Abstract
Purpose
The aim of this study was to explore the relationship between non-alcoholic fatty liver disease (NAFLD) and the two blood inflammatory markers including the systemic immune-inflammation (SII) index, and the system inflammation response index (SIRI).
Methods
The National Health and Nutrition Examination Survey data between the year of 2017–2018 was used for this cross-sectional study. In order to analyze the association of SII index, and SIRI and risk of NAFLD, we used multivariable logistic regression models, restricted cubic spline (RCS) plot, and subgroup analysis to analyze the data.
Results
In total, there were 1,199 individuals who participated in the survey. As shown by the RCS plot, SII index, and SIRI were linked with NAFLD risk in a U-shaped pattern. With regard to known confounding variables, when comparing the lowest quartile, the odds ratio with 95 % confidence interval for prevalence of NAFLD across the quartiles of SII index and SIRI were (0.89 (0.57, 1.41), 0.56 (0.35, 0.89) and 1.01 (0.64, 1.59)), and (0.77 (0.48, 1.23), 0.79 (0.50, 1.24) and 0.94 (0.60, 1.47)), respectively. Additionally, SII index, and SIRI and NAFLD risk also were U-curve correlated among the participants in age ≥60 years, female, without hypertension, and BMI of ≥30 kg/m2.
Conclusions
There was a U-shaped association of SII index and SIRI with prevalence of NAFLD, indicating that SII index and SIRI should be monitored dynamically.
Keywords: Cross-sectional study, Systemic immune-inflammation index, Non-alcoholic fatty liver disease, System inflammation response index, United States
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) still occupies an important place in adult chronic liver diseases, with a global prevalence estimated at 32.4 % and rising (Riazi et al., 2022). NAFLD refers to liver fat deposition and hepatocyte steatosis caused by metabolic disorders in addition to alcohol, immunity, infection and other factors, and thus develops into liver inflammation, fibrosis, cirrhosis and even liver cancer (Zafrani, 2004). Although its mechanism has not been clearly explained, it is associated with lipid metabolism disorder, insulin resistance decline, type 2 diabetes and so on (Yan et al., 2022).
The blood inflammatory index is an inexpensive and easily accessible biomarker. As an indicator of both local immune response and systemic inflammation, the SII index, integrating three of inflammatory cells (lymphocyte, neutrophil, and platelet), is considered to be a good and stable index (Hu et al., 2014, Tong et al., 2017). Several studies have shown that SII index can predict the outcome of patients with multiple cancers, acute ischemic stroke, heart failure, and acute kidney injuries (Jiang et al., 2022, Tang et al., 2021, Xie et al., 2021, Yang et al., 2018, Zhou et al., 2022). Additionally, SIRI, which is composed of lymphocyte, monocyte, and neutrophil counts, is a more comprehensive indicator of chronic low-grade inflammation (Jia et al., 2019). The previous studies suggested that SIRI has been widely recognized as the potential indicators for early diagnosis and prognosis monitoring in stroke, inflammatory diseases and cancers (Li et al., 2017, Ma et al., 2022, Qi et al., 2016, Zhang et al., 2021). The consideration of the harmful effects of NAFLD, the identification of risk factors, and the taking of measures to prevent or control the consequences, as soon as possible, is very helpful in reducing or preventing the consequences of NAFLD. There has been no evidence that the SII index and SIRI are associated with NAFLD risk in the general American population based on the epidemiological research to date. By analysing the data from the Nutrition and Health Examination Survey (NHANES) between the year of 2017–2018, we tried to examine the association of the SII index and SIRI with the prevalence of NAFLD.
2. Methods
2.1. Study population
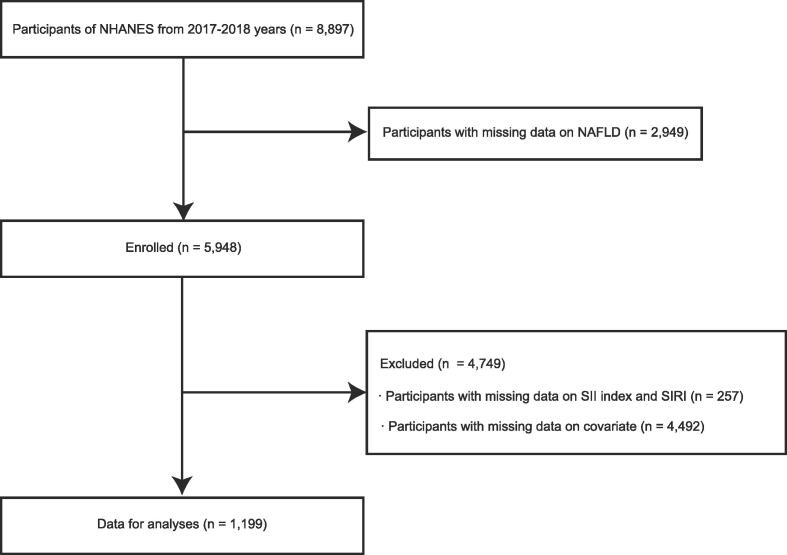
For the purpose of collecting information about the general population's health and nutrition, the National Health and Nutrition Examination Survey (NHANES) database (https://www.cdc.gov/nchs/nhanes/) uses a multistage stratified random sampling method (Xiao et al., 2023a). For the purpose of this study, we analysed the data from the NHANES data from the 2017 and 2018 years. In total, 8,897 participants in total sample were included in the study, however we excluded 2,949 participants without NAFLD data, and 257 participants without SII index and SIRI data. Further, participants who did not have data on missing covariate data (n = 4,492) were also excluded from the analysis. Finally, the research included 1,199 individuals (Fig. 1). Every participant was required to provide informed consent prior to participating in the study. In addition, the National Center for Health Statistics obtained institutional review board approval prior to data collection (Zipf et al., 2013).
