Abstract
Background
The spread of the coronavirus disease 2019 (COVID-19) in Peru has been reported at the regional level, few studies have evaluated its spread at the provincial level, in which the mechanisms could be different.
Methods
We conducted an analytical, cross-sectional, multistage observational population study to assess the seroprevalence of SARS-COV-2 at the provincial and urban/rural levels in a high-altitude setting. The sampling unit was the household, including a randomly selected family member. Sampling was performed using a data collection sheet on clinical and epidemiological variables. Chemiluminescence tests were used to detect total anti-SARS-COV-2 antibodies (IgG and IgM simultaneously). The percentages were adjusted to the sampling design.
Results
The overall prevalence in the region of Cusco was 25.9%, with considerably different prevalence between the 13 provinces (from 15.9% in Acomayo to 40.1% in Canchis) and between rural (21.1%) and urban (31.7%) areas. In multivariable model, living in a rural area was a protective factor (adjusted prevalence ratio [aPR], 0.68; 95% confidence interval [CI], 0.61–0.76).
Conclusions
Geographic diversity and population density determine different prevalence rates, typically lower in rural areas, possibly due to natural social distancing or limited interaction with people at risk.
Keywords: Seroprevalence, SARS-CoV-2, COVID-19
Graphical abstract
Highlights
-
•
Assessment of SARS-COV-2 seropositivity prevalence across all provinces within a high-altitude region exceeding 3000 meters above sea level (masl).
-
•
Examination of disparities in seroprevalence between urban and rural settings, with a focus on less urbanized cities or those characterized by lower purchasing power.
-
•
Identification of factors influencing the likelihood of contagion, exploring both potential amplifying and mitigating variables.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), has affected cities in different ways worldwide [1]. Peru's healthcare system is divided, with a public sector covering about 60% of the population, while the rest receive care through social security, the military, and the private sector, all operating independently. Consequently, the healthcare system worked at its limit for decades, unprepared for additional burdens. Moreover, Peru's healthcare investment is among the region's lowest, at 4% of GDP (Gross Domestic Product) in 1995, rising to 5.5% in 2017. This led to the construction of only a few hospitals and primary care centers to accommodate population growth.
Most studies and efforts were directed at the largest cities because they have a larger at-risk or susceptible population [2,3]. As a result, less urbanized cities or cities with lower purchasing power were underrepresented or misrepresented in the research through ecological designs with such biases that led to the hypothesis of a protective altitude effect in reducing the SARS-COV-2 contagion [[4], [5], [6], [7], [8]]. Peru was no exception, with most reports derived from records of patients treated in healthcare centers. Although such data can be gathered quickly, this information is not uniform because Peru suffers from major healthcare gaps, and the probability of identifying positive cases is inherently lower in areas with more limited access to healthcare. However, some population seroprevalence studies were conducted in the provinces of Lambayeque [9], Cusco [10] and Iquitos [11], which allows us to better understand the diversity of COVID-19 spread in the country, although there is limited data extrapolation to smaller population units. Therefore, this study aimed to determine the prevalence of SARS-COV-2 seropositivity in all the 13 provinces of the region of Cusco, assessing differences at the urban and rural levels, in addition to identifying specific factors that may increase or decrease the probability of contagion.
Materials and methods
Study
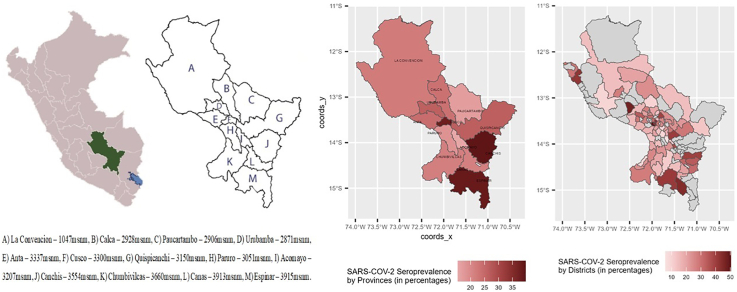
An observational, analytical, cross-sectional, population-based study was conducted in the region of Cusco, which consists of the following 13 provinces: Acomayo, Anta, Calca, Canas, Canchis, Cusco, Chumbivilcas, Espinar, La Convención, Paruro, Paucartambo, Quispicanchi and Urubamba, with varying degrees of urbanization (Fig. 1). The study was conducted from January 18 to March 28, 2021.
Fig. 1.
