Abstract
Sporadic Creutzfeldt-Jakob disease (sCJD) is an uncommon prion disease, also a fatal degenerative brain disorder. We aimed to illustrate 2 clinical cases, a 60-year-old female and a 57-year-old male, who came to the hospital due to rapidly progressive cognitive decline. A 1.5T brain MRI in both patients detected cortical and basal ganglia signal abnormalities with diffuse, asymmetrical features. The patient underwent electroencephalography and cerebrospinal fluid tests, which showed abnormal waves and a positive 14-3-3 protein test in the CSF samples of both patients. According to the 2018 US Centers for Disease Control and Prevention (CDC) diagnostic criteria, we finally diagnosed these patients with sCJD.
Keywords: Sporadic Creutzfeldt-Jakob disease, Prion, Asymmetric cortical abnormality, Pulvinar sign, Double hockey sign
Introduction
Sporadic Creutzfeldt-Jakob disease (sCJD) is a very rare prion disease with an incidence of 0.5-1 person/1 million, causing rapidly progressive dementia and death within 1 year of symptom onset [1,2]. CJD is caused by a type of abnormal protein known as a prion or scrapie PrP. Infectious prions are misfolded proteins that can affect normally folded proteins to also become misfolded, then have the effect on signaling processes, damaging neurons and resulting in degeneration of neurons. Clinical symptoms of this disease are nonspecific and can be seen in many other diseases. Brain biopsy is considered the gold standard for diagnosis, but is rarely used due to its invasive nature. The trio of laboratory tests commonly used in diagnosis are electroencephalography (EEG), cerebrospinal fluid (CSF), and brain magnetic resonance imaging (MRI).
Brain MRI plays an important role in providing early information for sCJD diagnosis, helping clinicians orientate appropriate tests, and excluding the differential diagnosis. Two important points that need to focus on brain MRI of suspected patients are the location and signal characteristics of lesions. With sCJD, FLAIR, and DWI are the most valuable sequences, with the typical characteristics of lesions being hyperintense on FLAIR and restricted diffusion on DWI. In this article, we intend to depict 2 cases diagnosed with sCJD at Hanoi Medical University.
Case report
Case 1
A 60-year-old female, with no medical history, was admitted to Hanoi Medical University Hospital due to psychiatric disorders such as unreasonable irritability, anxiety, and fear for 3 weeks, with no paranoia, or hallucinations. After 1 week, she presented with a memory disorder, often forgetting about recent events, movement repetition, and limited communication. Symptoms of myoclonus in the right hand and bilateral eyelids appeared 1 week later.
EEG presented with slowing activity, and generalized periodic triphasic sharp wave complexes with average amplitude. The CSF test had very few cells, no protein, and a negative culture. Blood tests for inflammation, and liver- kidney function are within normal limits.
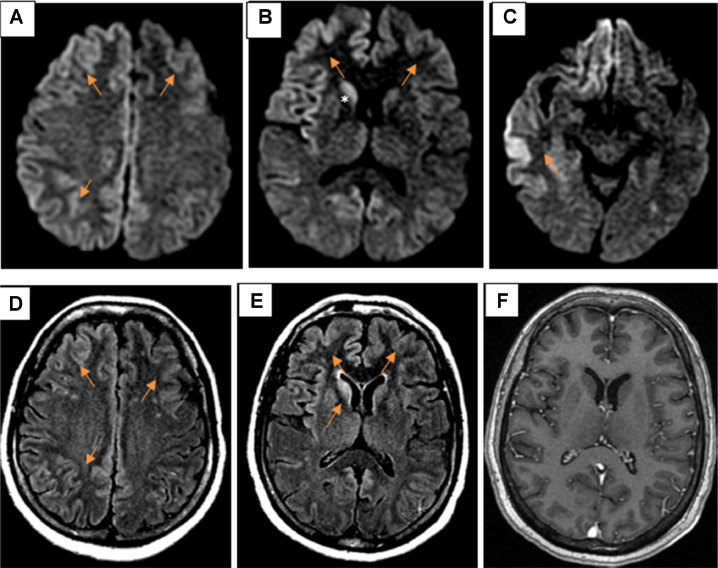
Brain MRI showed diffuse, asymmetric cortical abnormality in bilateral frontal-parietal-temporal lobes and cingulate gyrus, right insula, basal ganglia (right caudate and globus pallidus), and bilateral dorsomedial thalamus. These abnormalities were hyperintense on FLAIR, restricted diffusion on DWI/ADC, no enhancement after contrast injection, and no mass effect (Fig. 1).
