Abstract
Early-life stress negatively alters mammalian brain programming. Environmental enrichment (EE) has beneficial effects on brain structure and function. This study aimed to evaluate the effects of postnatal environmental enrichment on long-term potentiation (LTP) induction in the hippocampal CA1 area of prenatally stressed female rats. The pregnant Wistar rats were housed in a standard animal room and exposed to traffic noise stress 2 hours/day during the third week of pregnancy. Their offspring either remained intact (ST) or received enrichment (SE) for a month starting from postnatal day 21. The control groups either remained intact (CO) or received enrichment (CE). Basic field excitatory post-synaptic potentials (fEPSPs) were recorded in the CA1 area; then, LTP was induced by high-frequency stimulation. Finally, the serum levels of corticosterone were measured. Our results showed that while the prenatal noise stress decreased the baseline responses of the ST rats when compared to the control rats (P < 0.001), the postnatal EE increased the fEPSPs of both the CE and SE animals when compared to the respective controls. Additionally, high-frequency stimulation (HFS) induced LTP in the fEPSPs of the CO rats (P < 0.001) and failed to induce LTP in the fEPSPs of the ST animals. The enriched condition caused increased potentiation of post-HFS responses in the controls (P < 0.001) and restored the disrupted synaptic plasticity of the CA1 area in the prenatally stressed rats. Likewise, the postnatal EE decreased the elevated serum corticosterone of prenatally stressed offspring (P < 0.001). In conclusion, the postnatal EE restored the stress induced impairment of synaptic plasticity in rats' female offspring.
Keywords: environmental enrichment, prenatal exposure, synaptic plasticity, rats
1. Introduction
There is evidence that the intrauterine/fetal environment has long-term aftereffects on infant development [1]. Childhood is one of the sensitive periods in an individual's development. Exposure to stress during this period has a “programmed” effect on the structure and function of the central nervous system [2]. Studies have shown the effects of prenatal stress (PS) on the neuronal and synaptic development of several brain regions such as the neocortex, amygdala, hippocampus, and hypothalamus [3].
Acute or chronic fetal stress exposure induces hypothalamic-pituitary-adrenal (HPA) axis dysfunction and increased glucocorticoid secretion in animals [4]. In addition, maternal stress hormones such as adrenal steroids, catecholamines, and CRH reach the fetal brain and alter fetal neuronal structure and function [5]. Additionally, prenatal stress through the contraction of the placental artery due to activation of the mother's sympathetic nervous system reduces the supply of essential nutrients and oxygen, thereby negatively affecting the health of the fetus [6]. As we know, the hippocampus is very vulnerable to stress and plays a crucial role in spatial learning and memory [7]. It has been reported that prenatal stress impairs the spatial learning and memory of rats in a Morris water maze [8]. Theoretically, two types of hippocampal synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD), have been accepted as key mechanisms of certain types of learning and memory formation [9]. A previous study reported that prenatal stress impaired LTP but facilitated LTD within the CA1 area of the young rats' hippocampus [10].
In addition to understanding the harmful effects of prenatal stress on cognition, there is now considerable interest in developing a novel strategy to ameliorate these deficits. One such approach is utilizing environmental enrichment (EE) [11]. EE is a non-invasive therapy that creates strong changes in neuronal structure and behavior [12]. Within the central nervous system, EE enhances neurogenesis [13], synaptic plasticity [14], glutamate level [15], as well as decreases gamma-aminobutyric acid (GABA) [16]. At the molecular level, studies have demonstrated that exposure to EE alters various plasticity-related molecules such as synaptophysin [17], CaMKII, CREB [18], as well as N-methyl-D-aspartate (NMDA) receptor subunits GluR1, NR2B, and NR2A [19]. Furthermore, several studies have demonstrated that EE can counteract cognitive deficits caused by early life stress [20]. Dandi and colleagues reported that EE improved the cognitive decline associated with maternal separation and further reduced corticosterone levels after acute stress exposure [21]. Results of another study demonstrated that exposing mice to EE significantly enhanced hippocampal LTP and cognitive function at the Schaffer's Collateral CA1 synapse [22]. Additionally, researchers indicated that short-term exposure to EE improves working memory, facilitated hippocampal synaptic plasticity, and completely reverses the effects of stress on anxiety behavior [23]. Furthermore, studies have shown that environmental enrichment improves hippocampal-based memory in 60–80 years old adults [24], as well as cognitive dysfunction in developing and aging anesthetics-exposed brains [25].
The aim of this study is to evaluate the effects of postnatal EE on the synaptic plasticity of the hippocampus of noise-stressed prenatal female rats.
