Abstract
Background
The COVID-19 pandemic has impacted many people's activities of daily living and health. It has also created economic burdens and caused mental turmoil across the world. Musculoskeletal symptoms, especially low back pain, have been observed in subjects of post-COVID-19 infection and post-vaccination.
Aim
In this study, we aimed to investigate the relationship between low back pain and COVID-19 infection and vaccination, as well as associated factors and characteristics.
Methods
We conducted a questionnaire-based cross-sectional observational study at All India Institute of Medical Science (AIIMS) Bibinagar between September 2021 and March 2022. We collected data from individuals through physical and Google Forms (Google, Mountain View, California).
Results
We included a total of 535 individuals in the study: 274 (51.2%) were previously positive for COVID-19 infection (group A), and 261 (48.8%) were vaccinated against COVID-19 without a history of COVID-19 infection (group B). Each group was divided into two categories based on whether they had low back pain before COVID-19 infection or vaccination. In group A, 90.1% of individuals experienced an aggravation of low back pain after COVID-19 infection, which was found to be significant (p<0.001). In group B, there was an insignificant increase in low back pain following COVID-19 vaccination (p=0.275). The study also revealed a significant association between comorbidities and low back pain in both groups (p<0.001). Additionally, several differences were observed between the two groups, including duration (p<0.001), severity (p=0.012), and intensity (p<0.001) of low back pain, usage of a back support or brace (p=0.043), and intake of vitamin D (p=0.002).
Conclusion
Low back pain is an ignored feature of one of the musculoskeletal symptoms of COVID-19 and was aggravated by COVID-19 infection in our patients compared to those who received the vaccination. The findings of this study have implications for raising awareness, improving management and rehabilitation, and guiding future research in this area.
Keywords: musculoskeletal system, vaccination, infection, covid-19, low back pain
Introduction
COVID-19 infection had its origins in the Huanan seafood market of Southern China on December 29, 2019, with four index cases. Pneumonia of unknown etiology was detected in the city of Wuhan in China on December 31, 2019, and was subsequently reported to the World Health Organization (WHO). The coronavirus disease 2019 (COVID-19) outbreak was declared a pandemic on March 11, 2020 [1,2]. India reported an index case of COVID-19 in Kerala on January 30, 2020, a student who returned from Wuhan. The Ministry of Health and Family Welfare of the Indian Government declared community transmission on March 30, 2020 [3,4].
COVID-19 infection is caused by a novel human coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses. It is a new RNA virus strain that has not been identified in humans previously [5]. There is a substantial degree of homology in the genetic sequence and predicted viral human interaction between SARS-CoV-1 and SARS-CoV-2 strains [6,7]. In addition to direct infection of the cells, the airway's systemic inflammatory response to COVID-19 affects various organ systems, including the musculoskeletal system [8,9]. Most patients infected with SARS-CoV-2 have symptoms ranging from asymptomatic to mild or severe respiratory illness. SARS-CoV-2 resembles SARS-CoV-1, causing SARS and affecting multiple organ systems, including the musculoskeletal system. Myalgias, muscle dysfunction, osteoporosis, and osteonecrosis are common sequelae in patients with SARS infection, as revealed by epidemiological data of the 2002-2004 SARS pandemic [10]. Various studies of SARS-infected patients reported the musculoskeletal burden of the disease, including muscle, neurological, bone, and joint disorders [11,12]. On December 11, 2020, the Pfizer-BioNTech COVID-19 vaccine was approved for emergency use by the US Food and Drug Administration for those older than 16 years [13]. In India, the emergency use of Covishield (the name employed in India for the Oxford-AstraZeneca vaccine, which requires two doses recommended at a time interval of 12-16 weeks apart) and Covaxin, indigenously developed by Bharat Biotech in collaboration with the Indian Council of Medical Research and the National Institute of Virology to be given as two doses, 28 days apart. A free vaccination program was started in India on January 16, 2021 [14,15]. Studies advocated that inflammatory musculoskeletal symptoms may develop in close temporal association with COVID-19 vaccination despite a lack of a clear cause-effect relationship [16].
Mechanical/non-neurological low back pain is one of the most frequent and exorbitant illnesses of young people, leading to a significant loss of productivity. After six to eight weeks of management, 90% of those affected improve, with a recurrence of 60% in the following two years [17]. The economic burden of low back pain is also a big concern; in Western countries, it has been estimated to be 1-2% of the gross national product [18]. Of late, few research articles have been published on symptoms in post-COVID-19 patients. Specifically, only a few studies investigated the effect of COVID-19 on the musculoskeletal system in post-COVID-19 infected patients and post-vaccination populations [19-22].
We had a significant fraction of patients with low back pain after COVID-19 infection and post-vaccination subjects. We intended to investigate and establish/refute the relationship between COVID-19 infection and vaccination, and low back pain.
Materials and methods
Study design
We conducted a cross-sectional observational study at All India Institute of Medical Sciences (AIIMS) Bibinagar between September 28, 2021, and March 24, 2022. The AIIMS institutional ethics committee approved the study (approval number AIIMS/BBN/IEC/SEP/2021/87-A) on September 9, 2021.
Data collection
We designed a questionnaire-based study with both physical forms and virtual Google Forms (Google, Mountain View, California). The questionnaire was written in English, and the scientific terms were written and explained in the local language. We verbally explained the study to eligible subjects attending the hospital's outpatient department. Once the participants consented to the study, we provided either physical or Google Forms (depending on comfort with technology) to collect the information. For the virtual form, we collected the phone numbers of the COVID-19 patients and vaccinated participants at AIIMS Bibinagar, and we sent Google forms after explaining the purpose of the study.
