Abstract
Purpose:
The monarcHER trial has shown that abemaciclib, a cyclin-dependent kinase 4 and 6 inhibitor, combined with fulvestrant and trastuzumab, improves progression-free survival (PFS) in hormone receptor–positive (HR+), HER2-positive (HER2+) advanced breast cancer (ABC) compared with standard-of-care (SOC) chemotherapy combined with trastuzumab. We report the final overall survival (OS) analysis, updated safety and efficacy data, and exploratory biomarker results from monarcHER.
Patients and Methods:
monarcHER (NCT02675231), a randomized, multicenter, open-label, phase II trial, enrolled 237 patients across Arm A (abemaciclib, trastuzumab, fulvestrant), Arm B (abemaciclib, trastuzumab), and Arm C (SOC chemotherapy, trastuzumab). Following the statistical plan, OS and PFS were estimated in all arms. RNA sequencing (RNA-seq) was performed on archival tissue.
Results:
Median OS was 31.1 months in Arm A, 29.2 months in Arm B, and 20.7 months in Arm C [A vs. C: HR, 0.71; 95% confidence interval (CI), 0.48–1.05; nominal two-sided P value 0.086; B vs. C: HR 0.83 (95% CI, 0.57–1.23); nominal two-sided P value 0.365]. Updated PFS and safety findings were consistent with previous results. The most frequently reported treatment-emergent adverse events included diarrhea, fatigue, nausea, neutrophil count decrease, and anemia. In exploratory RNA-seq analyses, Luminal subtypes were associated with longer PFS [8.6 vs. 5.4 months (HR, 0.54; 95% CI, 0.38–0.79)] and OS [31.7 vs. 19.7 months (HR, 0.68; 95% CI, 0.46–1.00)] compared with non-Luminal.
Conclusions:
In this phase II trial, abemaciclib + trastuzumab ± fulvestrant numerically improved median OS in women with HR+, HER2+ ABC compared with SOC chemotherapy + trastuzumab.
Translational Relevance.
Hormone receptor–positive (HR+), HER2-positive (HER2+) breast cancer makes up 10% of all breast cancers in the United States. Abemaciclib, a potent oral cyclin-dependent kinase 4 and 6 (CDK4 and 6) inhibitor, is approved for HR+, HER2-negative early and advanced breast cancer (ABC), in combination with endocrine therapy. In this update to the phase II monarcHER study, we report that abemaciclib in combination with trastuzumab ± fulvestrant resulted in a numerical improvement in overall survival (OS) in patients with HR+, HER2+ ABC compared with patients treated with standard-of-care chemotherapy and trastuzumab. In addition, exploratory analysis of intrinsic subtypes suggested that Luminal subtypes were associated with longer progression-free survival and OS than non-Luminal subtypes. The efficacy and safety data indicate abemaciclib has potential as a therapy for HR+, HER2+ ABC.
Introduction
Breast cancer is the most frequently diagnosed cancer in females in the United States and globally (1–3), with hormone receptor–positive (HR+), HER2-positive (HER2+) breast cancer making up an estimated 10% of all breast cancer subtypes in the United States (4). Combining HER2-directed therapies with standard-of-care (SOC) chemotherapy has improved outcomes for patients with HER2+ breast cancer (5, 6). Current therapy for metastatic HER2+ breast cancer often consists of first-line therapy with a taxane combined with trastuzumab and pertuzumab, followed by therapy with trastuzumab deruxtecan, and then sequenced with either trastuzumab emtansine or capecitabine plus trastuzumab and pertuzumab. After progression on these standard therapies, patients then often receive sequential chemotherapy with trastuzumab or with margetuximab. (7–9). There are multiple mechanisms of resistance against HER2-directed therapies, notably those regulated by effectors downstream of the HER2 receptor (10). Patients with HR+, HER2+ tumors that progress on an anti-HER2 therapy, in combination with a cytotoxic or endocrine therapy (ET), should be offered additional anti-HER2 agents to achieve ongoing suppression of HER2 pathway signaling (11). In this setting, HER2-directed therapy combined with cytotoxic chemotherapy agents offer modest clinical benefit without increased toxicities (12, 13).
Abemaciclib is an oral, selective cyclin-dependent kinase 4 and 6 (CDK4 and 6) inhibitor, approved for HR+, HER2-negative early breast cancer in combination with ET and advanced breast cancer (ABC) as monotherapy for endocrine-refractory disease (United States; ref. 14) and in combination with ET for initial treatment and after progression on ET (15, 16). Abemaciclib is also active in HER2+ disease. In a phase I study of abemaciclib, 4 of 11 patients with HR+, HER2+ ABC (3 of whom received ET) achieved a partial response [PR, 36%; 95% confidence interval (CI), 10.9–69.2; ref. 17]. Median progression-free survival (PFS) for this subpopulation was 7.2 months (95% CI, 2.8–12.0). These results provided clinical rationale to investigate abemaciclib in HER2+ ABC. Preclinical studies also suggested a biological rationale supporting the study of abemaciclib in HER2+ ABC. For example, studies demonstrated the CDK4 and 6 pathway can mediate resistance to HER2-directed therapies, and that this can be overcome by abemaciclib (18, 19). In addition, it was demonstrated that adding ET to abemaciclib further enhances the efficacy of abemaciclib plus trastuzumab in models of HR+, HER2+ breast cancer (19).
In the monarcHER trial, with a median follow-up of 19.0 months, abemaciclib, trastuzumab, and fulvestrant improved PFS in patients with HR+, HER2+ ABC versus SOC chemotherapy plus trastuzumab, while also showing a tolerable safety profile (20). Here, we present overall survival (OS) results from the monarcHER trial comparing the efficacy of abemaciclib plus trastuzumab with or without fulvestrant versus SOC chemotherapy plus trastuzumab in women with HR+, HER2+ ABC, with a median follow-up of 52.9 months. This final analysis also reports updated PFS and safety outcomes, as well as exploratory biomarker analyses.
Patients and Methods
Study design and patients
The monarcHER trial is a phase II, randomized, 3-group, open-label trial across 14 countries, including 75 hospitals, clinics, and medical centers. Female patients ≥ 18 years of age with a confirmed diagnosis of HR+, HER2+ breast cancer and unresectable, locally advanced, recurrent or metastatic disease were eligible. Detailed inclusion and exclusion criteria have been previously published (20). Briefly, patients must have received ≥ 2 prior HER2-directed therapies for ABC. Prior trastuzumab emtansine and a taxane in any setting were required, and prior pertuzumab was permitted. Women were required to have postmenopausal status (pre- or perimenopausal patients received a gonadotropin-releasing hormone agonist initiated at least 28 days before Day 1, Cycle 1). All patients were CDK4 and 6 inhibitor- and fulvestrant-naïve and had no untreated or symptomatic central nervous system metastases.
