Abstract
This study’s objective was to compare detection rates of radiograph, computed tomography (CT), and positron emission tomography-contrast-enhanced computed tomography (PET-CECT) for pulmonary metastasis/synchronous primary lung tumors in head and neck squamous cell cancer (HNSCC) and its association with clinico-radio-pathological factors. Our retrospective study included 837 HNSCC patients from January 2012 to December 2017. Lung nodules were characterized on CT as benign, indeterminate, and metastatic. The true detection rate and statistical significance of associated risk factors were calculated. Risk factors for metastasis were determined using univariate and multivariate logistic regression models. Seventy-five (8.9%) patients had pulmonary metastasis and 3 (0.3%) had second lung primary. Detection rate of pulmonary metastasis by CT was higher (sensitivity-97.3%, specificity-97.2%) as compared to radiograph (sensitivity 49% and specificity 89%). Correlation was found between pulmonary and extra-pulmonary metastasis and N classification (P = 0.01, P = 0.02) and positive low jugular node (P = 0.001, P = 0.001). Using PET-CECT in place of CT costed an extra outlay of 7,033,805 INR (95,551.85 USD) while detecting distant metastasis in only 4 (0.47%) extra cases. Chest CT is a useful pulmonary metastases screening tool in advanced HNSCC patients with reasonable imaging cost as compared to PET-CT.
Keywords: Chest screening, PET CECT, CT scan, Pulmonary metastases, Head and neck squamous cell cancers, Chest radiographs
Introduction
The overall incidence of clinical metastasis in head-neck tumors ranges from 4 to 25% with a reported frequency of sites of metastases being 70–85% for lungs, 15–39% for bone, and 10–30% for liver depending on the study population and other factors [1, 2]. Over time with advancements in surgical techniques, different chemotherapy regimens and radiotherapy techniques there has been significant improvement in loco-regional control of head and neck cancers [3]. Advanced locoregional treatment, however, goes in vain if the patient develops distant metastases.
Most of these patients with distant metastasis are clinically asymptomatic at the time of diagnosis of the primary disease. Hence, radiological screening here plays a key role. Knowledge of independent prognostic factors for the presence or development of distant metastases like male gender, hypopharyngeal tumors, advanced T-stage, poor differentiation grade, regional lymph node metastasis [4], and extranodal extension [5] of regional lymph nodes metastasis can aid in selecting higher risk candidates for screening [6].
Conventionally, chest radiographs were used for pulmonary metastases screening. The sensitivity of chest radiographs for detecting nodules in patients with lung metastases is about 40 to 45% [7]. Not only the sensitivity and specificity of chest radiographs are low for the detection of lung nodules but also radiographs cannot differentiate pulmonary metastasis from second primary lung cancers. Second primary tumors can occur in 5–10% of HNSCC patients being notably the first cause of death with a critical impact on the overall survival rates of these patients making their detection crucial. These are more frequent in the head and neck region, esophagus, and lungs [8]. Other superior modalities available for screening are chest CT [9–13] and fluorine-18 fluorodeoxyglucose positron emission tomography-contrast-enhanced tomography (FDG PET-CECT) [14–16] of which FDG-PET/CT has the additional advantage of detecting extrathoracic metastatic disease. Using spiral CT with 5-mm collimation, a sensitivity of more than 80% has been reported for the detection of nodular metastases 5 mm or less in diameter; the sensitivity of CT in detecting metastases larger than 5 mm is 100% [17]. When comparing FDG-PET/CT with chest CT for detecting intra-thoracic metastases, the specificity and sensitivity of FDG-PET is found to be equal to chest CT [18].
Our study is aimed at building up evidence to incorporate chest CT as a screening modality in the workup as an alternative to PET-CT of all advanced head and neck cancers. The primary objective of this study was to determine the detection rate of lung metastasis or a synchronous lung primary tumor in patients with newly diagnosed advanced HNSCC with chest CT and to determine the associated clinicopathological factors. The secondary objective was to compare the sensitivity of chest radiographs, chest CT, and PET-CECT for metastasis detection.
