Abstract
Objective
This study aims to compare, through quantitative analysis, the effectiveness of different endurance training types on increasing lower limb strength and muscle cross-sectional area (MCSA) in concurrent training.
Methods
This systematic literature search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [PROSPERO ID: CRD42023396886]. Web of Science, SportDiscuss, Pubmed, Cochrane, and Scopus were systematically searched from their inception date to October 20, 2023.
Results
A total of 40 studies (841 participants) were included in this meta-analysis. MCSA analysis showed that, compared to resistance training alone, concurrent high-intensity interval running training and resistance training and concurrent moderate-intensity continuous cycling training and resistance training were more effective (SMD = 0.15, 95% CI = −0.46 to 0.76, and SMD = 0.07, 95% CI = −0.24 to 0.38 respectively), while other modalities of concurrent training not. Lower body maximal strength analysis showed that all modalities of concurrent training were inferior to resistance training alone, but concurrent high-intensity interval training and resistance training showed an advantage in four different concurrent training modalities (SMD = −0.08, 95% CI = −0.25 to 0.08). For explosive strength, only concurrent high-intensity interval training and resistance training was superior to resistance training (SMD = 0.06, 95% CI = −0.21 to 0.33).
Conclusion
Different endurance training types have an impact on the effectiveness of concurrent training, particularly on lower limb strength. Adopting high-intensity interval running as the endurance training type in concurrent training can effectively minimize the adverse effects on lower limb strength and MCSA.
Keywords: Concurrent training, Muscle hypertrophy, Muscle strength, Endurance training type
1. Introduction
Concurrent training, which combines strength and endurance modalities,1 is a widely recognized approach to enhance aerobic capacity and muscle strength among diverse age groups.2, 3, 4 However, distinct biological adaptations arise from strength and endurance training: strength training can decrease, while endurance training tends to increase muscle mitochondrial density and quantity.5,6 Such divergent adaptations may lead to the ‘interference effect,’ characterized by compromised gains in strength-related parameters such as muscle cross-sectional area (MCSA, which is defined as the number of muscle fibers within the physiological cross-section),7, 8, 9, 10, 11, 12, 13 lower body explosive strength (LBES, which is defined as the ability of lower limb muscles to generate maximum power in a short time),14, 15, 16, 17 and lower body strength (LBS, which is defined as the maximum force that lower body muscle can produce).18, 19, 20, 21
The scientific community has extensively investigated strategies to mitigate the interference effect over the last forty years.22, 23, 24 Currently, endurance training type, training load, training frequency, and the interval between endurance training and strength training are considered important variables affecting the effectiveness of concurrent training.6,25,26 In the design of endurance training type, modality (moderate-intensity continuous training and high-intensity interval training) and method (running and cycling) are key considerations. Moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT) have significant differences in load and duration, thus resulting in different training adaptations, mainly related to muscle strength, body composition, and aerobic capacity.27,28 However, it is worth noting that endurance training modality is an important variable that has been often neglected in past studies. There is no conclusive evidence yet as to which endurance training modality is suitable for reducing the interference effect in concurrent training.
Additionally, there is no consensus on the best endurance training method, and people have been hesitant about choosing cycling or running.25 Running and cycling are the most commonly used endurance training methods, and different studies have different conclusions on whether the endurance training method affects the concurrent training effect. Some studies have shown that running can impair the improvement of strength after concurrent training,6,13 while other studies suggest that the effect of concurrent training does not seem to be affected by the endurance training method.9,22,29 In the early stages, it was thought that the reason for this difference might be that compared to cycling, running involves more eccentric contractions, and the recruitment conditions of muscle fibers and the forms of exercise unit recruitment are different, which may lead to greater muscle damage after running.30,31 This view seems to explain why cycling is better than running. However, Sabag et al. (2018) researched a different conclusion. Their meta-analysis found that using high-intensity interval running as an endurance training method for concurrent training would reduce the interference of endurance training on strength training compared to high-intensity interval cycling.8 The view based on muscle contraction patterns seems unable to explain the phenomenon mentioned above. The difference in results may be caused by the characteristics of the studies included,8 as Sabag et al.'s (2018) study only included HIIT as the endurance training modality in concurrent training.
The different types of endurance training play a crucial role in the manifestation of interference effects. Combining HIIT and MICT for analysis may further exacerbate the heterogeneity of the studies included. Additionally, the amalgamation of different endurance training types for meta-analysis may result in diminished external validity since no single training type can encompass both HIIT and MICT or cycling and running. Therefore, this approach may not accurately infer the optimal concurrent training type, leaving coaches or fitness instructors without clear grounds for selecting a specific training modality.
Randomized controlled trials (RCTs) and pair meta-analyses are not sufficient to determine the optimal endurance training mode. However, Bayesian methods can rank all analyzed interventions through posterior probabilities. Compared with frequentist methods, Bayesian methods avoid biased and unstable results caused by continuously iterating to estimate the maximum likelihood function during parameter estimation. Consequently, the objective of this study is to perform a Bayesian network meta-analysis to quantitatively assess the impact of different endurance training modalities on LBS, LBES, and MCSA within a concurrent training framework, providing a more definitive guide for coaches and fitness professionals.
2. Methods
This Bayesian network meta-analysis was reported by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.32,33 The study protocol was registered in the international Prospective Register of Systematic Reviews (PROSPERO) [PROSPERO ID: CRD42023396886].
2.1. Search strategy
We performed a comprehensive systematic search, without language restrictions, using databases such as PubMed, Scopus, Cochrane Library, Sportdiscus, and Web of Science, covering their inception dates until October 20, 2023. The specific search strategies, including search terms, dates, and processes, were shown in Supplementary File 1. The reference lists of relevant studies and reviews were also screened for additional studies. Title/abstract and full-text screening were conducted independently and in duplicate by investigators, with disagreements resolved by discussion or adjudication by the third author.
2.2. Included criteria
The inclusion criteria were based on the PICOS (participants, interventions, comparators, outcomes, and study design) approach:33
(P) Population: healthy adults aged from ≥18 years to <45 years.34
(I) Intervention: concurrent aerobic training and strength training in which strength training is required to undergo whole-body strength training or lower-body strength training. It is commonly assumed that training interventions with a duration of less than 4 weeks may result in subjects being in a learning and adaptation phase, which could potentially affect the outcomes. Therefore, we only included experimental studies with intervention durations exceeding 4 weeks. The interventions were classified into the following four categories: (1) Concurrent high-intensity interval running training and resistance training (HIIRT + RT). (2) Concurrent high-intensity interval cycling training and resistance training (HIICT + RT). (3) Concurrent moderate-intensity continuous running training and resistance training (MICRT + RT). (4) Concurrent moderate-intensity continuous cycling training and resistance training (MICCT + RT).
High-intensity interval running training (HIIRT) or high-intensity interval cycling training (HIICT) were defined as repeated bouts of ≤5 min of running or cycling with>80% maximal heart rate (MHR) or >100% lactate threshold or >90% maximal oxygen uptake (VO2 max).
Moderate-intensity continuous running training and moderate-intensity continuous cycling training were defined as each session lasting more than 30 min running or cycling with >46% VO2max or >64% MHR or >RPE12.
(C) Comparator: for a comparison, eligible studies had to include a group performing strength training alone with an identical strength training prescription.
(O) Outcomes: To avoid confusion of results, we have classified the outcomes of interest into primary and secondary outcomes. (1) Primary outcome: MCSA. Included studies required the use of biopsy, ultrasound examination, computed tomography scan, dual X-ray absorptiometry, magnetic resonance imaging, and/or density measurement methods to detect and report changes in MCSA. (2) Secondary outcome: LBS. The included studies required the use of dynamic maximum repetition or maximum voluntary contraction to measure changes in LBS. (3) Secondary outcome: LBES. Included studies required measuring neuromuscular performance such as jump or sprint performance to reflect LBES.
(S) study type: RCTs.
2.3. Excluded criteria
-
1.
Studies that only presented abstracts or conference reports without sufficient data were excluded since relevant data could not be extracted.
-
2.
Due to the focus of this study on healthy adults, adolescents, elderly individuals, and individuals with diseases have been excluded.
-
3.
Specialized strength training methods may introduce bias in results; therefore, we excluded specialized strength training modes, such as blood flow restriction training and vibration training.
-
4.
The increase in protein breakdown rate is one of the reasons for the interference effect. However, specific protein nutritional supplements may interfere with this effect. Therefore, in the study, the use of additional nutritional supplements will be excluded.
2.4. Data extraction
A nine-item, standardized, and pre-piloted data extraction form was used to record data from the included studies under the following headings: (i) author, (ii) year of publication, (iii) sample size, (iv) sample training status (trained/moderate active/untrained), (v) sample mean physical characteristics, (vi) training frequency, (vii) training duration, (viii) the rest between endurance training and resistance training, (ix) details of training interventions, (x) following up MCSA, LBS and LBES.