This study was conducted in accordance with consensus ethics principles derived from the international ethics guidelines, the Declaration of Helsinki, and the International Conference on Harmonization of Good Clinical Practice guidelines. This study was approved by ethical and institutional review boards. All patients provided informed, written consent.
Random assignment and treatment
Patients were randomly assigned 1:1:1 among 3 groups; randomization was stratified by the number of previous systemic regimens excluding single-agent ET (2–3 vs. >3) and disease status (measurable vs. nonmeasurable). Arm A patients received abemaciclib, trastuzumab, and fulvestrant. Arm B patients received abemaciclib plus trastuzumab. Arm C patients received trastuzumab plus SOC single-agent chemotherapy (physician's choice). Abemaciclib was administered orally at 150 mg twice daily. Trastuzumab was administered intravenously at 8 mg/kg on Day 1 of Cycle 1, then maintained at 6 mg/kg on Day 1 of each subsequent 21-day cycle, per SOC at time of study design. Fulvestrant was intramuscularly administered at 500 mg on Days 1 and 15 of Cycle 1, and on Day 29 (Day 8 of Cycle 2 if no dose suspension for trastuzumab occurred), then once every 4 weeks, per fulvestrant label. SOC single-agent chemotherapy was selected from a set of approved breast cancer chemotherapies and administered per product label. Patients received study treatment in the assigned treatment arm until disease progression per RECIST version 1.1 or unacceptable toxicity.
Central hematology, chemistry, and cystatin C were completed before Day 1 of each cycle. Adverse events (AE) were monitored at each patient visit and graded according to the NCI Common Terminology Criteria version 4.03.
Endpoints
Investigator-assessed PFS, the primary study endpoint, was measured from randomization to the date of objective cancer progression, per RECIST 1.1, or death from any cause. Secondary endpoints included OS, overall response, duration of response, the proportion of patients achieving disease control, clinical benefit rate (CBR), and safety. OS was measured from randomization to the date of death from any cause. Patients alive at the analysis cut-off date were censored at the date of last contact. Overall response was a summary measure of best overall response (BOR) and defined by RECIST 1.1. BOR was derived from timepoint responses observed while on study treatment and during short-term follow-up, but prior to post-discontinuation therapy initiation, except for patients who received surgery, radiotherapy, or both for ABC. Duration of response was measured from the date of first evidence of complete response (CR) or PR to the date of objective progression or death due to any cause, whichever occurred first. CBR was defined as CR plus PR plus stable disease ≥6 months.
Biomarker analyses
Extracted RNA from formalin fixed, paraffin embedded tumor samples was sequenced utilizing Illumina's Truseq RNA sequencing (RNA-seq; San Diego, CA) as described previously (21). To facilitate the subtype classification (Basal-like, Luminal A, Luminal B, HER2-enriched, and normal-like), a published 2-step normalization process was applied using data from The Cancer Genome Atlas and Molecular Taxonomy of Breast Cancer International Consortium (22–24). Intrinsic subtyping was then performed using the published PAM50 classifier and Genefu (“pam50” and “ssp2006”), and voting made final predictions based on the most frequent one (25, 26). To identify candidate genomic features associated with response, we compared patients with clinical benefit (responders) to those without clinical benefit (nonresponders), across the abemaciclib-containing arms (Arms A and B). Responders were defined as having CR or PR by RECIST V.1.1; nonresponders were defined as having progressive disease (PD). Continuous molecular variables were compared between responder versus nonresponder groups using the non-parametric Mann-Whitney Wilcoxon test. The Cancer Hallmark gene set enrichment analysis (GSEA) was performed using the R package “fgsea” (27, 28).
Statistical analysis
The study was designed to test the superiority of abemaciclib, trastuzumab, and fulvestrant (Arm A) or abemaciclib and trastuzumab (Arm B) to SOC chemotherapy and trastuzumab (Arm C) in improving PFS in the intention-to-treat (ITT) population, using the log-rank test stratified by the randomization strata. OS, the key secondary endpoint, was tested inferentially for significance only if PFS was significantly improved in the abemaciclib arms (A and B), to control the overall type I error in the trial at the two-sided significance level of 0.20. Given that PFS was only improved in arm A versus C, the OS was not formally tested for statistical significance; PFS and OS were estimated using the Kaplan–Meier method. Final OS analysis was planned after approximately 158 deaths occurred in the ITT population. The Kaplan–Meier method was used to estimate the OS and PFS curves. HRs and 95% CIs were estimated by means of the Cox proportional hazards regression model stratified by randomization strata. The effects of prognostic variables (stratification factors, intrinsic, and extrinsic factors) on treatment response were established by means of an unstratified Cox proportional hazards regression model in a prespecified subgroup analysis. Efficacy analyses and a description of patient and disease characteristics were based on the ITT population. The safety analysis population included all enrolled patients receiving at least 1 dose of any study drug. SAS version 9.4 was used for all statistical analyses. This trial is registered at ClinicalTrials.gov, NCT02675231.
Data availability
Eli Lilly and Company provides access, after anonymization, to all individual participant data collected during the trial, except for pharmacokinetic and genetic data. Data can be requested 6 months after the indication studied has been approved in the United States and EU or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see instructions at https://vivli.org.
Results
The study enrolled 237 patients (1:1:1), with 79 patients in each arm and the 227 patients who received at least 1 dose of study treatment were included in the safety population. At the time of data cutoff (March 31, 2022), 157 deaths had occurred across treatment arms: 50 (63.3%) in Arm A, 54 (68.4%) in Arm B, and 53 (67.1%) in Arm C. Median follow-up was 52.9 months.