Material and Methods
We conducted a retrospective analytical study in a tertiary care hospital after clearance from the Institutional Ethics Committee, The Human Ethics Committee. Our study population included all treatment naïve head and neck squamous cancer patients from the year 2012 January to 2017 December who underwent chest CT or PET-CECT as part of their diagnostic workup. No prior sample size calculations or power analyses were conducted. All the patients who had a high probability of distant metastases in view of the clinical profile including factors like a bulky primary disease-T3/T4, multiple/bulky neck nodes, N2/N3, bulky lower level neck nodes, and sites like hypopharynx were evaluated for the presence of distant metastases with either a PET-CT or chest CT. Baseline chest radiographs were also evaluated for these patients when available. Patients with non-squamous cell head and neck carcinoma, primary tumor of salivary glands, thyroid, and nasopharynx and those with recurrent disease were also excluded.
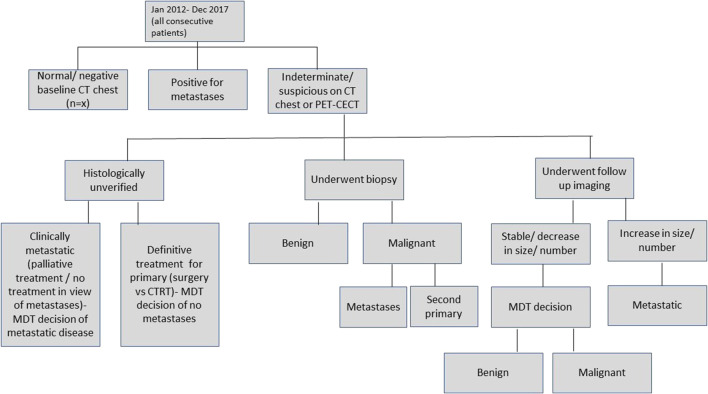
Lung nodules were identified on chest CT and then classified as benign/indeterminate/metastatic or second primary lung neoplasm by an expert sub-specialty onco-radiologist with more than 10 years of experience in head-neck onco-imaging (Fig. 1) [19]. The following parameters were used to categorize each nodule on chest CT: size (longest diameter), margins, presence of calcification/cavitation, attenuation coefficient/HU (Hounsfield Units), uni/bilateral, and multiplicity. Five-point ordinal Likert scale (Table 1) was used to score every lesion for suspiciousness of distant metastases in all patients (with or without a second primary tumor). Indeterminate nodules underwent either biopsy/fine needle aspiration (FNA)/follow-up imaging after at least 3–6 months. Nodules that were stable on follow-up imaging or decreased in size were considered benign whereas those nodules which increased in size were considered malignant (metastases/second primary in the lung).
Fig. 1.
Flowchart showing algorithm of analysis of data
Table 1.
Likert scale scoring of lung nodule
| Five-point ordinal Likert score | Radiological impression |
|---|---|
| 1 | Definitively benign |
| 2 | Probably benign |
| 3 | Equivocal |
| 4 | Probably malignant |
| 5 | Definitely malignant |
Clinical factors such as T and N classification, site/size of primary, and demographic variables such as age and gender were correlated. American Joint Committee on Cancer (AJCC) staging 7th edition was followed for T and N classification of the primary site and nodal disease. Corresponding chest radiograph findings were recorded and compared to determine their relative sensitivity. Standardized uptake value (SUV) max of the lung nodule and extrathoracic metastases present on PET-CECT were recorded.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) statistical software version 21 [IBM New York, United States] using Pearson’s chi-square test. Both univariate and multivariate logistic regression models were used to determine risk factors for HNSCC metastasis. A P value of < 0.05 was considered to be statistically significant.
Results
Lung Nodules
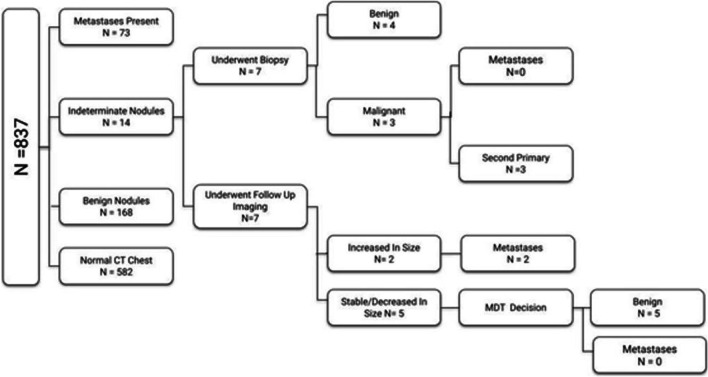
Out of 837 patients in our study, 582 patients (69.5%) had no lung nodule, 3 patients (0.4%) had biopsy-proven squamous cell carcinoma of lung, 177 patients (21.1%) had benign nodules, and 75 patients (9%) had metastatic nodules (Fig. 2). Of the 14 patients with indeterminate nodules, 7 underwent biopsy—3 of which came positive for second primary malignancy in the lung, and the rest 7 were evaluated on follow up CT with 2 of them considered metastatic in view of increase in size. The detection rate of pulmonary metastases in head and neck primary is 8.96% in our study.
