Abstract
Succinate-ubiquinone oxidoreductase (SQR) from Escherichia coli is expressed maximally during aerobic growth, when it catalyzes the oxidation of succinate to fumarate in the tricarboxylic acid cycle and reduces ubiquinone in the membrane. The enzyme is similar in structure and function to fumarate reductase (menaquinol-fumarate oxidoreductase [QFR]), which participates in anaerobic respiration by E. coli. Fumarate reductase, which is proficient in succinate oxidation, is able to functionally replace SQR in aerobic respiration when conditions are used to allow the expression of the frdABCD operon aerobically. SQR has not previously been shown to be capable of supporting anaerobic growth of E. coli because expression of the enzyme complex is largely repressed by anaerobic conditions. In order to obtain expression of SQR anaerobically, plasmids which utilize the PFRD promoter of the frdABCD operon fused to the sdhCDAB genes to drive expression were constructed. It was found that, under anaerobic growth conditions where fumarate is utilized as the terminal electron acceptor, SQR would function to support anaerobic growth of E. coli. The levels of amplification of SQR and QFR were similar under anaerobic growth conditions. The catalytic properties of SQR isolated from anaerobically grown cells were measured and found to be identical to those of enzyme produced aerobically. The anaerobic expression of SQR gave a greater yield of enzyme complex than was found in the membrane from aerobically grown cells under the conditions tested. In addition, it was found that anaerobic expression of SQR could saturate the capacity of the membrane for incorporation of enzyme complex. As has been seen with the amplified QFR complex, E. coli accommodates the excess SQR produced by increasing the amount of membrane. The excess membrane was found in tubular structures that could be seen in thin-section electron micrographs.
Succinate dehydrogenase (succinate-ubiquinone oxidoreductase [SQR]) and fumarate reductase (menaquinol-fumarate oxidoreductase [QFR]) are enzyme complexes similar both in composition and in subunit structure, even though in vivo they normally catalyze their enzymatic reactions in opposite directions (2, 11). Each enzyme is a membrane-bound complex that can catalyze a two-electron or two-proton transfer between succinate or fumarate and quinone or quinol. Succinate dehydrogenase plays an important role in cellular metabolism and directly connects the Krebs cycle with the aerobic respiratory chain. Fumarate reductase catalyzes the final step in anaerobic respiration with fumarate as a terminal electron acceptor (21). These enzyme complexes are thus examples of the high evolutionary adaptation of organisms to specialized environments (11).
In Escherichia coli, two distinct operons encoding the SQR (sdhCDAB) (7, 37) and QFR (frdABCD) subunits are found (6, 22). Both enzymes have a large domain extrinsic to the membrane composed of a flavoprotein subunit (SdhA or FrdA) which contains a covalently bound flavin adenine dinucleotide cofactor and the dicarboxylate binding site (2, 11, 27) and an iron-sulfur protein subunit (SdhB or FrdB) containing three distinct iron-sulfur clusters (17, 20). This domain is bound to two small integral membrane subunits (SdhC and -D or FrdC and -D) which are necessary to form the quinone binding site(s) (4, 36) found in both of these enzyme complexes. One difference between SQR and QFR in E. coli is that SQR contains an additional prosthetic group, a heme b556 cofactor, which bridges the SdhC and SdhD subunits (33).
It has been 35 years since the first demonstration that in E. coli there are two distinct enzyme systems catalyzing fumarate reduction and succinate oxidation (13). The majority of mechanistic studies on succinate dehydrogenase, however, have been done on the mammalian enzyme from bovine mitochondria (2, 12). Kinetic experiments on SQR have shown that the enzyme is much more capable of oxidizing succinate than of reducing fumarate, with the ratio of succinate oxidation to fumarate reduction on the order of 40:1 (9). In contrast, more recent cyclic voltammetry studies of soluble succinate dehydrogenase (SdhAB) adsorbed on a graphite electrode showed that rates of electron transfer between succinate or fumarate and the electrode depended on the applied potential and pH. These results indicated that, at a potential of −75 mV and at pH 7.5, SdhAB functions as proficiently in fumarate reduction as in succinate oxidation (14). As a neutrophilic bacterium, E. coli maintains a cytoplasmic pH of 7.6 to 7.8 (23). In the bacterial cell, the composition of the quinone pool is controlled by oxygen. In order to reduce more negative terminal substrates than oxygen under anaerobic conditions, menaquinones (−75 mV) and demethylmenaquinone (+36 mV) predominate in the cell membrane (29, 32). Aerobic growth or exposure of cells to oxygen induces ubiquinone (+112 mV) production and represses menaquinone biosynthesis (29). Taken together, the above results would indicate that SQR should function as a fumarate reductase under physiological conditions, similar to the demonstrated ability of QFR to function physiologically as a succino-oxidase in cases where SQR function has been disrupted (10).
The control of sdhCDAB and frdABCD gene expression is provided by two cellular regulatory proteins, ArcA and Fnr, and responds to aerobic-anaerobic conditions and the cellular growth medium (16, 24). Under anaerobic conditions, sdhCDAB gene expression is repressed and frdABCD expression levels are increased more than 10-fold, whereas aerobic conditions have the opposite effect on SQR and QFR levels (18). Thus, under anaerobic conditions the levels of SQR in E. coli are not sufficient to support anaerobic respiration, with succinate dehydrogenase acting in reverse as a fumarate reductase. The present studies were undertaken to determine if in vivo SQR could functionally replace QFR, when conditions are provided to express the sdhCDAB genes anaerobically. To accomplish this, vectors in which the PFRD promoter of the frdABCD (fumarate reductase) operon was used to express the sdhCDAB genes were constructed and the cells were grown anaerobically under conditions requiring fumarate reduction for growth. The results of these studies and the properties of the SQR complex obtained from membranes of anaerobically grown cells are described below.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli strains and plasmids used in this study are described in Table 1. Strain DW35 (35), a derivative of MC4100, contains a deletion of the frd operon (ΔfrdABCD) and an insertional mutation in sdh (sdhC::Kan) (36) that eliminates strain background expression of any enzymes capable of succinate oxidase activity. Plasmid pSDH15 encodes the sdhC+D+A+B+ operon (33), both pH3 and pGC1002 contain the frdA+B+C+D+ (3, 4) operon, and all are derivatives of pBR322. The construction of plasmids pFGS and pFAS, where the PFRD promoter is used to control expression of the sdhCDAB genes, is described below. The latter plasmids are identical, except that pFGS has a GTG initiation codon for sdhC, whereas pFAS has an ATG initiation codon in sdhC.