2. Methods
2.1. Animals
Wistar rats provided by the Kashan University of Medical Sciences were housed in a controlled room: humidity (55–60%), temperature (22–24 °C), 12 hours light/dark cycle, and with water and food ad libitum throughout the experiment. Two mature, virgin, female rats were housed together in a cage with a sexually experienced male overnight. If a vaginal plug was observed the next morning, the female rat was considered pregnant and entered the study. Gestation day one was defined as the day the vaginal smear was positive. At gestational day 15, pregnant female Wistar rats were randomly assigned to control and stress groups. After weaning, at postnatal day 21 (P21), half of the prenatally stressed (ST, n = 10) and control offspring (CO, n = 10) were kept in standard cages and the other half were kept in enrichment conditions (SE or CE groups, n = 10 for each).
2.2. Prenatal stress protocol
Pregnant rats within the stress group were exposed to 95 dB broadband traffic noise, previously recorded by a recorder (Panasonic RQ-L11) in a high-traffic square, for 2 hours once a day (between 08:00–12:00 am) from the 15th day after mating until the delivery of the pups [26]. A speaker was placed on the upper left of a Plexiglas chamber (25 × 35 × 70 cm) at a distance of 30 cm from the rat cage. A software set the amplitude of the recorded noise at 95 dB (Sonar, Cakewalk, USA), and a sound level meter (Extech Instruments, MA; USA) was used to measure the noise level during the experiments.
2.3. Environmental enrichment conditioning
Female offspring at P21 were subjected to EE. Rats were kept in groups of six in EE cages (80 × 40 × 50 cm) with plastic pipes, a steel box, and a wooden ladder. The locations of objects were altered weekly to maintain novelty [27]. The enrichment lasted for one month.
2.4. In vivo Electrophysiology
As previously described [28], for electrophysiology recording, the rats were anesthetized with urethane (1.5 g/kg, IP) and fixed in a head holder within a stereotaxic apparatus. (Borj Sanat, Iran). To place the stimulating and recording electrodes in the brain, a drill bit was used to produce two small holes (1 mm diameter) in the skull. A stimulating electrode was initially positioned into the Schaffer's collaterals at the stereotaxic coordinates 4.2 mm posterior to the bregma, 3.4 mm lateral to the midline, and 3.5 mm below the dura surface. The coordinates used for recording electrode were 3.8 mm posterior to the bregma, 2.5 mm lateral to the midline, and 2.8 mm below the dura. The electrodes were prepared from a Teflon-coated stainless-steel wire (A-M Systems, 0.008-inch diameter, USA) exposed only at the tip (tip separation 0.10 mm). The proper location of the electrodes was determined using electrophysiological and stereotaxic indicators. Using a computer software (eProbe, ScienceBeam, Iran), the field excitatory postsynaptic potentials (fEPSPs) were recorded from the CA1 region of the hippocampus in response to stimulation (two sweeps/min at 30-sec intervals) of the ipsilateral to the Schaffer's collateral region. An input-output curve was drawn using a range of stimulus currents when the response was stable. Then, the stimulation intensity was obtained to elicit an fEPSPs amplitude of 60% of the maximum response. Baseline fEPSPs were recorded over a 30-minute period and averaged for comparison with post-tetanus responses. Then, LTP was induced by a 100 Hz high frequency stimulation (HFS) (10 bursts of 10 stimulations, stimulus duration 0.2 ms and interval between bursts of 10 s). After tetanus stimulation, recordings continued for at least 2 hours. Data were considered for the percentage change in amplitude of pre- and post-tetanus recordings.
2.5. Serum corticosterone concentration
After electrophysiological recording, blood was sampled from the jugular vein. The plasma was separated into microcentrifuge tubes and stored at −80 ºC until assayed. Plasma corticosterone concentrations were quantified by a radioimmunoassay (RIA) kit (Zellbio, GmbH).
2.6. Statistics
All results are shown as means ± SEM. Statistical analysis was performed by a two-way ANOVA followed by Tukey's test. All statistical analyses were completed using the SPSS 20 software, and P values <0.05 were considered statistically significant.