Fig. 1.
Study flow chart. Abbreviations: NAFLD, non-alcoholic fatty liver disease; SII index, systemic immune-inflammation index; NHANES, National Health and Nutrition Examination Surveys; SIRI, system inflammation response index;
2.2. Covariates
In the study, the covariates were as follows: age, education level (less than high school, more than high school, and high school), sex (male and female), family poverty income ratio (PIR), marital status (no partner, having a partner and unmarried), smoker (no, former, now), race/ethnicity (Other Race, Mexican American, Non-Hispanic Black, Other Hispanic and Non-Hispanic White), the complication of angina pectoris, hypertension, coronary heart disease (CHD), diabetes mellitus (DM), heart attack, congestive heart failure (CHF), and stroke, drinker (never, mild, moderate, heavy), body mass index (BMI), fast glucose (FBG), waist circumference, hemoglobin (Hb), alanine aminotransferase (ALT), high-sensitivity C-reactive protein (hs CRP), aspartate amino transferase (AST), blood urea nitrogen (BUN), gamma-glutamyl transpeptidase (GGT), uric acid (UA), high-density lipoprotein-cholesterol (HDL-C), estimated glomerular filtration rate (eGFR), total cholesterol (TC), serum creatinine (Scr), and triglyceride (TG). A detailed explanation of the variables involved in this study can be found at the following link: www.cdc.gov/nchs/nhanes/.
2.3. Calculation of the SII index
In the study, the blood samples were collected from the participants who were fasted in the morning. The professional technicians used the automated hematology analyzing devices (Coulter® DxH 800 analyzer) to measure blood count (monocyte, lymphocyte (Lym), platelet counts and neutrophil (Neu)). We calculated SII index and SIRI according to the following formula for each participant:
SIRI (×109/L) = neutrophil count (×109/L) × monocyte(×109/L)/lymphocyte count (×109/L) (Qi et al., 2016).
SII index (×109/L) = neutrophil count (×109/L)/lymphocyte count (×109/L) × platelet count (×109/L) (Hu et al., 2014, Xiao et al., 2023b).
2.4. NAFLD measurement
NAFLD was defined using the U.S. fatty liver index (FLI), a well-validated diagnostic index (Ruhl and Everhart, 2015), which was employed utilizing NHANES III data and calculated as an equation according to the previous study (Bedogni et al., 2006, Kobyliak et al., 2018) that included information on GGT, BMI, TG, and waist circumference. All the information was collected concurrently with the status of iron metabolism. NAFLD was defined as an FLI score of ≥60. The FLI formula is expressed as follows:
FLI = (e0.953∗ln(TG)+0.139∗BMI+0.718∗ln (GGT)+0.053∗waist circumference−15.745)/(1 + e0.953∗ln (TG)+0.139∗BMI+0.718∗ln (GGT)+0.053∗waist circumference−15.745) ∗ 100 (Zhang et al., 2022).
2.5. Statistical analysis
In this study, we conducted all analyses by using the SPSS version 23.0 (SPSS Inc., Chicago, IL, USA) and R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria). The estimates were based on the weighted NHANES sample. The statistical significance of the study was determined by P-value < 0.05. The two systemic inflammatory biomarkers were all divided into quartiles, including SII index (Q1, 50.000–291.652; Q2, 292.653–423.059; Q3, 423.060–603.132; Q4, 603.133–3250.715;) and SIRI (Q1, 0.120–0.632; Q2, 0.633–0.924; Q3, 0.925–1.371; Q4, 1.372–10.890;), and the lowest quartile (Q1) of SII and SIRI served as the reference group (Q1). Variables that are continuous are expressed as means (standard deviations, SDs), while variables that are categorical are expressed as percentages (%). We used weighted Student’s t-test for continuous variables and weighted chi-square tests for categorical variables to calculate differences between groups. The association of SIRI, and SII index with prevalence of NAFLD were investigated using weighted multivariate logistic regression analysis. Firstly, model 1 was adjusted for sex, race/ethnicity, and age. Secondly, model 2 was adjusted for model 1 variables plus the complication of hypertension, smoke status, marital status, drink status, education level, the complication of DM, family PIR, and BMI. Finally, model 3 was adjusted for model 2 variables plus the complication of CHF, CHD, stroke, angina pectoris, and heart attack, ALT, TG, hs CRP, GGT, mean energy intake, UA, BUN, AST, waist circumference, FBG, Scr, Hb, eGFR, TC, and HDL-C.
