Abstract
Ferroptosis is a regulatory cell death (RCD) caused by iron-dependent lipid peroxidation, which is the backbone of regulating various diseases such as tumor, nervous system diseases and so on. Despite ferroptosis without specific detection methods currently, there are numerous types of detection technology commonly used, including flow cytometry, cell activity assay, microscopic imaging, western blotting, quantitative polymerase chain reaction (qPCR). In addition, ferroptosis could be detected by quantifying oxygen-free radicals reactive oxygen species (ROS), the lipid metabolite (malondialdehyde ((MDA)), related pathways and observing mitochondrial damage. In the face of numerous detection methods, how to choose appropriate detection methods based on experimental purposes has become a problem that needs to be solved at present. In this review, we summarized the commonly used detection methods of the critical substances in the process of ferroptosis, in the hope of facilitating the comprehensive study of ferroptosis, with a view to providing a guidance for subsequent related research.
Keywords: Ferroptosis, Cell death, Detection methods
1. Introduction
Cell death has been identified and classified as accidental death (uncontrolled passive process) and regulated cell death (active process) over the past decades [1,2]. Compared with accidental death, regulated cell death has been widely studied [3,4]. Ferroptosis is a form of nonapoptotic cell death, that is driven by iron-dependent lipid peroxidation [5]. Ferroptosis emerged from Dolma et al. identification in 2003 as a small molecule that induced a nonapoptotic form of cell death [6], which they found to be regulated in an iron-dependent manner [7]. In subsequent studies, scholars discovered a cell death method that is different from apoptosis in many aspects, such as morphology, biochemistry, and genetics [8]. For example, the morphological features of mitochondria are observed as include swelling, dense electrons, and the reduction or disappearance of mitochondrial cristae in ferroptosis [9]. In 2012, this special way of regulating cell death was named “"ferroptosis"” [5]. We will show the characteristics of ferroptosis, pyroptosis, necroptosis, autophagy, and apoptosis in Table 1. Although the history of research on ferroptosis is not so long, the research field of ferroptosis has been growing exponentially in the past 20 years and has been proven to play a vital role in the development of various organisms and diseases. The presence of ferroptosis has thus far been observed in different systems, revealing the universality of this mechanism. Ferroptosis is closely related to various diseases and even plays a key role. The study of ferroptosis in relation to cancer has received much attention because it is closely related to the development and metastasis of cancers [10,11], such as colorectal cancer [12], liver cancer [13], and gastric cancer [14]. The expression of ferroptosis-related genes can serve as a prognostic indicator for cancer patients. Ferroptosis may become a potential strategy for preventing cancer metastasis and drug resistance. In addition, research on ferroptosis in some inflammatory diseases, such as ischaemia-reperfusion injury [15], neuroinflammation [16], and acute kidney injury, has also received widespread attention [17]. As an independent form of cell death, ferroptosis is important in both regulatory pathways and its relationship with various diseases. Currently, research on ferroptosis is still in its early stages; therefore, exploring the pathogenesis of ferroptosis and its role in various diseases, and proposing effective and targeted treatment methods have significant theoretical significance and practical value.
Table 1.
Biochemical features, morphological features and regulatory pathways of ferroptosis, apoptosis, necroptosis, autophagy and pyroptosis.
| Type | Biochemical features | Morphological features | Regulatory pathways | Ref. |
|---|---|---|---|---|
| Ferroptosis | Iron accumulation and lipid peroxidation | Small mitochondria with increased mitochondrial membrane densities, reduction or vanishing of mitochondria crista, outer mitochondrial membrane rupture and normal nucleus | Positive: TFRC, ALOX, ACSL4, LPCAT3 Negative:GPX4, AIFM2, ESCRT-III |
[9] |
| Apoptosis | DNA fragmentation | Cellular and nuclear volume reduction chromatin agglutination, nuclear fragmentation, formation of apoptotic bodies and cytoskeletal disintegration, no significant changes in mitochondrial structure | Positive: Caspase Bax, Fas Negative: Bcl-2, PI3K |
[18] |
| Necroptosis | Drop in ATP levels | Plasma membrane breakdown, generalized swelling of the cytoplasm and organelles, moderate chromatin condensation, spillage of cellular constituents into the microen | Positive: RIPK1, RIPK3 and MLKL Negative: AURK4, ESCRT-III |
[19,20] |
| Autophagy | Formation of autophagosome, elevated autophagic flux, and lysosomal activity | Formation of double membraned autolysosomes, including macroautophagy, mircroautophagy and chaperone-mediated autophagy | Positive: AMKP, ULK, VP34 Negative: mTOR |
[21] |
| Pyroptosis | Caspase-dependent, gasdermin D cleavage, formation of inflammasome, IL-18 and IL-1β release | Cells swelling, formation of membrane proes and membrane rupture, peculiar from of chromatin condensation and intact nuclei, intact and swollen mitochondria with reduced matrix density and collapsed cristae | Positive: CASP1, CASP4, CASP5, CASP11, Gasdermin D Negative: ESCRT-III, GPX4 |
[22] |
Ferroptosis is part of a bodily defence that also includes by metabolism, iron regulation and ROS [[23], [24], [25]]. Therefore, the regulation of metabolism, iron homeostasis and ROS levels is crucial way to regulate the susceptibility of cells to ferroptosis. In recent decades, it has been reported that a variety of pharmacological or natural compounds and intrinsic cellular proteins can regulate the process and function of ferroptosis [1,26]. Therefore, a full understanding of the effective components in ferroptosis and their regulatory network might pave the way for the development of research on ferroptosis.
To demonstrate the role of the crucial effective components, detection approaches have been used and even invented, such as flow cytometry, cell activity assays, microscopic imaging, western blotting, and real-time qPCR. In this review, we provide a framework to summarize the latest advances and challenges in assays of genes, proteins and processes that have played a key role in ferroptosis in the past 5 years, thereby enhancing the development of research on ferroptosis.
2. Mechanisms of ferroptosis
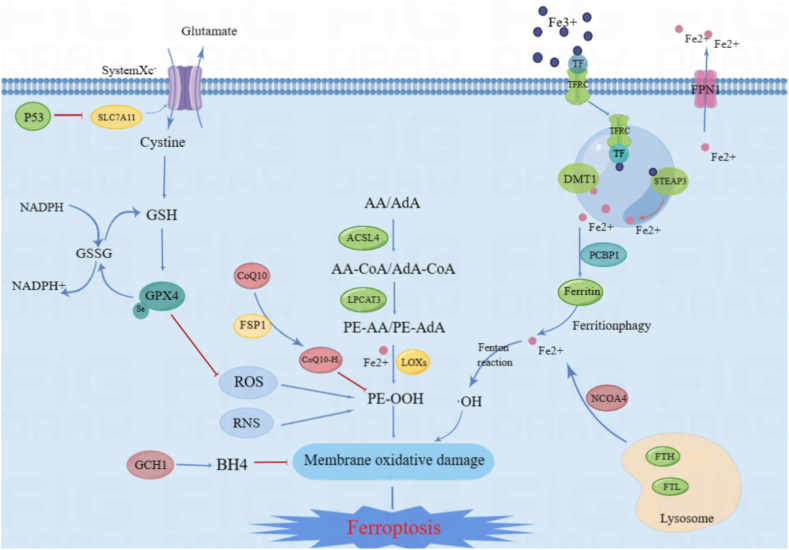
Ferroptosis is at the intersection of metabolism, ROS biology and iron regulation, which are the mechanisms governing ferroptosis [5]. The preconditions for ferroptosis are the oxidation of specific lipids and damage to natural mechanisms that could block the accumulation of oxidized lipids [[27], [28], [29], [30]]. In addition, the decrease in iron utilization may increase the sensitivity of ferroptosis and genes related to iron metabolism are generally upregulated during ferroptosis [[31], [32], [33]]. When ferroptosis occurs, the morphological, biochemical and genetic characteristics related to ferroptosis should be reflected. The morphological changes include loss of plasma membrane integrity, swelling of cytoplasmic and cytoplasmic organelles, and moderate chromatin condensation [6,34,35]. As an ROS-dependent form of cell death, ferroptosis mainly is associated with the biochemical process of iron accumulation and lipid peroxidation [23,24,36]. In addition, the overexpression of some genes and proteins has also been considered a biomarker of ferroptosis [37] (Fig. 1).
Fig. 1.
The mechanisms related to ferroptosis.
The figure briefly shows the representative pathways of ferroptosis. The GSH-GPX4, FSP1-CoQ10, and GCH1-BH4 pathways are considered the three major stand-alone mechanisms modulating ferroptosis. The antiporter System Xc− containing SLC7A11 and SLC3A2 mediates the uptake of cystine, which is consumed during the synthesis of intracellular GSH. Next, the antioxidant enzyme GPX4 reduces lipid hydroperoxides into lipid alcohols via GSH to protect cells from ferroptosis. The micronutrient selenium is needed for the biosynthesis of GPX4. Gpx4 also catalyses the reduction of oxidized biolipids, which convert toxic lipid hydroperoxides into nontoxic lipid alcohols through its cofactor GSH. CoQ10 is another important antioxidant molecule that can be reduced to CoQ10H2 by FSP1 and hence protect the cells from ferroptosis. The GCH1-BH4 axis suppresses ferroptosis by regulating the antioxidants BH4 and CoQ10, and lipid peroxidation. The production of PUFAs and subsequent lipid peroxidation play a major role in promoting ferroptosis. Fe3+ is imported into cells by Tf through TfR1, after which it is reduced to Fe2+ and imported via DMT1. Iron is stored as Fe3+ in ferritin, where it is not available to promote ferroptosis. The regulation of iron-abundance through controlling the level of the iron-storage protein ferritin via ferritinophagy dictates sensitivity to ferroptosis.