TABLE 1.
E. coli strains and plasmids
| Strain or plasmid | Origin | Genotype | Source or reference |
|---|---|---|---|
| Strains | |||
| MC4100 | F−araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | 30 | |
| DW35 | DW31 | zjd::Tn10 Δ(frdABCD)18 sdhC::kan araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR | 36 |
| HB101 | supE44 hsdS20(rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 26 | |
| Plasmids | |||
| pSDH15 | pBR322 | PSDHsdhC+D+A+B+ Ampr | 33 |
| pGC1002 | pBR322 | PFRDfrdA+B+C+D+ Ampr | 4 |
| pH3 | pBR322 | PFRDfrdA+B+C+D+ Ampr | 3 |
| pH3-177 | pACYC177 | PFRDfrdA+B+C+D+ Ampr | This study |
| pFGS | pBR322 | PFRDsdhC+(GTG)D+A+B+ Ampr | This study |
| pFGS-177 | pACYC177 | PFRDsdhC+(GTG)D+A+B+ Ampr Kanr | This study |
| pFAS | pBR322 | PFRDsdhC+(ATG)D+A+B+ Ampr | This study |
Construction of plasmids.
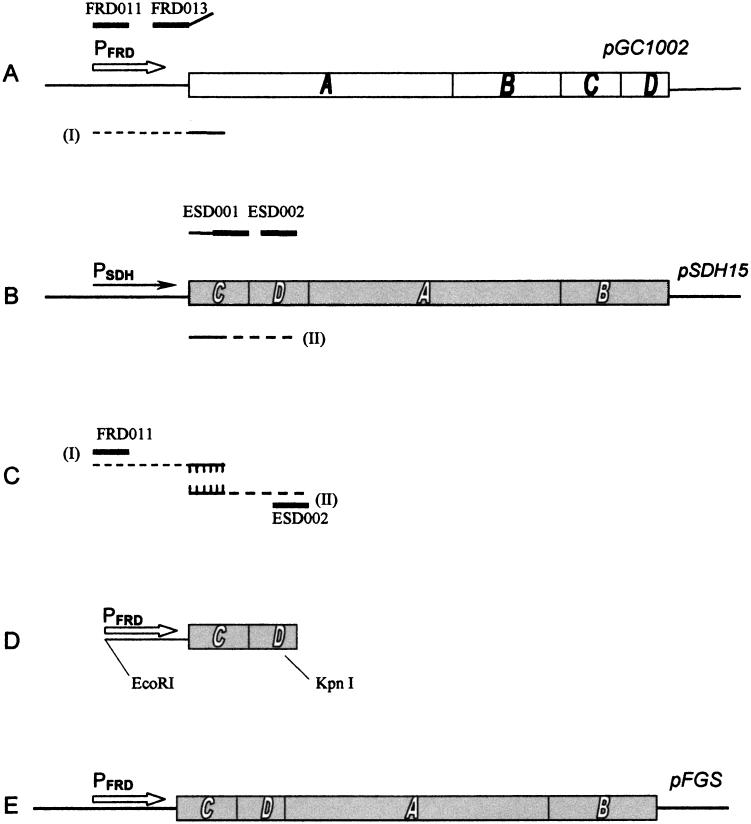
Molecular cloning and related procedures were carried out by standard methods (26). Promega kits (Promega Corporation, Madison, Wis.) were used for plasmid isolation. Plasmid pH3-177 was constructed by inserting the 4.44-kb HindIII-XhoI fragment of pH3 encompassing the frd operon into the HindIII-XhoI sites of pACYC177. The construction of plasmid pFGS is diagrammed in Fig. 1. The PFRD promoter was fused to the sdhCDAB genes by synthesizing two DNA fragments by PCR and then joining the fragments by the method of PCR overlap extension (15). The PFRD promoter was synthesized with pGC1002 (PFRD frdA+B+C+D+) as the template with oligonucleotide FRD011 (5′-CACGAATTCAAAGAACGACGG-3′) and either FRD012 (5′-CATTTCTTATCATGACATTCCTCCAG-3′) for plasmid pFAS or oligonucleotide FRD013 (5′-CATTTCTTATCACGACATTCCTCCAG-3′) for plasmid pFGS used as primer (Fig. 1A). Oligonucleotide FRD011 annealed 3′ of the NdeI site (5) in the noncoding region of the frd operon insert in pGC1002 and encodes an EcoRI extension. The first 13 nucleotides of FRD012 and FRD013 were complementary to the promoter sequence immediately 5′ of the GTG start codon of frdA, and the remaining nucleotides were complementary to the 5′ region of sdhC. The construction of pFAS was identical to that of pFGS except that oligonucleotide FRD012 was used as a primer in the PCRs in place of FRD013. FRD012 coded for an ATG initiation codon, as is found in wild-type sdhC, whereas for pFGS, FRD013 coded for a GTG start codon, as is normally found in frdA. A second PCR fragment (II) was synthesized, encompassing the complete sdhC gene and the 5′ end of the sdhD gene, with pSDH15 (sdhC+D+A+B+) as the template and oligonucleotide ESD001 (5′-TGATAAGAAATGTGAAAAAACAAAGAC-3′), which was complementary to the 5′ end of sdhC (excluding the first nucleotide), and ESD002 (5′-CATTGCGTCCTAATGCGGAGG-3′), which annealed within sdhD (Fig. 1B). The products from the PCRs (Fig. 1A and B, products I and II) were then used as the template for PCR overlap extension with FRD011 and ESD002 as the outside primers (Fig. 1C). The PCR product resulting from the reaction diagrammed in Fig. 1C was then digested with EcoRI and KpnI (a unique restriction site in the sdhD gene) and cloned into the appropriate sites in pSDH15 in order to reconstruct the sdh operon under the control of the PFRD promoter (Fig. 1D and E). This region was then sequenced to verify that no inadvertent mutations had been introduced into the coding region. Thus, the resulting plasmid pFGS has a PFRD-sdhC+D+A+B+ fusion and sdhC initiates with a GTG codon. Plasmid pFGS-177 was constructed by inserting the 4.39-kb AatII-BamHI fragment of pFGS, encompassing the complete frd promoter sdh gene region, into the AatII-BamHI sites of pACYC177.
