Abstract
An open reading frame located in the bisC-cspA intergenic region, or at 80.1 min on the Escherichia coli chromosome, encodes a hypothetical 2-hydroxyacid dehydrogenase, which was identified as a result of the E. coli Genome Sequencing Project. We report here that the product of the gene (yiaE) is a 2-ketoaldonate reductase (2KR). The gene was cloned and expressed with a C-terminal His tag in E. coli, and the protein was purified by metal-chelate affinity chromatography. The determination of the NH2-terminal amino acid sequence of the protein defined the translational start site of this gene. The enzyme was found to be a 2KR catalyzing the reduction of 2,5-diketo-d-gluconate to 5-keto-d-gluconate, 2-keto-d-gluconate (2KDG) to d-gluconate, 2-keto-l-gulonate to l-idonate. The reductase was optimally active at pH 7.5, with NADPH as a preferred electron donor. The deduced amino acid sequence showed 69.4% identity with that of 2KR from Erwinia herbicola. Disruption of this gene on the chromosome resulted in the loss of 2KR activity in E. coli. E. coli W3110 was found to grow on 2KDG, whereas the mutant deficient in 2KR activity was unable to grow on 2KDG as the carbon source, suggesting that 2KR is responsible for the catabolism of 2KDG in E. coli and the diminishment of produced 2KDG from d-gluconate in the cultivation of E. coli harboring a cloned gluconate dehydrogenase gene.
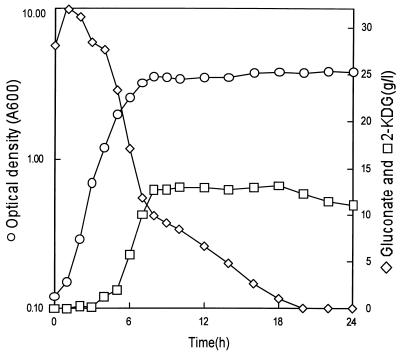
We previously reported the cloning and expression of a gene cluster encoding three subunits of membrane-bound gluconate dehydrogenase (GADH) from Erwinia cypripedii in Escherichia coli (26). In the course of further study on the conversion of d-gluconate to 2-keto-d-gluconate (2KDG) with a recombinant E. coli strain, we observed that the level of 2KDG produced in the medium gradually decreased after the exhaustion of d-gluconate in the medium (see Fig. 1). In an effort to find the reason, the NADPH-dependent reductase activity catalyzing the conversion of 2KDG to d-gluconate was detected in extracts of E. coli cells. This result suggested the existence of enzymes involved in ketogluconate metabolism in E. coli, as reported for several species of the genera Corynebacterium, Brevibacterium, Erwinia, Acetobacter, Gluconobacter, Serratia, and Pseudomonas (20, 23, 25). In Erwinia, Acetobacter, Gluconobacter, Serratia, and Pseudomonas, oxidation of glucose to ketogluconates such as 2KDG, 5-keto-d-gluconate (5KDG), and 2,5-diketo-d-gluconate (25DKG) has been shown to proceed via membrane-bound dehydrogenases, which are linked to the electron transport chain (2, 21). The ketogluconates or their phosphorylated forms are unique substrates in that they enter into central metabolism only after they are reduced by NADPH-dependent reductases (20, 23). NADPH-dependent 2-ketoaldonate reductase (2KR), which catalyzes the reduction of 2KDG to d-gluconate, 25DKG to 5KDG, and 2-keto-l-gulonate (2KLG) to l-idonate (IA), has been purified and characterized from Brevibacterium ketosoreductum (25) and Erwinia herbicola (23). Even if the substrate specificity has not been examined with 25DKG as a substrate, 2KDG reductases from acetic acid bacteria also catalyze the reduction of 2KLG to IA as well as of 2KDG to d-gluconate (1).
FIG. 1.
Time course of bioconversion of d-gluconate to 2KDG by E. coli harboring the cloned GADH gene. E. coli W3110(pGA313) was grown in a 2-liter fermentor at 37°C with aeration at 1 vvm and agitation at 500 rpm.