The questionnaire comprised four categories of questions. The first part of the questionnaire included demographic details, personnel habits, and co-morbidities. The second part included information about general well-being related to low back pain. The third part included COVID-19 infection data and their relationship with low back pain. The fourth part included COVID-19 vaccination-related data.
Study population
We screened patients admitted to the outpatient departments (OPDs) of AIIMS, Bibinagar, which included the post-covid clinic, orthopedic, and other OPDs.
Inclusion criteria
We included subjects who tested positive for COVID-19 infection or who were vaccinated with Covishield or Covaxin in the past without COVID-19 infection.
Exclusion criteria
We excluded subjects who were negative for COVID-19 infection and were not vaccinated against COVID-19, and those who were vaccinated with a history of COVID-19 infection. We also excluded subjects with neurological deficits or failed back syndrome.
Validity and reliability
The questionnaire was partly derived from other validated questionnaires available in the literature and modified for technical clarity and presentation [23,24]. The remaining part of the questionnaire was self-developed. We assessed the reliability of the questionnaire after obtaining responses to the self-administered questionnaire from respondents, including medical professionals of different broad and super-specialties. The entire set of questionnaire items was validated among a cluster (n=5) of medical experts working (interested) in the field. Each expert independently rated the relevance of each item using a four-point Likert scale (1 = not relevant, 2 = somewhat relevant, 3 = relevant, 4 = very relevant). Ratings of "3" and "4" were together considered a "favorable" response to the item, namely, the particular question was relevant; similarly, ratings of "1" and "2" were together considered an "unfavorable" response to the item, and the question was irrelevant. All the items were retained without major modifications, as the content validity index of the individual items was well above the cut-off, except for question number 2, which was deleted.
Study groups with sample size
We adopted a non-random convenience sampling technique for the study.
Sample size calculation
To calculate the sample size, we estimated a percentage or proportion in the case of the finite population. With a margin of error of 5%, a confidence level of 95%, and an available population size of 100,000, the minimum recommended sample size for the survey was estimated as 373 participants.
Ethical considerations
We began the study after obtaining prior approval from the Scientific Research Committee and Institutional Ethics Committee of AIIMS Bibinagar. The identity of the respondents was kept confidential.
Statistical analysis
We entered the data into a Microsoft Excel spreadsheet (Microsoft, Redmond, Washington), which was analyzed using SPSS 22 version (IBM Inc., Armonk, New York) software. We represented the categorical data in terms of frequencies and proportions. We used the chi-square test to determine the significance for qualitative data. We represented continuous data as means and standard deviations. We tested the normality of the continuous data with the Kolmogorov-Smirnov test and the Shapiro-Wilk test. We used the Student's independent t-test to determine significance and identify the mean difference between pairs of quantitative variables. A p-value (i.e., the probability that the result is true) of <0.05 was considered statistically significant after assuming all the rules of statistical tests.
Results
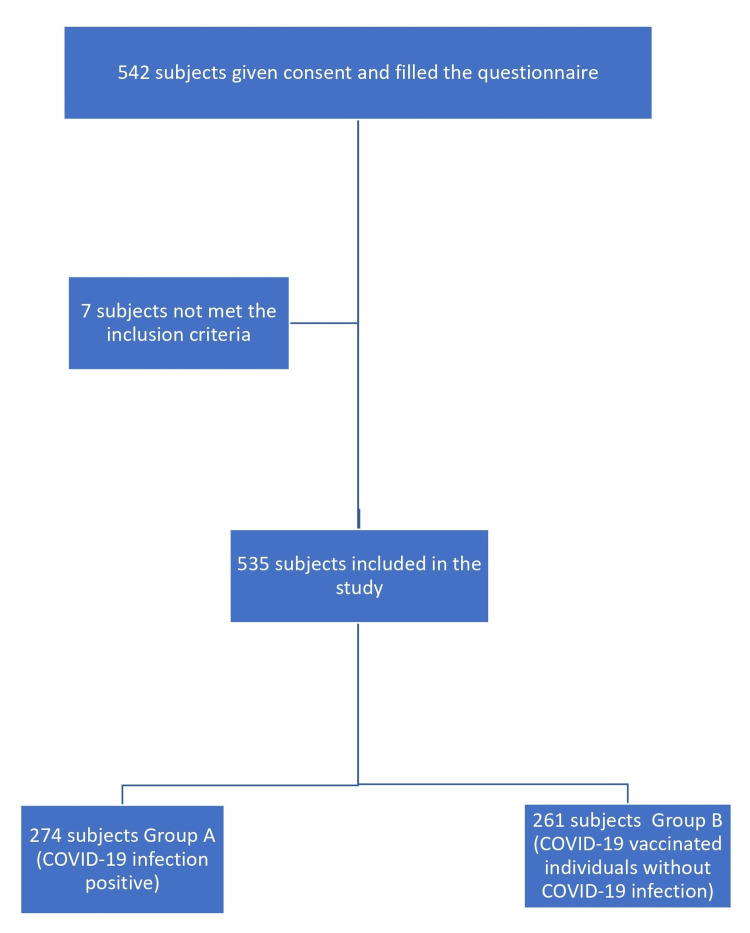
In this study, 542 participants provided consent and filled out the COVID-19 questionnaire. However, seven participants did not meet the inclusion criteria. Out of 535 participants who met the inclusion and exclusion criteria, 274 participants had a history of COVID-19 infection (group A), and 261 participants were vaccinated against COVID-19 (group B); these vaccinated individuals were not infected with COVID-19 disease (Figure 1).