Distribution of the provinces of the department of Cusco and seroprevalence of anti-SARS-CoV2 antibodies.
A) La Convencion – 1047msnm, B) Calca – 2928msnm, C) Paucartambo – 2906msnm, D) Urubamba – 2871msnm, E) Anta – 3337msnm, F) Cusco – 3300msnm, G) Quispicanchi – 3150msnm, H) Paruro – 3051msnm, I) Acomayo – 3207msnm, J) Canchis – 3554msnm, K) Chumbivilcas – 3660msnm, L) Canas – 3913msnm, M) Espinar – 3915msnm.
The 13 provinces of the Cusco region are geographically dispersed. Some have little direct interaction with each other, with the province of Cusco (the capital) mediating communication between them. Only the provinces of Cusco and Urubamba have strong tourist activity; meanwhile, in other provinces, the main activities are agriculture and animal husbandry or mining. They also present a wide climatic diversity; some provinces (i.e., La Convencion) are located in subtropical areas at <1500 m above sea level (masl), while other cities, such as Espinar, are located at 3915 masl, and their temperature decreases below 0 °C during summer nights.
Sample and sampling
According to the 2017 population census, the total population of Cusco was 1,205,527 inhabitants, 45% of whom were in rural areas. In this study, a two-stage complex sampling design by clusters was used, thus forming the primary sampling unit (PSUs), that is, clusters, consisting of one or more contiguous blocks. Each PSU had 120 homes on average. Each cluster was located on a digitized map to facilitate its physical location and contained information on house numbers, locality, district, province, region, urban or rural areas, and poverty and altitude levels. Secondary sampling units were households within the clusters. In each household, a family member was randomly invited to participate in the study.
The sample size required to obtain representativeness at the level of each province and in the urban/rural strata was 5232 people considering a design effect of 1.5, population prevalence of 40%, and 5% confidence level. The inclusion criteria are as follows: aged ≥18 years, voluntarily agreed to participate in the study and signed their informed consent form, had been living in the household for >6 months, and had the ability to understand the procedures. Initially, inclusion of adolescents was considered, but due to the high rejection rate, the study was continued with adults only. People with acute symptoms or who reported positive results on previous tests were not excluded from this study.
Study procedures
Approach to volunteers
The invitation process consisted of explaining the objectives of the project, handing out the information sheet and informed consent form, explaining the study procedures, clarifying doubts, and explaining the research methods. When one of the randomly selected volunteers did not agree to participate in the study, a previously randomly selected household was used.
Data collection
Participants responded to an ad hoc questionnaire with data on epidemiological variables (age, sex, isolation, contact with a person with suspected or confirmed SARS-CoV-2 infection) and self-reported comorbidities (hypertension, diabetes, obesity, bronchial asthma, cancer, tuberculosis, and chronic obstructive pulmonary disease, etc.), ultimately providing a sample for antibody detection.
Collection and transport of biological samples
For each chemiluminescence test, 3.5 ml of whole blood was collected in a serum separator tube at the participant's home. Each tube was encoded and stored for transportation. All healthcare staff who participated in collecting and transporting biological samples wore personal protective equipment (PPE) and were trained in biosafety to safely handle biological samples. According to the guideline of the World Health Organization, samples were stored at 2–8 °C for transfer and further processing [12]. All samples collected outside the province of Cusco were centrifuged to extract 1 ml of serum aliquots, which were sent as described in the protocol.
Chemiluminescence test for the detection of total anti-SARS-CoV-2 antibodies
The Elecsys Anti-SARS-CoV-2 test (ROCHE QFSA) was used to assess seropositivity. This test is based on a sandwich immunoassay, in which the recombinant protein N of SARS-CoV-2 is the target detected by the antibodies possibly present in the serum sample [13]. The Cobas analyzer (Roche) was used for automated data processing at the Adolfo Guevara Velasco National Hospital (Hospital Nacional Adolfo Guevara Velasco, HNAGV), Social Health Insurance (El Seguro Social de Salud, EsSalud) Cusco, which has a certified laboratory for these processes. Quantitative results reported by the team were expressed as “nonreactive or negative for antibodies” or “reactive or positive for antibodies.” This test has 99.8% specificity, and its sensitivity reaches 99.5% if the test is performed 14 days after a positive PCR result [13,14].
Data analysis
Data were entered as they were collected on a digital platform (Kobox) by the field staff, and their correct completion was verified through cross-review with a second entry. The resulting database was exported to STATA 16.1 software, where statistical analysis was performed. All calculations were made considering the multistage sampling and expansion factors using the svyset command.