Until now, no ketoaldonate reductase has been reported for E. coli. We report here that the product of the yiaE gene, located in the bisC-cspA intergenic region at 80.1 min on the E. coli chromosome, is a 2KR; in addition, the diminishment of produced 2KDG from d-gluconate in the cultivation of recombinant E. coli harboring a cloned membrane-bound GADH gene is due to 2KR as the cytosolic enzyme responsible for conversion of 2KDG to d-gluconate. We found also that E. coli W3110 grows on 2KDG as the sole carbon source.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli W3110 (22) and DH5α [F− endA1 hsdR17 (rK− mK+) supE44 thi-1 recA1 gyrA relA1 Δ(argF-lac)U169 deoR φ80lacZΔM15] (16) were used as host strains. Strain JC7623 (recBC sbcBC) (24) was used for site-directed insertion mutagenesis. Strain W3110 (yiaE::Km) was constructed by P1 transduction of a yiaE::Km allele into strain W3110. E. coli strains were routinely grown at 37°C in Luria broth (LB) or M9 minimal medium (16) with carbohydrate. For mutant characterization, M9 medium with 2KDG as the carbon source was used. Where appropriate, ampicillin (100 μg/ml) and kanamycin (15 μg/ml) were included in the growth media.
Bioconversion of d-gluconate to 2KDG by recombinant E. coli harboring the cloned GADH gene.
The seed culture of recombinant E. coli W3110(pGA313) (26) was grown in EP medium, which consists of 0.5 g of yeast extract (Difco), 0.3 g of peptone (Difco), 0.01 g of KH2PO4, 0.05 g of NaCl, and 0.1 g of NH4Cl (pH 7.0) in 100 ml of distilled water. The flask was inoculated with cells obtained from a fresh plate of a strain, followed by incubation at 37°C for 12 h on a rotary shaker. The 50 ml of seed culture of recombinant E. coli was inoculated into 1 liter of EP medium containing 30 g of d-gluconate/liter in a 2-liter fermentor and cultivated at 37°C for 24 h with aeration at 1 vvm and agitation at 500 rpm. Bacterial growth was measured by the optical density at 600 nm.
DNA preparation and manipulation.
Total DNA from E. coli was prepared by using QIAGEN Genomic Tips. DNAs of the vector plasmids were prepared by a rapid alkaline lysis procedure (5). General DNA manipulations were carried out as described by Maniatis et al. (16). DNA sequencing of both strands was performed with an ABI373 automated sequencer with dye-labelled terminators (Applied Biosystems Division of Perkin-Elmer). Oligonucleotides were synthesized by Bioneer (Chungweon, Korea).
Enzyme assay and determination of d-gluconate, 2KDG, 5KDG, 2KLG, 25DKG, and IA.
2KR activity was assayed as described previously (18). The reaction was monitored for an initial decrease in absorbance at 340 nm (ɛ = 6.22 mM−1 cm−1). One unit of activity corresponds to the production of 1 μmol of NADP+ per min. The protein concentration of each sample was determined by the BCA protein assay kit (Pierce). d-Gluconate, 2KDG, 5KDG, 2KLG, 25DKG, and IA in the reaction mixtures were determined by high-pressure liquid chromatography (HPLC) with an HPX-87C column (Bio-Rad) at 30°C at a flow rate of 0.5 ml of 0.008 N H2SO4 per min as the eluent.
Cloning of the yiaE gene.
The design of the primers (2KRA-5′ [5′ACGGGTGGTCACGACCTGAACAT3′] as the forward primer and 2KRA-3′ [5′ATGAACGGTTCGCTGGGTGTGCT3′] as the reverse primer [see Fig. 2]) for PCR was based on the published yiaE nucleotide sequence (GenBank accession no. AE000432) (6). PCR was carried out in a GeneAmp PCR system 2400 (Perkin-Elmer) with 30 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 65°C, and extension for 2 min at 72°C, followed by a 5-min extension period at 72°C. The PCR products were cleaved with BclI, and the 1.5-kb DNA fragment was ligated into pUC19 which had been digested with BamHI. The resulting plasmid, designated pHD2, was sequenced to confirm that the sequence of the insert was identical to that of the yiaE gene.