Figure 1. Flow chart showing the selection of study participants.
Demographic data
The ages of the participants ranged between 17 and 86 years, and most (78.87%, n=422) were between 21 and 50 years, with a mean age of 40.23 ± 12.25 years. Three hundred seventeen subjects (59.25%) were male, and 216 (40.37%) were female. Two participants preferred not to provide their gender. Thus, the male-to-female ratio was 1.47:1. The occupational data of the participants showed that 88 (16.45%) participants were healthcare workers, which included allopathic doctors, dental surgeons, veterinary doctors, nursing officers, physiotherapists, laboratory technicians, occupational therapy technicians, and X-ray technicians. Seventy-six (14.20%) subjects were housewives or homemakers, 60 (11.21%) were in service for the public and private sectors, 48 (8.97%) were business persons, 40 (7.48%) were agricultural workers, 23 (4.30%) were IT professionals, 18 (3.36%) were students, 11 (2.06%) were from the marketing field, 11 (2.06%) were drivers, 11 (2.06%) were daily laborers, 10 (1.87%) were retired employees, and the remaining were from various fields. One hundred fifteen (21.49%) participants did not provide their occupational details. Most of the participants in this study were from various places in the Telangana state, followed by Andhra Pradesh (Table 1).
Table 1. Demographic data of the study participants.
| Variable | Category | Frequency, n (%) |
| Age distribution (years) | 17-20 | 6 (1.12%) |
| 21-30 | 125 (23.36%) | |
| 31-40 | 184 (34.39%) | |
| 41-50 | 113 (21.12%) | |
| 51-60 | 75 (14.02%) | |
| 61-70 | 24 (4.49%) | |
| 71-80 | 6 (1.12%) | |
| 81-86 | 2 (0.37%) | |
| Gender | Male | 317 (59.25%) |
| Female | 216 (40.37%) | |
| Prefer not to say | 2 (0.37%) | |
| Occupation of participant | Healthcare worker | 88 (16.45%) |
| Homemaker/housewife | 76 (14.20%) | |
| Service in public/private sectors | 60 (11.21%) | |
| Business person | 48 (8.97%) | |
| Agriculture field | 40 (7.48%) | |
| IT professional | 23 (4.30%) | |
| Student | 18 (3.36%) | |
| Marketing field | 11 (2.06%) | |
| Driver | 11 (2.06%) | |
| Daily laborer | 11 (2.06%) | |
| Retired employee | 10 (1.87%) | |
| Other occupations | 24 (4.48%) | |
| Preferred not to mention | 115 (21.49%) | |
| Geographic location of the participants | Telangana | 457 (85.42%) |
| Andra Pradesh | 53 (9.90%) | |
| Maharashtra | 6 (1.12%) | |
| Tamil Nadu | 5 (0.93%) | |
| Karnataka | 3 (0.56%) | |
| Rajasthan | 2 (0.37%) | |
| Delhi | 2 (0.37%) | |
| Bihar | 2 (0.37%) | |
| Uttar Pradesh | 1 (0.19%) | |
| Himachal Pradesh | 1 (0.19%) | |
| West Bengal | 1 (0.19%) | |
| Odisha | 1 (0.19%) | |
| London | 1 (0.19%) |
51.2% (n=274) of our participants were previously positive for COVID-19 infection (group A), and 48.8% (n=261) were vaccinated subjects with no history of COVID-19 infection (group B) (Table 2).
Table 2. Group categorization of study participants.
| Number of participants (n=535) | Percentage (%) | |
| COVID-19 positive (group A) | 274 | 51.20% |
| Vaccinated without COVID-19 (group B) | 261 | 48.80% |
The mean age of the participants in groups A and B was 41.15 ± 12.32 years and 39.26 ± 12.11 years, and the mean body mass index (BMI) was 24.30 ± 4.08 and 24.16 ± 3.88, respectively. There was no significant difference in mean age and mean BMI between the two groups (Table 3).
Table 3. Age and BMI distribution comparison between groups A and B.
| Group A (COVID-19 positive) | Group B (vaccinated without COVID-19) | p-value | |||
| Mean | SD | Mean | SD | ||
| Age (in years) | 41.15 | 12.32 | 39.26 | 12.11 | 0.074 |
| BMI | 24.3 | 4.08 | 24.16 | 3.88 | 0.699 |
In groups A (COVID-19 positive) and B (vaccinated without COVID-19), 32.1% (n=88) and 49% (n=128) of participants were female, 67.2% (n=184) and 51% (n=133) were male, 47.4% (n=130) and 30.3% (n=79) consumed alcohol, and 32.1% (n=88) and 19.5% (n=51) had comorbidities, respectively. There was a significant difference in gender distribution (p<0.001), alcohol intake (p<0.001), and comorbidities (p=0.001) between groups A and B, whereas there was no significant difference in smoking (8.4%, n=23 and 5.7%,n=15) or BMI distribution (normal BMI of 67.5%, n=185 and 62.5%,n=163), respectively (Table 4).