2.5. Risk of bias of individual studies
The Physiotherapy Evidence Database (PEDro) Scale was employed to assess the risk of bias (ROB).35 The following eleven items were considered: (i) Eligibility criteria and source (ii) Random allocation, (iii) Concealed allocation, (iv) Baseline comparability, (v) Blinding of participants, (vi) Blinding of therapists, (vii) Blinding of assessors, (viii) Adequate follow-up (>85%), (ix) Intention-to-treat analysis, (x) Between-group statistical comparisons, (xi) Reporting of point measures and measures of variability. Each item represented one score, <4 was considered “poor”, 4 to 5 was considered “fair”, 6 to 8 was considered “good” and 9 to 10 was considered “excellent”.35 Since the first criterion only affects the external validity of the experiment and has no impact on the internal and statistical validity, it is commonly agreed that it should not be included in calculating the PEDro score. Meanwhile, all studies cannot be the default, complete “blinding of participants” and “blinding of therapists”, given it was impossible to blind participants and trainers to group assignment in exercise intervention protocols. Therefore, these components were not included in the overall ROB score. Two assessors independently assessed the ROB, and conflicts were resolved by a third reviewer.
2.6. Data analysis
We performed a random-effects network meta-analysis to calculate pooled estimates and 95% confidence intervals (95% CI). Since all of the included indicators were measured by different rating instruments, the effect size was tested by the standardized mean difference (SMD).36 SMD values of 0.2–0.5 represented small, values of 0.5–0.8 were considered medium, and values > 0.8 represented large.36
We utilized the “network plot” function of STATA software (version 14.0) to generate network plots that visually displayed the geometry of different forms of exercise.36,37 Specifically, different interventions were represented as nodes, and head-to-head comparisons between interventions were represented as edges.
The “chi2” test was applied to evaluate the global inconsistency,38 and perform a node-splitting method to assess inconsistency between direct and indirect comparisons.
In the case of possible important heterogeneity or inconsistency, we will explore the possible sources using subgroup analyses.
To evaluate the efficacy and safety of the interventions, we calculated the probabilities of the surface under the cumulative ranking curve (SUCRA) for both primary and secondary outcomes. The SUCRA values, represented as percentages in a range from 1 to 0, indicate the level of efficacy for each intervention. Those interventions that have SUCRA values closest to 1 are considered to be most effective as they reflect high levels of efficacy. Ultimately, we ranked the interventions according to this effectiveness measure. Pairwise comparisons were represented in the league's table. The network funnel plot was used to check for the presence of bias due to small-scale studies, which may lead to publication bias in NMA.
3. Results
3.1. Descriptions of included studies
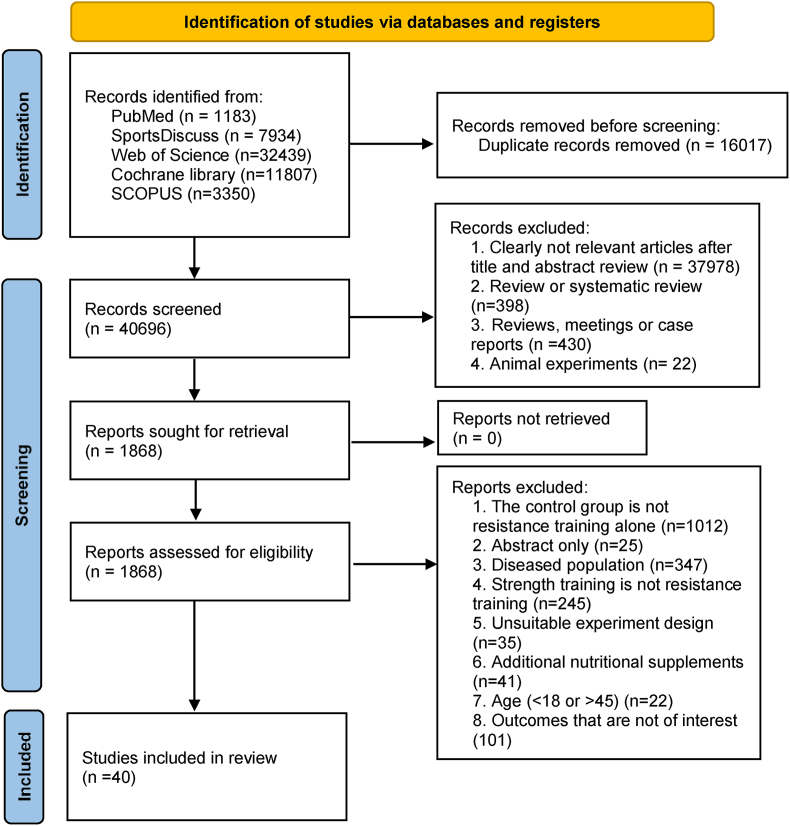
According to the predetermined search strategy, a total of 56,697 potential studies were obtained. After excluding duplicate studies, there were 42,694 studies, and 1866 were considered potential studies after excluding abstracts and titles. Finally, after applying the inclusion and exclusion criteria, a total of 40 studies were included (Fig. 1).10,16,17,19,21,22,39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 The agreement rate for study selection and data extraction between the two researchers was 87.8% and 86.5%, respectively.
Fig. 1.
PRISMA flowchart of literature search for eligible studies.
The characteristics of 40 studies were summarized in Table 1. Sample size ranged from 5 to 18 participants, training frequency ranged from 2 to 3 times per week, and training duration ranged from 5 to 22 weeks.
Table 1.
Training program of included studies.
| Study | Group | Aerobic training |
Resistance training |
Rest | Frequency (Days/Weeks) | Duration (Weeks) | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|
| Volume | Intensity | Prescription | Intensity | ||||||
| Balabinis 2003 | HIIRT + RT | 200 m*8, 100 m*8 | 85% VO2max | 4–6 reps, 1–2 sets | 75%–85% 1RM | 7 h | 2 | 7 | J, G, F |
| RT | – | As same above | |||||||
| Bell 2000 | MICCT + RT | 30–42 min | 90% VO2max | 4–12 reps, 2–6 sets | 72%–84% 1RM | 24 h | 3 | 12 | G, H |
| RT | – | As same above | |||||||
| Cantrell 2014 | HIICT + RT | 30 s*4–6 | modified Wingate protocol | 4–6 reps, 3 sets | 85% 1RM | 24 h | 2 | 12 | F, B, A |
| RT | – | As same above | |||||||
| Chtaha 2008 | HIIRT + RT | Running to failure*5 | 100% VO2max | 16–26 reps, 4 sets | 10%-25% body mass load | In the same session | 2 | 12 | J, N |
| RT | – | As same above | |||||||
| Craig 1991 | MICRT + RT | 30–35 min | 75% maximal heart rate | 8–10 reps, 3 sets | 75% 1RM | In the same session | 3 | 10 | G, A, D, E |
| RT | – | As same above | |||||||
| de Souza 2012 | HIICT + RT | 60 s*15–20 | 80%–100% VO2max | 10–12 reps, 3-5 sets | 6–12RM | In The same session | 2 | 8 | G, D |
| RT | – | As same above | |||||||
| Dolezal 1998 | MICRT + RT | 25–40 min | 65%–85% heart rate maximum | 4–15 reps, 3 sets | Exercise to failure | Less than 24 h, but not in the same session | 3 | 10 | F, A |
| RT | – | As same above | |||||||
| Fyfe 2016 | HIICT + RT | 2 min*5–11 | 120%–150% LT | 12 reps, 3 sets | 65%–90% 1RM | In the same session | 3 | 8 | J, G, B, A |
| MICCT + RT | 15–33 min | 80%–100% LT | As same above | ||||||
| RT | – | As same above | |||||||
| Gettman 1982 | HIIRT + RT | 30 s*10 | 60% maximum heart rate | 12–15 reps, 3 sets | 40%–50% 1RM | In the same session | 3 | 12 | G, A |
| RT | – | As same above | |||||||
| Glowacki 2004 | MICRT + RT | 20–40 min | 65%–80% heart rate reserve | 6–10 reps, 3 sets | 75%–85% 1RM | >24 h | 2 | 12 | H, J, G, A |
| RT | – | As same above | |||||||
| Hendrickson 2010 | MICRT + RT | 20–30 min | 70–85% maximum heart rate | 3–12 reps, 3 sets | 3–12RM | 2 h | 3 | 8 | F, F |
| RT | – | As same above | |||||||
| Hennessy 1994 | MICRT + RT | 20–40 min | 85% maximum heart rate | 6–25 reps, 2–6 sets | 65%–100% 1RM | In the same session | 3 | 8 | J, F, K |
| RT | – | As same above | |||||||
| Hickson 1980 | HIICT + RT | 5 min*6 | 100% VO2max | 5–20 reps, 3–5 sets | 80% 1RM | 2 h | 3 | 10 | F |
| RT | – | As same above | |||||||
| Izquierdo 2005 | MICCT + RT | 30–40 min | 70%–90% maximum heart rate | 5–15 reps, 3–5 sets | 50%–80% 1RM | >24 h | 2 | 16 | G, A |
| RT | – | As same above | |||||||
| Jones 2022 | HIICT + RT | 3 min*5–6 | 85% VO2max | 5–10 reps, 3 sets | 75%–90% 1RM | In the same session | 2 | 8 | A |
