex and atrial fibrillation. While K-means offers several advantages in terms of simplicity and ease of interpretation, its inability to incorporate these categorical variables could impact the comprehensiveness of our findings. The prevalence of more than moderate valvular diseases other than AS in our cohort was approximately 10%. Given this relatively low proportion, we decided not to include these conditions in the primary analysis. While our analysis was deliberately focused on AS to maintain specificity in assessing post-TAVR outcomes, we recognize that other valvular pathologies may also affect these outcomes. Future research may benefit from including a wider array of valvular heart diseases to enrich the understanding of the prognosis following TAVR. The sample size of this study, although substantial, was limited to a certain extent, which may limit the statistical power of the study. The follow-up period was relatively short, and longer-term outcomes of different phenotypes need to be studied in the future. An additional limitation of our study is the exclusion of detailed post-operative echocardiographic data. While our analysis focused on pre-operative patient phenotypes, the lack of detailed post-operative echocardiographic outcomes limits our ability to assess factors influencing post-procedural prognosis. Future research could benefit from a more comprehensive inclusion of both pre- and post-operative echocardiographic data to fully understand the impact of TAVR on patient outcomes. Despite these limitations, this study provides a valuable contribution to the understanding of the heterogeneity of patients undergoing TAVR and highlights the need for individualized treatment approaches based on patient phenotype.
Conclusion
The unsupervised cluster analysis of patients who had undergone TAVR uncovered three distinct groups for prognostic evaluation and demonstrated the association of Cluster 3 with worse outcomes. These findings have important clinical implications and highlight the importance of considering comorbidities and extravalvular cardiac dysfunction in the prognostic assessment of patients undergoing TAVR. Our study provides a new perspective in the categorization of patients undergoing TAVR and can inform clinical decision-making and resource allocation.
Supplementary Material
Acknowledgements
The JSE-TAVI study investigators are as follows: Masaki Izumo (St. Marianna Medical University Hospital, Kawasaki), Atsushi Okada, Chisato Izumi (National Cerebral and Cardiovascular Center, Osaka), Shu Inami (Dokkyo Medical University, Mibu), Yasuharu Takeda, Toshinari Onishi (Osaka University Graduate School of Medicine, Suita), Yuki Izumi, (Sakakibara Heart Institute, Tokyo), Akiko Kumagai (Iwate Medical University, Iwate), Tomoko Fukuda, Naohiko Takahashi (Oita University, Oita), Takeshi Kitai (Kobe City Medical Center General Hospital, Kobe), Hiroyuki Iwano (Hokkaido University, Sapporo), Shigeo Sugawara (Nihonkai General Hospital, Yamagata), Kazumi Akasaka (Asahikawa Medical University Hospital, Asahikawa), Kenji Harada (Jichi Medical University, Tochigi), Yoshiko Masaoka (Hiroshima City Hiroshima Citizens Hospital, Hiroshima), Kazuaki Tanabe, Takahiro Sakamoto (Shimane University Faculty of Medicine, Izumo), and Takeshi Takamura (Ise Red Cross Hospital, Ise).
Contributor Information
Kenya Kusunose, Department of Cardiovascular Medicine, Nephrology, and Neurology, Graduate School of Medicine, University of the Ryukyus, 207 Uehara, Nishihara Town, Okinawa 903-0215, Japan; Department of Cardiovascular Medicine, Tokushima University Hospital, 2-50-1 Kuramoto, Tokushima 770-8503, Japan.
Takumasa Tsuji, Department of Radiological Technology, Graduate School of Medical Care and Technology, Teikyo University, Tokyo, Japan.
Yukina Hirata, Ultrasound Examination Center, Tokushima University Hospital, Tokushima, Japan.
Tomonori Takahashi, Department of Cardiovascular Medicine, Tokushima University Hospital, 2-50-1 Kuramoto, Tokushima 770-8503, Japan.
Masataka Sata, Department of Cardiovascular Medicine, Tokushima University Hospital, 2-50-1 Kuramoto, Tokushima 770-8503, Japan.
Kimi Sato, Department of Cardiology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
Noor Albakaa, Department of Cardiology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
Tomoko Ishizu, Department of Cardiology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan.
Jun’ichi Kotoku, Department of Radiological Technology, Graduate School of Medical Care and Technology, Teikyo University, Tokyo, Japan.
