Abstract
Background
Human chorionic gonadotropin (HCG) is routinely used for final oocyte maturation triggering in in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) cycles, but the use of HCG for this purpose may have drawbacks. Gonadotropin‐releasing hormone (GnRH) agonists present an alternative to HCG in controlled ovarian hyperstimulation (COH) treatment regimens in which the cycle has been down‐regulated with a GnRH antagonist. This is an update of a review first published in 2010.
Objectives
To evaluate the effectiveness and safety of GnRH agonists in comparison with HCG for triggering final oocyte maturation in IVF and ICSI for women undergoing COH in a GnRH antagonist protocol.
Search methods
We searched databases including the Menstrual Disorders and Subfertility Group (MDSG) Specialised Register of Controlled Trials, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO, the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and trial registers for published and unpublished articles (in any language) on randomised controlled trials (RCTs) of gonadotropin‐releasing hormone agonists versus HCG for oocyte triggering in GnRH antagonist IVF/ICSI treatment cycles. The search is current to 8 September 2014.
Selection criteria
RCTs that compared the clinical outcomes of GnRH agonist triggers versus HCG for final oocyte maturation triggering in women undergoing GnRH antagonist IVF/ICSI treatment cycles were included.
Data collection and analysis
Two or more review authors independently selected studies, extracted data and assessed study risk of bias. Treatment effects were summarised using a fixed‐effect model, and subgroup analyses were conducted to explore potential sources of heterogeneity. Treatment effects were expressed as mean differences (MDs) for continuous outcomes and as odds ratios (ORs) for dichotomous outcomes, together with 95% confidence intervals (CIs). Primary outcomes were live birth and rate of ovarian hyperstimulation syndrome (OHSS) per women randomised. Grades of Recommendation, Assessment, Development and Evaluation (GRADE) methods were used to assess the quality of the evidence for each comparison.
Main results
We included 17 RCTs (n = 1847), of which 13 studies assessed fresh autologous cycles and four studies assessed donor‐recipient cycles. In fresh autologous cycles, GnRH agonists were associated with a lower live birth rate than was seen with HCG (OR 0.47, 95% CI 0.31 to 0.70; five RCTs, 532 women, I2 = 56%, moderate‐quality evidence). This suggests that for a woman with a 31% chance of achieving live birth with the use of HCG, the chance of a live birth with the use of an GnRH agonist would be between 12% and 24%.
In women undergoing fresh autologous cycles, GnRH agonists were associated with a lower incidence of mild, moderate or severe OHSS than was HCG (OR 0.15, 95% CI 0.05 to 0.47; eight RCTs, 989 women, I² = 42%, moderate‐quality evidence). This suggests that for a woman with a 5% risk of mild, moderate or severe OHSS with the use of HCG, the risk of OHSS with the use of a GnRH agonist would be between nil and 2%.
In women undergoing fresh autologous cycles, GnRH agonists were associated with a lower ongoing pregnancy rate than was seen with HCG (OR 0.70, 95% CI 0.54 to 0.91; 11 studies, 1198 women, I2 = 59%, low‐quality evidence) and a higher early miscarriage rate (OR 1.74, 95% CI 1.10 to 2.75; 11 RCTs, 1198 women, I² = 1%, moderate‐quality evidence). However, the effect was dependent on the type of luteal phase support provided (with or without luteinising hormone (LH) activity); the higher rate of pregnancies in the HCG group applied only to the group that received luteal phase support without LH activity (OR 0.36, 95% CI 0.21 to 0.62; I2 = 73%, five RCTs, 370 women). No evidence was found of a difference between groups in risk of multiple pregnancy (OR 3.00, 95% CI 0.30 to 30.47; two RCTs, 62 women, I2 = 0%, low‐quality evidence).
In women with donor‐recipient cycles, no evidence suggested a difference between groups in live birth rate (OR 0.92, 95% CI 0.53 to 1.61; one RCT, 212 women) or ongoing pregnancy rate (OR 0.88, 95% CI 0.58 to 1.32; three RCTs, 372 women, I² = 0%). We found evidence of a lower incidence of OHSS in the GnRH agonist group than in the HCG group (OR 0.05, 95% CI 0.01 to 0.28; three RCTs, 374 women, I² = 0%).
The main limitation in the quality of the evidence was risk of bias associated with poor reporting of methods in the included studies.
Authors' conclusions
Final oocyte maturation triggering with GnRH agonist instead of HCG in fresh autologous GnRH antagonist IVF/ICSI treatment cycles prevents OHSS to the detriment of the live birth rate. In donor‐recipient cycles, use of GnRH agonists instead of HCG resulted in a lower incidence of OHSS, with no evidence of a difference in live birth rate.
Evidence suggests that GnRH agonist as a final oocyte maturation trigger in fresh autologous cycles is associated with a lower live birth rate, a lower ongoing pregnancy rate (pregnancy beyond 12 weeks) and a higher rate of early miscarriage (less than 12 weeks). GnRH agonist as an oocyte maturation trigger could be useful for women who choose to avoid fresh transfers (for whatever reason), women who donate oocytes to recipients or women who wish to freeze their eggs for later use in the context of fertility preservation.
Plain language summary
Gonadotropin‐releasing hormone agonist versus HCG for oocyte triggering in antagonist‐assisted reproductive technology cycles
Review question
We reviewed the evidence on the effects of GnRH agonists on final oocyte maturation triggering in GnRH antagonist IVF/ICSI treatment cycles.
Background
Oocyte maturation triggering is the final differentiation process of an immature oocyte before fertilisation in unstimulated or stimulated cycles with assisted reproductive techniques. Two hormones can be used to trigger oocyte maturation: human chorionic gonadotropin (HCG), which is the standard treatment, and gonadotropin‐releasing hormone agonist (GnRH agonist). In this review, we assessed the benefits and harms of GnRH agonists as oocyte maturation triggers. Evidence is current to September 2014.
Study characteristics
We included 17 studies of 1817 women. Researchers assessed fresh or donor cycles in women at varying risk of ovarian hyperstimulation syndrome (OHSS). The authors of four studies stated that the studies were commercially funded. Most studies failed to disclose their funding source.
Key results
GnRH agonist triggers significantly reduce the risk of ovarian hyperstimulation but also lower the chance of pregnancy in fresh autologous IVF/ICSI treatment cycles compared with HCG. GnRH agonist use as an oocyte maturation trigger could be useful for women who choose to avoid fresh transfers (for whatever reason), women who donate oocytes to recipients or women who wish to freeze their eggs for later use in the context of fertility preservation.
Quality of the evidence
The overall quality of the evidence was moderate for most comparisons. The main limitation in the quality of the evidence was risk of bias associated with poor reporting of study methods.
Summary of findings
Summary of findings for the main comparison. GnRH agonist compared with HCG for oocyte maturation triggering in antagonist‐assisted reproductive technology.
| GnRH agonist compared with HCG for oocyte maturation triggering in antagonist‐assisted reproductive technology | ||||||
| Population: subfertile women Settings: assisted reproductive technology: autologous cycles Intervention: GnRH agonist Comparison: HCG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| HCG for oocyte maturation triggering | GnRH agonist | |||||
| Live birth | 313 per 1000 | 176 per 1000 (124 to 242) | OR 0.47 (0.31 to 0.70 ) | 532 (5 studies) | ⊕⊕⊕⊝ Moderatea,d | |
| OHSS (mild, moderate or severe): overall risk | 5 per 1000 | 1 per 1000 (0 to 2) | OR 0.15 (0.05 to 0.47 ) | 989 (8 studies) | ⊕⊕⊕⊝ Moderateb | |
| OHSS (moderate or severe): overall risk | 5 per 1000 | 1 per 1000 (0 to 3) | OR 0.21 (0.07 to 0.66 ) | 989 (8 studies) | ⊕⊕⊕⊝ Moderatec | Low event rate: 4 of 9 RCTs reported no events in either arm |
| OHSS (mild, moderate or severe) in women at high risk of OHSS | 308 per 1000 | 26 per 1000 (4 to 131) |
OR 0.06 (0.01 to 0.34) | 212 women (3 studies) |
⊕⊕⊕⊝ Moderateb | |
| Ongoing pregnancy | 256 per 1000 | 194 per 1000 (157 to 238) | OR 0.7 (0.54 to 0.91 ) | 1198 (11 studies) | ⊕⊕⊝⊝ Lowd,e | |
| Miscarriage | 67 per 1000 | 111 per 1000 (73 to 165) | OR 1.74 (1.10 to 2.75 ) | 1198 (11 studies) | ⊕⊕⊕⊝ Moderatee | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOne of the studies at high risk of bias because of premature termination.
bAll studies at high risk of bias in 1 or more domains. None clearly reported blinded outcome assessment.
cMost studies at high risk of bias in 1 or more domains. None clearly reported blinded outcome assessment.
dSubstantial heterogeneity: I2 = 59% to 66%.
e5/11 studies at high risk of bias because of early termination and/or inadequate allocation concealment. None clearly reported blinded outcome assessment.
Background
Description of the condition
After oocyte growth is stimulated by gonadotropins, the next step in in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) treatment consists of triggering the oocytes to go through the last stage of maturation, so that they can be retrieved and fertilised. This final oocyte maturation is usually triggered by human chorionic gonadotropin (HCG), but use of HCG for this purpose may have drawbacks. Some studies have suggested a negative impact of HCG on endometrial receptivity (Simon 1995; Forman 1998; Simon 1998) and embryo quality (Valbuena 2001; Tavaniotou 2002). In addition, the sustained luteotrophic effect of HCG is associated with increased chances of ovarian hyperstimulation syndrome (OHSS), which is an iatrogenic complication of assisted reproductive technology (ART).
OHSS may be associated with massive ovarian enlargement, ascites, hydrothorax, liver dysfunction and renal failure. It can lead to cancellation of an IVF cycle and the need for prolonged bed rest or hospitalisation, which may have a significant emotional, social and economic impact or—in its most severe form—may even result in mortality (Delvigne 2003).
Gonadotropin‐releasing hormone (GnRH) agonists present an alternative to HCG for triggering endogenous luteinising hormone (LH) release (Gonen 1990; Olivennes 1996; Olivennes 2001; Tay 2002). Use of GnRH agonist triggering is applicable only in IVF with controlled ovarian hyperstimulation (COH) treatment regimens in which the cycle has been down‐regulated by a GnRH antagonist. Because of the specific mode of action of the antagonist, the pituitary remains responsive to a GnRH agonist, provided that the GnRH antagonist treatment utilised standard doses (Felberbaum 1995; Orvieto 2006).
Description of the intervention
A midcycle single bolus of GnRH agonist may be injected subcutaneously (0.2 to 0.5 mg of triptorelin, leuprorelin or buserelin) (Itskovitz‐Eldor 2000; Humaidan 2005) or administered intranasally (200 µg buserelin) (Pirard 2006).
How the intervention might work
A single injection of a GnRH agonist results in an acute release of LH and follicle‐stimulating hormone (FSH)—the so‐called flare‐up. Serum LH and FSH levels rise after four hours and 12 hours, respectively, and are elevated for 24 to 36 hours. The amplitude of the surges is similar to that seen in the normal menstrual cycle, but, in contrast to the natural cycle, the LH surge consists of two phases: a short ascending limb (> 4 hours) and a long descending limb (> 20 hours). This has no bearing on luteal phase steroid levels, which are qualitatively similar to those observed in the natural cycle (Segal 1992; Itskovitz‐Eldor 2000; Fauser 2002; Nevo 2003; Kol 2004).
Consequently, oocyte maturation triggering with GnRH agonists may provide several advantages over that achieved with HCG. First, GnRH agonists reduce the risk of OHSS due to quick and irreversible luteolysis (Kol 2004). Second, a more physiological LH and FSH surge is induced by the agonists, which may result in better oocyte and embryo quality (Humaidan 2005). Third, GnRH agonists may improve endometrial quality as a result of the lower luteal phase steroid levels (Forman 1998; Simon 1998).
Why it is important to do this review
This is an update of a review first published in 2010 (Youssef 2010). HCG is the standard medication for final oocyte maturation triggering. More recently, GnRH agonists have been proposed, especially as they may prevent OHSS to a large extent. Summarising the available evidence shows what is known about the effectiveness and safety of GnRH agonists in comparison with HCG and hence will help fertility experts and women to make informed decisions on final oocyte maturation triggering by GnRH antagonists in IVF/ICSI treatment cycles.
Objectives
To evaluate the effectiveness and safety of GnRH agonists in comparison with HCG for triggering final oocyte maturation in IVF and ICSI for women undergoing COH in a GnRH antagonist protocol.
Methods
Criteria for considering studies for this review
Types of studies
Only published and unpublished randomised controlled trials (RCTs) were included in the review.
Non‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days and participant numbers), as they are associated with high risk of bias, were excluded from the review.
Cross‐over trials were excluded, as the design is not valid in this context.
Types of participants
Inclusion criteria
Subfertile couples undergoing IVF or ICSI for therapeutic reasons or for oocyte donation and randomly assigned to receive a GnRH agonist or HCG for final oocyte maturation triggering.
Exclusion criteria
Women who were not undergoing IVF or ICSI (i.e. those undergoing intrauterine insemination (IUI)).
Types of interventions
GnRH agonists in comparison with HCG for final oocyte maturation triggering in GnRH antagonist–controlled hyperstimulation cycles, IVF or ICSI followed by embryo transfer (ET) with or without luteal phase support, in autologous or donor cycles.
Types of outcome measures
Primary outcomes
Live birth rate (LBR) per woman randomised: live birth defined as delivery of a live fetus after 20 completed weeks of gestation.
Incidence of OHSS per woman randomised (mild, moderate or severe): detected by clinical, laboratory or imaging grading of OHSS.
Secondary outcomes
Ongoing pregnancy rate (OPR) per woman randomised: ongoing pregnancy defined as pregnancy beyond 12 weeks.
Clinical pregnancy rate (CPR) per woman randomised: clinical pregnancy defined as presence of a fetal heart rate with transvaginal ultrasound.
Early miscarriage rate per woman randomised.
Multiple pregnancy rate per woman randomised.
Search methods for identification of studies
All published and unpublished RCTs of GnRH agonists versus HCG for final oocyte maturation triggering were sought, without language restriction and in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator, using the following search strategy.
Electronic searches
2014 update
We searched the following electronic databases, trial registers and websites to 8 September 2014: the MDSG Specialised Register of Controlled Trials (Appendix 1), the Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 2), MEDLINE (Appendix 3), EMBASE (Appendix 4), PsycINFO (Appendix 5) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL)(Appendix 6). Other electronic sources of trials included the following.
Trial registers for ongoing and registered trials: http://www.controlled‐trials.com, http://clinicaltrials.gov/ct2/home, http://www.who.int/trialsearch/Default.aspx.
Citation indexes: http://scientific.thomson.com/products/sci/ Conference abstracts.
Conference abstracts in the Web of Knowledge: http://www.wokinfo.com
Latin American and Caribbean Health Science Information Database (LILACS) database, for trials from the Portuguese and Spanish‐speaking world: http://bases.bireme.br/cgi ‐bin/ wxislind.exe/iah/online/?IsisScript=iah/i ah.xis&base=LILACS&lang=i&form=F.
PubMed: www.ncbi.nlm.nih.gov/pubmed/.
Open System for Information on Grey Literature in Europe (OpenSIGLE) database (http://opensigle.inist.fr/) and Google for grey literature.
MEDLINE and EMBASE search strategies use different filters for identifying randomised trials. The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0.1, Chapter 6, 6.4.11). EMBASE and CINAHL searches were combined with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/methodology/filters.html#random).
Searching other resources
Reference lists of relevant clinical practice guidelines, review articles and studies.
Letters seeking information about unpublished or incomplete RCTs sent to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search, conducted by MAFMY and MVW, the full texts of all potentially eligible studies were retrieved. These full‐text articles were examined for compliance with the inclusion criteria, and review authors selected studies eligible for inclusion in the review. We corresponded with study investigators as required to clarify study eligibility. The selection process was documented on a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 1).
1.
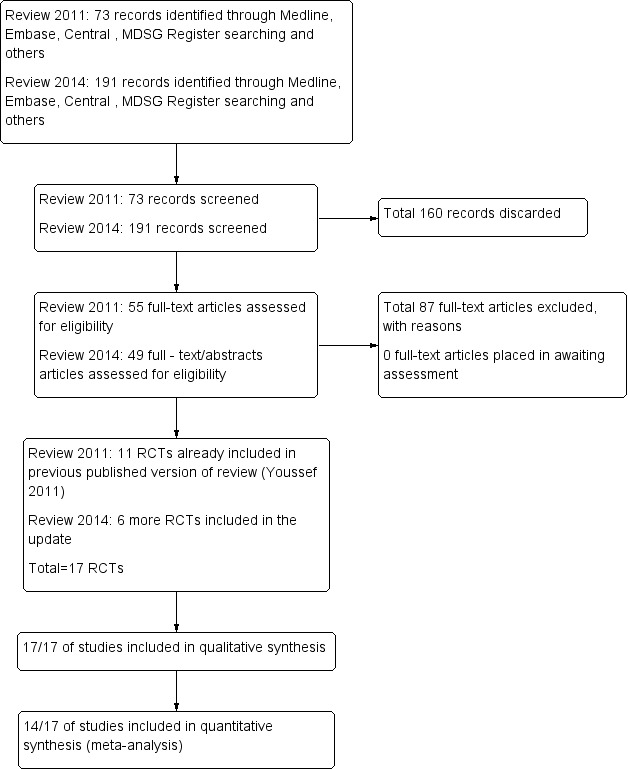
Study flow diagram.
Data extraction and management
Two review authors independently extracted data from eligible studies using a standard data extraction form that they designed and pilot‐tested. Disagreements were resolved by discussion or by consultation with a third review author. Extracted data included study characteristics and outcome data (see data extraction table for details, Characteristics of included studies).
Data entry was carried out by the same two review authors.
Studies were analysed for the following quality criteria and methodological details.
Trial characteristics
Study design
Method of randomisation.
Multi‐centre or single‐centre design.
Presence or absence of blinding to treatment allocation.
Number of participants randomised, excluded or lost to follow‐up.
Presence of intention‐to‐treat (ITT) analysis.
Presence of a power calculation.
Characteristics of study participants
Subfertile women undergoing IVF/ICSI treatment cycles.
At high or low risk to develop OHSS.
Interventions used
Types of ovarian hyperstimulation protocols used.
Types of final oocyte maturation triggering used: route of administration, duration and dose.
Types of luteal phase support provided: dose, duration and route of administration.
Outcomes
LBR.
Incidence of OHSS.
Ongoing pregnancy rate.
Clinical pregnancy rate.
Miscarriage rate.
Multiple pregnancy rate.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias using the risk of bias assessment tool of The Cochrane Collaboration (Higgins 2011) to assess allocation (random sequence generation and allocation concealment); blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; and other bias. Disagreements were resolved by discussion or by consultation with a third review author.
