Abstract
Following 3R (reduction, refinement, and replacement) principles, we employed the rat liver S9 fraction to mimic liver metabolism of curcumol having high in vitro IC50 on cancer cells. In HCT116 and HT29 colon cancer cells, the metabolites of curcumol by S9 fraction exerted more enhanced activity in inducing cell cycle arrest and apoptosis via regulating the expression of cyclin D1, CDK1, p21, PARP and Bcl-2 than curcumol. In addition, oral administration of curcumol at 4 mg/kg BW significantly suppressed the development of colon tumor induced by azoxymethane/dextran sulfate sodium, and induced cell cycle arrest and apoptosis in tumor tissues. In mass analysis, curcumenol and curzerene were identified as the metabolites of curcumol by S9 fraction metabolism. Taken together, curcumol metabolites showed the enhanced suppressive effect on colon cancer, suggesting that S9 fraction can be considered as simple, fast, and bio-mimicking platform for the screening of chemical libraries on different chronic diseases.
Keywords: Colon cancer, Curcumol, Liver metabolism, S9 fraction
Introduction
Liver metabolism is composed of phase I and phase II metabolism, converting the parent molecules into more polar metabolites by oxidation/reduction process and transforming the phase I metabolites by conjugation, respectively (Rathaur and SR, 2019). After metabolism, the metabolites travel following the circulatory system and exhibit various physiological functions (Karn et al., 2021). For example, demethylated metabolites of 5-demethylnobiletin exerted stronger inhibitory effects on colon cancer cells than 5-demethylnobiletin (Zheng et al., 2013) and 6,7,4′-trihydroxyisoflavone, the hepatic metabolites of daidzein, was more effective in inhibiting the growth of estrogen receptor negative breast cancer than daidzein (Chen et al., 2020). Therefore, it is critical to consider the effect of liver metabolism to evaluate the health beneficial efficacies of natural and dietary phytochemicals (Underhill and Khetani, 2018), indicating that direct treatment of phytochemicals in in vitro cell culture system is limited to screen their activities.
Although animal experiments have been performed for demonstrating biological effects of different potent phytochemicals, there are drawbacks including time-consuming, expensive, and labor-intensive characteristics (Huang et al., 2021). In addition, after the first discussion in 1959 about 3Rs indicating reduction, refinement, and replacement, the concern of ethical problems is growing and different alternative methods such as cell-based tests, in silico biomodeling, and ex vivo models have been developed (Burden et al., 2021; Huang et al., 2021). For the liver metabolism of chemicals, in vitro systems using hepatocyte-like cells, microsomes, and S9 fractions have been applied (Richardson et al., 2016). However, microsomes cannot perform the full hepatic metabolism due to lack of phase II enzymes, and hepatocyte-like cells from embryonic stem cells or induced pluripotent stem cells exerted the unstable phenotype in culture system (Rashidi et al., 2016). S9 fraction prepared from rodent liver after treatment of enzyme inducing agents is a post-mitochondrial fraction supplemented with cofactors and is the most widely used external metabolization system in mutagenicity/genotoxicity assays of different chemicals (Brendt et al., 2021; Hendriksen, 2009). Recently, it was reported that S9 fraction was effective to improve the predictivity of endocrine disrupting compounds (EDCs) in cell-based reporter gene assay (van Vugt-Lussenburg et al., 2018). In drug discovery, it was also suggested that S9 fraction is more appropriate and comprehensive system for high throughput metabolic screening (Richardson et al., 2016).
Curcumol, a bioactive sesquiterpenoid isolated from numerous plants of family Zingiberaceae (Wei et al., 2019), has been demonstrated to exert the tumor suppressive activity (Hashem et al., 2021). However, the concentration of curcumol treated in in vitro cell culture system was relatively high and the IC50 was up to 200 µM (Chen et al., 2014; Liu et al., 2019; Yu et al., 2021), which might not be feasible in physiological circulatory system. In a recent study, curcumol did not show the significant inhibition of lung cancer cells’ proliferation even at 50 μg/mL, corresponding to about 212 μM, but oral administration of curcumol at 20 mg/kg body weight (BW) every 2 days exerted more than 70% suppression of tumor growth (Sheng et al., 2022), suggesting that the biological activity of curcumol may be enhanced by in vivo metabolism.
Here, we employed rat liver S9 fraction for in vitro hepatic metabolism and compared the inhibitory effect on the proliferation of colon cancer cells. In addition, we also confirmed whether the oral administration of curcumol at low concentrations (2 mg/kg BW and 4 mg/kg BW) suppressed the development of colon tumors in azoxymethane (AOM)/dextran sulfate sodium (DSS)-induced colon tumorigenesis model.
