Abstract
We report a clinical case of preoperative three-dimensional reconstruction combined with fluorescence imaging in simultaneous bilateral single-hole thoracoscopic treatment of pulmonary nodules. Fluorescent staining is widely used in clinics to show the interface between lung segments, and there are many reports in the literature. Indocyanine green (ICG), fluorescent staining method has the advantages of no need for repeated lung inflation and short time consumption. Moreover, the development of imaging three-dimensional reconstruction technology also provides convenience for accurate positioning of anatomical structures. Finally, we think that the application of preoperative three-dimensional reconstruction combined with fluorescence imaging for simultaneous double-lung single-hole thoracoscopic pneumonectomy can quickly and clearly display the interface between lung segments, provide technical support for the operation, shorten the operation time, reduce intraoperative bleeding, and have relatively high safety, and may shorten the learning curve for the growth of clinical thoracic surgeons, which may be worth popularizing and applying.
Keywords: Bilateral multiple pulmonary nodules, one-stage bilateral video-assisted thoracic surgery, three-dimensional reconstruction, fluorescence thoracoscope, anatomic segmentone-stage bilateral video-assisted thoracic surgeryectomy
Introduction
Ground-glass opacities (GGOs), also known as ground-glass nodules, are a radiological manifestation seen on chest X-rays or lung CT (Computer Tomography) scans. Surgery is usually the primary treatment for suspected malignant or progressive GGOs. However, surgeons often encounter difficulties in localizing small GGOs and determining their borders during the surgical procedure because minimal visual and tactile differences exist between GGOs and the surrounding lung tissue.
Imaging with indocyanine green (ICG) and intraoperative near infrared fluorescence has become an accurate and effective method to locate pulmonary nodules [1]. ICG is a fluorescent contrast agent that emits light in the near infrared spectrum and has been approved by the United States (US) Food and Drug Administration as an intravenous drug [2]. Near infrared fluorescence imaging of intravenous ICG has found many successful clinical applications in areas including ophthalmic angiography [3], cerebral perfusion assessment [4], retinal lymph node identification [5], and arterial perfusion assessment [2]. It is also widely used in resection of various tumor types because of its high contrast of specific targets.
In recent years, fluorescence staining has been widely used in clinical settings for displaying the interface between lung segments. It has been reported in much of the literature that indocyanine green (ICG) fluorescence staining method has the advantages of no need for repeated lung inflation and it has a short time consumption [6-8]. The development of imaging three-dimensional reconstruction technology has also enabled convenient and accurate location of anatomical structures [9,10].
With increasing attention being paid to health examination and the development of imaging technology, bilateral multiple pulmonary nodules (comprising two or more bilateral nodules) are increasingly being screened simultaneously, and are often confirmed to be malignant [11-13]. The surgical treatment of bilateral multiple pulmonary nodules typically involves either simultaneous operation or staged operation. Traditional bilateral simultaneous thoracotomy is considered highly invasive, and is associated with many complications and high mortality, particularly bilateral lobectomy, which has not been recommended in previous reports because it may cause substantial trauma [14-16]. Therefore, in the past, staging operation or unilateral operation with observation on the other side has often been used. As thoracoscopic technology has developed and matured, its notable advantage of minimal invasiveness has been widely recognized. Its application scope has gradually expanded from bilateral pulmonary bullae to lung cancer, and the number of reported cases is increasing. Bilateral video-assisted thoracic surgery (VATS) has little effect on cardiopulmonary function and is generally tolerated by patients and is safe and feasible in clinical settings [11,17].
Mimics is a set of highly integrated and easy-to-use 3Dimensions (3D) image generation and editing software, which can be used to generate 3D reconstruction images of patients’ pulmonary arteries and veins before operation to guide clinical surgery [18].
However, few clinical studies have examined preoperative three-dimensional reconstruction combined with fluorescence staining technology. Therefore, we sought to explore the application and clinical value of preoperative Mimics three-dimensional reconstruction combined with fluorescence imaging in simultaneous bilateral single-port thoracoscopic anatomical lung segmentectomy.