Fig. 2.
Flowchart showing characteristics of lung nodules in study patients
Character of Lung Nodule on Chest CT
Size
All of the patients with second primary size of the nodule was > 6 mm size. Nodules with > 10 mm are more likely metastatic. Thirty-eight patients with nodule size more than 10 mm were found to have pulmonary metastasis whereas 37 patients with nodule size ≤ 10 mm have pulmonary metastasis. P value is found significant (P < 0.001).
Margins
61 patients with smooth nodules had pulmonary metastasis, whereas 14 patients with spiculated/irregular margin had pulmonary metastasis. Among the metastatic nodules, 81.3% were of smooth margin, compared to 13.5% of irregular margins and 2.7% of smooth margins. There is a significant correlation between nodule margin and pulmonary metastasis (P value < 0.001).
Number of Nodules
There was a significant correlation between the number of lung nodules and pulmonary metastasis (P < 0.001). Thirty-five patients with ≥ 5 nodules were metastatic whereas 40 patients with less than 5 nodules had metastatic disease.
Uni/Bilaterality
80% of unilateral lung nodules were benign, whereas among metastatic nodules, 62.7% were bilateral in location. There is a statistical correlation between bilateral lung nodules and metastasis (P < 0.001).
Patient Characteristics and Pulmonary Metastases (Tables 2 and 3)
Table 2.
Relation of pulmonary metastases with patient/disease characteristics. Total no. of patient (N) = 837, n = number of patients in each category
| Variables | With metastases (n = 75) | (%) | Without metastases (n = 762) | (%) | P value |
|---|---|---|---|---|---|
| Gender (male/female) | 4.3:1 | 81.3/18.7 | 4.6:1 | 91.1/17.8 | .860 |
| Age < 58/ ≥ 58 | 40/35 | 53.3/46.7 | 376/386 | 49.3/50.7 | .595 |
| Site of primary disease | Metastatic nodules | Benign nodules | .549 | ||
| Oral cavity | 26 | 34.6 | 82 | 45.1 | |
| Oropharynx | 13 | 17.3 | 33 | 18.6 | |
| Hypopharynx | 23 | 30.6 | 29 | 16.3 | |
| Larynx | 13 | 17.3 | 36 | 19.7 | |
| T stage | |||||
| T1/T2/T3/T4 | 3/10/21/41 | 4.0/13.3/48.0/54.7 | 43/110/162/47 | 5.6/14.4/21.3/58.7 | .838 |
| (T1, T2)/(T3, T4) | 13/62 | 19.3/102.7 | 153/209 | 20.0/80.0 | .569 |
| (T1,T2,T3)/(T4) | 34/41 | 45.3/54.7 | 315/47 | 41.3/58.7 | .503 |
| N stage | |||||
| N0/N1/N2a/N2b/N2c/N3 | 5/11/1/17/14/27 | 6.7/14.7/1.3/22.7/18.7/36.0 | 167/109/38/176/160/112 | 21.9/14.3/5.0/23.1/21.0/14.7 | > .05 |
| Lower jugular node (present/absent) | 25/50 | 33.3/67.7 | 132/630 | 17.3/82.7 | .001 |
| Total | 75 | 8.9 | 762 | 91.1 |
Table 3.