FIG. 2.
Nucleotide sequence of the yiaE gene coding for 2KR. The putative ribosome-binding site (SD), the putative −10 and −35 regions of the promoter site, and the 22 residues of 2KR determined by NH2-terminal amino acid sequencing are underlined. Facing arrows show an inverted repeat. Primers 2KRA-5′ and 2KRA-3′ were used for PCR.
Disruption of the yiaE gene.
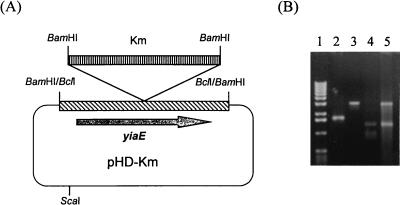
To construct the yiaE disruption strain, plasmid pHD-Km, with kanamycin resistance, was constructed. To introduce the 1.2-kb BamHI fragment (Km, Tn903) of pUC4K (Pharmacia) in the middle of the yiaE gene, a new BamHI site was generated by PCR with oligonucleotides 5′TGCGCACGTGGATCCAGCGCCAT3′ and 5′CTTTGGCTTCAACATGCCCATCCTC3′ (the nucleotide positions correspond to nucleotides [nt] 838 to 860 and 861 to 885, respectively; the BamHI restriction site is underlined, and the point-mutated position is shown in boldface type). PCR was carried out with pHD2 as a template. The PCR product was ligated after polynucleotide kinase treatment. The resulting plasmid, pHD-Bam, was digested with BamHI and ligated with the 1.4-kb BamHI fragment (Km) of pUC4K. An insertion mutation generated in the plasmid-encoded yiaE gene was used to generate a chromosomal mutation in E. coli. Plasmid pHD-Km, linearized with ScaI, was used to transform JC7623 (recBC sbcBC) to Kmr by the procedure outlined by Winans et al. (24). The Kmr-carrying fragment integrated into the chromosome was transferred by P1-mediated transduction (17) to strain W3110. Transductants were screened for Kmr colonies, and the yiaE::Km disruption in the chromosome was confirmed by PCR with primers 2KRA-5′ and 2KRA-3′ (see Fig. 4B).
FIG. 4.
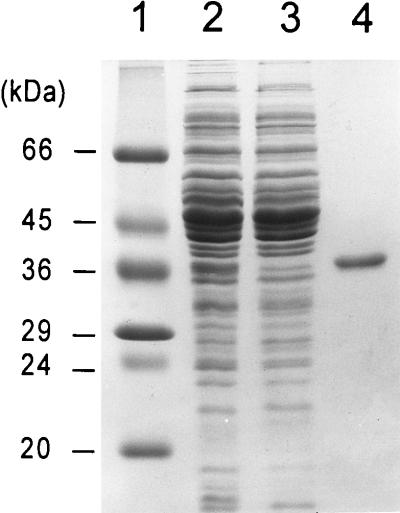
SDS–12.5% PAGE monitoring purification of E. coli His6-tagged 2KR from E. coli DH5α(pUCHisC), as described in Materials and Methods. Lane 1, molecular mass markers (Sigma); lane 2, whole-cell lysate; lane 3, fraction passed through column; lane 4, eluate from Ni-NTA column.
Purification of C-terminal His6-tagged 2KR.
For purification of 2KR in E. coli, a plasmid which adds a six-histidine tag onto the C terminus of the yiaE gene product was constructed. 2KRA-5′, the primer for cloning, was used as a forward primer. The reverse PCR primer, 5′GGGgaattcAGTGATGGTGATGGTGATGGTCCGCGACGTGCGGATTCACAC3′, contained an EcoRI restriction site (lowercase), a complementary C-terminal nucleotide sequence of yiaE (underlined), and an additional sequence encoding His6 (boldface type, including the sequence tcA, which is complementary to the stop codon). PCR was carried out under the same conditions as used for cloning. The PCR product was cleaved with BclI and EcoRI, and the 1.4-kb DNA fragment was ligated into pUC19 which had been digested with BamHI and EcoRI. The resulting plasmid, designated pUCHisC, was verified to contain the published yiaE nucleotide sequences.