| MICCT + RT | 40 min | 65% VO2max | As same above | In the same session | |||||
| Kazior 2016 | MICCT + RT | 30–60 min | 60% VO2max | 8–15 reps, 4–6 sets | 70% 1RM | In the same session | 2 | 7 | G |
| RT | – | As same above | |||||||
| Kotzamanidis 2005 | HIIRT + RT | 30 m*15 | the best effort | 60 min | 3–8RM | In the same session | 2 | 9 | J, L, T, M |
| RT | – | As same above | |||||||
| Kraemer 1995 | MICRT + RT | 40 min | 80%–85% VO2max | 5–15 reps, 2–5 sets | 5–15RM | 5–6 h | 2 | 12 | H, G |
| RT | – | As same above | |||||||
| Kraemer 2004 | MICRT + RT | 40 min | 70%–80% VO2max | 5–10 reps, 3–5 sets | 5–10RM | 5–6 h | 4 | 12 | J, C |
| RT | – | As same above | |||||||
| Laird 2016 | HIICT + RT | 20 s*8 | 110%–120% VO2max | 3–10 reps, 3–5 sets | 75% 1RM | 4 h | 3 | 11 | F, A |
| RT | – | As same above | |||||||
| Leveritt 2003 | HIICT + RT | 5 min*5 | 40–100% VO2max | Exercise to failure, 3 sets | 4–10RM | In the same session | 3 | 6 | F |
| RT | – | As same above | |||||||
| Lundberg 2013 | MICCT + RT | 40 min | RPE10 | 4 reps, 7 sets | 100% 1RM | 6 h | 3 | 5 | D |
| RT | – | As same above | |||||||
| McCarthy 1995 | MICCT + RT | 30–45 min | 70% heart rate reserve | 5–7 reps, 3 sets | 5–7RM | In the same session | 3 | 10 | J, G, F, A |
| RT | – | As same above | |||||||
| Mikkola 2012 | MICRT + RT | 30–90 min | From below aerobic threshold to above aerobic threshold | 8–15 reps, 2–3 sets | 50%–80% 1RM | >24 h | 2 | 21 | H |
| RT | – | As same above | |||||||
| Mirghani 2014 | MICRT + RT | 16–30 min | 65–80% maximal heart rate | 6–10 reps, 2–3 sets | 55%–85% 1RM | In the same session | 3 | 8 | G, F |
| RT | – | As same above | |||||||
| Panissa 2018 | HIIRT + RT | 1 min, untill complete 5 km | 100% maximal aerobic speed | 8–12 reps, 3 sets | 8–12RM | In the same session | 2 | 12 | F |
| RT | – | As same above | |||||||
| Petré 2018 | HIICT + RT | 20 s*8*2–3 | 150% VO2max | 2–5 reps, 2–5 sets | 80%–90% 1RM | In the same session | 3 | 6 | J, F |
| MICCT + RT | 40–80 min | 70% VO2max | As same above | In the same session | |||||
| Putman 2004 | MICCT + RT | 30–42 min | power output which elicited ventilation threshold | 4–10 reps, 2–6 sets | 70%–85% 1RM | >24 h | 3 | 12 | G |
| RT | – | As same above | |||||||
| Prieto-González 2022 | MICRT + RT | 45–50 min | 117–180 heart rate | 6–12 reps, 4–5 sets | 64%–86% 1RM | >24 h | 3 | 12 | J, F, A |
| RT | – | As same above | |||||||
| Robineau 2016 | HIIRT + RT | 15 s*12*3 | 100% VO2max | 3–10 reps, 3–4 sets | 70%–90% 1RM | 0/6/24 h | 2 | 7 | I, J, F |
| RT | – | As same above | |||||||
| Robineau 2017 | HIIRT + RT | 30 s* | |||||||
| 8-12*2 | Maximal velocity | 3–10 reps, 2–3 sets | 70%–90% 1RM | >24 h | 2 | 8 | I, J, F | ||
| RT | – | As same above | |||||||
| Ross 2009 | HIIRT + RT | 40–60 m *2–12 reps *1–3 sets | 0%–25% body mass load | 4–10 reps, 2–4 sets | 6–10RM | In the same session | 2 | 7 | F, L |
| RT | – | As same above | |||||||
| Sale 1990 | HIICT + RT | 3min*5 | 90%–100% VO2max | 15-20reps, 6 sets | 15-20RM | In the same session | 3 | 22 | G |
| RT | – | As same above | |||||||
| Shamim 2018 | HIICT + RT | 40–60s *3–6 | Maximum aerobic power | 8–15 reps, 4 sets | 60%–98% 1RM | 24 h | 3 | 12 | G, H, C, A |
| RT | – | As same above | |||||||
| Shaw 2009 | MICRT + RT | 22min | 65% maximal heart rate | 15 reps, 3 sets | 60% 1RM | In the same session | 2 | 16 | H, G, A |
| RT | – | As same above | |||||||
| Silva 2012 | HIIRT + RT | 1 min* 10–15 | 100% VO2max | 4 sets | Exercise to failure | In the same session | 2 | 11 | H, G |
| MICRT + RT | 20–30 min | 95 % HRVT2 | As same above | In the same session | |||||
| MICCT + RT | 20–30 min | 95 % HRVT2 | As same above | In the same session | |||||
| RT | – | As same above | |||||||
| Spiliopoulou 2021 | HIICT + RT | 1 min*10 | 100% VO2max | 2–3 reps, 8 sets | 40%–65% 1RM | In the same session | 3 | 6 | J, F |
| RT | – | As same above | |||||||
| Timmins 2020 | MICCT + RT | 20–30 min | 25%–110% maximum aerobic power cycling | 2–15 reps, 2–5 sets | 60%–97.5% 1RM | 24 h | 3 | 12 | A, B |
| RT | – | As same above | |||||||
| Tsitkanou 2017 | HIICT + RT | 60 s*10 | 100% maximal heart rate | 10 reps, 2 sets | 6RM | In the same session | 2 | 8 | G, L, D |
| RT | – | As same above | |||||||
| Volpe 1993 | MICRT + RT | 25 min | 75% maximum heart rate | 8–12 reps, 3 sets | 60%–75% 1RM | In the same session | 3 | 9 | H, G, A, E |
| RT | – | As same above | |||||||
LT, lactate threshold; reps, repetitions; 1RM, one repetition maximum; A, free fat mass; B, lower body free fat mass; C, leg free fat mass; D, leg cross-sectional area; E, thigh thickness; F, half squat one repetition maximum G, leg press one repetition maximum; H, leg extension one repetition maximum; I, quadriceps maximum voluntary contraction movement; J, counter movement jump height; K, 20 m sprint; L, 30 m sprint; M, drop jump.
The characteristics of individuals were summarized in Supplementary File 2. Among these included studies, 12 included studies explored the effects of concurrent training for individuals with trained status in 12 RCTs, the effects of concurrent training for individuals with moderate active status were explored in 18 RCTs, and the effects of concurrent training for individuals with untrained status were revealed in 11 RCTs. In the included trials, 10 studies (90 participants) examined the effects of HIICT + RT, 12 studies (141 participants) examined the effects of HIIRT + RT, 11 studies (86 participants) examined the effects of MICCT + RT, 13 studies (143 participants) examined the effects of MICRT + RT, 38 studies (381 participants) examined the effects of RT. 4 studies were compared the effects of two different concurrent training, and two study of which adopted 3-arm design and 4-arm design respectively.
3.2. Risk of bias
Details of the ROB assessment in each study included were provided in Supplementary File 3. Overall, 2 studies were judged to be of excellent, and 38 studies were judged to be of good. In terms of each ROB domain, 5 studies were lack of the random allocation and there was no blinding of participants, therapists and assessors in all included studies.
3.3. Network meta-analysis
MCSA, LBS, and LBES were included in the NMA. Supplementary File 4 provided details of the pre-post data for all outcomes included in NMA.
4. Primary outcome: muscle cross-sectional area
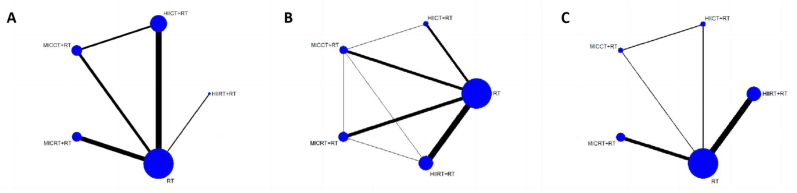
Twenty-one studies involving 428 participants showed the effect of concurrent training on MCSA which included lean body mass, lean lower body mass, thigh cross-sectional area, lean leg mass, and thigh thickness. All available comparisons from the included trials were shown in the network plot for MCSA (Fig. 2A).
Fig. 2.
Network meta-analysis of eligible comparison for (A) muscle cross-sectional area, (B) lower body strength, (C) lower body explosive strength.