Materials and methods
Reagents and cell culture
Curcumol (purity > 98%, HPLC) was purchased from Chengdu Alfa Biotechnology Co., Ltd (Chengdu, China) and dissolved in DMSO (Sigma Aldrich, St. Louis, Missouri, USA). Rat liver S9 fraction was purchased from Corning (Thermo Fisher Scientific Inc., Waltham, USA). β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate (NADPH) was purchased from Roche (Darmstadt, Germany). Glucose-6-phosphate (G-6-P), glucose-6-phosphate dehydrogenase (G-6-PD), magnesium chloride hexahydrate (MgCl2), l-glutathione reduced (GSH), Uridine 5′-diphosphoglucuronic acid (UDPGA), 3′-phosphoadenosine-5′-phosphosulfate (PAPS), AOM, and DSS were purchased from HCT116 and HT29 cell lines were obtained from Korean Cell Line Bank (Seoul, Korea) and cultured in DMEM with 10% fetal bovine serum, 1% l-Glutamine, and 1% penicillin/streptomycin (P/S) (Life Technologies, Grand Island, NY, USA) at 37 °C, 5% CO2. High performance liquid chromatography (HPLC) grade acetonitrile (ACN), water, and methanol (MeOH) were purchased from Honeywell (Burdick & Jackson, Muskegon, MI, USA) for measurement of curcumol metabolites by liver S9 fraction. Mass spectrometry (MS) grade formic acid, ACN, and water were purchased from Thermo Fisher Scientific (Waltham, MA, USA) to perform metabolites profiling using LC–MS/MS analysis.
Cell viability assay
Cells were seeded in 96-well plate (1 × 104 cells/well) and incubated with compounds at different concentrations for 24 h, then 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma Aldrich) was added and incubated together for 4 h at 37 °C. The formed formazan crystals were dissolved with DMSO and absorbance was measured at 570 nm using ELISA plate reader (Molecular Devices, Sunnyvale, CA, USA). Cell viability was defined as the percentage absorbance compared with control.
Colony formation assay
HCT116 (1 × 103 cells/well) and HT29 (2 × 103 cells/well) cells were seeded into 12-well plate and treated with different concentrations curcumol for 1–2 weeks. The media was replaced every 3 days. After treatment, cells were washed with PBS, fixed with methanol for 15 min at room temperature and stained with 0.5% crystal violet for 10 min. Then visible colonies were counted.
Metabolism of curcumol by rat liver S9 fraction
Rat liver S9 fraction stock was prepared with S9 fraction supplemented with cofactors NADPH (2 mM), G-6-P (30 mM), MgCl2 (50 mM), and G-6-PD (3 U/mL) to mimic hepatic phase I metabolism. For phase II metabolism, GSH (2 mM), UDPGA (5 mM) and PAPS (0.02 mM) were additionally supplemented. Curcumol was incubated with S9 fraction with or without cofactors for 6 h, and then treated on HCT116 and HT29 cells for 24 h at 37 °C, 5% CO2.
Western blot
After treatment, the total protein was extracted with a RIPA buffer with 1% protease inhibitor (AMRESCO, OH, USA). As described previously (Zhou et al., 2022), the cell lysates were centrifuged at 13,000 rpm for 15 min at 4 °C and supernatant was collected. Then the proteins were separated in SDS-PAGE and transferred to the polyvinylidene fluoride (PVDF) membrane. After blocking process, the membrane was incubated with primary and secondary antibodies, respectively. Primary antibodies for proliferation- proliferating cell nuclear antigen (PCNA), p21, Cyclin D1, Cyclin-dependent kinase 1 (CDK1), B-cell lymphoma 2 (Bcl-2), and poly (ADP-ribose) polymerase (PARP) were purchased from Santa Cruz Biotechnology (CA, USA). The secondary antibodies were obtained from Cell Signaling Technology (Boston, MA, USA). The blots were visualized by Clarity Western Enhanced chemiluminescence solution (Bio-rad, Hercules, California, USA) and determined by EZ capture imaging system (ATTO, Tokyo, Japan).
Animal experiment
Five-week-old male C57BL/6 mice were purchased from Doo-yeol Biotech (Seoul, Korea). After 1 week adaptation, animals were randomly divided into four groups (n = 10 each): Control (Con), AOM/DSS, AOM/DSS + 2 mg/kg BW of curcumol (AOM/DSS + CurL), and AOM/DSS + 4 mg/kg BW of curcumol (AOM/DSS + CurH), and mice were intraperitoneally injected with AOM (10 mg/kg BW) except Con group. One week later, 2% DSS solution was provided for 1 week followed by providing water for 2 weeks, and this cycle repeated three times. Curcumol was orally administered 5 days per week for 9 weeks. Body weight and food intake were measured two times a week. After sacrifice, the colon lengths and number of colon tumor were measured, and tumor tissues were collected for the protein analysis. Animal experiments were approved by the Institutional Animal Care and Use Committee at Chung-Ang University (Approval number: A2021039).