The study subjects were characterized by calculating the absolute and relative frequencies of the categorical variables and the mean and standard error (SE) of the quantitative variables. Point prevalence rates of positivity in chemiluminescence tests were calculated for the entire Cusco region, for each of the 13 provinces, broken down by sex and urbanization (urban/rural). Calculations were performed using the Poisson distribution model with robust variance, which presented the results with 95% CIs. An additional calculation was made using the altitude of the city (Fig. 1), which was analyzed as a quantitative variable; since its impact (in meters) was small, the results were expressed per thousand masl.
Associations were assessed using the chi-square test and Student's t-test, where appropriate, and the significance level was set at p < 0.05. The magnitudes of the association were measured with prevalence ratios (PR); the result of the chemiluminescence test was considered the response variable, and all other variables were considered exposure variables. Crude (cPR) and adjusted (aPR) models were constructed, including the variables sex, province, and urbanization as adjustment variables.
Ethical aspects
The project was evaluated by the HNAGV ethics committee (resolution 52-GRACU-ESSALUD-2021), the data were collected by the researchers under strict confidentiality rules, and informed consent was obtained from each adult who participated in the study (supplementary section).
Results
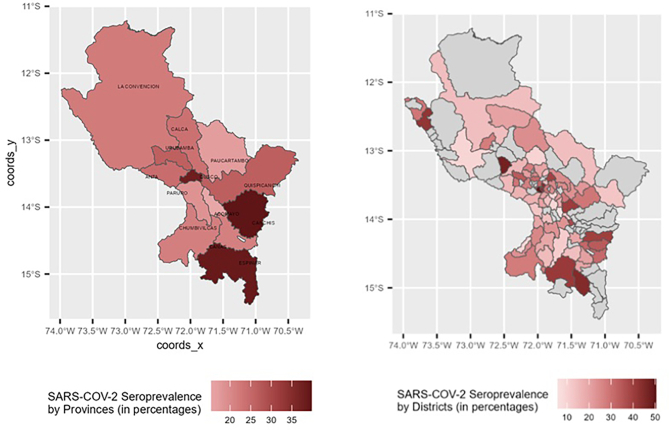
In total, 5394 volunteers from the 13 provinces of Cusco were included in this study, 60.9% of whom were females, with a mean age of 44.9 years (SE, 0.28). All other characteristics of the population are listed in Table 1. The overall prevalence of anti-SARS-CoV-2 antibodies was 29.9%, with considerable differences between provinces. The provinces of Acomayo (15.4%) and Paucartambo (16.9%) had the lowest PR, and the province of Canchis (39.8%) had the highest PR (Fig. 1, Fig. 2 and Table 2). The district seroprevalence at district level, showed a geographical dispersion of SARS-CoV-2. This suggest that there seems to be no correlation between neighbor places.
Table 1.
Population characteristics according to the results of anti-SARS-COV-2 antibodies and factors associated with seropositivity.
| Total | Reactive | Nonreactive | Crude PR | 95% CI | Adjusted PR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Origin | |||||||
| Urban | 55.2% (2727/4937) |
65.6% (972/1482) |
50.8% (1754/3455) |
1 | 0.58–0.72 | 1 | 0.61–0.76 |
| Rural | 44.8% (2210/4937) |
34.4% (510/1482) |
49.2% (1701/3455) |
0.65 | 0.68 | ||
| Sex | |||||||
| Female | 60.9% (3014/4941) |
63.7% (944/1481) |
59.8% (2070/3460) |
1 | 0.79–0.99 | 1 | 0.81–1.01 |
| Male | 39.0% (1927/4941) |
36.3% (537/1481) |
40.2% (1390/3460) |
0.89 | 0.91 | ||
| Age group (years) | |||||||
| ≥18–30 | 19.9% (1017/5110) |
21.4% (328/1532) |
19.3% (689/3578) |
1 | 0.85–1.10 0.63–0.90 |
1 | 0.83–1.08 0.64–0.91 |
| 30–59 | 58.7% (3001/5110) |
61.3% (939/1532) |
57.7% (2063/3578) |
0.96 | 0.95 | ||
| ≥60 | 21.4% (1092/5110) |