FIG. 1.
Schematic diagram showing relevant features of construction of plasmid pFGS. The methodology is described in Materials and Methods. The PFRD promoter is indicated by the open arrow, and the PSDH promoter is indicated by the solid arrow. Oligonucleotide primers used in PCRs are indicated by thick solid lines. The resulting PCR products I and II are indicated by the dashed lines and, as shown in panel C, were joined by the method of PCR overlap extension (15). The frd genes in pGC1002 are indicated by solid lettering (A), whereas the sdh genes are indicated by open lettering (B). The EcoRI and KpnI restriction sites used to create the constructs are indicated.
Growth conditions.
Cells were grown overnight in Luria-Bertani medium with appropriate antibiotics, and then a 1:500 dilution was used as the inoculum for anaerobic minimal medium. Anaerobic growth was carried out at 37°C with constant moderate stirring with a magnet in 1-liter sealed bottles filled to the top with minimal glycerol-fumarate medium (31) supplemented with 0.05% (wt/vol) Casamino Acids and ampicillin (100 μg per ml). Fumarate and glycerol were used at 25 mM concentrations. Growth was monitored by determining cell optical density at 600 nm with a Uvikon 10 spectrophotometer (Kontron Instruments, Zurich, Switzerland).
Thin-section electron microscopy.
Cells were collected by centrifugation after 60 h of anaerobic growth and fixed with 2.7% glutaraldehyde–0.8% paraformaldehyde–0.2 M sodium cacodylate (pH 7.2). They were then washed with phosphate-buffered sucrose, postfixed in 2% OsO4, reduced with 1.5% potassium ferricyanide, and block stained in 2% uranyl acetate. The samples were dehydrated in ethanol and embedded in Spurr’s resin (Pelco, Inc., Redding, Calif.). Sections 70 nm thick were poststained with uranyl acetate and lead citrate and mounted on grids for electron microscopy.
Preparation of membrane fraction and enzyme purification.
Cells from 1 liter of culture were collected by centrifugation at 4,500 × g for 10 min, then suspended with 200 ml of 100 mM potassium phosphate–5 mM EDTA (pH 7.6), centrifuged again as described above, and then frozen at −70°C. Cells were then suspended in 40 ml of the same buffer containing the “Complete” protease inhibitor tablets (Boehringer Mannheim, Indianapolis, Ind.) and disrupted by sonication. Unbroken cells were removed by centrifugation (15 min at 10,000 × g), and the supernatant was centrifuged for 90 min at 100,000 × g to collect the membranes. The membrane fraction was suspended in 30 ml of the same buffer, and both centrifugation steps were repeated. Membranes were suspended in 50 mM potassium phosphate–0.2 mM EDTA (pH 7.8) to approximately 15 mg of protein/ml and frozen at −70°C. SQR was purified according to a published procedure (19). The purification of QFR was essentially the same as that for SQR, except that a linear gradient of 0.1 to 0.2 M NaCl was used for the DEAE fast-flow chromatography step.
Measurement of enzymatic activity and analytical procedures.
Membranes were thawed and diluted with 50 mM potassium phosphate–0.2 mM EDTA (pH 7.8) to a concentration of 1 mg/ml. In order to measure the full activity of SQR and QFR, it was necessary to remove bound oxaloacetate from the active site of the enzymes. Therefore, 10 mM malonate was added and samples were incubated for 15 min at 37°C and stored at 4°C until used. All enzyme assays were carried out at 30°C in 2-ml cuvettes with 50 mM potassium phosphate–0.2 mM EDTA (pH 7.8)–3 mM KCN. Measurement of succinate oxidation by phenazine ethosulfate (PES) and ubiquinone-2 (Q2) in the presence of dichlorophenolindophenol (ɛ600 = 21.8 mM−1 cm−1; pH 7.8) was performed as previously described (1). The substrates were used at the following concentrations: 10 mM succinate (pH 7.0), 50 μM dichlorophenolindophenol, and 1.5 mM PES or 20 μM Q2. The succinate-ferricyanide reductase activity of QFR was determined with 0.45 mM potassium ferricyanide (ɛ420 = 1 mM−1 cm−1). The quinol-fumarate reductase reaction was assayed in a coupled system with rat liver NADH-quinone reductase (DT Diaphorase), with Q2 and menaquinone-1 (MQ1) as described elsewhere (9). The succinate-quinone reductase and quinol-fumarate reductase reactions of purified complexes were determined in the presence of 0.005% (wt/vol) of the nonionic detergent Thesit (Boehringer Mannheim).