Mass spectrometry for the identification of metabolite
The metabolites by S9 fraction were placed at − 20 °C for 1 h for protein precipitation and centrifuged for 15 min at 15,115×g. Then the supernatant was taken and dehydrated with SpeedVac Vacuum Concentrator (EYELA CVE-3000, Tokyo, JAPAN). Dehydrated samples were dissolved with 50% ACN and analyzed using a Vanquish UHPLC system (VF-D11-A, Thermo Fisher Scientific, Waltham, USA) connected to Q Exactive Orbitrap mass spectrometer. For separation, Hypersil GOLD™ aQ C18 HPLC Column (100 × 2.1 mm, 1.9 μm) was used at 36 °C. Mobile phases were consisted of 0.1% formic acid in water (A) and 0.1% formic acid in ACN (B). Gradient elution was as followed: 0–2 min 10%B, 2–20 min 10–95%B, 20–22 min 95%B, 22–22.1 min 95–10%B, 22.1–25 min 10%B. Sample (5 μL) was injected and flowed at 0.3 mL/min. The Orbitrap mass spectrometer was connected a heat electro spray ionization (HESI) interface and the ion source spray voltage was set as 3.80 kV. Full MS scan mode with positive ionization mode was screened 100–1200 m/z. The UV absorption spectra was determined between 190 and 500 nm. MS data was processed with Compound Discoverer 3.1 software (Thermo Fisher Scientific, Waltham, USA) and SIMCA 17.0 software (Umetrics, Umeå, Sweden). The metabolites were screened (p < 0.05) and identified using Human Metabolome Database (https://hmdb.ca/metabolites) and related papers based on their MS/MS spectra.
Statistical analysis
The data were presented as mean ± standard deviation (SD). IBM SPSS Statistics v.20 software (New York, USA) was applied to analyze the statistical difference. Mean differences were analyzed using one-way analysis of variance (ANOVA) with Dunnett’s or Duncan’s post hoc test. p < 0.05 was considered statistically significant.
Results and discussion
Curcumol metabolized by the liver S9 fraction model inhibited the proliferation of colon cancer cells at low concentrations
In in vivo biotransformation, small molecules, especially aglycons are known to be absorbed through small intestine and metabolized by phase I and phase II enzymes from liver and small intestine (Karn et al., 2021), indicating that in vitro culture system is not fully matched with in vivo activities of the chemicals. Therefore, to evaluate the toxicities of xenobiotics or EDCs, the Organization for Economic Co-operation and Development (OECD) proposed five different levels for in vitro and in vivo experiments, among which they are trying to combine level 2 (in vitro assay) and level 3 (in vivo assay) by using alternative methods such as S9 fraction to follow the 3R principles (Gelbke et al., 2004; Grignard et al., 2020; van Vugt-Lussenburg et al., 2018). S9 fraction has been employed in the study of phytochemicals and plant extracts, but most of them focused to determine their toxicity characteristics such as mutagenicity and genotoxicity (Ben Sghaier et al., 2011; de Mello Silva Oliveira et al., 2016; Gontijo et al., 2020). In this study, we employed the liver metabolism of phytochemicals by rat liver S9 fraction and evaluated the physiological efficacy on cancer.
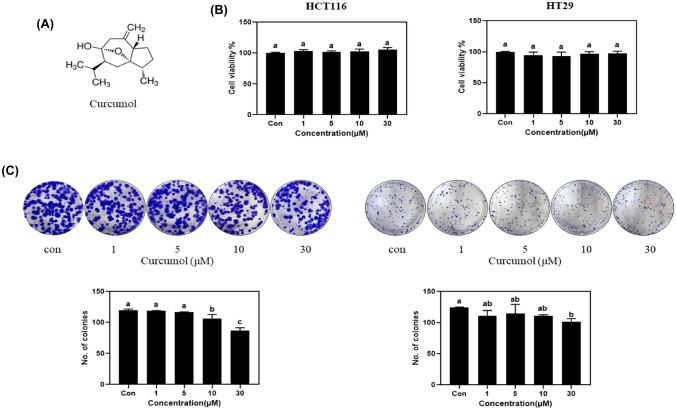
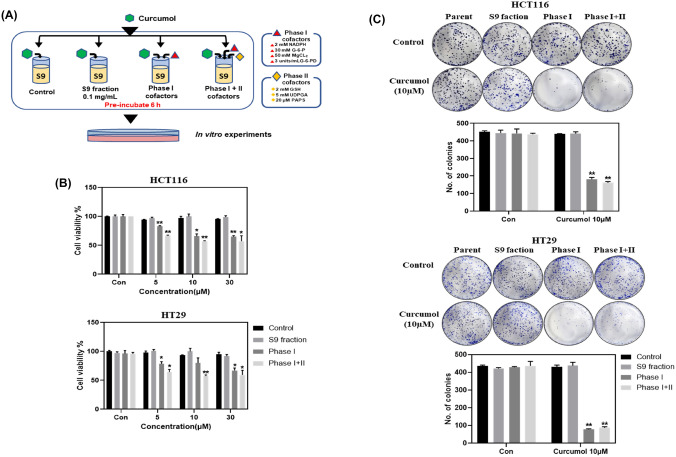
To confirm the effect of curcumol on the proliferation of colon cancer proliferation, we performed in vitro experiments in HCT116 and HT29 colon cancer cells. In MTT assay, curcumol did not show the inhibitory activity of proliferation in HCT116 and HT29 colon cancer cells even at 30 µM [Fig. 1(B)]. In colony formation assay, curcumol at 30 µM significantly reduced the number of colonies in HCT116 and HT29 cells, but it showed only marginal effect at 10 µM or lower concentration [Fig. 1(C)]. These results indicated that curcumol has weak or no inhibitory effects on proliferation of colon cancer cells as the parent form. However, after 6 h pre-incubation with rat liver S9 fraction with or without cofactors [Fig. 2(A)], curcumol metabolites were treated in HCT116 and HT29 cells. As shown in Fig. 2(B), S9 fraction without cofactors did not show any significant changes compared to the control, but curcumol metabolites by phase I metabolism and those by phase I + phase II metabolism significantly inhibited the viability of HCT116 and HT29 cells in a dose-dependent manner [Fig. 2(B)]. In colony formation assay, we also confirmed that curcumol metabolites by phase I metabolism and phase I + phase II metabolism dramatically reduced the number of colonies by 2.4 ± 0.2-fold and 2.7 ± 0.2-fold in HCT116 cells, respectively. [Fig. 2(C)]. In HT29 cells, they also exerted the similar effects, 5.6 ± 0.5-fold and 5.0 ± 0.4-fold reduction of colonies, respectively [Fig. 2(C)]. These results suggested that liver metabolism of curcumol by S9 fraction could modulate the inhibitory effects on the proliferation of colorectal cancer cells.