3. Results
3.1. Effects of prenatal sound stress and postnatal environment enrichment on synaptic plasticity of the Schaffer's collaterals - CA1 pathway
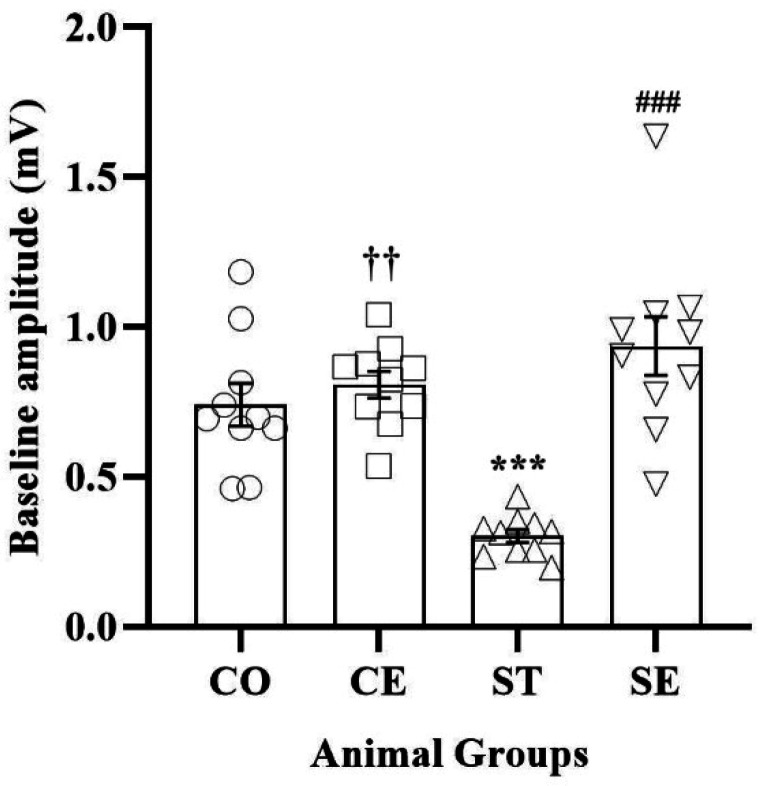
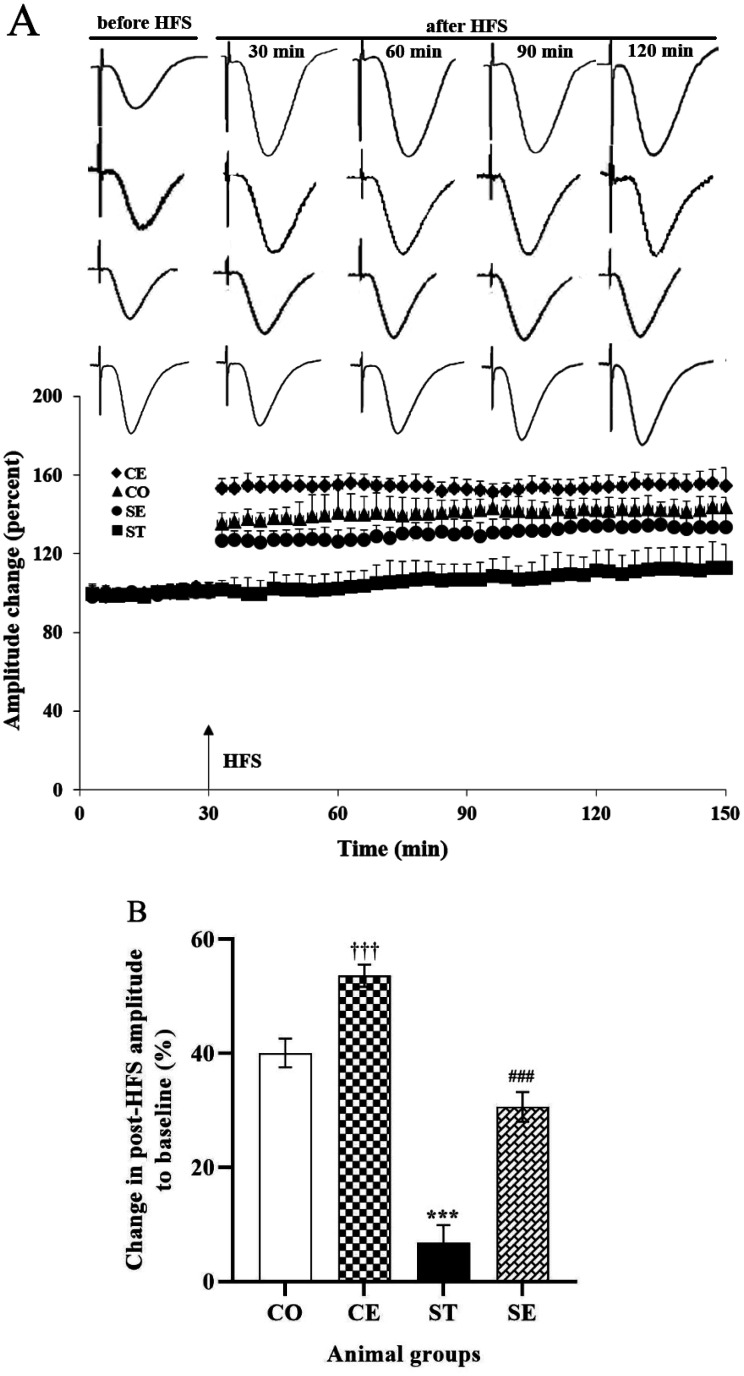
Statistical analyses revealed that the interaction of exposure to prenatal sound stress and postnatal environment enrichment changes the amplitude of fEPSPs of female offspring (F7,5992 = 19.425; P < 0.001). The mean amplitude size of the baseline response recorded in the CA1 neurons of the rats decreased from 0.74 ± 0.01 mV in the CO rats to 0.30 ± 0.004 mV in the ST rats (P < 0.001) (Figure 1). The EE strikingly reversed the effect of prenatal sound stress on the baseline activity of synapses, where the mean amplitude of the fEPSPs of the SE rats increased to more than three times the fEPSPs of the ST animals (P < 0.001). Additionally, a statistical difference was observed between the mean fEPSPs' amplitude of the CO animals as compared to the CE rats (P < 0.01). In the electrophysiological recordings, basal fEPSPs were evoked by stimulation of the Schaffer's collaterals within the CA1 area of the hippocampus, whereas HFS induced LTP of the excitatory synapses (Figure 2A). There was a statistical difference in the post-HFS potentiation between the CO and ST groups (P < 0.001). On the other hand, LTP induction increased the mean amplitude of the fEPSPs recorded from the CA1 region of CO animals (up to 40%); however, in the ST group, the mean amplitude of the fEPSPs only increased by 6% (Figure 2B). The environmental enrichment deeply affected LTP induction in the CA1 neurons of CE and SE animals, where tetanic stimulation of Schaffer's collaterals induced approximately 53% of LTP in the control group (P < 0.001). Moreover, the EE condition successfully reversed the impairment of the synaptic plasticity for the stressed group, and high-frequency stimulation of the CA3-CA1 pathway induced a significant LTP (~30%) in the fEPSPs recorded for the SE group (P < 0.001). In addition, a two-way ANOVA showed a significant difference between the amplitudes of post-tetanus fEPSPs of the CE and CO animals (P < 0.001).