Yoshihiro Seo, Department of Cardiology, Graduate School of Medical Sciences, Nagoya City University, Nagoya, Japan.
JSE-TAVI investigators:
Masaki Izumo, Atsushi Okada, Chisato Izumi, Shu Inami, Yasuharu Takeda, Toshinari Onishi, Yuki Izumi, Akiko Kumagai, Tomoko Fukuda, Naohiko Takahashi, Takeshi Kitai, Hiroyuki Iwano, Shigeo Sugawara, Kazumi Akasaka, Kenji Harada, Yoshiko Masaoka, Kazuaki Tanabe, Takahiro Sakamoto, and Takeshi Takamura
Lead author biography
 As the fourth professor at Ryukyu University’s Department of Cardiovascular Medicine, Nephrology, and Neurology, Dr. Kenya Kusunose specializes in heart failure, pulmonary hypertension, valvular heart disease, and innovative applications of artificial intelligence in the medical field. His career is marked by several awards, including being a finalist for the Young Investigation Award by the American Society of Echocardiography (2012 and 2013) and receiving multiple honors from various Japanese cardiology societies. Dr. Kusunose also contributes as an editorial board member and associate editor for several journals like Circulation: Cardiovascular Imaging, Heart, and the International Journal of Cardiology.
As the fourth professor at Ryukyu University’s Department of Cardiovascular Medicine, Nephrology, and Neurology, Dr. Kenya Kusunose specializes in heart failure, pulmonary hypertension, valvular heart disease, and innovative applications of artificial intelligence in the medical field. His career is marked by several awards, including being a finalist for the Young Investigation Award by the American Society of Echocardiography (2012 and 2013) and receiving multiple honors from various Japanese cardiology societies. Dr. Kusunose also contributes as an editorial board member and associate editor for several journals like Circulation: Cardiovascular Imaging, Heart, and the International Journal of Cardiology.
Data availability
Data are available on reasonable request.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Ethical approval
The study was approved by the local ethics committee and Institutional Review Board of the University of Tokushima (protocol: 3499).
Authors’ contribution
K.K. conceived the idea for this study. Y.H., T.T., and J.K. conducted the data analyses. The initial draft of the manuscript was produced by K.K.. All authors were involved in interpreting the results and writing the manuscript. All authors read and approved the final manuscript.
Funding
This work was partially supported by grants from Japan Society for the Promotion of Science Kakenhi Grants (number 23K07509 to K.K.) and the Japan Agency for Medical Research and Development (AMED, JP22uk1024007 to K.K.). This study was approved as an academic project of the Japanese Society of Echocardiography and was supported by a grant from the Japanese Society of Echocardiography.
References
- 1. Members WC, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O’Gara PT, Rigolin VH, Sundt TM, Thompson A, Toly C. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol 2021;77:e25–e197. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, Neumann F-J, Myers P, Abdelhamid M, Achenbach S, Asteggiano R, Barili F, Borger MA, Carrel T, Collet J-P, Foldager D, Habib G, Hassager C, Irs A, Iung B, Jahangiri M, Katus HA, Koskinas KC, Massberg S, Mueller CE, Nielsen JC, Pibarot P, Rakisheva A, Roffi M, Rubboli A, Shlyakhto E, Siepe M, Sitges M, Sondergaard L, Sousa-Uva M, Tarantini G, Zamorano JL, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, Benchabi Y, Chilingaryan A, Metzler B, Rustamova Y, Shumavets V, Lancellotti P, Smajic E, Trendafilova-Lazarova D, Samardzic J, Karakyriou M, Palecek T, Sanchez Dahl J, Meshaal MS, Palm K, Virtanen M, Bouleti C, Bakhutashvili Z, Achenbach S, Boutsikou M, Kertész AB, Danielsen R, Topilsky Y, Golino P, Tuleutayev R, Elezi S, Kerimkulov A, Rudzitis A, Glaveckaite S, Sow R, Demarco DC, Bulatovic N, Aouad A, van den Brink R, Antova E, Beitnes JO, Ochala A, Ribeiras R, Vinereanu D, Irtyuga O, Ivanovic B, Simkova I, González Gómez A, Sarno G, Pedrazzini GB, Bsata W, Zakhama L, Korkmaz L, Cherniuk S, Khanji MY, Sharipov I. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2022;43:561–632.34453165 [Google Scholar]
- 3. Izumi C, Eishi K, Ashihara K, Arita T, Otsuji Y, Kunihara T, Komiya T, Shibata T, Seo Y, Daimon M, Takanashi S, Tanaka H, Nakatani S, Ninami H, Nishi H, Hayashida K, Yaku H, Yamaguchi J, Yamamoto K, Watanabe H, Abe Y, Amaki M, Amano M, Obase K, Tabata M, Miura T, Miyake M, Murata M, Watanabe N, Akasaka T, Okita Y, Kimura T, Sawa Y, Yoshida K. JCS/JSCS/JATS/JSVS 2020 guidelines on the management of valvular heart disease. Circ J 2020;84:2037–2119. [DOI] [PubMed] [Google Scholar]