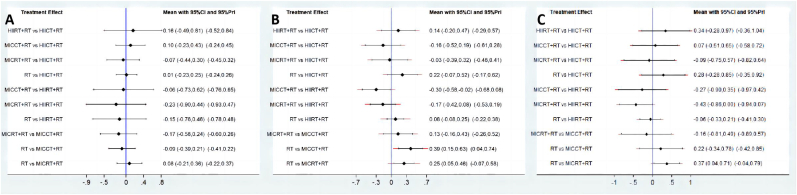
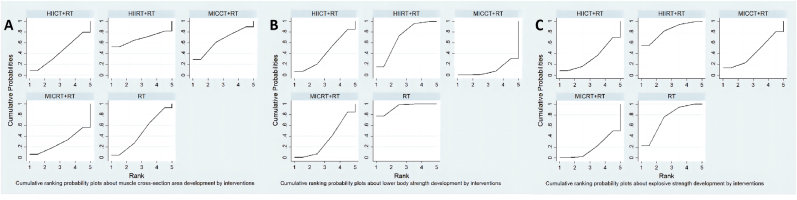
The results of pairwise comparisons were shown in the forest (Fig. 3A and Table 3A). All four concurrent training modalities increased the MCSA, while HIIRT + RT training (SUCRA = 67.3%, SMD = 0.15, 95% CI = −0.46 to 0.76) and MICCT + RT training (SUCRA = 63.8%, SMD = 0.07, 95% CI = −0.24 to 0.38) were superior to RT. Other two concurrent trainings (HIICT + RT: SUCRA = 42.8%, SMD = −0.01, 95% CI = −0.25 to 0.22; MICRT + RT: SUCRA = 28.9%, SMD = −0.08, 95% CI = −0.36 to 0.21) were inferior than RT (SUCRA = 47.1%) (Fig. 4A and Table 2). HIIRT + RT was the best concurrent training in the network comparison for MCSA development. However, MCSA was improved in all interventions, however, there were no significant differences.
Fig. 3.
Forest for (A) muscle cross-sectional area, (B) lower body strength, (C) lower body explosive strength.
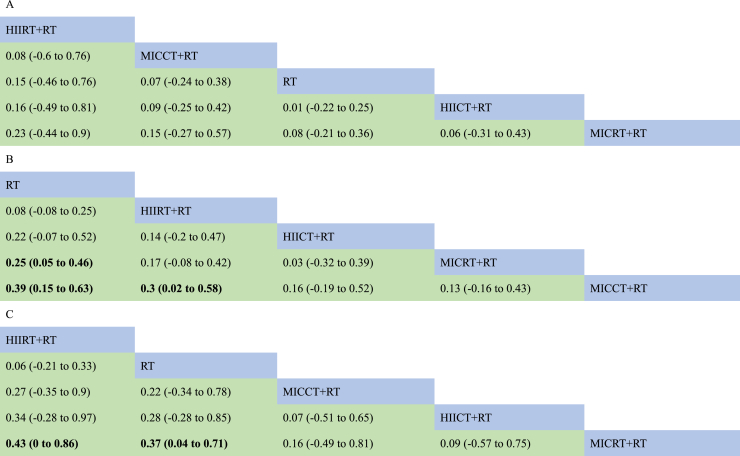
Table 3.
Comparative effectiveness results for (A) muscle cross-sectional area, (B) lower body strength, (C) lower body explosive strength.
Fig. 4.
Cumulative ranking probability plots for (A) muscle cross-sectional area (B) lower body strength (C) lower body explosive strength. The surface under the cumulative ranking curves for different outcomes, a larger area under the line represents a better intervention effect.
Table 2.
SUCRA ranking.
| Interventions |
Muscle Cross-sectional Area |
Lower Body Strength |
Lower Body Explosive Strength |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SUCRA | PrBest | Mean Rank | SUCRA | PrBest | Mean Rank | SUCRA | PrBest | Mean Rank | |
| HIICT + RT | 42.8 | 8.4 | 3.3 | 41.7 | 6.7 | 3.3 | 32.8 | 8.1 | 3.7 |
| HIIRT + RT | 67.3 | 52 | 2.3 | 71.0 | 15.3 | 2.2 | 82.7 | 54.7 | 1.7 |
| MICCT + RT | 63.8 | 28.7 | 2.4 | 9.7 | 0.1 | 4.6 | 42.2 | 13.4 | 3.3 |
| MICRT + RT | 28.9 | 6.3 | 3.8 | 33.6 | 0.6 | 3.7 | 18.9 | 0.6 | 4.2 |
| RT | 47.1 | 4.6 | 3.1 | 94.0 | 77.5 | 1.2 | 73.5 | 24.2 | 2.1 |
Global inconsistency was measured through chi2 test, prob > chi2 = 0.9169. The results showed that there is no inconsistency in included studies. A node-splitting test showed that there was no difference between direct comparison and indirect comparison (p > 0.05) (Supplementary Table 1).
The distribution of the dots demonstrated no apparent publication bias among the trials (Supplementary Fig. 1).
5. Secondary outcomes: lower body strength
Thirteen-four studies, including 799 participants, reported LBS which included one repetition maximum in half squat 1RM (kg), leg press 1RM (kg), leg extension 1RM (kg), and quadriceps maximum voluntary contraction (MVC) (Nm). The network plot for LBS (Fig. 2B) showed all available comparisons from the included trials.
The results of pairwise comparisons were shown in the forest (Fig. 3B and Table 3B). The lower body maximal strength analysis showed that compared RT alone (SUCRA = 94.0%), all four concurrent training including HIIRT + RT (SUCRA = 71.0%, SMD = −0.08, 95% CI = −0.25 to 0.08), HIICT + RT (SUCRA = 41.7%, SMD = −0.18, 95% CI = −0.49 to 0.13), MICRT + RT (SUCRA = 33.6%, SMD = −0.25, 95% CI = −0.46 to −0.05) and MICCT (SUCRA = 9.7%, SMD = −0.38, 95% CI = −0.62 to −0.14), were less than RT (Fig. 4B and Table 2). HIIRT + RT was the best concurrent training protocol in these four interventions to increase the lower body maximal strength. RT was significantly different for MICRT + RT and MICCT + RT, but not for HIIRT + RT and HIICT + RT.
Global inconsistency was measured though the chi2 test, prob > chi2 = 0.9657, there is no inconsistency in included studies. A node-splitting test indicated that there was no difference between direct comparison and indirect comparison (p > 0.05) (Supplementary Table 1).
The distribution of the dots demonstrated no apparent publication bias among the trials (Supplementary Fig. 1).
6. Secondary outcomes: lower body explosive strength
Fifteen studies including 355 participants reported LBES which included counter movement jump (cm), 30 m sprint (s), 20 m sprint (s), drop jump (cm), and peak power (W). The network plot for LBES (Fig. 2C) showed all available comparisons from the included trials.
The results of pairwise comparisons were shown in the forest (Fig. 3C and Table 3C). HIIRT + RT group increased the explosive strength (SUCRA = 82.7%, SMD = 0.06, 95% CI = −0.21 to 0.33) compared to RT alone (SUCRA = 73.5%). Other three different modality concurrent training decreased the explosive strength (MICCT + RT: SUCRA = 42.2%, SMD = −0.22, 95% CI = −0.78 to 0.34; HIICT + RT: SUCRA = 32.8%, SMD = −0.28, 95% CI = −0.85 to 0.28; MICRT: SUCRA = 18.9%, SMD = −0.37, 95% CI = −0.71 to −0.04) (Fig. 4C and Table 2). HIIRT + RT had the highest likelihood of improving explosive strength in both direct and indirect comparisons. All interventions improved the explosive strength, only MICRT + RT was significantly different from HIIRT + RT and RT, while other pairwise comparisons showed no difference in statistics.
Global inconsistency was measured though the chi2 test, prob > chi2 = 0.2984, there was no inconsistency in included studies. A node-splitting test indicated that there was no difference between direct comparison and indirect comparison (p > 0.05) (Supplementary Table 1).
The dots distribution showed an absence of conspicuous publication bias across the trials (Supplementary Fig. 1).
7. Discussion
This study aims to determine the effects of concurrent training using different endurance training types on muscle strength and hypertrophy. A network meta-analysis was used to synthesize existing original studies, and according to the SUCRA ranking, HIIRT + RT showed greater advantages in the development of LBS, LBES, and MCSA, and could reduce the interference effect of concurrent training. MCSA analysis showed that there were no significant differences between interventions on the endurance training modality. While, this study focuses on the comparison between different interventions, it should be cautious when discussing the research results. The study was considered to have moderate methodological quality.
HIIT encompasses various endurance training modalities, such as long-duration HIIT (L-HIIT), and has been shown to confer superior benefits in lean body mass (LBM), LBES, and MCSA compared to MICT. While previous meta-analyses have indicated that outcomes are contingent upon both the modality and methodology of the interventions,7,8,70 Schumann et al. (2022) reported that the specific endurance training method (cycling versus running) did not significantly alter the results of concurrent training modalities. Nevertheless, our findings diverge, potentially due to Schumann et al.'s exclusion of a direct comparison between HIIT and MICT modalities.7 We contend that subgroup analyses, which do not differentiate between training modalities, may conflate findings and yield misleading conclusions. This view is bolstered by a recent narrative review that identified training modality as a pivotal variable accounting for disparities in research outcomes.71 Aligning with Sabag et al. (2018), our research corroborates that HIIT, particularly high-intensity interval running, emerges as the most efficacious endurance training approach within the sphere of concurrent training.8 By expanding upon Sabag et al.'s work through a network meta-analysis, our study not only compares multiple endurance training methodologies but also reinforces the preeminence of HIIRT.