The His6-tagged fusion protein was purified from recombinant E. coli cells by using Ni-nitrilotriacetic acid (NTA) resin (QIAGEN). Centrifugations were carried out at 4°C, and column chromatographies were carried out at room temperature. For 2KR purification, the cell pellet from a 500-ml culture of E. coli DH5α(pUCHisC) was suspended in a solution of 20 ml of 50 mM Na-phosphate (pH 8.0) and 0.3 M NaCl and sonicated on ice. The resulting cell lysate was centrifuged at 16,000 × g and was passed directly over a column containing 1.6 ml of Ni-NTA resin (QIAGEN). After the column was washed with a solution of 30 ml of 50 mM Na-phosphate (pH 8.0), 0.3 M NaCl, and 10% glycerol, the C-terminal His6-tagged 2KR was eluted with 4 ml of 50 mM Na-citrate buffer (pH 6.0). Sodium dodecyl sulfate (SDS)-polyacrylamide slab gel electrophoresis was done by Laemmli’s method (14).
NH2-terminal amino acid sequence of 2KR.
Purified protein on SDS-polyacrylamide gels was electroblotted onto a polyvinylidene difluoride (PVDF) transfer membrane (Millipore) for 1 h at 100 V in a Bio-Rad Trans-Blot apparatus. The PVDF membrane was stained with 0.1% Coomassie blue R-250 in 50% (vol/vol) methanol for 30 s and then destained for 3 min with 10% (vol/vol) acetic acid in 50% methanol. The NH2-terminal amino acid sequence of the 2KR, immobilized on a PVDF membrane, was determined with an Applied Biosystems model 470A sequencer.
RESULTS AND DISCUSSION
Bioconversion of d-gluconate to 2KDG by recombinant E. coli harboring the GADH gene and 2KDG reduction activity.
Since the ketogluconate metabolism in E. coli is not known, we considered the possibility of using E. coli as an efficient host strain for bioconversion processing of d-gluconate to 2KDG with the cloned GADH gene (26). Unexpectedly, as shown in Fig. 1, the produced 2KDG in the medium decreased slightly after 18 h of cultivation. This time point coincided with the point at which d-gluconate was depleted in the medium, which indicated that 2KDG could be used as a carbon source. In Erwinia sp., 2KDG can be converted to d-gluconate by NAD(P)H-dependent reduction, and d-gluconate is phosphorylated to 6-phosphogluconate and further metabolized through the pentose phosphate pathway (23). Therefore, we checked the 2KDG reduction activity of E. coli cell extracts. As a result, NADPH-dependent reductase activity catalyzing the conversion of 2KDG to d-gluconate was detected in the extracts of E. coli cells. The activity also catalyzed the reduction of 25DKG to 5KDG and 2KLG to IA. Therefore, we expected the existence of 2KR in E. coli.
Characterization of the putative 2-hydroxyacid dehydrogenase gene (yiaE) of E. coli.