17.3% (265/1532) |
23.1% (826/3578) |
0.75 | 0.76 | ||
| Education level (year) | |||||||
| <7a | 32.4% (1590/4907) |
26.5% (390/1472) |
34.9% (1200/3435) |
1 | 1.08–1.42 1.25–1.64 |
1 | 0.99–1.30 1.02–1.38 |
| 7-11a | 36.0% (1768/4907) |
36.7% (539/1472) |
35.8% (1230/3435) |
1.24 | 1.14 | ||
| 12a or more | 31.6% (1549/4907) |
36.9% (543/1472) |
29.3% (1005/3435) |
1.43 | 1.19 | ||
| People living in the same household: Mean (SE) | 3.98 (0.04) | 4.13 (0.08) | 3.90 (0.05) | 1.02 | 1.01–1.04 | 1.02 | 0.99–1.04 |
| Type of work | |||||||
| In-person | 85,9% (4248/4941) |
87.2% (1291/1481) |
85.5% (2957/3460) |
1 | 0.73–1.13 0.69–1.15 |
1 | 0.64–1.00 0.66–1.11 |
| Remote | 8.8% (435/4941) |
8.1% (120/1481) |
9.1% (315/3460) |
0.91 | 0.80 | ||
| Hybrid | 5.2% (258/491) |
4.7% (70/1481) |
5.5% (188/3460) |
0.89 | 0.86 | ||
| Deceased at home (all causes) | |||||||
| Yes | 3.4% (170/4941) |
3.8% (56/1481) |
3.3% (114/3460) |
1 | 0.81–1.48 | 1 | 0.77–1.39 |
| No | 96.6% (4771/4941) |
96.2% (1425/1481) |
96.7% (3346/3460) |
1.10 | 1.04 | ||
| Hypertension | 4.5% (219/4783) |
4.7% (68/1452) |
4.5% (151/3331) |
1.02 | 0.77–1.35 | 0.97 | 0.74–1.28 |
| Diabetes | 2.5% (119/4769) |
2.5% (36/1444) |
2.5% (83/3325) |
0.98 | 0.66–1.47 | 0.89 | 0.60–1.31 |
| Asthma | 0.8% (36/4750) |
0.9% (12/1442) |
0.7% (24/3308) |
1.14 | 0.59–2.17 | 1.04 | 0.56–1.95 |
| Heart disease | 0.6% (29/4770) |
0.8% (11/1451) |
0.5% (18/3319) |
1.27 | 0.69–2.31 | 1.25 | 0.69–2.25 |
| Cancer | 0.2% (7/4765) |
0.01% (1/1446) |
0.2% (6/3319) |
0.30 | 0.04–2.38 | 0.29 | 0.04–2.35 |
| Respiratory symptoms in the last 14 days | 8.2% (391/4791) |
9.4% (136/1449) |
7.6% (255/3342) |
1.17 | 0.97–1.39 | 1.14 | 0.95–1.37 |
SE, standard error; PR, prevalence ratio; CI, confidence interval;
sex-, urban/rural area- and province-adjusted model.
Fig. 2.
Distribution of the provinces and districts of the department of Cusco and seroprevalence of anti-SARS-CoV2 antibodies.
Table 2.
Seroprevalence differences between provinces of the department of Cusco, 2021; sex- and urban/rural area-adjusted model.
| Provincia | Prevalence % (95% IC) | Crude PR | (IC 95%) | Adjusted PR | (IC 95%) |
|---|---|---|---|---|---|
| Cusco | 37.8 (33.9–41.8) | 1 | 1 | ||
| Acomayo | 15.4 (11.8–19.9) | 0.41 | 0.31–0.54 | 0.45 | 0.34–0.60 |
| Anta | 24.4 (20.3–29.1) | 0.65 | 0.52–0.79 | 0.77 | 0.62–0.96 |
| Calca | 23.6 (19.3–28.6) | 0.62 | 0.50–0.79 | 0.74 | 0.59–0.93 |
| Canas | 21.4 (17.6–25.8) | 0.67 | 0.46–0.71 | 0.69 | 0.55–0.87 |
| Canchis | 39.8 (34.3–45.5) | 1.05 | 0.89–1.25 | 1.18 | 0.99–1.41 |
| Chumbivilcas | 21.5 (17.7–26.0) | 0.57 | 0.46–0.71 | 0.68 | 0.54–0.85 |
| Espinar | 38.6 (33.7–43.8) | 1.02 | 0.86–1.21 | 1.16 | 0.97–1.38 |
| La Convención | 22.1 (18.0–26.8) | 0.59 | 0.47–0.73 | 0.69 | 0.55–0.87 |
| Paruro | 18.4 (14.3–23.3) | 0.49 | 0.37–0.64 | 0.58 | 0.44–0.76 |
| Paucartambo | 16.9 (12.8–22.0) | 0.45 | 0.33–0.59 | 0.55 | 0.41–0.74 |
| Quispicanchis | 26.4 (22.1–31.2) | 0.69 | 0.57–0.86 | 0.80 | 0.65–0.99 |
| Urubamba | 26.3 (21.9–31.3) | 0.69 | 0.57–0.86 | 0.80 | 0.65–0.99 |
| TOTAL | 29.9 (28.3–31.5) |
Model adjusted by gender and urban/rural area.