As training intensity increases, the recruited muscle fibers gradually shift from type I muscle fibers to type II muscle fibers. Long-term high-intensity endurance training leads to hypertrophy of type II muscle fibers, and meanwhile, the recruitment capacity of the neuromuscular system is enhanced. Type II muscle fibers are innervated by larger motor neurons with thicker nerve fibers and faster conduction velocities. Compared to MICT, HIIT modalities of endurance training will recruit more type II muscle fibers, which is more conducive to the development of lower limb strength. Conversely, MICT will recruit more type I muscle fibers, leading to hypertrophy of type I muscle fibers with long-term training. LBES requires a higher level of neuromuscular recruitment, and a higher proportion of type II muscle fibers is more conducive to the development of lower limb strength. Meanwhile, after training, type II muscle fibers are more likely to undergo hypertrophy than type I muscle fibers,72 so it can be inferred that HIIT is more conducive to increasing MCSA.
However, a new concept has recently been proposed in the field of concurrent training that when HIIT and resistance training are performed concurrently, an “additive effect"may occur,26 which means that endurance training can lead to a certain degree of improvement in lower limb strength. In certain training settings, endurance training and strength training can together promote the improvement of strength quality, but this additive effect has not yet been confirmed. High-intensity strength training can recruit high-threshold motor units, while compared to MICT, HIIT utilizes sprints or cycling at near-maximal intensity to complete exercises with high power output and fast muscle contraction speeds, which can enhance muscle activation and generate greater force, similar to resistance training. Currently, research on the effects of HIIT on MCSA and muscle strength is very limited.73,74 However, it has been observed that HIIT is a better way to alleviate muscle atrophy in middle-aged and elderly populations who experience muscle atrophy.75,76 Meanwhile, for the adolescent population, HIIT is also an essential way to improve cardiovascular health and muscle strength. Some studies suggest that HIIT may increase MCSA to some extent, but this still requires further research to confirm. One study indicates that compared to MICT (10%), only HIIT (50%) can promote a greater increase in muscle fiber protein synthesis rate, and only HIIT can increase myofibrillar protein synthesis rate,77 which suggests that HIIT has the potential to promote muscle hypertrophy.78 Although endurance training seems to reduce MCSA in mouse experiments,79 in human studies, concurrent strength and endurance training does not seem to affect the development of MCSA,6 especially in concurrent training studies using HIIT as the endurance training mode, where no decrease in MCSA has been observed.16,41,67,80, 81, 82 This study's analysis of maximal lower limb strength reveals a notable finding: the interference effect surpasses the combined effect. Conversely, in the context of lower limb explosive power, the combined effect predominates. This discrepancy could be attributed to variations in loading parameters. Endurance training typically involves resistance from the body's own weight, whereas strength training employs substantially higher resistance. These observations suggest that while HIIT can improve certain aspects of muscular strength, both HIIT and MICT may exhibit inherent limitations in the recruitment of high-threshold motor units. This differential impact underscores the need for tailored training regimens to optimize specific strength and power adaptations.
Early studies suggested that when endurance training precedes strength training and is separated by a short period (<6 h), residual neuromuscular fatigue or depleted muscle glycogen and creatine phosphate from endurance training may impair the quality of the subsequent strength training session.14,83,84 Muscle glycogen is a storage form of glucose in muscles, and endurance training at 65%–85% of maximum intensity results in the greatest glycogen depletion. HIIT, with its high intensity and short duration, results in lower glycogen depletion compared to MICT. Muscle glycogen can take up to 24 h to fully recover to baseline levels after depletion. Therefore, MICT appears to produce greater energy expenditure. Markov et al. (2022) found that longer-duration aerobic training at moderate to high intensity may have an acute negative effect on muscle strength, while shorter-duration aerobic training may reduce the negative effect.85 However, this negative effect is significantly associated with the methods of endurance training (cycling or running).
In this study, endurance training modes were categorized into HIIT and MICT. However, the SUCRA rankings were not arranged in sequence, indicating that neither of the two HIIT methods is entirely superior to the two MICT training methods. This suggests the presence of an interactive effect between endurance training modes and training methods. Therefore, caution is warranted when explaining why running is superior to cycling. Although both running and cycling primarily involve lower limb muscles, they differ significantly in their movement patterns. Cycling involves only concentric contractions, while running involves both concentric and eccentric contractions.86 These differences in movement patterns result in substantial disparities between cycling and running in terms of muscle activation, fatigue accumulation, and skeletal muscle signaling pathways.87 Muscle activation levels may reflect the characteristics of muscle activity during a particular movement. According to relevant studies, it has been demonstrated that during running, the activation level of muscles involved in eccentric contractions is higher than that of muscles involved in concentric contractions.86 However, during cycling, primarily muscles involved in concentric functions are activated. Considering that most lower limb strength training focuses on the quadriceps as the agonist muscle, cycling may lead to greater local fatigue in the quadriceps, which could potentially compromise the quality of subsequent strength training sessions.85 The differences between cycling and running also manifest in skeletal muscle signaling pathways. Eccentric exercise, such as running, may increase the levels of cytoskeletal desmin and alpha-crystallin B proteins,88 which serve to protect muscle fiber integrity and maintain cellular stability.89 Consequently, running may result in higher muscle fiber integrity and cellular stability compared to cycling, which is more conducive to subsequent planning of strength training, minimizing the degree of interference effects.
The interference effect of concurrent training may be related to the incompatibility of adaptation and metabolic pathways of different training modalities.78 Strength training can promote the elevation of insulin-like growth factor-I (IGF-1), thereby activating the IGF/PI3K/Akt pathway, which is an upstream signaling pathway of the mTOR signaling pathway. Therefore, the activation of this pathway can result in the activation of mTOR and downstream signaling pathways, ultimately promoting protein synthesis.71,90, 91, 92 On the other hand, significant changes occur in calcium, reactive oxygen species, adenosine monophosphate (AMP), lactate, nicotinamide adenine dinucleotide (NAD+), inorganic phosphate, and glycogen concentration in skeletal muscles during endurance exercise. In sawtooth animals, AMP increased by endurance exercise can activate AMP-dependent protein kinase (AMPK).93 However, AMPK may inhibit the signaling transduction of the protein synthesis mechanism by inhibiting the activity of mTOR and its downstream targets.71,93,94 Notably, many studies on human research have not found changes in mTOR signaling after strength training caused by endurance training,44,71,95, 96, 97, 98, 99 and even genes and protein synthesis signals related to muscle mass were found to be upregulated after HIIT.78 The study by Apró et al. (2015) found that the AMPK pathway activated by high-intensity interval cycling did not inhibit the mTORC1 signaling pathway.95 However, MuRF1 induced by endurance training may be accompanied by the breakdown of myofibers.100 Therefore, further research is needed to determine the potential mediators that cause interference effects.
7.1. Limitation
While interpreting the results of this study, it is crucial to consider several limitations. Firstly, the methodological constraints of this study precluded a detailed analysis of training interval time. A significant portion (67.5%) of the included studies implemented concurrent training within the same session. Prior research indicates that short interval times between strength and endurance training could significantly influence interference effects.34,51,71,101 This factor warrants further investigation for a comprehensive understanding.
Secondly, despite systematic screening efforts, our study confronts the challenge of limited large-scale research. Notably, studies focusing on female participants were sparse, representing only 20% of the total population examined.100 This limitation curtails the generalizability of our findings to broader, more diverse populations.
We also acknowledge that our sample predominantly comprised trained male individuals, which may limit the applicability of our findings to untrained individuals or female populations. Physiological and hormonal differences between these groups can significantly influence training outcomes and adaptations. Therefore, it is imperative for future research to delve into these population-specific responses to training.
Furthermore, the scarcity of long-term intervention studies in our research limits the ability to conclusively determine the effects of extended training durations. Most studies provided a snapshot rather than a longitudinal view, which is essential to understand the full spectrum of training adaptations and outcomes over time.
Lastly, our analysis included only one study that utilized high-intensity interval training (HIIT) as the endurance modality in untrained individuals, potentially introducing a bias in the results. The underrepresentation of various training modalities and demographic groups suggests that our conclusions may not fully encapsulate the diversity of training responses.
These limitations highlight the need for caution in applying our results to different training designs, especially those varying in interval times between strength and endurance sessions, and to underrepresented populations like females or untrained individuals. Future research should thus integrate our findings with studies employing more robust methodological designs, larger and more diverse sample sizes, and a wider range of training modalities. This approach will be pivotal in determining the universal applicability of our conclusions and in understanding the nuances of interference effects across different populations and training variables.
8. Conclusion
In synthesizing the findings of prior research, the present study offers significant insights for practical application in the field of sports science and coaching. It indicates that HIIRT, as a mode of endurance training in a concurrent training regimen, may mitigate the detrimental interference effects commonly observed on strength performance and muscle mass. This suggests a strategic advantage for coaches and fitness instructors in employing HIIRT to prioritize muscle strength enhancement. However, when the training objective is the augmentation of MCSA, the specific modality of endurance training may be of less critical consideration.
Notwithstanding these findings, the conclusions drawn must be approached with caution due to the study's inherent limitations and the relatively small number of studies evaluated. Future research with a more extensive array of studies may provide further validation and clarity regarding the impact of high-intensity interval running on muscle strength and MCSA in the context of concurrent training.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies
Yonghui Chen: Gender male, age 23. Master's student at Beijing Sport University, majoring in Physical Education and Training, with a research direction in strength training theory and practice.