Because the E. coli Genome Sequencing Project has been completed (6, 19), we tried to find putative hydroxyacid reductases or dehydrogenases among unidentified proteins encoded by E. coli genes to find the gene encoding an enzyme catalyzing the conversion of 2KDG to d-gluconate. As a result, a putative 2-hydroxyacid dehydrogenase gene (yiaE) (6, 19), showing homology with phosphoglycerate dehydrogenase and hydroxypyruvate reductase, was found in the bisC-cspA intergenic region, or at 80 min on the E. coli chromosome. To characterize the protein encoded by yiaE, the gene was amplified by PCR with chromosomal DNA from E. coli W3110 as the template. Cell extracts of recombinant E. coli harboring pHD2 showed 2KDG reductase activity at about 10 times the level of wild-type E. coli. This activity was also found to catalyze the reduction of 25DKG to 5KDG and 2KLG to IA. As a result, the enzyme encoded by yiaE was designated 2KR. The open reading frame corresponding to yiaE might start with either the ATG at nt 355 to 357 or the ATG at nt 367 to 369 (Fig. 2). The ATG at nt 367 to 369 appears to be a functional initiator because it is preceded by a possible ribosome-binding sequence, GGAG (nt 357 to 360). The gene consists of 972 bp, encoding a polypeptide of 324 amino acids and a calculated molecular weight of 35,399. In the GenBank/EMBL/DDBJ database, the sequence has 328 amino acids, rather than the 324 amino acids that we have determined. The deduced amino acid sequence of 2KR showed 69.4% identity to that of 2KR (3) (the sequence has not been deposited in the databases) from E. herbicola (Fig. 3). The amino acid sequence of a Bacillus subtilis hypothetical protein (EMBL accession no. Z99121) similar to glycerate dehydrogenase showed a high level of similarity (44.4%) to that of 2KR. Therefore, we are characterizing the protein of B. subtilis to examine the possibility that it could also be a 2KR. Comparison of the predicted amino acid sequence of 2KR with those of other oxidoreductases, including glycerate dehydrogenase of Hyphomicrobium metylovorum (12), d-3-phosphoglycerate dehydrogenase of Haemophilus influenzae (10), formate dehydrogenase of Solanum tuberosum (9), and d-lactate dehydrogenase of E. coli (8), showed identities of 26.8, 25.0, 23.1, and 18.8%, respectively, for the entire amino acid sequences. Other reductases related to ketogluconate metabolism, two 25DKG reductases from Corynebacterium sp. (4, 11) and a 5KDG reductase from Gluconobacter oxydans (13), did not show significant similarity to 2KR.
FIG. 3.
Sequence alignment of 2KRs from E. coli and E. herbicola. Identical amino acids are indicated by asterisks.
Purification and characterization of His6-tagged 2KR.
The His-tagged protein was purified by metal-chelate affinity chromatography on a Ni-NTA column. Figure 4 shows SDS-polyacrylamide gel electrophoresis (PAGE) of various fractions during purification of the His-tagged E. coli 2KR. The elution volumes of the purified enzyme were compared with those of standard proteins following Sephacryl S-200 gel filtration, and this comparison showed that the molecular weight of the protein was about 74,000 (data not shown). Following SDS-PAGE, the protein yielded a single band when stained with Coomassie blue (Fig. 4). The molecular weight was estimated to be 36,000, which coincided with the calculated molecular weight of the protein deduced from the nucleotide sequence. These results indicate that the native enzyme may exist as a dimer. The molecular weight and subunit structure of 2KR from E. coli are similar to those of 2KR from B. ketosoreductum (25). The NH2-terminal 22-amino-acid sequence was determined to be NH2-Met-Lys-Pro-Ser-Val-Ile-Leu-Tyr-Lys-Ala-Leu-Pro-Asp-Asp-Leu-Leu-Gln-Arg-Leu-Gln-Glu-His, which was identical to deduced amino acid residues 1 to 22 (Fig. 2). This result shows that the ATG at nt 367 to 369, preceded by a possible ribosome-binding sequence, GGAG (nt 357 to 360), rather than the ATG at nt 355 to 357, is a functional initiator. The reductase was optimally active at pH 7.5, with NADPH as a preferred electron donor. The 2KR in this work was found to catalyze the reduction of 25DKG to 5KDG, 2KDG to d-gluconate, and 2KLG to IA. The reductase was inactive toward 5KDG, d-fructose, and l-sorbose in the presence of NADPH or NADH. The substrate specificity of 2KR is similar to those from E. herbicola (23) and B. ketosoreductum (25).
Disruption and complementation of the yiaE gene.