3. Results
3.1. Baseline characteristics
Table 1 provides an overview of the laboratory examinations and basic clinical characteristics. There was a prevalence of 28.0 % of NAFLD in this study. We computed that the number of participants in this study is likely representative of the total U.S. population of 64,397,408. There was a statistically significant difference in age, sex Marital status, hypertension, DM, alcohol user, CHF, angina pectoris, BMI, Neu, mean energy intake, GGT, Lym, waist circumference, Ast, platelet, Hb, Alt, hs CRP, and eGFR between non-NAFLD and NAFLD.
Table 1.
Demographic characteristics of the study adults in the United States from NHANES 2017–2018 (n = 1,199).
| Variables | Overall (n = 1,199) | Non-NAFLD (n = 863) | NAFLD (n = 336) | P-value |
|---|---|---|---|---|
| Age, years | 45.87±0.85 | 44.02±0.87 | 51.24±1.60 | 0.001 |
| Sex, n (%) | 0.002 | |||
| Male | 585 (48.8) | 467 (38.9) | 118 (9.8) | |
| Female | 614 (51.2) | 396 (33.0) | 218 (18.2) | |
| Race, n (%) | 0.533 | |||
| Mexican American | 165 (13.8) | 110 (9.2) | 55 (4.6) | |
| Other Hispanic | 108 (9.0) | 82 (6.8) | 26 (2.2) | |
| Non-Hispanic Black | 288 (24.0) | 209 (17.4) | 79 (6.6) | |
| Non-Hispanic White | 430 (35.9) | 302 (25.2) | 128 (10.7) | |
| Other race | 208 (17.3) | 160 (13.3) | 48 (4.0) | |
| Family PIR | 3.22±0.09 | 3.25±0.08 | 3.14±0.18 | 0.521 |
| Education level, n (%) | 0.414 | |||
| Less than high school | 172 (14.3) | 118 (9.8) | 54 (4.5) | |
| High school | 276 (23.0) | 188 (15.7) | 88 (7.3) | |
| More than high school | 751 (62.6) | 557 (46.5) | 194 (16.2) | |
| Marital status, n (%) | 0.009 | |||
| Having a partner | 724 (60.4) | 502 (41.9) | 222 (18.5) | |
| No partner | 257 (21.4) | 181 (15.1) | 76 (6.3) | |
| Unmarried | 218 (18.2) | 180 (15.0) | 38 (3.2) | |
| Hypertension, n (%) | 0.002 | |||
| No | 709 (59.1) | 537 (44.8) | 172 (14.3) | |
| Yes | 490 (40.9) | 326 (27.2) | 164 (13.7) | |
| DM, n (%) | 0.007 | |||
| No | 966 (80.6) | 716 (59.7) | 250 (20.9) | |
| Yes | 233 (19.4) | 147 (12.3) | 86 (7.2) | |
| Smoker, n (%) | 0.561 | |||
| No | 705 (58.8) | 495 (41.3) | 210 (17.5) | |
| Former | 270 (22.5) | 195 (16.3) | 75 (6.3) | |
| Now | 224 (18.7) | 173 (14.4) | 51 (4.3) | |
| Alcohol user, n (%) | < 0.001 | |||
| No | 117 (9.9) | 51 (4.3) | 66 (5.5) | |
| Mild | 560 (46.7) | 425 (35.4) | 135 (11.2) | |
| Moderate | 259 (21.6) | 185 (15.4) | 74 (6.2) | |
| Heavy | 263 (21.9) | 202 (16.8) | 61 (5.1) | |
| CHD, n (%) | 0.131 | |||
| No | 1160 (96.7) | 842 (70.2) | 318 (26.5) | |
| Yes | 39 (3.3) | 21 (1.8) | 18 (1.5) | |