Figure 1. The amplitude of the baseline fEPSPs recorded in the CA1 area of the hippocampus of rats' offspring. Whereas the prenatal noise stress decreased the baseline responses significantly (*** P < 0.001; CO group vs. ST group), the postnatal EE increased fEPSPs more than three times (### P < 0.001; SE group vs. ST group). Also, the postnatal EE increased the baseline responses of the CO animals (†† P < 0.01; CE group vs. CO group).
Figure 2. Pre- and post-HFS fEPSPs recorded in the CA1 area of the hippocampus of rats' offspring. While HFS induced LTP in the fEPSPs of the CO rats (*** P < 0.001) it was failed to induced LTP in the fEPSPs of the ST animals (A). The postnatal EE induced LTP about 53% in the controls (††† P < 0.001; CE group vs. CO group) and also by about 30% in the prenatally stressed rats (### P < 0.001; SE group vs. ST group) (B).
3.2. Effects of prenatal sound stress and postnatal environment enrichment on serum concentration of corticosterone
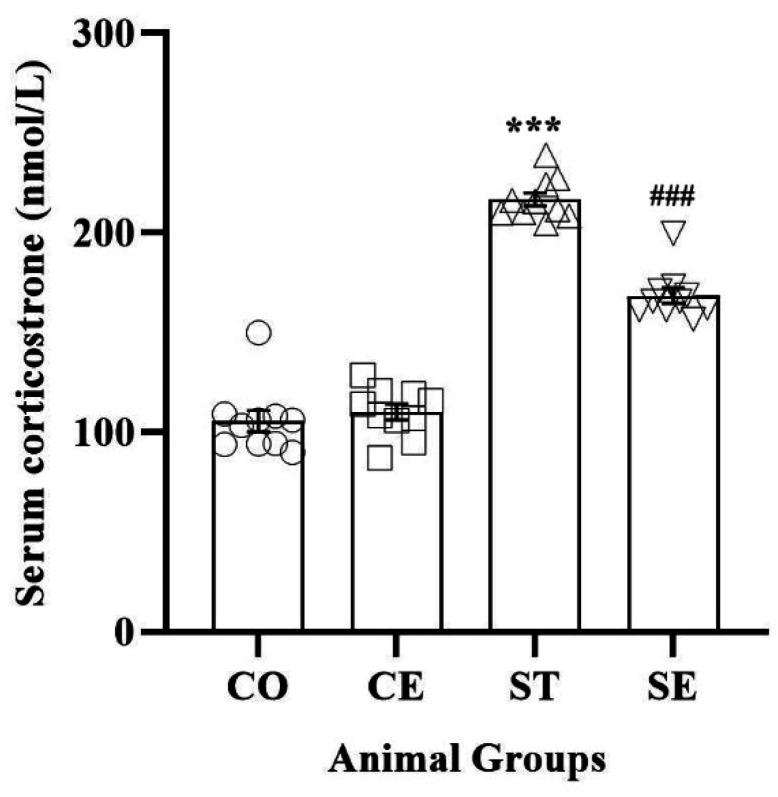
An analysis of variance indicated a substantial difference among the four groups (F3,36 = 159.229; P < 0.0001). As shown in Figure 3, two hours of prenatal sound stress significantly increased the serum corticosterone levels to 216.64 ± 3.31 (nmol/L), which was significantly different compared to the CO group (P < 0.001). The statistical analysis revealed that the variation observed between the CO animals (105.51 ± 5.41 nmol/L) and the CE animals (110.16 ± 3.93 nmol/L) was not significant. However, comparing the ST (216.64 ± 3.31 nmol/L) and SE (168.5 ± 3.79 nmol/L) groups showed that postnatal EE decreases the serum level of corticosterone (P < 0.001).