CI, confidence interval.
In the bivariate analysis, several variables with differences in SARS-COV-2 seropositivity were identified when breaking down the results by urban/rural area, age, sex, level of education, and number of household members. No differences were found in self-reported comorbidities or type of work (in-person, remote, or hybrid). In the multivariable analysis, living in a rural area (aPR, 0.68; 95% CI, 0.61–0.76) and aged ≥60 years (aPR, 0.76; 95% CI, 0.64–0.91) persisted as protective variable while having >12 years of education increased the probability of being seropositive (aPR, 1.19; 95% CI, 1.02–1.38). The remaining results are outlined in Table 1.
Variables altitude (per 1000 masl) and population density (per 100 people/m2) were analyzed separately, including sex, urban/rural area, and age in the adjustment model. Altitude and population density were weakly positively associated with the probability of testing positive for anti-SARS-CoV-2 antibodies (altitude: aPR, 1.13; 95% CI, 1.04–1.23; population density: aPR, 1.03; 95% CI, 1.01–1.06) (Fig. 2,).
Discussion
The overall prevalence in the region of Cusco was 25.9%, with 21.1% seroprevalence in rural areas and 31.7% in urban areas. The main protective factors among the general population of Cusco were living in a rural area (aPR, 0.68; 95% CI, 0.61–0.76) and aged ≥60 years (aPR, 0.76; 95% CI, 0.64–0.91). In addition, all provinces (except Canchis and Espinar) had a lower probability of SARS-CoV-2 seropositivity than the province of Cusco, the capital of the region. Seroprevalence studies overcome the limitations of case reports that only consider symptomatic cases [15] or ecological studies that collect data on cases identified by healthcare systems [[4], [5], [6]], correcting infection rates, which are underestimated because they do not consider asymptomatic cases that can play a key role in community viral transmission, in addition to reducing selection bias, since the selection process is probabilistic [[16], [17], [18]], which is one of the main strengths of our study.
Our study showed an overall seroprevalence of 25.9% in the region of Cusco, a finding that was framed at the beginning of the second wave of SARS-CoV-2 transmission. In Peru, the seroprevalence of SARS-CoV-2 varied between regions, with SARS-CoV-2 mortality peaking in Iquitos, where the seroprevalence during the first wave of SARS-CoV-2 reached 70% [11], a much higher seroprevalence than that found in our study. On the contrary, in Lambayeque, a province on the coast of Peru, the seroprevalence was 29.5% [9], and in Lima (the capital of Peru), the seroprevalence was 25.2% [19]. However, our results are consistent with another study conducted at the end of the first wave in Cusco, which reported a seroprevalence of 38.8% in the province of Cusco [10].
In contrast to seroprevalence rates ranging from 1 to 10% at the end of the first wave in Europe [20,21] from 0.07 to 15.6% in Asia, and 0.2 to 12.5% in North America [22], in South America, the seroprevalence rates were higher, averaging 33.6% [23]. All these studies have few methodological differences from ours because, as probabilistic population studies without major inclusion biases, the tests used may differ (rapid tests, chemiluminescence tests, and enzyme-linked immunosorbent assay, etc.), and the diagnostic performance between them does not justify such differences. Our seroprevalence is much higher, most likely due to structural factors present before the pandemic, such as informality, cultural practices, and misinformation in the media that favored the increased spread of this disease. Unlike European studies, Indian studies have reported much higher seroprevalence rates than those found in this study, reaching 54.1% in marginalized neighborhoods and 16.1% in non-marginalized neighborhoods [24].