Fig. 1.
Curcumol has almost no inhibitory effect on the proliferation of colon cancer cells. (A) Chemical structure of curcumol. (B) HCT116 and HT29 cells (3000 cells/96-well plate) were treated with curcumol (0, 1, 5, 10, and 30 μM) for 24 h and followed by MTT assay. (C) HCT116 (1 × 103 cells/well) and HT29 (2 × 103 cells/well) were incubated with different concentrations of curcumol for 2 weeks. The colonies were stained with 0.5% crystal violet and the numbers were counted. Values represent means ± SD (n = 3). The letters a–c indicate statistically significant differences between different groups at p < 0.05 calculated by one-way ANOVA followed by Duncan’s post hoc test
Fig. 2.
Curcumol metabolized by the liver S9 fraction model showed strong inhibitory effects on the proliferation of colon cancer cells at low concentrations. (A) The process of pre-incubating curcumol with the rat liver S9 fraction model. (B) HCT116 and HT29 cells (3000 cells /96-well plate) were treated with rat S9 liver fraction model pre-incubated curcumol (0, 5, 10, and 30 μM) for 24 h and followed by MTT assay. (C) HCT116 (1 × 103 cells/well) and HT29 (2 × 103 cells/well) were treated with rat S9 liver fraction model pre-incubated curcumol (0, 10 μM) for 2 weeks. The colonies were stained the colonies with 0.5% crystal violet and the numbers were counted. Values represent means ± SD (n = 3). *p < 0.05, **p < 0.01 calculated by one-way ANOVA followed by Dunnett’s post hoc test compared to the control
Curcumol metabolized by the liver S9 fraction regulated the cell cycle and apoptosis-related markers in colon cancer cells
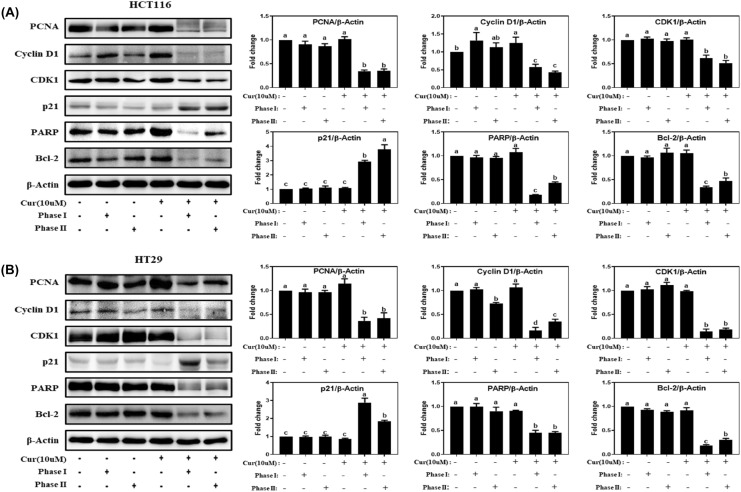
Cell proliferation is well known to be regulated by cell cycle and apoptosis (Evan and Vousden, 2001). To determine whether there was an association between liver metabolites of curcmol and cell proliferation regulatory pathways, we examined the protein levels of the major markers related to proliferation (PCNA), cell cycle (p21, cyclin D1, and CDK1) and apoptosis (Bcl-2 and PARP) in colon cancer cells. In HCT116 cells, compared to the control group, the expression levels of PCNA, cyclin D1, CDK1, PARP, and Bcl-2 decreased to 34.7 ± 2.4%, 58.1 ± 7.5%, 61.7 ± 6.5%, 18.4 ± 0.5%, and 33.9 ± 2.7%, respectively, after phase I metabolism and decreased to 35.3 ± 4.1%, 43.0 ± 3.4%, 51.6 ± 5.5%, 43.3 ± 2.2%, and 47.5 ± 6.2%, respectively, after phase I + II metabolism by S9 fraction [Fig. 3(A)]. In addition, the expression level of p21 was increased to 2.9 ± 0.1-fold, 3.8 ± 0.3-fold in HCT116 cells by phase I and phase I + II metabolism, respectively [Fig. 3(A)]. In HT29 cells, the curcumol metabolites by phase I and phase I + II metabolism exerted the similar significant regulation in PCNA, cyclin D1, CDK1, PARP, Bcl-2, and p21, compared to those in control [Fig. 3(B)]. These results demonstrated that curcumol metabolites by S9 fraction showed the enhanced effect on cell proliferation by regulating cell cycle and apoptosis although the parent curcumol at 10 μM did not show the significant changes (Fig. 3). It suggests that rat liver S9 fraction can be applied as a platform to screen biological efficacies of dietary and natural compounds, which is closer to in vivo system.