Efficacy
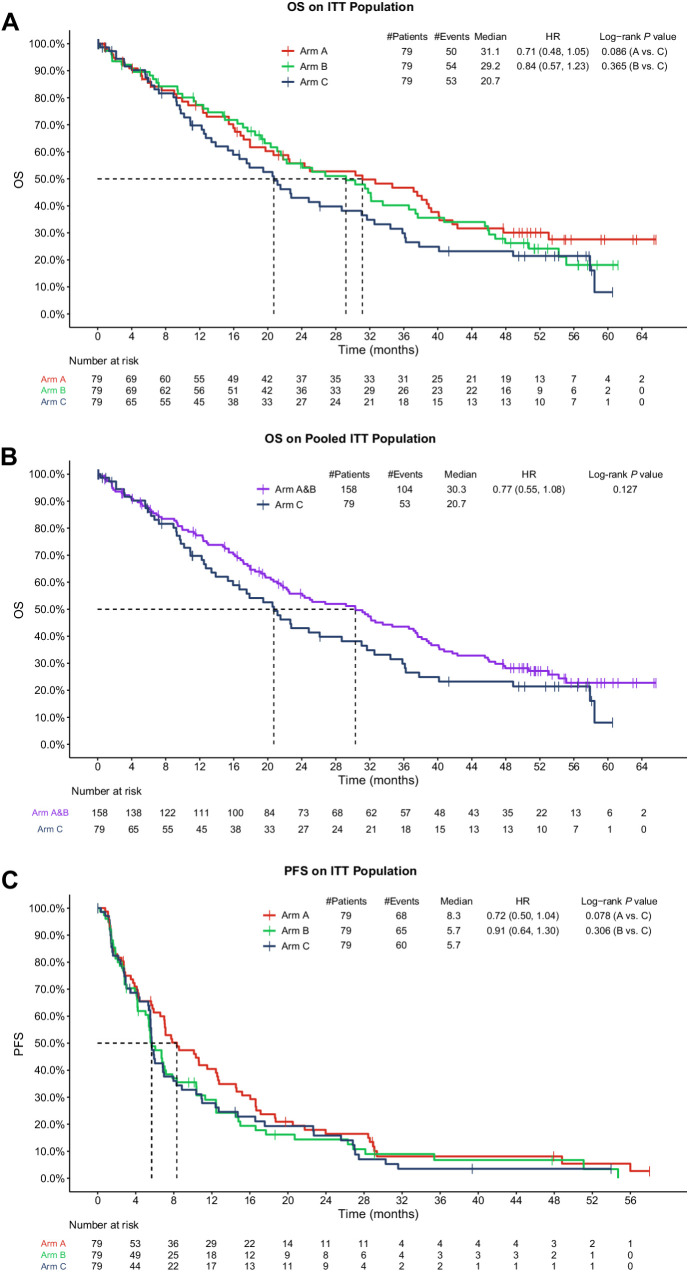
OS was estimated in all three arms (Fig. 1A). Median OS was 31.1 months in Arm A (HR, 0.71; 95% CI, 0.48–1.05; two-sided nominal P value 0.086 Arm A versus Arm C) and 29.2 months in Arm B, versus 20.7 months in Arm C (HR, 0.84; 95% CI, 0.57–1.23; two-sided nominal P value 0.365). The arms in which abemaciclib was administered (Arms A and B) showed numerical OS improvement of 10.4 months and 8.5 months, respectively, over SOC single-agent chemotherapy (Arm C). Similar OS results were observed when OS was estimated in pooled abemaciclib Arms (A + B) compared with SOC in Arm C (Fig. 1B). Median OS was 30.3 months in Arms A+B versus 20.7 months in Arm C (HR, 0.77; 95% CI, 0.55–1.08; two-sided nominal P value 0.127), giving an OS improvement of approximately 10 months.
Figure 1.
OS and PFS in monarcHER for the ITT population. A, Shows all study arms separately. B, Shows the pooled abemaciclib arms (Arms A + B) versus Arm C. C, Shows the updated PFS in all study arms separately. HR, hazard ratio.
Updated investigator-assessed PFS data in an ITT analysis indicated the hazard of progression in Arm A was reduced by approximately 28.0% compared with Arm C (8.3 months vs. 5.7 months; HR, 0.72; 95% CI, 0.50–1.04; two-sided nominal P value 0.077). The updated median PFS in both Arms B and C was 5.7 months (Fig. 1C).
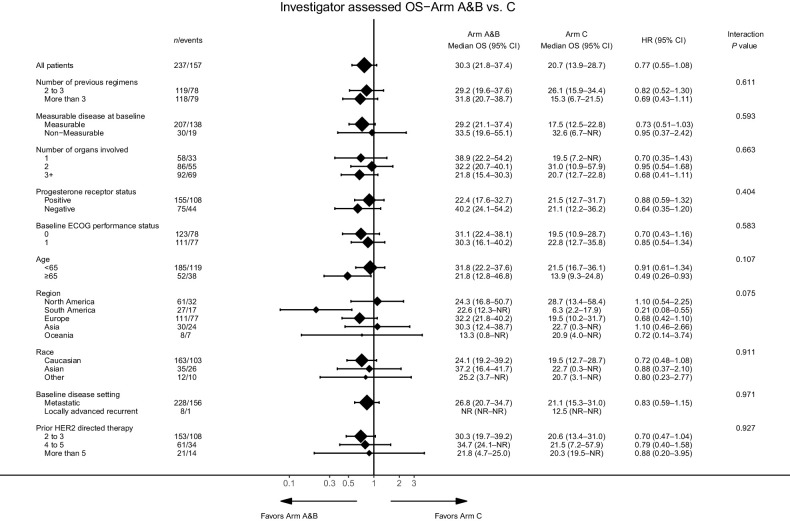
Additional subgroup analyses of Arms A + B versus Arm C were generally consistent with overall results (Fig. 2).
Figure 2.
OS by subgroups of interest. Subgroups include number of previous regimens, measurable disease at baseline, number of organs involved, progesterone receptor status, baseline ECOG performance status, age, region, race, baseline disease setting, and prior HER2-directed therapy. ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
In the analysis of overall response in the ITT population, patients in Arm A had a PR rate of 34.2%, patients in Arm B had a PR rate of 13.9%, and patients in Arm C had a PR rate of 12.7% (Supplementary Table S1). One patient in both Arms A and C (1.3%) achieved a CR. The CBR was 58.2%, 45.6%, and 38.0%, and the duration of response was 9.9, 7.6, and 4.2 months in Arms A, B and C, respectively. Finally, the ORR, defined as CR plus PR, was 35.4% (95% CI, 24.9–46.0) in Arm A, and 13.9% (95% CI, 6.3–21.6) in both Arms B and C (Supplementary Table S1).
Safety
Most patients in all arms had at least 1 treatment-emergent adverse event (TEAE; Supplementary Table S2), and 98.7%, 98.7%, and 94.4% of patients in Arms A, B, and C, respectively, had at least 1 TEAE, regardless of causality. In Arm A, 59 (75.6%) patients had ≥ 1 Grade ≥ 3 TEAE, regardless of causality, compared with 43 (55.8%) in Arm B, and 39 (54.2%) in Arm C. The most frequently reported TEAEs, regardless of arm and grade, included diarrhea (62.1%), fatigue (50.7%), nausea (41.9%), neutrophil count decrease (40.5%), and anemia (30.0%; Table 1). Twenty-four (30.8%) patients in Arm A had at least 1 serious adverse event (SAE), regardless of causality, compared with 15 (19.5%) in Arm B, and 14 (19.4%) in Arm C. Four patients (5.1%) in Arm A discontinued study treatment due to an SAE, compared with 2 patients in Arm B (2.6%) and 3 patients in Arm C (4.2%).