Xinmiao Feng: Gender male, age 24. Master's student at Beijing Sport University, majoring in Physical Education and Training, with a research direction in Exercise Physiology and Biochemistry.
Lanmin Huang: Gender female, age 23. Master's student at Beijing Sport University, majoring in Physical Education and Training, with a research direction in youth strength training and practice.
Keli Wang: Gender female, age 23. Master's student at Beijing Sport University, majoring in Physical Education and Training, with a research direction in football strength training and practice.
Jing Mi: Gender male, age 49. PhD Candidate at Beijing Sport University, majoring in Physical Education and Training, with a research direction in youth strength training and practice.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jesf.2023.12.005.
Contributor Information
Yonghui Chen, Email: chenyonghui0601@bsu.edu.cn.
Xinmiao Feng, Email: fengxinmiao666@163.com.
Lanmin Huang, Email: Huanglanmin1106@163.com.
Keli Wang, Email: wangkeli98@126.com.
Jing Mi, Email: taishanmijing@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Leveritt M., Abernethy P.J., Barry B.K., Logan P.A. Concurrent strength and endurance training. A review. Sports Med. 1999;28:413–427. doi: 10.2165/00007256-199928060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Markov A., Hauser L., Chaabene H. Effects of concurrent strength and endurance training on measures of physical fitness in healthy middle-aged and older adults: a systematic review with meta-analysis. Sports Med. 2023;53:437–455. doi: 10.1007/s40279-022-01764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Hermoso A., Ramírez-Vélez R., Ramírez-Campillo R., Peterson M.D., Martínez-Vizcaíno V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: a systematic review and meta-analysis. Br J Sports Med. 2018;52:161–166. doi: 10.1136/bjsports-2016-096605. [DOI] [PubMed] [Google Scholar]
- 4.Khalafi M., Sakhaei M.H., Rosenkranz S.K., Symonds M.E. Impact of concurrent training versus aerobic or resistance training on cardiorespiratory fitness and muscular strength in middle-aged to older adults: a systematic review and meta-analysis. Physiol Behav. 2022;254 doi: 10.1016/j.physbeh.2022.113888. [DOI] [PubMed] [Google Scholar]
- 5.Fyfe J.J., Bishop D.J., Stepto N.K. Interference between concurrent resistance and endurance exercise: molecular bases and the role of individual training variables. Sports Med. 2014;44:743–762. doi: 10.1007/s40279-014-0162-1. [DOI] [PubMed] [Google Scholar]
- 6.Murach K.A., Bagley J.R. Skeletal muscle hypertrophy with concurrent exercise training: contrary evidence for an interference effect. Sports Med. 2016;46:1029–1039. doi: 10.1007/s40279-016-0496-y. [DOI] [PubMed] [Google Scholar]
- 7.Schumann M., Feuerbacher J.F., Sunkeler M., et al. Compatibility of concurrent aerobic and strength training for skeletal muscle size and function: an updated systematic review and meta-analysis. Sports Med. 2022;52:601–612. doi: 10.1007/s40279-021-01587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabag A., Najafi A., Michael S., Esgin T., Halaki M., Hackett D. The compatibility of concurrent high intensity interval training and resistance training for muscular strength and hypertrophy: a systematic review and meta-analysis. J Sports Sci. 2018;36:2472–2483. doi: 10.1080/02640414.2018.1464636. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg T.R., Feuerbacher J.F., Sunkeler M., Schumann M. The effects of concurrent aerobic and strength training on muscle fiber hypertrophy: a systematic review and meta-analysis. Sports Med. 2022;52:2391–2403. doi: 10.1007/s40279-022-01688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsitkanou S., Spengos K., Stasinaki A.N., et al. Effects of high-intensity interval cycling performed after resistance training on muscle strength and hypertrophy. Scand J Med Sci Sports. 2017;27:1317–1327. doi: 10.1111/sms.12751. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki H., Kitada T., Nakagata T., Naito H. Combination of body mass-based resistance training and high-intensity walking can improve both muscle size and V˙O(2) peak in untrained older women. Geriatr Gerontol Int. 2017;17:779–784. doi: 10.1111/ggi.12786. [DOI] [PubMed] [Google Scholar]
- 12.Küüsmaa M., Schumann M., Sedliak M., et al. Effects of morning versus evening combined strength and endurance training on physical performance, muscle hypertrophy, and serum hormone concentrations. Appl Physiol Nutr Metabol. 2016;41:1285–1294. doi: 10.1139/apnm-2016-0271. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J.M., Marin P.J., Rhea M.R., Wilson S.M., Loenneke J.P., Anderson J.C. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Condit Res. 2012;26:2293–2307. doi: 10.1519/JSC.0b013e31823a3e2d. [DOI] [PubMed] [Google Scholar]
- 14.Dragutinovic B., Feuerbacher J.F., Jacobs M.W., Bloch W., Schumann M. Acute effects of concurrent high-intensity interval cycling and bench-press loading on upper- and lower-body explosive strength performance. Int J Sports Physiol Perform. 2022;17:1077–1084. doi: 10.1123/ijspp.2021-0571. [DOI] [PubMed] [Google Scholar]
- 15.Liu M., Zhou K., Li B., et al. Effect of 12 weeks of complex training on occupational activities, strength, and power in professional firefighters. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.962546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotzamanidis C., Chatzopoulos D., Michailidis C., Papaiakovou G., Patikas D. The effect of a combined high-intensity strength and speed training program on the running and jumping ability of soccer players. J Strength Condit Res. 2005;19:369–375. doi: 10.1519/R-14944.1. [DOI] [PubMed] [Google Scholar]
- 17.Chtara M., Chaouachi A., Levin G.T., et al. Effect of concurrent endurance and circuit resistance training sequence on muscular strength and power development. J Strength Condit Res. 2008;22:1037–1045. doi: 10.1519/JSC.0b013e31816a4419. [DOI] [PubMed] [Google Scholar]
- 18.Taipale R.S., Forssell J., Ihalainen J.K., Kyröläinen H., Häkkinen K. A 10-week block of combined high-intensity endurance and strength training produced similar changes in dynamic strength, body composition, and serum hormones in women and men. Front Sports Act Living. 2020;2 doi: 10.3389/fspor.2020.581305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw B.S., Shaw I., Brown G.A. Comparison of resistance and concurrent resistance and endurance training regimes in the development of strength. J Strength Condit Res. 2009;23:2507–2514. doi: 10.1519/JSC.0b013e3181bc191e. [DOI] [PubMed] [Google Scholar]
- 20.Gergley J.C. Comparison of two lower-body modes of endurance training on lower-body strength development while concurrently training. J Strength Condit Res. 2009;23:979–987. doi: 10.1519/JSC.0b013e3181a0629d. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy J.P., Agre J.C., Graf B.K., Pozniak M.A., Vailas A.C. Compatibility of adaptive responses with combining strength and endurance training. Med Sci Sports Exerc. 1995;27:429–436. [PubMed] [Google Scholar]
- 22.Silva R.F., Cadore E.L., Kothe G., et al. Concurrent training with different aerobic exercises. Int J Sports Med. 2012;33:627–634. doi: 10.1055/s-0031-1299698. [DOI] [PubMed] [Google Scholar]
- 23.Davis W.J., Wood D.T., Andrews R.G., Elkind L.M., Davis W.B. Concurrent training enhances athletes' strength, muscle endurance, and other measures. J Strength Condit Res. 2008;22:1487–1502. doi: 10.1519/JSC.0b013e3181739f08. [DOI] [PubMed] [Google Scholar]
- 24.Baar K. Using molecular biology to maximize concurrent training. Sports Med. 2014;44(Suppl 2):S117–S125. doi: 10.1007/s40279-014-0252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berryman N., Mujika I., Bosquet L. Concurrent training for sports performance: the 2 sides of the medal. Int J Sports Physiol Perform. 2019;14:279–285. doi: 10.1123/ijspp.2018-0103. [DOI] [PubMed] [Google Scholar]
- 26.Vechin F.C., Conceição M.S., Telles G.D., Libardi C.A., Ugrinowitsch C. Interference phenomenon with concurrent strength and high-intensity interval training-based aerobic training: an updated model. Sports Med. 2021;51:599–605. doi: 10.1007/s40279-020-01421-6. [DOI] [PubMed] [Google Scholar]
- 27.Milanović Z., Sporiš G., Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45:1469–1481. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z.J., Wang Z.Y., Gao H.E., Zhou X.F., Li F.H. Impact of high-intensity interval training on cardiorespiratory fitness, body composition, physical fitness, and metabolic parameters in older adults: a meta-analysis of randomized controlled trials. Exp Gerontol. 2021;150 doi: 10.1016/j.exger.2021.111345. [DOI] [PubMed] [Google Scholar]