Table 4. Sociodemographic profile comparison between groups A and B.
| Group A (COVID-19 positive) | Group B (Vaccinated without COVID-19) | p-value | ||||
| Count (n) | Percentage (%) | Count (n) | Percentage (%) | |||
| Gender | Female | 88 | 32.10% | 128 | 49.00% | <0.001* |
| Male | 184 | 67.20% | 133 | 51.00% | ||
| Prefer not to say | 2 | 0.70% | 0 | 0.00% | ||
| Alcohol intake | No | 144 | 52.60% | 182 | 69.70% | <0.001* |
| Yes | 130 | 47.40% | 79 | 30.30% | ||
| Smoking | No | 251 | 91.60% | 246 | 94.30% | 0.233 |
| Yes | 23 | 8.40% | 15 | 5.70% | ||
| Comorbidity | Yes | 88 | 32.10% | 51 | 19.50% | 0.001* |
| No | 186 | 67.90% | 210 | 80.50% | ||
| BMI | <18.5 | 7 | 2.60% | 9 | 3.40% | 0.465 |
| 18.5–24.9 | 185 | 67.50% | 163 | 62.50% | ||
| 25–29.9 | 57 | 20.80% | 70 | 26.80% | ||
| 30–34.9 | 17 | 6.20% | 12 | 4.60% | ||
| >35 | 8 | 2.90% | 7 | 2.70% | ||
There was a significant difference (p<0.001) in backache before COVID-19 between groups A and B at 81.4% (n=223) and 41% (n=107), respectively (Table 5).
Table 5. Backache before COVID-19 comparison between groups A and B.
χ2 =2.26, df =1, p<0.001* (Chi-squared test)
| Group A (COVID-19 positive) | Group B (Vaccinated without COVID-19) | Total | |||||
| Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | ||
| Backache before COVID-19 | Yes | 223 | 81.40% | 107 | 41.00% | 330 | 61.70% |
| No | 51 | 18.60% | 154 | 59.00% | 205 | 38.30% | |
| Total | 274 | 100.00% | 261 | 100.00% | 535 | 100.00% | |
In group A, out of 223 participants with backache before COVID-19, 90.1% (n=201) had aggravation of low back pain after COVID-19 infection, and 9.90% (n=22) had no aggravation. In 51 participants without low back pain before COVID-19, 100% had no low back pain after COVID-19 infection. There was a significant increase in low back pain after COVID-19 infection (Table 6).
Table 6. Comparison of backache before and after COVID-19 infection in group A.
χ2 =172.5, df =1, p<0.001* (Chi-squared test)
| Backache Before COVID-19 | |||||||
| Yes | No | Total | |||||
| Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | ||
| Backpain after COVID-19 infection | Aggravated | 201 | 90.1% | 0 | 0.0% | 201 | 73.4% |
| Not aggravated or absent | 22 | 9.9% | 51 | 100.0% | 73 | 26.6% | |
| Total | 223 | 100.0% | 51 | 100.0% | 274 | 100.0% | |
In group B, out of 107 participants with low back pain before COVID-19, 37.4% (n=40) had low back pain after COVID-19 vaccination, and out of 154 subjects without low back pain before COVID-19, 44.2% (n=68) had backache after vaccination. However, there was no significant difference in low back pain before COVID-19 and after vaccination (Table 7).
Table 7. Comparison of backache before and after COVID-19 vaccination in group B.
χ2 =1.194, df =1, p=0.275 (Chi-squared test)
| Backache before COVID-19 | |||||||
| Yes | No | Total | |||||
| Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | ||
| Backpain after COVID-19 vaccination | Backache present | 40 | 37.4% | 68 | 44.2% | 108 | 41.4% |
| Backache absent | 67 | 62.6% | 86 | 55.8% | 153 | 58.6% | |
| Total | 107 | 100.0% | 154 | 100.0% | 261 | 100.0% | |
In this study, there was no significant difference in gender, alcohol intake, smoking, or BMI between the two groups with low back pain. We observed a significant association between comorbidities and low back pain in the two groups. Of those in group A with low back pain, 35.4% (n=79) had comorbidities, whereas of those in group B with low back pain, 11.1% (n=12) had comorbidities (Table 8).
Table 8. Association between demographic profile and low back pain in groups A and B.
| Group A (COVID-19 positive low back pain group) (n=223) | Group B (vaccinated without COVID-19 low back pain group) (n=108) | p-value | ||||
| Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | |||
| Gender | Female | 79 | 35.40% | 40 | 37.00% | 0.759 |
| Male | 143 | 64.10% | 68 | 63.00% | ||
| Prefer not to say | 1 | 0.40% | 0 | 0.00% | ||
| Alcohol intake | No | 120 | 53.80% | 63 | 58.30% | 0.438 |
| Yes | 103 | 46.20% | 45 | 41.70% | ||
| Smoking | No | 207 | 92.80% | 104 | 96.30% | 0.214 |
| Yes | 16 | 7.20% | 4 | 3.70% | ||
| Comorbidity | Yes | 79 | 35.40% | 12 | 11.10% | <0.001* |
| No | 144 | 64.60% | 96 | 88.90% | ||
| BMI | <18.5 | 6 | 2.70% | 7 | 6.50% | 0.071 |
| 18.5-24.9 | 154 | 69.10% | 80 | 74.10% | ||
| 25-29.9 | 43 | 19.30% | 19 | 17.60% | ||
| 30-34.9 | 13 | 5.80% | 1 | 0.90% | ||
| >35 | 7 | 3.10% | 1 | 0.90% | ||
In group A with low back pain, the majority had backache over months (57%, n=127), moderate in severity (72.6%, n=162), and waxing and waning in intensity (80.7%, n=180); in the vaccinated low back pain group, the majority had backache over weeks (42.5%, n=17), moderate in severity (50%, n=20), and constant in intensity (72.5%, n=29). There was a significant difference in the duration (p<0.001), severity (p=0.012), and intensity (p<0.001) of backache between the two groups (Table 9).