Table 1.
Updated TEAEs.
| Abemaciclib + Trastuzumab + Fulvestrant (N = 78) | Abemaciclib + Trastuzumab (N = 77) | Trastzumab + Chemotherapy (N = 72) | Total (N = 227) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3/4/5 | Any grade | Grade 3/4/5 | Any grade | Grade 3/4/5 | Any grade | Grade 3/4/5 | |||||||||
| MedDRA preferred term | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % |
| Subjects with ≥ 1 TEAE | 77 | 98.7 | 59 | 75.6 | 76 | 98.7 | 43 | 55.8 | 68 | 94.4 | 39 | 54.2 | 221 | 97.4 | 141 | 62.1 |
| Diarrhea | 63 | 80.8 | 7 | 9.0 | 60 | 77.9 | 6 | 7.8 | 18 | 25.0 | 2 | 2.8 | 141 | 62.1 | 15 | 6.6 |
| Fatigue | 43 | 55.1 | 3 | 3.8 | 40 | 51.9 | 5 | 6.5 | 32 | 44.4 | 1 | 1.4 | 115 | 50.7 | 9 | 4.0 |
| Nausea | 37 | 47.4 | 3 | 3.8 | 32 | 41.6 | 2 | 2.6 | 26 | 36.1 | 0 | 0.0 | 95 | 41.9 | 5 | 2.2 |
| Neutrophil count decreased | 38 | 48.7 | 21 | 26.9 | 27 | 35.1 | 18 | 23.4 | 27 | 37.5 | 17 | 23.6 | 92 | 40.5 | 56 | 24.7 |
| Anemia | 30 | 38.5 | 8 | 10.3 | 21 | 27.3 | 3 | 3.9 | 17 | 23.6 | 4 | 5.6 | 68 | 30.0 | 15 | 6.6 |
| Abdominal pain | 23 | 29.5 | 1 | 1.3 | 18 | 23.4 | 0 | 0.0 | 14 | 19.4 | 1 | 1.4 | 55 | 24.2 | 2 | 0.9 |
| Vomiting | 21 | 26.9 | 1 | 1.3 | 22 | 28.6 | 2 | 2.6 | 11 | 15.3 | 1 | 1.4 | 54 | 23.8 | 4 | 1.8 |
| Platelet count decreased | 22 | 28.2 | 8 | 10.3 | 23 | 29.9 | 5 | 6.5 | 5 | 6.9 | 2 | 2.8 | 50 | 22.0 | 15 | 6.6 |
| Decreased appetite | 18 | 23.1 | 0 | 0.0 | 17 | 22.1 | 0 | 0.0 | 13 | 18.1 | 1 | 1.4 | 48 | 21.1 | 1 | 0.4 |
| Cough | 21 | 26.9 | 0 | 0.0 | 12 | 15.6 | 0 | 0.0 | 9 | 12.5 | 0 | 0.0 | 42 | 18.5 | 0 | 0.0 |
| Headache | 14 | 17.9 | 1 | 1.3 | 12 | 15.6 | 0 | 0.0 | 15 | 20.8 | 2 | 2.8 | 41 | 18.1 | 3 | 13.3 |
| White blood cell count decreased | 18 | 23.1 | 8 | 10.3 | 9 | 11.7 | 3 | 3.9 | 10 | 13.9 | 7 | 9.7 | 37 | 16.3 | 18 | 7.9 |
| Dyspnea | 14 | 17.9 | 4 | 5.1 | 8 | 10.4 | 1 | 1.3 | 13 | 18.1 | 2 | 2.8 | 35 | 15.4 | 7 | 3.1 |
| Constipation | 10 | 12.8 | 1 | 1.3 | 8 | 10.4 | 0 | 0.0 | 16 | 22.2 | 0 | 0.0 | 34 | 15.0 | 1 | 0.4 |
| Stomatitis | 9 | 11.5 | 2 | 2.6 | 10 | 13.0 | 0 | 0.0 | 15 | 20.8 | 1 | 1.4 | 34 | 15.0 | 3 | 1.3 |
| Pyrexia | 15 | 19.2 | 2 | 2.6 | 6 | 7.8 | 0 | 0.0 | 11 | 15.3 | 2 | 2.8 | 32 | 14.1 | 4 | 1.8 |
| Upper respiratory tract infection | 16 | 20.5 | 1 | 1.3 | 5 | 6.5 | 0 | 0.0 | 8 | 11.1 | 0 | 0.0 | 39 | 12.8 | 1 | 0.4 |
| Myalgia | 8 | 10.3 | 0 | 0.0 | 8 | 10.4 | 0 | 0.0 | 10 | 13.9 | 0 | 0.0 | 26 | 11.5 | 0 | 0.0 |
| Pain | 9 | 11.5 | 0 | 0.0 | 8 | 10.4 | 1 | 1.3 | 8 | 11.1 | 0 | 0.0 | 25 | 11.0 | 1 | 0.4 |
| Pruritus | 12 | 15.4 | 0 | 0.0 | 10 | 13.0 | 0 | 0.0 | 3 | 4.2 | 0 | 0.0 | 25 | 11.0 | 0 | 0.0 |
Abbreviations: N, number of subjects in I3Y_MC_JPBZ; n, number of subjects in the specified category; MedDRA, Medical Dictionary for Regulatory Activities.
N = 78 (abemaciclib 150 mg + trastuzumab 8 mg/kg + fulvestrant 500 mg), N = 77 (abemaciclib 150 mg + trastuzumab 8 mg/kg), N = 72 (trastuzumab 8 mg/kg + chemotherapy), N = 227 (total).
Thirty-nine patients (50.0%) in Arm A had at least 1 dose reduction of abemaciclib, compared with 32 (41.6%) in Arm B (Supplementary Table S3). Reasons leading to dose reduction included AEs for 38 (48.7%) patients in Arm A, and 31 (40.3%) patients in Arm B; protocol was cause for dose reduction in 1 (1.3%) patient in both Arms A and B. The AEs leading to dose reduction included diarrhea (10 patients in Arm A; 12 patients in Arm B) and neutropenia (8 patients in Arm A; 9 patients in Arm B) in both arms, anemia (4 patients) in Arm A, and leukopenia (3 patients) in Arm B. Three (3.8%) deaths due to AE while on study treatment, regardless of causality, were reported in Arm A, and 1 each was reported in Arms B (1.3%, respiratory failure) and C (1.4%, febrile neutropenia).