The likelihood ratio for estimating the risk of pulmonary metastasis in presence of high-risk factors such as low jugular node and advanced nodal staging
| Dependent variable—presence of pulmonary metastasis | |||||||
| Independent variable | Univariate P value | Multivariate P value | Likelihood ratio | Lower boundary of 95% CI | Upper boundary of 95% CI | ||
| Low jugular node | .001 | .029 | 1.846 | 1.064 | 3.203 | ||
| New N staging | .002 | .028 | 1.951 | 1.073 | 3.548 | ||
| In, new N staging, we grouped N staging as N0/N1/N2a and N2b/N2c/N3 | |||||||
| Independent variable | Univariate P value | Multivariate P value | Likelihood ratio | Lower boundary of 95% CI | Upper boundary of 95% CI | ||
| Low jugular node | .001 | .013 | .513 | .303 | .868 | ||
| N staging | .002 | .014 | .318 | .120 | .789 | ||
| In N staging, we classified N0 in one group and N1, N2, and N3 in another group | |||||||
This study included 837 patients including 687 (82.1%) males and 150 (17.9%) females. Most of the patients were in the age range of 45–74 years. The median age was 56.65 years, and the mean age was 58 years. Number of patients with/without pulmonary metastases in relation to patient’s characteristics—gender, age, site of primary disease, T stage, N stage, presence of positive jugular node—has been given in detail in Table 2. A significant positive correlation was found between the presence of positive low jugular nodes and pulmonary metastases (P < 0.001). The likelihood ratio for estimating the risk of pulmonary metastasis in presence of high-risk factors such as low jugular node and advanced nodal staging has been discussed in Table 3.
Extrapulmonary Metastases and Their Relation with Primary Disease Characteristics (Table 4)
Table 4.
Relation of extra-pulmonary metastases with primary disease characteristics. Total no. of patient (N) = 837. n = number of patients in each category
| Variables | With metastases (n) | (%) | Without metastases (n) | (%) | P value |
|---|---|---|---|---|---|
| T stage | |||||
| T1/T2/T3/T4 | 4/9/10/33 | 7.1/16.1/17.9/58.9 | 42/111/173/455 | 5.4/14.2/22.2/58.3 | .833 |
| (T1, T2)/(T3,T4) | 13/62 | 19.3/102.7 | 153/209 | 20.0/80.0 | .511 |
| (T1,T2,T3)/T4 | 23/33 | 30.6/69.4 | 327/455 | 42.9/57.1 | .922 |
| N stage | |||||
| N0,N1,N2a,N2b,N2c,N3 | 4/9/1/11/13/18 | 7.1/16.1/1.8/19.6/23.2/32.1 | 168/111/38/182/161/121 | 21.5/14.2/4.9/23.3/20.6/15.5 | .008 |
| (N0,N1,N2a)/(N2b,N2c,N3) | 14/42 | 18.6/81.3 | 317/464 | 41.6/58.4 | .021 |
| (N0,N1)/(N2,N3) | 13/43 | 25/75 | 279/502 | 35.7/64.3 | .06 |
| Lower jugular node (present/absent) | 20/36 | 35.7/64.3 | 137/644 | 17.5/82.5 | .001 |
| Total | 56 | 6.7 | 781 | 93.3 | |
In our study, a total of 56 (6.7%) patients had extra-pulmonary metastasis. Seven hundred eighty-one patients (93.3%) had no extra-pulmonary metastasis. Out of 56 patients with extrapulmonary metastasis, 20 patients also had pulmonary metastatic nodules, 27 patients had no nodules, and the rest of the patients had benign nodules. Nineteen patients had mediastinal and pleural metastasis (out of which 14 patients had mediastinal nodal metastases, and 5 patients had malignant pleural/pericardial effusion), 33 had skeletal metastasis, and 11 had hepatic metastasis. Rare sites of metastasis included gastro-hepatic node, spleen, subcutaneous tissue, adrenal, and thyroid (single patient each). A total of 12 patients (1.4%) had extrathoracic metastases. Only 2 (0.2%) cases had distant metastases at sites that are not covered in the routine chest CT study—thyroid and femur (one each). There was a significant positive correlation between N classification and extra-pulmonary metastasis. There was also a significant positive correlation between the presence of low jugular nodes and extra-pulmonary metastasis (P < 0.001).
Correlation Between Pulmonary and Extrapulmonary Metastases (Table 5)
Table 5.
Relation of extra-pulmonary metastases with pulmonary metastases. Total no. of patient (N) = 837
| Pulmonary metastasis present | Pulmonary metastasis absent | |
|---|---|---|
| Extra-pulmonary metastasis present | 20 (26.6%) | 36 (4.72%) |
| Extra-pulmonary metastasis absent | 55 (73.3%) | 726 (95.2%) |
| Total | 75 | 762 |
Presence of extra-pulmonary metastasis is a risk factor for pulmonary metastasis (P < 0.001).
Second Primary Neoplasm
Eleven patients had second primary malignancy of which one patient had three concomitant primary malignancies at the time of presentation. Most common site of second primary malignancy was esophagus seen in 5 patients (45%). Second most common site was lung seen in 3 patients (27%). Next common sites of second primary malignancy were colon and hypopharynx (2 patients each), breast, and prostate (one patient each). Incidence of second primary malignancy in lung is 0.03%.