2.1. Regulation of ferroptosis
2.1.1. Lipid metabolism
Ferroptosis is driven by the peroxidation of specific membrane lipids. It is important to identify the specific lipids and enzymes that play critical roles in ferroptosis. It has been reported that polyunsaturated fatty acids (PUFAs) serve as essential peroxidation substrates for ferroptosis [38,39]. Acylcoenzyme A (CoA) synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) were identified to facilitate PUFAs incorporation into membrane lipids [[40], [41], [42], [43]]. This is a critical step in the occurrence of ferroptosis. The arachidonic acid lipoxygenase (ALOX) family exists in mammals and plays a crucial role in PUFA mediated peroxidation [44]. In the ALOX family, ALOX15 was identified as the main mediator of phospholipid peroxidation caused by ischaemia, which was further confirmed by chemical genetics [45]. On the other hand, the live or death of cells in response to ferroptotic stimuli depends on the balance of injury and the anti-injury response [46]. Furthermore, ALOX12 is critical for p53-mediated ferroptosis [44]. In addition, the activity of PUFAs in ferroptosis is competitively affected by monounsaturated fatty acids (MUFAs), and MUFA-induced ferroptosis depends on acylcoenzyme A (CoA) synthetase long-chain family member 3 (ACSL3) or stearoyl-CoA desaturase 1 (SCD1) [43,47]. Prostaglandin-endoperoxide synthase 2 (PTGS2) encodes cyclooxygenase-2 (COX-2), is the key enzyme in prostaglandin biosynthesis, and acts both as a peroxidase and as a dioxygenase [48]. PTGS2 is usually considered as a biomarker, but not a driver, of ferroptosis [37]. However, PTGS2 may mediate ferroptosis in neural cells after traumatic injury in the brain [49].
2.1.2. Iron metabolism
Ferroptosis is an RCD caused by iron-dependent lipid peroxidation. Fe3+ and Fe2+ are the two oxidation states of iron, which affect the sensitivity of ferroptosis [50,51]. Fe2+ plays an important role in metabolism and biochemical processes, and the abnormal increase in Fe2+ in the endoplasmic reticulum (ER) and lysosomes could enhance ferroptosis [52,53]. Transferrin receptor protein (TFRC) is located in the cell membrane, which and can mediate Holo-transferrin uptake and increase intracellular iron content [54]. After being absorbed by TFRC, the six transmembrane epithelial antigen 3 (STEAP3) metal reductase in the endosome reduces Fe3+ to Fe2+, and then solute carrier family 11 member 2/divalent metal-ion transporter-1 (SLC11A2/DMT1) releases Fe2+ from the endosome into the cytoplasm [55]. The iron-storage protein ferritin includes ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1), which can be degraded by lysosomes to increase free iron levels. The labile iron pool (LIP) is a storage place for intracellular iron, which provides raw material for the synthesis, assembly and insertion of iron cofactors. Generally, LIP is composed of Fe2+, while some scholars consider LIP to be a buffer system composed of small and large molecules [56,57]. LIP exists not only in the cytoplasm, but also in mitochondria, lysosomes and the nucleus [58]. Inhibiting nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy, a type of selective autophagy for the degradation of ferritin by lysosomes, decreases iron storage and limits ferroptosis in cancer cells [59,60]. Poly (RC)-binding proteins (PCBPs) act as iron chaperones, PCBP1 and PCBP2 are the major iron chaperones that play integral roles in intracellular iron trafficking [56]. Ferroportin (FPN) is a transporter protein, that can excrete excessive cellular iron to maintain the balance of iron homeostasis.
2.1.3. Oxidant system
ROS and reactive nitrogen species (RNS) are considered to be markers of ferroptosis and take part in the regulation of cell survival and death [61,62]. On the other hand, the live or death of cells in response to ferroptotic stimuli depend on the balance of injury and the anti-injury response. Glutathione peroxidase 4 (GPX4) has been demonstrated to be a glutathione (GSH)-dependent peroxidase, that can resist the oxidation of lipids in membranes [63]. Suppresses solute carrier family 7 member 11 (SLC7A11) has also been reported as a ferroptosis inhibitor, which is a unit of the glutamate-cystine antiporter xc− [64]. Ferroptosis-suppressor-protein 1 (FSP1) regenerates reductive ubiquinol (CoQ10H2) by oxidizing ubiquinone (CoQ10) with nicotinamide adenine dinucleotide phosphate (NADPH) [40,63,65]. CoQ10H2 plays a key role in inhibiting ferroptosis and lipid peroxide production. Tetrahydrobiopterin (BH4), the leading figure in the GTP cyclohydrolase 1(GCH1)/BH4 pathway, works as an antioxidant for trapping free radicals and supports the synthesis of CoQ10 by converting phenylalanine into tyrosine [66,67]. Erythroid 2-related Factor 2 (NRF2) is a stress-inducible transcription factor, and the target genes of NRF2 are involved in preventing lipid peroxidation and ferroptosis [68]. NRF2 plays a key role in mediating iron not only by regulating solute carrier family 40 member 1 (SLC40A1) but also by regulating SLC7A11 [69,70]. Hypoxic inducible factor (HIF) is a transcriptional complex, and HIF subunits can be divided into three types: HIF-1α, HIF-2α, and HIF-3α [71]. Current studies have reported that HIF-1α can limit ferroptosis by storing lipids in droplets and influencing lipid metabolism, which can attenuate peroxidation-mediated damage [72,73].
2.2. The hallmarks of ferroptosis
2.2.1. Morphological features
Cells underging ferroptosis usually show features such as the loss of plasma membrane integrity, cytoplasmic oncosis, swelling of cytoplasmic organelles and moderate chromatin condensation [74]. At the ultrastructural level, mitochondria-induced cysteine starvation, ER-related oxidative stress, lysosomal dysfunction and Golgi stress-related lipid peroxidation all contribute to induction of ferroptosis [75]. Recent evidence suggests that mitochondria-mediated ROS production, deoxyribonucleic acid (DNA) stress and metabolic reprogramming are necessary for the induction of lipid peroxidation and ferroptosis [9,76]. Mitochondria in ferroptotic cells usually exhibit swelling, increased membrane density, reduced or missing crests, and rupture of the outer membrane [77]. The ER in ferroptotic cells is the most critical site of lipid peroxidation during ferroptosis, in which the compounds that regulate ferroptosis by affecting lipid peroxidation are mainly located [78]. Lysosomes are reservoirs of iron, which can cause ferroptosis in principle, and the abnormal increase in Fe2+ in lysosomes may promote ferroptosis [79]. There is a close interaction between the excessive accumulation of lipid peroxidation and Golgi dysfunction, which is involved in the regulation of oxidative stress, lipid peroxidation and ferroptosis [80,81].
2.2.2. Biochemical features
Lipid peroxidation, as a marker of ferroptosis, has undergone extensive research. Lipid peroxidation is a reaction of oxidative degradation of lipids caused by oxidative fracture and shortening of lipid carbon chains under the effect of lipid peroxidase or free radicals, resulting in cytotoxic substances such as lipid free radicals, lipid hydroperoxides and reactive aldehydes, and eventually causing cell damage [82]. Lipid peroxidation mainly affects unsaturated fatty acids in the cell membrane. Lipid peroxidation produces lipid hydroperoxides (LOOHs) and reactive aldehydes (e.g., 4-hydroxynonenal (4HNE) and MDA), which increase during ferroptosis [1]. Hydroperoxides are not stable, so for the products of lipid oxidation, 4HNE and MDA are mainly detected [83].
Fe2+ is important for metabolic and biochemical processes, such as oxygen transport, energy metabolism and iron-sulfur protein production in mitochondria. As a cofactor, iron regulates the activity of iron-requiring enzymes by forming redox-active (loosely-bound) and redox-silent complexes, thereby playing a complex role in oxidative stress [84]. However, the exact effect of iron on ferroptosis remains unclear [85]. As we know, Iron is mainly stored as a complexe with ferritin (FT) or stored in LIPs in cells [40]. FPN is a transporter protein that can excrete excessive cellular iron to maintain the balance of iron homeostasis. Once iron homeostasis is disrupted, excessive amounts of Fe2+ can trigger the Fenton reaction and produce large amounts ROS [86], followed by lipid peroxidation and the induction of ferroptosis [87]. In summary, excess iron ions, can be one of the indicators for the occurrence of iron death in cells.
Ferroptosis is a peroxidation-induced nonapoptotic cell death, and its process is strongly associated with iron and ROS content [83]. ROS are produced by normal physiological processes and are crucial to cell signalling and tissue homeostasis [88]. ROS are oxygen-containing molecules with incomplete low valence, including superoxide (O2•-) peroxides (H2O2 and ROOH) and free radicals (HO• and RO•). Excess ROS can be neutralized by reactions catalysed by antioxidants, superoxide dismutase (SOD), GPX4 and catalase. When the balance between the generation and neutralization rates of ROS is broken, oxidative stress leads to the generation of free radicals, impairing DNA proteins and lipids [89]. Elevated levels of intracellular iron and the consumption of the antioxidant GSH are now believed to increase ROS, which in turn triggers lipid peroxidation and ultimately leads to ferroptosis [90].
3. Detection approaches for ferroptosis
As an independent RCD, ferroptosis has a unique cell morphology, which is different from that of other RCDs, such as autophagy, pyroptosis and necroptosis [91]. The process of ferroptosis is complex and involves a great number of metabolites and enzymes. Although there are no characteristic testing methods for ferroptosis yet, many approaches are commonly used in ferroptosis research. We summarized the detection approaches to provide guidance for follow-up research.
3.1. Detection of the cellular state in ferroptosis
At the cellular level, researchers often use these reagents to detect reductions in cell number or changes in cell state, for instance,3-(4, 5-dimethylthiazole-2)-2, 5-diphenyltetrazolium bromide (MMT), cell counting kit-8 (CCK-8), propidium iodide (PI), lactate dehydrogenase (LDH) and adenosine triphosphate (ATP) kits. The MMT, CCK8, LDH and APT kits are colorimetric or stain, and PI can be used with flow cytometry or fluorescence microscopy. The reason for using these methods is that, as an RCD, cell death and cell state changes occur as a consequence of ferroptosis. However, cell viability assays are not specific. Cell viability testing is essential in studies that prove the presence of ferroptosis in the discovery process of disease. The detection approaches are summarized in Table 2.
Table 2.