Randomisation
Randomisation was considered adequate if any random method of allocation was described and was verifiable, that is,
using a computerised random number generator; or
referring to a number table.
Concealment of allocation (selection bias)
This was considered adequate if a third‐party system; serially numbered sealed, opaque envelopes; or a similar system was described. Concealment was stated as 'unclear ' if no information was available pertaining to allocation concealment.
Blinding of participants and personnel (performance bias)
This was examined with regard to likelihood of bias influencing primary outcomes. We considered it unlikely that blinding would influence findings for live birth, but likely that blinding could influence findings for OHSS, so unblinded studies were rated as having high risk of bias for this outcome.
Blinding of outcome assessors (detection bias)
This was examined with regard to likelihood of bias influencing primary outcomes. We considered it unlikely that blinding would influence findings for live birth, but likely that blinding could influence findings for OHSS, so unblinded studies were rated as having high risk of bias for this outcome.
Incomplete outcome data
Low risk of bias was allocated if no outcome data were missing, or if missing outcome data were balanced in numbers across intervention groups with similar reasons provided for missing data across groups.
Selective outcome reporting
Low risk of bias was allocated if all of a study's primary, secondary and additional outcomes of interest in the review were reported in a prespecified way; when fewer outcome measures were reported than planned, this was considered to be a source of bias.
Other potential sources of bias
We considered other potential forms of bias (e.g. baseline imbalance of groups, premature discontinuation of study).
Measures of treatment effect
For dichotomous data (e.g. live birth rates), the numbers of events in control and intervention groups of each study were used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) for each individual trial.
Unit of analysis issues
The primary analysis was per woman randomised (e.g. live birth rate or miscarriage rate per woman randomised, defined as the number of women achieving a live birth divided by the number of women treated). Data per cycle were not included in the analysis.
Dealing with missing data
When possible, data were extracted to allow for an ITT analysis, defined as including all randomised participants in the denominator. When appropriate, study authors were contacted to provide further information or missing data. Data obtained in this manner were included in our analyses. Women lost to follow‐up were assumed to be not pregnant.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by the measure of the I2 statistic. An I2 measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2011). We tested the effect of using a random‐effects model when heterogeneity was substantial.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If 10 or more studies were included in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
Data from primary studies were combined using the fixed‐effect model in the following comparisons.
GnRH agonist versus HCG in fresh autologous cycles.
GnRH agonist versus HCG in donor‐recipient cycles.
An increase in the odds of a particular outcome, which may be beneficial (e.g. live birth) or detrimental (e.g. OHSS, miscarriage), was displayed graphically in the meta‐analyses to the right of the centre‐line (i.e. in favour of GnRH agonist), and a decrease in the odds of an outcome to the left of the centre‐line (i.e. in favour of HCG).
For the meta‐analysis, the number of women experiencing the event in each group of the trial was recorded. Meta‐analysis of binary data was performed using the Mantel‐Haenszel method with a fixed‐effect model, and the OR and the 95% CI were calculated using RevMan 5 software.
We performed a separate analysis for oocyte donor‐recipient cycles.
Subgroup analysis and investigation of heterogeneity
We considered clinical and methodological differences between studies that might account for any heterogeneity.
When data were available, we conducted subgroup analyses to determine separate evidence within the following subgroups in studies of autologous cycles.
Type of luteal phase support (for the outcomes of live birth, OHSS and ongoing pregnancy)
Luteal phase support with LH activity (single or two doses of HCG, recLH and repeated GnRH doses)
Luteal phase support without LH activity (progesterone only or progesterone plus oestradiol).
Risk of OHSS (for the outcome of OHSS)
Studies of women with low OHSS risk: Low risk was defined as studies excluding women with polycystic ovary syndrome (PCOS) or women with high numbers of ovarian follicles (≥ 14 follicles) ≥ 11 mm in diameter.
Studies of women with high OHSS risk: High risk was defined as studies including women with PCOS or women with high numbers of ovarian follicles (≥ 14 follicles) ≥ 11 mm in diameter.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding study eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
we had used a random‐effects model for the primary outcomes;
we had reported risk ratios rather than odds ratios; or
we had included only moderate or severe OHSS as an outcome (not including mild OHSS).
Results
Description of studies
For details about the studies, please see: Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
In searches to date (2011 and 2014), a total of 264 references were identified. Most references identified by the search were excluded at the first screening step, as they were clearly irrelevant (n = 160). The most frequent reasons for exclusion were the following: The article was a review or a commentary/editorial, or the study was clearly of a non‐randomised design or reported comparisons of no interest (n = 87); 17 RCTs fulfilled the inclusion criteria (Figure 1).
Included studies
Design
Seventeen RCTs, 13 in fresh autologous cycles and four in donor‐recipient cycles, including 1847 randomly assigned women, met the inclusion criteria and were fully reviewed. Randomisation was done as soon as oocyte maturation triggering was planned in all except one trial. In this trial, randomisation was timed to occur at the beginning of stimulation (Kolibianakis 2005). Three abstracts (Segal 1992; Ossina 2004; Peňa 2007) were published in conference proceedings. As it was not possible to obtain further information from the authors of these abstracts, they were excluded from the quantitative analysis. See Characteristics of included studies.
Ten studies were randomised controlled single‐centre studies (Segal 1992; Acevedo 2006; Babayof 2006; Humaidan 2006; Pirard 2006; Peňa 2007; Engmann 2008; Galindo 2009; Melo 2009; Papanikolaou 2010). Three studies were two‐centre studies (Beckers 2003; Humaidan 2005; Kolibianakis 2005), one was a three‐centre study (Humaidan 2010), one was a four‐centre study (Humaidan 2013) and two studies were six‐centre studies (Fauser 2002; Ossina 2004).
Nine studies reported sample size calculations for the primary outcome (Beckers 2003; Humaidan 2005; Kolibianakis 2005; Babayof 2006; Engmann 2008; Galindo 2009; Melo 2009; Humaidan 2010; Humaidan 2013). No sample size calculation was performed in three studies (Fauser 2002; Acevedo 2006; Pirard 2006); in five studies, this information was not provided (Segal 1992; Ossina 2004; Humaidan 2006; Peňa 2007; Papanikolaou 2010).
Three studies failed to achieve the intended sample size (Humaidan 2005; Kolibianakis 2005; Humaidan 2013). Nine studies recruited only a small number of women (Fauser 2002, n = 57; Beckers 2003, n = 40; Humaidan 2005, n = 45; Acevedo 2006, n = 60; Babayof 2006, n = 28; Pirard 2006, n = 30; Engmann 2008, n= 66; Melo 2009, n = 100; Papanikolaou 2010; n = 39).
Fourteen RCTs were published as full‐text articles (Fauser 2002; Beckers 2003; Humaidan 2005; Kolibianakis 2005; Acevedo 2006; Babayof 2006; Humaidan 2006; Pirard 2006; Engmann 2008; Galindo 2009; Melo 2009; Humaidan 2010; Papanikolaou 2010; Humaidan 2013) and three as abstracts (Segal 1992; Ossina 2004; Peňa 2007) in conference proceedings.
For details of study risk of bias, see the Characteristics of included studies table.
Source of funding (Lundh 2012): Four studies (28%) reported that they received industry funding (Beckers 2003; Engmann 2008; Papanikolaou 2010; Humaidan 2013).
Participants
Analysed studies (14/17) included 791 women in the intervention groups and 779 in the control groups. All were women with subfertility from 18 to 40 years of age. All participants were undergoing IVF/ICSI treatment cycles followed by fresh ET in autologous or donor cycles.
The number of randomly assigned participants ranged from 23 (Pirard 2006) to 302 (Humaidan 2010), including both GnRH agonist and HCG groups.
Baseline characteristics were comparable between groups (Characteristics of included studies).
Ten studies included women at low risk of developing OHSS (Fauser 2002; Beckers 2003; Humaidan 2005; Kolibianakis 2005; Acevedo 2006; Humaidan 2006; Galindo 2009; Melo 2009; Humaidan 2010; Papanikolaou 2010), and only three studies randomised women with PCOS or with retrieved oocytes with more than 14 follicles (Babayof 2006; Engmann 2008; Humaidan 2013). Risk of OHSS was reported unclearly in four studies (Segal 1992; Ossina 2004; Pirard 2006; Peňa 2007).
Intervention
All included studies compared GnRH agonist versus HCG for final oocyte maturation triggering in GnRH antagonist down‐regulated IVF and ICSI cycles.
Five studies used 250 μg of recombinant HCG (rHCG) for final oocyte maturation triggering in the control group (Acevedo 2006; Babayof 2006; Galindo 2009; Melo 2009; Papanikolaou 2010). A three‐arm study compared LH versus rHCG versus GnRH (Beckers 2003). Other included studies used 10,000 IU of urinary HCG for final oocyte maturation triggering, except one (Engmann 2008), which used a dose ranging from 3300 to 10,000 IU, depending on follicular response.
Luteal phase support: Five studies used progesterone (P) plus oestradiol (E2) in fresh autologous cycles (Kolibianakis 2005; Humaidan 2005; Babayof 2006; Humaidan 2006; Engmann 2008) and one study in donor‐recipient cycles (Acevedo 2006). Two studies used the combination of P + E2 + single dose of 1500 IU hCG (Humaidan 2010) or two doses of 1500 IU HCG (Humaidan 2013); one study used P only in fresh autologous cycles (Fauser 2002) and two studies in donor‐recipient cycles (Galindo 2009; Melo 2009); one study used the combination of P + six doses of recLH (Papanikolaou 2010); one study used repeated administration of GnRH agonist (Pirard 2006); and one study provided no luteal phase support (Beckers 2003).
Outcomes
Five studies reported live birth rate in fresh autologous cycles (Humaidan 2005; Babayof 2006; Humaidan 2006; Humaidan 2010; Papanikolaou 2010) and one study in donor‐recipient cycles (Galindo 2009).
Eight studies reported OHSS incidence in fresh autologous cycles (Kolibianakis 2005; Babayof 2006; Humaidan 2006; Pirard 2006; Engmann 2008; Humaidan 2010; Papanikolaou 2010; Humaidan 2013) and three studies in donor‐recipient cycles (Acevedo 2006; Galindo 2009; Melo 2009).
All included studies reported ongoing pregnancy rate, clinical pregnancy rate and early miscarriage rate in both groups.
Multiple pregnancy rate was reported in all donor‐recipient cycles and in two studies in fresh autologous cycles (Babayof 2006; Papanikolaou 2010).
Three studies were published as abstracts with no details on outcome measures (Segal 1992; Ossina 2004; Peňa 2007); therefore they were included only in the qualitative research—not in the meta‐analysis.
Excluded studies
In searches to date (2011 and 2014), a total of 87 studies were excluded. Reasons for exclusion are explained in the Characteristics of excluded studies table.
Risk of bias in included studies
Risk of bias in the included studies is summarised in Figure 2 and Figure 3.
2.
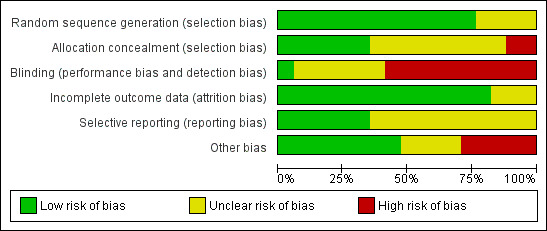
Risk of bias graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
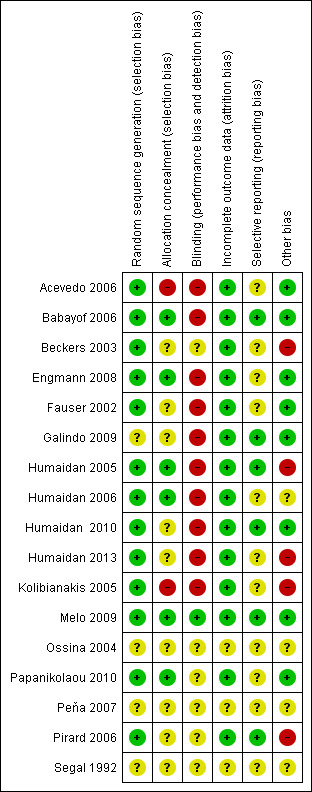
Methodological risk of bias summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Thirteen studies were rated as having low risk of bias related to sequence generation, and four were rated as having unclear risk of bias in this domain.
Six studies were rated as having low risk of bias related to allocation concealment, and nine were rated as having unclear risk of this bias. In two trials, the allocation was not adequately concealed; these studies were rated as having high risk of bias (Kolibianakis 2005; Acevedo 2006).
Blinding
One study clearly reported blinding of assessors (Melo 2009) and was deemed to be at low risk of bias related to blinding. Six studies did not clearly report on blinding and were rated as having unclear risk of bias related to assessment of OHSS. Ten reported lack of blinding and were rated as having high risk of bias related to assessment of OHSS.
Incomplete outcome data
Fourteen studies were rated as having low risk of attrition bias. Three were rated as having unclear risk of bias in this domain.
Intention‐to‐treat analysis
We contacted the following investigators of individual studies via email to ask for additional information, so we could perform analyses on an ITT basis (Fauser 2002; Humaidan 2005; Acevedo 2006; Humaidan 2006; Humaidan 2010). We could not identify contact details for the authors of two abstracts (Ossina 2004; Peňa 2007); therefore we excluded these studies from analysis on the basis of missing relevant data.
Only five studies performed an ITT analysis (Humaidan 2006; Galindo 2009; Humaidan 2010; Papanikolaou 2010; Humaidan 2013).
In seven studies, no ITT analysis was performed (Fauser 2002; Beckers 2003; Humaidan 2005; Kolibianakis 2005; Acevedo 2006; Pirard 2006; Engmann 2008), and it was unclear whether ITT was used in two studies (Babayof 2006; Melo 2009). However, for all of these studies, the number of women randomised was known; therefore the ITT data could be imputed.
Selective reporting
Six studies were rated as having low risk of selective reporting bias; 11 were rated as having unclear risk of bias in this domain, in most cases because live birth and/or OHSS was not reported.
Other potential sources of bias
For eight studies, no additional potential sources of bias were noted. Four studies were rated as having unclear risk of other bias because they were reported only as abstracts and provided insufficient details on methods.
Five studies were deemed at high risk of other potential bias. All of these studies were prematurely discontinued. In one case (Kolibianakis 2005), study discontinuation was triggered by preplanned stopping rules. In other cases (Beckers 2003; Humaidan 2005; Pirard 2006), the interim analysis was unplanned and/or stopping rules were unclear. Three of these studies were stopped prematurely as the result of a significantly lower pregnancy rate in the GnRH agonist triggering group, and in one trial with six arms, two arms were stopped prematurely for the same reason (Pirard 2006). One study was stopped prematurely before the estimated sample size had been obtained as a result of the death of one of the local principal investigators and job rotations among other investigators (Humaidan 2013).
Effects of interventions
See: Table 1
Primary outcomes
1.1 Live birth rate per woman randomised
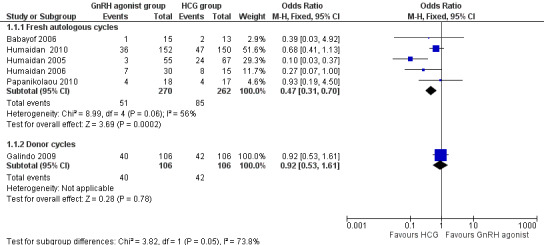
1.1.1 Fresh autologous cycles
GnRH agonist trigger was associated with a lower live birth rate than was seen with HCG (OR 0.47, 95% CI 0.31 to 0.70; five RCTs, 532 women, I² = 56%, moderate‐quality evidence). This means that for a woman with a 31% chance of achieving live birth with the use of HCG, the chance of a live birth with the use of a GnRh agonist will be between 12% and 24%. Use of a random‐effects model did not substantially affect the results (OR 0.38, 95% CI 0.17 to 0.89), nor did use of risk ratios have a substantial effect. Statistical heterogeneity for this outcome was moderate. The live birth rate varied from 15% to 53% in the HCG group and from 5% to 24% in the agonist group (Analysis 1.1; Figure 4).
1.1. Analysis.
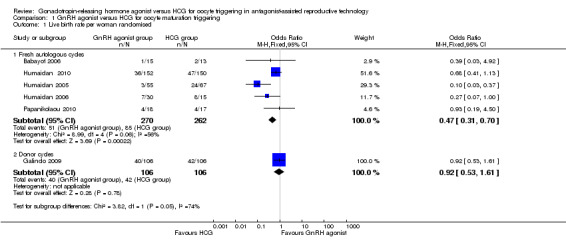
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 1 Live birth rate per woman randomised.
4.
GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.1 Live birth rate per women randomly assigned.
1.1.2 Donor‐recipient cycles
No evidence of a difference in live birth rate was noted between GnRH agonist and HCG groups in donor‐recipient cycles (OR 0.92, 95% CI 0.53 to 1.61; one RCT, 212 women) (Analysis 1.1; Figure 4).
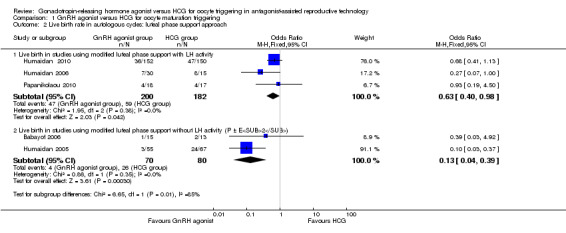
1.2 Live birth rate in autologous cycles: subgroup analysis on luteal support approach
The subgroup analysis based on luteal phase support methods used in the included studies revealed differences in live birth rates between trials that used luteal phase support with LH activity and trials that used luteal phase support without LH activity. Both groups showed evidence of differences in live birth rate in favour of HCG, but this difference was significantly greater in studies that used luteal support without LH activity (studies with luteal phase support with LH activity: OR 0.63, 95% CI 0.40 to 0.98; three RCTs, 382 women, I2 = 0%; studies with luteal phase support without LH activity: OR 0.13, 95% CI 0.04 to 0.39; two RCTS, 150 women, I2 = 73%; test for subgroup differences: Chi² = 6.65, df = 1 (P value 0.010), I² = 85.0%) (Analysis 1.2).
1.2. Analysis.
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 2 Live birth rate in autologous cycles: luteal phase support approach.