Chest Radiographs in Detecting Pulmonary Metastases
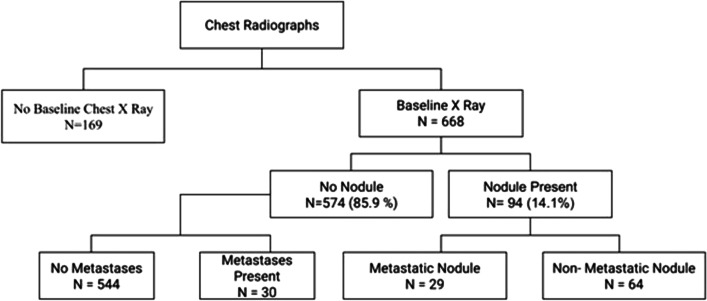
In our study, sensitivity, specificity, positive predictive value, and negative predictive value of chest radiographs for detection of pulmonary metastases were 49%, 88%, 30%, and 94.7%, respectively. Chest radiograph observations are described in Fig. 3.
Fig. 3.
Flowchart showing observations on chest radiographs
Chest CT in Detecting Pulmonary Metastases
We used two scoring methods by two independent radiologists to calculate sensitivity and specificity of chest CT in detecting pulmonary metastases. Taking Likert scale value 3.5 as cut off, we obtained sensitivity = 97.3% and specificity = 97.2%. Here, we have taken values ≥ 4 metastatic and < 4 benign (higher the Likert scale value, higher is the chance of nodule benign malignant—Likert 1 is completely benign, whereas Likert 5 is completely malignant). In the other method, we used 1.5 as cut off. So, considering indeterminate value as the cut off sensitivity and specificity is 100% and 93.3%. The positive predictive and negative predictive values were 93.5% and 98.8%, respectively.
PET-CECT in Detecting Metastases
Mean SUV max of benign nodule is 0.25 (95% confidence interval of − 0.1110 to 0.6110) and median value 0.00, whereas for metastatic nodule, mean SUV max value is 5.22 (95% confidence interval 3.77 to 6.67) and median value 5.00. One hundred and seven patients underwent PET-CECT in our study. One of the potential advantages of PET-CECT is in detecting distant metastases beyond the area covered in chest CT. However, in our study such additional lesions were picked up by PET-CT in only 4 (0.47%) cases. Of these, 3 were in the pelvic bones (ilium) and one in the thyroid. For the rest of the patients (99.8%), there was no added advantage of PET-CT.
Another expected role of PET-CECT is in the characterization of pulmonary nodules into benign/indeterminate/malignant. In our study, SUV max value was available for only 107 patients. Out of them, 23 (88.5%) patients with SUV max value ≥ 3 had metastases. Eight (9.9%) patients with SUV max < 3 had metastases (P < 0.001). Lung nodules less than 8 mm in size were those which posed diagnostic challenges. PET CECT could not categorize these nodules as they had borderline SUV max values. One of the biggest drawbacks of PET-CECT is that it is a costly imaging modality. The superfluous expense becomes a substantial burden on the patient, insurance companies, and funds of the government hospitals, especially in resource-constrained countries like ours. To estimate the financial burden associated with metastases screening with PET-CECT, we worked out the formula for calculating the extra cost borne by the patient per each PET-CECT. This formula can be used universally in all government and private institutional settings.
Let x be the extra cost borne by PET-CECT, a be the cost of PET-CECT, b be the cost of CECT head and neck, and c be the cost of chest CECT.
| Cohort 1: patients who underwent CECT for head and neck and chest screening | x = a − (b + c) |
| Cohort 2: patients who underwent CECT for chest screening and CE-MRI for head and neck imaging | x = a − c |
We calculated the extra cost borne by a patient in undergoing PET-CECT over chest CT in the private category in our Institute using the above formula. Cost of CT in our institute are as follows:
| Cost (INR*) | |
| Chest CECT | 4720 |
| CECT head and neck | 5980 |
| CECT neck + thorax | 7740 |
| CE-MRI head and neck | 7130 |
| PET-CECT whole body | 19,025 |
Extra cost borne by one patient in our institute:
| Cohort 1 | x = a − (b + c) = 19,025 − (5980 + 4720) = 8325 INR (113.85 USD**) |
| Cohort 2 | x = (a − c) = 19,025 − 4720 = 14,305 INR (194.33 USD**) |
*Indian national rupee (INR), ** US dollar
Hence, patients in both cohorts have to bear additional expenses for diagnostic investigations, the difference in cohort 2 being notable. On calculating the extra cost that would have been borne by the patient/institution/insurance companies in getting PET-CECT of 837 patients in our study, the total monetary drain equals 7,033,805 INR (95,551.85 USD), which is a huge sum. Using chest CT instead of PET-CECT as a metastases screening tool, the drain on the patient and hospital’s finances can be limited with no compromise of sensitivity.