Seropositivity rates in different geographic areas were consistent with the early spread of SARS-CoV-2 in the region of Cusco. Seroprevalence varied markedly between urban and rural areas, which can be attributed to many factors, such as cultural practices, population density and increased urbanization, levels of social interaction, economic activity and movement due to tourism, and mitigation efforts [3,25]. Therefore, the dispersion of dwellings, as well as cultural practices and outdoor agricultural work, has likely favored measures, i.e., social distancing by reducing the effective reproduction number with a decrease in SARS-CoV-2 transmission of ≤60%, which might have mitigated SARS-CoV-2 infection in rural areas [3]. This finding is consistent with other studies; thus, a global meta-analysis showed that maintaining a physical distance of at least 1 m and using PPE reduced the transmission of SARS-CoV-2 [26]. In addition, the study by Xu [15] showed that people living in areas outside the epicenter of the outbreak had a lower seroprevalence, in line with the spread of this respiratory virus.
All provinces in the region of Cusco, Peru, except for the provinces of Canchis and Espinar, showed a protective factor against SARS-CoV-2. This protective factor may have been due to the increase in the number of cases identified in the second wave of SARS-CoV-2, which coincided with the study period, thus reflecting the geographical movement in the spread of infection that was initially located in urban areas and with a high movement of the population. The provinces of Canchis and Espinar are located in the south of Peru, on the border with the region of Arequipa, whose seroprevalence could also have been affected by the economic and migratory movement of the region of Arequipa, which experienced a growth in the incidence of cases during this study. These findings may also be associated with the increase in household transmission because the provinces of Canchis and Espinar have a higher population density than the other provinces of the region of Cusco, and households are an important source of transmission.
Aged ≥60 years was a protective factor against SARS-CoV-2 seropositivity. These data are also like those reported in another study, which found that individuals aged ≥65 years had a lower risk of SARS-CoV-2 seropositivity than individuals aged 20–49 years [22]. These findings are likely related to the combination of different measures, such as reduced exposure, as this age group is more susceptible to serious diseases with a higher risk of death due to senescence of the immune system [27].
Study limitations
Our study has some limitations. The first is that this research is based only on the adult population; thus, the behavior of the younger population cannot be described. However, in Cusco, during the initial waves, nearly all activities involving minors were restricted, entailing limited access to public spaces and attendance at schools. This restriction resulted in a lower rate of COVID-19 transmission among children compared to adults [28,29]. Moreover, these restrictions on children adversely impacted their mental health, increasing the risk of anxiety and depression [30]. Consequently, the likelihood of contagion among children is expected to be lower than that observed in adults.
In addition, the data were collected over a longer period (2 months), which could have affected the prevalence. Moreover, although the second wave began in mid-February 2021 in Peru, its impact was delayed in the provinces of Cusco. Therefore, in many of them, the second wave likely only peaked after this study.
Furthermore, the disparity between the expected seroprevalence (40%) and the observed seroprevalence (25.9%) may have introduced selection bias in the examined population, potentially leading to an underestimation of both the seroprevalence and the findings [31]. However, the transmission of COVID-19 depends on serotypes [32], which could have influenced the disease transmission patterns and seroprevalence during different study periods. Additionally, the expected seroprevalence was determined in three urban settings (Cusco, the peripheral area of the city, and Quillabamba) [10], which may have overestimated the seroprevalence in Cusco region, but this was the only preliminary study conducted in the region.
On the other hand, there could have been challenges in interpreting regression coefficients due to the evaluation of COVID-19, a communicable disease that generated interdependence among individuals through transmission chains, resulting in increased transmissibility and total number of infections. This situation posed difficulties in interpreting confounding factors and assessing the overrepresentation of other associations [33].
Conclusions
The overall prevalence in the region of Cusco was 25.9%, although there were considerable differences between the provinces studied. The geographical diversity of the region of Cusco determines the different prevalence rates between its provinces, and rural areas typically have a lower prevalence, possibly due to natural social distancing or limited interaction with people at risk. The high percentages of susceptible people in rural areas suggest that these areas could face sudden increases in the number of cases of shortages in the hospital system.
Ethical approval and consent to participate
The project was evaluated by the HNAGV ethics committee (resolution 52-GRACU-ESSALUD-2021), the data were collected by the researchers under strict confidentiality rules, and informed consent was obtained from each adult who participated in the study.
Consent for publication
The authors declare their consent for publication.
Funding
This work was partially supported by FONDECYT (Fondo Nacional de Ciencia y Tecnología, Peru): 071-2020.