Detection of cellular state in ferroptosis.
| Reagent | Reaction principle | Detection method | Variation | Ref. |
|---|---|---|---|---|
| CCK8/MMT | Reagent is reduced to blue purple crystal methyl black by succinate dehydrogenase in the mitochondria of living cells | Microplate plate analyzer, Microplate reader | Decline | [92] |
| Trypan blue dye | Trypan blue dye can enter inactivated cells or incompleted cell membranes | Microscope | Increase | [[93], [94], [95]] |
| Hoechst33342 and SYTOX Green | SYTOX Green marks the nucleic acid of dead cells, and Hoechst33342 marks the DNA of living cells | Fluorescence microscope, fluorescence microplate reader, flow cytometer | Green fluorescent increase, blue fluorescent decline | [54] |
| PI | PI is a small flurescent molecule, can enter the cell and bind to DNA | Fluorescence microscope, Flow cytometer | Increase | [96,97] |
| LDH | The plasma membrane ruptures and LDH is released | Microplate reader | Increase | [[98], [99], [100]] |
| CellTiter-Glo Luminescent Assay Kit | Content of ATP can reflect cell activity | Luminometer, Liquid scintillation meter | Decline | [101] |
MMT is reduced to blue purple crystal methyl black by succinate dehydrogenase in the mitochondria of living cells, depositing in cells. A microplate plate analyzer measured the optical density (OD) value at 570 nm. With more viable cells, more deposits are generated, and the OD value is higher. However, MTT is not suitable for suspended cells because the culture medium needs to be removed before dissolving formazan. This step can easily cause the loss of formazan, leading to deviations in the experimental results. However if the culture medium is not removed, the serum and phenol red will affect the experimental results. The main advantage of CCK-8 is that the reaction product is water-soluble and does not require the use of lysis buffer to dissolve the precipitate or the need to absorb the supernatant. It is suitable for both adherent and suspended cells. The detection time and the number of processing steps are reduced, which also greatly improves the detection efficiency and experimental sensitivity. The disadvantage is that the cost of CCK-8 is high, and the color of CCK-8 is light red. cck8 is close to the color of the culture medium containing phenol red, and it is easy to miss or add too much. As a substance similar to MMT, CCK-8 has a parallel application principle, whereas the product can be directly dissolved in water, making it easier to operate. When a ferroptosis inducer is applied to the cells, the OD value will decrease using CCK-8 or MMT to detect cell viability [92]. Trypan blue dye enters cells that are inactivated or have incomplete cell membranes, and blue cells were observed with the aid of a microscope. However, trypan blue staining is generally not used alone but is used together with CCK-8 and MMT [93,94]. In addition, trypan blue acts as an indicator of membrane permeability in combination with flow cytometry, and red fluorescence is tested under the red filter to assess cell survival [95].
Cell death can also be assessed using Hoechst 33,342 and SYTOX Green dual staining. It was then detected using a microscope and the percentage of dead cells was calculated by ImageJ software [54]. SYTOX Green can label the DNA of dead cells to produce green fluorescence, while Hoechst 33,342 can label the DNA of living cells to produce blue fluorescence. Fluorescence microscopes, fluorescence microplate readers, and flow cytometry can all be used to examine these fluorescent probes.
PI, which is a small flurescent molecule, can be used to discriminate dead cells from live cells with intact membranes. PI enters cells that do not possess an intact plasma membrane and bind to DNA [96]. Using PI staining in the study of ferroptosis, the more fluorescence produced, the more cells died [97].
LDH is a stable cytoplasmic enzyme that is present in all cells. When the plasma membrane is damaged, LDH is rapidly released into the cell culture supernatant, which is an important manifestation of apoptosis, necrosis and other forms of cellular damage. The LDH kit is based on the principle that the amount of formazan is proportional to the amount of lactate dehydrogenase in the culture, which in turn is proportional to the number of dead or damaged cells [98]. Therefore, when ferroptosis occurs in cells, the results of the assay will show higher levels of LDH [99,100]. Comparing LDH and MTT, the results show that LDH is simpler, more sensitive, and faster to measure. Since endogenous ATP, a source of energy metabolism for cells, is rapidly hydrolysed upon cell death, measuring its content can reflect cell activity and the number of viable cells [101]. The higher the fluorescence intensity, the stronger the cell activity.
Overall, the detection of cell status or cell activity is commonly used in the study of ferroptosis. CCK-8 should be the most widely used method at present. This may be because the reagent is simple to operate and researchers mainly focus on studying regulatory pathways and mechanisms. In terms of instrument selection, microplate readers and fluorescence microscopes are relatively common in laboratories and are simple to operate, so we recommend using colorimetric methods or fluorescence detection to detect the cellular state. Flow cytometry is an ideal method to study the effects of different factors on the cell cycle and cell activity. Its disadvantage is that the sample must be single cells, and must be processed and detected immediately after staining. At the same time, the operation process of flow cytometry is more complicated than that of ordinary microscopes, microplate readers, etc., requiring dedicated personnel to operate and maintain.
3.2. Detection of subcellular organelles in ferroptosis
The accumulation of lipid peroxide leads to ferroptosis. In recent years, researchershave studied the subcellular membrane containing these oxidized lipids and clarified the contribution of different organelles to ferroptosis. Therefore, detecting changes insubcellular organelles in the process of ferroptosis is helpful to prompte the occurrence of ferroptosis. The detection approaches are summarized in Table 3.
Table 3.
Detection of subcellular orgenells in ferroptosis.
| Reagent | Reaction principle | Detection method | Variation | Ref. | |
|---|---|---|---|---|---|
| Mitochondrial | / | The morphological features of mitochondria are changed. | TEM | Swelling, dense electron, reduction or disappearance of cristae | [102,103] |
| Mito-Tracker Green, MitoSox Red | Increased mitochondrial ROS promote ferroptosis | Confocal microscope | Fluorescence weaken | [80] | |
| MitoSox Red | Increased mitochondrial ROS promote ferroptosis | Fluorescence microscope, Flow cytometer | Fluorescence weaken | [74,104] | |
| DIOC6/MitoTracker Deep Red FM | Increased mitochondrial membrane permeability | Confocal microscope, Fluorescence microscope, Flow cytometer | Fluorescence weaken | [95] | |
| Tetramethylrhodamine methyl ester (TMRM)/JC-1 | Decreased mitochondrial membrane potential | Flow cytometer, fluorescence microscope, fluorescent microplate reader | Fluorescence weaken | [105,106] | |
| Endoplasmic reticulum | ER-targeting fluorescent probe (PV1) | Endoplasmic reticulum viscosity increase | two-photon phosphorescence lifetime imaging | Viscosity increase | [78] |
| Confocal microscope, Fluorescence microscope, Flow cytometer | Fluorescence enhancement | [107] | |||
| ATF4, eukaryotic initiation factor 2, α subunit (eIF2α) | Endoplasmic reticulum stress | western blotting | Increased expression | [108] | |
| Lysosomes | LysoTracker Green | Iron accumulate in lysosomal | Confocal microscope, Fluorescence microscope, Flow cytometer | Fluorescence enhancement | [109] |
| – | Cathepsin B (CTSB) is considered as an executioner of ferroptosis | Western blotting | Increased expression | [110] | |
| Golgi | Cis-Golgi marker GM130 | Golgi dispersal diminish | Fluorescence microscopy. | Fluorescence weaken | [111] |
Although the mechanism of the role of mitochondria in ferroptosis is not well defined, we cannot ignore the importance of mitochondria in ferroptosis [112]. Mitochondrial ROS, mitochondrial iron, mitochondrial DNA (mtDNA) and the tricarboxylic acid (TCA) cycle have been found to be strongly associated with ferroptosis [80]. The morphological features of mitochondria are observed as swelling, dense electrons, and reduction or disappearance of mitochondrial cristae [113]. Transmission electron microscopy (TEM) is an ideal method for studying the internal structure of cells. Therefore, using TEM to observe changes in mitochondrial structure is one of the most commonly used methods in ferroptosis research [102,103]. Mitochondria are an essential source of ROS in most of the mammalian cells, and increased mitochondrial ROS promote ferroptosis [114]. Mitochondria-associated antioxidant proteins protect mitochondria from oxidative damage in ferroptosis such as SOD2 [115], GPX4 [114], and microsomal glutathione S-transferase 1 (MGST1) [116]. Mito-Tracker Green and MitoSox Red were used to label mitochondria and mitochondrial ROS, and the mitochondria and mitochondrial ROS were observed under a confocal microscope [80]. MitoSOX Red treats cells, and the relative level of mitochondrial fluorescence was quantified by flow cytometry [74,104]. Intracellular iron is transported into the mitochondria to synthesize haem and Fe–S. Haem and Fe–S have been shown to be involved in ferroptosis [117,118]. In research, mtDNA stress activates GAS-STING1 pathway-dependent autophagy to mediate ferroptosis [76]. Increased mitochondrial membrane permeability and decreased mitochondrial membrane potential are alterations characteristic of mitochondrial membranes in ferroptosis [119]. Therefore, the occurrence of ferroptosis can be verified not only by observing the changes in mitochondrial morphology, but also by evaluating the change of mitochondrial membrane potential and permeability. 3,3' -Diethyloxyheterocarbonylcyanine iodine (DIOC6) is a green fluorescent lipophilic dye with cell membrane permeability. It can selectively label mitochondria in living cells, and green fluorescence is weakened when the mitochondrial membrane potential decreases due to ferroptosis [95]. Mito Tracker Deep Red FM can also text the changes in mitochondrial membrane permeability. The principle is that the probe enters cells and gathers on active mitochondria, emitting red fluorescence. When mitochondrial permeability is increased and red fluorescence weakens, the possibility of ferroptosis is further proven. Tetramethylrhodamine methyl ester (TMRM) is a permeable dye that accumulates in active mitochondria with intact membrane potential, which is a common method to detect changes in mitochondrial membrane potential [105]. When ferroptosis occurs in cells and mitochondrial membrane potential is lost, TMRM no longer accumulates, and the fluorescence signal becomes weak or disappears. The fluorescence probe JC-1 can determine the mitochondrial membrane potential △Ψm [120,106].
The ER is an important organelle in eukaryotes and plays a key role in numerous cellular functions. Previous studies have found that the use of ferroptosis inducers can increase ER viscosity, lipid peroxidation and ER stress, and reduce MUFAs. Zinc is transported from the ER to the cytosol, which is also one of the manifestations of ferroptosis [80]. Quantitative measurement of viscosity increase in the ER during ferroptosis using two-photon phosphorescence lifetime imaging [78]. An alternative method to detect viscosity changes in the ER is the ER-targeting fluorescent probe (PV1) [107]. Activating transcription Factor 4 (ATF4) is a key factor in ER stress, and the diversity of ATF4 target genes leads toe multiple biological functions in ferroptosis. Detecting the expression of ER stress-related proteins, such as ATF4, eukaryotic initiation Factor 2, and α subunit (eIF2α), by western blotting is a common way to demonstrate ER stress [108].