Absorption spectra were recorded at room temperature by using a diode array rapid-scanning spectrophotometer (8451A; Hewlett-Packard, Palo Alto, Calif.) in a 1-ml anaerobic cuvette. The spectrum of cytochrome b556 attributed to SQR was determined essentially as previously described (19) in 50 mM potassium phosphate–0.2 mM EDTA (pH 7.8) at a protein concentration of 0.25 mg/ml. To subtract the background of heme associated with bd oxidase, the distribution of this heme was measured in the presence of 0.5 mM N,N,N,N-tetramethyl-4-p-phenylenediamine (TMPD) and 0.5 M ascorbate (pH 7.0). Cytochrome b556 (ɛ558–575 = 22.8 mM−1 cm−1) associated with SQR was reduced with 10 mM succinate and sodium dithionite. Protein content was determined by the biuret method with bovine serum albumin as a standard.
RESULTS AND DISCUSSION
The rates of quinone reduction and quinol oxidation catalyzed by SQR and the QFR isolated and purified from aerobically and anaerobically grown cells, respectively, are shown in Table 2. It can be seen that the potential of the quinones affected the maximal rates of the reactions. The low-potential donor MQ1H2 was a better substrate for fumarate reduction. With the higher-potential acceptor Q2, the enzymes demonstrated higher rates of succinate oxidation. The data show that SQR catalyzes succinate oxidation by ubiquinone 40 times faster than it does fumarate reduction by ubiquinol (Q2H2). SQR catalyzed the reduction of fumarate 2.4 times faster with MQ1 than Q2. Moreover, when the enzyme operates with MQ1 it behaves as a fumarate reductase; the rate of fumarate reduction is more than 20 times higher than that of succinate oxidation. The activity of isolated QFR with quinone-quinols shows the opposite effects from that seen with SQR with respect to quinone potential. Thus, the data support the observation that QFR can functionally replace SQR when expressed aerobically (10). The data in Table 2 thus provide evidence that in vivo SQR should be able to support anaerobic respiration, assuming that conditions are found where the enzyme complex is able to be synthesized.
TABLE 2.
Succinate-quinone reductase and quinol-fumarate reductase activity catalyzed by isolated E. coli SQRa and QFRb (pH 7.8, 30°C)
| Quinone | Turnover no. (s−1) catalyzed by:
|
|||
|---|---|---|---|---|
| SQR
|
QFR
|
|||
| Succinate-quinone reductase | Quinol-fumarate reductase | Succinate-quinone reductase | Quinol-fumarate reductase | |
| Q2 | 80 | 27 | ||
| MQ1 | <0.2 | 11 | ||
| Q2H2 | 2 | <0.2 | ||
| MQ1H2 | 4.8 | 170 | ||
SQR was isolated from aerobically grown DW35 transformed with pSDH15.
QFR was isolated from anaerobically grown DW35 transformed with pH3.
The first gene in the frd operon (frdA) belongs to the small group of genes in E. coli which use a GUG initiation codon instead of the more common AUG (5). In order to investigate the ability of SQR to support anaerobic growth of E. coli, a PFRD-sdhCDAB fusion was constructed, with the PFRD promoter driving expression of the sdhCDAB genes. Constructs were made with either ATG (pFAS) or GTG (pFGS) as the start codon in sdhC and then introduced into vector pBR322 or pACYC177. Initial growth studies using anaerobic minimal glycerol-fumarate medium demonstrated that DW35 (Δfrd sdhC::kan) transformed with plasmid pFGS would grow with a doubling time of about 3.0 h after a long lag phase (data not shown). In order to reduce this lag phase, the medium was supplemented with varying amounts of tryptone and yeast extract. It was found that 0.025% (wt/vol) tryptone and yeast extract were sufficient to stimulate growth without allowing strain DW35 to grow anaerobically on glycerol-fumarate medium in the absence of QFR- or SQR-encoding plasmids. This medium was used for all further studies.
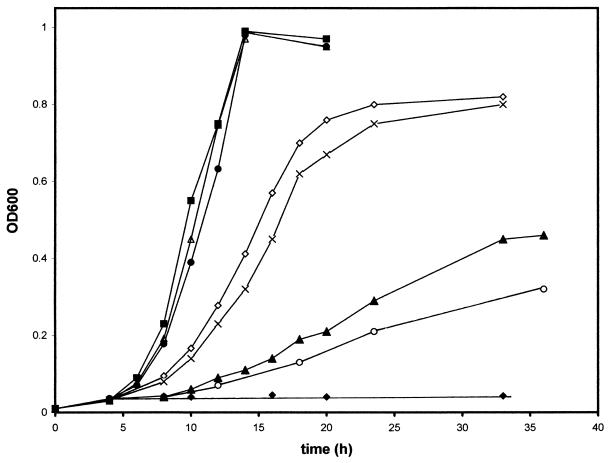
The results of anaerobic growth are depicted in Fig. 2. To maintain the plasmid in the cell, antibiotics were always present in the growth medium and the growth rates and final culture density were highly reproducible over numerous experiments. DW35, like the wild-type strain MC4100, grew with a doubling time of approximately 1.6 to 1.8 h when transformed with the wild-type QFR-encoding plasmid pH3 or pH3-177. Additionally, the SQR-encoding plasmid pFGS or pFGS-177 allowed the E. coli strain to grow with a 3.0- to 3.3-h doubling time, and the final cell density was approximately the same as that with the frdABCD plasmids. As a control, DW35 transformed with pSDH15 (sdhCDAB driven from the PSDH promoter in pBR322) was grown anaerobically. It can be seen that some growth is possible, although the doubling time is significantly slower than that with the pH3 or pFGS plasmid. It has been shown that the PSDH-lacZ fusions can be expressed anaerobically at about 3 to 5% of their aerobic expression level (28), and thus, this low-level expression from a multicopy plasmid with the PSDH promoter is able to support growth with a doubling time of greater than 8 h. The DW35/pSDH15 culture reproducibly stops growing at an optical density at 600 nm of about 0.35 after 48 h of growth, in contrast to results seen with other plasmids. As seen in Fig. 2, cells transformed with the SQR-encoding pFAS plasmid (sdhC initiates with an ATG codon) also supported growth but at a significantly slower doubling time of approximately 7.6 h, and the final cell density reached was only about half of that with the pFGS or pFGS-177 plasmid. A similar pattern of inhibition of growth by pFAS was also observed for anaerobically grown cultures on rich medium such as Terrific Broth (26) (data not shown). Aerobic cultures grown on either rich or minimal medium, however, do not show any inhibition by pFAS. The specific reason for inhibition of anaerobic growth by pFAS remains unknown; however, it may reflect the high level of amplification of the membrane-bound SQR from this plasmid (see below). It is possible that the overproduction of membrane-bound proteins from multicopy plasmids may be deleterious to host strains or that growth in the presence of pFAS may deplete the cultures of necessary nutrients for efficient growth. It should be noted, however, that this was not observed for overproduction of QFR in the experiments reported here or by others (2, 8, 34) or for SQR from pFGS (Fig. 2).