Exploratory RNA-seq analysis
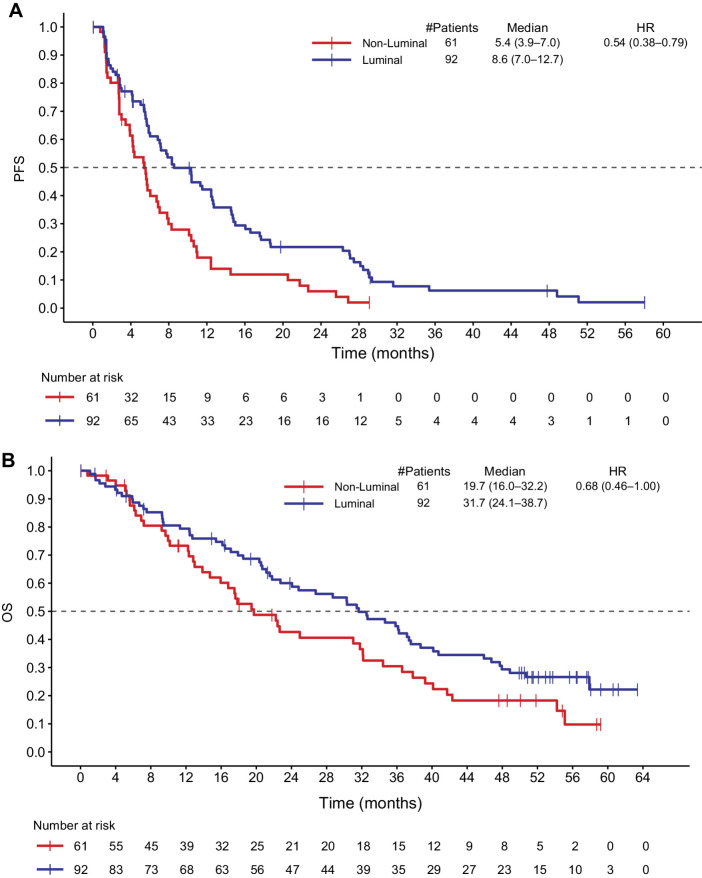
RNA-seq data were available for 153 patients within the ITT population, including 51 patients in Arm A, 56 patients in Arm B, and 46 patients in Arm C. A Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) diagram is available in Supplementary Fig. S1. The RNA-seq data were then used to determine intrinsic subtypes using the Parker PAM50 classifier and Genefu. The final consensus subtype was assigned on the basis of the subtype having the most frequent prediction across the methods. A high concordance was observed between the predictions from the Parker PAM50 classifier and Genefu, with higher concordance for Basal, HER2-enriched, and Luminal B subtypes compared with Luminal A and normal-like subtypes. The predicted intrinsic subtypes were analyzed to determine if there was an association between subtype and clinical efficacy. Of 153 samples, 8% were of the normal-like subtype, 8% were Basal, 24% were HER2-enriched, 34% were Luminal A, and 26% were Luminal B. Luminal subtypes were associated with longer PFS and OS compared with non-Luminal. The median PFS in Luminal subtypes was 8.6 months versus 5.4 months in non-Luminal subtypes (HR, 0.54; 95% CI, 0.38–0.79); median OS was 31.7 months in Luminal subtypes versus 19.7 months in non-Luminal subtypes (HR, 0.68; 95% CI, 0.46–1.00; Figs. 3A and B). Supplementary Figure S2 shows the PFS and OS for each intrinsic subtype in Arms A and B or Arms A, B, and C combined; Supplementary Fig. S3 shows the PFS and OS for each intrinsic subtype in Arm C alone.
Figure 3.
PFS and OS in Luminal versus non-Luminal disease. A, Shows the PFS in Luminal and non-Luminal breast cancer subtypes. B, Shows the OS in Luminal and non-Luminal breast cancer subtypes. HR, hazard ratio.
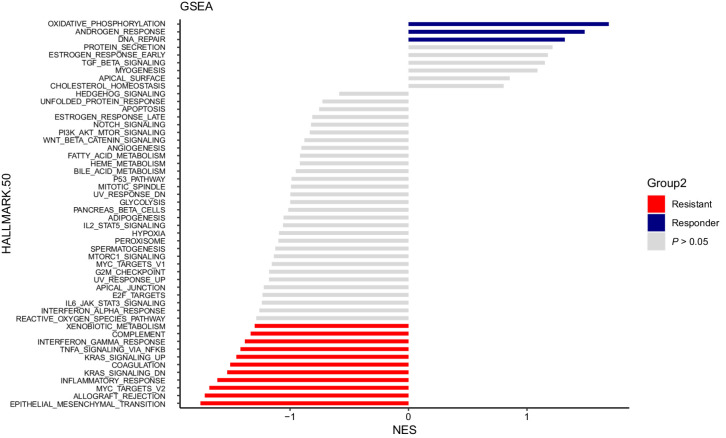
GSEA was also performed, including an assessment of 50 hallmark pathways in responders (defined as patients with CR or PR as BOR versus nonresponders (defined as patients with PD as BOR) in the abemaciclib-containing arms (Arms A and B). Oxidative phosphorylation, androgen response, and DNA repair were highly enriched in tumors from the patients with response, while epithelial-to-mesenchymal transition (EMT) and multiple immune and inflammatory response gene sets were highly enriched in tumors from patients with resistance (Fig. 4).
Figure 4.
GSEA identifies DEG sets in responder tumors compared with nonresponder tumors in Arms A and B. GSEA results for NES versus the total list of GSEA hallmark upregulated (in blue lines) and downregulated (in red lines) categories, with significantly enriched terms (P < 0.05) among non-enriched (P ≥ 0.05, in grey lines). The GSEA analysis was performed using the “fgsea” package in R for the hallmark collection (H; Broad Institute), with n = 1,000 permutations. DN, downregulated; KRAS, Kirsten rat sarcoma virus; NES, normalization enrichment score; WNT, Wingless Int-1.