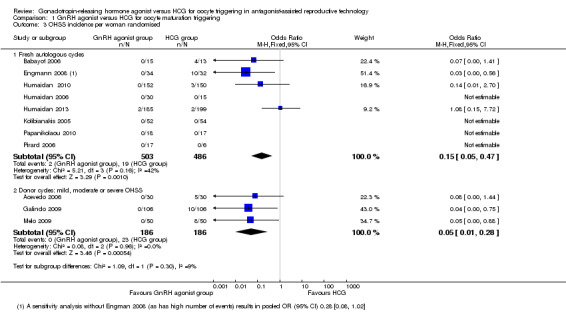
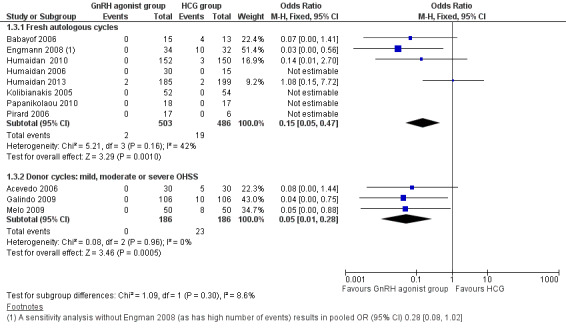
1.3 Ovarian hyperstimulation syndrome (OHSS)
1.3.1 Fresh autologous cycles
GnRH agonist was associated with lower risk of OHSS (mild, moderate or severe) than was seen with HCG (OR 0.15, 95% CI 0.05 to 0.47; eight RCTs, 989 women, I² = 42%, moderate‐quality evidence; Analysis 1.3). This suggests that for a woman with a 5% risk of OHSS using HCG, the rate would be between nil and 2% with use of a GnRH agonist. Use of a random‐effects model did not substantially affect the results (OR 0.17, 95% CI 0.03 to 0.98; I2 = 42%) (Analysis 1.3; Figure 5).
1.3. Analysis.
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 3 OHSS incidence per woman randomised.
5.
GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.2 OHSS incidence per women randomly assigned.
1.3.2 Donor‐recipient cycles
We found evidence of a lower incidence of OHSS in the GnRH agonist group than in the HCG group (OR 0.05, 95% CI 0.01 to 0.28; three RCTs, 374 women, I² = 0%) (Analysis 1.3; Figure 5).
1.4 Incidence of OHSS in autologous cycles: subgroup analysis on luteal support approach
The subgroup analysis based on luteal phase support methods used in the included studies found no evidence of a difference in OHSS rates between trials that used luteal phase support with LH activity and trials that used luteal phase support without LH activity (test for subgroup differences: Chi² = 3.39, df = 1 (P value 0.07), I² = 71%). No evidence was found of a difference between GnRH agonist and HCG groups among women who had luteal phase support with LH activity (OR 0.47, 95% CI 0.11 to 2.09; I2 = 25%, five RCTs), but the OHSS rate was lower in the GnRH agonist group among women who had luteal phase support without LH activity (OR 0.04, 95% CI 0.01 to 0.34; I2 = 0%) (Analysis 1.4).
1.4. Analysis.
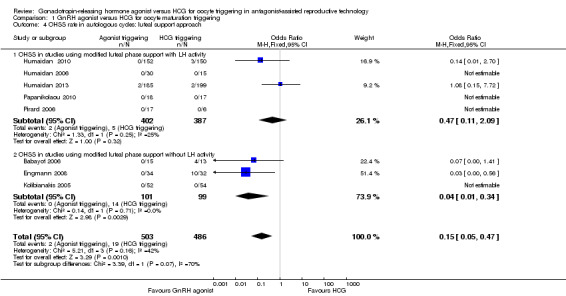
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 4 OHSS rate in autologous cycles: luteal support approach.
Secondary outcomes
1.5 Ongoing pregnancy rate per woman randomised
1.5.1 Fresh autologous cycles
GnRH agonist trigger was associated with a lower ongoing pregnancy rate when compared with HCG (OR 0.70, 95% CI 0.54 to 0.91; 11 RCTs, 1198 women, I² = 54%, moderate‐quality evidence) (Analysis 1.5; Figure 6).
1.5. Analysis.
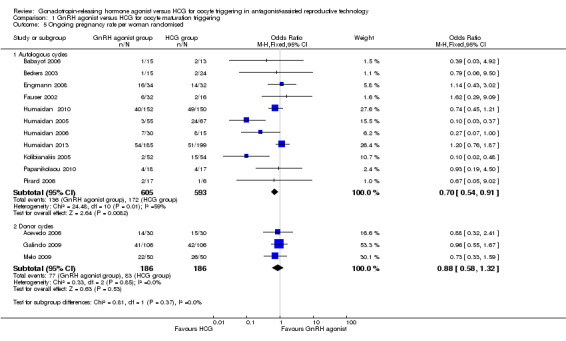
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 5 Ongoing pregnancy rate per woman randomised.
6.
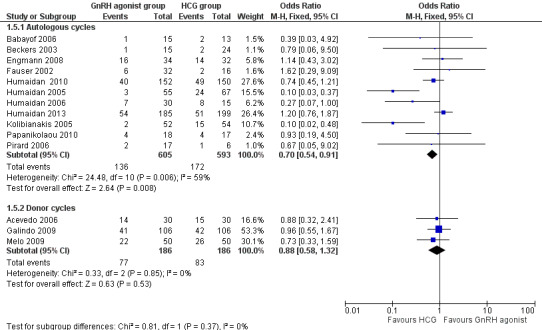
GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.3 Ongoing pregnancy rate per women randomly assigned.
1.5.2 Donor‐recipient cycles
We observed no evidence of differences between groups in ongoing pregnancy rate (OR 0.88, 95% CI 0.58 to 1.32; three RCTs, 374 women, I² = 0%) (Figure 5).
1.6 Ongoing pregnancy rate in autologous cycles: subgroup analysis on luteal support approach
The subgroup analysis based on luteal phase support methods used in the included studies indicated differences in ongoing pregnancy rate between trials that used luteal phase support with LH activity and those that used luteal phase support without LH activity (test for subgroup differences: Chi² = 8.1, df = 1 (P value 0.004), I² = 88%). No evidence was found of differences between groups among women who had luteal phase support with LH activity (OR 0.89, 95% CI 0.65 to 1.21; I2 = 27%, five RCTs), but the ongoing pregnancy rate in the HCG group was higher among women who had luteal phase support without LH activity (OR 0.36, 95% CI 0.21 to 0.62; I2 = 73%, five RCTs, 370 women) (Analysis 1.6).
1.6. Analysis.
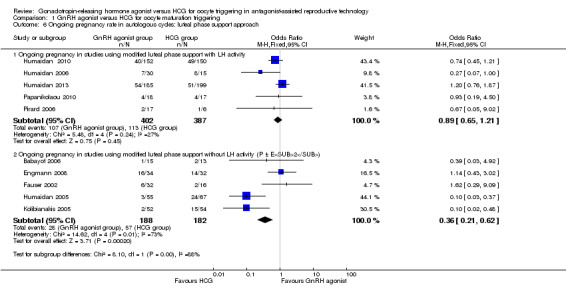
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 6 Ongoing pregnancy rate in autologous cycles: luteal phase support approach.
1.7 Clinical pregnancy rate per woman randomised
1.7.1 Fresh autologous cycles
We found no evidence of a difference between groups in clinical pregnancy rate (OR 0.81, 95% CI 0.61 to 1.04; 11 RCTs, 1198 women, I² = 49%) (Analysis 1.7).
1.7. Analysis.
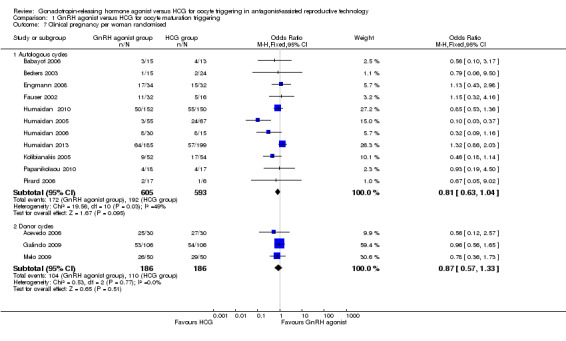
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 7 Clinical pregnancy per woman randomised.
1.7.2 Donor‐recipient cycles
We found no evidence of a difference between groups in clinical pregnancy rate (OR 0.87, 95% CI 0.57 to 1.33; three RCTs, 372 women, I² = 0%) (Analysis 1.7).
1.8 Miscarriage rate per woman randomised
1.8.1 Fresh autologous cycles
GnRH agonist trigger was associated with a higher early miscarriage rate when compared with HCG (OR 1.74, 95% CI 1.10 to 2.75; 11 RCTs, 1198 women, I² = 1%) (Analysis 1.8).
1.8. Analysis.
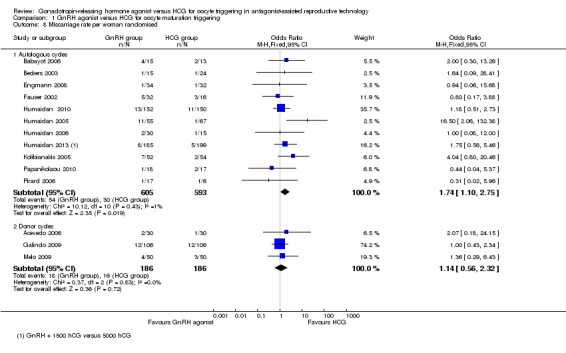
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 8 Miscarriage rate per woman randomised.
1.8.2 Donor‐recipient cycles
We found no evidence of differences between groups in miscarriage rate (OR 1.14, 95% CI 0.56 to 2.32; three RCTs, 372 women, I² = 0%) (Analysis 1.8).
1.9 Multiple pregnancy per woman randomised
1.9.1 Fresh autologous cycles
We found no evidence of differences between groups in multiple pregnancy rate (OR 3.00, 95% CI 0.30 to 30.47; two RCTs, 62 women, I² = 0%) (Analysis 1.9).
1.9. Analysis.
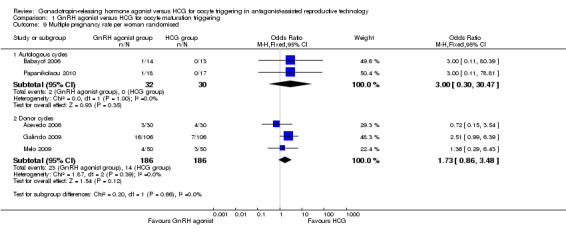
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 9 Multiple pregnancy rate per woman randomised.
1.9.2 Donor‐recipient cycles
We found no evidence of differences between groups in multiple pregnancy rate (OR 1.73, 95% CI 0.86 to 3.48; three RCTs, 374 women, I² = 0%) (Analysis 1.9).
Additional analyses
Subgroup and sensitivity analyses
10.1 OHSS incidence: effect of risk
OHSS in women at low risk of OHSS
No evidence of a difference between GnRh agonist and HCG was noted in the rate of OHSS among women at low risk of OHSS (OR 0.79, 95% CI 0.18 to 3.47; six RCTs, 777 women, I2 = 66%; Analysis 1.10). Heterogeneity for this analysis was substantial, probably as a result of the low event rate, with four of the six RCTs reporting no events in either arm.
1.10. Analysis.
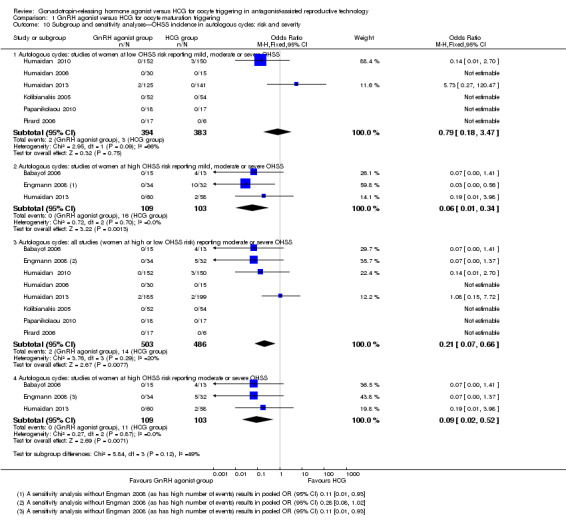
Comparison 1 GnRH agonist versus HCG for oocyte maturation triggering, Outcome 10 Subgroup and sensitivity analyses—OHSS incidence in autologous cycles: risk and severity.
OHSS in women at high risk of OHSS
GnRH agonist was associated with a significantly lower risk of OHSS when compared with HCG among women at high risk of OHSS (OR 0.06, 95% CI 0.01 to 0.34; three RCTs, 212 women, I2 = 0%; Analysis 1.10).
10.2 Effect of including only moderate or severe OHSS as an outcome
After cases with mild OHSS were excluded, GnRH agonist was associated with lower risk of moderate or severe OHSS when compared with HCG (OR 0.21, 95% CI 0.07 to 0.66; four RCTs, 112 women, I2 = 20%; Analysis 1.2). The analysis included only 16 events reported by four RCTs. A further five RCTs reported no events in either arm.
Results were similar among women at high risk of OHSS: GnRH agonist was associated with significantly lower risk of moderate or severe OHSS when compared with HCG (OR 0.09, 95% CI 0.02 to 0.52; four RCTs, 112 women, I2 = 0%; Analysis 1.10).
10.3 Use of risk ratios rather than odds ratios
Use of risk ratios rather than odds ratios did not materially affect our findings.
Findings of other subgroup and sensitivity analyses are described above, under the section on relevant comparisons.
Assessment of publication bias
A funnel plot was constructed for the outcome of ongoing pregnancy (Figure 7). This plot was not symmetrical, as a greater number of effect estimates were placed on the left side of the graph. This could imply publication bias, but in this case it seems more likely that the effect was due to the fact that the more extreme effect estimates were derived from studies that did not use luteal support with LH.
7.
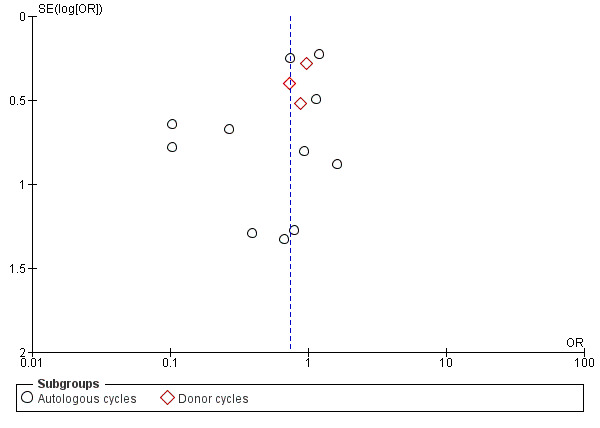
Funnel plot of comparison: 1 GnRH agonist versus HCG for oocyte maturation triggering, outcome: 1.5 Ongoing pregnancy rate per woman randomised.
Discussion
Summary of main results
This review update on the benefits and harms of GnRH agonist trigger in subfertile women treated with GnRH antagonist in IVF/ICSI treatment cycles found that use of GnRH agonist trigger compared with HCG triggering was associated with a markedly reduced live birth rate and an increased early miscarriage rate but was beneficial in preventing OHSS in fresh autologous cycles among women at high risk of OHSS. No differences between interventions in OHSS incidence were noted among women at low risk of OHSS. Overall (regardless of underlying risk) for a woman with a 5% risk of mild, moderate or severe OHSS with use of HCG, the risk of OHSS with use of a GnRh agonist was between nil and 2%, and for women with a 5% risk of developing moderate or severe OHSS with use of HCG, the risk with use of a GnRH antagonist was between nil and 3% (Table 1).
In donor‐recipient cycles, use of GnRH agonist instead of HCG also resulted in a lower incidence of OHSS. No evidence was found of a difference in live birth or ongoing pregnancy rate, although the results were consistent with those for fresh autologous cycles.
Overall completeness and applicability of evidence
Quality of the evidence
GRADE assessment found that evidence for most review outcomes was of moderate quality. Exceptions included ongoing pregnancy and multiple pregnancy, which were rated as having low‐quality evidence. Reasons for downgrading evidence quality included poor reporting of study methods, premature study termination, failure to blind outcome assessment and statistical heterogeneity. For some outcomes, confidence intervals were wide as the result of low event rates (Table 1).
The authors of four studies stated that the studies were commercially funded. The authors of most studies failed to disclose their funding source.
Potential biases in the review process
Strengths of this review include comprehensive systematic searching for eligible studies, rigid inclusion criteria for RCTs and data extraction and analysis by two independent review authors. Furthermore, the possibility of publication bias was minimised by inclusion of both published and unpublished studies (such as abstracts from meetings). However, as with any review, we cannot guarantee that we found all eligible studies.
Agreements and disagreements with other studies or reviews
Our results are in agreement with those of a previous review (Griesinger 2006). However, that review included only three small randomised controlled studies (Fauser 2002; Humaidan 2005; Kolibianakis 2005) involving 275 randomised women.
How can poor reproductive outcomes following oocyte triggering with GnRH agonist be explained? In previous studies, oocyte maturity, fertilisation rate and embryo development were comparable between GnRH agonist and HCG‐induced final oocyte maturation. This was found both in fresh autologous cycles (Griesinger 2006) and in donor cycles (Bodri 2009; Erb 2009). Furthermore, frozen‐thawed cycles with embryos obtained after oocyte triggering with GnRH agonist resulted in high pregnancy rates (Griesinger 2007a; Griesinger 2007b). Hence, oocyte triggering with GnRH agonist appears to have no major impact on oocyte and embryo quality.
It seems more likely that GnRH agonist induces a luteal phase defect. This luteal phase defect may result from the short half‐life of the induced LH surge, leading to premature luteolysis of corpus luteum and significantly lower steroidal and non‐steroidal hormones, thus affecting endometrial receptivity (Lanzone 1994; Peñarrubia 1998; Nevo 2003; Emperaire 2004; Humaidan 2005). Consequently, further studies have been conducted to evaluate different modified luteal phase strategies with LH activity supplementation in terms of administration of small dosages of HCG around the time of oocyte maturation trigger (Humaidan 2010; Humaidan 2013) or with repeated administration of recLH (Papanikolaou 2010), or without LH supplementation but with the help of progesterone and oestradiol administration (Engmann 2008). Our subgroup analysis shows that, although modified luteal phase support with LH was associated with pregnancy rates almost comparable with those of HCG, the difference in OHSS risk was no longer present. Apparently, available regimens could not compensate for the induced luteal phase defect in GnRH agonist–triggered cycles.
Our meta‐analysis of fresh autologous cycles and donor‐recipient cycles found that use of a GnRH agonist trigger is associated with a significantly reduced incidence of OHSS when compared with HCG, as none of the women in the pooled studies developed any form of OHSS when in the GnRH agonist group. The shorter half‐life of the endogenous LH surge and subsequent pituitary suppression and withdrawal of LH support for the corpora luteum may lead to early luteolysis (Kol 2004; Kol 2008). Moreover, significantly lower luteal levels of inhibins and steroid hormones suggest that the corpora luteum may secrete lower levels of other non‐steroidal substances, and the vasoactive properties of vascular endothelial growth factor (VEGF) may be involved in OHSS. This may explain the mechanism of OHSS prevention with the use of GnRH agonists (Nevo 2003; Cerrillo 2011).
Authors' conclusions
Implications for practice.
Evidence suggests that GnRH agonist as a final oocyte maturation trigger in fresh autologous cycles is associated with a lower live birth rate, a lower ongoing pregnancy rate (pregnancy beyond 12 weeks) and a higher rate of early miscarriage (less than 12 weeks). GnRH agonist as an oocyte maturation trigger could be useful for women who choose to avoid fresh transfers (for whatever reason), women who donate oocytes to recipients or women who wish to freeze their eggs for later use in the context of fertility preservation.