Table 9. Association of low back pain characteristics between groups A and B.
| Group A (COVID-19 positive low back pain group) (n=223) | Group B (vaccinated without COVID-19 low back pain group) (n=40) | Total | p-value | |||||
| Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | |||
| How long have you been suffering from backache? | Over days | 11 | 4.90% | 12 | 30.00% | 23 | 8.70% | <0.001* |
| Over weeks | 49 | 22.00% | 17 | 42.50% | 66 | 25.10% | ||
| Over months | 127 | 57.00% | 11 | 27.50% | 138 | 52.50% | ||
| Over years | 36 | 16.10% | 0 | 0.00% | 36 | 13.70% | ||
| Severity of backache | Mild | 25 | 11.20% | 11 | 27.50% | 36 | 13.70% | 0.012* |
| Moderate | 162 | 72.60% | 20 | 50.00% | 182 | 69.20% | ||
| Severe | 33 | 14.80% | 9 | 22.50% | 42 | 16.00% | ||
| Excruciating | 3 | 1.30% | 0 | 0.00% | 3 | 1.10% | ||
| How does the backache vary? | Constant | 40 | 17.90% | 29 | 72.50% | 69 | 26.20% | <0.001* |
| Increases on bending | 1 | 0.40% | 0 | 0.00% | 1 | 0.40% | ||
| Rarely | 1 | 0.40% | 0 | 0.00% | 1 | 0.40% | ||
| Standing continuously for 30 minutes | 1 | 0.40% | 0 | 0.00% | 1 | 0.40% | ||
| Waxing and waning | 180 | 80.70% | 11 | 27.50% | 191 | 72.60% | ||
There was no significant difference in disturbed sleep, consultation of a doctor, medication for backache, injections to the spine, surgery for backache, congenital (birth) or developmental (growth) defect, infectious or inflammatory pathology of the spine, history of significant trauma to the spine, neoplastic/ tumorous spine pathology, diagnosis of a degenerative/age-related spine disorder, physiotherapy for backache, or back-strengthening exercises between the two groups. There was a significant difference in the investigation for backache (p=0.002), ever using a back support/brace (p=0.043), and vitamin D supplementation (p=0.002) between the two groups. Group A with low backache had higher rates of being investigated for backache, using a back support/brace, and vitamin D supplementation, compared with group B low back pain (Table 10).
Table 10. Low back pain affecting routine activities among the subjects within groups A and B.
| Group A (COVID-19 with low back pain group) (n=223) | Group B (Vaccinated without COVID-19 low back pain group) (n=40) | Total | p-value | |||||
| Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | Number of participants (n) | Percentage (%) | |||
| Sleep disturbed | No | 190 | 85.20% | 35 | 87.50% | 225 | 85.60% | 0.703 |
| Yes | 33 | 14.80% | 5 | 12.50% | 38 | 14.40% | ||
| Consulted a doctor for backache | No | 73 | 32.70% | 12 | 30.00% | 85 | 32.30% | 0.733 |
| Yes | 150 | 67.30% | 28 | 70.00% | 178 | 67.70% | ||
| Investigated for backache | No | 96 | 43.00% | 28 | 70.00% | 124 | 47.10% | 0.002* |
| Yes | 127 | 57.00% | 12 | 30.00% | 139 | 52.90% | ||
| Used any medication for backache | No | 39 | 17.50% | 5 | 12.50% | 44 | 16.70% | 0.436 |
| Yes | 184 | 82.50% | 35 | 87.50% | 219 | 83.30% | ||
| Injections to spine for backache | No | 216 | 96.90% | 39 | 97.50% | 255 | 97.00% | 0.828 |
| Yes | 7 | 3.10% | 1 | 2.50% | 8 | 3.00% | ||
| Ever suggested to go for surgery for backache | No | 212 | 95.10% | 40 | 100.00% | 252 | 95.80% | 0.151 |
| Yes | 11 | 4.90% | 0 | 0.00% | 11 | 4.20% | ||
| Ever used a back support/ brace | No | 202 | 90.60% | 40 | 100.00% | 242 | 92.00% | 0.043* |
| Yes | 21 | 9.40% | 0 | 0.00% | 21 | 8.00% | ||
| Vitamin D supplementation | No | 86 | 38.60% | 26 | 65.00% | 112 | 42.60% | 0.002* |
| Yes | 137 | 61.40% | 14 | 35.00% | 151 | 57.40% | ||
| Congenital (Birth defect) or Developmental (Growth defect) abnormality of spine | I don’t know | 4 | 1.80% | 0 | 0.00% | 4 | 1.50% | 0.393 |
| No | 219 | 98.20% | 40 | 100.00% | 259 | 98.50% | ||
| Suffered any infectious or inflammatory pathology of spine | I don’t know | 5 | 2.20% | 0 | 0.00% | 5 | 1.90% | 0.577 |
| No | 217 | 97.30% | 40 | 100.00% | 257 | 97.70% | ||
| Yes | 1 | 0.40% | 0 | 0.00% | 1 | 0.40% | ||
| Any history of significant trauma to spine | I don’t know | 2 | 0.90% | 0 | 0.00% | 2 | 0.80% | 0.577 |
| No | 217 | 97.30% | 40 | 100.00% | 257 | 97.70% | ||
| Yes | 4 | 1.80% | 0 | 0.00% | 4 | 1.50% | ||
| History of neoplastic/ tumorous Spine pathology | I don’t know | 3 | 1.30% | 0 | 0.00% | 3 | 1.10% | 0.695 |
| No | 219 | 98.20% | 40 | 100.00% | 259 | 98.50% | ||
| Yes | 1 | 0.40% | 0 | 0.00% | 1 | 0.40% | ||
| Diagnosed with a degenerative/ age-related spine disorder | I don’t know | 5 | 2.20% | 0 | 0.00% | 5 | 1.90% | 0.339 |
| No | 218 | 97.80% | 40 | 100.00% | 258 | 98.10% | ||
| Physiotherapy for backache | No | 199 | 89.20% | 39 | 97.50% | 238 | 90.50% | 0.101 |
| Yes | 24 | 10.80% | 1 | 2.50% | 25 | 9.50% | ||
| Back-strengthening exercises | No | 172 | 77.10% | 33 | 82.50% | 205 | 77.90% | 0.658 |
| Not anymore | 4 | 1.80% | 1 | 2.50% | 5 | 1.90% | ||
| Yes | 47 | 21.10% | 6 | 15.00% | 53 | 20.20% | ||
Discussion