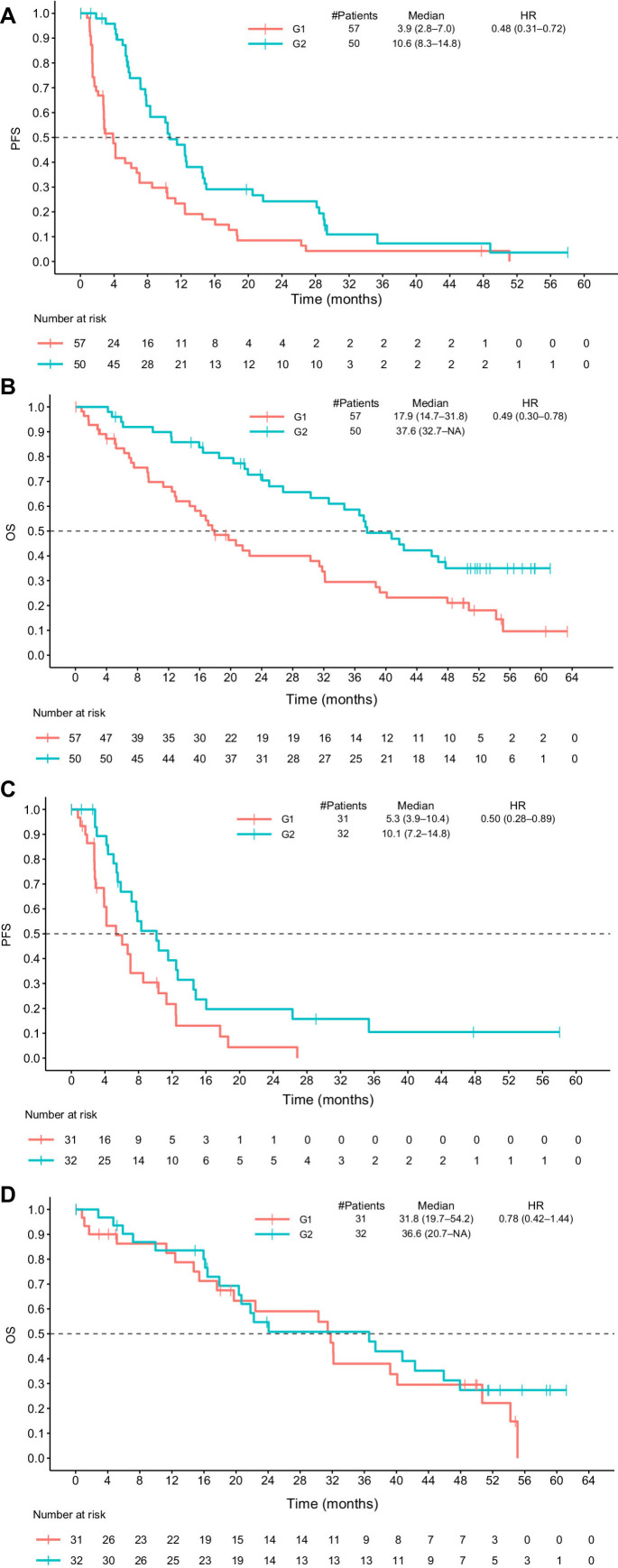
Differential gene expression was assessed between responders and nonresponders in the abemaciclib-containing arms (Arms A and B), resulting in the identification of 216 differentially expressed genes (DEG). Upregulation of 104 genes was seen in responsive tumors, while 112 genes were upregulated in the progressive tumors. Pathway overrepresentation analysis revealed differentially upregulated genes in sensitive tumors were mainly involved in Hallmark Androgen Response. Upregulated genes in resistant tumors were not significantly enriched in any of the hallmark gene sets, according to our criteria (FDR < 0.1; Supplementary Table S4). This gene set was then applied to assign Arm A and Arm B tumor samples to 1 of 2 groups, G1 or G2, using the single sample prediction method, with the nearest centroid generated from monarcHER. The G1 group had a median PFS of 3.9 months (95% CI, 2.8–7.0) compared with 10.6 months for the G2 group (95% CI, 8.3–14.8; HR, 0.48; 95% CI, 0.31–0.72; Fig. 5A) and a median OS for the G1 group of 17.9 months (95% CI, 14.7–31.8) compared with 37.6 months for the G2 group (95% CI, 32.7–NA; HR, 0.49; 0.30–0.78; Fig. 5B).
Figure 5.
PFS and OS for Arm A and Arm B after differential gene expression analysis of tumor samples. A and B, Show PFS (A) and OS (B) in G1 and G2 groups within responders and nonresponders in Arms A and B. C and D, Show PFS (C) and OS (D) in G1 and G2 groups in the stable disease and not-evaluable subgroups in Arms A and B. HR, hazard ratio.
The above analysis was repeated in tumor samples from patients with a BOR of stable disease (SD) and those for whom response was not evaluable, resulting in a median PFS of 5.3 months for the G1 group (95% CI, 3.9–10.4) compared with 10.1 months for the G2 group (95% CI, 7.2–14.8; HR, 0.50; 0.28–0.89; Fig. 5C). The G1 group also had a median OS of 31.8 months (95% CI, 19.7–54.2) compared with 36.6 months in the G2 group (95% CI, 20.7–NA; HR, 0.78; 0.42–1.44; Fig. 5D). When repeating the above analysis for Arm C, there was no significant difference in median PFS or OS between the G1 and G2 groups, indicating that the gene set had no prognostic utility in Arm C (Supplementary Fig. S4). Finally, a table describing the representativeness of study participants is available in Supplementary Table S5.
Discussion
In a previous monarcHER report, abemaciclib, a CDK4 and 6 inhibitor, in combination with trastuzumab and fulvestrant, was shown to improve PFS for patients with HR+, HER2+ ABC, compared with patients who received SOC chemotherapy and trastuzumab (20). In this pre-specified final analysis of monarcHER, abemaciclib treatment numerically improved median OS in both abemaciclib-containing arms: Arm A (abemaciclib + trastuzumab + fulvestrant) and Arm B (abemaciclib + trastuzumab), versus Arm C (SOC chemotherapy + trastuzumab). The updated PFS results in this current analysis, with an additional median 34.0 months of follow-up, showed a consistent relationship, demonstrating improved PFS in Arm A compared with Arm C. The updated ORR was improved in Arm A versus C, similar to the previous report (20). The duration of response was higher in Arms A and B compared with Arm C. The disconnect between improved PFS in the abemaciclib, fulvestrant, and trastuzumab triplet and increased OS seen in both the triplet and the abemaciclib + trastuzumab doublet could potentially be due to immune-mediated effects of abemaciclib as a CDK4 and 6 inhibitor, leading to a carryover benefit not seen while patients are on therapy (29). Safety findings in this analysis were also consistent with previously reported results (20).