Discussion
Cancers of the lip and oral cavity are highly frequent in South Central Asia becoming a leading cause of cancer death [20]. It is a significant concern in these countries as most patients here present with advanced-stage disease. In patients with known head and neck primary, pulmonary nodule is the most common thoracic manifestation of distant metastasis [21]. Our study showed the rate of detection of lung metastasis and second primary in lung in newly diagnosed HNSCC to be 8.96% and 0.3%, respectively, indicating a need to screen these patients with chest CT which has a higher rate of detection (sensitivity 97.3% and specificity 97.2%) as compared to chest radiographs (sensitivity 49% and specificity 89%) and better availability and economical as compared to PET-CECT.
Age and gender did not show any significant correlation with pulmonary metastases. On comparing results of our study with Surveillance, Epidemiology, and End Results (SEER) database from 1974 to 1999, we found a higher incidence (34.6%) of metastasis among patients with oral cavity as the primary site of disease which can be explained by more smokeless tobacco consumption and poor dental hygiene among our patients as compared to more smoking-related pharyngeal cancers in other populations. The relation between N stage of disease and pulmonary metastases was statistically significant with the observation that risk of a positive chest CT in those with N2/N3 disease was 7.16 times more than in those with N0/N1 disease. Significant correlation was also found between positive low jugular node status and pulmonary metastasis.
We found the detection rate of pulmonary metastases to be 8.96%. Reported incidence of pulmonary metastases ranges from 5 to 40% [22]. Detection rate of distant metastasis (overall) in our study was 13.2%. Among them, 67% pulmonary, 29.7% skeletal, 0.09% hepatic, and 17% were mediastinal and pleural metastasis. We found the presence of extra-pulmonary metastasis to be a risk factor for pulmonary metastasis.
As compared to western literature, the percentage of benign nodules was more common in our study amounting to 69.4%. This is due to increased prevalence of chronic lung infections like tuberculosis in our population. This is in coherence with our finding of strong correlation between calcification and benign nodules. Our results showed that nodules smaller than 10 mm are more likely to be benign (n = 105/111, 95%), whereas those measuring 10 mm or greater in size are more likely to be malignant (n = 22/26, 85%). Also, 30% of 5 mm or greater nodules and 85% of 10 mm or greater nodules were malignant. Also, we found that calcified nodules are more likely benign. Nodules with smooth margin and nodules > 5 in number and bilateral nodules are more likely metastatic.
Reported sensitivity and specificity of chest radiographs in detecting lung metastases ranges between 20 and 50% and between 90 and 98%, respectively [23, 24]. In our study, we found that in 574 patients who had no lung nodule on radiograph, chest CT detected metastases in 30 cases and led to a change in the plan of management. On comparison of sensitivity, specificity, positive predictive value, the negative predictive value of chest radiographs, and chest CT in the detection of pulmonary metastasis (chest CT-sensitivity 97.3% and specificity 97.2%; chest radiograph-sensitivity 49% and specificity 89%), the advantage of chest CT over chest radiograph in screening for metastasis is well found in our study. This is because chest CT is able to detect smaller lesions and gives much better visualization of the lungs than a radiograph.
Positron emission tomography scans have been reported to be effective in detecting lung metastasis or synchronous lung primary tumors, but it comes with the demerit of being costly and unavailable universally. Also, often PET-CT fails to distinguish between metastasis and infection. For diagnosing a nodule as malignant, PET has a sensitivity and specificity of 96% and 88%, respectively [25]. In our study, only 12 patients (1.44%) had extrathoracic metastasis out of which only 4 (0.47%) cases had distant metastases at sites that are not covered in the routine chest CT study. Of these, 3 were in the pelvic bones (ilium) and one in the thyroid. For the rest of the patients (99.8%), there was no added advantage of PET-CT. This observation was concordant with a similar study by Yehree Kim et al. which reported the advantage of PET-CT in detecting more synchronous lung cancers but not more distant metastases than chest CT in patients with head and neck cancers [26]. This is because the most common site of distant metastasis in head and neck malignancies is the lung, and the majority of hepatic parenchyma is visible on chest CT.