| CHF, n (%) | 0.013 | |||
| No | 1181 (98.5) | 850 (70.9) | 331 (27.6) | |
| Yes | 18 (1.5) | 13 (1.1) | 5 (0.4) | |
| Angina pectoris, n (%) | 0.048 | |||
| No | 1177 (98.2) | 850 (70.9) | 327 (27.3) | |
| Yes | 22 (1.8) | 13 (1.1) | 9 (0.8) | |
| Heart attack, n (%) | 0.050 | |||
| No | 1159 (96.7) | 836 (69.7) | 323 (26.9) | |
| Yes | 40 (3.3) | 27 (2.3) | 13 (1.1) | |
| Stroke, n (%) | 0.611 | |||
| No | 1162 (96.9) | 839 (70.0) | 323 (26.9) | |
| Yes | 37 (3.1) | 24 (2.0) | 13 (1.1) | |
| BMI, kg/m2 | 29.24±0.37 | 28.72±0.36 | 30.73±0.77 | 0.019 |
| Waist circumference, cm | 99.56±0.82 | 98.10±0.81 | 103.78±1.69 | 0.005 |
| Mean energy | 2141.06±30.69 | 2164.97±36.09 | 2071.52±30.60 | 0.022 |
| Intake (kcal/day) | ||||
| Neu, 1000 cells/uL | 3.85±0.07 | 3.78±0.08 | 4.04±0.11 | 0.038 |
| Lym, 1000 cells/uL | 2.06±0.03 | 2.03±0.04 | 2.15±0.04 | 0.044 |
| Monocyte, 1000 cells/uL | 0.54±0.01 | 0.54±0.01 | 0.54±0.02 | 0.888 |
| Platelet, 10^9/L | 238.03±3.28 | 234.17±3.52 | 249.24±5.27 | 0.012 |
| Hb, g/dL | 14.44±0.08 | 14.58±0.09 | 14.03±0.12 | 0.001 |
| FBG, mg/dL | 108.89±1.43 | 107.27±1.00 | 113.61±3.56 | 0.078 |
| Hs CRP, mg/L | 3.37±0.23 | 2.83±0.16 | 4.95±0.76 | 0.018 |
| Alt, U/L | 23.88±0.79 | 26.82±1.09 | 15.33±0.52 | < 0.001 |
| Ast, U/L | 22.76±0.63 | 24.69±0.84 | 17.14±0.33 | < 0.001 |
| GGT, iu/L | 29.25±1.18 | 31.52±1.63 | 22.64±0.95 | < 0.001 |
| TC, mg/dL | 187.43±2.19 | 186.53±2.39 | 190.07±3.19 | 0.307 |
| TG, mg/dL | 108.94±3.39 | 108.20±4.42 | 111.10±4.08 | 0.649 |
| HDL, mg/dL | 54.78±0.79 | 54.86±0.84 | 54.55±1.13 | 0.780 |
| BUN, mg/dL | 14.49±0.20 | 14.34±0.17 | 14.93±0.41 | 0.137 |
| UA, mg/dL | 5.41±0.06 | 5.40±0.08 | 5.46±0.08 | 0.596 |
| Scr, mg/dL | 0.86±0.01 | 0.87±0.01 | 0.83±0.01 | 0.111 |
| eGFR, ml/min/1.73 m2 | 96.93±1.11 | 98.20±1.25 | 93.26±1.31 | 0.007 |
| SII index | 475.65±11.45 | 463.97±12.28 | 509.63±18.88 | 0.033 |
| SIRI | 1.11±0.03 | 1.10±0.03 | 1.13±0.05 | 0.619 |
Abbreviations: DM, diabetes mellitus; CHD, coronary heart disease; CHF, congestive heart failure; BMI, body mass index; Hb, hemoglobin; FBG, fast glucose; Hs CRP, hypersensitive C-reactive protein; Alt, alanine aminotransferase; Ast, aspartate amino transferase; GGT, gamma glutamyl transferase; Alk, alkaline phosphatase; TC, total cholesterol; TG, triglycerides; HDL-cholesterol, high density lipoprotein-cholesterol; BUN, blood urea nitrogen; UA, uric acid; Scr, serum creatinine; eGFR, estimated glomerular filtration rate; SII index, systemic immune inflammation index; SIRI, system inflammation response index.