Lysosomes are signalling hubs and degradation centresin cells and play significant roles in cellular homeostasis, development, and ageing [121]. Stimulation of cells with erastin or glutamate, a ferroptosis inducer, increases lipid peroxidation, nitric oxide (NO), chthepsins, and iron within lysosomes [80]. Currently, lysosomes act through three mechanisms: (i) accumulation of lysosomal iron or NO; (ii) release of lysosomal cathepsins; and (iii) activation of autophagy [122,123]. The lysosome is a master regulator of iron metabolism. The accumulation of lysosomal NO or iron can promote lysosome-dependent ferroptosis by inducing lipid peroxidation. We will describe later how to detect iron or NO using reagents or probing. Cathepsin B (CTSB) is a lysosomal cathepsin that is considered an executioner of ferroptosis [110]. At present, western blotting is widely used to detect the expression of CTSB [110]. Among several selective autophagy pathways associated with lysosomes, lysosomes reduce ferroptosis through GPX4, SLC40A1, Recombinant Aryl Hydrocarbon Receptor Nuclear Translocator Like Protein (ARNTL), ferritin and lipid droplet (LDs) reduction, etc [80]. These ferroptosis-related genes and proteins can be detected by--qPCR or western blotting.
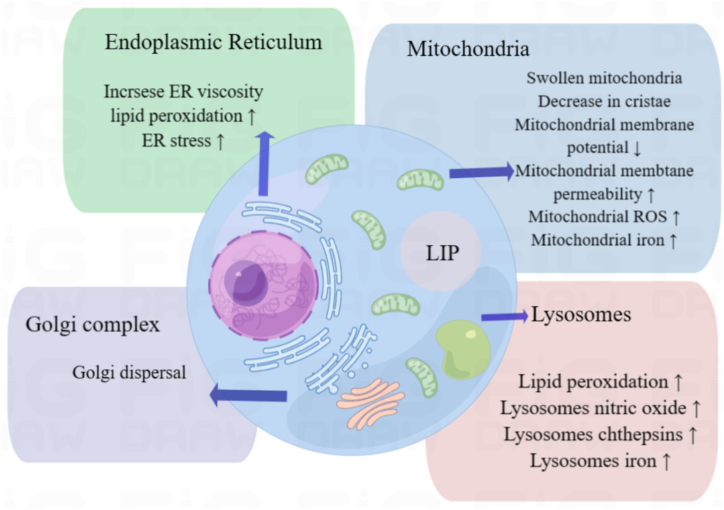
There is a close interaction between excessive accumulation of lipid peroxidation and Golgi dysfunction, although the mechanism is not yet clear. Several Golgi-dispersing compounds, including AMF-26/M-COPA, brefeldin A, and golgicide A, have also been shown to induce ferroptosis [111]. Golgi morphology was analysed by immunofluorescence staining using the cis-Golgi marker GM130 followed by fluorescence microscopy. Golgi dispersal was substantially diminished during ferroptosis [111] (Fig. 2).
Fig. 2.
Detection of subcellular organelles in ferroptosis.
In the current study, mitochondria played a very important role in ferroptosis. In studies on detecting ferroptosis, researchers have prioritizedto detecting changes in mitochondria, whether using TEM to observe changes in mitochondrial biomorphology or detecting changes in mitochondrial membrane potential. Although TEM technology is the most direct technology, it also has shortcomings. During the process of making slices, it is easy to change the membrane structure due to human factors, and other double-membrane organelles will also interfere with the results, leaving great deficiencies in quantitative analysis. However, which organelle changes to focus on is closely related to the researcher's research content.
3.3. Detection of genes, proteins and transcription factors in ferroptosis
There are many genes, transcription factors, and proteins involved in ferroptosis, which is a complex metabolic process. Real-time quantitative reverse transcription-PCR (RT qPCR) and western blotting are widely used in ferroptosis research as conventional detection techniques. RT qPCR adds fluorophores to the PCR and analyses the initial amount of target gene by continuously monitoring the fluorescence signal and signal strength. By binding specific antibodies to particular proteins in cell or biological tissue samples, Western blot analyses the colouring position and depth to obtain the expression of these special proteins [124]. Ferroptosis is caused by iron-dependent lipid peroxidation. The genes and proteins involved in ferroptosis are primarily related to lipid metabolism, iron metabolism, and the antioxidant system, as listed in Table 4.
Table 4.
Detection of gene and protein in ferroptosis.
| Gene/Protein | Function | Variation | Ref. |
| Gene and protein of lipid metabolism | |||
| ACSL4 | Key enzyme of catalyses the activation of long-chain fatty acids | Increase | [125] |
| LPCAT3 | Catalysed FUFA-CoA produce PE-AA and PE-AdA | Increase | [126] |
| LOXs | Assist lipid peroxidation | Increase | [126] |
| SCD1 | Increases tumor resistance to ferroptosis inducers | Increase | [127] |
| ACSL3 | Promoting LD formation and maturation and activating MUFA | Decline | [128] |
| PTGS2 | Marker of ferroptosis | Increase | [129] |
| Gene and protein of iron metabolism | |||
| TFR1 | Transports Fe 3+ from extracellular to intracellular | Increase | [130] |
| NCOA4 | Mediates autophagic degradation of ferritin to maintain intracellular iron homeostasis | Increase | [131] |
| FTL/FTH1 | Store iron ions | Decline | [132] |
| FPN | Transports Fe 2+ from intracellular to extracellular | Decline | [133] |
| Gene and protein of antioxidant system | |||
| SLC7A11 | Transport cystine | Decline | [126] |
| GPX4 | Important antioxidant | Decline | [125] |
| GSH | Primary cofactor for GPX4 synthesis | Decline | [134,135] |
| FSP1 | Regenerate the reduced form of CoQ10 | Decline | [136] |
| CoQ10 | Protecting against the lipid peroxidation that drives ferroptosis. | Decline | [63] |
| GCH1 | Influence initiation and rate-limiting of BH4 synthesis | Decline | [66,137] |
| BH4 | Inhibit lipid peroxidation | Decline | [66] |
ACSL4 and LPCAT3, as the first identified promoters of ferroptosis, have been extensively studied in ferroptosis experiments. ACSL4 is a key enzyme that catalyses the activation of long-chain fatty acids [138]. ACSL4 determines ferroptosis sensitivity by altering the cellular lipid composition and acts as a specific biomarker and driver of ferroptosis. ACSL4 is more biased towards PUFAs such as adrenaline (ADA) and arachidonic acid (AA) and catalyses them into ADA-CoA and AA-CoA [139]. PE-AA and PE-AdA mainly catalysed FUFA-CoA through LPCAT3. These products can trigger lipid peroxidation under the action of LOXs or free ferrous iron. However, the contribution of LOXs to ferroptosis and lipid peroxidation is still controversial [140]. ACSL3 is a negative regulatory gene of ferroptosis that can reduce the sensitivity of cancer cells to ferroptosis [85]. This may be related to ACSL3 promoting LD formation and maturation and activating MUFAs [43,141]. SCD1 is a lipid-modifying enzyme that is upregulated in many malignancies, especially ovarian cancer [47,142]. In tumours, high expression of SCD1 increases tumor resistance to ferroptosis inducers and may promote tumor recurrence [127]. In summary, the increased expression of ACSL4, LPCAT3 and LOXs is a favourable factor for promoting ferroptosis, among which ACSL4 has been studied the most. Increased expression of ACSL3 and SCD1 can inhibit ferroptosis, and research on SCD1 has mainly focused on cancer. PTGS2 is usually considered a biomarker but not a driver of ferroptosis [37]. PTGS2 expression increases when ferroptosis occurs. Commonly used detection methods are mainly qPCR and western blotting, and some scholars use immunohistochemistry.
Transferrin receptor 1 (TFR1), a carrier protein that transports Fe3+ from the extracellular space to the intracellular space, is the first step in causing iron overload. Currently, TFR1 is mainly used as a marker of ferroptosis. Scholars have made an important discovery in their research: the accumulation of TfR1 on the cell surface is a feature of ferroptosis [143]. When ferroptosis occurs, there is usually an increase in TFR1, and the most common detection method is western blotting, followed by qPCR [144,145]. NCOA4 is a selective cargo receptor that mediates autophagic degradation of ferritin to maintain intracellular iron homeostasis. In NCOA4-mediated ferritinophagy, NCOA4 increases, accompanied by decreases in FTL and FTH1 [132]. FPN is a protein that maintains intracellular iron balance by transporting Fe2+ out of the cell. Ferroptosis can be induced by inhibiting the expression of FPN [146]. Regarding the genes and proteins related to iron metabolism, the most studied are FTH1 and NCOA4, followed by TFR1 and FTL.
The system xc−/GSH/GPX4 pathway is the earliest and most important regulatory pathway. The source of cysteine, the biosynthesis of GSH, and the function of GPX4 are central to controlling ferroptosis [147]. The system xc− exchanges the extracellular and intracellular cystine and glutamate in a1:1 ratio, in which solute carrier family 3 member 2 (SLC3A2) transports glutamate out of the cell, and SLC7A11 transports cystine into the cell for GSH biosynthesis [148,149]. Then, under the action of glutamylcysteine synthetase (GCS) and glutathione synthetase (GSS), glutamate, cysteine and glycine are synthesized into GSH. GSH is the primary cofactor for GPX4 synthesis. GSH can improve the reaction of GPX4 with intracellular phospholipid hydroperoxides (PLOOHs) to reduce the corresponding phospholipid alcohols (PLOHs), thereby reducing the occurrence of ferroptosis [150]. The study found that the expression or activity of GPX4 is controlled by selenium and GSH. Therefore, researchers most commonly use qPCR, western blotting and immunohistochemistry to demonstrate that ferroptosis may have occurred, by verifying decreased expression of GPX4 or SLC7A11 [151]. Quantification of GSH using the GSH-GSH/Glutathione, Oxydized (GSSG) Ratio Detection Assay Kit allows assessment of intracellular GSH levels [134,135]. NADPH is an essential electron donor in all living organisms and is a biomarker of ferroptosis sensitivity. NADP is the oxidized form of NADPH. The NADP/NADPH system is used by many ferroptosis regulators to regulate electron transfer, such as GPX4 and NOX, [152]. The available evidence suggests that a lower NADP/NADPH ratio or higher levels of NADPH promote resistance to ferroptosis. For the research of NADPH, scholars mainly use the NADP/NADPH-Glo Assay Kit to detect the ratio of NADP/NADPH [135,153]. The FSP1/CoQ10 pathway and GCH1/BH4 pathway are pathways that inhibit ferroptosis and are independent of the GPX4 pathway [66,154]. At present, their regulatory mechanism for ferroptosis still needs to be further studied, and ferroptosis is detected by western blotting and qPCR [34,155]. Determination of BH4 levels was measured using high-performance liquid chromatography (HPLC) [156].