Figure 3. The serum level of corticosterone in the offspring. Although the prenatal noise stress significantly increased corticosterone in the serum of rats' offspring (*** P < 0.001; CO group vs. ST group), the postnatal EE decreased it (### P < 0.001; SE group vs. ST group).
4. Discussion
Exposure to stress during pregnancy has long-term effects on offspring because it is crucial for the brain circuitry to form. In agreement with animal data, the results of a study on children whose mothers received exogenous glucocorticoids, faced psychological stress or adverse events during pregnancy, show long-term neurodevelopmental effects [12]. The results of our study demonstrated that exposure to prenatal noise stress between days 14 to 21 of gestation increased the level of serum corticosterone and disrupted the hippocampus-dependent synaptic plasticity of young adult female rats. Furthermore, we found that post-weaning EE positively affects this ameliorated synaptic plasticity and potentiates post-HFS responses of the control rats. Similarly, it has been reported that exposure to PS has a negative effect on LTP induction but enhanced LTD in the hippocampal slices of young rat offspring [29]. Consistent with our results, Barzegar and colleagues reported that exposure to noise stress during pregnancy impaired basal synaptic activity and synaptic plasticity in hippocampal circuits of the rats' male offspring [26].
The exact mechanisms of prenatal stress that affects the synaptic plasticity of the hippocampal circuits in young adult offspring remains to be determined. Although numerous studies have focused on the effects of the HPA-axis and glucocorticoids on learning, memory, and synaptic plasticity, little is known about the downstream mechanism underlying the HPA-axis. In mammals, during embryogenesis, both genetic codes and environmental factors affect the CNS [30], and exposure to any stress chronically affects the programming and development of the offspring's nervous system [31]. Similar to our findings, in one study where rats were exposed to restrainer stress at three weeks of gestation, there was a 50& decrease in the density of the glucocorticoid receptors (GRs) in the hippocampi of their female offspring compared to those of the non-stressed offspring [32]. Mifsud et al. indicated that only exposure to 15 minutes of force swimming stress reduced the number of GRs in the hippocampus of rats [33]. Moreover, it has been reported that either giving glucocorticoid to the fetus [34] or maternal stress [35] leads to the downregulation of GRs in the hippocampus and the development of HPA reactivity; these might be reasons for the alteration in the stress-induced behavior of the offspring [36]. It is well known that prenatal stress can reduce neurogenesis, increase cell death, and enhance hippocampal neurons' oxidative stress in offspring rats [37]. Additionally, prenatal stress reduces the expression and maturation of brain derived neurotrophic factor (BDNF) in the offspring neurons, which is a necessary factor that supports the persistence of long-term memory storage [38]. Furthermore, alterations in GABAergic and glutamatergic systems have been observed in the rats prenatally exposed to stress. There is evidence that PS can reduce glutamate receptor-dependent neuronal synaptic plasticity, thereby leading to impaired learning and memory function [39]. We know that both ionotropic receptors of glutamate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and NMDA are key modulators of hippocampal synaptic plasticity. PS inhibits the expression levels of the NMDA receptor subunits NR2A and NR1 [40] and GluA1-3 subunits of AMPA receptors [41] in the animal's hippocampus. Moreover, PS changes the arrangement of the NMDA receptor subunits that affect function of the NMDA receptors [42]. Feng and colleagues demonstrated that the female offspring of mothers exposed to stress during gestation days 8–20 are more pronounced to PS-induced cognitive dysfunction because of PS-induced changes in the expression of NR2A and NR2B subunits are more prevalent than the male offspring [43]. Furthermore, Adrover et al. revealed that placing rats in a restrainer three times a day for 45 minutes/day during the third week of pregnancy changes glutamate transporters expression, as well as alters glutamate transmission in the offspring brain, which may cause cognitive dysfunction [44]. On one hand, about 10–15 percent of the hippocampal interneurons are GABAergic, and the GABAergic system play critical roles in synaptic plasticity and memory formation [45]. On the other hand, stress has an adverse effect on the GABAergic network structure and function in the hippocampus [46]. Veerawatananan and colleagues showed that maternal forced stress delayed the maturation of the GABAergic system, as well as altered the expression of the GABAA receptor α1 and α5 subunits in the hippocampus of rat pups [47]. Lussiera and Stevens's study revealed that PS reduced the GABAergic cell number and delayed the maturation in the mice hippocampus, which resulted in behavioral dysfunction [48]. Therefore, PS may have long-lasting effects on glutamate and GABA levels and the function of their receptors in the hippocampus, leading to impaired LTP and promotion of LTD in young adult offspring.