Fig. 3.
Curcumol metabolized by the liver S9 fraction model suppressed proliferation-related markers in colon cancer cells. (A) HCT116 (8 × 105 cells in 6 cm dishes) and (B) HT29 cells (1 × 106 cells in 6 cm dishes) were treated with the rat S9 liver fraction model pre-incubated curcumol (0, 10 μM) for 24 h, and the levels of proliferation-related proteins were determined by Western blot. The relative abundance of each band was normalized by β-actin. Values represent means ± SD (n = 3). The letters a–d indicate statistically significant differences between different groups at p < 0.05 calculated by one-way ANOVA followed by Duncan’s post hoc test
Curcumol inhibited tumorigenesis in AOM/DSS induced colon cancer animal model and regulated cell cycle and apoptosis-related markers
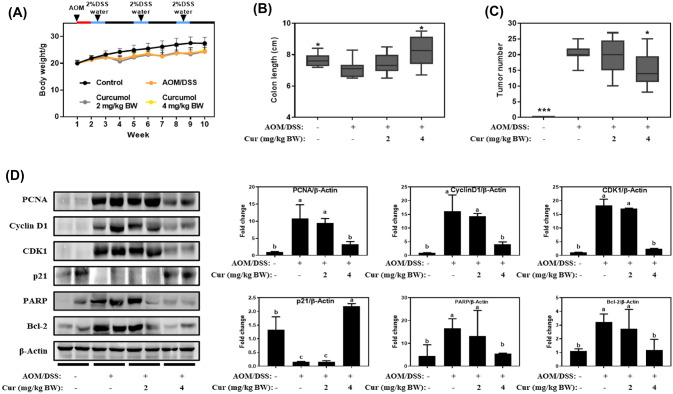
Based on the in vitro studies, we hypothesized that curcumol may reduce tumor growth in vivo after the liver metabolism and investigated the effect of curcumol at lower concentrations (2 mg/kg BW and 4 mg/kg BW) in AOM/DSS induced colitis-associated colon cancer mouse model. All mice treated with AOM/DSS showed a significant weight loss compared to Con, but there was no significant difference between AOM/DSS and AOM/DSS + curcumol group [Fig. 4(A)]. As shown in Fig. 4(B, C), the colon length decreased by AOM/DSS was recovered from 7.1 ± 0.6 to 8.2 ± 0.9 cm by oral administration of curcumol at 4 mg/kg BW, and the number of colon tumors in AOM/DSS was significantly reduced from 20.3 ± 2.6 to 15.2 ± 5.5 by administration of curcumol at 4 mg/kg BW. Administration of curcumol at 2 mg/kg BW exerted the marginal effect on colon length recovery and tumor number reduction, but it was not statistically significant [Fig. 4(B, C)]. From the tumor tissues, we analyzed the protein expression level of cell cycle and apoptosis related markers. In line with in vitro results, the increased expression levels of PCNA, cyclin D1, CDK1, Bcl-2, and PARP by AOM/DSS treatment were significantly suppressed by oral administration of cucurmol at 4 mg/kg BW to 3.1 ± 0.7-fold, 4.0 ± 0.9-fold, 2.2 ± 0.2-fold, 1.1 ± 0.7-fold, and 1.2 ± 0.1-fold, respectively [Fig. 4(D)]. These results indicate that curcumol had beneficial effects in inhibiting colon tumorigenesis induced by AOM/DSS, which might be derived from curcumol metabolism.
Fig. 4.