Our study showed the rate of detection of lung metastasis in newly diagnosed HNSCC to be 8.96% which is significantly high indicating a need to screen for lung metastasis and synchronous lung primary tumor in these patients. In our study, chest radiographs missed metastatic lung nodules in 30 patients which were later detected on chest CT. Also, its sensitivity was found to be only 49% indicating poor reliability of chest x-ray as screening tool. In studies before 2017, there was concern regarding cost and logistics of using CT as a screening tool. Previous National Comprehensive Cancer Network (NCCN) guidelines recommended using chest radiographs only. However, we found a need to follow a rational approach to identify patients at risk for distant metastasis and synchronous lung primary tumors, so that these patients can be selected for chest CT over radiographs—certain predictors being T and N classification and grade of tumor as also shown in previous few studies. Taking our observations into consideration, we believe that chest CT should be the method of choice for lung screening in patients with newly diagnosed HNSCC.
Our data showed no relation between the site of primary tumor and chest CT positivity. It showed significant correlation between N2 or N3 disease and positive chest CT. The implication of these findings is that head and neck mucosal SCC arising from any site and having N2/N3 positivity should get a chest CT to screen the lungs. It was noteworthy that T stage did not correlate with a positive chest CT, providing evidence that we could avoid chest CT in those with N0 disease with any T stage.
Conclusion
Chest CT is a useful screening tool in newly diagnosed advanced HNSCC patients for the detection of occult metastasis. There is an imperative role of baseline chest CT as the detection rate of chest radiographs is low, and very few patients had extra-thoracic metastasis at distant sites that could only be detected by PET-CET which comes with additional demerits of being costly and unavailable universally. Chest CT when used as a screening tool substantially contributes to changing management intent from curative to palliative in asymptomatic patients with distant metastasis and avoids unnecessary surgical interventions. In addition, the cost incurred in screening with chest CT is more reasonable in resource-constrained countries like India. We recommend baseline chest CT in all patients with nodal stage N2 and above and/or the presence of low jugular nodes irrespective of T classification or primary site.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL. 2001;63(4):202–207. doi: 10.1159/000055740. [DOI] [PubMed] [Google Scholar]
- 2.Leemans CR, Tiwari R, Nauta JJ, Van der Waal I, Snow GB. Regional lymph node involvement and its significance in the development of distant metastases in head and neck carcinoma. Cancer. 1993;71(2):452–456. doi: 10.1002/1097-0142(19930115)71:2<452::AID-CNCR2820710228>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Burri RJ, Kao J, Navada S, Packer S. Nonsurgical treatment of head and neck cancer. In: Som PM, Curtin HD, editors. Head and neck imaging. 5. St Louis, Mo: Mosby; 2011. pp. 2893–2914. [Google Scholar]
- 4.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405–409. doi: 10.1016/S0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 5.Mahajan A, Chand A, Agarwal U, Patil V, Vaish R, Noronha V, Joshi A, Kapoor A, Ahuja A, Shukla S, Menon N. Prognostic value of radiological extranodal extension (rENE) detected by computed tomography (CT) for predicting outcomes in locally advanced head and neck squamous cell cancer (LAHNSCC) patients treated with radical concurrent chemoradiotherapy (CCRT) Front Oncol. 2022;27:2029. doi: 10.3389/fonc.2022.814895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Kamp MF, Muntinghe FO, Iepsma RS, Plaat BE, van der Laan BF, Algassab A, et al. Predictors for distant metastasis in head and neck cancer, with emphasis on age. Eur Arch Otorhinolaryngol. 2021;278(1):181–190. doi: 10.1007/s00405-020-06118-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oshiro Y, Kusumoto M, Moriyama N, Kaneko M, Suzuki K, Asamura H, et al. Intrapulmonary lymph nodes: thin-section CT features of 19 nodules. J Comput Assist Tomogr. 2002;26(4):553–557. doi: 10.1097/00004728-200207000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Narayana A, Vaughan AM, Fisher S, Reddy S. Second primary tumors in laryngeal cancer: results of long-term follow-up. Int J Radiat Oncol* Biol* Phys. 1998;42(3):557–62. doi: 10.1016/S0360-3016(98)00250-8. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin MS, Drucker EA, McLoud TC, Shepard JA. Small pulmonary nodules: detection at chest CT and outcome. Radiology. 2003;226:489–493. doi: 10.1148/radiol.2262010556. [DOI] [PubMed] [Google Scholar]