We showed that post-weaning EE positively affected both basic synaptic transmission and LTP induction in neural circuits within the CA1 region for both control and PS animals. In line with our results, it has been shown that EE enhances hippocampal LTP and improves learning and memory performance in rats [49]. Additionally, Yang et al. demonstrated that a one-month growth in EE significantly counteracts abnormal alterations in synaptic plasticity induced by PS [50]. It has been shown that EE induces certain hippocampal changes such as increased glial cell number, synaptic density, neurogenesis, and the dendrite branching [51]. For example, EE increases the expression of nerve growth factors including GDNF, NGF, and BDNF in the hippocampus [51]. EE enhances synaptic plasticity and improves various hippocampal-related learning abilities by activating both AMPA and NMDA receptors [52]. On the one hand, Hullinger et al. showed that EE improved learning, memory and hippocampal LTP by increasing mGluR5 activity [53]. On the other hand, Montes et al. indicated that housing in EE for four weeks ameliorated toluene-induced memory impairment in mice and reduced hippocampal GABA levels in these animals [16]. Begenisic and colleagues reported that amelioration of cognitive deficits and synaptic plasticity defects in a mouse model of Down syndrome exposed to EE was associated with decreased hippocampal GABA release [54]. Moreover, EE decreases serum levels of corticosterone and inhibits the anxiety-like behavior provoked immediately after one hour exposure to acute restraint stress [55]. Dandi et al. reported that EE protects against cognitive dysfunction caused by maternal separation and decreases corticosterone levels [56].
5. Conclusions
In conclusion, we showed that postnatal EE restored the stress induced impairment of synaptic plasticity within the CA1 area of rats' female offspring. It appears that the reduction of the stress hormone corticosterone and alterations in the function and structure of the hippocampus, especially the balance between excitatory and inhibitory transmission, are possible mechanisms through which EE favors synaptic plasticity.
Acknowledgments
This study was supported financially by Grant No. 9767, Deputy of Research and Technology, Kashan Medical Sciences University (KAUMS). We would like to thank A. Vatankhah for her technical support. Thanks also to KAUMS Animal Breeding Center for supplying the animals.
Abbreviations
- AMPA
α amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- BDNF
brain derived neurotrophic factor
- EE
environmental enrichment
- fEPSPs
field excitatory post-synaptic potentials
- GRs
glucocorticoid receptors
- GABA
gamma-Aminobutyric acid
- HPA
hypothalamic–pituitary–adrenal
- HFS
high-frequency stimulation
- LTD
long-term depression
- LTP
long-term potentiation
- NMDA
N-methyl-D-aspartate
- PS
prenatal stress
- RIA
radioimmunoassay
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Zijlmans MA, Riksen-Walraven JM, de Weerth C. Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neurosci Biobehav R. 2015;53:1–24. doi: 10.1016/j.neubiorev.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The foetal and infant origins of inequalities in health in Britain. J Public Health. 1991;13(2):64–68. [PubMed] [Google Scholar]
- 3.Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse. 2012;66(4):308–314. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Shang Y, Xiao X, et al. Prenatal stress-induced impairments of cognitive flexibility and bidirectional synaptic plasticity are possibly associated with autophagy in adolescent male-offspring. Exp Neurol. 2017;298:68–78. doi: 10.1016/j.expneurol.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav R. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Myers RE. Maternal psychological stress and fetal asphyxia: a study in the monkey. Am J Obstet Gynecol. 1975;122(1):47–59. doi: 10.1016/0002-9378(75)90614-6. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro ML, Riceberg JS, Seip-Cammack K, et al. Functional interactions of prefrontal cortex and the hippocampus in learning and memory, in Space, Time and Memory in the Hippocampal Formation. Springer. 2014:517–560. doi: 10.1007/978-3-7091-1292-2_19. [DOI] [Google Scholar]
- 8.de los Angeles GAM, del Carmen ROM, Wendy PM, et al. Tactile stimulation effects on hippocampal neurogenesis and spatial learning and memory in prenatally stressed rats. Brain Res Bull. 2016;124:1–11. doi: 10.1016/j.brainresbull.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Andersen N, Krauth N, Nabavi S. Hebbian plasticity in vivo: relevance and induction. Curr Opin Neurobiol. 2017;45:188–192. doi: 10.1016/j.conb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Yeh CM, Huang CC, Hsu KS. Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. J Physiol. 2012;590(4):991–1010. doi: 10.1113/jphysiol.2011.222042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girbovan C, Plamondon H. Environmental enrichment in female rodents: considerations in the effects on behavior and biochemical markers. Behav Brain Res. 2013;253:178–190. doi: 10.1016/j.bbr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 12.McCreary JK, Metz GA. Environmental enrichment as an intervention for adverse health outcomes of prenatal stress. Environ Epigenetics. 2016;2(3) doi: 10.1093/eep/dvw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veena J, Srikumar BN, Mahati K, et al. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009;87(4):831–843. doi: 10.1002/jnr.21907. [DOI] [PubMed] [Google Scholar]