Breast cancer can be classified into 5 major intrinsic subtypes: Luminal A or B, HER2 over-expression, Basal, or normal-like (25). Recently, palbociclib in combination with trastuzumab with or without ET was assessed in patients with HER2+ breast cancer, and a longer median PFS was reported among patients with Luminal disease compared with patients with non-Luminal disease (median PFS 10.6 vs. 4.2 months, adjusted HR 0.40, P = 0.003; ref. 30). Another study analyzed the genomic and molecular features of triple-positive breast cancers (TPBC) treated with trastuzumab, revealing patients with Luminal A–like TPBC had a better prognosis compared with other TPBC subtypes (multivariate survival analysis HR, 0.33; 95% CI, 0.11–0.97), and a reduced benefit when compared with patients with Luminal A–like TPBC not treated with trastuzumab (31). The results from monarcHER are consistent with these reports, as intrinsic subtype analysis demonstrated Luminal breast cancer subtypes were associated with longer PFS and OS, compared with non-Luminal subtypes, indicating a possible prognostic role for intrinsic subtypes. Exploratory GSEA in this study also identified that genes associated with oxidative phosphorylation, androgen response, and DNA repair pathways were associated with response in the abemaciclib arms, while genes associated with EMT and immune and inflammatory responses were associated with resistance. Analysis of differential gene expression further identified a set of genes differentially expressed between responders and nonresponders, which also was enriched for genes in the androgen response hallmark.
The results of this study should be considered within the context of potential limitations. Study design did not allow assessment of the isolated treatment effect of fulvestrant, which would have required a fourth treatment group examining the combined treatment of fulvestrant and trastuzumab. Although the administration of abemaciclib numerically improved OS, it had a greater impact on response rate when given in combination with fulvestrant and trastuzumab than when it was given in combination with trastuzumab alone. Of note, the response rate in Arm A is almost double the response rates in both Arms B and C. Given the fulvestrant plus trastuzumab doublet was not a SOC therapy when this trial was designed, such a treatment arm was not included. Recently, a study investigating fulvestrant and trastuzumab combination therapy found fulvestrant and trastuzumab combined led to a median PFS of 6.4 months (95% CI, 3.5–8.2), median OS of 35.3 months (95% CI, 20.0–46.7), and a disease control rate of 64% (32). These results are similar to those seen in Arm A of the updated monarcHER study: abemaciclib, trastuzumab, and fulvestrant combination therapy led to a median PFS of 8.3 months, and a median OS of 31.1 months (Fig. 1A amd C). An additional study design limitation was monarcHER was designed with an experimental two-sided alpha of 0.2, rather than the more rigorous alpha of 0.05 often used in phase III clinical trials. Another limitation of the study could be the samples for biomarker analyses were not available from all patients. Moreover, the inclusion criterion did not mandate previous pertuzumab treatment for patients before enrollment into the monarcHER study. When the study was enrolling between May 31, 2016 and February 28, 2018, pertuzumab was not widely available to patients outside of the United States. As a result, 119 (50%) patients had previous exposure to pertuzumab, reflecting the global availability of pertuzumab at study enrollment time. In addition, other novel strategies to treat metastatic HER2+ breast cancer have emerged since this trial was enrolled, including agents like trastuzumab deruxtecan and tucatinib (9, 33, 34). While patients in this trial had not received these prior therapies, this study demonstrates that in heavily pretreated patients, a non-chemotherapy–based strategy can lead to improvements in PFS and a clinically meaningful difference in OS compared with standard chemotherapy and trastuzumab.
Conclusions
In the updated monarcHER study results, abemaciclib + trastuzumab ± fulvestrant numerically improved OS in patients with HR+, HER2+ ABC compared with SOC chemotherapy plus trastuzumab. In exploratory analysis of intrinsic subtypes, Luminal subtypes were associated with longer PFS and OS compared with non-Luminal subtypes. Exploratory gene analysis identified pathways enriched in patients deemed as responders and demonstrated potential prognostic utility for patients in the abemaciclib arms (Arm A and Arm B).
Supplementary Material
REMARK (Reporting Recommendations for Tumor Marker Prognostic Studies) diagram for patients in the monarcHER trial.
Progression-free survival (PFS) and overall survival (OS) for intrinsic subtypes.
Progression-free survival (PFS) and overall survival (OS) for intrinsic subtypes in Arm C.
S4A, B: : Progression-free survival and overall survival for Arm C after differential gene expression analysis of tumor samples.
Updated objective response rate for the ITT population.
Updated adverse event overview.
Summary of abemaciclib dose adjustments in the safety population of the monarcHER trial.
ORA for 216 differentially expressed genes.
Representativeness of Study Participants.
Acknowledgments
This work was funded by Eli Lilly and Company. Fulvestrant was provided by AstraZeneca. Eli Lilly and Company contracted with Syneos Health for medical writing assistance from Ndidi Uzor, PhD, and editorial assistance from Dana Schamberger, MA. The authors and Eli Lilly and Company would like to thank all patients and their families for participating in this trial, as well as the study sites, study investigators and their staff, the Assessment Committee members, and the clinical trial team, without whom monarcHER would not have been possible.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
S.M. Tolaney reports grants, personal fees, and nonfinancial support from Eli Lilly and Company during the conduct of the study. S.M. Tolany also reports grants and personal fees from Genentech/Roche, Merck, Pfizer, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, Gilead, and Seattle Genetics; grants from Exelixis, NanoString Technologies, and OncoPep; and personal fees from Sanofi, CytomX Therapeutics, Daiichi Sankyo, Ellipses Pharma, 4D Pharma, OncoSec Medical Inc., BeyondSpring Pharmaceuticals, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics, Infinity Therapeutics, Myovant, Zetagen, Umoja Biopharma, Artios Pharma, Menarini/Stemline, Aadi Biopharma, Bayer, Incyte Corp, and Jazz Pharmaceuticals outside the submitted work. S. Goel reports grants from G1 Therapeutics, Incyclix Bio, and Lilly, as well as personal fees from Pfizer, Novartis, and Lilly outside the submitted work. J. Nadal reports grants and nonfinancial support from Eli Lilly during the conduct of the study, as well as personal fees from Eli Lilly outside the submitted work. H. Denys reports nonfinancial support and other support from AstraZeneca, MSD, GSK, Teva, Seagen, PharmaMar, Roche, Eli Lilly, and Novartis, as well as grants, nonfinancial support, and other support from Gilead outside the submitted work. L.M. Litchfield reports personal fees from Eli Lilly and Company during the conduct of the study; personal fees from Eli Lilly and Company outside the submitted work; and ownership of Eli Lilly and Company stock. J. Liu reports personal fees from Eli Lilly and Company during the conduct of the study, as well as personal fees from Eli Lilly and Company (employment) outside the submitted work. Y. Chen reports employment at Eli Lilly and Company as well as ownership of Eli Lilly and Company stock. F. André reports grants from AstraZeneca, Guardant Health, Novartis, Owkin, Pfizer, Lilly, Roche, and Daiichi Sankyo, as well as personal fees from Lilly outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
S.M. Tolaney: Conceptualization, investigation, methodology, writing–review and editing. S. Goel: Conceptualization, methodology, writing–review and editing. J. Nadal: Writing–review and editing. H. Denys: Formal analysis, writing–review and editing. M.R. Borrego: Conceptualization, writing–review and editing. L.M. Litchfield: Conceptualization, formal analysis, methodology, writing–review and editing. J. Liu: Conceptualization, formal analysis, methodology, writing–review and editing. A.K. Appiah: Methodology, writing–review and editing. Y. Chen: Conceptualization, formal analysis, writing–review and editing. F. André: Conceptualization, writing–review and editing.