3.4. Detection of various reaction products in ferroptosis
When ferroptosis occurs, reaction products, such as ROS, Fe, and lipid peroxidation, usually change in the cytoplasm. The detection approaches for these products are summarized in Table 5.
Table 5.
Detection of various reaction products in ferroptosis.
| Reagent | Reaction principle | Detection method | Variation | Ref. | |
|---|---|---|---|---|---|
| Fe2+ | FerroOrange, PGSK, FeRhoNox-1 | Increase of intracellular Fe2+ will trigger ferroptosis | Confocal microscope, Fluorescence microscope, Flow cytometer | Fluorescence enhancement | [[157], [158], [159]] |
| Iron Assay Kit | Microplate reader | Increase | [145] | ||
| ROS | DCFH-DA, DHE | ROS leads to oxidative stress | Confocal microscope, Fluorescence microscope, Flow cytometer | Fluorescence enhancement | [109,160,161] |
| RNS | DAF-FM Diacetate | NO mediate lipid peroxidation | Confocal microscope | Fluorescence enhancement | [61] |
| HKYellow-1, BTMO-PN | ONOO- mediate lipid peroxidation and lipid peroxidation | Confocal microscope, Flow cytometry, Fluorescence microscope | Fluorescence enhancement | [61,162] | |
| Lipid peroxidization products | C11-BODIPY | Lipid peroxidation is increased in ferroptosis | Fluorescence microscope, fluorescence microplate reader, flow cytometer | Increase | [163] |
| MDA Assay Kit | MDA is main lipid peroxidization products | Microplate reader | Increase | [164] | |
| 4-HNEAssay Kit | 4-HNE is main lipid peroxidization products | Microplate reader | Increase | [164] | |
| TBARS | TBARS can detect MDA and some minor related compounds | Microplate reader | Increase | [165] |
The increase in intracellular iron ions will trigger ferroptosis, and excessive ROS will be generated through the Fenton reaction [23]. Part of the iron ions in cells is stored in ferritin in the form of compounds, and the other part is stored in LIP. We have briefly described the metabolism of iron in cells. Now we will mainly describe the detection method and reagents used for intracellular iron ions. In research on ferroptosis, FerroOrange or Phen green SK (PGSK) are commonly used to detect Fe2+, as observed by fluorescence microscopy or flow cytometry [[157], [158], [159]]. FeRhoNox-1 is a probe that combines with Fe2+ to produce an irreversible orange-red fluorescent substance, and the change in intracellular iron is observed by flow cytometry or microscopy [54]. The Iron Assay Kit detects ferrous iron in tissues and cells, and the content of iron ions is reflected by the od value of the microplate reader [145]. And LysoTracker Green is a probe that labels iron ions in lysosomes. The cell photographs were captured by a confocal microscope to acquire green fluorescent lysosomes [109]. In other studies, ferrous ions can be detected using these probes, such as FIP-1, IP-1, Probe 3, RhoNox-1, SiRhoNox-1, and ICL-1. CP655, FD1, Sensor 1, FS1, BOD-NHOH, etc., can be used to detect ferric ions [58].
Ferroptosis is a peroxidation-induced nonapoptotic cell death, and its process is strongly associated with iron and ROS content [83]. ROS are produced by normal physiological processes and are essential for cell signalling and tissue homeostasis [88]. ROS detection is chiefly divided into three categories, namely, intracellular ROS, lipid ROS and mitochondrial ROS. Intracellular ROS are often detected by 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), a probe specific for hydrogen peroxide (H2O2), and green fluorescence is observed when ROS increase [109,166]. Another method to test cellular ROS is dihydroethidium (DHE), which can freely enter the cell through the living cell membrane and is oxidized by intracellular ROS to produce ethidium oxide. Then, ethidium oxide is incorporated into chromosomal DNA to produce red fluorescence [167,160].
In addition to ROS, RNS also cause ferroptosis [168,169]. Peroxynitrite (ONOO-) and NO can react with unsaturated fatty acids to form nitrated oxidation products [170]. ONOO- and NO are representative RNS [171]. RNS can attack PUFAs, which in intracellular organelles and plasma membranes produce lipid peroxides [68]. 4-Amino-5-Methylamino-2′,7′-Difluorofluorescein Diacetate (DAF-FM Diacetate) can combine with NO production to produce a fluorescent product visualizing the fluorescent imaging by using inverted confocal microscopy [61]. HKYellow-1 and BTMO-PN are newly developed highly selective and sensitive ONOO- probes that which were used to detect the intracellular ONOO- level. Using instruments such as confocal microscopy, flow cytometry and fluorescence microscopy, the intracellular ONOO- level can be detected [61,162].
Ferroptosis is a form of regulated cell death that relies on iron and is characterized by the accumulation of lipid peroxides, resulting in oncotic cell swelling and eventual disruption of cellular membranes. Lipid peroxidation, a hallmark of ferroptosis, refers to the oxidative deterioration of lipids that contain carbon double bonds, particularly polyunsaturated fatty acids [172]. The cytotoxicity of lipid peroxidation products is manifested in two aspects: on the one hand, it influences the physical properties of the lipid bilayer, thereby changing membrane fluidity and membrane permeability; on the other hand, the degradation products naturally formed by lipid peroxidation cause toxicity to cells [1]. Currently, one of the most commonly used and effective methods is the C11-BODIPY assay, which utilizes a fluorescent probe that selectively sensitizes lipid peroxidation in cell membranes [172]. C11-BODIPY, as a lipid-soluble ratio fluorescent probe, can indicate lipid peroxidation and antioxidant properties in membrane systems and living cells, and is commonly used to measure intracellular lipid peroxides [173]. When intracellular lipid peroxides gaining, the increasing oxidation state of C11-BODIPY can be detected and the fluorescence color changes from red to green. Another way to detect lipid oxidation is to examine their products [174]. The two lipid peroxidation products investigated are 4-HNE and MDA. The reaction of MDA with thiobarbituric acid produces a chromophore whose concentration can be quantified by absorbance, and then the test kit can measure lipid peroxidation [[175], [176], [177]]. Moreover, the reaction of the aldehyde group of 4-HNE with 2, 4-dinitrophenylhydrazine can measure the degree of carbonylation of proteins in biological samples [164]. Some scholars use thiobarbituric acid-reactive substance (TBARS) to measure lipid peroxidation in cells. TBARS was measured at 532 nm using a microplate reader [165]. The MDA detection kit is the most widely used in the study of lipid peroxidation. The MDA kit can detect MDA not only in cells, but also in tissues. Since cells contain less MDA, the number of cells should be as large as possible so that the trend in the test results may be more obvious.
During the occurrence and development of ferroptosis, not all metabolites produced within cells are specific. For example, ROS are a common metabolites in CRD, such as apoptosis [178] and autophagy [179]. Therefore, detecting the increase in ROS alone cannot determine the occurrence of ferroptosis. Ferroptosis is an RCD caused by iron-dependent lipid peroxidation. Detection of iron ions and lipid peroxidation is important for ferroptosis, especially lipid peroxidation.
4. Summary and prospects
Ferroptosis is a new RCD and has been reported to be related to many diseases [12,180,181]. Therefore, it is of great significance to detect the occurrence and development of ferroptosis in diseases. Brent R. Stockwell, who is an authoritative scholar of ferroptosis, proposed the following suggestions: 1) markers should be detected prior to cell death; 2) a minimum of three markers need to be detected, and lipid peroxidation should be detected; 3) markers of lipid peroxidation and expression of TFR1 can be inhibited by ferroptosis inhibitors; and 4) expression of some gene markers should not be suppressed by ferroptosis inhibitors [5]. Ferroptosis is driven by iron-dependent lipid peroxidation, and it is critical to detect such lipid peroxidation events during ferroptosis. While some markers may be activated by these other types of stress, if at least three markers are used or a suitable combination of two markers (such as lipid peroxidation and TfR1 mobilization), ferroptosis can be distinguished from these other stress conditions [5]. The markers may mitochondria typically exhibit shrunken, dense morphologies, and specific gene expression changes can be detected, such as PTGS2 SLC7A11, ACSL4, and TfR1 upregulation and movement to the plasma membrane.
In this review, we presented ferroptosis from the perspectives of cells, subcellular organelles and gene proteins and summarized the most commonly used detection methods, principles and reagents of ferroptosis in past 5 years to provide more choices for ferroptosis studies.
Although ferroptosis has been proven to play an important role in the development of various organisms and diseases, there is no gold standard for the detection of ferroptosis. In addition, it is more challenging to monitor ferroptosis in vivo than in vitro. However, we still hope to provide some reference for scholars who are studying or want to study ferroptosis. The choice of ferroptosis detection method is not fixed and is related to the experimental purpose, equipment, resources and professional knowledge. If the purpose of the experiment is to demonstrate a disease that has not yet been shown to be associated with ferroptosis. Detection of cell viability, lipid peroxidation, mitochondrial changes,/and changes in ferroptosis-related genes or proteins is necessary. In the detection of lipid peroxidation, we hope that it is not a single detection method, but should try to detect lipid peroxidation from multiple aspects and angles, such as using C11-BODIPY and MDA kits. For changes in mitochondria, researchers who do not have the conditions to use TEM can also consider testing mitochondrial membrane potential and mitochondrial membrane permeability, both of which are often tested together. There are many genes and regulatory factors involved in ferroptosis. The best way is to complete it through transcriptomic sequencing. This method covers all genes, reduces the workload and is more convincing, but it is expensive. Researchers with limited research funds also choose representative genes or proteins as detection targets. The final point is that using inhibitors of ferroptosis can reverse the results we detected previously. Those who want to develop new treatment options by inhibiting or promoting ferroptosis need to detect relevant indicators that reflect the progression of the disease in addition to the detection methods mentioned above.
5. Conclusion
As a new route of RCD, ferroptosis plays an important role in the prevention and treatment of various diseases. The process of ferroptosis is complex and involves a great number of metabolites and enzymes. However, the gold standard marker or detection method of ferroptosis has not yet been established. Therefore, it is crucial to choose the most suitable detection approaches for ferroptosis according to the purpose and experimental conditions. In this review, we summarize the commonly used reagents and detection methods in ferroptosis research to provide some guidance for follow-up research.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Zheyi Chen: Writing – original draft. Hongbing Lin: Writing – original draft. Xiaoyu Wang: Writing – review & editing. Guiqi Li: Software. Na Liu: Software. Manli Zhang: Supervision. Yuqin Shen: Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Contributor Information
Manli Zhang, Email: ZML1020@163.com.