Implications for research.
In women with high risk of OHSS, the utility of GnRH agonist as a final oocyte maturation trigger in fresh autologous cycles should be evaluated in the context of effectiveness versus safety. For these studies, it is important that trial authors clearly report their funding source.
What's new
| Date | Event | Description |
|---|---|---|
| 8 September 2014 | New citation required but conclusions have not changed | 6 new studies added (Engmann 2008; Humaidan 2013; Ossina 2004; Papanikolaou 2010; Peňa 2007; Segal 1992), but we have made no change to our conclusions |
| 8 September 2014 | New search has been performed | Updated. No change to conclusions |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 11, 2010
| Date | Event | Description |
|---|---|---|
| 16 November 2010 | New citation required but conclusions have not changed | Two new authors added |
Acknowledgements
Marian Showell, Trial Search Co‐ordinator for the Cochrane Menstrual Disorders and Subfertility Group.
Appendices
Appendix 1. MDSG specialised register search strategy
Keywords CONTAINS "GnRH a" or "GnRH agonist" or "GnRH agonists" or "GnRHa" or "GnRHa‐gonadotropin" or "Gonadorelin" or "Gonadotrophin releasing agonist" or "gonadotropin releasing hormone agonist" or "Goserelin" or "goserelin acetate" or "Gosereline " or "buserelin" or "Buserelin Acetate" or "buserelin naferelin" or "busereline" or "Leuprolide" or "leuprolide acetate" or "leuprolin" or "leuprorelin" or "leuprorelin acetate" or "Nafarelin" or "triptoielin" or "triptorelin" or "triptoreline" or "triptoreline pamoat" or "triptorelyn" or "triptrolein" or "Lupron" or "deslorelin" or "Zoladex" or Title CONTAINS"GnRH a" or "GnRH agonist" or "GnRH agonists" or "GnRHa" or "GnRHa‐gonadotropin"
AND
Keywords CONTAINS "trigger" or "triggered ovulation" or "*Ovulation Induction" or "ovulation trigger" or "oocyte maturation" or Title CONTAINS "trigger" or "triggered ovulation" or "*Ovulation Induction" or "ovulation trigger" or "oocyte maturation"
AND
Keywords CONTAINS "human chorionic gonadotrophin" or "human chorionic gonadotropin" or "HCG" or "r‐HCG" or "chorionic gonadotrophins" or Title CONTAINS "human chorionic gonadotrophin" or "human chorionic gonadotropin" or "HCG" or "r‐HCG" or "chorionic gonadotrophins"
Appendix 2. Cochrane Central Register of Controlled Trials
1 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin/ (1664) 2 gonadotropin‐releasing hormone$.tw. (656) 3 (buserelin or Suprefact).tw. (266) 4 (goserelin or Zoladex).tw. (445) 5 (leuprolide or lupron).tw. (391) 6 (nafarelin or Synarel).tw. (101) 7 (histrelin or Supprelin).tw. (0) 8 (deslorelin or Suprelorin or Ovuplant).tw. (8) 9 triptorelin$.tw. (171) 10 gonadotropin‐releasing hormone agonist$.tw. (294) 11 gonadotrophin releasing hormone agonist$.tw. (130) 12 GnRH agonist$.tw. (611) 13 GnRH a.tw. (1025) 14 GnRHa.tw. (186) 15 or/1‐14 (2712) 16 trigger$.tw. (1848) 17 (oocyte adj5 matur$).tw. (142) 18 (ovulat$ adj2 induc$).tw. (516) 19 or/16‐18 (2452) 20 19 and 15 (246) 21 HCG$.tw. (909) 22 $HCG.tw. (903) 23 exp Chorionic Gonadotropin/ (560) 24 chorionic gonadotropin$.tw. (364) 25 chorionic gonadotrophin$.tw. (227) 26 or/21‐25 (1215) 27 26 and 20 (138)
Appendix 3. MEDLINE
1 exp gonadotropin‐releasing hormone/ or exp buserelin/ or exp goserelin/ or exp leuprolide/ or exp nafarelin/ or exp triptorelin/ (26342) 2 gonadotropin‐releasing hormone$.tw. (9697) 3 (buserelin or Suprefact).tw. (1201) 4 (goserelin or Zoladex).tw. (887) 5 (leuprolide or lupron).tw. (1419) 6 (nafarelin or Synarel).tw. (251) 7 (histrelin or Supprelin).tw. (36) 8 (deslorelin or Suprelorin or Ovuplant).tw. (142) 9 triptorelin$.tw. (481) 10 gonadotropin‐releasing hormone agonist$.tw. (1436) 11 gonadotrophin releasing hormone agonist$.tw. (400) 12 GnRH agonist$.tw. (2891) 13 GnRH a.tw. (822) 14 GnRHa.tw. (930) 15 or/1‐14 (30322) 16 trigger$.tw. (124349) 17 (oocyte adj5 matur$).tw. (5092) 18 (ovulat$ adj2 induc$).tw. (6042) 19 or/16‐18 (134873) 20 19 and 15 (1781) 21 HCG$.tw. (19418) 22 $HCG.tw. (19020) 23 exp Chorionic Gonadotropin/ (27317) 24 chorionic gonadotropin$.tw. (12107) 25 chorionic gonadotrophin$.tw. (3924) 26 or/21‐25 (36144) 27 26 and 20 (549) 28 randomized controlled trial.pt. (300724) 29 controlled clinical trial.pt. (82572) 30 randomized.ab. (214782) 31 placebo.tw. (129299) 32 clinical trials as topic.sh. (151348) 33 randomly.ab. (158665) 34 trial.ti. (92324) 35 (crossover or cross‐over or cross over).tw. (49488) 36 or/28‐35 (730928) 37 exp animals/ not humans.sh. (3540159) 38 36 not 37 (676083) 39 27 and 38 (125)
Appendix 4. EMBASE
1 gonadorelin derivative/ or buserelin/ or buserelin acetate/ or deslorelin/ or folligen/ or exp gonadorelin/ or exp gonadorelin acetate/ or exp gonadorelin agonist/ or exp goserelin/ or exp histrelin/ or exp leuprorelin/ or exp lutrelin/ or exp nafarelin/ or exp nafarelin acetate/ or exp ovurelin/ or exp triptorelin/ (45421) 2 gonadorelin$.tw. (231) 3 gonadotropin‐releasing hormone$.tw. (9950) 4 (buserelin or Suprefact).tw. (2053) 5 (goserelin or Zoladex).tw. (2250) 6 (leuprolide or lupron).tw. (2646) 7 (nafarelin or Synarel).tw. (540) 8 (histrelin or Supprelin).tw. (87) 9 (deslorelin or Suprelorin or Ovuplant).tw. (155) 10 triptorelin$.tw. (616) 11 gonadotropin‐releasing hormone agonist$.tw. (1537) 12 gonadotrophin releasing hormone agonist$.tw. (443) 13 GnRH agonist$.tw. (3405) 14 GnRH a.tw. (908) 15 GnRHa.tw. (1065) 16 or/1‐14 (47694) 17 trigger$.tw. (137076) 18 (oocyte adj5 matur$).tw. (5447) 19 (ovulat$ adj2 induc$).tw. (6441) 20 or/17‐19 (148250) 21 20 and 16 (2510) 22 HCG$.tw. (20224) 23 $HCG.tw. (19726) 24 exp Chorionic Gonadotropin/ (32112) 25 chorionic gonadotropin$.tw. (11662) 26 chorionic gonadotrophin$.tw. (3838) 27 or/22‐26 (41192) 28 27 and 21 (1052) 29 Clinical Trial/ (809202) 30 Randomized Controlled Trial/ (281780) 31 exp randomization/ (52523) 32 Single Blind Procedure/ (13353) 33 Double Blind Procedure/ (99325) 34 Crossover Procedure/ (29336) 35 Placebo/ (168562) 36 Randomi?ed controlled trial$.tw. (56492) 37 Rct.tw. (5973) 38 random allocation.tw. (989) 39 randomly allocated.tw. (14666) 40 allocated randomly.tw. (1671) 41 (allocated adj2 random).tw. (677) 42 Single blind$.tw. (10411) 43 Double blind$.tw. (113453) 44 ((treble or triple) adj blind$).tw. (225) 45 placebo$.tw. (150973) 46 prospective study/ (156030) 47 or/29‐46 (1087729) 48 case study/ (10327) 49 case report.tw. (191485) 50 abstract report/ or letter/ (755276) 51 or/48‐50 (953548) 52 47 not 51 (1056074) 53 28 and 52 (301)
Appendix 5. PsycINFO
1 exp Gonadotropic Hormones/ (3254) 2 gonadotropin‐releasing hormone$.tw. (349) 3 (buserelin or Suprefact).tw. (4) 4 (goserelin or Zoladex).tw. (13) 5 (leuprolide or lupron).tw. (54) 6 (nafarelin or Synarel).tw. (0) 7 (histrelin or Supprelin).tw. (1) 8 (deslorelin or Suprelorin or Ovuplant).tw. (2) 9 triptorelin$.tw. (17) 10 gonadotropin‐releasing hormone agonist$.tw. (41) 11 gonadotrophin releasing hormone agonist$.tw. (2) 12 GnRH agonist$.tw. (34) 13 GnRH a.tw. (7) 14 GnRHa.tw. (14) 15 or/1‐14 (3390) 16 trigger$.tw. (13815) 17 (oocyte adj5 matur$).tw. (15) 18 (ovulat$ adj2 induc$).tw. (66) 19 or/16‐18 (13890) 20 19 and 15 (50) 21 HCG$.tw. (61) 22 $HCG.tw. (55) 23 chorionic gonadotropin$.tw. (63) 24 chorionic gonadotrophin$.tw. (8) 25 or/21‐24 (93) 26 20 and 25 (2)
Appendix 6. CINAHL
CINAHL search strategy for MM1690 29.05.14
| # | Query | Results |
| S26 | S11 AND S25 | 39 |
| S25 | S12 OR S13 or S14 or S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 | 892,353 |
| S24 | TX allocat* random* | 3,910 |
| S23 | (MH "Quantitative Studies") | 12,053 |
| S22 | (MH "Placebos") | 8,750 |
| S21 | TX placebo* | 31,617 |
| S20 | TX random* allocat* | 3,910 |
| S19 | (MH "Random Assignment") | 37,302 |
| S18 | TX randomi* control* trial* | 73,175 |
| S17 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 716,730 |
| S16 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 105 |
| S15 | TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 0 |
| S14 | TX clinic* n1 trial* | 163,504 |
| S13 | PT Clinical trial | 76,024 |
| S12 | (MH "Clinical Trials+") | 175,230 |
| S11 | S6 AND S10 | 86 |
| S10 | S7 OR S8 OR S9 | 12,370 |
| S9 | TX (ovulat* N2 induc*) | 516 |
| S8 | TX (oocyte N3 matur*) | 42 |
| S7 | TX trigger* | 11,843 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 1,298 |
| S5 | TX GnRHa | 36 |
| S4 | TX GnRH | 283 |
| S3 | TX gonadotrophin releasing hormone* | 77 |
| S2 | TX gonadotropin‐releasing hormone* | 318 |
| S1 | (MH "Gonadorelin+") | 1,061 |
Data and analyses
Comparison 1. GnRH agonist versus HCG for oocyte maturation triggering.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate per woman randomised | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Fresh autologous cycles | 5 | 532 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.31, 0.70] |
| 1.2 Donor cycles | 1 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.61] |
| 2 Live birth rate in autologous cycles: luteal phase support approach | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Live birth in studies using modified luteal phase support with LH activity | 3 | 382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 2.2 Live birth in studies using modified luteal phase support without LH activity (P ± E2) | 2 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.04, 0.39] |
| 3 OHSS incidence per woman randomised | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fresh autologous cycles | 8 | 989 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.47] |
| 3.2 Donor cycles: mild, moderate or severe OHSS | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.28] |
| 4 OHSS rate in autologous cycles: luteal support approach | 8 | 989 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.47] |
| 4.1 OHSS in studies using modified luteal phase support with LH activity | 5 | 789 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.11, 2.09] |
| 4.2 OHSS in studies using modified luteal phase support without LH activity | 3 | 200 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.34] |
| 5 Ongoing pregnancy rate per woman randomised | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Autologous cycles | 11 | 1198 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.91] |
| 5.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.58, 1.32] |
| 6 Ongoing pregnancy rate in autologous cycles: luteal phase support approach | 10 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Ongoing pregnancy in studies using modified luteal phase support with LH activity | 5 | 789 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
| 6.2 Ongoing pregnancy in studies using modified luteal phase support without LH activity (P ± E2) | 5 | 370 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.21, 0.62] |
| 7 Clinical pregnancy per woman randomised | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Autologous cycles | 11 | 1198 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.04] |
| 7.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.57, 1.33] |
| 8 Miscarriage rate per woman randomised | 14 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Autologous cycles | 11 | 1198 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.10, 2.75] |
| 8.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.56, 2.32] |
| 9 Multiple pregnancy rate per woman randomised | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Autologous cycles | 2 | 62 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.30, 30.47] |
| 9.2 Donor cycles | 3 | 372 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.86, 3.48] |
| 10 Subgroup and sensitivity analyses—OHSS incidence in autologous cycles: risk and severity | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Autologous cycles: studies of women at low OHSS risk reporting mild, moderate or severe OHSS | 6 | 777 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.18, 3.47] |
| 10.2 Autologous cycles: studies of women at high OHSS risk reporting mild, moderate or severe OHSS | 3 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.34] |
| 10.3 Autologous cycles: all studies (women at high or low OHSS risk) reporting moderate or severe OHSS | 8 | 989 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.66] |
| 10.4 Autologous cycles: studies of women at high OHSS risk reporting moderate or severe OHSS | 3 | 212 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.02, 0.52] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acevedo 2006.
| Methods | Randomised, controlled, single‐centre, donor‐recipient study | |
| Participants | 60 oocyte donors 18 to 35 years of age with normal menstrual cycle: no PCOS, endometriosis, hydrosalpinges or severe male factor. 98 recipients 34–47 years of age received oocyte, but only 60 participants were analysed. Baseline characteristics: Most donors had similar basal ovarian conditions: basal FSH 5.2 vs 2.3 mIU/mL; E2 44.1 vs 32.5 pg/mL | |
| Interventions |
Ovarian stimulation: fixed dose of 150 IU rFSH on cd 3/4 f + 0.25 mg/d sc orgalutran + 75 IU/d of LH Intervention: 0.2 mg, SC triptorelin vs 250 μg/mL SC rHCG Luteal phase support (recipients): E2 plus 600 mg/d natural progesterone |
|
| Outcomes |
Donors Primary outcome: OHSS Secondary outcomes: FSH and LH units (IU), GnRH antagonist ampoules, E2 levels, follicle numbers on day 5 of COH and on HCG day Recipients Pregnancy rates, implantation rates |
|
| Notes | 98 recipients were included in the study, but for statistical techniques, only one participant of those who received oocytes from the same donor was included in the analysis Participants received embryos originating from donors; some donors gave oocytes to 2 recipients. Only 1 recipient was randomly included in the statistical analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | High risk | Reported that allocation was not concealed (after contact was made with study author) |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Participants, those administering the interventions and those assessing the outcomes were not blinded to group assignment. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. Live birth rates were not reported |
| Other bias | Low risk | No other potential bias was identified |
Babayof 2006.
| Methods | Randomised, controlled, single‐centre study | |
| Participants | 28 women with PCOS for IVF. Basic clinical characteristics: Both groups were similar in age: 30.1 vs 29.3, BMI 24.1 vs 27.1 and cause of infertility; 5.8 vs 5.3 and basal FSH (IU/L) 7.8 vs 4.3 | |
| Interventions |
Ovarian stimulation: adjustable dose of 225 IU SC rFSH + 0.25 mg SC cetrotide Intervention: 0.2 mg decapeptyl vs 250 μg rHCG Number of embryos transferred: GnRH agonist group vs HCG group (mean: 2.3 ± 0.2 vs 2.2 ± 0.4) Luteal phase support: 50 mg/d of progesterone IM ± 4 mg/d E2 PO (if serum E2 concentration was below 200 pmol/L ± doubled dose of progesterone if serum progesterone concentration was below 40 nmol/L) |
|
| Outcomes |
Primary outcome: serum levels of inhibin A, VEGF, TNFa, E2 and progesterone and incidence of OHSS Secondary outcomes: ovarian size and pelvic fluid accumulation, live birth, ongoing, chemical, miscarriage, number of oocytes retrieved, number of MII oocytes, fertilisation rate and number of embryos transferred |
|
| Notes |
OHSS classification:Golan 1989 HCG group: 2 cases of ET were cancelled, and all embryos were frozen as the result of severe OHSS with accumulation of large amount of free fluid in the pelvis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list |
| Allocation concealment (selection bias) | Low risk | An independent nurse dispensed HCG or GnRH agonist according to a randomisation list |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Participants, those administering interventions and those assessing outcomes were not blinded to group assignment. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include most expected outcomes |
| Other bias | Low risk | No other potential bias was identified |
Beckers 2003.
| Methods | Randomised, controlled, 3‐arm, 2‐centre study | |
| Participants | 40 participants for IVF/ICSI. 38 years of age or younger, regular menstrual cycle, both ovaries present, absence of uterine abnormalities, BMI 18 to 29 kg/m2, no history of poor ovarian response or moderate or severe OHSS Baseline characteristics: comparable between the 2 groups (data not shown) |
|
| Interventions |
Ovarian stimulation: fixed dose of 150 IU r‐hFSH on cd 2 or 3 using + 1 mg daily SC antide Intervention: 0.2 mg SC triptorelin vs 250 μg/mL SC rHCG vs 1 mg SC r‐LH Number of embryos transferred: GnRH agonist group vs HCG group: maximum of 2 embryos were transferred after 2 to 5 days of culture Luteal phase support: none |
|
| Outcomes |
Primary outcomes: LH (day of oocyte retrieval), day of progesterone maximal level, day of decrease in progesterone Secondary outcomes: duration of follicular phase (days), number of days of GnRH antagonist, number of follicles ≥ 11 mm, number of oocytes retrieved, number of participants achieving embryo transfer pregnancy, ongoing pregnancy |
|
| Notes | Study was cancelled prematurely because of observed premature luteal phase bleeding and extremely low pregnancy rates Commercial funding: This investigator‐driven study was supported by a research grant from Serono International SA, and by ‘Stichting Voortplantingsgeneeskunde’ Rotterdam |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated with sealed envelopes for both centres; a separate stratified randomisation list was generated by computer |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No further details were reported |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | Unclear risk | Blindedness was not reported clearly. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. No data were provided on live birth rate, incidence of OHSS |
| Other bias | High risk | Study was terminated prematurely because of observed premature luteal phase bleeding and extremely low pregnancy rates |
Engmann 2008.