3.2. Association of SII index and SIRI with NAFLD
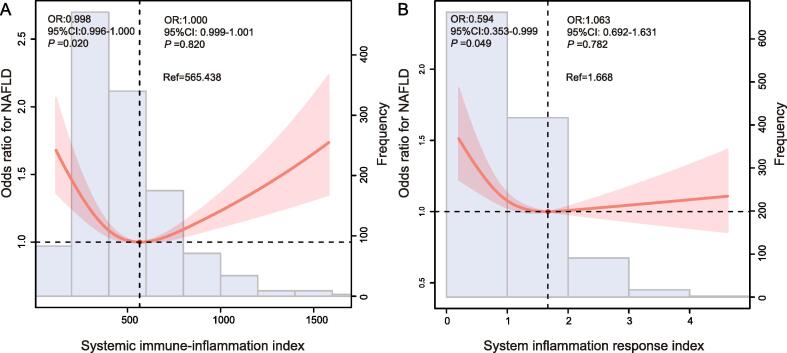
Based on the restricted cubic spline plot (RCS), we can notice that there is a U-shaped association between SII index and SIRI coexisting with NAFLD prevalence (P for nonlinearity < 0.05, Fig. 2A and B). With the increase of SII index and SIRI, NAFLD risk was significantly reduced. The prevalence of NAFLD was lowest when the SII index and SIRI reached 565.438 and 1.668, respectively, and then the curve showed an upward trend. The association of SII index and SIRI with prevalence of NAFLD was investigated employing three multivariate logistic regression models (Models 1, 2, and 3) (Table 2, Table 3). With regard to known confounding variables, when comparing the lowest quartile, the odds ratio with 95 % confidence interval for prevalence of NAFLD across the quartiles of SII index and SIRI were (0.89 (0.57, 1.41), 0.56 (0.35, 0.89) and 1.01 (0.64, 1.59)), and (0.77 (0.48, 1.23), 0.79 (0.50, 1.24) and 0.94 (0.60, 1.47)), respectively.
Fig. 2.
The restricted cubic spline curve for the association of (a) SII index and (b) SIRI with the prevalence of NAFLD in adults in the United States from NHANES 2017–2018. Abbreviations: OR, odd ratio; CI, confidence interval; SII index, systemic immune-inflammation index; NAFLD, non-alcoholic fatty liver disease; SIRI, system inflammation response index.
Table 2.
The adjusted ORs for the association of SII index with the prevalence of NAFLD in adults in the United States from NHANES 2017–2018.
| SII index | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR (95 %CI) | OR (95 %CI) | OR (95 %CI) | |
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 0.98 (0.67, 1.44) | 0.97 (0.71, 1.61) | 0.89 (0.57, 1.41) |
| Q3 | 0.85 (0.58, 1.26) | 0.75 (0.50, 1.14) | 0.56 (0.35, 0.89) * |
| Q4 | 1.74 (1.21, 2.51) ** | 1.58 (1.07, 2.34) * | 1.01 (0.64, 1.59) |
| P for trend | 0.006 | 0.071 | 0.668 |
Abbreviations: SII index, systemic immune inflammation index; NAFLD, non-alcoholic fatty liver disease; Q1, 50.000–291.652; Q2, 292.653–423.059; Q3, 423.060–603.132; Q4, 603.133–3250.715; *P < 0.05; **P < 0.01; OR, odd ratio; CI, confidence interval. Model 1: age, sex and race/ethnicity. Model 2: model 1 variables plus education level, marital status, family poverty income ratio, the complication of hypertension, and diabetes mellitus, smoke status, drink status, and body mass index. Model 3 was adjusted for model 2 variables plus the complication of coronary heart disease, congestive heart failure, angina pectoris, heart attack, and stroke, mean energy intake, waist circumference, fast glucose, hemoglobin, high-sensitivity C-reactive protein, alanine aminotransferase, aspartate amino transferase, gamma-glutamyl transpeptidase, high-density lipoprotein-cholesterol, total cholesterol, triglyceride, blood urea nitrogen, uric acid, serum creatinine, and estimated glomerular filtration rate.
Table 3.
The adjusted ORs for the association of SIRI with the prevalence of NAFLD in adults in the United States from NHANES 2017–2018.
| SIRI | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR (95 %CI) | OR (95 %CI) | OR (95 %CI) | |
| Q1 | Ref. | Ref. | Ref. |
| Q2 | 0.88 (0.60, 1.28) | 0.90 (0.60, 1.34) | 0.77 (0.48, 1.23) |
| Q3 | 1.04 (0.72, 1.52) | 0.91 (0.61, 1.36) | 0.79 (0.50, 1.24) |
| Q4 | 1.13 (0.77, 1.64) | 0.96 (0.64, 1.45) | 0.94 (0.60, 1.47) |
| P for trend | 0.379 | 0.895 | 0.214 |
Abbreviations: SIRI, system inflammation response index; NAFLD, non-alcoholic fatty liver disease; Q1, 0.120–0.632; Q2, 0.633–0.924; Q3, 0.925–1.371; Q4, 1.372–10.890; OR, odd ratio; CI, confidence interval. Model 1: age, sex and race/ethnicity. Model 2: model 1 variables plus education level, marital status, family poverty income ratio, the complication of hypertension, and diabetes mellitus, smoke status, drink status, and body mass index. Model 3 was adjusted for model 2 variables plus the complication of coronary heart disease, congestive heart failure, angina pectoris, heart attack, and stroke, mean energy intake, waist circumference, fast glucose, hemoglobin, high-sensitivity C-reactive protein, alanine aminotransferase, aspartate amino transferase, gamma-glutamyl transpeptidase, high-density lipoprotein-cholesterol, total cholesterol, triglyceride, blood urea nitrogen, uric acid, serum creatinine, and estimated glomerular filtration rate.