Yuqin Shen, Email: 2020686041@gzhmu.edu.cn.
Abbreviations
- RCD
regulatory cell death
- ROS
reactive oxygen species
- MDA
malondialdehyde
- qPCR
quantitative polymerase chain reaction
- RT-qPCR
Real-time quantitative reverse transcription-PCR
- PUFAs
polyunsaturated fatty acids
- ACSL4
Acylcoenzyme A (CoA) synthetase long-chain family member 4
- LPCAT3
lysophosphatidylcholine acyltransferase 3
- ALOX
arachidonic acid lipoxygenase
- MUFA
monounsaturated fatty acid
- ACSL3
Acylcoenzyme A (CoA) synthetase long-chain family member 3
- SCD1
stearoyl-CoA desaturase 1
- PTGS2
Prostaglandin-endoperoxide synthase 2
- COX-2
cyclooxygenase-2
- ER
endoplasmic reticulum
- TFRC
Transferrin receptor protein
- STEAP3
six transmembrane epithelial antigen 3
- SLC11A2
solute carrier family 11 member 2
- DMT1
divalent metal-ion transporter-1
- FTL
ferritin light chain
- FTH1
ferritin heavy chain 1
- NCOA4
nuclear receptor coactivator 4
- PCBPs
Poly (RC)-binding proteins
- FPN
Ferroportin
- RNS
reactive nitrogen species
- GPX4
Glutathione peroxidase 4
- GSH
glutathione
- SLC7A11
family 7 member 11
- FSP1
Ferroptosis-suppressor-protein 1
- CoQ10H2
ubiquinol
- CoQ10
ubiquinone
- NADPH
nicotinamide adenine dinucleotide phosphate
- BH4
Tetrahydrobiopterin
- GCH1
GTP cyclohydrolase 1
- NRF2
Erythroid 2-related factor 2
- HIF
Hypoxic inducible factor
- DNA
Deoxyribonucleic acid
- LOOHs
lipid hydroperoxides
- 4HNE
4-hydroxynonenal
- FT
ferritin
- LIP
labile iron pool
- SOD
superoxide dismutase (SOD)
- MMT
3-(4, 5-dimethylthiazole-2)-2, 5-diphenyltetrazolium bromide
- CCK-8
cell counting kit-8
- PI
propidium iodide
- LDH
lactate dehydrogenase
- ATP
adenosine triphosphate
- OD
optical density
- mtDNA
Mitochondrial DNA
- TCA
tricarboxylic acid
- TEM
Transmission Electron Microscopy
- MGST1
microsomal glutathione S-transferase 1
- DIOC6
3,3' -Diethyloxyheterocarbonylcyanine iodine
- TMRM
Tetramethylrhodamine methyl ester
- ATF4
Activating transcription factor 4 (ATF4)
- eIF2α
eukaryotic initiation factor 2, α subunit
- NO
nitric oxide
- CTSB
Cathepsin B
- ARNTL
Recombinant Aryl Hydrocarbon Receptor Nuclear Translocator Like Protein
- LDs
lipid droplets
- ADA
adrenaline
- AA
arachidonic acid
- TFR1
Transferrin Receptor 1
- SLC3A2
solute carrier family 3 member 2
- GSS
glutathione synthetase
- PLOOHs
phospholipid hydroperoxides
- PLOHs
phospholipid alcohols
- GSSG
- Glutathione, Oxydized
- HPLC
high-performance liquid chromatography
- PGSK
Phen green SK
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- H2O2
hydrogen peroxide
- DHE
dihydroethidium
- DAF-FM Diacetate
4-Amino-5-Methylamino-2′,7′-Difluorofluorescein Diacetate
- TBARS
thiobarbituric acid-reactive substance
References
- 1.Tang D., et al. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang D., et al. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galluzzi L., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockwell B.R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185(14):2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolma S., et al. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 7.Yang W.S., Stockwell B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., et al. The function and mechanism of ferroptosis in cancer. Apoptosis. 2020;25(11–12):786–798. doi: 10.1007/s10495-020-01638-w. [DOI] [PubMed] [Google Scholar]
- 9.Gao M., et al. Role of mitochondria in ferroptosis. Mol. Cell. 2019;73(2):354–363.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng L., et al. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023;72(2):281–299. doi: 10.1007/s00011-022-01672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang R., et al. Ferroptosis in cancer progression. Cells. 2023;12(14) doi: 10.3390/cells12141820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan H., Talty R., Johnson C.H. Targeting ferroptosis to treat colorectal cancer. Trends Cell Biol. 2023;33(3):185–188. doi: 10.1016/j.tcb.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y., et al. Ferroptosis and its interaction with tumor immune microenvironment in liver cancer. Biochim. Biophys. Acta Rev. Canc. 2023;1878(1) doi: 10.1016/j.bbcan.2022.188848. [DOI] [PubMed] [Google Scholar]
- 14.Li D., et al. CST1 inhibits ferroptosis and promotes gastric cancer metastasis by regulating GPX4 protein stability via OTUB1. Oncogene. 2023;42(2):83–98. doi: 10.1038/s41388-022-02537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai W., et al. Alox15/15-HpETE aggravates myocardial ischemia-reperfusion injury by promoting cardiomyocyte ferroptosis. Circulation. 2023;147(19):1444–1460. doi: 10.1161/CIRCULATIONAHA.122.060257. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y., et al. Melatonin ameliorates neurological deficits through MT2/IL-33/ferritin H signaling-mediated inhibition of neuroinflammation and ferroptosis after traumatic brain injury. Free Radic. Biol. Med. 2023;199:97–112. doi: 10.1016/j.freeradbiomed.2023.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Sanz A.B., et al. Regulated cell death pathways in kidney disease. Nat. Rev. Nephrol. 2023;19(5):281–299. doi: 10.1038/s41581-023-00694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts J.Z., Crawford N., Longley D.B. The role of ubiquitination in apoptosis and necroptosis. Cell Death Differ. 2022;29(2):272–284. doi: 10.1038/s41418-021-00922-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyake S., et al. Identification of the hallmarks of necroptosis and ferroptosis by transmission electron microscopy. Biochem. Biophys. Res. Commun. 2020;527(3):839–844. doi: 10.1016/j.bbrc.2020.04.127. [DOI] [PubMed] [Google Scholar]
- 20.Bertheloot D., Latz E., Franklin B.S. Necroptosis, pyroptosis and apoptosis: an intricate game of cell death. Cell. Mol. Immunol. 2021;18(5):1106–1121. doi: 10.1038/s41423-020-00630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54(3):437–453. doi: 10.1016/j.immuni.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu P., et al. Pyroptosis: mechanisms and diseases. Signal Transduct. Targeted Ther. 2021;6(1):128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X., et al. Iron metabolism in ferroptosis. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.590226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapralov A.A., et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 2020;16(3):278–290. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H., et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020;22(2):225–234. doi: 10.1038/s41556-020-0461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon S.J., et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 2015;10(7):1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doll S., et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H.L., et al. PKCbetaII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat. Cell Biol. 2022;24(1):88–98. doi: 10.1038/s41556-021-00818-3. [DOI] [PubMed] [Google Scholar]
- 30.Sun W.Y., et al. Phospholipase iPLA(2)beta averts ferroptosis by eliminating a redox lipid death signal. Nat. Chem. Biol. 2021;17(4):465–476. doi: 10.1038/s41589-020-00734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eagle H. Nutrition needs of mammalian cells in tissue culture. Science. 1955;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- 32.Dixon S.J., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alvarez S.W., et al. Author Correction: NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2022;609(7929):E12. doi: 10.1038/s41586-022-05323-7. [DOI] [PubMed] [Google Scholar]
- 34.Müller F., et al. Elevated FSP1 protects KRAS-mutated cells from ferroptosis during tumor initiation. Cell Death Differ. 2022 doi: 10.1038/s41418-022-01096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie Y., et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagan V.E., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W.S., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ubellacker J.M., et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585(7823):113–118. doi: 10.1038/s41586-020-2623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dierge E., et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metabol. 2021;33(8):1701–1715 e5. doi: 10.1016/j.cmet.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Ma J., et al. The role of macrophage iron overload and ferroptosis in atherosclerosis. Biomolecules. 2022;12(11) doi: 10.3390/biom12111702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J., et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769):402–406. doi: 10.1038/s41586-019-1426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beatty A., et al. Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1. Nat. Commun. 2021;12(1):2244. doi: 10.1038/s41467-021-22471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magtanong L., et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. 2019;26(3):420–432.e9. doi: 10.1016/j.chembiol.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu B., et al. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019;21(5):579–591. doi: 10.1038/s41556-019-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma X.H., et al. ALOX15-launched PUFA-phospholipids peroxidation increases the susceptibility of ferroptosis in ischemia-induced myocardial damage. Signal Transduct. Targeted Ther. 2022;7(1):288. doi: 10.1038/s41392-022-01090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lamade A.M., et al. Inactivation of RIP3 kinase sensitizes to 15LOX/PEBP1-mediated ferroptotic death. Redox Biol. 2022;50 doi: 10.1016/j.redox.2022.102232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tesfay L., et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79(20):5355–5366. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua X., et al. ROS-Induced oxidative injury involved in pathogenesis of fungal keratitis via p38 MAPK activation. Sci. Rep. 2017;7(1) doi: 10.1038/s41598-017-09636-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao X., et al. miR-212-5p attenuates ferroptotic neuronal death after traumatic brain injury by targeting Ptgs2. Mol. Brain. 2019;12(1):78. doi: 10.1186/s13041-019-0501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Z., et al. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano. 2018;12(11):11355–11365. doi: 10.1021/acsnano.8b06201. [DOI] [PubMed] [Google Scholar]
- 51.Liu T., et al. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano. 2018;12(12):12181–12192. doi: 10.1021/acsnano.8b05860. [DOI] [PubMed] [Google Scholar]
- 52.Hong X., et al. The lipogenic regulator SREBP2 induces transferrin in circulating melanoma cells and suppresses ferroptosis. Cancer Discov. 2021;11(3):678–695. doi: 10.1158/2159-8290.CD-19-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song J.X., et al. Liraglutide attenuates hepatic iron levels and ferroptosis in db/db mice. Bioengineered. 2022;13(4):8334–8348. doi: 10.1080/21655979.2022.2051858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pei Z., et al. Inhibition of ferroptosis and iron accumulation alleviates pulmonary fibrosis in a bleomycin model. Redox Biol. 2022;57 doi: 10.1016/j.redox.2022.102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi Q., et al. Application of genomic selection and experimental techniques to predict cell death and immunotherapeutic efficacy of ferroptosis-related CXCL2 in hepatocellular carcinoma. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.998736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philpott C.C., Patel S.J., Protchenko O. Management versus miscues in the cytosolic labile iron pool: the varied functions of iron chaperones. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867(11) doi: 10.1016/j.bbamcr.2020.118830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chakrabarti M., et al. Speciation of iron in mouse liver during development, iron deficiency, IRP2 deletion and inflammatory hepatitis. Metallomics. 2015;7(1):93–101. doi: 10.1039/c4mt00215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lv H., Shang P. The significance, trafficking and determination of labile iron in cytosol, mitochondria and lysosomes. Metallomics. 2018;10(7):899–916. doi: 10.1039/c8mt00048d. [DOI] [PubMed] [Google Scholar]