We disrupted the chromosomal yiaE gene by homologous recombination to make a host strain in which 2KDG is not metabolized when the conversion of d-glucose or d-gluconate to 2KDG by recombinant E. coli harboring the cloned GADH gene (26) is attempted. For this purpose, we constructed pHD-Km (Fig. 5A), containing a kanamycin resistance gene in the coding sequence of yiaE on pUC19. Insertional disruption generated in the plasmid-encoded yiaE was used to generated chromosomal disruption. The linearized pHD-Km was used to transform E. coli JC7623. About 50 kanamycin-resistant colonies were isolated, and 5 colonies were picked. Insertion of the kanamycin resistance gene into the yiaE gene was confirmed by PCR with primers 2KRA-5′ and 2KRA-3′ (Fig. 5B). The inserted DNA sequence was transferred to strain W3110 by bacteriophage P1-mediated transduction. The functional disruption of the yiaE chromosomal gene was confirmed by an in vitro activity assay for 2KR, in vitro conversion of 2KDG to d-gluconate, and PCR. Disruption of the gene on the chromosome resulted in the loss of 2KR activity in strains JC7623 (yiaE::Km) and W3110 (yiaE::Km) (Table 1). Further confirmation of chromosomal disruption was evident based on complementation experiments. In order to test for the ability of the yiaE gene to complement the 2KR-deficient phenotype of strain W3110 (yiaE::Km), plasmid pHD2 was introduced into the mutant. Plasmid pHD2 restored 2KR activity (Table 1).
FIG. 5.
Disruption of the chromosomal yiaE gene of E. coli. (A) Structure of pHD-Km. A BamHI site was generated in the middle of the yiaE gene, and a 1.4-kb BamHI fragment containing the kanamycin resistance gene was inserted at the BamHI site of pHD-Bam. The resulting plasmid, pHD-Km, was linearized with ScaI before P1 transduction. (B) Confirmation of correct disruption of the yiaE gene by PCR. PCR with primers 2KRA-5′ and 2KRA-3′ (Fig. 2) was done with chromosomal DNAs from E. coli W3110 and its mutant strain as templates. Lane 1, 1-kb ladder; lane 2, PCR product with W3110 genomic DNA as a template; lane 3, PCR product with W3110 (yiaE::Km) genomic DNA as a template; lane 4, BamHI-digested PCR product with W3110 (yiaE::Km) genomic DNA; lane 5, pUC4K digested with BamHI.
TABLE 1.
Disruption and complementation analysis of the yiaE gene
| Straina | 2KR activity (U/mg of protein) | Conversion of 2KDG to d-gluconateb | Growth on a 2KDG platec |
|---|---|---|---|
| JC7623 | 0.042 | + | − |
| JC7623 (yiaE::Km) | 0 | − | − |
| W3110 | 0.009 | + | + |
| W3110 (yiaE::Km) | 0 | − | − |
| W3110 (yiaE::Km)(pHD2) | 0.390 | + | + |
Strains were cultured for 18 h at 37°C in LB. Cells were then harvested, and 2KR activity was measured by using 2KDG as a substrate.
Conversion was done with crude cell extracts and the reaction products were determined by HPLC, as described in Materials and Methods.
M9 medium with 2KDG (5 g/liter) as the carbon source.
Growth of E. coli on 2KDG.
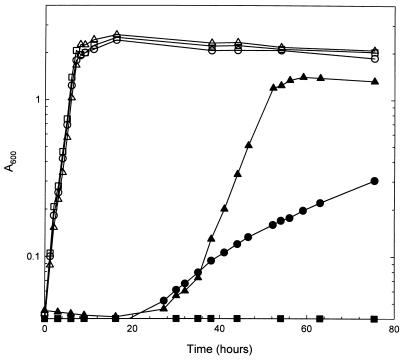
The metabolism related to ketogluconate use has been characterized in acetic acid bacteria and in Erwinia sp., which produce ketogluconates as incomplete oxidation products of glucose via membrane-bound dehydrogenases (2, 21). The metabolic pathways involved in the use of such ketogluconates in Corynebacterium and Erwinia spp. have been studied (20, 23). The pathways in the two microorganisms are quite similar except that in Erwinia sp., 25DKG is converted to 5KDG, but in Corynebacterium sp., 25DKG is converted to 2KDG before being converted to gluconate. As ketogluconates are used through the pentose phosphate pathway in acetic acid bacteria, the ketogluconate reductases have been presumed to function in regenerating NADP+ rather than in providing carbon (1, 15). The ketogluconate metabolism in E. coli has been unknown, and it has been reported that no strain of E. coli utilizes 2KDG as the sole carbon source (7). However, in our experiment, E. coli W3110 grew on M9 medium containing 2KDG as the sole carbon source while the mutant W3110 (yiaE::Km), deficient in 2KR activity, was unable to grow on 2KDG (Table 1). This result indicated that 2KDG enters into central metabolism only after it is reduced to d-gluconate by 2KR. Thus, 2KR is responsible for the catabolism of 2KDG in E. coli and the diminishment of 2KDG produced from d-gluconate in the cultivation of E. coli harboring a cloned gluconate dehydrogenase gene. The generation time of E. coli W3110 on minimal medium containing 2KDG was about 27.8 h, compared to a generation time of about 1.3 h on glucose (Fig. 6). In the mutant W3110 (yiaE::Km) harboring plasmid pHD2, the growth rate was about six times higher than in W3110.