Curcumol inhibited tumorigenesis in AOM/DSS induced colon cancer mouse model. (A) Body weight changes (n = 10/group) were measured once a week. After 1-week adaptation, except the control group, all mice were intraperitoneally injected with AOM (10 mg/kg BW). One week later, the mice injected AOM were fed with water containing 2% DSS for 1 week followed by feeding with normal water for 2 weeks. This cycle is repeated three times. (B) Colon length in each group. (C) Tumor number in each group. (D) The levels of cell cycle and apoptosis-related proteins in colon tissue were determined by Western blot. The relative abundance of each band was normalized by β-actin. Values represent means ± SD. The letters a–d indicate statistically significant differences between different groups at p < 0.05 calculated by one-way ANOVA followed by Duncan’s post hoc test
Although curcumol was suggested as a potent agent for inhibiting numerous cancer cells by targeting different signaling pathways (Wei et al., 2019), it has been reported that IC50 of curcumol in colorectal cancer cell lines was 76.15 μg/mL corresponding to 322.18 μM in HCT116 cells, 93.59 μg/mL corresponding to 395.96 μM in LoVo cells, 209.09 μg/mL corresponding to 884.63 μM in SW480 cells (Liu et al., 2019), which is too high concentration to achieve in physiological level. We also confirmed that curcumol to 30 μM showed no or marginal inhibitory activities in the proliferation of HCT116 and HT29 colon cancer cells (Fig. 1). In addition, direct treatment of curcumol at 10 μM did not affect the expression of cell cycle and apoptosis related markers (Fig. 3). Based on these results, curcumol would not be considered for further study in the screening process of chemical library. Interestingly, however, the metabolites of curcumol by S9 fraction significantly suppressed the proliferation of cancer cells and regulated molecular markers of cell proliferation (Figs. 2, 3). In AOM/DSS induced colon cancer mouse model, orally administered curcumol at 4 mg/kg BW also exerted the inhibitory effect on colon tumorigenesis and regulated the protein expression of cell cycle and apoptosis markers (Fig. 4), which are in line with in vitro study. These results suggest that curcumol is metabolized to more active components, and S9 fraction is simply applicable to consider the liver metabolism and their health beneficial functions.
Curcumenol and curzerene were identified as the major metabolites of curcumol by MS analysis
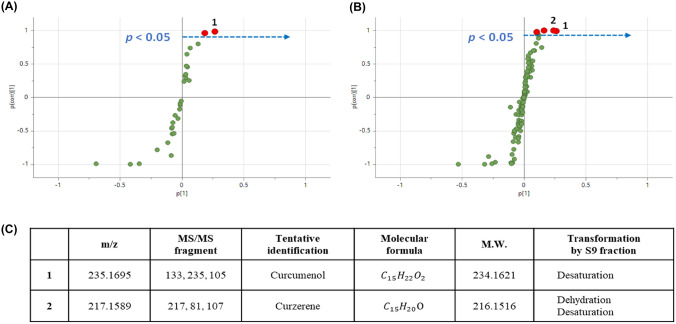
There are few studies on the identification of curcumol metabolites by liver S9 fraction and its metabolites were profiled using Orbitrap-LC–MS/MS with multivariate statistical analysis. Metabolomic variation by phase I and phase I + II reactions were analyzed through orthogonal partial least squares discriminant analysis (OPLS-DA) and the key metabolites were screened and identified (Fig. 5). The characteristic metabolite in phase I reaction group was tentatively identified as curzerene by dehydration and desaturation reaction with m/z 217 of the parent molecular. Through phase I + II reaction, two distinctive metabolites were screened and identified as curcumenol and curzerene as indicated in Fig. 5. Curzerene was supposed to be a significant metabolite by dehydration and desaturation of curcumol as in phase I reaction, while curcumenol was formed through desaturation metabolism of phase I + II reaction of liver S9 fraction.
Fig. 5.
Curcumenol and curzerene were identified as the major metabolites of curcumol by Orbitrap-LC–MS/MS analysis after incubated with the liver S9 fraction model. (A) Metabolites of curcumol after phase I metabolism. 1, curcumenol. (B) Metabolites of curcumol after phase I + II metabolism. 1, curcumenol. 2, curzerene. (C) The main metabolites and reactions of curcumol after phase I and phase I + II metabolism. p < 0.05 were analyzed through orthogonal partial least squares discriminant analysis (OPLS-DA)
There are few studies identifying the metabolites of curcumol, among which Lou et al. identified the metabolites in urine of rats after oral administration of curcumol at 40 mg/kg BW (Lou et al., 2010). We performed mass analysis of curcumol metabolites by S9 fraction and identified curcumenol and curzerene (Fig. 5). Although there is no report identifying curcumenol and curzerene as liver metabolites of curcumol, curcumenol exerted the suppressive activity of tumor growth in gastric cancer (Jung et al., 2018), breast cancer (Han et al., 2012), and liver cancer (Zhang et al., 2022). In addition, curzerene inhibited the proliferation of HCT116 colon cancer cells where the IC50 was approximately 9.2 (8.2–10.3) μM (Figueiredo et al., 2019). Therefore, the suppressive effect of curcumol metabolites on in vitro and in vivo proliferation of colon cancer may be at least in part derived from curcumenol and curzerene.