Case report
We report the case of a 60-year-old woman with no clear respiratory symptoms. Her physical examination findings and laboratory test results were normal. She had no history of smoking, personal history of tumors, or relevant surgical treatment. However, her two uncles had a history of lung cancer.
We adopted the strategy of combining preoperative imaging discussion, follow-up records and intraoperative freezing to implement the corresponding surgical methods. The right apical pulmonary nodule of the patient was only 5 mm in size. The perfect Positron emission tomography/computed tomography (PET/CT) examination before operation showed that there was no active high glucose metabolism, and early lung malignant tumor was not ruled out. After the collection of family medical history before operation, combined with auxiliary examination and follow-up records within the most recent two years, with the lung tumor markers being normal, and after discussion in the department we tended to suspect early lung cancer, so we took partial wedge resection of the upper lobe of the right lung. The left upper lobe lingual pulmonary nodule was about 12*9 mm in size, and the lingual artery had variation, and a segmental vein originated from the inferior pulmonary vein. Combined with the imaging findings, it was possible to imagine adenocarcinoma before operation, so we choose to do an anatomical lingual resection of the left upper lung. It has been reported that lobectomy plus lymph node dissection was the main surgical treatment for early lung cancer in the past, but with the deepening of research, especially the results of JCOG0802 study [19] and CALGB140503 study [20] which were published one after another, they provided high-level evidence for the implementation of sub-lobectomy. For some patients with early lung cancer, thoracoscopic segmental resection can achieve similar clinical effects as lobectomy, and at the same time, it can better protect lung function.
A small GGO was detected in the left S4-5 segment (left upper lobe lingular segment). High-resolution axial computed tomography revealed a marginated mixed GGO with clear boundaries and uneven density, without any evidence of distant metastasis (Figure 1). A lung nodule in the left upper lobe lingular segment, which was highly suspected to be adenocarcinoma, was assessed preoperatively.
Figure 1.

Thin-slice chest CT of the upper lobe of the left lung. A 60-year-old woman was found to have a mixed ground-glass opacity (GGO) in the upper left lung on high-resolution computed tomography (CT). A. CT image obtained with mediastinal window settings, showing solid components visible in the GGO. B. Axial CT image (5 mm thick slice), showing a circular mixed GGO with a diameter of 10 mm (orange arrow). C. Magnified image, showing details of the well-defined GGO border (blue arrow).
On the right side, a 5 mm GGO was observed in the S1 segment (right upper lobe apical segment) (Figure 2). Preoperative PET/CT examination suggested no evidence of increased glucose metabolism. However, on the basis of the preoperative collection of family medical history, auxiliary examinations, and the patient’s follow-up records over the prior 2 years, we believed that the possibility of early stage malignant lung tumor could not be ruled out for the right lung nodule.
Figure 2.

Thin-slice chest CT of the upper lobe of the right lung. A 60-year-old woman was found to have a pure ground-glass opacity (GGO) nodule in the upper right lung on high-resolution computed tomography (CT). A. CT image obtained with mediastinal window settings, showing no solid components in the GGO. B. Axial CT image (5 mm thick slice), showing a circular pure GGO with a diameter of 5 mm (orange arrow). C. Magnified image, showing details of the well-defined GGO border (blue arrow).
Preoperatively, Digital Imaging and Communications in Medicine (DCM) format images were imported into the MIMICS 21.0 system for three-dimensional reconstruction. The nodules were identified, and structures such as bronchi, arteries, and veins were reconstructed in three dimensions. The areas of the lung segments to be removed and preserved were marked on the reconstructed model, and the interface between them was delineated (Figure 3). Fluorescence thoracoscopy was chosen, and anatomical lung segmentectomy was performed intraoperatively, on the basis of the preoperative three-dimensional reconstruction model (Figure 4). The mode was switched to fluorescence mode, and the attending nurse was instructed to dissolve 25 mg of indocyanine green in 10 mL of normal saline and to administer 5 mL intravenously peripherally. The interface was marked through electrocoagulation (Figure 3), and precise subsegmental lung resection was subsequently performed at the interface. Concurrently, wedge resection was performed on the right lung nodule.