CRediT authorship contribution statement
Huamaní Charles: Conceptualization, Data curation, Formal analysis, Project administration, Writing – original draft, Writing – review & editing. Concha-Velasco Fátima: Conceptualization, Methodology, Project administration, Supervision, Visualization, Writing – original draft – review & editing. Velásquez Lucio: Conceptualization, Supervision, Visualization, Writing – review & editing. K. Antich María: Data curation, Writing – original draft, Writing – review & editing. Cassa Johar: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Palacios Kevin: Supervision, Writing – review & editing. Bernable-Villasante Luz: Supervision, Writing – review & editing. Giraldo-Alencastre Guido: Supervision, Writing – review & editing. Benites-Calderon Eduarda: Supervision, Writing – review & editing. Mendieta-Nuñez Sebastian: Supervision, Writing – review & editing. Quispe-Jihuallanca Heber: Supervision, Writing – review & editing. Quispe-Yana Matilde: Supervision, Writing – review & editing. Zavala-Vargas Karla: Supervision, Writing – review & editing. Hinojosa-Florez Liesbeth: Supervision, Writing – review & editing. Ramírez-Escobar Javier: Conceptualization, Supervision, Visualization, Writing – review & editing. Spelucin-Runciman Juan: Conceptualization, Supervision, Visualization, Writing – review & editing. Bernabe-Ortiz Antonio: Formal analysis, Methodology, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflicts of interest. The funders nor authorities of the Gerencia Regional de Salud del Cusco had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Acknowledgments
We would like to thank all the health redes staff and rapid response teams that participated collecting data for this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gloepi.2023.100131.
Contributor Information
Concha-Velasco Fátima, Email: fconcha@continental.edu.pe.
Cassa Johar, Email: johar.cassa@emory.edu.
Bernabe-Ortiz Antonio, Email: antonio.bernabe@upch.pe.
Appendix A. Supplementary data
Supplementary material: Appendix 1: Data collection sheet. Appendix 2: Informed consent form.
Data availability
The information data for this publication is available for any person.
References
- 1.“WHO Coronavirus (COVID-19) Dashboard.” Accessed: Aug. 20, 2023. [Online]. Available: https://covid19.who.int.
- 2.Lai C.-C., Wang C.-Y., Hsueh P.-R. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. Aug. 2020;53(4):505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rostami A., et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. Mar. 2021;27(3):331–340. doi: 10.1016/j.cmi.2020.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segovia-Juarez J., Castagnetto J.M., Gonzales G.F. High altitude reduces infection rate of COVID-19 but not case-fatality rate. Respir Physiol Neurobiol. Oct. 2020;281:103494. doi: 10.1016/j.resp.2020.103494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arias-Reyes C., et al. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir Physiol Neurobiol. Jun. 2020;277:103443. doi: 10.1016/j.resp.2020.103443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cano-Pérez E., Torres-Pacheco J., Fragozo-Ramos M.C., García-Díaz G., Montalvo-Varela E., Pozo-Palacios J.C. Negative correlation between altitude and COVID-19 pandemic in Colombia: a preliminary report. Am J Trop Med Hyg. Dec. 2020;103(6):2347–2349. doi: 10.4269/ajtmh.20-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolcott O.O., Bergman R.N. Mortality attributed to COVID-19 in high-altitude populations. High Alt Med Biol. Dec. 2020;21(4):409–416. doi: 10.1089/ham.2020.0098. [DOI] [PubMed] [Google Scholar]
- 8.Thomson T.M., Casas F., Guerrero H.A., Figueroa-Mujíca R., Villafuerte F.C., Machicado C. Potential protective effect from COVID-19 conferred by altitude: a longitudinal analysis in Peru during full lockdown. High Alt Med Biol. Jun. 2021;22(2):209–224. doi: 10.1089/ham.2020.0202. [DOI] [PubMed] [Google Scholar]
- 9.Díaz-Vélez C., et al. SARS-CoV-2 seroprevalence study in Lambayeque, Peru. June-July 2020. PeerJ. 2021;9:e11210. doi: 10.7717/peerj.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huamaní C., Velásquez L., Montes S., Mayanga-Herrera A., Bernabé-Ortiz A. SARS-CoV-2 seroprevalence in a high-altitude setting in Peru: adult population-based cross-sectional study. PeerJ. 2021;9:e12149. doi: 10.7717/peerj.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Álvarez-Antonio C., et al. Seroprevalence of anti-SARS-CoV-2 antibodies in Iquitos, Peru in July and august, 2020: a population-based study. Lancet Glob Health. Jul. 2021;9(7):e925–e931. doi: 10.1016/S2214-109X(21)00173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . World Health Organization; 2020. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases: interim guidance, 19 March 2020. WHO/COVID-19/laboratory/2020.5. Accessed: Aug. 20, 2023. [Online]. Available: https://apps.who.int/iris/handle/10665/331501. [Google Scholar]
- 13.“Reactivo para anticuerpos IGM/IGG CONTRA SARS-COV-2 Método Quimioluminiscencia.” Accessed: Aug. 20, 2023. [Online]. Available: https://www.gob.pe/institucion/ins/informes-publicaciones/1377347-reactivo-para-anticuerpos-igm-igg-contra-sars-cov-2-metodo-quimioluminiscencia.