FIG. 2.
Anaerobic cell growth. DW35 (Δfrd sdhC::kan) cells carrying pBR322 (Ampr) (⧫), pH3 (PFRD frdABCD in pBR322) (•), pH3-177 (PFRD frdABCD in pACYC177) (▵), pFGS (PFRD sdhCGTG DAB in pBR322) (◊), pFGS-177 (PFRD sdhCGTGDAB in pACYC177) (×), pFAS (PFRD sdhCATGDAB in pBR322) (▴), and pSDH15 (PSDH sdhCDAB in pBR322) (○) and strain MC4100 (wild type) with pBR322 (■) were grown on glycerol-fumarate medium supplemented with 0.025% tryptone and 0.025% yeast extract as described in Materials and Methods. OD600, optical density at 600 nm.
The levels of SQR expression were monitored by measuring succinate dehydrogenase activities and heme b556 content in the membrane fraction of cells grown anaerobically (Table 3). The data show that the enzyme has the same turnover number regardless of the plasmid source used for its expression. Based on the amount of SQR-specific heme b556 produced from the PFRD promoter with pFGS, the data suggest that succinate dehydrogenase is amplified to the same levels as wild-type QFR from similar vectors. In addition, the SQR complex isolated and purified from both aerobically and anaerobically grown E. coli showed no differences in its kinetic properties, rate of heme reduction, composition, and/or stability compared with aerobically expressed wild-type SQR (data not shown). Based on the level of heme b556 in the membrane, it can be seen in Table 3 that the anaerobic expression of the SQR complex is some seven- to ninefold higher with plasmid pFGS or pFAS, respectively, than when expressed from its own promoter (pSDH15). It should also be noted that, based upon heme content, the levels of SQR produced from anaerobically grown E. coli containing pFAS or pFGS are approximately twofold higher than that found in aerobic cells where SQR (pSDH15) is expressed from its native promoter (Table 3). This result is supported by the observation that a three- to fivefold-higher yield of purified SQR enzyme complex is obtained from an equivalent cell mass of anaerobically grown E. coli containing either pFAS or pFGS than from aerobically grown cells containing pSDH15 (data not shown).
TABLE 3.
Specific activity and heme contents of membranes obtained from the anaerobically grown cellsa
| Strain or plasmid | Succinate-acceptor reductase activity (2 electron equivalents/min/mg of protein) (10−6)
|
Heme b556b (nmol/mg) | Turnover numberc (S−1) | Amplification leveld | Doubling time (h) | ||
|---|---|---|---|---|---|---|---|
| PES | K3Fe(CN)6 | Q2 | |||||
| frdABCD plasmid | |||||||
| MC4100 (wild type) | 0.6 | 0.6 | 0.3 | 25 | 1 | 1.6 | |
| pH3-177 | 6.1 | 6.6 | 3.3 | 27 | 11 | 1.6 | |
| pH3 | 7.6 | 9.4 | 4.6 | 28 | 15 | 1.8 | |
| sdhCDAB plasmid | |||||||
| pFGS-177 | 14 | —f | 12.8 | 2 | 102 | 11 | 3.3 |
| pFGS | 18.8 | — | 17.5 | 2.7 | 105 | 15 | 3.0 |
| pFAS | 24 | — | 22 | 3.6 | 102 | 19 | 7.6 |
| pSDH15 | 2.7 | — | 2.3 | 0.4 | 96 | 2 | >8 |
| Aerobically grown cellse (pSDH15) | 10.5 | — | 9.4 | 1.5 | 100 | NDg | ND |
All cells were DW35 except the MC4100 wild type.
Determined as level reduced with dithionite minus level reduced with ascorbate-TMPD.
Based on succinate-Q2 reductase reaction.
Compared to wild-type expression of QFR in MC4100.
Cultures were grown aerobically on the same medium as anaerobically grown cultures except that 25 mM succinate replaced fumarate.
SQR does not show succinate-ferricyanide reductase activity.
ND, not determined.