3.3. Subgroup analyses
Using subgroup analyses stratified based on age, hypertension, sex, DM, and BMI, we investigated the relationship between SII index as well as SIRI and NAFLD risk in various populations (Supplementary Figs. 1 and 2; Supplementary Tables 1 and 2). The stratified analysis revealed that SII index, and SIRI and NAFLD risk also were U-curve correlated among participants in age ≥ 60 years, female, without hypertension, and BMI of ≥30 kg/m2.
4. Discussion
In the study, firstly, we found that a U-shaped correlation between the SII index and prevalence of NAFLD in the RCS plot. The SII index is a marker of the comprehensive evaluation system and is derives from peripheral neutrophils, lymphocytes, and platelets counts, which probably indicate three pathways, such as the formation of inflammation, thrombus formation, and adaptive immunity, that contribute to the disease process (Azab et al., 2010, Kurtul and Ornek, 2019). It is well known that inflammation plays an important role in the occurrence and progression of NAFLD, which can lead to a change in the levels of peripheral blood leukocytes as a result of such a process (Tilg et al., 2021). Additionally, it is important to point out that the presence of the neutrophils in the liver is a hallmark of inflammation that can occur in the several different types of liver disease. A common feature of NAFLD is the presence of neutrophil infiltration, which results in the recruitment of macrophages and cell damage resulting from the release of inflammatory mediators or reactive oxygen species (Rensen et al., 2009). A systemic inflammatory condition will result in an increase in the total circulating neutrophils and platelets, but a decrease in lymphocytes. Previous studies have shown that hepatic stellate cells were shown to increase the survival of neutrophils, which, in turn, contributes to the formation of reactive oxygen species in the liver, which will lead to liver fibrosis (Zhou et al., 2018). The platelets interplay with inflammatory cells, which results in the release of chemokines, which promotes the accumulation of immune mediators, which plays a crucial role in promoting the formation of NAFLD (Ye et al., 2023). Song et al. found that a positive correlation was found between SII index and the hepatic steatosis among U.S. adults. Thus, the SII index may be an effective and affordable tool for detecting hepatic steatosis in a simple and straightforward manner (Song et al., 2022). This is a good explanation for the increased risk of NAFLD as SII increases. Zhao et al. also showed that in population with NAFLD identified by ultrasound, SII index had a J-shaped curve associated with all-cause deaths and higher SII levels were associated with increased mortality. Despite the fact that multiple confounding variables were adjusted for, this association still persisted (Zhao et al., 2023). This conclusion is consistent with the findings of the study. Additionally, this study is the first to find a correlation between SIRI and NAFLD. We also found a U-shaped correlation between the SIRI and prevalence of NAFLD. As mentioned above, inflammation plays a crucial role in the development and progression of NAFLD. Zhao et al. revealed that as the level of continuous SIRI increases by a unit, there is a 30 % increase in the hazard of mortality due to all causes as well as cardiovascular disease among hypertensive patients (Zhao et al., 2022). Additionally, a few studies have shown that in certain types of inflammation-related disorders like acne vulgaris, and chronic spontaneous urticaria, SIRI is with the potential to reliably predict the effectiveness of anti-inflammatory therapies (Coşansu et al., 2022, Cosansu et al., 2022). However, broader investigations are necessary to explore the relationship between SIRI and prevalence of NAFLD.
This study has strong strengths in terms of its large sample size and representative sample selection. As a result, the findings are generalizable. It is important to note, however, that our study has several limitations that should be taken into account. Firstly, due to year limitations, we only included general population data from NHANES 2017–2018. Secondly, the determination of some covariates was derived from self-report questionnaires, so self-reported confounders would emerge. Thirdly, we could not rule out the possibility that NAFLD may be caused by drug-induced hepatotoxicity. Due to this, we cannot determine a causal relationship between a history of drug use and NAFLD. Finally, due to the fact that this was a cross-sectional study, it was limited to identifying the associations rather than causal relationships.
5. Conclusion
To conclude, there is a U-shaped curve in the American population when it comes to the SII index, and SIRI in relation to the prevalence of NAFLD. The SII index or SIRI can serve as a biomarker for NAFLD and a diagnostic or therapeutic target for the systemic inflammation. The potential mechanisms underlying the systemic inflammatory biomarkers (SII index and SIRI), in relation to NAFLD need to be further explored.
Author contributions
Wu Sun, and Yan Fang contributed to hypothesis development and manuscript preparation. Bing Zhou, and Guoliang Mao contributed to the study design. Wu Sun, Yan Fang, Jiao Cheng, and Xinxin Zhang undertook data analyses. Yinhua Liu contributed to the conception and design of the work. Hao Chen supervised the study and wrote the manuscript. All authors approved the final draft of the manuscript for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to acknowledge and thank the staff and participants of the NHANES study for their valuable contributions to the research project.