Fig. 1.
Axial DWI (A–C) showed diffuse, asymmetric restricted diffusion in the bilateral cortex (arrows), and right caudate nucleus (*). Axial FLAIR (D and E) hyperintense on FLAIR (arrows). Axial enhanced T1W (F) no enhancement after contrast injection.
The result of brain MRI, EEG, and clinical symptoms suggested a case of sCJD. So a CSF 14-3-3 protein assay was performed and showed positive in this patient. Multiple tests to exclude the other causes such as infection, toxic or metabolic disease, and autoimmune encephalitis were also performed.
Following the diagnostic criteria of the United States of America's Center for Disease Control and Prevention (CDC) in 2018, this patient was diagnosed with probable CJD.
Case 2
A 57-year-old male, with no medical history, was admitted to Hanoi Medical University Hospital due to dizziness, confusion, poor memory, spatial and time orientation disorders, decreased cognitive ability, and myoclonus for 1 month.
EEG presented with triphasic wave complexes with an average amplitude, and frequency of 2 cycles/s, appearing to spread over 2 hemispheres. The CSF test has very few white blood cells, protein and glucose values within normal limits, and a negative microbial culture. Other tests were performed such as inflammatory bilan tests, liver- kidney function within normal limits, and negative paraneoplastic autoantibodies.
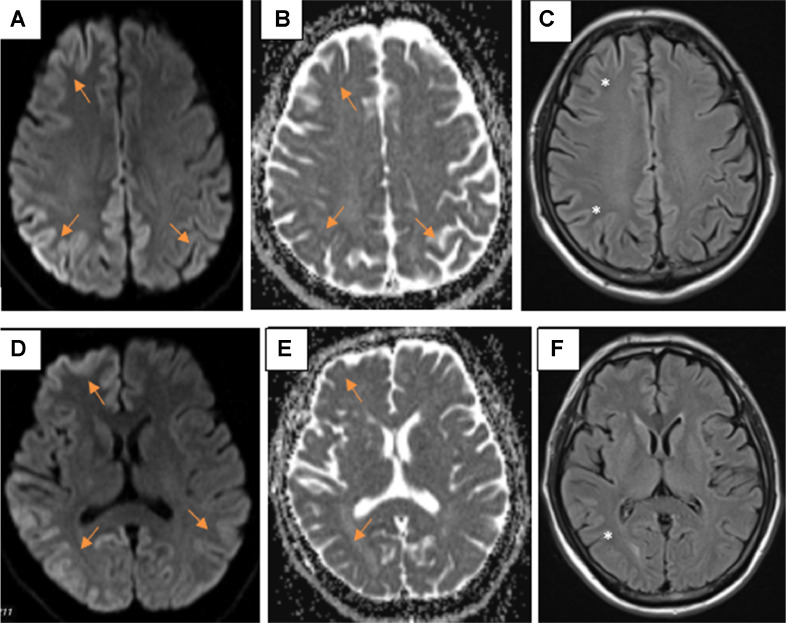
Brain MRI showed diffuse cortical abnormalities in the right frontal lobe, bilateral parietal–temporal-occipital lobes, cingulate gyrus, and right caudate. The lesion has asymmetrical characteristics, restricted diffusion on DWI, and slight hyperintense on FLAIR (Fig. 2).
Fig. 2.
Diffuse, asymmetrical cortical abnormalities in 2 hemispheres restricted diffusion on DWI/ADC (A, B, D, and E) (arrows), and slightly hyperintense on FLAIR (C and F) (*).
The result of brain MRI, EEG, and clinical symptoms suggested a case of sCJD. A CSF 14-3-3 protein assay was performed and showed positive. Similar to the first case, multiple tests to exclude the other causes such as infection, toxic or metabolic disease, and autoimmune encephalitis were also performed.
Following the diagnostic criteria of the CDC in 2018, this patient was diagnosed with probable CJD.
Discussion
sCJD is a rare prion disease, associated with fatal degenerative brain disorder. Research in Thailand revealed the median age of onset of 60 and mean survival of 4 months with CJD cases [3]. It is rarely seen in young people, less than 30 years old, and more common in middle age [4].