- 59.Hou W., et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao M., et al. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen Y., et al. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic. Biol. Med. 2021;173:41–51. doi: 10.1016/j.freeradbiomed.2021.07.019. [DOI] [PubMed] [Google Scholar]
- 62.Kruger C., et al. The importance of aquaporin-8 for cytokine-mediated toxicity in rat insulin-producing cells. Free Radic. Biol. Med. 2021;174:135–143. doi: 10.1016/j.freeradbiomed.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Bersuker K., et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lang X., et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 2019;9(12):1673–1685. doi: 10.1158/2159-8290.CD-19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doll S., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 66.Kraft V.A.N., et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 2020;6(1):41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soula M., et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 2020;16(12):1351–1360. doi: 10.1038/s41589-020-0613-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23 doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harada N., et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch. Biochem. Biophys. 2011;508(1):101–109. doi: 10.1016/j.abb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Zheng J., et al. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs. 2014;12(7):4214–4230. doi: 10.3390/md12074214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo N., et al. Potential role of APEX1 during ferroptosis. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.798304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M., et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci. Adv. 2019;5(7):eaaw2238. doi: 10.1126/sciadv.aaw2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai Y., et al. Lipid storage and lipophagy regulates ferroptosis. Biochem. Biophys. Res. Commun. 2019;508(4):997–1003. doi: 10.1016/j.bbrc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 74.Jiang J.J., et al. Targeting mitochondrial ROS-mediated ferroptosis by quercetin alleviates high-fat diet-induced hepatic lipotoxicity. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.876550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Y., et al. The epigenetic regulators and metabolic changes in ferroptosis-associated cancer progression. Mol. Cancer. 2020;19(1):39. doi: 10.1186/s12943-020-01157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C., et al. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy. 2021;17(4):948–960. doi: 10.1080/15548627.2020.1739447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Y., et al. The toxicity mechanism of different sized iron nanoparticles on human breast cancer (MCF7) cells. Food Chem. 2021;341(Pt 2) doi: 10.1016/j.foodchem.2020.128263. [DOI] [PubMed] [Google Scholar]
- 78.Hao L., et al. Quantitative tracking of endoplasmic reticulum viscosity during ferroptosis by an iridium complex via TPPLM. Chem. Commun. 2021;57(41):5040–5042. doi: 10.1039/d1cc01062j. [DOI] [PubMed] [Google Scholar]
- 79.Tian R., et al. Genome-wide CRISPRi/a screens in human neurons link lysosomal failure to ferroptosis. Nat. Neurosci. 2021;24(7):1020–1034. doi: 10.1038/s41593-021-00862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X., et al. Organelle-specific regulation of ferroptosis. Cell Death Differ. 2021;28(10):2843–2856. doi: 10.1038/s41418-021-00859-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alborzinia H., et al. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun. Biol. 2018;1:210. doi: 10.1038/s42003-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su L.J., et al. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/5080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Outten F.W., Theil E.C. Iron-based redox switches in biology. Antioxidants Redox Signal. 2009;11(5):1029–1046. doi: 10.1089/ars.2008.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li D., Li Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Targeted Ther. 2020;5(1):108. doi: 10.1038/s41392-020-00216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S., et al. Double-edge sword roles of iron in driving energy production versus instigating ferroptosis. Cell Death Dis. 2022;13(1):40. doi: 10.1038/s41419-021-04490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 88.Ferreira C.A., et al. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018;11(10):4955–4984. doi: 10.1007/s12274-018-2092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Latunde-Dada G.O. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017;1861(8):1893–1900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 90.Stockwell B.R., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao W., et al. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Targeted Ther. 2022;7(1):196. doi: 10.1038/s41392-022-01046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng J., et al. Carnosic acid protects against ferroptosis in PC12 cells exposed to erastin through activation of Nrf2 pathway. Life Sci. 2021;266 doi: 10.1016/j.lfs.2020.118905. [DOI] [PubMed] [Google Scholar]
- 93.Yan B., et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol. Cell. 2021;81(2):355–369.e10. doi: 10.1016/j.molcel.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 94.Song Z., et al. Ruscogenin induces ferroptosis in pancreatic cancer cells. Oncol. Rep. 2020;43(2):516–524. doi: 10.3892/or.2019.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma S., et al. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7(7):e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crowley L.C., et al. Measuring cell death by propidium iodide uptake and flow cytometry. Cold Spring Harb. Protoc. 2016;2016(7) doi: 10.1101/pdb.prot087163. [DOI] [PubMed] [Google Scholar]
- 97.Liu Y., et al. Aquaporin 4 depolarization-enhanced transferrin infiltration leads to neuronal ferroptosis after subarachnoid hemorrhage in mice. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/8808677. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Kumar P., Nagarajan A., Uchil P.D. Analysis of cell viability by the lactate dehydrogenase assay. Cold Spring Harb. Protoc. 2018;2018(6) doi: 10.1101/pdb.prot095497. [DOI] [PubMed] [Google Scholar]
- 99.Xu P., et al. MicroRNA-15a promotes prostate cancer cell ferroptosis by inhibiting GPX4 expression. Oncol. Lett. 2022;23(2):67. doi: 10.3892/ol.2022.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang J., et al. Platycodin D regulates high glucose-induced ferroptosis of HK-2 cells through glutathione peroxidase 4 (GPX4) Bioengineered. 2022;13(3):6627–6637. doi: 10.1080/21655979.2022.2045834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sun L., et al. Lapatinib induces mitochondrial dysfunction to enhance oxidative stress and ferroptosis in doxorubicin-induced cardiomyocytes via inhibition of PI3K/AKT signaling pathway. Bioengineered. 2022;13(1):48–60. doi: 10.1080/21655979.2021.2004980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuan F., et al. The dual role of p62 in ferroptosis of glioblastoma according to p53 status. Cell Biosci. 2022;12(1):20. doi: 10.1186/s13578-022-00764-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mi Y., et al. Melatonin inhibits ferroptosis and delays age-related cataract by regulating SIRT6/p-Nrf2/GPX4 and SIRT6/NCOA4/FTH1 pathways. Biomed. Pharmacother. 2023;157 doi: 10.1016/j.biopha.2022.114048. [DOI] [PubMed] [Google Scholar]
- 104.Sun L., et al. Herceptin induces ferroptosis and mitochondrial dysfunction in H9c2 cells. Int. J. Mol. Med. 2022;49(2) doi: 10.3892/ijmm.2021.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Creed S., McKenzie M. Measurement of mitochondrial membrane potential with the fluorescent dye tetramethylrhodamine methyl ester (TMRM) Methods Mol. Biol. 2019;1928:69–76. doi: 10.1007/978-1-4939-9027-6_5. [DOI] [PubMed] [Google Scholar]
- 106.Tan Y., et al. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis. 2022;13(4):319. doi: 10.1038/s41419-022-04764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei H., et al. An endoplasmic reticulum-targeting fluorescent probe for the visualization of the viscosity fluctuations during ferroptosis in live cells. Anal. Chim. Acta. 2022;1232 doi: 10.1016/j.aca.2022.340454. [DOI] [PubMed] [Google Scholar]
- 108.Zhao C., et al. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic. Biol. Med. 2021;175:236–248. doi: 10.1016/j.freeradbiomed.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Zhang J., et al. YAP1 alleviates sepsis-induced acute lung injury via inhibiting ferritinophagy-mediated ferroptosis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.884362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuang F., et al. Cathepsin B is a mediator of organelle-specific initiation of ferroptosis. Biochem. Biophys. Res. Commun. 2020;533(4):1464–1469. doi: 10.1016/j.bbrc.2020.10.035. [DOI] [PubMed] [Google Scholar]
- 111.Alborzinia H., et al. Golgi stress mediates redox imbalance and ferroptosis in human cells. Commun. Biol. 2018;1:210. doi: 10.1038/s42003-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Javadov S. Mitochondria and ferroptosis. Curr Opin Physiol. 2022;25 doi: 10.1016/j.cophys.2022.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deng L., et al. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2022:1–19. doi: 10.1007/s00011-022-01672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tadokoro T., et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5(9) doi: 10.1172/jci.insight.132747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma C.S., et al. NRF2-GPX4/SOD2 axis imparts resistance to EGFR-tyrosine kinase inhibitors in non-small-cell lung cancer cells. Acta Pharmacol. Sin. 2021;42(4):613–623. doi: 10.1038/s41401-020-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuang F., et al. MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic cancer cells. Cell Chem. Biol. 2021;28(6):765–775.e5. doi: 10.1016/j.chembiol.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 117.Abe K., et al. Doxorubicin causes ferroptosis and cardiotoxicity by intercalating into mitochondrial DNA and disrupting Alas1-dependent heme synthesis. Sci. Signal. 2022;15(758):eabn8017. doi: 10.1126/scisignal.abn8017. [DOI] [PubMed] [Google Scholar]
- 118.Du J., et al. Identification of Frataxin as a regulator of ferroptosis. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang P., et al. Mitochondria-related ferroptosis drives cognitive deficits in neonatal mice following sevoflurane administration. Front. Med. 2022;9 doi: 10.3389/fmed.2022.887062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carrageta D.F., et al. Evaluation of human spermatozoa mitochondrial membrane potential using the JC-1 dye. Curr Protoc. 2022;2(9):e531. doi: 10.1002/cpz1.531. [DOI] [PubMed] [Google Scholar]