Figure 3.

Three-dimensional reconstruction image of the upper lobe of the left lung before operation. (A-C) Three-dimensional reconstruction image of the right upper lobe artery, vein, bronchus, and lesion. (D-F) Three-dimensional reconstruction image of bilateral pulmonary arteries, veins, bronchi, and lesions. (G-I) Three-dimensional reconstruction images of arteries, veins, bronchi, and lesions in the upper lobe of the left lung. The red arrow in (G) shows the variant lingual vein originating from the inferior pulmonary vein.
Figure 4.

Intraoperative fluorescence staining of the lung segment. A-C. Image obtained by thoracoscopic surgery, showing the target site of surgery, on the basis of fluorescence defects (blue arrow) and the normal lung tissue in fluorescent purple (red arrow).
Finally, the patient underwent intraoperative frozen biopsy and pathological confirmation. The intraoperative frozen biopsy suggested: adenocarcinoma in situ in the right upper lung; Tongue adenocarcinoma of the left upper lung (Figure 5), we cleaned the lymph nodes in groups 5, 7, 9, 10, 11 and 12 at the same time. The patient’s operation lasted for 6 hours, with a bleeding volume of about 500 ml, and she was monitored in the ward after operation.
Figure 5.
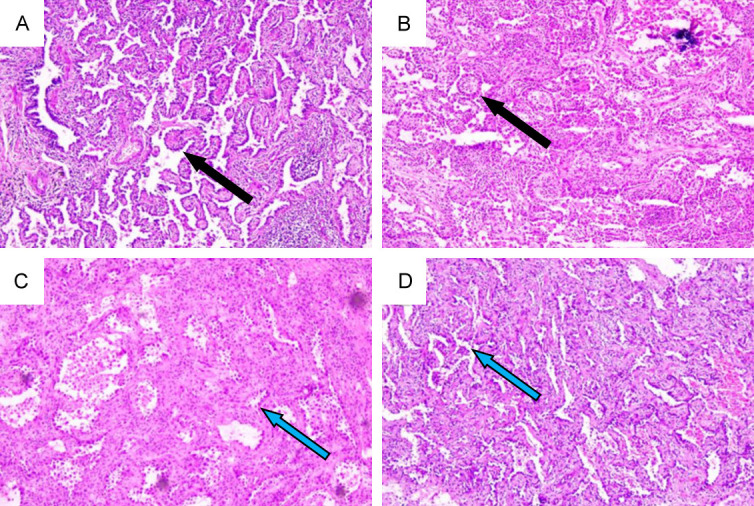
Postoperative pathological immunohistochemistry. A, B. Histological specimen, showing infiltrating adenocarcinoma in the left lung. The tumor cells exhibit invasive growth around the segmental bronchus, with predominant growth along the alveolar epithelium, partly forming papillary structures. A small number of micro-papillary structures can be observed. (black arrow) The tumor stroma shows elevated lymphoid tissue proliferation, with the formation of lymphoid follicles. The surgical margins are negative (IHC ×40). C, D. Histological specimen, showing micro-invasive adenocarcinoma in the right lung. Elevated atypical alveolar epithelial proliferation is observed, with enlarged, deeply stained tumor cell nuclei exhibiting nail-like growth (blue arrow). The tumor foci exhibit a glandular infiltration growth pattern, accompanied by stromal proliferation and infiltration of inflammatory cells. The infiltrative foci measure <0.2 cm (IHC ×40).