In India, the first case of COVID-19 infection was detected on January 30, 2021, and WHO declared COVID-19 to be a pandemic, followed by a community transmission declaration in India [25]. Apart from respiratory symptoms ranging from mild to moderate to severe in intensity, COVID-19 affects various other organ systems and exhibits a variety of manifestations, including musculoskeletal symptoms [26]. Despite musculoskeletal manifestations being common in the early phase of COVID-19 infection for various reasons, it has been underreported because of disease severity in other organ systems [27]. In a study by Bakilan et al. [28], fatigue was reported in 72% of post-acute COVID-19 patients, low back pain was reported in 71% of patients, and myalgias and arthralgias were reported in 61% and 44% of patients, respectively. The angiotensin-converting enzyme 2 (ACE2) receptors, which are homologous in configuration and genetic makeup, are targets of both SARS-CoV-1 and SARS-CoV-2. Although the precise mechanism is unclear due to the expression of ACE2 receptors in skeletal muscle, adipocytes, and endothelial cells within the musculoskeletal system, they are target sites for SARS-CoV-2 infection, which might also explain the musculoskeletal manifestations of the disease. The principal pathophysiology of musculoskeletal manifestations related to COVID-19 includes cytokine storm, high expression of IL-6 and other proinflammatory mediators, development of a prothrombotic state, and autoimmunity [28-31]. In this study, we aimed to determine the frequency of low back pain of post-COVID-19 positive individuals (group A) and post-vaccination individuals without COVID-19 infection (group B). We also aimed to compare the analyzed demographic factors associated with low back pain and to evaluate the characteristics of low back pain in these two groups.
To the best of our knowledge, this is the only study conducted to determine low back pain related to COVID-19 in chronic settings, including COVID-19 positive and COVID-19 vaccination subjects. The ages of our 535 participants ranged from 17 to 86 years, and 78.87% (n=422) were 21 to 50 years of age, with a mean age of 40.23 ± 12.25 years. 59.25% (n=317) of our participants were male. Most of the study participants were from a single geographical area. Having divided the participant data into two groups, 51.2% (n=274) were categorized as group A (COVID-19 infection positive), and 48.8% (n=261) were categorized as group B (vaccinated group without COVID-19 infection), with mean ages of 41.15 ± 12.32 years and 39.26 ± 12.11 years, and mean BMIs of 24.30 ± 4.08 and 24.16 ± 3.88, respectively. Cipollaro et al. [32] analyzed data from retrospective single-center studies in 12,046 COVID-19 patients and reported various musculoskeletal manifestations and epidemiological characteristics. The average age of the patients was 52.13 years; 54% were male, and 46% were female. Cipollaro et al. observed that myalgia, arthralgia, and fatigue symptoms were continuously present from the initial stage to the most severe stage of the COVID-19 disease. Our study differed from that of Cipollaro et al., as we analyzed the post-COVID-19 symptoms and compared them with those with pre-COVID-19 status. In group A (COVID-19 infection positive), 223 individuals had backache before COVID-19 infection, in which 90.1% (n=201) had experienced the aggravation of their low back pain after COVID-19 infection, and 9.90% (n=22) of individuals had no aggravation of their low back pain after infection. Our statistical analysis suggested that there was a significant increase in low back pain after COVID-19 infection. A systematic review and meta-analysis by Abdullahi et al. [33] included 11,069 subjects, of which 5168 were male, and the mean age ranged from 24 to 95 years. They reported that the pooled prevalence of low back pain in COVID-19 patients was 10% (95% confidence interval: 0.01-0.23). However, in that study, 2377 patients were in critically ill condition. Uz et al. [34] conducted a study on 99 COVID-19 polymerase chain reaction-positive inpatients. The mean age of the patients (53 male and 46 female) was 48.80 ± 14.64 years, and 59 (60%) patients had at least one comorbidity. Low back pain was observed in 50.5% (n=50) of the patients. Jena et al. [35] conducted a study on 182 hospitalized COVID-19 patients and reported low back pain in 22.53% (n=41) of patients.
In our study, out of 107 participants with low back pain before COVID-19 in group B, 37.4% (n=40) had low back pain after COVID-19 vaccination. Out of 154 participants without low back pain before COVID-19, 44.2% (n=68) had backache after vaccination, which suggested that there was no significant difference in low back pain before COVID-19 and after vaccination. In comparison with group B (vaccinated individuals), group A (COVID-19 infection) had significantly higher levels of low back pain. However, there was a significant difference in pre-COVID-19 and pre-vaccination low back pain status, gender distribution, and alcohol intake between the groups.