- 121.Yang C., Wang X. Lysosome biogenesis: regulation and functions. J. Cell Biol. 2021;220(6) doi: 10.1083/jcb.202102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J., et al. Tumor heterogeneity in autophagy-dependent ferroptosis. Autophagy. 2021;17(11):3361–3374. doi: 10.1080/15548627.2021.1872241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rizzollo F., et al. The lysosome as a master regulator of iron metabolism. Trends Biochem. Sci. 2021;46(12):960–975. doi: 10.1016/j.tibs.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 124.Pillai-Kastoori L., Schutz-Geschwender A.R., Harford J.A. A systematic approach to quantitative Western blot analysis. Anal. Biochem. 2020;593 doi: 10.1016/j.ab.2020.113608. [DOI] [PubMed] [Google Scholar]
- 125.Shi L., et al. MiR-20a-5p alleviates kidney ischemia/reperfusion injury by targeting ACSL4-dependent ferroptosis. Am. J. Transplant. 2023;23(1):11–25. doi: 10.1016/j.ajt.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 126.Feng H., et al. Human umbilical cord mesenchymal stem cells ameliorate erectile dysfunction in rats with diabetes mellitus through the attenuation of ferroptosis. Stem Cell Res. Ther. 2022;13(1):450. doi: 10.1186/s13287-022-03147-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luis G., et al. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xie Y., et al. Mammary adipocytes protect triple-negative breast cancer cells from ferroptosis. J. Hematol. Oncol. 2022;15(1):72. doi: 10.1186/s13045-022-01297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ren T., et al. Zoledronic acid induces ferroptosis by reducing ubiquinone and promoting HMOX1 expression in osteosarcoma cells. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1071946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen J., et al. Ginkgolide B alleviates oxidative stress and ferroptosis by inhibiting GPX4 ubiquitination to improve diabetic nephropathy. Biomed. Pharmacother. 2022;156 doi: 10.1016/j.biopha.2022.113953. [DOI] [PubMed] [Google Scholar]
- 131.Santana-Codina N., Mancias J.D. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals. 2018;11(4) doi: 10.3390/ph11040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou H., et al. NCOA4-mediated ferritinophagy is involved in ionizing radiation-induced ferroptosis of intestinal epithelial cells. Redox Biol. 2022;55 doi: 10.1016/j.redox.2022.102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bao W.D., et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer's disease. Cell Death Differ. 2021;28(5):1548–1562. doi: 10.1038/s41418-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Feng Z., et al. NMN recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation induced skin injury. Biochim. Biophys. Acta, Mol. Basis Dis. 2022;1868(1) doi: 10.1016/j.bbadis.2021.166287. [DOI] [PubMed] [Google Scholar]
- 135.Yuan Y., et al. Kaempferol ameliorates oxygen-glucose deprivation/reoxygenation-induced neuronal ferroptosis by activating nrf2/slc7a11/GPX4 Axis. Biomolecules. 2021;11(7) doi: 10.3390/biom11070923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Koppula P., et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 2022;13(1):2206. doi: 10.1038/s41467-022-29905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang Y., et al. CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J. Exp. Clin. Cancer Res. 2022;41(1):307. doi: 10.1186/s13046-022-02518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hou J., et al. ACSL4 as a potential target and biomarker for anticancer: from molecular mechanisms to clinical therapeutics. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.949863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Küch E.M., et al. Differentially localized acyl-CoA synthetase 4 isoenzymes mediate the metabolic channeling of fatty acids towards phosphatidylinositol. Biochim. Biophys. Acta. 2014;1841(2):227–239. doi: 10.1016/j.bbalip.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 140.Lee J.Y., et al. Lipid metabolism and ferroptosis. Biology. 2021;10(3) doi: 10.3390/biology10030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Quan J., Bode A.M., Luo X. ACSL family: the regulatory mechanisms and therapeutic implications in cancer. Eur. J. Pharmacol. 2021;909 doi: 10.1016/j.ejphar.2021.174397. [DOI] [PubMed] [Google Scholar]
- 142.Tang S., et al. Olaparib synergizes with arsenic trioxide by promoting apoptosis and ferroptosis in platinum-resistant ovarian cancer. Cell Death Dis. 2022;13(9):826. doi: 10.1038/s41419-022-05257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feng H., et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 2020;30(10):3411–3423.e7. doi: 10.1016/j.celrep.2020.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang H., et al. Ferrostatin-1 ameliorates liver dysfunction via reducing iron in thioacetamide-induced acute liver injury in mice. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.869794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li T., et al. Resveratrol protects against myocardial ischemia-reperfusion injury via attenuating ferroptosis. Gene. 2022;808 doi: 10.1016/j.gene.2021.145968. [DOI] [PubMed] [Google Scholar]
- 146.Li Y., et al. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod. 2021;36(4):951–964. doi: 10.1093/humrep/deaa363. [DOI] [PubMed] [Google Scholar]
- 147.Seibt T.M., Proneth B., Conrad M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019;133:144–152. doi: 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 148.Kobayashi S., et al. Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function. J. Biol. Chem. 2015;290(14):8778–8788. doi: 10.1074/jbc.M114.625053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mandal P.K., et al. System x(c)- and thioredoxin reductase 1 cooperatively rescue glutathione deficiency. J. Biol. Chem. 2010;285(29):22244–22253. doi: 10.1074/jbc.M110.121327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang J., et al. Qing-Xin-Jie-Yu Granule inhibits ferroptosis and stabilizes atherosclerotic plaques by regulating the GPX4/xCT signaling pathway. J. Ethnopharmacol. 2023;301 doi: 10.1016/j.jep.2022.115852. [DOI] [PubMed] [Google Scholar]
- 152.Tang D., Kroemer G. Ferroptosis. Curr Biol. 2020;30(21):R1292–r1297. doi: 10.1016/j.cub.2020.09.068. [DOI] [PubMed] [Google Scholar]
- 153.Leu J.I., Murphy M.E., George D.L. Targeting ErbB3 and cellular NADPH/NADP(+) abundance sensitizes cutaneous melanomas to ferroptosis inducers. ACS Chem. Biol. 2022;17(5):1038–1044. doi: 10.1021/acschembio.2c00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Doll S., et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 155.Hu Q., et al. Blockade of GCH1/BH4 Axis activates ferritinophagy to mitigate the resistance of colorectal cancer to erastin-induced ferroptosis. Front. Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.810327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang L., et al. Transferrin receptor-mediated reactive oxygen species promotes ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN induced kinase 1/acyl-CoA synthetase long chain family member 4 signaling. Bioengineered. 2021;12(1):4983–4994. doi: 10.1080/21655979.2021.1956403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang M., et al. COMMD10 inhibits HIF1α/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J. Hepatol. 2022;76(5):1138–1150. doi: 10.1016/j.jhep.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 158.Ouyang S., et al. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022;52 doi: 10.1016/j.redox.2022.102317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Z., et al. Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis. Biomed. Pharmacother. 2022;154 doi: 10.1016/j.biopha.2022.113572. [DOI] [PubMed] [Google Scholar]
- 160.Chen C., et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021;12(1):65. doi: 10.1038/s41419-020-03362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.He G.N., et al. Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des. Dev. Ther. 2021;15:3965–3978. doi: 10.2147/DDDT.S332847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ma L., et al. A benzothiazole-based fluorescence probe for imaging of peroxynitrite during ferroptosis and diagnosis of tumor tissues. Anal. Bioanal. Chem. 2022;414(27):7753–7762. doi: 10.1007/s00216-022-04307-w. [DOI] [PubMed] [Google Scholar]
- 163.He F., et al. Regulation of ACSL4-catalyzed lipid peroxidation process resists cisplatin ototoxicity. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/3080263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu P., et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett. 2020;25:10. doi: 10.1186/s11658-020-00205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Han F., et al. Interleukin-6 promotes ferroptosis in bronchial epithelial cells by inducing reactive oxygen species-dependent lipid peroxidation and disrupting iron homeostasis. Bioengineered. 2021;12(1):5279–5288. doi: 10.1080/21655979.2021.1964158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eruslanov E., Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol. Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 167.Yazdani M. Concerns in the application of fluorescent probes DCDHF-DA, DHR 123 and DHE to measure reactive oxygen species in vitro. Toxicol. Vitro. 2015;30(1 Pt B):578–582. doi: 10.1016/j.tiv.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 168.Zeng T., et al. Indoleamine 2, 3-dioxygenase 1enhanceshepatocytes ferroptosis in acute immune hepatitis associated with excess nitrative stress. Free Radic. Biol. Med. 2020;152:668–679. doi: 10.1016/j.freeradbiomed.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 169.Wang F., et al. Fundamental mechanisms of the cell death caused by nitrosative stress. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.742483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rubbo H., et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994;269(42):26066–26075. [PubMed] [Google Scholar]
- 171.O'Donnell V.B., et al. Nitration of unsaturated fatty acids by nitric oxide-derived reactive species. Methods Enzymol. 1999;301:454–470. doi: 10.1016/s0076-6879(99)01109-x. [DOI] [PubMed] [Google Scholar]
- 172.Dai Z., et al. Probing lipid peroxidation in ferroptosis: emphasizing the utilization of C11-BODIPY-based protocols. Methods Mol. Biol. 2023;2712:61–72. doi: 10.1007/978-1-0716-3433-2_6. [DOI] [PubMed] [Google Scholar]
- 173.Shao C.J., et al. Downregulation of miR-221-3p promotes the ferroptosis in gastric cancer cells via upregulation of ATF3 to mediate the transcription inhibition of GPX4 and HRD1. Transl Oncol. 2023;32 doi: 10.1016/j.tranon.2023.101649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gaschler M.M., Stockwell B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017;482(3):419–425. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal. Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 176.Wang Y., et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021;28:231–243. doi: 10.1016/j.jare.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wu Y., et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard Mater. 2022;426 doi: 10.1016/j.jhazmat.2021.127807. [DOI] [PubMed] [Google Scholar]
- 178.Florido J., et al. Melatonin drives apoptosis in head and neck cancer by increasing mitochondrial ROS generated via reverse electron transport. J. Pineal Res. 2022;73(3) doi: 10.1111/jpi.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kma L., Baruah T.J. The interplay of ROS and the PI3K/Akt pathway in autophagy regulation. Biotechnol. Appl. Biochem. 2022;69(1):248–264. doi: 10.1002/bab.2104. [DOI] [PubMed] [Google Scholar]
- 180.Yang F., et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metabol. 2023;35(1):84–100.e8. doi: 10.1016/j.cmet.2022.09.021. [DOI] [PubMed] [Google Scholar]
- 181.Fang X., et al. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023;20(1):7–23. doi: 10.1038/s41569-022-00735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.