In the present study, E. coli succinate-quinone oxidoreductase, normally a component of the aerobic respiratory chain, was expressed during anaerobic cell growth on glycerol-fumarate medium by using the PFRD promoter to achieve expression. Under these conditions where quinol-fumarate reductase activity is essential for respiration, SQR functionally replaced QFR in the simplified anaerobic respiratory chain consisting of the anaerobic glycerol-3-phosphate dehydrogenase and menaquinones. Previous work from the Guest laboratory (10) has shown that fumarate reductase can offset the metabolic consequences of a deficiency of succinate dehydrogenase and thus replace the physiological function of succinate dehydrogenase. This was achieved by allowing amplification of fumarate reductase production aerobically through titration of a specific repressor (10). A similar observation was made when E. coli DW35 was grown with plasmid pH3 or pGC1002 on aerobic succinate minimal medium (3, 27, 36). The results described in this work directly demonstrate that the converse can occur, i.e., succinate dehydrogenase can physiologically replace fumarate reductase when conditions allow it to be expressed anaerobically. These results are also consistent with in vitro studies, using soluble beef heart succinate dehydrogenase, which suggest that the soluble enzyme (SdhAB domain) is physiologically capable of catalyzing fumarate reduction at pH values below 7.64 (14) and support the contention that complex II will also function as a fumarate reductase.
In spite of a significant difference in turnover numbers of menaquinol-fumarate reductase reaction of SQR and QFR (Table 2), the cells encoding succinate dehydrogenase from pFGS and pFGS-177 grew only about two times slower (Fig. 2 and Table 3) than cells with frdABCD. In the case of QFR, up to 15-fold amplification of enzyme levels did not affect the culture’s doubling time during anaerobic growth. These results suggest that the levels of fumarate reductase are not in themselves rate limiting for cell growth under the conditions tested. As shown in Table 3, the 10- to 15-fold overproduction of SQR obtained from plasmid pFGS and pFGS-177 is similar to the overproduction obtained from the QFR plasmids pH3-177 and pH3, which were constructed from the same vectors. This difference in SQR amplification in the membrane did not affect growth, as seen from the similar doubling times. It is significant to note, however, that although the level of enzyme produced in the membrane from plasmid pFAS is about 30% higher than that from pFGS, growth was impaired from this plasmid (Fig. 2). It has been found that the translation efficiency of E. coli proteins depends on the initiation codon, and the level of gene product is increased by changing the first codon from GUG to AUG (25). Furthermore, it has been shown that a promoter fusion, PFRD-lacZ+, starting with the ATG codon, gave a fivefold-higher expression level than that with protein fusion frdA′-lacZ+, which starts from a GTG codon (18). The data presented here are consistent with the higher level of expression when translation of the sdh genes begins with the AUG codon.
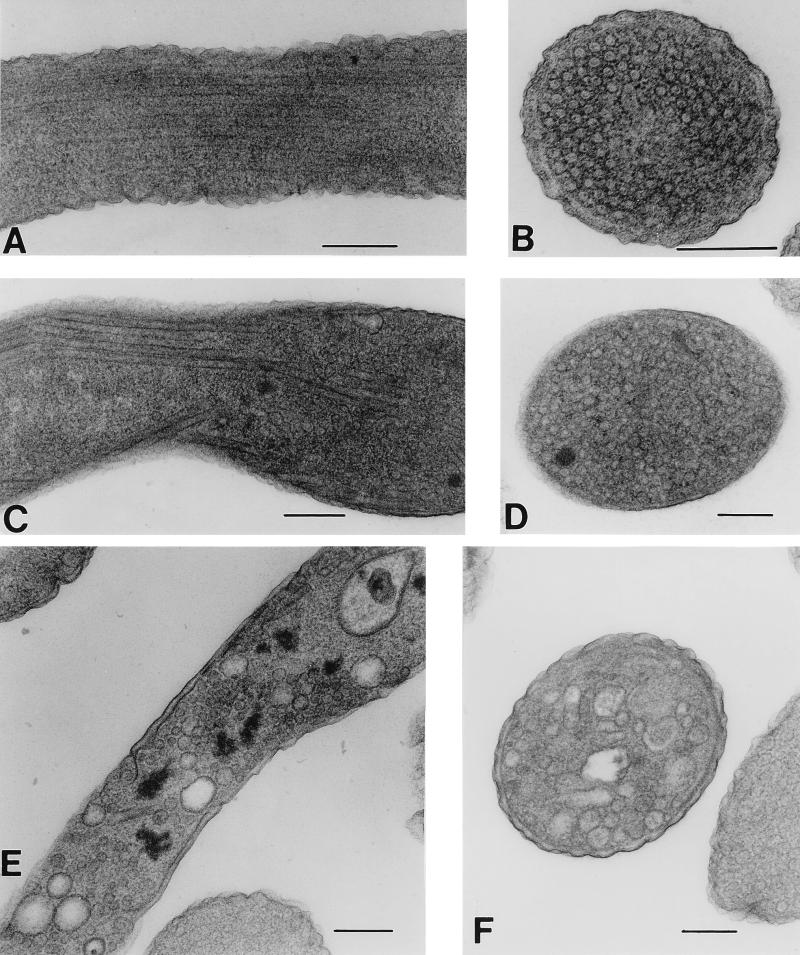
It has previously been shown that, in stationary-phase E. coli cells harboring a fumarate reductase-encoding plasmid similar to pH3, QFR production was amplified 20-fold (34). Because of the limited capacity of the inner membrane, the cells produced novel tubular structures rather than accumulating the extra protein in soluble form, or as inclusion bodies. The lipid-protein tubular structure was composed of QFR and enriched for cardiolipin compared to normal membranes (8, 34). Since SQR and QFR are similar in composition and function and because they are being expressed under similar growth conditions, it seemed likely that similar structures would be formed in cells containing the PFRD-sdhCDAB expression system. As shown in Fig. 3, structures resembling the QFR tubules are formed in DW35 transformed with pFAS when cells are grown anaerobically on glycerol-fumarate medium. While QFR overproduction induced the formation of tubular structures in the cytoplasm (Fig. 3A and B) (8, 34), cells harboring pFAS showed both tubular structures and vesicule-like structures in the cytoplasm (Fig. 3C to F). These differences are reproducible; however, the reason for the difference in type of structure formed is unknown. These structures are seen in either DW35 or a frd+ sdh+ strain of E. coli such as HB101. The overproduction of SQR causes tubule formation with either plasmid pFGS (not shown) or pFAS; however, it is more prevalent with the latter. It should also be noted that pFAS inhibits the growth rate of HB101 (similar to DW35 [Fig. 1]) even though this strain contains a wild-type copy of frdABCD. The significant decrease in cell doubling time seen in cultures containing pFAS may thus reflect the depletion of the resources necessary for efficient growth by enzyme overproduction.