Funding
This work was supported by Anhui Provincial Natural Science Foundation (2308085QH245), the Major Projects of Natural Science Research in Colleges and Universities in Anhui Province (2023AH040261), and the Scientific Research Project at School-level of Wannan Medical College (WK2022ZF02).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2023.102536.
Contributor Information
Yinhua Liu, Email: liuyinhua@yjsyy.com.
Hao Chen, Email: ha0chen@wnmc.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Azab B., Zaher M., Weiserbs K.F., Torbey E., Lacossiere K., Gaddam S., Gobunsuy R., Jadonath S., Baldari D., et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. doi: 10.1016/j.amjcard.2010.03.062. [DOI] [PubMed] [Google Scholar]
- Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., Tiribelli C. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterology. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coşansu N.C., Kara R., Yaldiz M., Dikicier B.S. New markers to predict the response to omalizumab in chronic spontaneous urticaria. Dermatologic Therapy. 2022;35:e15589. doi: 10.1111/dth.15589. [DOI] [PubMed] [Google Scholar]
- Cosansu N.C., Yuksekal G., Turan U., Umitfer F., Yaldiz M., Sevimli Dikicier B. Investigation of systemic immune-inflammation index and systemic inflammation response index as an indicator of the anti-inflammatuary effect of isotretinoin in patients with acne vulgaris. Cutaneous and Ocular Toxicology. 2022;41:174–218. doi: 10.1080/15569527.2022.2081700. [DOI] [PubMed] [Google Scholar]
- Hu B., Yang X.R., Xu Y., Sun Y.F., Sun C., Guo W., Zhang X., Wang W.M., Qiu S.J., et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- Jia C.P., Chen H., Sun B. Research advances on the value of preoperative systemic inflammatory response index in predicting the prognosis of patients with resectable pancreatic cancer. Zhonghua Wai Ke Za Zhi [chinese Journal of Surgery] 2019;57:862–885. doi: 10.3760/cma.j.issn.0529-5815.2019.11.013. [DOI] [PubMed] [Google Scholar]
- Jiang H., Li D., Xu T., Chen Z., Shan Y., Zhao L., Fu G., Luan Y., Xia S., et al. Systemic Immune-Inflammation Index Predicts Contrast-Induced Acute Kidney Injury in Patients Undergoing Coronary Angiography: A Cross-Sectional Study. Frontiers in Medicine. 2022;9 doi: 10.3389/fmed.2022.841601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobyliak N., Abenavoli L., Mykhalchyshyn G., Kononenko L., Boccuto L., Kyriienko D., Dynnyk O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J Gastrointestin Liver Dis. 2018;27:41–49. doi: 10.15403/jgld.2014.1121.271.kby. [DOI] [PubMed] [Google Scholar]
- Kurtul A., Ornek E. Platelet to Lymphocyte Ratio in Cardiovascular Diseases: A Systematic Review. Angiology. 2019;70:802–818. doi: 10.1177/0003319719845186. [DOI] [PubMed] [Google Scholar]
- Li S., Lan X., Gao H., Li Z., Chen L., Wang W., Song S., Wang Y., Li C., et al. Systemic Inflammation Response Index (SIRI), cancer stem cells and survival of localised gastric adenocarcinoma after curative resection. J Cancer Res Clin Oncol. 2017;143:2455–2468. doi: 10.1007/s00432-017-2506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S.J., Yu H., Khan M., Gill J., Santhosh S., Chatterjee U., Iovoli A., Farrugia M., Mohammadpour H., et al. Evaluation of Optimal Threshold of Neutrophil-Lymphocyte Ratio and Its Association With Survival Outcomes Among Patients With Head and Neck Cancer. JAMA Network Open. 2022;5:e227567. doi: 10.1001/jamanetworkopen.2022.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q., Zhuang L., Shen Y., Geng Y., Yu S., Chen H., Liu L., Meng Z., Wang P., et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer. 2016;122:2158–2167. doi: 10.1002/cncr.30057. [DOI] [PubMed] [Google Scholar]
- Rensen S.S., Slaats Y., Nijhuis J., Jans A., Bieghs V., Driessen A., Malle E., Greve J.W., Buurman W.A. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol. 2009;175:1473–1482. doi: 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazi K., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. Race and Ethnicity in Non-Alcoholic Fatty Liver Disease (NAFLD): A Narrative Review. Nutrients. 2022;14 doi: 10.3390/nu14214556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl C.E., Everhart J.E. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Alimentary Pharmacology & Therapeutics. 2015;41:65–76. doi: 10.1111/apt.13012. [DOI] [PubMed] [Google Scholar]