References
- 1. Islami F, Ward EM, Sung H, Cronin KA, Tangka FKL, Sherman RL, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. J Natl Cancer Inst 2021;113:1648–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Winters S, Martin C, Murphy D, Shokar NK. Breast cancer epidemiology, prevention, and screening. Prog Mol Biol Transl Sci 2017;151:1–32. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. [DOI] [PubMed] [Google Scholar]
- 4. Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, et al. Breast cancer statistics, 2022. CA Cancer J Clin 2022;72:524–41. [DOI] [PubMed] [Google Scholar]
- 5. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- 6. Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 2005;23:4265–74. [DOI] [PubMed] [Google Scholar]
- 7. Kümler I, Tuxen MK, Nielsen DL. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat Rev 2014;40:259–70. [DOI] [PubMed] [Google Scholar]
- 8. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med 2022;386:1143–54. [DOI] [PubMed] [Google Scholar]
- 10. Thery JC, Spano JP, Azria D, Raymond E, Penault Llorca F. Resistance to human epidermal growth factor receptor type 2—targeted therapies. Eur J Cancer 2014;50:892–901. [DOI] [PubMed] [Google Scholar]
- 11. Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733–43. [DOI] [PubMed] [Google Scholar]
- 13. Krop IE, Kim SB, Martin AG, LoRusso PM, Ferrero JM, Badovinac-Crnjevic T, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomized open-label phase III trial. Lancet Oncol 2017;18:743–54. [DOI] [PubMed] [Google Scholar]
- 14. Dickler MN, Tolaney SM, Rugo HS, Cortes J, Dieras V, Patt D, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res 2017;23:5218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–46. [DOI] [PubMed] [Google Scholar]
- 16. Sledge GW Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol 2020;6:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–53. [DOI] [PubMed] [Google Scholar]
- 18. Goel S, Wang Q, Watt AC, Tolaney SM, Dillon DA, Li W, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 2016;29:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Brien N, Conklin D, Beckmann R, Luo T, Chau K, Thomas J, et al. Preclinical activity of abemaciclib alone or in combination with antimitotic and targeted therapies in breast cancer. Mol Cancer Ther 2018;17:897–907. [DOI] [PubMed] [Google Scholar]
- 20. Tolaney SM, Wardley AM, Zambelli S, Hilton JF, Troso-Sandoval TA, Ricci F, et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor–positive, HER2-positive advanced breast cancer (monarcHER): a randomized, open-label, phase II trial. Lancet Oncol 2020;21:763–75. [DOI] [PubMed] [Google Scholar]
- 21. Valle JW, Vogel A, Denlinger CS, He AR, Bai LY, Orlova R, et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: a randomized, double-blind, multicenter, phase II study. Lancet Oncol 2021;22:1468–82. [DOI] [PubMed] [Google Scholar]
- 22. Carey LA, Berry DA, Cirrincione CT, Barry WT, Pitcher BN, Harris LN, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol 2016;34:542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumors reveals novel subgroups. Nature 2012;486:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gendoo DM, Ratanasirigulchai N, Schroder MS, Pare L, Parker JS, Prat A, et al. Genefu: an R/Bioconductor package for computation of gene expression-based signatures in breast cancer. Bioinformatics 2016;32:1097–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011;27:1739–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Korotkevich G, Sukhov V, Budin N, Shpak B, Artyomov MN, Sergushichev A. Fast gene set enrichment analysis. bioRxiv 2021. Available from: https://www.biorxiv.org/content/10.1101/060012v3.
- 29. Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, et al. CDK4/6 inhibition triggers antitumor immunity. Nature 2017;548:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ciruelos E, Villagrasa P, Pascual T, Oliveira M, Pernas S, Pare L, et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clin Cancer Res 2020;26:5820–9. [DOI] [PubMed] [Google Scholar]
- 31. Zhao S, Liu XY, Jin X, Ma D, Xiao Y, Shao ZM, et al. Molecular portraits and trastuzumab responsiveness of estrogen receptor-positive, progesterone receptor-positive, and HER2-positive breast cancer. Theranostics 2019;9:4935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozaki Y, Aoyama Y, Masuda J, Inagaki L, Kawai S, Shibayama T, et al. Trastuzumab and fulvestrant combination therapy for women with advanced breast cancer positive for hormone receptor and human epidermal growth factor receptor 2: a retrospective single-center study. BMC Cancer 2022;22:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah M, Wedam S, Cheng J, Fiero MH, Xia H, Li F, et al. FDA approval summary: tucatinib for the treatment of patients with advanced or metastatic HER2-positive breast cancer. Clin Cancer Res 2021;27:1220–6. [DOI] [PubMed] [Google Scholar]
- 34. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 2020;382:597–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
REMARK (Reporting Recommendations for Tumor Marker Prognostic Studies) diagram for patients in the monarcHER trial.
Progression-free survival (PFS) and overall survival (OS) for intrinsic subtypes.
Progression-free survival (PFS) and overall survival (OS) for intrinsic subtypes in Arm C.
S4A, B: : Progression-free survival and overall survival for Arm C after differential gene expression analysis of tumor samples.
Updated objective response rate for the ITT population.
Updated adverse event overview.
Summary of abemaciclib dose adjustments in the safety population of the monarcHER trial.
ORA for 216 differentially expressed genes.
Representativeness of Study Participants.
Data Availability Statement
Eli Lilly and Company provides access, after anonymization, to all individual participant data collected during the trial, except for pharmacokinetic and genetic data. Data can be requested 6 months after the indication studied has been approved in the United States and EU or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data-sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data-sharing environment. For details on submitting a request, see instructions at https://vivli.org.