- 14.Artola A, Von Frijtag JC, Fermont PCJ, et al. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23(1):261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 15.Segovia G, Del Arco A, De Blas M, et al. Environmental enrichment increases the in vivo extracellular concentration of dopamine in the nucleus accumbens: a microdialysis study. J Neural Transm. 2010;117(10):1123–1130. doi: 10.1007/s00702-010-0447-y. [DOI] [PubMed] [Google Scholar]
- 16.Montes S, del Carmen Solís-Guillén R, García-Jácome D, et al. Environmental enrichment reverses memory impairment induced by toluene in mice. Neurotoxicol Teratol. 2017;61:7–16. doi: 10.1016/j.ntt.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Nithianantharajah J, Levis H, Murphy M. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol Learn Mem. 2004;81(3):200–210. doi: 10.1016/j.nlm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Huang FL, Huang K-P, Wu J, et al. Environmental enrichment enhances neurogranin expression and hippocampal learning and memory but fails to rescue the impairments of neurogranin null mutant mice. J Neurosci. 2006;26(23):6230–6237. doi: 10.1523/JNEUROSCI.1182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y-P, Wang H, Feng R, et al. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41(6):779–790. doi: 10.1016/S0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- 20.Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Dev Brain Res. 2004;150(2):103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Dandi E, Kalamari A, Touloumi O, et al. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. Int J Dev Neurosci. 2018;67:19–32. doi: 10.1016/j.ijdevneu.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Benito E, Kerimoglu C, Ramachandran B, et al. RNA-dependent intergenerational inheritance of enhanced synaptic plasticity after environmental enrichment. Cell Rep. 2018;23(2):546–554. doi: 10.1016/j.celrep.2018.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhagya VR, Srikumar BN, Veena J, et al. Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J Neurosci Res. 2017;95(8):1602–1610. doi: 10.1002/jnr.23992. [DOI] [PubMed] [Google Scholar]
- 24.Clemenson GD, Stark SM, Rutledge SM, et al. Enriching hippocampal memory function in older adults through video games. Behav Brain Res. 2020;390:112667. doi: 10.1016/j.bbr.2020.112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang X, Tian Y. Environmental enrichment holds promise as a novel treatment for anesthesia-induced neurocognitive disorders. Neurotoxicol Teratol. 2022;94:107133. doi: 10.1016/j.ntt.2022.107133. [DOI] [PubMed] [Google Scholar]
- 26.Barzegar M, Sajjadi FS, Talaei SA, et al. Prenatal exposure to noise stress: anxiety, impaired spatial memory, and deteriorated hippocampal plasticity in postnatal life. Hippocampus. 2015;25(2):187–196. doi: 10.1002/hipo.22363. [DOI] [PubMed] [Google Scholar]
- 27.Hullinger R, O'Riordan K, Burger C. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem. 2015;125:126–134. doi: 10.1016/j.nlm.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talaei S, Azami A, Salami M. Postnatal development and sensory experience synergistically underlie the excitatory/inhibitory features of hippocampal neural circuits: glutamatergic and GABAergic neurotransmission. Neuroscience. 2016;318:230–243. doi: 10.1016/j.neuroscience.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Han H, Cao J, et al. Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus. 2006;16(5):431–436. doi: 10.1002/hipo.20181. [DOI] [PubMed] [Google Scholar]
- 30.Alyamani RAS, Murgatroyd C. Epigenetic programming by early-life stress. Prog Mol Biol Transl. 2018;157:133–150. doi: 10.1016/bs.pmbts.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Jafari Z, Mehla J, Kolb BE, et al. Prenatal noise stress impairs HPA axis and cognitive performance in mice. Sci Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-09799-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szuran TF, Pliška V, Pokorny J, et al. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71(3–4):353–362. doi: 10.1016/S0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- 33.Mifsud KR, Saunderson EA, Spiers H, et al. Rapid down-regulation of glucocorticoid receptor gene expression in the dentate gyrus after acute stress in vivo: role of DNA methylation and microRNA activity. Neuroendocrinology. 2017;104(2):157–169. doi: 10.1159/000445875. [DOI] [PubMed] [Google Scholar]