FIG. 6.
Growth of E. coli on 2KDG and d-glucose. E. coli W3110 (circles), W3110 (yiaE::Km) (squares), and W3110 (yiaE::Km) harboring pHD2 (triangles) were grown on M9 minimal medium containing 2KDG (solid symbols) or d-glucose (open symbols).
To check whether the reductase is inducible by ketogluconate, the specific 2KR activities in E. coli W3110 cells were assayed after cultivation in media containing ketogluconate (Table 2). 2KR activities were found in cells cultivated both in LB and in LB containing carbohydrate, and there was no significant induction of 2KR by carbohydrate except that higher activity in the presence of d-glucose or d-gluconate was found. This result suggests that the yiaE gene is expressed constitutively in E. coli.
TABLE 2.
Specific 2KR activity in E. coli W3110 cellsa
| Carbohydrate (1.0%) | Sp act of 2KR (U/mg of protein) |
|---|---|
| None | 0.012 |
| d-Glucose | 0.027 |
| d-Gluconate | 0.027 |
| 2KLG | 0.014 |
| 2KDG | 0.021 |
| 25DKG | 0.017 |
| 5KDG | 0.013 |
Strains were cultivated in LB containing 1.0% carbohydrate for 20 h at 37°C. Cells were then harvested, and 2KR activity was measured by using 2KDG as a substrate.
The existence of 2KR in E. coli suggests strongly that the other ketogluconate reductases, 5KDG reductase or 25DKG reductase, and the related ketogluconate metabolism may also exist. Further studies on the identification of other ketogluconate reductases and their physiological roles in E. coli are in progress.
ACKNOWLEDGMENT
This investigation was supported by grant HS1810 from the Ministry of Science and Technology of Korea (MOST).
REFERENCES
- 1.Ameyama M, Adachi O. 2-Keto-d-gluconate reductase from acetic acid bacteria. Methods Enzymol. 1982;89:203–209. doi: 10.1016/s0076-6879(82)89033-2. [DOI] [PubMed] [Google Scholar]
- 2.Ameyama M, Matsushita K, Shinagawa E, Adachi O. Sugar-oxidizing respiratory chain of Gluconobacter suboxydans. Evidence for a branched respiratory chain and characterization of respiratory chain-linked cytochromes. Agric Biol Chem. 1987;51:2943–2950. [Google Scholar]
- 3.Anderson, S., R. A. Lazarus, H. I. Miller, and R. K. Stafford. July 1991. U.S. patent 5,032,514.