Three types of prions disease have been described: sporadic (sporadic CJD, sporadic fatal insomnia, and variably protease-sensitive prionopathy), inherited (inherited CJD, familial fatal insomnia, Gerstmann-Sträussler-Scheinker syndrome), acquired (acquired CJD, Kuru, variant CJD). sCJD accounts for at least 90% of cases [2]. At present, it's not known what exactly triggers sporadic CJD. Familial or inherited CJD is a rare form of CJD caused by a mutation in the gene for PrP. The variant form is caused by eating food from infected animals having bovine spongiform encephalopathy, and blood transfusion. Iatrogenic or acquired CJD is where the infection is spread from patients with CJD through the use of human-derived pituitary growth hormones, gonadotropin hormone therapy, transplants, and infected neurosurgical devices [5].
In the diagnosis of sCJD, a brain biopsy is considered the gold standard, but this is an invasive method, so it is not often preferred. The current diagnosis is mainly based on clinical symptoms and 3 less invasive paraclinical methods: brain MRI, EEG, and CSF testing. Clinically, rapidly progressive dementia and myoclonus are the most important and typical symptoms that suggest sCJD; visual or cerebellar disorders and pyramidal and extrapyramidal dysfunction may also be encountered [6]. The typical EEG of sCJD is a short-period periodic triphasic sharp wave complex. Protein 14-3-3, RT-QuIC and CSF tau protein are valuable tests in the diagnosis of sCJD, of which the 14-3-3 protein test is most commonly used. The 14-3-3 protein in the CSF is noted as a typical and suggestive biomarker for sCJD, with specificity and sensitivity are 80% and 92%, respectively [6]. However, the 14-3-3 protein is also present in some normal tissues, its presence in the CSF can be seen in some cases of severe destruction of brain tissue such as stroke, and meningitis.
Brain MRI plays an important role in providing early diagnosis of sCJD, with clinical presentations, EEG, and CSF tests helping clinicians orientate a case of probable sCJD according to the CDC (2018). Brain MRI with DWI sequence has a high sensitivity and specificity of 83%-92% and 87%-95%, respectively, of which sensitivity is higher than EEG and similar protein 14-3-3 [7,8]. In early stages, when abnormalities are predominant in gray matter, signal changes on DWI occur earlier and more dominantly than on FLAIR, and may even appear before clinical symptoms, EEG, and CSF [9,10].
In contrast, in the later stages, the gliosis process predominates, and the characteristic of hyperintense on the FLAIR will be more sensitive to DWI [11]. The most common site includes basal ganglia and cortical cortex, especially the cortex in the insula, cingulate gyrus, upper frontal gyrus, and around the midline [12,13]. Recent studies indicate that single lesions of the limbic system and perirolandic area are not common in sCJD. Pulvinar sign (lesion in the pulvinar thalamic nuclei) and double hockey sign (lesions in the dorsomedial thalamic nuclei) are more common in the variant CJD, but can also be seen in sCJD. Hyperintense on T1W of globus pallidus can also be encountered. There is an interesting point that although cerebellar damage is typical on histopathology and causes clinical symptoms, the abnormalities on MRI are often unclear, the most common feature of the infratentorial parenchyma is brain atrophy, hypointense on ADC, difficult to evaluate on nonquantitative DWI [13,14].
Another important point in the diagnosis of sCJD is to exclude other causes that have similar clinical manifestations of rapid dementia or muscle twitching such as infection, toxicity-metabolism, autoimmunity, malignancy. In these situations, clinical conditions, blood tests, cerebrospinal fluid, electroencephalography as well and imaging findings are effective in distinguishing.
According to CDC's diagnostic criteria for CJD, 2018 [15], our cases were diagnosed with probable CJD. At present, unfortunately, there is no curative, effective treatment for sCJD. The condition is universally fatal with a mean survival of only several months to a year after onset [16]. Treatment focuses on palliative care and symptom management.
Conclusion
sCJD is a very rare disease with a typical presentation of rapidly progressive dementia. The diagnosis is mainly based on clinical symptoms, brain MRI, EEG, and protein 14-3-3 in CSF. On brain MRI, abnormalities are predominant in the cerebral cortex and basal ganglia. The most sensitive sequences to identify characteristic changes are DWI and FLAIR. There is currently no curative treatment and the disease is invariably fatal with a mean survival of several months to a year, so early diagnostics helps to economize medical resources and orient clinicians in patient management.