- 29.Mathieu B., Robineau J., Piscione J., Babault N. Concurrent training programming: the acute effects of sprint interval exercise on the subsequent strength training. Sports. 2022;10 doi: 10.3390/sports10050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabiner M.D., Owings T.M. EMG differences between concentric and eccentric maximum voluntary contractions are evident prior to movement onset. Exp Brain Res. 2002;145:505–511. doi: 10.1007/s00221-002-1129-2. [DOI] [PubMed] [Google Scholar]
- 31.Babault N., Pousson M., Ballay Y., Van Hoecke J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91:2628–2634. doi: 10.1152/jappl.2001.91.6.2628. [DOI] [PubMed] [Google Scholar]
- 32.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 34.Petré H., Hemmingsson E., Rosdahl H., Psilander N. Development of maximal dynamic strength during concurrent resistance and endurance training in untrained, moderately trained, and trained individuals: a systematic review and meta-analysis. Sports Med. 2021;51:991–1010. doi: 10.1007/s40279-021-01426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cashin A.G., McAuley J.H. Clinimetrics: Physiotherapy evidence database (PEDro) scale. J Physiother. 2020;66:59. doi: 10.1016/j.jphys.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Chaimani A., Higgins J.P., Mavridis D., Spyridonos P., Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daly C., Soobiah C. Software to conduct a meta-analysis and network meta-analysis. Methods Mol Biol. 2022;2345:223–244. doi: 10.1007/978-1-0716-1566-9_14. [DOI] [PubMed] [Google Scholar]
- 38.Watt J., Del Giovane C. Network meta-analysis. Methods Mol Biol. 2022;2345:187–201. doi: 10.1007/978-1-0716-1566-9_12. [DOI] [PubMed] [Google Scholar]
- 39.Balabinis C.P., Psarakis C.H., Moukas M., Vassiliou M.P., Behrakis P.K. Early phase changes by concurrent endurance and strength training. J Strength Condit Res. 2003;17:393–401. doi: 10.1519/1533-4287(2003)017<0393:epcbce>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Bell G.J., Syrotuik D., Martin T.P., Burnham R., Quinney H.A. Effect of concurrent strength and endurance training on skeletal muscle properties and hormone concentrations in humans. Eur J Appl Physiol. 2000;81:418–427. doi: 10.1007/s004210050063. [DOI] [PubMed] [Google Scholar]
- 41.Cantrell G.S., Schilling B.K., Paquette M.R., Murlasits Z. Maximal strength, power, and aerobic endurance adaptations to concurrent strength and sprint interval training. Eur J Appl Physiol. 2014;114:763–771. doi: 10.1007/s00421-013-2811-8. [DOI] [PubMed] [Google Scholar]
- 42.de Souza E.O., Tricoli V., Roschel H., et al. Molecular adaptations to concurrent training. Int J Sports Med. 2013;34:207–213. doi: 10.1055/s-0032-1312627. [DOI] [PubMed] [Google Scholar]
- 43.Dolezal B.A., Potteiger J.A. Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals. J Appl Physiol. 1998;85:695–700. doi: 10.1152/jappl.1998.85.2.695. [DOI] [PubMed] [Google Scholar]
- 44.Fyfe J.J., Bishop D.J., Zacharewicz E., Russell A.P., Stepto N.K. Concurrent exercise incorporating high-intensity interval or continuous training modulates mTORC1 signaling and microRNA expression in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2016;310:R1297–R1311. doi: 10.1152/ajpregu.00479.2015. [DOI] [PubMed] [Google Scholar]
- 45.Glowacki S.P., Martin S.E., Maurer A., Baek W., Green J.S., Crouse S.F. Effects of resistance, endurance, and concurrent exercise on training outcomes in men. Med Sci Sports Exerc. 2004;36:2119–2127. doi: 10.1249/01.mss.0000147629.74832.52. [DOI] [PubMed] [Google Scholar]
- 46.Hendrickson N.R., Sharp M.A., Alemany J.A., et al. Combined resistance and endurance training improves physical capacity and performance on tactical occupational tasks. Eur J Appl Physiol. 2010;109:1197–1208. doi: 10.1007/s00421-010-1462-2. [DOI] [PubMed] [Google Scholar]
- 47.Izquierdo M., Häkkinen K., Ibáñez J., Kraemer W.J., Gorostiaga E.M. Effects of combined resistance and cardiovascular training on strength, power, muscle cross-sectional area, and endurance markers in middle-aged men. Eur J Appl Physiol. 2005;94:70–75. doi: 10.1007/s00421-004-1280-5. [DOI] [PubMed] [Google Scholar]
- 48.Jones T.W., Eddens L., Kupusarevic J., et al. Effects of cycling intensity on acute signaling adaptations to 8-weeks concurrent training in trained cyclists. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.852595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kazior Z., Willis S.J., Moberg M., et al. Endurance exercise enhances the effect of strength training on muscle fiber size and protein expression of Akt and mTOR. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laird RHt, Elmer D.J., Barberio M.D., Salom L.P., Lee K.A., Pascoe D.D. Evaluation of performance improvements after either resistance training or sprint interval-based concurrent training. J Strength Condit Res. 2016;30:3057–3065. doi: 10.1519/JSC.0000000000001412. [DOI] [PubMed] [Google Scholar]
- 51.Leveritt M., Abernethy P.J., Barry B., Logan P.A. Concurrent strength and endurance training: the influence of dependent variable selection. J Strength Condit Res. 2003;17:503–508. doi: 10.1519/1533-4287(2003)017<0503:csaett>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 52.Lundberg T.R., Fernandez-Gonzalo R., Gustafsson T., Tesch P.A. Aerobic exercise does not compromise muscle hypertrophy response to short-term resistance training. J Appl Physiol. 2013;114:81–89. doi: 10.1152/japplphysiol.01013.2012. [DOI] [PubMed] [Google Scholar]
- 53.Mikkola J., Rusko H., Izquierdo M., Gorostiaga E.M., Hakkinen K. Neuromuscular and cardiovascular adaptations during concurrent strength and endurance training in untrained men. Int J Sports Med. 2012;33:702–710. doi: 10.1055/s-0031-1295475. [DOI] [PubMed] [Google Scholar]
- 54.Panissa V.L.G., Fukuda D.H., de Oliveira F.P., et al. Maximum strength development and volume-load during concurrent high intensity intermittent training plus strength or strength-only training. J Sports Sci Med. 2018;17:623–632. [PMC free article] [PubMed] [Google Scholar]
- 55.Petré H., Löfving P., Psilander N. The effect of two different concurrent training programs on strength and power gains in highly-trained individuals. J Sports Sci Med. 2018;17:167–173. [PMC free article] [PubMed] [Google Scholar]
- 56.Putman C.T., Xu X., Gillies E., MacLean I.M., Bell G.J. Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur J Appl Physiol. 2004;92:376–384. doi: 10.1007/s00421-004-1104-7. [DOI] [PubMed] [Google Scholar]
- 57.Prieto-González P., Sedlacek J. Effects of running-specific strength training, endurance training, and concurrent training on recreational endurance athletes' performance and selected Anthropometric parameters. Int J Environ Res Publ Health. 2022;19 doi: 10.3390/ijerph191710773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robineau J., Babault N., Piscione J., Lacome M., Bigard A.X. Specific training effects of concurrent aerobic and strength exercises depend on recovery duration. J Strength Condit Res. 2016;30:672–683. doi: 10.1519/JSC.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 59.Robineau J., Lacome M., Piscione J., Bigard X., Babault N. Concurrent training in rugby sevens: effects of high-intensity interval exercises. Int J Sports Physiol Perform. 2017;12:336–344. doi: 10.1123/ijspp.2015-0370. [DOI] [PubMed] [Google Scholar]
- 60.Shamim B., Devlin B.L., Timmins R.G., et al. Adaptations to concurrent training in combination with high protein Availability: a comparative trial in healthy, recreationally active men. Sports Med. 2018;48:2869–2883. doi: 10.1007/s40279-018-0999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Timmins R.G., Shamim B., Tofari P.J., Hickey J.T., Camera D.M. Differences in lower limb strength and structure after 12 Weeks of resistance, endurance, and concurrent training. Int J Sports Physiol Perform. 2020:1–8. doi: 10.1123/ijspp.2019-0788. [DOI] [PubMed] [Google Scholar]
- 62.Volpe S.L., Walberg-Rankin J., Rodman K.W., Sebolt D.R. The effect of endurance running on training adaptations in women participating in a weight lifting program. J Strength Condit Res. 1993;7:101–107. [Google Scholar]
- 63.Hickson R.C. Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol. 1980;45:255–263. doi: 10.1007/BF00421333. [DOI] [PubMed] [Google Scholar]
- 64.Gettman L.R., Ward P., Hagan R.D. A comparison of combined running and weight training with circuit weight training. Med Sci Sports Exerc. 1982;14:229–234. [PubMed] [Google Scholar]
- 65.Kraemer W.J., Patton J.F., Gordon S.E., et al. Compatibility of high-intensity strength and endurance training on hormonal and skeletal muscle adaptations. J Appl Physiol. 1995;78:976–989. doi: 10.1152/jappl.1995.78.3.976. [DOI] [PubMed] [Google Scholar]