- 14.Riester E., et al. Performance evaluation of the Roche Elecsys anti-SARS-CoV-2 S immunoassay. J Virol Methods. Nov. 2021;297:114271. doi: 10.1016/j.jviromet.2021.114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X., et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. Aug. 2020;26(8):1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 16.Franceschi V.B., et al. Population-based prevalence surveys during the Covid-19 pandemic: a systematic review. Rev Med Virol. Jul. 2021;31(4):e2200. doi: 10.1002/rmv.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal R., Ranganathan P. Study designs: part 2 - descriptive studies. Perspect Clin Res. 2019;10(1):34–36. doi: 10.4103/picr.PICR_154_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gnaldi M., Tomaselli V., Forcina A. Ecological fallacy and covariates: new insights based on multilevel modelling of individual data. Int Stat Rev. 2018;86(1):119–135. doi: 10.1111/insr.12244. [DOI] [Google Scholar]
- 19.Reyes-Vega M.F., et al. SARS-CoV-2 prevalence associated to low socioeconomic status and overcrowding in an LMIC megacity: a population-based seroepidemiological survey in Lima, Peru. EClinicalMedicine. Apr. 2021;34:100801. doi: 10.1016/j.eclinm.2021.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollán M., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet Lond Engl. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward H., et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. Feb. 2021;12(1):905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai C.-C., Wang J.-H., Hsueh P.-R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: an up-to-date review. Int J Infect Dis. Dec. 2020;101:314–322. doi: 10.1016/j.ijid.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Núñez-Zapata S.F., Benites-Peralta B., Mayta-Tristan P., Rodríguez-Morales A.J. High seroprevalence for SARS-CoV-2 infection in South America, but still not enough for herd immunity! Int J Infect Dis IJID Off Publ Int Soc Infect Dis. Aug. 2021;109:244–246. doi: 10.1016/j.ijid.2021.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malani A., et al. Seroprevalence of SARS-CoV-2 in slums versus non-slums in Mumbai, India. Lancet Glob Health. Feb. 2021;9(2):e110–e111. doi: 10.1016/S2214-109X(20)30467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet Lond Engl. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet Lond Engl. Jun. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawelec G., Weng N. Can an effective SARS-CoV-2 vaccine be developed for the older population? Immun Age A. Apr. 2020;17:8. doi: 10.1186/s12979-020-00180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverberg S.L., et al. Child transmission of SARS-CoV-2: a systematic review and meta-analysis. BMC Pediatr. Apr. 2022;22(1):172. doi: 10.1186/s12887-022-03175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumm L., et al. Lower SARS-CoV-2 household transmission in children and adolescents compared to adults. Sci Rep. Dec. 2022;12(1) doi: 10.1038/s41598-022-24643-2. Art. no. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballena C.L., et al. Impacto del confinamiento por COVID-19 en la calidad de vida y salud mental. Rev Cuerpo Méd Hosp Nac Almanzor Aguinaga Asenjo. Jan. 2021;14(1):87–89. doi: 10.35434/rcmhnaaa.2021.141.904. [DOI] [Google Scholar]
- 31.Jones S.R., Carley S., Harrison M. An introduction to power and sample size estimation. Emerg Med J EMJ. Sep. 2003;20(5):453–458. doi: 10.1136/emj.20.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quispe-Ricalde M.A., et al. Evidence of natural selection and dominance of SARS-CoV-2 variant Lambda (C.37) over variants of concern in Cusco, Peru. Arch Virol. Feb. 2023;168(3):88. doi: 10.1007/s00705-022-05645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engebretsen S., Rø G., de Blasio B.F. A compelling demonstration of why traditional statistical regression models cannot be used to identify risk factors from case data on infectious diseases: a simulation study. BMC Med Res Methodol. May 2022;22(1):146. doi: 10.1186/s12874-022-01565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Appendix 1: Data collection sheet. Appendix 2: Informed consent form.
Data Availability Statement
The information data for this publication is available for any person.