Author's contributions
Hoang DA and Nguyen MD: Case file retrieval and case summary preparation. Hoang DA and Nguyen MD: preparation of manuscript and editing. All authors read and approved the final manuscript.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our institution does not require ethical approval for reporting individual cases or case series. Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Consent for publication
Not applicable.
Patient consent
Informed consent for patient information to be published in this article was obtained.
Footnotes
Acknowledgments: None to declare.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Klug GM, Wand H, Simpson M, Boyd A, Law M, Masters CL, et al. Intensity of human prion disease surveillance predicts observed disease incidence. J Neurol Neurosurg Psychiatry. 2013;84(12):1372–1377. doi: 10.1136/jnnp-2012-304820. [DOI] [PubMed] [Google Scholar]
- 2.Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618–628. doi: 10.1016/S1474-4422(12)70063-7. [DOI] [PubMed] [Google Scholar]
- 3.Lolekha P, Rasheed A, Yotsarawat C. Creutzfeldt-Jakob disease in a tertiary care hospital in Thailand: a case series and review of the literature. J Mov Disord. 2015;8(3):136–140. doi: 10.14802/jmd.15014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikorska B, Knight R, Ironside JW, Liberski PP. Creutzfeldt-Jakob disease. Adv Exp Med Biol. 2012;724:76–90. doi: 10.1007/978-1-4614-0653-2_6. [DOI] [PubMed] [Google Scholar]
- 5.Creutzfeldt-Jakob disease- UpToDate. [accessed 6.4.23] https://www.uptodate.com/contents/creutzfeldt-jakob-disease.
- 6.Muayqil T, Gronseth G, Camicioli R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease. Neurology. 2012;79(14):1499–1506. doi: 10.1212/WNL.0b013e31826d5fc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitali P, Maccagnano E, Caverzasi E, Henry RG, Haman A, Torres-Chae C, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology. 2011;76(20):1711–1719. doi: 10.1212/WNL.0b013e31821a4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young GS, Geschwind MD, Fischbein NJ, Martindale JL, Henry RG, Liu S, et al. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol. 2005;26(6):1551–1562. [PMC free article] [PubMed] [Google Scholar]
- 9.Warden DR, Dennison JV, Limback J, Shroff SM, Messina SA. Imaging manifestations of Creutzfeldt-Jakob disease and case series. Cureus. 10(12):e3725. doi:10.7759/cureus.3725 [DOI] [PMC free article] [PubMed]
- 10.Meissner B, Körtner K, Bartl M, Jastrow U, Mollenhauer B, Schröter A, et al. Sporadic Creutzfeldt-Jakob disease: magnetic resonance imaging and clinical findings. Neurology. 2004;63(3):450–456. doi: 10.1212/01.wnl.0000136225.80445.c9. [DOI] [PubMed] [Google Scholar]
- 11.Morgan C, Gupta M, El-Feky W, Shamim S, Opatowsky M. Creutzfeldt-Jakob disease: case discussion and imaging review. Proc (Bayl Univ Med Cent) 2009;22(1):69–71. doi: 10.1080/08998280.2009.11928476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tschampa HJ, Kallenberg K, Kretzschmar HA, Meissner B, Knauth M, Urbach H, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2007;28(6):1114–1118. doi: 10.3174/ajnr.A0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fragoso DC, Gonçalves Filho AL, Pacheco FT, Barros BR, Aguiar Littig I, Nunes RH, et al. Imaging of Creutzfeldt-Jakob Disease: Imaging patterns and their differential diagnosis. Radiographics. 2017;37(1):234–257. doi: 10.1148/rg.2017160075. [DOI] [PubMed] [Google Scholar]
- 14.Cohen OS, Hoffmann C, Lee H, Chapman J, Fulbright RK, Prohovnik I. MRI detection of the cerebellar syndrome in Creutzfeldt-Jakob disease. Cerebellum. 2009;8(3):373–381. doi: 10.1007/s12311-009-0106-8. [DOI] [PubMed] [Google Scholar]
- 15.Diagnostic Criteria | Creutzfeldt-Jakob Disease, Classic (CJD) | Prion Disease | CDC. 2021. [accessed 05.26.23]. https://www.cdc.gov/prions/cjd/diagnostic-criteria.html.
- 16.Iwasaki Y. Creutzfeldt-Jakob disease. Neuropathology. 2017;37(2):174–188. doi: 10.1111/neup.12355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.