Taken together, we employed the rat liver S9 fraction model to demonstrate the health beneficial activity of curcumol in vitro and in vivo. Although the parent curcumol barely inhibited the proliferation of colon cancer cells, the metabolites induced by S9 fraction significantly enhanced the activity of curcumol at low concentrations and oral administration of curcumol at 4 mg/kg BW suppressed the colon tumorigenesis in AOM/DSS induced mice model, indicating that liver metabolism needs to be considered to characterize the physiological efficacy of phytochemicals. To our best knowledge, it is the first report that the rat liver S9 fraction was applied to mimic in vivo liver metabolism of phytochemical and its effect on in vivo animal model was confirmed. Therefore, the rat liver S9 fractions can be considered as simple, fast, and bio-mimicking platform for the screening of chemical libraries on different chronic diseases following the 3R principles.
Acknowledgements
This research was supported by a Grant (19162MFDS099 and 20163MFDS120) from Ministry of Food and Drug Safety in 2021. This research was also supported by the Chung-Ang University Graduate Research Scholarship in 2023.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yimeng Zhou and Ji Hyun Moon have contributed to this work equally.
References
- Ben Sghaier M, Boubaker J, Skandrani I, Bouhlel I, Limem I, Ghedira K, Chekir-Ghedira L. Antimutagenic, antigenotoxic and antioxidant activities of phenolic-enriched extracts from Teucrium ramosissimum: combination with their phytochemical composition. Environmental Toxicology and Pharmacology. 2011;31:220–232. doi: 10.1016/j.etap.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Brendt J, Crawford SE, Velki M, Xiao H, Thalmann B, Hollert H, Schiwy A. Is a liver comparable to a liver? A comparison of different rat-derived S9-fractions with a biotechnological animal-free alternative in the Ames fluctuation assay. Science of the Total Environment. 2021;759:143522. doi: 10.1016/j.scitotenv.2020.143522. [DOI] [PubMed] [Google Scholar]
- Burden N, Embry MR, Hutchinson TH, Lynn SG, Maynard SK, Mitchell CA, Pellizzato F, Sewell F, Thorpe KL, Weltje L, Wheeler JR. Investigating endocrine-disrupting properties of chemicals in fish and amphibians: opportunities to apply the 3Rs. Integrated Environmental Assessment and Management. 2021;18:442–458. doi: 10.1002/ieam.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Wang Y, Li M, Xu T, Wang X, Hong B, Niu Y. Curcumol induces HSC-T6 cell death through suppression of Bcl-2: involvement of PI3K and NF-kappaB pathways. European Journal of Pharmaceutical Sciences. 2014;65:21–28. doi: 10.1016/j.ejps.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Chen J, Qiu S, Kim JT, Cho JS, Moon JH, Zhou Y, Auh JH, Lee HJ. Garcinone C suppresses colon tumorigenesis through the Gli1-dependent hedgehog signaling pathway. Phytomedicine. 2020;79:153334. doi: 10.1016/j.phymed.2020.153334. [DOI] [PubMed] [Google Scholar]
- de Mello Silva Oliveira N, Reis Resende M, Alexandre Morales D, de Ragão Umbuzeiro G, Boriollo MFG. In vitro mutagenicity assay (Ames test) and phytochemical characterization of seeds oil of Helianthus annuus Linné (sunflower) Toxicology Reports. 2016;3:733–739. doi: 10.1016/j.toxrep.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Figueiredo PLB, Pinto LC, da Costa JS, da Silva ARC, Mourao RHV, Montenegro RC, da Silva JKR, Maia JGS. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. Journal of Ethnopharmacology. 2019;232:30–38. doi: 10.1016/j.jep.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Gelbke HP, Kayser M, Poole A. OECD test strategies and methods for endocrine disruptors. Toxicology. 2004;205:17–25. doi: 10.1016/j.tox.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Gontijo DC, Nunes LG, Farias LM, Duarte MGR, Carvalho AF, Fietto LG, Leite JPV. Assessment of the phenolic content, mutagenicity and genotoxicity of ethanolic extracts of stem bark and leaves from Strychnos pseudoquina A. St.-hil. Drug and Chemical Toxicology. 2020;43:539–545. doi: 10.1080/01480545.2018.1515218. [DOI] [PubMed] [Google Scholar]
- Grignard E, Håkansson H, Munn S. Regulatory needs and activities to address the retinoid system in the context of endocrine disruption: the European viewpoint. Reproductive Toxicology. 2020;93:250–258. doi: 10.1016/j.reprotox.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XH, Ye YY, Guo BF, Liu S. Effects of platycodin D in combination with different active ingredients of Chinese herbs on proliferation and invasion of 4T1 and MDA-MB-231 breast cancer cell lines. Journal of Chinese Integrative Medicine. 2012;10:67–75. doi: 10.3736/jcim20120111. [DOI] [PubMed] [Google Scholar]
- Hashem S, Nisar S, Sageena G, Macha MA, Yadav SK, Krishnankutty R, Uddin S, Haris M, Bhat AA. Therapeutic effects of curcumol in several diseases; an overview. Nutrition and Cancer. 2021;73:181–195. doi: 10.1080/01635581.2020.1749676. [DOI] [PubMed] [Google Scholar]