FIG. 3.
Thin-section electron microscopy of E. coli. Cultures were grown to stationary phase as described in Materials and Methods. (A and B) Longitudinal and lateral sections, respectively, of HB101/pH3 expressing fumarate reductase. (C to F) DW35/pFAS cells expressing SQR complex: (C and D) tubule formation; (E and F) cytoplasmic vesicle formation from amplified expression of SQR. Bars, 0.2 μm.
The results described in this work are the first direct demonstration that succinate dehydrogenase can physiologically replace fumarate reductase when conditions allow it to be expressed anaerobically. An additional finding is that the isolated SQR complex obtained from anaerobically grown E. coli is produced in a fully active form and at a greater yield in the membrane (based on heme content) than aerobically produced enzyme. This is an aid in obtaining material for biochemical investigation of E. coli succinate dehydrogenase. By using the PFRD-sdhCDAB fusion with single-copy vectors, it should now also be possible to isolate SQR mutants that are altered in their ability to grow anaerobically. Questions remain as to the cause of the differences in catalytic activity seen with QFR and SQR; therefore, isolation of mutant forms of both enzyme complexes under physiological growth conditions will aid in understanding these differences.
ACKNOWLEDGMENTS
We thank Sandra L. Huling of the Morphology Core of the San Francisco VA Medical Center for her electron microscopy expertise. We also thank Orlena Chuck for assistance with the PCRs.
This research was supported by the Department of Veterans Affairs, National Institutes of Health grant HL-16251, and National Science Foundation grant MCB-9728778.
REFERENCES
- 1.Ackrell B A C, Kearney E B, Singer T P. Mammalian succinate dehydrogenase. Methods Enzymol. 1978;53:466–483. doi: 10.1016/s0076-6879(78)53050-4. [DOI] [PubMed] [Google Scholar]
- 2.Ackrell B A C, Johnson M K, Gunsalus R P, Cecchini G. Structure and function of succinate dehydrogenase and fumarate reductase. In: Muller F, editor. Chemistry and biochemistry of flavoenzymes. III. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 229–297. [Google Scholar]
- 3.Blaut M, Whittaker K, Valdovinos A, Ackrell B A C, Gunsalus R P, Cecchini G. Fumarate reductase mutants of Escherichia coli that lack covalently bound flavin. J Biol Chem. 1989;264:13599–13604. [PubMed] [Google Scholar]
- 4.Cecchini G, Thompson C R, Ackrell B A C, Westenberg D J, Dean N, Gunsalus R P. Oxidation of reduced menaquinone by the fumarate reductase complex in Escherichia coli requires the hydrophobic FrdD peptide. Proc Natl Acad Sci USA. 1986;83:8898–8902. doi: 10.1073/pnas.83.23.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole S T. Nucleotide sequence coding for the flavoprotein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982;122:479–484. doi: 10.1111/j.1432-1033.1982.tb06462.x. [DOI] [PubMed] [Google Scholar]
- 6.Cole S T, Guest J R. Genetic and physical characterization of lambda transducing phages (lambda frdA) containing the fumarate reductase gene of Escherichia coli K12. Mol Gen Genet. 1980;178:409–418. doi: 10.1007/BF00270492. [DOI] [PubMed] [Google Scholar]
- 7.Darlison M G, Guest J R. Nucleotide sequence encoding the iron-sulphur protein subunit of the succinate dehydrogenase of Escherichia coli. Biochem J. 1984;223:507–517. doi: 10.1042/bj2230507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elmes M L, Scraba D G, Weiner J H. Isolation and characterization of the tubular organelles induced by fumarate reductase overproduction in Escherichia coli. J Gen Microbiol. 1986;132:1429–1439. doi: 10.1099/00221287-132-6-1429. [DOI] [PubMed] [Google Scholar]
- 9.Grivennikova V G, Gavrikova E V, Timoshin A A, Vinogradov A D. Fumarate reductase activity of bovine heart succinate-ubiquinone reductase. New assay system and overall properties of the reaction. Biochim Biophys Acta. 1993;1140:282–292. doi: 10.1016/0005-2728(93)90067-p. [DOI] [PubMed] [Google Scholar]
- 10.Guest J R. Partial replacement of succinate dehydrogenase function by phage- and plasmid-specified fumarate reductase in Escherichia coli. J Gen Microbiol. 1981;122:171–179. doi: 10.1099/00221287-122-2-171. [DOI] [PubMed] [Google Scholar]
- 11.Hägerhäll C. Succinate:quinone oxidoreductases; variations on a conserved theme. Biochim Biophys Acta. 1997;1320:107–141. doi: 10.1016/s0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 12.Hederstedt L, Ohnishi T. Progress in succinate:quinone oxidoreductase research. In: Ernster L, editor. Molecular mechanisms in bioenergetics. Amsterdam, The Netherlands: Elsevier; 1992. pp. 133–198. [Google Scholar]
- 13.Hirsch C A, Rasminsky M, Davis B D, Lin E C C. A fumarate reductase in Escherichia coli distinct from succinate dehydrogenase. J Biol Chem. 1963;238:3770–3774. [PubMed] [Google Scholar]
- 14.Hirst J, Sucheta A, Ackrell B A C, Armstrong F A. Electrocatalytic voltammetry of succinate dehydrogenase: direct quantification of the catalytic properties of a complex electron-transport enzyme. J Am Chem Soc. 1996;118:5031–5038. [Google Scholar]