- 4. Lantelme P, Aubry M, Peng JC, Riche B, Souteyrand G, Jaafar P, Rabilloud M, Harbaoui B, Muller O, Cosset B, Pagnoni M, Manigold T. Comorbidities may offset expected improved survival after transcatheter aortic valve replacement. Eur Heart J Open 2022;2:oeac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Díez-Villanueva P, Salamanca J, Rojas A, Alfonso F. Importance of frailty and comorbidity in elderly patients with severe aortic stenosis. J Geriatr Cardiol 2017;14:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJV, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015;17:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez C, Tucker S, Salameh T, Tucker C. An unsupervised machine learning method for discovering patient clusters based on genetic signatures. J Biomed Inform 2018;85:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Segar MW, Patel KV, Ayers C, Basit M, Tang WW, Willett D, Berry J, Grodin JL, Pandey A. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur J Heart Fail 2020;22:148–158. [DOI] [PubMed] [Google Scholar]
- 9. Kwak S, Lee Y, Ko T, Yang S, Hwang I-C, Park J-B, Yoon YE, Kim H-L, Kim H-K, Kim Y-J, Cho G-Y, Sohn D-W Won S, Lee S-P. Unsupervised cluster analysis of patients with aortic stenosis reveals distinct population with different phenotypes and outcomes. Circ Cardiovasc Imaging 2020;13:e009707. [DOI] [PubMed] [Google Scholar]
- 10. Bisson A, Fawzy AM, Wahbi E-B, Angoulvant D, Gregory Y, Fauchier L, Clementy N. Clinical phenotypes and atrial fibrillation recurrences after catheter ablation: an unsupervised cluster analysis. Curr Probl Cardiol 2023;48:101732.8. [DOI] [PubMed] [Google Scholar]
- 11. Sato K, Seo Y, Ishizu T, Albakaa NK, Izumo M, Okada A, Izumi C, Inami S, Takeda Y, Onishi T, Izumi Y, Kumagai A, Fukuda T, Takahashi N, Kitai T, Iwano H, Sugawara S, Akasaka K, Harada K, Masaoka Y, Kusunose K, Tanabe K, Sakamoto T, Takamura T, Ieda M. Cardiac reversibility and survival after transcatheter aortic valve implantation in patients with low-gradient aortic stenosis. J Am Heart Assoc 2023;12:e029717. PubMed PMID: 37581389. Epub 2023/08/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J-U. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. PubMed PMID: 25559473. Epub 2015/01/07. [DOI] [PubMed] [Google Scholar]
- 13. Ohte N, Ishizu T, Izumi C, Itoh H, Iwanaga S, Okura H, Otsuji Y, Sakata Y, Shibata T, Shinke T, Seo Y, Daimon M, Takeuchi M, Tanabe K, Nakatani S, Nii M, Nishigami K, Hozumi T, Yasukochi S, Yamada H, Yamamoto K, Izumo M, Inoue K, Iwano H, Okada A, Kataoka A, Kaji S, Kusunose K, Goda A, Takeda Y, Tanaka H, Dohi K, Hamaguchi H, Fukuta H, Yamada S, Watanabe N, Akaishi M, Akasaka T, Kimura T, Kosuge M, Masuyama T. JCS 2021 guideline on the clinical application of echocardiography. Circ J 2022;86:2045–2119. PubMed PMID: 36328514. Epub 2022/11/04. [DOI] [PubMed] [Google Scholar]
- 14. Dubey A, Gupta U, Jain S. Medical data clustering and classification using TLBO and machine learning algorithms. Comput Mater Contin 2021;70:4523–4543. [Google Scholar]
- 15. Ahmad T, Lund LH, Rao P, Ghosh R, Warier P, Vaccaro B, Dahlström U, O'Connor CM, Felker GM, Desai NR. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J Am Heart Assoc 2018;7:e008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manikandan P. Medical big data classification using a combination of random forest classifier and k-means clustering. Int J Intell Syst Appl 2018;10:11–19. [Google Scholar]
- 17. Pasin O, Gonenc S. An investigation into epidemiological situations of COVID-19 with fuzzy K-means and K-prototype clustering methods. Sci Rep 2023;13:6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ketchen DJ, Shook CL. The application of cluster analysis in strategic management research: an analysis and critique. Strateg Management J 1996;17:441–458. [Google Scholar]
- 19. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 1987;20:53–65. [Google Scholar]
- 20. Arthur D, Vassilvitskii S. k-means++: the advantages of careful seeding. In: SODA '07: Proceedings of the Eighteenth Annual ACM-SIAM Symposium on Discrete Algorithms, 2007. p.1027–1035. [Google Scholar]
- 21. Akiba T, Sano S, Yanase T, Ohta T, Koyama M. Optuna: a next–generation hyperparameter optimization framework. In: Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, 2019. p.2623–2631.