- 66.Ross R.E., Ratamess N.A., Hoffman J.R., Faigenbaum A.D., Kang J., Chilakos A. The effects of treadmill sprint training and resistance training on maximal running velocity and power. J Strength Condit Res. 2009;23:385–394. doi: 10.1519/JSC.0b013e3181964a7a. [DOI] [PubMed] [Google Scholar]
- 67.Sale D.G., MacDougall J.D., Jacobs I., Garner S. Interaction between concurrent strength and endurance training. J Appl Physiol. 1990;68:260–270. doi: 10.1152/jappl.1990.68.1.260. [DOI] [PubMed] [Google Scholar]
- 68.Spiliopoulou P., Zaras N., Methenitis S., et al. Effect of concurrent power training and high-intensity interval cycling on muscle morphology and performance. J Strength Condit Res. 2021;35:2464–2471. doi: 10.1519/JSC.0000000000003172. [DOI] [PubMed] [Google Scholar]
- 69.Kraemer W.J., Vescovi J.D., Volek J.S., et al. Effects of concurrent resistance and aerobic training on load-bearing performance and the Army physical fitness test. Mil Med. 2004;169:994–999. doi: 10.7205/milmed.169.12.994. [DOI] [PubMed] [Google Scholar]
- 70.Wilson J.M., Marin P.J., Rhea M.R., Wilson S.M.C., Loenneke J.P., Anderson J.C. Concurrent training: a meta-analysis examining interference of aerobic and resistance exercises. J Strength Condit Res. 2012;26:2293–2307. doi: 10.1519/JSC.0b013e31823a3e2d. [DOI] [PubMed] [Google Scholar]
- 71.Nader G.A. Concurrent strength and endurance training: from molecules to man. Med Sci Sports Exerc. 2006;38:1965–1970. doi: 10.1249/01.mss.0000233795.39282.33. [DOI] [PubMed] [Google Scholar]
- 72.Fry A.C. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34:663–679. doi: 10.2165/00007256-200434100-00004. [DOI] [PubMed] [Google Scholar]
- 73.Osawa Y., Azuma K., Tabata S., et al. Effects of 16-week high-intensity interval training using upper and lower body ergometers on aerobic fitness and morphological changes in healthy men: a preliminary study. Open Access J Sports Med. 2014;5:257–265. doi: 10.2147/OAJSM.S68932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Linossier M.T., Dormois D., Geyssant A., Denis C. Performance and fibre characteristics of human skeletal muscle during short sprint training and detraining on a cycle ergometer. Eur J Appl Physiol Occup Physiol. 1997;75:491–498. doi: 10.1007/s004210050194. [DOI] [PubMed] [Google Scholar]
- 75.Jiménez-García J.D., Martínez-Amat A., De la Torre-Cruz M.J., et al. Suspension training HIIT improves gait speed, strength and quality of life in older adults. Int J Sports Med. 2019;40:116–124. doi: 10.1055/a-0787-1548. [DOI] [PubMed] [Google Scholar]
- 76.Alzar-Teruel M., Aibar-Almazán A., Hita-Contreras F., et al. High-intensity interval training among middle-aged and older adults for body composition and muscle strength: a systematic review. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.992706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bell K.E., Séguin C., Parise G., Baker S.K., Phillips S.M. Day-to-Day changes in muscle protein synthesis in recovery from resistance, aerobic, and high-intensity interval exercise in older men. J Gerontol A Biol Sci Med Sci. 2015;70:1024–1029. doi: 10.1093/gerona/glu313. [DOI] [PubMed] [Google Scholar]
- 78.Callahan M.J., Parr E.B., Hawley J.A., Camera D.M. Can high-intensity interval training promote skeletal muscle Anabolism? Sports Med. 2021;51:405–421. doi: 10.1007/s40279-020-01397-3. [DOI] [PubMed] [Google Scholar]
- 79.Thomson D.M., Gordon S.E. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol. 2005;98:557–564. doi: 10.1152/japplphysiol.00811.2004. [DOI] [PubMed] [Google Scholar]
- 80.Fyfe J.J., Bartlett J.D., Hanson E.D., Stepto N.K., Bishop D.J. Endurance training intensity does not mediate interference to maximal lower-body strength gain during short-term concurrent training. Front Physiol. 2016;7:487. doi: 10.3389/fphys.2016.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones T.W., Eddens L., Kupusarevic J., et al. Aerobic exercise intensity does not affect the anabolic signaling following resistance exercise in endurance athletes. Sci Rep. 2021;11 doi: 10.1038/s41598-021-90274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wong P.L., Chaouachi A., Chamari K., Dellal A., Wisloff U. Effect of preseason concurrent muscular strength and high-intensity interval training in professional soccer players. J Strength Condit Res. 2010;24:653–660. doi: 10.1519/JSC.0b013e3181aa36a2. [DOI] [PubMed] [Google Scholar]
- 83.de Souza E.O., Tricoli V., Franchini E., Paulo A.C., Regazzini M., Ugrinowitsch C. Acute effect of two aerobic exercise modes on maximum strength and strength endurance. J Strength Condit Res. 2007;21:1286–1290. doi: 10.1519/R-20686.1. [DOI] [PubMed] [Google Scholar]
- 84.Burgomaster K.A., Howarth K.R., Phillips S.M., et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Markov A., Chaabene H., Hauser L., et al. Acute effects of aerobic exercise on muscle strength and power in trained male individuals: a systematic review with meta-analysis. Sports Med. 2022;52:1385–1398. doi: 10.1007/s40279-021-01615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bijker K.E., de Groot G., Hollander A.P. Differences in leg muscle activity during running and cycling in humans. Eur J Appl Physiol. 2002;87:556–561. doi: 10.1007/s00421-002-0663-8. [DOI] [PubMed] [Google Scholar]
- 87.Millet G.Y., Lepers R. Alterations of neuromuscular function after prolonged running, cycling and skiing exercises. Sports Med. 2004;34:105–116. doi: 10.2165/00007256-200434020-00004. [DOI] [PubMed] [Google Scholar]
- 88.Frankenberg N.T., Lamb G.D., Overgaard K., Murphy R.M., Vissing K. Small heat shock proteins translocate to the cytoskeleton in human skeletal muscle following eccentric exercise independently of phosphorylation. J Appl Physiol. 2014;116:1463–1472. doi: 10.1152/japplphysiol.01026.2013. [DOI] [PubMed] [Google Scholar]
- 89.Jacko D., Bersiner K., Schulz O., et al. Coordinated alpha-crystallin B phosphorylation and desmin expression indicate adaptation and deadaptation to resistance exercise-induced loading in human skeletal muscle. Am J Physiol Cell Physiol. 2020;319:C300–c312. doi: 10.1152/ajpcell.00087.2020. [DOI] [PubMed] [Google Scholar]
- 90.Glass D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 91.Léger B., Cartoni R., Praz M., et al. Akt signalling through GSK-3 beta, mTOR and Foxo 1 is involved in human skeletal muscle hypertrophy and atrophy. J Physiol. 2006;576:923–933. doi: 10.1113/jphysiol.2006.116715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glass D.J. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–350. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 93.Coffey V.G., Hawley J.A. The molecular bases of training adaptation. Sports Med. 2007;37:737–763. doi: 10.2165/00007256-200737090-00001. [DOI] [PubMed] [Google Scholar]
- 94.Ogasawara R., Sato K., Matsutani K., Nakazato K., Fujita S. The order of concurrent endurance and resistance exercise modifies mTOR signaling and protein synthesis in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2014;306:E1155–E1162. doi: 10.1152/ajpendo.00647.2013. [DOI] [PubMed] [Google Scholar]
- 95.Apró W., Moberg M., Hamilton D.L., et al. Resistance exercise-induced S6K1 kinase activity is not inhibited in human skeletal muscle despite prior activation of AMPK by high-intensity interval cycling. Am J Physiol Endocrinol Metab. 2015;308:E470–E481. doi: 10.1152/ajpendo.00486.2014. [DOI] [PubMed] [Google Scholar]
- 96.Lundberg T.R., Fernandez-Gonzalo R., Gustafsson T., Tesch P.A. Aerobic exercise alters skeletal muscle molecular responses to resistance exercise. Med Sci Sports Exerc. 2012;44:1680–1688. doi: 10.1249/MSS.0b013e318256fbe8. [DOI] [PubMed] [Google Scholar]
- 97.Pugh J.K., Faulkner S.H., Jackson A.P., King J.A., Nimmo M.A. Acute molecular responses to concurrent resistance and high-intensity interval exercise in untrained skeletal muscle. Phys Rep. 2015;3 doi: 10.14814/phy2.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Apró W., Wang L., Pontén M., Blomstrand E., Sahlin K. Resistance exercise induced mTORC1 signaling is not impaired by subsequent endurance exercise in human skeletal muscle. Am J Physiol Endocrinol Metab. 2013;305:E22–E32. doi: 10.1152/ajpendo.00091.2013. [DOI] [PubMed] [Google Scholar]
- 99.Donges C.E., Burd N.A., Duffield R., et al. Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men. J Appl Physiol. 2012;112:1992–2001. doi: 10.1152/japplphysiol.00166.2012. [DOI] [PubMed] [Google Scholar]
- 100.Nakashima K., Yakabe Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem. 2007;71:1650–1656. doi: 10.1271/bbb.70057. [DOI] [PubMed] [Google Scholar]
- 101.Coffey V.G., Hawley J.A. Concurrent exercise training: do opposites distract? J Physiol. 2017;595:2883–2896. doi: 10.1113/JP272270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.