- 4.Anderson S, Marks C B, Lazarus R, Miller J, Stafford K, Seymour J, Light D, Rastetter W, Estell D. Production of 2-keto-l-gulonate, an intermediate in l-ascorbate synthesis, by a genetically modified Erwinia herbicola. Science. 1985;230:144–149. doi: 10.1126/science.230.4722.144. [DOI] [PubMed] [Google Scholar]
- 5.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner F R, Plunkett III G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 7.Buissiere J, Brault G, Le Minor L. Utilization and fermentation of 2-ketogluconate by Enterobacteriaceae. Ann Microbiol (Paris) 1981;132:191–195. [PubMed] [Google Scholar]
- 8.Bunch P K, Mat-Jan F, Lee N, Clark D P. The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology. 1997;143:187–195. doi: 10.1099/00221287-143-1-187. [DOI] [PubMed] [Google Scholar]
- 9.Colas des Francs-Small C, Ambard-Bretteville F, Small I D, Remy R. Identification of a major soluble protein in mitochondria from nonphotosynthetic tissues as NAD-dependent formate dehydrogenase. Plant Physiol. 1993;102:1171–1177. doi: 10.1104/pp.102.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 11.Grindley J F, Payton M A, van de Pol H, Hardy K G. Conversion of glucose to 2-keto-l-gulonate, an intermediate in l-ascorbate synthesis, by a recombinant strain of Erwinia citreus. Appl Environ Microbiol. 1988;54:1770–1775. doi: 10.1128/aem.54.7.1770-1775.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi Y, Yoshida T, Kanzaki H, Toki S, Miyazaki S S, Yamada H. Purification and characterization of hydroxypyruvate reductase from a serine-producing methylotroph, Hyphomicrobium methylovorum GM2. Eur J Biochem. 1990;190:279–284. doi: 10.1111/j.1432-1033.1990.tb15573.x. [DOI] [PubMed] [Google Scholar]
- 13.Klasen R, Bringer-Meyer S, Sahm H. Biochemical characterization and sequence analysis of the gluconate:NADP 5-oxidoreductase gene from Gluconobacter oxydans. J Bacteriol. 1995;177:2637–2643. doi: 10.1128/jb.177.10.2637-2643.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Levering P R, Weenk G, Olijve W, Dijkhuizen L, Harder W. Regulation of gluconate and ketogluconate production in Gluconobacter oxydans ATCC 621-H. Arch Microbiol. 1988;149:534–539. [Google Scholar]
- 16.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 17.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 18.Miller J V, Estell D A, Lazarus R A. Purification and characterization of 2,5-diketo-d-gluconate reductase from Corynebacterium sp. J Biol Chem. 1987;262:9016–9020. [PubMed] [Google Scholar]
- 19.Sofia H J, Burland V, Daniels D L, Plunkett III G, Blattner F R. Analysis of the Escherichia coli genome. V. DNA sequence of the region from 76.0 to 81.5 minutes. Nucleic Acids Res. 1994;22:2576–2586. doi: 10.1093/nar/22.13.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonoyama T, Kageyama B, Yagi S, Mitsushima K. Biochemical aspects of 2-keto-l-gulonate accumulation from 2,5-diketo-d-gluconate by Corynebacterium sp. and its mutants. Agric Biol Chem. 1987;51:3039–3047. [Google Scholar]
- 21.Sonoyama T, Kageyama B, Yagi S, Mitsushima K. Facultatively anaerobic bacteria showing high productivities of 2,5-diketo-d-gluconate from d-glucose. Agric Biol Chem. 1988;52:667–674. [Google Scholar]
- 22.Tabata S, Higashitani A, Takanami M, Akiyama K, Kohara Y, Nishimura Y, Nishimura A, Yasuda S, Hirota Y. Construction of an ordered cosmid collection of the Escherichia coli K-12 W3110 chromosome. J Bacteriol. 1989;171:1214–1218. doi: 10.1128/jb.171.2.1214-1218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truesdell S J, Sims J C, Boerman P A, Seymour J L, Lazarus R A. Pathways for metabolism of ketoaldonic acids in an Erwinia sp. J Bacteriol. 1991;173:6651–6656. doi: 10.1128/jb.173.21.6651-6656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winans S C, Elledge S J, Krueger J H, Walker G C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985;161:1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yum D-Y, Bae S-S, Pan J-G. Purification and characterization of the 2-ketoaldonate reductase from Brevibacterium ketosoreductum ATCC 21914. Biosci Biotechnol Biochem. 1998;62:154–156. doi: 10.1271/bbb.62.154. [DOI] [PubMed] [Google Scholar]
- 26.Yum D-Y, Lee Y-P, Pan J-G. Cloning and expression of a gene cluster encoding three subunits of membrane-bound gluconate dehydrogenase from Erwinia cypripedii ATCC 29267 in Escherichia coli. J Bacteriol. 1997;179:6566–6572. doi: 10.1128/jb.179.21.6566-6572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]