- Hendriksen CF. Replacement, reduction and refinement alternatives to animal use in vaccine potency measurement. Expert Review of Vaccines. 2009;8:313–322. doi: 10.1586/14760584.8.3.313. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Lee YH, Hsu YH, Liao CT, Lin YF, Chiu HW. Current strategies in assessment of nanotoxicity: alternatives to in vivo animal testing. International Journal of Molecular Sciences. 2021;22:4216. doi: 10.3390/ijms22084216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung EB, Trinh TA, Lee TK, Yamabe N, Kang KS, Song JH, Choi S, Lee S, Jang TS, Kim KH, Hwang GS. Curcuzedoalide contributes to the cytotoxicity of Curcuma zedoaria rhizomes against human gastric cancer AGS cells through induction of apoptosis. Journal of Ethnopharmacology. 2018;213:48–55. doi: 10.1016/j.jep.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Karn A, Zhao C, Yang F, Cui J, Gao Z, Wang M, Wang F, Xiao H, Zheng J. In-vivo biotransformation of citrus functional components and their effects on health. Critical Reviews in Food Science and Nutrition. 2021;61:756–776. doi: 10.1080/10408398.2020.1746234. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang J, Tao Y, Li X, Qin J, Bai Z, Chi B, Yan W, Chen X. Curcumol inhibits colorectal cancer proliferation by targeting miR-21 and modulated PTEN/PI3K/Akt pathways. Life Sciences. 2019;221:354–361. doi: 10.1016/j.lfs.2019.02.049. [DOI] [PubMed] [Google Scholar]
- Lou Y, Zhang H, He H, Peng K, Kang N, Wei X, Li X, Chen L, Yao X, Qiu F. Isolation and identification of phase 1 metabolites of curcumol in rats. Drug Metabolism and Disposition. 2010;38:2014–2022. doi: 10.1124/dmd.110.034215. [DOI] [PubMed] [Google Scholar]
- Rashidi H, Alhaque S, Szkolnicka D, Flint O, Hay DC. Fluid shear stress modulation of hepatocyte-like cell function. Archives of Toxicology. 2016;90:1757–1761. doi: 10.1007/s00204-016-1689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathaur P, SR KJ. Metabolism and pharmacokinetics of phytochemicals in the human body. Current Drug Metabolism. 2019;20:1085–1102. doi: 10.2174/1389200221666200103090757. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Bai A, Kulkarni AA, Moghaddam MF. Efficiency in drug discovery: liver S9 fraction assay as a screen for metabolic stability. Drug Metabolism Letters. 2016;10:83–90. doi: 10.2174/1872312810666160223121836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W, Ding J, Liu L, Wang N, Lu B, You X, He Q, Zhou Q. Curcumol inhibits the development of prostate cancer by miR-125a/STAT3 axis. Evidence-Based Complementary and Alternative Medicine. 2022;2022:9317402. doi: 10.1155/2022/9317402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill GH, Khetani SR. Advances in engineered human liver platforms for drug metabolism studies. Drug Metabolism and Disposition. 2018;46:1626–1637. doi: 10.1124/dmd.118.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt-Lussenburg BMA, van der Lee RB, Man HY, Middelhof I, Brouwer A, Besselink H, van der Burg B. Incorporation of metabolic enzymes to improve predictivity of reporter gene assay results for estrogenic and anti-androgenic activity. Reproductive Toxicology. 2018;75:40–48. doi: 10.1016/j.reprotox.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Wei W, Rasul A, Sadiqa A, Sarfraz I, Hussain G, Nageen B, Liu X, Watanabe N, Selamoglu Z, Ali M, Li X, Li J. Curcumol: from plant roots to cancer roots. International Journal of Biological Sciences. 2019;15:1600–1609. doi: 10.7150/ijbs.34716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Liu H, Qin J, Huangfu M, Guan X, Li X, Zhou L, Dou T, Liu Y, Wang L, Fu M, Wang J, Chen X. Curcumol inhibits the viability and invasion of colorectal cancer cells via miR-30a-5p and Hippo signaling pathway. Oncology Letters. 2021;21:299. doi: 10.3892/ol.2021.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Pan T, Xiang Y, Zhang M, Xie H, Liang Z, Chen B, Xu C, Wang J, Huang X, Zhu Q, Zhao Z, Gao Q, Wen C, Liu W, Ma W, Feng J, Sun X, Duan T, Lai-Han Leung E, Xie T, Wu Q, Sui X. Curcumenol triggered ferroptosis in lung cancer cells via lncRNA H19/miR-19b-3p/FTH1 axis. Bioactive Materials. 2022;13:23–36. doi: 10.1016/j.bioactmat.2021.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Song M, Dong P, Qiu P, Guo S, Zhong Z, Li S, Ho CT, Xiao H. Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Molecular Nutrition and Food Research. 2013;57:1999–2007. doi: 10.1002/mnfr.201300211. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Kim JT, Qiu S, Lee SB, Park HJ, Soon MJ, Lee HJ. 1,3,5,8-Tetrahydroxyxanthone suppressed adipogenesis via activating Hedgehog signaling in 3T3-L1 adipocytes. Food Science and Biotechnology. 2022;31:1473–1480. doi: 10.1007/s10068-022-01130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]