| Methods | Open‐label, parallel, university‐based tertiary fertility centre, RCT | |
| Participants | 66 women were included. Inclusion criteria: age 20 to 39 years, basal FSH concentration ≤ 10.0 IU/L and undergoing first cycle of IVF with PCOS or PCOM, or undergoing subsequent cycle with a history of high response in previous IVF cycles. Exclusion criteria: women with hypogonadotropic hypogonadism Baseline characteristics: 32.0 ± 3.7 vs 33.1 ± 3.6 years |
|
| Interventions |
Ovarian stimulation: Control group: OCP + 112 to 225 IU recFSH on CD2 + midluteal 1 mg leuprolide acetate (SC). Study group: OCP + 112 to 225 IU recFSH on CD2 + flexible GnRH antagonist protocol (SC) Intervention: SC leuprolide in a dose of 1 mg approximately 12 hours after last dose of ganirelix vs SC hCG (Profasi; Serono, Randolph, MA) in a dose ranging from 3300 to 10,000 IU, depending on follicular response Number of embryos transferred: GnRH agonist group vs HCG group (mean ± SD: 2.0 ± 0.2 vs 2.2 ± 0.6) LPS: study group: 50 mg IM P in oil + 0.1 mg transdermal E2 patches every other day, starting the day after oocyte retrieval. Both doses were adjusted according to E2 and P levels on the day of embryo transfer and 1 week after oocyte retrieval. Control group: 0 mg IM P in oil |
|
| Outcomes | Primary outcome measures: OHSS occurrence assessed 1 week after oocyte retrieval and implantation rate assessed at 7 weeks' gestation Secondary outcome measures: clinical pregnancy rate assessed at time of ultrasound, mature oocytes assessed at time of retrieval and ovarian volume assessed 1 week after oocyte retrieval | |
| Notes | Supported in part by an unrestricted educational grant from Organon USA, Roseland, New Jersey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 by means of computer‐generated random numbers with separate randomisation for women undergoing first cycle and for women with a previous high response by the use of stratified randomised blocks |
| Allocation concealment (selection bias) | Low risk | Research nurse by using a series of consecutively numbered sealed opaque envelopes (1 for each category of previous cycle) |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Not blinded. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most randomly assigned women were analysed using per‐protocol (PP) and intention‐to‐treat analysis (ITT) |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available and outcomes were prespecified; OHSS, implantation rate (IR), MII, CPR, ovarian volume 1 week after oocyte retrieval. Study reported extra outcomes not stated in the protocol, such as serum luteal phase E2, P levels, fertilisation rate (FR). Live birth rate not reported |
| Other bias | Low risk | No other source of potential bias was identified |
Fauser 2002.
| Methods | Randomised, controlled, open‐label, 3‐arm, 6‐international centre study | |
| Participants | 57 women for IVF/ICSI. 18 to 39 years of age, regular menstrual cycle (24 to 35 days) and BMI 18 to 29 kg/m2. Baseline characteristics were comparable between the 3 treatment groups: mean age 30.4 years, height 1.67, BMI 23.3; 98% were Caucasian | |
| Interventions |
Ovarian stimulation: adjustable dose of 150 to 225 IU rFSH, SC on cd 2 to 3 for the first 5 days + 0.25 mg ganirelix on day 6 of FSH stimulation Intervention: 0.2 mg triptorelin vs 0.5 mg leuprorelin vs 10,000 IU HCG Number of embryos transferred: GnRH agonist group vs HCG group: No more than 3 embryos were transferred Luteal phase support: progestin 50 mg daily, from the day of embryo transfer (ET) for at least 2 weeks or until menses |
|
| Outcomes |
Primary outcomes: FSH, LH, E2, HCG and P in the luteal phase Secondary outcomes: FSH consumption (IU); duration of FSH treatment (days); duration of ganirelix treatment (days); number of oocytes/participant on day of HCG or GnRH agonist proportion of metaphase II oocytes; fertilisation rate; number of embryos obtained/participant; embryo quality; implantation rate; ongoing pregnancy rate |
|
| Notes | Sample calculation not performed 57 of 200 participants; only 47 were randomly assigned. Eight participants were not randomly assigned because ovarian response to stimulation was not sufficient. Two participants were not randomly assigned because of high response. One participant in the hCG group did not undergo ET because of fertilisation failure. Duration of fertility was not stated, no data on live birth rate and on OHSS incidence and multiple pregnancy rates were provided Commercial funding: supported by NV Organon |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive telephone randomisation system that stratified for age, primary or secondary infertility and number of follicles. Participants were randomly assigned in a ratio of 1:1:1 |
| Allocation concealment (selection bias) | Unclear risk | Adequate |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Outcome assessors and participants were not blind to the intervention. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. Live birth rate was not reported |
| Other bias | Low risk | No other potential bias was identified |
Galindo 2009.
| Methods | Randomised, controlled, open‐label, single‐centre study | |
| Participants | 257 oocyte donors 18 to 35 years of age, BMI < 30 kg/m2, regular (26 to 35 days) menstrual cycles. Patients with previous history of low response to ovarian stimulation, PCO or using OCP were excluded. Baseline characteristics: age 25.8 vs 26.6 years, BMI 22.9 vs 22.8, fertilisation rate 70.1 vs 67.8 274 recipient cycles: ≤ 50 years with POF, reduced ovarian reserve or a history of previous failed IVF cycles. Baseline characteristics: 40.6 vs 40.6 years of age, menopause16% vs 21%, previous failed IVF 23% vs 28% |
|
| Interventions |
Ovarian stimulation: 225 IU of rFSH on cd 2 + 0.25 mg/d cetrotide Intervention: 0.2 mg triptorelin SC vs 250 μg rHCG Luteal phase support: 800 mg of micronised vaginal progesterone daily |
|
| Outcomes |
Donors: stimulation duration, total FSH dose, final E2 level and follicular count, fertilisation rate, OHSS incidence Recipients: clinical, ongoing and live birth rates, implantation rate and twinning rate |
|
| Notes |
Excluded patients: donors with a final E2 4.500 pg/mL and/or 20 follicles 14 mm at last control were excluded from randomisation and donors who needed coasting OHSS classification:Navot 1992 No conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not reported clearly |
| Allocation concealment (selection bias) | Unclear risk | Sealed, opaque envelopes. No further details were reported |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Participants, those administering interventions and those assessing outcomes were not blinded to group assignment. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available. Live birth rate was not reported |
| Other bias | Low risk | No other potential bias was identified |
Humaidan 2010.
| Methods | Randomised, controlled, 3‐centre study | |
| Participants | 302 normo‐gonadotrophic IVF/ICSI participants, 25 to 40 years of age, BMI 18 to 30 kg/m2, basal FSH < 12 IU/L, menstrual cycle 25 to 34 days, both ovaries present, absence of uterine abnormalities. Each participant contributed with only 1 cycle Baseline characteristics: 31.5 vs 30.9 years of age, BMI 23.8 vs 23.5, basal FSH 6.7 vs 6.7 |
|
| Interventions |
Ovarian stimulation: adjustable dose of 150 to 200 IU rFSH + 0.25 mg ganirelix Intervention: 0.5 mg buserelin SC plus 1500 IU HCG IM 35 hours after triggering of ovulation vs 10,000 IU HCG Luteal phase support: 90 mg/d progesterone vaginal plus 4 mg/d oestradiol, beginning on the day after OPU and continuing until the day of the pregnancy test |
|
| Outcomes |
Primary outcomes: reduction in high early pregnancy loss rate Secondary outcomes: MII oocytes retrieved, OHSS incidence, ongoing pregnancy rate |
|
| Notes | A total of 305 participants were included in the study, but 3 were not randomly assigned because of inadequate ovarian response Not stated whether investigators received commercial funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No further details were reported |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Participants, those administering interventions and those assessing outcomes were not blinded to group assignment. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include most expected outcomes |
| Other bias | Low risk | No other potential bias was identified |
Humaidan 2005.
| Methods | Randomised, controlled, open‐label, 2‐centre study | |
| Participants | 122 normo‐gonadotrophic women for IVF or ICSI. 25 to 40 years of age, baseline FSH and LH 12 IU/L, menstrual cycles between 25 and 34 days, BMI 18 to 30 kg/m2, both ovaries present, absence of uterine abnormalities. Each participant contributed with 1 cycle only Baseline characteristics: 33.4 vs 32.3 years of age, BMI 23.6 vs 23.5, FSH 6.8 vs 6.7 |
|
| Interventions |
Ovarian stimulation: adjusted dose of 150 or 200 IU rFSH on cd 2 + 0.25 mg ganirelix Intervention: 0.5 mg buserelin SC vs 10,000 IU HCG SC Number of embryos transferred: Maximum of 2 embryos were transferred. Mean number of embryos transferred: mean and range: 1.71 (1 to 2) vs 1.64 (1 to 2) Luteal phase support: 90 mg/d progesterone vaginally plus oestradiol 4 mg/d per os, commencing from the day following oocyte retrieval and continuing until the day of the pregnancy test |
|
| Outcomes |
Primary outcomes: positive HCG per ET. Clinical pregnancy. Early pregnancy loss Secondary outcomes: rate of embryo transfer (ET), numbers of embryos transferred, implantation rate, oocytes retrieved, MII oocytes, pronuclear oocytes, embryos (%); E2, FSH and LH levels on sd1, day 6 and ovulation induction day; progesterone on ovulation induction day |
|
| Notes | Terminated because of differences in clinical outcomes between groups Embryo transfer was cancelled in 7 patients in the GnRH agonist group and in 10 patients in the HCG group as the result of total fertilisation failure or poor embryo development Commercial funding: unclear whether investigators received commercial funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | By a study nurse; using computer‐generated random numbers in sealed, unlabelled envelopes, each containing a unique study number |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Participants, those administering interventions and those assessing outcomes were not blinded to group assignment. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include most expected outcomes |
| Other bias | High risk | Terminated early because of differences in clinical outcomes between groups |
Humaidan 2006.
| Methods | Randomised, controlled, open‐label, single‐centre study | |
| Participants | 45 normo‐gonadotrophic women for IVF/ICSI, 25 to 40 years of age, baseline FSH and LH < 12 IU/L, menstrual cycles between 25 and 34 days, BMI 18 to 30 kg/m2, both ovaries present, absence of uterine abnormalities. Each participant contributed with only 1 cycle Baseline characteristics: The 3 groups were comparable and 100% Caucasian |
|
| Interventions |
Ovarian stimulation: adjusted dose of 150 to 200 IU r‐hFSH on cd 2 + 0.25 mg ganirelix Intervention: 0.5 mg buserelin SC plus HCG 1500 IU IM 12 hours vs 0.5 mg buserelin SC 1500 IU IM 35 hours after buserelin injection vs 10,000 IU HCG SC Number of embryos transferred (mean ± SD): 1.9 ± 0.3 vs 1.9 ± 0.3 vs 1.8 ± 1.5 Luteal phase support: 90 mg/d progesterone vaginally plus 4 mg/d oestradiol orally |
|
| Outcomes |
Primary outcomes: serum P and inhibin A concentration Secondary outcomes: total dose of FSH (IU), duration of FSH stimulation (days), total dose of antagonist (mg), serum oestradiol (n mol/L) on SI, S6, day of ovulation induction, serum FSH, day of ovulation induction (IU/L), number of oocytes, number of embryos, rate of transfer, number of embryos transferred, positive HCG per embryo transfer, clinical pregnancy per embryo transfer, clinical pregnancy per cycle, implantation rate, early pregnancy loss |
|
| Notes | Unclear whether investigators received commercial funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes, each containing a unique study number. Allocation by a study nurse |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Participants, those administering interventions and those assessing outcomes were not blinded to group assignment. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available, but published reports include most expected outcomes |
| Other bias | Unclear risk | Serum P and inhibin A as primary outcomes, small group of women, funding unclear |
Humaidan 2013.
| Methods | 2 randomised, controlled studies; multi‐centre study (4 centres) | |
| Participants | 384 infertile women 25 to 40 years of age; normal menstrual cycles of 25 to 34 days or oligomenorrhoea/amenorrhoea or polycystic ovary syndrome, defined according to Rotterdam criteria (2004), BMI 18 to 35 kg/m2 and absence of uterine abnormalities. Exclusion criteria included women with hypothalamic dysfunction, diabetes, epilepsy, liver, renal or heart disease or metabolic disorders | |
| Interventions |
Ovarian stimulation: fixed dose of 150 to 200 IU/d recFSH (Puregon; Organon, Skovlunde, Denmark) from cd 2 or 3 and for the first 4 days, then dose adjusted according to ovarian response. Fixed GnRH antagonist protocol, bolus of 0.25 mg/d ganirelix (Orgalutran; Organon, Skovlunde, Denmark), was initiated on stimulation day 5 Intervention: As soon as 2 follicles had reached a diameter of 17 mm, 2 different randomisation lists were available, depending on the number of follicles seen on transvaginal ultrasound examination on the final day of ovarian stimulation: 1 for participants with 14 follicles ≥ 11 mm in diameter (at risk of OHSS) and 1 for participants with ≤ 14 follicles ≥ 11 mm (OHSS low‐risk group)
Number of embryos transferred: GnRH agonist group vs HCG group (median 1 to 5 vs 1 to 6) embryos transferred Luteal phase support: micronised progesterone vaginally, 90 mg twice daily (Crinone; Serono Nordic, Copenhagen, Denmark) and oestradiol (E2) 4 mg a day per os (Estrofem; Novo Nordisk, Copenhagen, Denmark), commencing on the day following oocyte retrieval and continuing until 7 weeks of gestation |
|
| Outcomes |
|
|
| Notes | Study was discontinued before the estimated sample size had been obtained as a result of the death of 1 of the local principal investigators and job rotations among other investigators Commercially funded by MSD, Denmark |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a study nurse, using computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Sealed, unlabelled envelopes, each containing a unique study number. No further details were reported |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Not blinded. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomly assigned were included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is available, but published reports did not include most expected outcomes (LBR) |
| Other bias | High risk | Study was discontinued early |
Kolibianakis 2005.
| Methods | RCT, 2‐armed, 1:1 randomisation ratio, open‐label, parallel design; 2‐centre study | |
| Participants | 106 women for IVF/ICSI. 39 years of age or younger, normal day 3 serum FSH levels, ≤ 3 previous assisted reproduction technology (ART) attempts, BMI 18 to 29 kg/m2, regular menstrual cycles, no PCOS or previous poor response to ovarian stimulation, both ovaries present, fresh ejaculated sperm and no embryo biopsy. Participants could enter the study only once Baseline characteristics: 32.4 vs 32.3 years of age, BMI 22.9 vs 23.7, basal FSH 8.2 vs 8.l |
|
| Interventions |
Ovarian stimulation: fixed dose of 200 IU rFSH started on cd 2 + 0.25 mg orgalutran Intervention: 0.2 mg triptorelin vs 10,000 IU of HCG Luteal phase support: 600 mg/d natural micronised progesterone in 3 separate doses vaginally plus daily 2 × 2 mg oral oestradiol starting 1 day after oocyte retrieval and continued until 7 weeks of gestation in the presence of a positive HCG test. At centre 2, vaginal and intramuscular progesterone was administered, if conception occurred, until 7 weeks of pregnancy |
|
| Outcomes |
Primary outcome: fertilisation rate Secondary outcomes: ongoing pregnancy, implantation rates, days of stimulation, total units of rFSH, number of COCs, follicles ≥ 11 mm on the day of triggering, number of follicles ≥ 17 mm on the day of triggering, proportion of MII oocytes, number of 2 PN oocytes, number of embryos transferred, E2 (pg/mL), progesterone (ng/L) |
|
| Notes | Stopped because of differences in pregnancy rate in favour of HCG No data on live birth rate and miscarriage rate Commercial funding: unclear whether investigators received commercial funding Stopping rule after interim analysis: If a difference in pregnancy rates was detected at a probability level of 0.03 at the second interim analysis, the study should be stopped for ethical reasons Funding source unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | High risk | Sequence of randomisation was not concealed |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | High risk | Outcome assessors and participants were not blinded to the intervention. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is not available. Live birth rate was not reported |
| Other bias | High risk | Stopped early because of differences in pregnancy rate in favour of HCG |
Melo 2009.
| Methods | Randomised, controlled, assessor‐blinded, parallel‐group, single‐centre study | |
| Participants | 100 oocyte donors, 18 to 34 years of age, regular menstrual cycles, no family history of hereditary or chromosomal disease, normal karyotype, BMI 18 to 29 kg/m2 and negative screening for sexually transmitted disease. PCOS was excluded. Basic clinical donor characteristics show no differences in age, BMI and antral follicle 96 recipients were women with menopause, 32 (33%); low response, 28 (29%); premature ovarian failure, 27 (28%); and female advanced age, 9 (10%). 18 to 49 years of age, BMI 18 to 29 kg/m2, male partner without severe male factor (< 5 million fresh spermatozoa/mm3, < 5% normal forms and/or non‐obstructive azoospermia). Exclusion criteria: cases with uterine pathology (submucous or intramural fibroids > 2 cm, polyps, adhesions, adenomyosis or müllerian defects), implantation failure and recurrent miscarriage |
|
| Interventions |
Oocyte donors Ovarian stimulation: OCP + adjustable dose of 225 IU rFSH + 0.25 mg cetrotide Intervention: 0.2 mg triptorelin SC vs 250 μg rHCG SC Luteal phase support (recipients): 800 mg/d micronised intravaginal progesterone |
|
| Outcomes |
Donors: oocytes retrieved, proportion of MII oocytes, fertilisation rate, cleavage rate, top‐quality embryos, number of embryos transferred, OHSS rate Recipients: implantation rate, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate |
|
| Notes | Funding source is unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Third party random assignment by a nurse |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | Low risk | All investigators, embryologists, laboratory personnel and sponsor staff, including the statistician responsible for statistical analysis, were blinded to treatment allocation throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available. Live birth rate was not reported |
| Other bias | Low risk | No other potential bias was identified |
Ossina 2004.
| Methods | RCT, multi‐centre study (6 centres) | |
| Participants | 101 participants (101 IVF/ICSI cycles) analysed | |
| Interventions | COH included recombinant FSH (recFSH; Puregon) in flexible multi‐dose GnRH antagonist protocol (orgalutran). Triggering was randomly performed by 10,000 IU HCG (Pregnyl) or 0.1 mg GnRH agonist (triptorelin) | |
| Outcomes | Serum concentrations of LH, FSH, E2 and P4 were measured at 0, 12, 36 and 108 hours after triggering | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | Unclear risk | Insufficient information: Full text was unavailable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information: Full text was unavailable |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Other bias | Unclear risk | Insufficient information: Full text was unavailable |
Papanikolaou 2010.