In our study, we observed a significant association (p<0.001) between comorbidities (e.g., hypertension, diabetes mellitus, and hypothyroidism) and low back pain in the two groups. 35.4% (n=79) had comorbidities in the COVID-19-positive (group A) low back pain group, whereas 11.1% (n=12) had comorbidities among the vaccinated low back pain group (group B). The COVID-19 patients with comorbidities had a higher chance of developing a severe progression of the disease. Low back pain has been associated with the presence and severity of COVID-19 [36]. In our study, with respect to low back pain duration (p<0.001), severity (p=0.012), and intensity (p<0.001), we observed a significant difference between the two groups. In the COVID-19 infection low back pain group, 57.0% (n=127) had low back pain over the course of months, whereas in the vaccinated low back pain group, 42.5% (n=17) had it over the course of weeks. In both the COVID-19 infected and the vaccinated low back pain groups, the majority of patients had moderate-type low back pain (72.6%,n=162 and 50%,n=20 respectively); 80.7% (n=180) had intermittent-type low back pain in the COVID-19 infection low back pain group, and 72.5% (n=29) had constant-type low back pain in the vaccinated low back pain group. In both groups, 57.0% (n=127) and 30.0% (n=12) were investigated for low back pain (p=0.002); 9.4% (n=21) and 0% used brace support (p=0.043); and 61.4% (n=137) and 35.0% (n=14) had taken vitamin D3 supplementation (p=0.002), respectively. Therefore, significant differences were observed between the groups.
In post-COVID-19 patients, we must give significance to complaints of low back pain because it can be due to intramuscular edema in multifidus and erector spinae, and it may be associated with raised c-reactive protein, erythrocyte sedimentation rate, creatinine kinase, and D-dimer levels. These features of paraspinal myositis can be obvious in magnetic resonance imaging and may be absent in the dorsal and cervical regions. Along with muscular viral load, an immune-mediated para-infectious inflammatory response, adverse effects of drugs, and critical illness related to myopathy could be causes of low back pain [37,38].
Our study had certain limitations, such as recall and selection bias. The data of low back pain and characteristics in the vaccinated low back pain group were significantly less compared with the COVID-19-infected low back pain group. We did not include the association between low back pain characteristics and disease characteristics in the study. We recommend further studies with a larger sample size to validate the results of our research and arrive at a more precise estimate of predicting the low back pain manifestation and its characteristics in the COVID-19 infected and vaccinated population to create a structured follow-up protocol for its effective management.
Conclusions
Our results showed that low back pain was more common in post-COVID-19 and post-vaccination individuals. Low back pain was much more aggravated in those who had low back pain previously. However, when compared with vaccinated individuals, infected individuals were affected more. We observed a significant association between comorbidities (e.g., hypertension, diabetes mellitus, and hypothyroidism) and low back pain. The duration of low back pain was also longer in the infected group. Therefore, orthopedic doctors should determine a history of exposure to COVID-19 in cases of low back pain as a matter of routine. This study provides a foundation to assess low back pain in post-COVID-19 and vaccinated individuals and its associated factors. Despite the limitations of this study, our results should hopefully inspire others within the scientific community to conduct further research on low back pain as a symptom of long COVID in post-COVID-19 survivors.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. The All India Institute of Medical Science (AIIMS) Institutional Ethics Committee issued approval AIIMS/BBN/IEC/SEP/2021/87-A
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Rolling updates on coronavirus disease (COVID-19) . 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 2.The coronavirus disease (COVID-19) pandemic. Rampal L, Liew BS. https://www.researchgate.net/publication/340629981_Coronavirus_disease_COVID-19_pandemic. Med J Malays. 2020;75:95–97. [PubMed] [Google Scholar]
- 3.WHO India situation report. 2020. https://www.who.int/india/emergencies/india-situation-report https://www.who.int/india/emergencies/india-situation-report
- 4.Standard operating procedure (SOP) for transporting a suspect/confirmed case of COVID-19. Coronavirus Disease. 2019. https://www.mohfw.gov.in/pdf/StandardOperatingProcedureSOPfortransportingasuspectorconfirmedcaseofCOVID19.pdf https://www.mohfw.gov.in/pdf/StandardOperatingProcedureSOPfortransportingasuspectorconfirmedcaseofCOVID19.pdf
- 5.Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Zhang JJ, Dong X, Cao YY, et al. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 6.A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Gordon DE, Jang GM, Bouhaddou M, et al. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Master regulator analysis of the SARS-CoV-2/human interactome. Guzzi PH, Mercatelli D, Ceraolo C, Giorgi FM. J Clin Med. 2020;9:982. doi: 10.3390/jcm9040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. Cheng H, Wang Y, Wang GQ. J Med Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Huang C, Wang Y, Li X, et al. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musculoskeletal Consequences of COVID-19. Disser NP, De Micheli AJ, Schonk MM, et al. J Bone Joint Surg Am. 2020;102:1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Leung TW, Wong KS, Hui AC, To KF, Lai ST, Ng WF, Ng HK. Arch Neurol. 2005;62:1113–1117. doi: 10.1001/archneur.62.7.1113. [DOI] [PubMed] [Google Scholar]