- 34.Herman JP, Spencer R. Regulation of hippocampal glucocorticoid receptor gene transcription and protein expression in vivo. J Neurosci. 1998;18(18):7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry C, Kabbaj M, Simon H, et al. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6(3):341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 36.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59(3):279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Mandyam CD, Crawford EF, Eisch AJ, et al. Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol. 2008;68(5):575–589. doi: 10.1002/dneu.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badihian N, Daniali SS, Kelishadi R. Transcriptional and epigenetic changes of brain derived neurotrophic factor following prenatal stress: A systematic review of animal studies. Neurosci Biobehav Rev. 2019;117:211–231. doi: 10.1016/j.neubiorev.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Tavassoli E, Saboory E, Teshfam M, et al. Effect of prenatal stress on density of NMDA receptors in rat brain. Int J Dev Neurosci. 2013;31(8):790–795. doi: 10.1016/j.ijdevneu.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Sun H, Guan L, Zhu Z, et al. Reduced levels of NR1 and NR2A with depression-like behavior in different brain regions in prenatally stressed juvenile offspring. PLOS ONE. 2013;8(11):e81775. doi: 10.1371/journal.pone.0081775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y, Zhang J, Zhang L, et al. Hippocampal Acetylation may Improve Prenatal-Stress-Induced Depression-Like Behavior of Male Offspring Rats Through Regulating AMPARs Expression. Neurochem Res. 2017;42(12):3456–3464. doi: 10.1007/s11064-017-2393-7. [DOI] [PubMed] [Google Scholar]
- 42.Hu L, Han B, Zhao X, et al. Chronic early postnatal scream sound stress induces learning deficits and NMDA receptor changes in the hippocampus of adult mice. Neuroreport. 2016;27(6):397–403. doi: 10.1097/WNR.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 43.Fang Y, Li H, Chang L, et al. Prenatal stress induced gender-specific alterations of N-methyl-d-aspartate receptor subunit expression and response to Abeta in offspring hippocampal cells. Behav Brain Res. 2018;336:182–190. doi: 10.1016/j.bbr.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 44.Adrover E, Pallarés ME, Baier CJ, et al. Glutamate neurotransmission is affected in prenatally stressed offspring. Neurochem Int. 2015;88:73–87. doi: 10.1016/j.neuint.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Lau PY-P, Katona L, Saghy P, et al. Long-term plasticity in identified hippocampal GABAergic interneurons in the CA1 area in vivo. Brain Struct Funct. 2017;222(4):1809–1827. doi: 10.1007/s00429-016-1309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu W, Zhang M, Czéh B, et al. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35(8):1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veerawatananan B, Surakul P, Chutabhakdikul N. Maternal restraint stress delays maturation of cation-chloride cotransporters and GABAA receptor subunits in the hippocampus of rat pups at puberty. Neurobiol Stress. 2016;3:1–7. doi: 10.1016/j.ynstr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lussier SJ, Stevens HE. Delays in GABAergic interneuron development and behavioral inhibition after prenatal stress. Dev Neurobiol. 2016;76(10):1078–1091. doi: 10.1002/dneu.22376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang AC, Zou B. Neonatal exposure to novelty enhances long-term potentiation in CA1 of the rat hippocampus. Hippocampus. 2002;12(3):398–404. doi: 10.1002/hipo.10017. [DOI] [PubMed] [Google Scholar]
- 50.Yang J, Hou C, Ma N, et al. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiol Learn Mem. 2007;87(2):257–263. doi: 10.1016/j.nlm.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nat Rev Neurosci. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 52.Lee EH, Hsu WL, Ma YL, et al. Enrichment enhances the expression of sgk, a glucocorticoid-induced gene, and facilitates spatial learning through glutamate AMPA receptor mediation. Eur J Neurosci. 2003;18(10):2842–2852. doi: 10.1111/j.1460-9568.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- 53.Hullinger R, O'Riordan K, Burger C. Environmental enrichment improves learning and memory and long-term potentiation in young adult rats through a mechanism requiring mGluR5 signaling and sustained activation of p70s6k. Neurobiol Learn Mem. 2015;125:126–134. doi: 10.1016/j.nlm.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Begenisic T, Spolidoro M, Braschi C, et al. Environmental enrichment decreases GABAergic inhibition and improves cognitive abilities, synaptic plasticity, and visual functions in a mouse model of Down syndrome. Front Cell Neurosci. 2011;5:29. doi: 10.3389/fncel.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novaes LS, dos Santos NB, Batalhote RFP, et al. Environmental enrichment protects against stress-induced anxiety: Role of glucocorticoid receptor, ERK, and CREB signaling in the basolateral amygdala. Neuropharmacology. 2017;113:457–466. doi: 10.1016/j.neuropharm.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Dandi E, Kalamari A, Touloumi O, et al. Beneficial effects of environmental enrichment on behavior, stress reactivity and synaptophysin/BDNF expression in hippocampus following early life stress. Int J Dev Neurosci. 2018;67:19–32. doi: 10.1016/j.ijdevneu.2018.03.003. [DOI] [PubMed] [Google Scholar]