| Methods | Proof‐of‐concept, single‐centre RCT | |
| Participants | 35 participants seeking IVF treatment. 4 participants refrained from further treatment (2 for personal problems, 1 became pregnant and 1 as the result of poor response). Inclusion criteria were as follows: younger than 36 years of age, elective single embryo transfer on day 5 and basal FSH less than 12 mIU/mL Exclusion criteria were as follows: polycystic ovary syndrome (PCOS); use of testicular sperm; and endometriosis stages III and IV. Age was 30.6 ±.0.8 vs 30.1 ± 0.7 years | |
| Interventions |
Ovarian stimulation: fixed dose 187.5 IU of recFSH (Gonal‐F; Merck‐Serono NV SA, Overijse, Belgium) starting on cd 2 of the cycle with GnRH antagonist, 0.25 mg cetrorelix (Cetrotide; Merck‐Serono) on cycle day 7 and continued daily until the day of trigger
Intervention: 17 participants were randomly assigned to standard treatment group. They received 250 mg recombinant HCG (Ovitrelle, Merck‐Serono, Geneva, Switzerland) for ovulation triggering and standard luteal P (600 mg micronised P vaginally administered from day after oocyte retrieval and maintained until 7 weeks of gestation). 18 participants were randomly assigned to the novel protocol. They received 0.2 mg of triptorelin (Ipsen, Boulogne Billancourt, France) for ovulation triggering Luteal phase support: standard P luteal support plus 6 doses every other day of 300 IU recombinant LH (Luveris, Merck‐Serono), starting on the day of oocyte retrieval up to day 10 after oocyte retrieval |
|
| Outcomes |
Primary outcomes: implantation rates. Clinical pregnancy (defined as cardiac activity at 7 weeks) is similar to implantation rate, as a single blastocyst was transferred Secondary outcomes: OHSS incidence |
|
| Notes | Medications used in the study were offered by Merck‐Serono, Overijse, Belgium | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Research nurse randomly assigned women to 1 of the 2 arms |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by the research nurse |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | Unclear risk | Treating physician was blinded to the allocation group until the day of trigger. Unclear whether outcome assessment was blinded. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | TPP and ITT were provided |
| Selective reporting (reporting bias) | Unclear risk | Protocol was available, outcomes were as described. Live birth rate was not reported |
| Other bias | Low risk | No other potential bias was identified |
Peňa 2007.
| Methods | RCT, single‐centre study | |
| Participants | 41 egg donors | |
| Interventions |
GnRH agonist group: GnRH antagonist/triggering oocyte maturation with HCG; and GnRH antagonist/triggering ovulation with leuprolide acetate (0.6 mg 35.5 hours before egg retrieval (ER), followed 10 hours later by a second dose of 0.6 mg) HCG group: down‐regulation with leuprolide acetate/triggering oocyte maturation with HCG (5000 to 10,000 IU) 35.5 hours before egg retrieval Ovulation induction: carried out in all groups with a combination of recombinant FSH and recombinant LH or urinary HMG; response to treatment was monitored by transvaginal ultrasound and blood oestradiol levels as needed. Initial dose was selected on the basis of ovarian volume and basal antral follicle count and varied between 150 and 225 IU. Dose was then adjusted according to individual response from day 3 of stimulation Oocyte maturation: triggered when at least 2 follicles reached a mean diameter of 18 mm. In most cases, eggs from a given donor were shared by 2 recipients, occasionally by 3 |
|
| Outcomes | Mean number of mature eggs per recipient, mean number of embryos transferred, clinical pregnancy rate, ongoing pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | Unclear risk | Insufficient information: Full text was unavailable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information: Full text was unavailable |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Other bias | Unclear risk | Insufficient information: Full text was unavailable |
Pirard 2006.
| Methods | Randomised, controlled, open, parallel‐group, pilot, single‐centre trial | |
| Participants | 30 infertile participants for IVF/ICSI Baseline characteristics: age and number retrieved were comparable between all groups |
|
| Interventions |
Ovarian stimulation: OCP + 150 to 300 IU HMG/FSH on cd 3 + 0.25 mg orgalutran Intervention and luteal phase support: Group A (n = 6) 10,000 IU HCG, followed by vaginal administration of 200 mg micronised progesterone 3 times daily (Group B) (n = 2) (discontinued) 200 μg intranasal (IN) buserelin (Suprefact; Aventis, Brussels, Belgium), followed by 100 μg IN buserelin/2 d; Group C (n = 3) (discontinued) 200 μg IN buserelin, followed by 100 μg IN buserelin/d; Group D (n = 6) 200 μg IN buserelin, followed by 100 μg IN buserelin twice a day (group E) (n = 6) 200 μg IN buserelin, followed by 100 μg IN buserelin 3 times a day Progesterone supplementation for luteal phase support in HCG group |
|
| Outcomes | Luteal phase duration in non‐pregnant participants (days), number of participants with luteal phase > 10 days, positive pregnancy test, clinical pregnancy rate, OHSS incidence, retrieved oocytes, retrieved oocytes/follicles > 10 mm, cleaved embryos, cleaved embryos/retrieved oocytes, transferred embryos | |
| Notes | During the course of the study, it became apparent that administration of buserelin every 2 days and every day was associated with severe luteal deficiency; these 2 treatment arms were stopped before completion. Participants who normally would have been included in Group B or C received a further sealed envelope, with new allocation instructions, after discontinuation of these study arms Source of funding was not clearly reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated by an independent statistician |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. No further details were reported |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | Unclear risk | Blinding was not reported clearly. Risk applies to assessment of OHSS |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available. Live birth rate was not reported |
| Other bias | High risk | During the course of the study, it became apparent that administration of buserelin every 2 days and every day was associated with severe luteal deficiency; these 2 treatment arms were stopped before completion |
Segal 1992.
| Methods | RCT, single‐centre study | |
| Participants | 179 women in the IVF programme | |
| Interventions | Subcutaneous injection of leuprolide acetate (500 micrograms) or intramuscular injection of HCG (5000 IU) 34 to 36 hours before oocyte retrieval. Vaginal progesterone (P) suppositories (50 mg) were used 2 times a day for luteal phase support. Subgroup of 41 women had serum oestradiol (E2) and P levels determined 2 and 7 days after embryo transfer (ET) | |
| Outcomes | Pregnancy rates and luteal phase E2 and P were compared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Blinding (performance bias and detection bias) FOR OHSS OUTCOME | Unclear risk | Insufficient information: Full text was unavailable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information: Full text was unavailable |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information: Full text was unavailable |
| Other bias | Unclear risk | Insufficient information: Full text was unavailable |
Abbreviations:
ART: assisted reproductive technology.
BMI: body mass index.
COH: controlled ovarian hyperstimulation.
CPR: clinical pregnancy rate.
ER: egg retrieval.
ET: embryo transfer.
FR: fertilisation rate.
FSH: follicle‐stimulating hormone.
GnRH: gonadotropin‐releasing hormone.
HCG: human chorionic gonadotropin.
ICSI: intracytoplasmic sperm injection.
IR: implantation rate.
ITT: intention‐to‐treat.
IVF: in vitro fertilisation.
LH: luteinising hormone.
LPS: luteal phase support
OCP: oral contraceptive pills
OHSS: ovarian hyperstimulation syndrome.
P: progesterone.
PCOM: polycystic ovary morphology
PCOS: polycystic ovary syndrome.
PP: per‐protocol.
RCT: randomised controlled trial.
rHCG: recombinant human chorionic gonadotropin.
TNFa: tumor necrosis factor‐alpha.
TPP: treatment per protocol
VEGF: vascular endothelial growth factor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 2006 | Overlap with Humaidan 2006 |
| Andreyko 2011 | Non‐RCT comparison |
| Awaad 2012 | IUI cycles |
| Bankowaski 2004 | Comparative, non‐randomised study |
| Beckers 2002 | Overlap with Beckers 2003 |
| Bennett 1997 | Retrospective study |
| Bodri 2009 | Retrospective cohort study |
| Bodri 2010 | Prospective, follow‐up study |
| Bodri 2013 | Not an RCT |
| Bracero 2001 | Retrospective cohort study |
| Bukulmez 2005 | Retrospective analysis of oocyte donation cycles |
| Carone 2005 | Observational uncontrolled trial |
| Castillo 2007 | Observational trial |
| Cerrillo 2011 | Prospective cohort study evaluating effects of GnRH agonist and HCG treatment on VEGF, angiopoietin‐2 and VE‐cadherin |
| Check 1993 | Non‐RCT |
| Chen 1998 | Incomplete data |
| Chen 2012 | Prospective cohort study |
| Cunha 2002 | RCT, incomplete data |
| Daneshmand 2006 | Retrospective study |
| De Jong 2001 | Case report |
| Diaz 2003 | Randomised, cross‐over, non‐IVF study |
| DiLuigi 2010 | Non‐RCT compared GnRH agonist vs coasting plus HCG |
| Egbase 2002 | IUI treatment |
| Eldar‐Geva 2007 | Retrospective study |
| Emperaire 1992 | Study design unclear and data incomplete |
| Engmann 2005 | Retrospective study |
| Engmann 2006 | Overlap with Engmann 2005 |
| Engmann 2006a | Retrospective analysis |
| Engmann 2011 | Subset analysis of participants included in RCT (Engmann 2008) |
| Engmann 2012 | Commentary |
| Erb 2009 | Retrospective study in donor cycles |
| Fatemi 2013 | RCT, randomly assigned 4 donors for 16 cycles and evaluated early luteal phase endocrine profile and double publication of Humaidan 2012 |
| Galera 2005 | Non‐randomised, uncontrolled study |
| Garcia‐Velasco 2012 | Commentary |
| Garcia‐Velasco 2010 | RCT in egg donors, all women triggered with GnRH agonist and randomly assigned to receive traditional LPS with or without small dose of HCG |
| Goto 2003 | Non‐randomised comparative cohort study |
| Griesinger 2005 | Review |
| Griesinger 2007a | Randomised observational study, double publication |
| Griesinger 2007b | Prospective, observational proof‐of‐concept study |
| Griesinger 2010 | Prospective, clinical cohort study |
| Griesinger 2011 | Prospective, clinical cohort study |
| Griffin 2012 | Retrospective cohort study |
| Herrero 2010 | Observational study |
| Humaidan 2011 | Levels of epidermal growth factor–like peptide amphiregulin in follicular fluid RCT overlap with Humaidan 2013 |
| Humaidan 2012 | RCT in 4 donors with different final oocyte maturation and oocyte triggering and luteal phase regimens |
| Imbar 2012 | Cohort study |
| Itskovitz‐Eldor 2000 | Preliminary report |
| Johnston‐MacAnanny 2007 | Retrospective comparative study |
| Joo 2012 | Non‐RCT study |
| Kaur 2012 | Prospective, non‐RCT study |
| Kol 2012 | Commentary |
| Krause 2006 | RCT, all women triggered with GnRH agonist, then randomly assigned to different LPS protocols Group A received 5 × 1000 IU HCG, Group B received 5 × 500 IU HCG and Group C received 5 × 250 mg progesterone intramuscularly |
| Kummer 2013 | Retrospective chart review |
| LaMonica2007 | Retrospective comparative study |
| Lanzone 1994 | Case control study |
| Lanzone 1994a | Study design unclear |
| Lewit 1996 | IUI treatment |
| Lin 2013 | Retrospective observational study |
| Lin MH 2013 | Retrospective cohort study |
| lliodromiti 2013 | Retrospective analysis |
| Loumaye 2004 | Control group: GnRH agonist/HCG |
| Loumaye 2007 | Observational uncontrolled study |
| Melo 2007 | Initial results of Melo 2009 |
| Meltzer 2002 | Overlap with Fauser 2002 |
| Nelson 2013 | Commentary |
| Nevo 2003 | Part of large RCT evaluating levels of inhibin A and pro‐αC during luteal phase |
| Olivennes 2001 | Overlap with Fauser 2002 |
| Orvieto 2006 | Prospective observational study |
| Orvieto 2013 | Retrospective study |
| Parneix 2001 | Study design unclearly reported in the abstract |
| Peñarrubia 1998 | Prospective non‐randomised study |
| Ricciarelli 2006 | Overlap with Acevedo 2006 |
| Schachter 2007 | Used GnRH analogue only for luteal phase support |
| Schmidt 1995 | RCT compared GnRH agonist with HCG in clomiphene citrate–stimulated cycles |
| Schmidt‐Sarosi 1995 | Randomised, controlled, IUI treatment |
| Seyhan 2013 | Retrospective analysis |
| Shalev 1995 | RCT, non‐IVF cycles |
| Shanis 1995 | No available data |
| Shapiro 2007 | Retrospective study of oocyte donor IVF cycles |
| Shapiro 2008 | Retrospective preliminary study in fresh autologous cycles of IVF |
| Shapiro 2011 | Retrospective study |
| Shapiro 2011a | Retrospective study |
| Sismangoul 2009 | Prospective randomised cross‐sectional study in egg donors |
| Toner 2006 | Retrospective cohort study |
| Westergaard 2004 | Duplicate publication (preliminary result of Humaidan 2005) |
| Wilkinson 2007 | Retrospective analysis of anonymous donor oocyte cycles |
| Yding 1993 | RCT, evaluated endocrine composition of follicular fluid, comparing human chorionic gonadotropin versus a gonadotropin‐releasing hormone agonist for ovulation induction |
Abbreviations:
GnRH: gonadotropin‐releasing hormone.
HCG: human chorionic gonadotropin.
IUI: intrauterine insemination.
IVF: in vitro fertilisation.
LPS: luteal phase support
RCT: randomised controlled trial.
VE: vascular endothelial
VEGF: vascular endothelial growth factor.
Differences between protocol and review
In this update, the following protocol changes were made.
We conducted subgroup analyses of the studies on autologous cycle, grouped by baseline risk of OHSS (low or high).
We conducted sensitivity analysis for the outcome of OHSS, including only studies of autologous cycles that reported moderate or severe OHSS as an outcome.
We subgrouped studies with modified luteal phase support: luteal phase support with LH activity (single dose or two doses of HCG, recLH and repeated GnRH doses) and luteal phase support without LH activity (progesterone only or progesterone plus oestradiol).
Differences between original review and review update
The study by Engmann 2008 (excluded in the original review because of lack of standardisation between regimens of treatment in both groups in terms of dual pituitary suppression instead of the GnRH antagonist protocol for the control group and lack of E2 supplementation in the control group) is now incorporated in the qualitative and quantitative analyses. Also, the studies of Ossina 2004, Peňa 2007 and Segal 1992 were included in the section on studies awaiting classification; they have been moved to the section on included studies but because of lack of full publication were excluded from the qualitative analysis.
Contributions of authors
Mohamed Youssef: developed and wrote the draft of the protocol, developed the title and intended methods of the review, entered the protocol and review into RevMan and responded to peer reviewers' comments.
Madelon van Wely: helped to develop the protocol, the title and the intended methods of the review; took part in writing the review and responding to peer reviewers' comments; and served as our consultant on statistical issues.
Monique Mochtar, Fulco van der Veen, Hesham Al‐Inany and Georg Griesinger: helped to develop the protocol, took part in interpretation of the data and writing of the review and served as consultants on clinical issues.
Ismail Aboulfoutouh and Mohamed Nagi Mohesen: took part in interpretation of the data and writing of the review.
Sources of support
Internal sources
University of Amsterdam, Netherlands.