- Song Y., Guo W., Li Z., Guo D., Li Z., Li Y. Systemic immune-inflammation index is associated with hepatic steatosis: Evidence from NHANES 2015–2018. Front Immunol. 2022;13:1058779. doi: 10.3389/fimmu.2022.1058779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Zeng X., Feng Y., Chen Q., Liu Z., Luo H., Zha L., Yu Z. Association of Systemic Immune-Inflammation Index With Short-Term Mortality of Congestive Heart Failure: A Retrospective Cohort Study. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.753133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H., Adolph T.E., Dudek M., Knolle P. Non-alcoholic fatty liver disease: the interplay between metabolism, microbes and immunity. Nature Metabolism. 2021;3:1596–1607. doi: 10.1038/s42255-021-00501-9. [DOI] [PubMed] [Google Scholar]
- Tong Y.S., Tan J., Zhou X.L., Song Y.Q., Song Y.J. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15:221. doi: 10.1186/s12967-017-1326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Wang X., Zhang G., Tong M., Chen J., Zhou Y., Ji Q., Liu N. Association of Systemic Immune Inflammation Index with Estimated Pulse Wave Velocity, Atherogenic Index of Plasma, Triglyceride-Glucose Index, and Cardiovascular Disease: A Large Cross-Sectional Study. Mediators Inflamm. 2023;2023:1966680. doi: 10.1155/2023/1966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Wang Z., Zuo R., Zhou Y., Yang Y., Chen T., Liu N. Association of Systemic Immune Inflammation Index with All-Cause, Cardiovascular Disease, and Cancer-Related Mortality in Patients with Cardiovascular Disease: A Cross-Sectional Study. Journal of Inflammation Research. 2023;16:941–961. doi: 10.2147/JIR.S402227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Zhuang T., Ping Y., Zhang Y., Wang X., Yu P., Duan X. Elevated systemic immune inflammation index level is associated with disease activity in ulcerative colitis patients. Clin Chim Acta. 2021;517:122–216. doi: 10.1016/j.cca.2021.02.016. [DOI] [PubMed] [Google Scholar]
- Yan M., Man S., Ma L., Gao W. Comprehensive molecular mechanisms and clinical therapy in nonalcoholic steatohepatitis: An overview and current perspectives. Metabolism. 2022;134 doi: 10.1016/j.metabol.2022.155264. [DOI] [PubMed] [Google Scholar]
- Yang R., Chang Q., Meng X., Gao N., Wang W. Prognostic value of Systemic immune-inflammation index in cancer: A meta-analysis. J Cancer. 2018;9:3295–3302. doi: 10.7150/jca.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye C., Yuan L., Wu K., Shen B., Zhu C. Association between systemic immune-inflammation index and chronic obstructive pulmonary disease: a population-based study. BMC Pulmonary Medicine. 2023;23:295. doi: 10.1186/s12890-023-02583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafrani E.S. Non-alcoholic fatty liver disease: an emerging pathological spectrum. Virchows Arch. 2004;444:3–12. doi: 10.1007/s00428-003-0943-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Xing Z., Zhou K., Jiang S. The Predictive Role of Systemic Inflammation Response Index (SIRI) in the Prognosis of Stroke Patients. Clinical Interventions in Aging. 2021;16:1997–2007. doi: 10.2147/CIA.S339221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zuo R., Xiao S., Wang L. Association between iron metabolism and non-alcoholic fatty liver disease: results from the National Health and Nutrition Examination Survey (NHANES 2017–2018) and a controlled animal study. Nutrition & Metabolism. 2022;19:81. doi: 10.1186/s12986-022-00715-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao E., Cheng Y., Yu C., Li H., Fan X. The systemic immune-inflammation index was non-linear associated with all-cause mortality in individuals with nonalcoholic fatty liver disease. Ann Med. 2023;55:2197652. doi: 10.1080/07853890.2023.2197652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Dong S., Qin Y., Wang Y., Zhang B., Liu A. Inflammation index SIRI is associated with increased all-cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. 2022;9:1066219. doi: 10.3389/fcvm.2022.1066219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.X., Li W.C., Xia S.H., Xiang T., Tang C., Luo J.L., Lin M.J., Xia X.W., Wang W.B. Predictive Value of the Systemic Immune Inflammation Index for Adverse Outcomes in Patients With Acute Ischemic Stroke. Frontiers in Neurology. 2022;13 doi: 10.3389/fneur.2022.836595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Xu M.J., Cai Y., Wang W., Jiang J.X., Varga Z.V., Feng D., Pacher P., Kunos G., et al. Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018;5:399–413. doi: 10.1016/j.jcmgh.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipf, G., Chiappa, M., Porter, K.S., Ostchega, Y., Lewis, B.G., Dostal, J., 2013. National health and nutrition examination survey: plan and operations, 1999-2010. Vital and health statistics. Ser. 1, Programs and collection procedures:1-37. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.