- 22. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, Vanderplas J. Scikit-learn: machine learning in python. J Mach Learn Res 2011;12:2825–2830. [Google Scholar]
- 23. Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodés-Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation 2014;129:2682–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maes F, Lerakis S, Ribeiro HB, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L, Amat-Santos I, Muñoz-García AJ, Garcia del Blanco B, Zajarias A, Lisko JC, Hayek S, Babaliaros V, Le Ven F, Gleason TG, Chakravarty T, Szeto W, Clavel M-A, de Agustin A, Serra V, Schindler JT, Dahou A, Salah-Annabi M, Pelletier-Beaumont E, Côté M, Puri R, Pibarot P, Rodés-Cabau J. Outcomes from transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis and left ventricular ejection fraction less than 30%: a substudy from the TOPAS-TAVI registry. JAMA Cardiol 2019;4:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zelis JM, van ‘t Veer M, Houterman S, Pijls NHJ, Tonino PAL; Netherlands Heart Registration Transcatheter Heart valve Implantation Registration C . Survival and quality of life after transcatheter aortic valve implantation relative to the general population. Int J Cardiol Heart Vasc 2020;28:100536. PubMed PMID: 32478166. Pubmed Central PMCID: PMC7251765. Epub 2020/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Minamino-Muta E, Kato T, Morimoto T, Taniguchi T, Shiomi H, Nakatsuma K, Shirai S, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Miyake M, Izumi C, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Inoko M, Ikeda T, Komasa A, Tada E, Ishii K, Hotta K, Higashitani N, Jinnai T, Kato Y, Inuzuka Y, Maeda C, Morikami Y, Saito N, Sakata R, Minatoya K, Kimura T. Causes of death in patients with severe aortic stenosis: an observational study. Sci Rep 2017;7:14723. PubMed PMID: 29116212. Pubmed Central PMCID: PMC5676690. Epub 2017/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genereux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, Svensson LG, Kapadia S, Tuzcu EM, Thourani VH, Babaliaros V, Herrmann HC, Szeto WY, Cohen DJ, Lindman BR, McAndrew T, Alu MC, Douglas PS, Hahn RT, Kodali SK, Smith CR, Miller DC, Webb JG, Leon MB. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J 2017;38:3351–3358. PubMed PMID: 29020232. Pubmed Central PMCID: PMC5837727. Epub 2017/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosano GMC, Moura B, Metra M, Bohm M, Bauersachs J, Ben Gal T, Adamopoulos S, Abdelhamid M, Bistola V, Čelutkienė J, Chioncel O, Farmakis D, Ferrari R, Filippatos G, Hill L, Jankowska EA, Jaarsma T, Jhund P, Lainscak M, Lopatin Y, Lund LH, Milicic D, Mullens W, Pinto F, Ponikowski P, Savarese G, Thum T, Volterrani M, Anker SD, Seferovic PM, Coats AJS. Patient profiling in heart failure for tailoring medical therapy. A consensus document of the heart failure association of the European Society of Cardiology. Eur J Heart Fail 2021;23:872–881. PubMed PMID: 33932268. Epub 2021/05/02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.