External sources
None, Other.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Acevedo 2006 {published data only}
- Acevedo B, Jose Gomez‐Palomares L, Ricciarelli E, Hernández ER. Triggering ovulation with gonadotropin‐releasing hormone agonists does not compromise embryo implantation rates. Fertility and Sterility 2006;86(6):1682‐7. [DOI] [PubMed] [Google Scholar]
Babayof 2006 {published data only}
- Babayof R, Margalioth J E, Huleihel M, Amash A, Zylber‐Haran E, Gal M, et al. Serum inhibin A, VEGF and TNFa levels after triggering oocyte maturation with GnRH agonist compared with HCG in women with polycystic ovaries undergoing IVF treatment: a prospective randomized trial. Human Reproduction 2006;21(5):1260–5. [DOI] [PubMed] [Google Scholar]
Beckers 2003 {published data only}
- Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, et al. Non supplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin‐releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle‐stimulating hormone and GnRH antagonist cotreatment. The Journal of Clinical Endocrinology and Metabolism 2003;88(9):4186–92. [DOI: 10.1210/jc.2002-021953] [DOI] [PubMed] [Google Scholar]
Engmann 2008 {published data only}
- Engmann L, DiLuigi A, Schmidt D, Nulsen J, Maier D, Benadiva C. The use of gonadotropin‐releasing hormone (GnRH) agonist to induce oocyte maturation after co‐treatment with GnRH antagonist in high‐risk patients undergoing in vitro fertilization prevents the risk of ovarian hyperstimulation syndrome: a prospective randomized controlled study. Fertility and Sterility 2008;89(1):84‐91. [DOI: ] [DOI] [PubMed] [Google Scholar]
Fauser 2002 {published data only}
- Fauser BC, Jong D, Olivennes F, Warmsby H, Tay CJ, Itskovitz‐Eldor J, Hooren HG. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. The Journal of Clinical Endocrinology and Metabolism 2002;87(2):709‐15. [DOI] [PubMed] [Google Scholar]
Galindo 2009 {published data only}
- Galindo A, Bodri D, Guillén JJ, Colodrón M, Vernaeve V, Coll O. Triggering with HCG or GnRH agonist in GnRH antagonist treated oocyte donation cycles: a randomised clinical trial. Gynecology Endocrinolology 2009;25(1):60‐6. [DOI] [PubMed] [Google Scholar]
Humaidan 2005 {published data only}
- Humaidan P, Bredkjær HE, Bungum L, Bungum M, Grøndahl ML, Westergaard L, Andersen CY. GnRH agonist (buserelin) or hCG for ovulation induction in GnRH antagonist IVF/ICSI cycles: a prospective randomized study. Human Reproduction 2005;20(5):1213–20. [DOI: 10.1093/humrep/deh765] [DOI] [PubMed] [Google Scholar]
Humaidan 2006 {published data only}
- Humaidan P, Bungum M, Andersen CY. Rescue of corpus luteum function with periovulatory HCG supplementation in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist: a pilot study. Reproductive BioMedicine Online 2006;13(2):173‐8. [DOI] [PubMed] [Google Scholar]
Humaidan 2010 {published data only}
- Humaidan P, Bredkjær HE, Westergaard L, Andersen CY. 1500 IU hCG secures a normal clinical pregnancy outcome in IVF/ICSI GnRH antagonist cycles in which ovulation was triggered with a GnRH agonist. Fertility and Sterility 2010;93(3):847‐54. [DOI: 10.1016/j.fertnstert.2008.12.042] [DOI] [PubMed] [Google Scholar]
Humaidan 2013 {published data only}
- Humaidan P, Polyzos NP, Alsbjerg B, Erb K, Mikkelsen AL, Elbaek HO, et al. GnRHa trigger and individualized luteal phase hCG support according to ovarian response to stimulation: two prospective randomized controlled multi‐centre studies in IVF patients. Human Reproduction 2013;28(9):2511‐21. [DOI] [PubMed] [Google Scholar]
Kolibianakis 2005 {published data only}
- Kolibianakis EM, Schultze‐Mosgau A, Schroer A, Steirteghem A, Devroey P, Diedrich K, et al. A lower ongoing pregnancy rate can be expected when GnRH agonist is used for triggering final oocyte maturation instead of HCG in patients undergoing IVF with GnRH antagonists. Human Reproduction 2005;20(10):2887–92. [DOI: 10.1093/humrep/dei150; PUBMED] [DOI] [PubMed] [Google Scholar]
Melo 2009 {published data only}
- Melo M, Busso CE, Bellver J, Alama P, Garrido N, Meseguer M, et al. GnRH agonist versus recombinant HCG in an oocyte donation programme: a randomized, prospective, controlled, assessor‐blind study.. Reproductive BioMedicine Online 2009;19(4):486‐92. [DOI] [PubMed] [Google Scholar]
Ossina 2004 {published data only}
- Ossina E, Yavorovskaya K, Kuzmichev L, Kornilov N, Belikov V, Belikova O, et al. Triggering of final oocyte maturation in GnRH antagonist IVFprotocols: triptorelin 0.1 mg versus hCG. A randomized multi centre trial. Abstracts of the 20th Annual Meeting of the ESHRE, Berlin, Germany, 27‐30 June 2004;19(1):i99‐i102. [Google Scholar]
Papanikolaou 2010 {published data only}
- Papanikolaou EG, Verpoest W, Fatemi H, Tarlatzis B, Devroey P, Tournaye H. A novel method of luteal supplementation with recombinant luteinizing hormone when a gonadotropin‐releasing hormone agonist is used instead of human chorionic gonadotropin for ovulation triggering: a randomized prospective proof of concept study. Fertility and Sterility 2011;95(3):1174‐7. [DOI] [PubMed] [Google Scholar]
Peňa 2007 {published data only}
- Peña V, Chinea E, Sanabria V, Hernandez J, Palumbo A. Triggering final oocyte maturation with a GnRH agonist in egg donors does not reduce implantation and pregnancy rates and eliminates the risk of OHSS. Abstracts of the 23rd Annual Meeting of the ESHRE,Lyon, France, 1‐4 July 2007;22(1):i123. [Google Scholar]
Pirard 2006 {published data only}
- Pirard C, Donnez J, Loumaye E. GnRH agonist as luteal phase support in assisted reproduction technique cycles: results of a pilot study. Human Reproduction 2006;21(7):1894‐900. [DOI: 10.1093/humrep/del072] [DOI] [PubMed] [Google Scholar]
Segal 1992 {published data only}
- Segal S, Casper RF. Gonadotropin‐releasing hormone agonist versus human chorionic gonadotropin for triggering follicular maturation in in vitro fertilization. Fertility and Sterility 1992; Vol. 57, issue 6:1254‐8. [PubMed]
References to studies excluded from this review
Andersen 2006 {published data only}
- Andersen CY, Humaidan P, Ejdrup HP, Bungum L, Grøndah ML, Westergaard LG. Hormonal characteristics of follicular fluid from women receiving either GnRH agonist or hCG for ovulation induction. Human Reproduction 2006;21(8):2126–30. [DOI] [PubMed] [Google Scholar]
Andreyko 2011 {published data only}
- Murray AM, Soto‐Albors C. Use of combined oral and vaginal estradiol and IM and vaginal progesterone in luteal phase of antagonist cycles triggered with GnRH agonist results in good clinical pregnancy rates. Fertility and Sterility 2011;45:S25. [Google Scholar]
Awaad 2012 {published data only}
- Awwad JT, Hannoun AB, Khalil A, Younes ZM, Ghazeeri GS. Induction of final follicle maturation with a gonadotropin‐releasing hormone agonist in women at risk of ovarian hyperstimulation syndrome undergoing gonadotropin stimulation and intrauterine insemination: proof‐of‐concept study. Clinical and Experimental Obstetrics and Gynecology 2012;39(4):436‐9. [PubMed] [Google Scholar]
Bankowaski 2004 {published data only}
- Bankowski B, Bracero N, King J, Garcia J, Wallach E, Vlahos N. Triggering ovulation with leuprolide acetate is associated with lower pregnancy rates. Abstracts of the 20th Annual Meeting of the ESHRE, Berlin, Germany, 27–30 June 2004;19 Suppl 1:i103. [Google Scholar]
Beckers 2002 {published data only}
- Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum R, Bustion S, et al. Comparison of the non supplemented luteal phase characteristics after recombinant luteinizing hormone (r) HCG, rLH or GnRH agonist for oocyte maturation in IVF. Abstracts of the 18th annual meeting of the ESHRE. 2002; Vol. 17, issue 7:55.
Bennett 1997 {published data only}
- Bennett RA, Vidali A, Walker J, Navot D. Triggering ovulation with GnRH agonist to avoid ovarian hyperstimulation, frequently results in profound corpus luteum insufficiency. Fertility and Sterility 1997;68 Suppl 1:174‐5. [Google Scholar]
Bodri 2009 {published data only}
- Bodri D, Guillen JJ, Galind A, Mataro D, Pujol A, Coll O. Triggering with human chorionic gonadotropin or a gonadotropin‐releasing hormone agonist in gonadotropin‐releasing hormone antagonist‐treated oocyte donor cycles: findings of a large retrospective cohort study. Fertility and Sterility 2009;91(2):365‐71. [DOI: 10.1016/j.fertnstert.2007.11.049] [DOI] [PubMed] [Google Scholar]
Bodri 2010 {published data only}
- Bodri D, Guillén JJ, Trullenque M, Schwenn K, Esteve C, Coll O. Early ovarian hyperstimulation syndrome is completely prevented by gonadotropin releasing‐hormone agonist triggering in high‐risk oocyte donor cycles: a prospective, luteal‐phase follow‐up study. Fertil Steril. 2010;93(7):2418‐20. [DOI] [PubMed] [Google Scholar]
Bodri 2013 {published data only}
- Bodri D. Low‐dose hCG supplementation after GnRH agonist triggering: don't be too quick on the trigger.. Hum Reprod 2013;28(9):2315‐7. [DOI] [PubMed] [Google Scholar]
Bracero 2001 {published data only}
- Bracero NJ, Jurema MW, Posada MN, Whelan JG, Garcia JE, Vlahos NP. Triggering ovulation with leuprolide acetate (LA) instead of human chorionic gonadotropin (hCG) after the use of ganirelix for in vitro fertilization‐embryo transfer (IVF‐ET) does not compromise cycle outcome and may prevent ovarian hyperstimulation syndrome. Fertility and Sterility 2001;76 Suppl 3:93. [Google Scholar]
Bukulmez 2005 {published data only}
- Bukulmez O, Rehman KS, Langley M, Carr BR, Doody KM, Doody KJ. Triggering ovulation by GnRH agonist leuprolide acetate does not adversely affect the number and quality of the oocytes as compared to recombinant hCG in oocyte donation cycles. Abstract of the annual meeting of ASRM 2005;84 Suppl(1):314‐5. [Google Scholar]
Carone 2005 {published and unpublished data}
- Carone D, Vizziello GM, Schonauer LM, D’Amato G. Safety and efficacy of GnRH agonist to trigger ovulation in controlled ovarian hyperstimulation for ART with recombinant FSH and GnRH‐antagonist in high responders (PCOD) patients. Abstracts of the 8th International Symposium on GnRH‐analogues in Cancer and Human Reproduction.Salzburg, Austria 2005:A24.
Castillo 2007 {published data only}
- Castillo JC, Dolz M, Evangelio B, Abad de Velasco L, Bonilla‐Musoles F. Efficacy and security of luteal phase support with low doses of HCG in OHSS high risk patients triggered with GnRH agonists. Abstracts of the 23rd Annual Meeting of the ESHRE, Lyon, France, 1–4 July 2007;22(1):i 81. [Google Scholar]
Cerrillo 2011 {published data only}
- Cerrillo M, Pacheco A, Rodríguez S, Mayoral M, Ruiz M, García Velasco JA. Differential regulation of VEGF, cadherin, and angiopoietin 2 by trigger oocyte maturation with GnRHa vs hCG in donors: try to explain the lower OHSS incidence. Abstracts of the 25th annual meeting of the ESHRE, Amsterdam, June 28‐1 July, 2009;24(1):i60. [Google Scholar]
Check 1993 {published data only}
Chen 1998 {published data only}
- Chen SL, Dong H, Xing FQ. Clinical study of buserelin, instead of hCG, used for triggering follicular maturation in infertile patients with PCOS. Fertility and Sterility 1998; Vol. 70, issue 3:S396.
Chen 2012 {published data only}
- Chen SL, Ye DS, Chen X, Yang XH, Zheng HY, Tang Y, et al. Circulating luteinizing hormone level after triggering oocyte maturation with GnRH agonist may predict oocyte yield in flexible GnRH antagonist protocol. Human Reproduction 2012;27(5):1351‐6. [DOI] [PubMed] [Google Scholar]
Cunha 2002 {published data only}
- Cunha Filho J, Gratao A, Souza C, Freitas F, Vettori D, Passos E. Prospective randomized clinical trial to evaluate embryo quality (score) after GnRH agonist or HCG administration to induce oocyte maturation. Human Reproduction 2002; Vol. 17, issue 1:150, Abstract no: P‐440.
Daneshmand 2006 {published data only}
- Daneshmand ST, Shapiro BS, Garner FC, Aguirre M, Ross R. Cumulative pregnancy rates when using GnRH agonists instead of HCG for final oocyte maturation. Abstract of the annual meeting of ASRM 2006;86 Suppl(2):184‐5. [Google Scholar]
De Jong 2001 {published data only}
- Jong D, Hooren EG, Macklon NS, Mannaerts BM, Fauser B. Pregnancy and birth after GnRH agonist treatment for induction of final oocyte maturation in a woman undergoing ovarian stimulation for ICSI, using a GnRH antagonist (Orgalutran/Antagon) to prevent a premature LH surge: a case report. Journal of Assisted Reproduction and Genetics 2001;18:30‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz 2003 {published data only}
- Diaz I, Guillen A, Pacheco A, Requena A, Simon C, Garcia Velasco J. Final oocyte maturation with GnRH‐agonist versus hCG in intrauterine insemination. Abstracts of the 19th annual meeting of the ESHRE.Madrid, Spain, June 29–July 2 2003;18(1):134. [Google Scholar]
DiLuigi 2010 {published data only}
- DiLuigi AJ, Engmann L, Schmidt DW, Maier DB, Nulsen JC, Benadiva CA. Gonadotropin‐releasing hormone agonist to induce final oocyte maturation prevents the development of ovarian hyperstimulation syndrome in high‐risk patients and leads to improved clinical outcomes compared with coasting. Fertility and Sterility 2010;94(3):1111‐4. [DOI] [PubMed] [Google Scholar]
Egbase 2002 {published data only}
- Egbase PE, Grudzinskas JG, Al Sharhan M, Ashkenani L. HCG or GnRH agonist to trigger ovulation in GnRH antagonist‐treated intrauterine insemination cycles: a prospective randomized study. Abstracts of the 18th Annual Meeting of the ESHRE, Vienna, Austria 2002 2002;17(1):2. [Google Scholar]
Eldar‐Geva 2007 {published data only}
- Eldar‐Geva T, Zylber‐Haran E, Babayof R, Halevy‐Shalem T, Ben‐Chetrit A, Tsafrir A, et al. Similar outcome for cryopreserved embryo transfer following GnRH antagonist/GnRH agonist, GnRH ‐antagonist/HCG or long protocol ovarian stimulation. Reproductive BioMedicine Online 2007;14(2):148‐54. [DOI] [PubMed] [Google Scholar]
Emperaire 1992 {published data only}
- Emperaire JC, Ruffie A, Audebert AJ. [Ovulation induction by endogenous LH released by the administration of an LHRH agonist after follicular stimulation for in vitro fertilization]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1992; Vol. 21:489‐94. [PubMed]
Engmann 2005 {published data only}
- Engmann LA, Diluigi D, Schmidt J, Nulsen D, Maier C, Benadiva. Prevention of ovarian hyperstimulation syndrome (OHSS) with the use of gonadotropin releasing hormone (GnRH) agonist to trigger final oocyte maturation after cotreatment with GnRH antagonist in patients with polycystic ovarian syndrome (PCOS) or previous high response undergoing IVF treatment. A prospective randomized clinical trial. Abstract of the annual meeting of ASRM 2005;84 Suppl(1):96. [Google Scholar]
Engmann 2006 {published data only}
- Engmann L, Siano L, Weitzman V, Nulsen J, Maier D, Benadiva C. Induction of oocyte maturation with GnRH agonists in high‐risk patients undergoing IVF treatment does not adversely affect implantation rate. Fertility and Sterility 2006;86(1):797‐8. [Google Scholar]
Engmann 2006a {published data only}
Engmann 2011 {published data only}
- Engmann L, Romak J, Nulsen J, Benadiva C, Peluso C. In vitro viability and secretory capacity of human luteinized granulosa cells after gonadotropin‐releasing hormone agonist trigger of oocyte maturation. Fertility and Sterility 2011;96(1):198‐202. [DOI] [PubMed] [Google Scholar]
Engmann 2012 {published data only}
- Engmann L, Benadiva C. Agonist trigger: what is the best approach? Agonist trigger with aggressive luteal support. Fertility and Sterility 2012;97(3):531‐3. [DOI] [PubMed] [Google Scholar]
Erb 2009 {unpublished data only}
Fatemi 2013 {published data only}
- Fatemi HM, Polyzos NP, Vaerenbergh I, Bourgain C, Blockeel C, Alsbjerg B, et al. Early luteal phase endocrine profile is affected by the mode of triggering final oocyte maturation and the luteal phase support used in recombinant follicle‐stimulating hormone‐gonadotropin‐releasing hormone antagonist in vitro fertilization cycles. Fertility and Sterility 2013;100(3):742‐7. [DOI] [PubMed] [Google Scholar]
Galera 2005 {published data only}
- Galera F, Rayward J, Verdu V, Villafanez V, Manzanares MA, Garijo E. GnRH agonist triggered LH surge: P4, E2 levels. IVF outcome. Abstracts of the 21st Annual meeting of the ESHRE , Copenhagen, Denmark, 19‐22, 2005;20(1):i 109. [Google Scholar]
Garcia‐Velasco 2010 {published data only}
- Garcia‐Velasco JA, Motta L, López A, Mayoral M, Cerrillo M, Pacheco A. Low‐dose human chorionic gonadotropin versus estradiol/progesterone luteal phase support in gonadotropin‐releasing hormone agonist‐triggered assisted reproductive technique cycles: understanding a new approach. Fertility and Sterility 2010;94(7):2820‐3. [DOI] [PubMed] [Google Scholar]
Garcia‐Velasco 2012 {published data only}
- Garcia‐Velasco JA. Agonist trigger: what is the best approach? Agonist trigger with vitrification of oocytes or embryos. Fertility and Sterility 2012;97(3):527‐8. [DOI] [PubMed] [Google Scholar]
Goto 2003 {published data only}
- Goto T, Oka C, Tatsuhiro T, Mukaida T, Takahashi K. Single dose nasal spray of gonadotropin releasing hormone (GnRH) agonist effectively matures oocytes for in vitro fertilization in an ovarian stimulation protocol using clomiphene citrate, gonadotropin, and GnRH antagonist. Abstracts of the Annual Meeting of the ASRM 2003;80 Suppl(3):6. [Google Scholar]
Griesinger 2005 {published data only}
- Griesinger G, Schultze‐Mosgau A, Schroer A, Steirteghem A, Devroey P, Diedrich K, et al. GnRH‐agonist instead of hCG for final oocyte maturation in GnRH‐antagonist cycles. 21st Annual Meeting of the European Society of Human Reproduction and Embryology, Copenhagen, Denmark, 19‐22 June 2005 2005:i91.