- 15.Horton R M, Pease L R. Recombination and mutagenesis of DNA sequences using PCR. In: McPherson M J, editor. Directed mutagenesis: a practical approach. Oxford, United Kingdom: Oxford University Press; 1991. pp. 217–247. [Google Scholar]
- 16.Iuchi S, Lin E C C. ArcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA. 1988;85:1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson M K, Kowal A T, Morningstar J E, Oliver M E, Whittaker K, Gunsalus R P, Ackrell B A C, Cecchini G. Subunit location of the iron-sulfur clusters in fumarate reductase from Escherichia coli. J Biol Chem. 1988;263:14732–14738. [PubMed] [Google Scholar]
- 18.Jones H M, Gunsalus R P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987;169:3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kita K, Vibat C R, Meinhardt S, Guest J R, Gennis R B. One-step purification from Escherichia coli of complex II (succinate:ubiquinone oxidoreductase) associated with succinate-reducible cytochrome b556. J Biol Chem. 1989;264:2672–2677. [PubMed] [Google Scholar]
- 20.Kowal A T, Werth M T, Manodori A, Cecchini G, Schröder I, Gunsalus R P, Johnson M K. Effect of cysteine to serine mutations on the properties of the [4Fe-4S] center in Escherichia coli fumarate reductase. Biochemistry. 1995;34:12284–12293. doi: 10.1021/bi00038a024. [DOI] [PubMed] [Google Scholar]
- 21.Kröger A. Fumarate as terminal acceptor of phosphorylative electron transport. Biochim Biophys Acta. 1978;505:129–145. doi: 10.1016/0304-4173(78)90010-1. [DOI] [PubMed] [Google Scholar]
- 22.Lohmeier E, Hagen D S, Dickie P, Weiner J H. Cloning and expression of fumarate reductase gene of Escherichia coli. Can J Biochem. 1981;59:158–164. doi: 10.1139/o81-023. [DOI] [PubMed] [Google Scholar]
- 23.Padan E, Schuldiner S. Intracellular pH regulation in bacterial cells. Methods Enzymol. 1986;125:337–352. doi: 10.1016/s0076-6879(86)25029-6. [DOI] [PubMed] [Google Scholar]
- 24.Park S J, Tseng C P, Gunsalus R P. Regulation of succinate dehydrogenase (sdhCDAB) operon expression in Escherichia coli in response to carbon supply and anaerobiosis: role of ArcA and Fnr. Mol Microbiol. 1995;15:473–482. doi: 10.1111/j.1365-2958.1995.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 25.Reddy P, Peterkofsky A, McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci USA. 1985;82:5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Schröder I, Gunsalus R P, Ackrell B A C, Cochran B, Cecchini G. Identification of active site residues of Escherichia coli fumarate reductase by site-directed mutagenesis. J Biol Chem. 1991;266:13572–13579. [PubMed] [Google Scholar]
- 28.Shen J, Gunsalus R P. Role of multiple ArcA recognition sites in anaerobic regulation of succinate dehydrogenase (sdhCDAB) gene expression in Escherichia coli. Mol Microbiol. 1997;26:223–236. doi: 10.1046/j.1365-2958.1997.5561923.x. [DOI] [PubMed] [Google Scholar]
- 29.Shestopalov A, Bogachev A V, Murtazina R A, Viryasov M B, Skulachev V P. Aeration-dependent changes in composition of the quinone pool in Escherichia coli. FEBS Lett. 1997;404:272–274. doi: 10.1016/s0014-5793(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 30.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 31.Spencer M E, Guest J R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973;114:563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unden G. Differential roles for menaquinone and demethylmenaquinone in anaerobic electron transport of E. coli and their fnr-independent expression. Arch Microbiol. 1988;150:499–503. doi: 10.1007/BF00422294. [DOI] [PubMed] [Google Scholar]
- 33.Vibat C R T, Cecchini G, Nakamura K, Kita K, Gennis R B. Localization of histidine residues responsible for heme axial ligation in cytochrome b556 of complex II (succinate:ubiquinone oxidoreductase) in Escherichia coli. Biochemistry. 1998;37:4148–4159. doi: 10.1021/bi9716635. [DOI] [PubMed] [Google Scholar]
- 34.Weiner J H, Lemire B D, Elmes M L, Bradley R D, Scraba D G. Overproduction of fumarate reductase in Escherichia coli induces a novel intracellular lipid-protein organelle. J Bacteriol. 1984;158:590–596. doi: 10.1128/jb.158.2.590-596.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westenberg D J, Gunsalus R P, Ackrell B A C, Cecchini G. Electron transfer from menaquinol to fumarate. Fumarate reductase anchor polypeptide mutants of Escherichia coli. J Biol Chem. 1990;265:19560–19567. [PubMed] [Google Scholar]
- 36.Westenberg D J, Gunsalus R P, Ackrell B A C, Sices H, Cecchini G. Escherichia coli fumarate reductase frdC and frdD mutants. Identification of amino acid residues involved in catalytic activity with quinones. J Biol Chem. 1993;268:815–822. [PubMed] [Google Scholar]
- 37.Wood D, Darlison M G, Wilde R J, Guest J R. Nucleotide sequence encoding the flavoprotein and hydrophobic subunits of the succinate dehydrogenase of Escherichia coli. Biochem J. 1984;222:519–534. doi: 10.1042/bj2220519. [DOI] [PMC free article] [PubMed] [Google Scholar]