- 12.Neurological manifestations in severe acute respiratory syndrome. Tsai LK, Hsieh ST, Chang YC. http://www.ant-tnsjournal.com/Mag_Files/14-3/14-3_p113.pdf. Acta neurologica Taiwanica. 2005;14:113–119. [PubMed] [Google Scholar]
- 13.Pfizer-BioNTech COVID-19 vaccine. 2020. http://www.fda.gov/media/144245/download http://www.fda.gov/media/144245/download
- 14.Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial. Ella R, Reddy S, Blackwelder W, et al. Lancet. 2021;398:2173–2184. doi: 10.1016/S0140-6736(21)02000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strategy for COVID-19 vaccination in India: the country with the second highest population and number of cases. Kumar VM, Pandi-Perumal SR, Trakht I, Thyagarajan SP. NPJ Vaccines. 2021;6:60. doi: 10.1038/s41541-021-00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spectrum of short-term inflammatory musculoskeletal manifestations after COVID-19 vaccine administration: a report of 66 cases. Ursini F, Ruscitti P, Raimondo V, et al. Ann Rheum Dis. 2022;81:440–441. doi: 10.1136/annrheumdis-2021-221587. [DOI] [PubMed] [Google Scholar]
- 17.Cost, controversy, crisis: low back pain and the health of the public. Deyo RA, Cherkin D, Conrad D, Volinn E. Annu Rev Public Health. 1991;12:141–156. doi: 10.1146/annurev.pu.12.050191.001041. [DOI] [PubMed] [Google Scholar]
- 18.Personal and societal impact of low back pain: the Groningen spine cohort. Dutmer AL, Schiphorst Preuper HR, Soer R, et al. Spine (Phila Pa 1976) 2019;44:0–51. doi: 10.1097/BRS.0000000000003174. [DOI] [PubMed] [Google Scholar]
- 19.6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Huang C, Huang L, Wang Y, et al. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persistent symptoms in patients after acute COVID-19. Carfì A, Bernabei R, Landi F. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: prospective follow-up by phone interviews. Karaarslan F, Demircioğlu Güneri F, Kardeş S. Rheumatol Int. 2021;41:1263–1271. doi: 10.1007/s00296-021-04882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musculoskeletal symptoms and its associated factors among post‐COVID‐19 patients attended in a rehabilitation centre. Numan SM. Int J Med Sci Clin Invent. 2021;8:5251–5257. [Google Scholar]
- 23.Estimating cost of care for patients with acute low low back pain: a retrospective review of patient records. Crow WT, Willis DR. https://www.degruyter.com/document/doi/10.7556/jaoa.2009.109.4.229/html. J Am Osteopath Assoc. 2009;109:229–233. [PubMed] [Google Scholar]
- 24.Coenen P. Amsterdam, the Netherlands: VU University Amsterdam; 2013. On the Origin of Low Back Pain. [Google Scholar]
- 25.Situation of India in the COVID-19 Pandemic: India's initial pandemic experience. Siddiqui AF, Wiederkehr M, Rozanova L, Flahault A. Int J Environ Res Public Health. 2020;17:8994. doi: 10.3390/ijerph17238994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musculoskeletal complications of severe acute respiratory syndrome. Griffith JF. Semin Musculoskelet Radiol. 2011;15:554–560. doi: 10.1055/s-0031-1293500. [DOI] [PubMed] [Google Scholar]
- 27.Musculoskeletal manifestations of COVID-19: currently described clinical symptoms and multimodality imaging findings. Omar IM, Weaver JS, Samet JD, Serhal AM, Mar WA, Taljanovic MS. Radiographics. 2022;42:1415–1432. doi: 10.1148/rg.220036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musculoskeletal symptoms and related factors in postacute COVID-19 patients. Bakılan F, Gökmen İG, Ortanca B, et al. Int J Clin Pract. 2021;75:0. doi: 10.1111/ijcp.14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. J Med Virol. 2001;64:262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 30.Clinical features of the first cases and a cluster of coronavirus disease 2019 (COVID-19) in Bolivia imported from Italy and Spain. Escalera-Antezana JP, Lizon-Ferrufino NF, Maldonado-Alanoca A, et al. Travel Med Infect Dis. 2020;35:101653. doi: 10.1016/j.tmaid.2020.101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Is a "cytokine storm" relevant to COVID-19? Sinha P, Matthay MA, Calfee CS. JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 32.Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. Cipollaro L, Giordano L, Padulo J, Oliva F, Maffulli N. J Orthop Surg Res. 2020;15:178. doi: 10.1186/s13018-020-01702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neurological and musculoskeletal features of COVID-19: a systematic review and meta-analysis. Abdullahi A, Candan SA, Abba MA, et al. Front Neurol. 2020;11:687. doi: 10.3389/fneur.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low back pain and related factors in patients with COVID-19 [PREPRINT] Uz C, Umay E, Gundogdu I, Uz F. 2020 [Google Scholar]
- 35.Musculoskeletal and neurological pain symptoms among hospitalized COVID-19 patients. Jena D, Sahoo J, Barman A, Gupta A, Patel V. Am J Phys Med Rehabil. 2022;101:411–416. doi: 10.1097/PHM.0000000000001969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Comorbidity and its impact on patients with COVID-19. Sanyaolu A, Okorie C, Marinkovic A, et al. SN Compr Clin Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Into the looking glass: post-viral syndrome post COVID-19. Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Med Hypotheses. 2020;144:110055. doi: 10.1016/j.mehy.2020.110055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paraspinal myositis in patients with COVID-19 infection. Mehan WA, Yoon BC, Lang M, Li MD, Rincon S, Buch K. AJNR Am J Neuroradiol. 2020;41:1949–1952. doi: 10.3174/ajnr.A6711. [DOI] [PMC free article] [PubMed] [Google Scholar]