- 10.Schaner EG, Chang AE, Doppman JL, Conkle DM, Flye MW, Rosenberg SA. Comparison of computed and conventional whole lung tomography in detecting pulmonary nodules: a prospective radiologic-pathologic study. Am J Roentgenol. 1978;131:51–54. doi: 10.2214/ajr.131.1.51. [DOI] [PubMed] [Google Scholar]
- 11.Proto AV, Thomas SR. Pulmonary nodules studied by computed tomography. Radiology. 1985;156:149–153. doi: 10.1148/radiology.156.1.4001402. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Sone S, Abe H, MacMahon H, Doi K. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology. 2004;233:793–798. doi: 10.1148/radiol.2333031018. [DOI] [PubMed] [Google Scholar]
- 13.Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 14.Coca-Pelaz A, Rodrigo JP, Suárez C. Clinicopathologic analysis and predictive factors for distant metastases in patients with head and neck squamous cell carcinomas. Head Neck. 2012;34(6):771–775. doi: 10.1002/hed.21804. [DOI] [PubMed] [Google Scholar]
- 15.Mahajan A, Cook G (2017) Physiologic and molecular basis of PET in cancer imaging. Basic Science of PET Imaging, pp 399–427
- 16.Mahajan A, Cook G (2017) Clinical applications of PET/CT in oncology. Basic Science of PET Imaging, pp 429–50
- 17.Webb WR, Higgins CB. Thoracic imaging
- 18.Tan LK, Greener CC, Seikaly H, Rassekh CH, Calhoun KH. Role of screening chest computed tomography in patients with advanced head and neck cancer. Otolaryngology—Head Neck Surg. 1999;120(5):689–92. doi: 10.1053/hn.1999.v120.a91767. [DOI] [PubMed] [Google Scholar]
- 19.Mahajan A, Shukla S, Mali R, Agarwal U, Sable N, Vaish R, Ankathi SK, Patil V, Janu AK, Prabhash K, Noronha V. Second opinion interpretations by specialty radiologists in head-and-neck oncology and their impact on clinical management: a retrospective observational study. Cancer Res Stat Treat. 2022;5(4):652–659. doi: 10.4103/crst.crst_36_22. [DOI] [Google Scholar]
- 20.Mahajan A, Ahuja A, Sable N, Stambuk HE. Imaging in oral cancers: a comprehensive review. Oral Oncol. 2020;1(104):104658. doi: 10.1016/j.oraloncology.2020.104658. [DOI] [PubMed] [Google Scholar]
- 21.Mesurolle B, Mignon F, Meingan P, Domenge C, Vasile M, Sigal R. Head and neck cancer patients with pulmonary nodules: value and role of CT-guided transthoracic needle aspiration biopsies. Head Neck. 2003;25(11):889–894. doi: 10.1002/hed.10303. [DOI] [PubMed] [Google Scholar]
- 22.Zbären P, Lehmann W. Frequency and sites of distant metastases in head and neck squamous cell carcinoma: an analysis of 101 cases at autopsy. Arch Otolaryngol-Head Neck Surg. 1987;113(7):762–764. doi: 10.1001/archotol.1987.01860070076020. [DOI] [PubMed] [Google Scholar]
- 23.Troell RJ, Terris DJ. Detection of metastases from head and neck cancers. Laryngoscope. 1995;105(3):247–250. doi: 10.1288/00005537-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 24.De Bree R, Deurloo EE, Snow GB, Leemans CR. Screening for distant metastases in patients with head and neck cancer. Laryngoscope. 2000;110(3):397–401. doi: 10.1097/00005537-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Girvin F, Ko JP. Pulmonary nodules: detection, assessment, and CAD. Am J Roentgenol. 2008;191(4):1057–1069. doi: 10.2214/AJR.07.3472. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y, Roh JL, Kim JS, Lee JH, Choi SH, Nam SY, et al. Chest radiography or chest CT plus head and neck CT versus 18F-FDG PET/CT for detection of distant metastasis and synchronous cancer in patients with head and neck cancer. Oral Oncol. 2019;1(88):109–114. doi: 10.1016/j.oraloncology.2018.11.026. [DOI] [PubMed] [Google Scholar]