Griesinger 2007a {published data only}
- Griesinger G, Kolibianakis EM, Papanikolaou EG, Diedrich K, Steirteghem A, Devroey PC. Triggering of final oocyte maturation with gonadotropin‐releasing hormone agonist or human chorionic gonadotropin. Live birth after frozen‐thawed embryo replacement cycles. Fertility and Sterility 2007;88(3):616‐21. [DOI: 10.1016/j.fertnstert.2006.12.006] [DOI] [PubMed] [Google Scholar]
Griesinger 2007b {published data only}
- Griesinger G, Otte S, Schroer A, Ludwig AK, Diedrich K, Al‐Hasani S, et al. Elective cryopreservation of all pro nuclear oocytes after GnRH agonist triggering of final oocyte maturation in patients at risk of developing OHSS: a prospective, observational proof‐of‐concept study. Human Reproduction 2007;22(5):1348–52. [DOI: 10.1093/humrep/dem006] [DOI] [PubMed] [Google Scholar]
Griesinger 2010 {published data only}
- Griesinger G, Berndt H, Schultz L, Depenbusch M, Schultze‐Mosgau A. Cumulative live birth rates after GnRH‐agonist triggering of final oocyte maturation in patients at risk of OHSS: a prospective, clinical cohort study. European Journal of Obstetric and Gynecologic Reproduction Biology 2010;149(2):190‐4. [DOI] [PubMed] [Google Scholar]
Griesinger 2011 {published data only}
- Griesinger G1, Schultz L, Bauer T, Broessner A, Frambach T, Kissler S. Ovarian hyperstimulation syndrome prevention by gonadotropin‐releasing hormone agonist triggering of final oocyte maturation in a gonadotropin‐releasing hormone antagonist protocol in combination with a "freeze‐all" strategy: a prospective multicentric study. Fertility and Sterility 2011;95(6):2029‐33. [DOI] [PubMed] [Google Scholar]
Griffin 2012 {published data only}
- Griffin D, Benadiva C, Kummer N, Budinetz T, Nulsen J, Engmann L. Dual trigger of oocyte maturation with gonadotropin‐releasing hormone agonist and low‐dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertility and Sterility 2012;97(6):1316‐20. [DOI] [PubMed] [Google Scholar]
Herrero 2010 {published data only}
- Herrero L, Pareja S, Losada C, et al. Avoiding the use of human chorionic gonadotropin combined with oocyte vitrification and GnRH agonist triggering versus coasting: a new strategy to avoid ovarian hyperstimulation syndrome. Fertility and Sterility 2010;95(3):1137‐40. [DOI] [PubMed] [Google Scholar]
Humaidan 2011 {published data only}
- Humaidan P, Westergaard LG, Mikkelsen AL, Fukuda M, Yding Andersen C. Levels of the epidermal growth factor‐like peptide amphiregulin in follicular fluid reflect the mode of triggering ovulation: a comparison between gonadotrophin‐releasing hormone agonist and urinary human chorionic gonadotrophin. Fertility and Sterility 2011; Vol. 95, issue 6:2034‐8. [DOI] [PubMed]
Humaidan 2012 {published data only}
- Humaidan P, Vaerenbergh I, Bourgain C, Alsbjerg B, Blockeel C, Schuit F, et al. Endometrial gene expression in the early luteal phase is impacted by mode of triggering final oocyte maturation in recFSH stimulated and GnRH antagonist co‐treated IVF cycles. Human Reproduction 2012; Vol. 27, issue 11:3259‐72. [DOI] [PubMed]
Imbar 2012 {published data only}
- Imbar T, Kol S, Lossos F, Bdolah Y, Hurwitz A, Haimov‐Kochman R. Reproductive outcome of fresh or frozen‐thawed embryo transfer is similar in high‐risk patients for ovarian hyperstimulation syndrome using GnRH agonist for final oocyte maturation and intensive luteal support. Human Reproduction 2012;27(3):753‐9. [DOI] [PubMed] [Google Scholar]
Itskovitz‐Eldor 2000 {published data only}
- Itskovitz‐Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndrome: preliminary report. Human Reproduction 2000;15(9):1965‐8. [DOI] [PubMed] [Google Scholar]
Johnston‐MacAnanny 2007 {published data only}
- Johnston‐MacAnanny EB, DiLuigi AJ, Benadiva CA, Maier DB, Nulsen JC, Engmann LL. Choice of medication for oocyte maturation for in vitro fertilization (IVF) gonadotropin releasing hormone (GnRH) antagonist cycles; Human chorionic gonadotropin (HCG) vs leuprolide acetate (LA). Abstract of the annual meeting of ASRM 2007;88 Suppl(1):142. [Google Scholar]
Joo 2012 {published data only}
- Joo JK, Choi JR, Son JB, Ko GR, Lee KS. Preliminary clinical outcome of novel strategy for the maximization of cumulative pregnancy rates per retrieval in normal responders. Clinical and Experimental Reproductive Medicine 2012;39(1):33‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaur 2012 {published data only}
- Kaur H, Krishna D, Shetty N, Krishnan S, Srinivas MS, Rao KA. Effect of pre‐ovulatory single dose GnRH agonist therapy on IVF outcome in GnRH antagonist; a prospective study. Journal of Reproduction and Infertility 2012;13(4):225‐31. [PMC free article] [PubMed] [Google Scholar]
Kol 2012 {published data only}
- Kol S, Homburg R, Alsbjerg B, Humaidan P. The gonadotropin‐releasing hormone antagonist protocol—the protocol of choice for the polycystic ovary syndrome patient undergoing controlled ovarian stimulation. Acta Obstetricia et Gynecologica Scandinavica 2012;91(6):643‐7. [DOI] [PubMed] [Google Scholar]
Krause 2006 {published data only}
- Krause BT, Ohlinger O. Safety and efficacy of low dose hCG for luteal support after triggering ovulation with a GnRH agonist in cases of polyfollicular development. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2006;126:87–92. [DOI] [PubMed] [Google Scholar]
Kummer 2013 {published data only}
- Kummer NE, Feinn RS, Griffin DW, Nulsen JC, Benadiva CA, Engmann LL. Predicting successful induction of oocyte maturation after gonadotropin‐releasing hormone agonist (GnRHa) trigger. Human Reproduction 2013;28(1):152‐9. [DOI] [PubMed] [Google Scholar]
LaMonica2007 {published data only}
- LaMonica R, Siano LJ, Nulsen JC, Maier DB, Benadiva CA, Engmann LL. Elective cryopreservation of all embryos after GnRH agonist trigger is not justified because of the comparable success rates between fresh and cryo‐thawed embryo transfers. Abstracts of the annual meeting of ASRM 2007;88 Suppl(1):135. [Google Scholar]
Lanzone 1994 {published data only}
- Lanzone A, Fulghesu AM, Villa P, Guida C, Guido M, Nicoletti MC, et al. Gonadotropin‐releasing hormone agonist versus human chorionic gonadotropin as a trigger of ovulation in polycystic ovarian disease gonadotropin hyperstimulated cycles. Fertility and Sterility 1994;62(1):35‐41. [DOI] [PubMed] [Google Scholar]
Lanzone 1994a {published data only}
- Lanzone A, Fulghesu AM, Villa P, Guida C, Guido M, Nicoletti MC, et al. Gonadotropin‐releasing hormone agonist versus human chorionic gonadotropin as a trigger of ovulation in polycystic ovarian disease gonadotropin hyperstimulated cycles [erratum appears in Fertil Steril 1995 Mar 63(3):684‐5]. Fertility and Sterility 1994; Vol. 62, issue 1:35‐41. [DOI] [PubMed]
Lewit 1996 {published data only}
- Lewit N, Kol S, Manor D, Itskovitz‐Eldor J. Comparison of gonadotrophin‐releasing hormone analogues and human chorionic gonadotrophin for the induction of ovulation and prevention of ovarian hyperstimulation syndrome. Human Reproduction 1996;11(7):1399‐402. [DOI] [PubMed] [Google Scholar]
Lin 2013 {published data only}
- Lin YH, Huang MZ, Hwang JL, Chen HJ, Hsieh BC, Huang LW, et al. Combination of cabergoline and embryo cryopreservation after GnRH agonist triggering prevents OHSS in patients with extremely high estradiol levels—a retrospective study. Journal of Assisted Reproduction and Genetics 2013;30(6):753‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin MH 2013 {published data only}
- Lin MH, Shao‐Ying Wu F, Kuo‐Kuang lee R, Li SH, Lin SY, Hwu YM. Dual trigger with combination of gonadotropin‐releasing hormone agonist and human chorionic gonadotropin significantly improves the live‐birth rate for normal responders in GnRH‐antagonist cycles. Fertilty and Sterility 2013;100(5):1296‐302. [DOI] [PubMed] [Google Scholar]
lliodromiti 2013 {published data only}
- Iliodromiti S, Blockeel C, Tremellen KP, Fleming R, Tournaye H, Humaidan P, et al. Consistent high clinical pregnancy rates and low ovarian hyperstimulation syndrome rates in high‐risk patients after GnRH agonist triggering and modified luteal support: a retrospective multicentre study. Human Reproduction 2013;28(9):2529‐36. [DOI] [PubMed] [Google Scholar]
Loumaye 2004 {published data only}
- Loumaye E, Pirard C, Donnez J. GnRH agonist as a novel luteal support: results of a pilot study. Abstracts of the 20th Annual Meeting of the ESHRE, Berlin, Germany, 27 ‐ 30 June 2004;19(1):i4. [Google Scholar]
Loumaye 2007 {published data only}
- Loumaye E, Pirard C, Donnez J. Efficacy of GnRH agonist as luteal support: results of a prospective, randomized, comparative study. Abstracts of the 23rd Annual Meeting of the ESHRE, Lyon,France 1‐4 July 2007;22(1):i82. [Google Scholar]
Melo 2007 {published data only}
- Melo M, Busso CE, Bellver J, Alama P, Garrido N, Meseguer M, et al. GnRH agonist versus recombinant HCG in an oocyte donation programme: a randomized, prospective, controlled, assessor‐blind study [Melo M, Busso CE, Bellver J, Alama P, Garrido N, Meseguer M, et al. A randomized, prospective, controlled, assessor‐blind study, comparing triptorelin vs rhCG as trigger oocyte maturation in oocyte donors]. 2007 Fertility and Sterility; Vol. 88, issue 1:34.
Meltzer 2002 {published data only}
- Meltzer S, Girsh E, Shults A, Katz N, Zohav E, Tur‐Kaspa I. Prevention of ovarian hyperstimulation syndrome in high responders undergoing IVF treatment with GnRH antagonist combined with single dose of GnRH agonist, instead of HCG, for the induction of oocyte maturation. Abstracts of the 18th Annual Meeting of the ESHRE 2002;17 Suppl1:89. [Google Scholar]
Nelson 2013 {published data only}
- Nelson SM. Venous thrombosis during assisted reproduction: novel risk reduction strategies. Thrombosis Research 2013;Suppl 1:S1‐S3. [DOI] [PubMed] [Google Scholar]
Nevo 2003 {published data only}
- Nevo O, Eldar‐Geva T, Kol BS, Itskovitz‐Eldor J. Lower levels of inhibin A and pro‐C during the luteal phase after triggering oocyte maturation with a gonadotropin releasing hormone agonist versus human chorionic gonadotropin. Fertility and Sterility 2003;79(5):1123‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Olivennes 2001 {published data only}
- Olivennes F. Induction of final oocyte maturation by a single dose of GNRH agonist after ganirelix treatment. Gynecological Endocrinology 2001;15(Suppl 2):7. [Google Scholar]
Orvieto 2006 {published data only}
- Orvieto R, Zagatsky I, Yullzari‐Roll V, Marca A, Fisch B. Substituting human gonadotropin by gonadotropin‐releasing hormone to trigger final follicular maturation, during controlled ovarian hyperstimulation, results in less systemic inflammation. Gynecological Endocrinology 2006;22(8):437‐40. [DOI] [PubMed] [Google Scholar]
Orvieto 2013 {published data only}
- Orvieto R, Nahum R, Zohav E, Liberty G, Anteby EY, Meltcer S. GnRH‐agonist ovulation trigger in patients undergoing controlled ovarian hyperstimulation for IVF with ultrashort flare GnRH‐agonist combined with multidose GnRH‐antagonist protocol. Gynecological Endocrinology 2013;29(1):51‐3. [DOI] [PubMed] [Google Scholar]
Parneix 2001 {published data only}
Peñarrubia 1998 {published data only}
- Peñarrubia J, Balasch J, Fábregues F, Creus M, Casamitjana R, Ballescá JL, et al. Human chorionic gonadotrophin luteal support overcomes luteal phase inadequacy after gonadotrophin‐releasing hormone agonist‐induced ovulations in gonadotrophin‐stimulated cycles. Human Reproduction 1998;13(12):3315‐8. [DOI] [PubMed] [Google Scholar]
Ricciarelli 2006 {published data only}
- Ricciarelli E, Acevedo‐Martin B, Gomez‐Palomares JL, Pareja A, Hernandes ER. Triggering ovulation with GnRH agonists does not compromise embryo implantability. Human Reproduction 2006; Vol. 21, issue Suppl:i35.
Schachter 2007 {published data only}
- Schachter M, Friedler S, Ron‐El R, Zimmerman AL, Strassburger D, Bern O, et al. Can pregnancy rate be improved in gonadotropin ‐releasing hormone (GnRH) antagonist cycles by administering GnRH agonist before oocyte retrieval? A prospective, randomized study. Fertility and Sterility 2008;90(4):1087‐93. [DOI: 10.1016/j.fertnstert.2007.07.1316] [DOI] [PubMed] [Google Scholar]
Schmidt 1995 {published data only}
- Schmidt Sarosi C, Kaplan DR, Sarosi P, Essig MN, Licciardi FL, Keltz M, et al. Ovulation triggering in clomiphene citrate stimulating cycles: human chronic gonadotropin versus a gonadotropin releasing hormone agonist. Journal of Assisted Reproduction & Genetics 1995; Vol. 12, issue 3:167‐73. [DOI] [PubMed]
Schmidt‐Sarosi 1995 {published data only}
- Schmidt‐Sarosi C, Kaplan DR, Sarosi P, Essig MN, Licciardi FL, Keltz M, et al. Ovulation triggering in clomiphene citrate‐stimulated cycles: human chorionic gonadotropin versus a gonadotropin releasing hormone agonist. Journal of Assisted Reproduction and Genetics 1995;12(3):167‐74. [DOI] [PubMed] [Google Scholar]
Seyhan 2013 {published data only}
- Seyhan A, Ata B, Polat M, Son WY, Yarali H, Dahan MH. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Human Reproduction 2013;28(9):2522‐8. [DOI] [PubMed] [Google Scholar]
Shalev 1995 {published data only}
Shanis 1995 {published data only}
Shapiro 2007 {published data only}
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Comparison of human chorionic gonadotropin and gonadotropin‐releasing hormone agonist for final oocyte maturation in oocyte donor cycles. Fertility and Sterility 2007;88(1):237. [DOI: 10.1016/j.fertnstert.2006.11.069] [DOI] [PubMed] [Google Scholar]
Shapiro 2008 {published data only}
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Ross R. Gonadotropin‐releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertility and Sterility 2008;90(1):231‐3. [DOI] [PubMed] [Google Scholar]
Shapiro 2011 {published data only}
- Shapiro BS, Daneshmand ST, Garner FC, et al. Comparison of "triggers" using leuprolide acetate alone or in combination with low‐dose human chorionic gonadotropin. Fertility and Sterility 2011;95(8):2715‐7. [DOI] [PubMed] [Google Scholar]
Shapiro 2011a {published data only}
- Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Efficacy of induced luteinizing hormone surge after "trigger" with gonadotropn‐releasing hormone agonist. Fertility and Sterility 2011;95(2):826‐8. [DOI] [PubMed] [Google Scholar]
Sismangoul 2009 {published data only}
- Sismanoglu A1, Tekin HI, Erden HF, Ciray NH, Ulug U, Bahceci M. Ovulation triggering with GnRH agonist vs. hCG in the same egg donor population undergoing donor oocyte cycles with GnRH antagonist: a prospective randomized cross‐over trial. Journal of Assisted Reproduction and Genetics 2009;26(5):251‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toner 2006 {published data only}
- Toner JP, Denis AL, Hasty LA, Carpenter S, Bates W. Lupron trigger experience in GnRH antagonist ART cycles. Abstracts of the Annual meeting of the ASRM 2006;86(3):S118‐9. [Google Scholar]
Westergaard 2004 {published data only}
- Westergaard L, Andersen CY, Humaidan P, Bredkjær HE, Bungum L, Bungum M, et al. Significant reduction of clinical pregnancy and implantation rates by use of GnRH agonist (Buserelin) as compared to hCG to induce ovulation in FSH/GnRH antagonist treated IVF/ICSI cycles. Abstracts of the 20th Annual Meeting of the ESHRE,Berlin,Lyon, 27‐30 June 2004;19(1):i3. [Google Scholar]
Wilkinson 2007 {published data only}
- Wilkinson SC, Walker SA, Hayes BA, Donesky BW, Bird JS, Anderson AR. Gonadotropin releasing hormone (GnRH) agonist trigger following antagonist protocols in anonymous oocyte donors minimizes ovarian hyperstimulation syndrome. Abstract of the annual meeting of ASRM 2007;88(1):S131. [Google Scholar]
Yding 1993 {published data only}
Additional references
Delvigne 2003
- Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome. Human Reproduction Update 2003;9:77‐96. [DOI] [PubMed] [Google Scholar]
Emperaire 2004
- Emperaire JC, Parneix I, Ruffie A. Luteal phase defects following agonist‐triggered ovulation: a patient‐dependent response. Reproductive Biomedicine Online 2004;9:22‐7. [DOI] [PubMed] [Google Scholar]
Felberbaum 1995
- Felberbaum PE, Reissmannb T, Kiipkera W, Bauera O, Al Hasania S, Diedrich C, et al. Preserved pituitary response under ovarian stimulation with HMG and GnRH antagonists (cetrorelix) in women with tubal infertility. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1995;61:151‐5. [DOI] [PubMed] [Google Scholar]
Forman 1998
- Forman R, Fries N, Tastart J, Belaisch J, Hazout A, Frydman R. Evidence of an adverse effect of elevated serum estradiol concentrations on embryo implantation. Fertility and Sterility 1998;49:118‐22. [DOI] [PubMed] [Google Scholar]
Golan 1989
- Golan A, Ron‐el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstetrical & Gynecological Survey 1989;44(6):430‐40. [DOI] [PubMed] [Google Scholar]
Gonen 1990
- Gonen Y, Balakier H, Powell W, Casper RF. Use of GnRH agonist to trigger follicular maturation for in vitro fertilization. The Journal of Clinical Endocrinology and Metabolism 1990;71:918–23. [DOI] [PubMed] [Google Scholar]
Griesinger 2006
- Griesinger G, Diedrich K, Dovroey P, Kolibianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta‐analysis. Human Reproduction Update 2006;12(2):159‐68. [DOI: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:www.cochrane‐handbook.org.
Humaidan 2009
- Humaidan P, Papanikolaou EG, Tarlatzis BC. GnRHa to trigger final oocyte maturation: a time to reconsider. Human Reproduction 2009;24(10):2389‐94. [DOI] [PubMed] [Google Scholar]
Kol 2004
- Kol S. Luteolysis induced by a gonadotropin‐releasing hormone agonists the key to prevention of ovarian hyperstimulation syndrome. Fertility and Sterility 2004;81(1):1–5. [DOI] [PubMed] [Google Scholar]
Kol 2008
- Kol S. GnRH agonist for triggering final oocyte maturation in patients at risk of ovarian hyperstimulation syndrome: still a controversy?. Journal of Assisted Reproduction and Genetics 2008;25:63‐6. [DOI: 10.1007/s10815-008-9198-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kol 2013
- Kol S, Humaidan P. GnRH agonist triggering: recent developments. Reproductive Biomedicine Online 2013;26(3):226‐30. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2012;2012 Dec 12;12:MR000033:doi: 10.1002/14651858.MR000033.pub2. Review.. [DOI] [PubMed] [Google Scholar]
Navot 1992
- Navot D, Bergh PA, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertility and Sterility 1992;58:249‐61. [DOI] [PubMed] [Google Scholar]
Olivennes 1996
- Olivennes F, Fanchin R, Bouchard P, Taieb J, Frydman R. Triggering of ovulation by a gonadotropin‐releasing hormone (GnRH) agonist in patients pretreated with GnRH antagonist. Fertility and Sterility 1996;66:151‐3. [DOI] [PubMed] [Google Scholar]
Simon 1995
- Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum estradiol concentrations in high and normal responders. Human Reproduction 1995;10:2432‐7. [DOI] [PubMed] [Google Scholar]
Simon 1998
- Simon C, Garcia Velasco JJ, Valbuena D, Peinado JA, Moreno C, Rehmohi J, et al. Increasing uterine receptivity by decreasing estradiol levels during the preimplantation period in high responders with the use of a follicle‐stimulating hormone step‐down regimen. Fertility and Sterility 1998;70:234‐9. [DOI] [PubMed] [Google Scholar]
Tavaniotou 2002
- Tavaniotou A, Albano C, Smitz J, Devroey P. Impact of ovarian stimulation on corpus luteum function and embryonic implantation. Journal of Reproductive Immunology 2002;55:123‐30. [DOI] [PubMed] [Google Scholar]
Tay 2002
- Tay CC. Use of gonadotropin‐releasing hormone agonist to trigger ovulation. Human Fertility 2002;5:G35‐7. [DOI] [PubMed] [Google Scholar]
Valbuena 2001
- Valbuena D, Martin J, Palbo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertility and Sterility 2001;76:962‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Youssef 2010
- Youssef MA, Veen F, Al‐Inany HG, Griesinger G, Mochtar MH, Wely M. Gonadotropin‐releasing hormone agonist versus HCG for oocyte triggering in antagonist assisted reproductive technology cycles. Cochrane Database Syst Rev 2010;11(CD008046):doi: 10.1002/14651858.CD008046.pub2.. [DOI] [PubMed] [Google Scholar]