Discussion
We reported a clinical case in which three-dimensional reconstruction combined with fluorescence imaging technology was used for GGO resection under 4K fluorescence thoracoscopy after intravenous injection of ICG. The success of this case expands the application of ICG to simultaneous bilateral pulmonary nodule resection.
For multiple bilateral lung lesions, individualized surgical approaches should be taken according to the characteristics of the lesions and the overall condition of the patient. With rigorous preoperative screening and meticulous perioperative management, simultaneous bilateral thoracoscopic surgery is safe and feasible.
In thoracic surgery, three-dimensional reconstruction technology has found applications including preoperative planning in lung cancer treatment [21], respiratory motion analysis of lung cancer tumors, and calculation of the degree of lung tissue compression in pneumothorax. In lung segment surgery, this technology enables precise three-dimensional reconstruction of relevant pulmonary vessels, bronchi, tumor locations, etc., thus clarifying the need for resection of specific segmental vessels and bronchi, and helping thoracic surgeons identify target vessels and bronchi. Consequently, this modality provides important surgical references for lung segment surgery, thereby decreasing surgical difficulty and time. ICG fluorescence thoracoscopy has been applied in fields such as thoracic surgery, hepatobiliary surgery, general surgery, and neurosurgery [22]. This technology has also been used for preoperative localization of pulmonary nodules, intraoperative inter-segmental plane display, sentinel lymph node tracing, visualization of sympathetic nerves and the thoracic duct, and evaluation of the blood supply to the gastric conduit in esophageal cancer [23].
The technique of 4K fluorescence thoracoscopy can rapidly and accurately display intersegmental planes in anatomical segmentectomy. When combined with MIMICS three-dimensional reconstruction, this technique can decrease the time required for identifying target segment vessels and the trachea during surgery, as well as for identifying intersegmental planes. Consequently, the surgical difficulty and duration can be decreased, rapid patient recovery can be facilitated, and contemporary fast-track, minimally invasive, precision surgery can be achieved.
In this case, the variation in the lingual vein originating from the lower pulmonary vein was discovered during preoperative planning. Ultimately, precise resection of the lingual segment of the left upper lobe and wedge resection of the right upper lobe were successfully performed. Therefore, we believe that the application of preoperative three-dimensional reconstruction combined with fluorescence imaging for simultaneous single-port thoracoscopic pulmonary segmentectomy can rapidly and clearly display the intersegmental boundaries, provide technical support for surgery, potentially shorten the surgical time, decrease intraoperative bleeding, and have relatively high safety. Moreover, it may shorten the learning curve for clinical thoracic surgeons, and thus may be worthy of promoting and applying.
However, there are some limitations in this study. First of all, the sample size is small, and the three-dimensional reconstruction technology combined with ICG based on fluorescence imaging did not compare the number of cases sufficiently to verify the clinical benefits of this new preoperative and intraoperative strategy for simultaneous bilateral pulmonary nodule resection. Follow-up needs large samples for further clinical trials. Secondly, this is a single-center study, which has limitations and needs further study in multi-center clinical trials. Finally, the combination of preoperative three-dimensional reconstruction planning and indocyanine green fluorescence staining localization. Long-term follow-up has not been carried out on the influence on lung function of patients in the later stage, and further clinical trials are still needed in the follow-up.
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. The present study was supported in part by the Dean’s Fund of the Fifth Affiliated Hospital of Southern Medical University (grant Nos. YZ2022ZX08 and YZ2022ZX10) and the 2019 Heilongjiang Provincial Health and Health Commission Scientific Research Project (grant No. 2019-445).
Disclosure of conflict of interest
None.
References
- 1.Mao Y, Chi C, Yang F, Zhou J, He K, Li H, Chen X, Ye J, Wang J, Tian J. The identification of sub-centimetre nodules by near-infrared fluorescence thoracoscopic systems in pulmonary resection surgeries. Eur J Cardiothorac Surg. 2017;52:1190–1196. doi: 10.1093/ejcts/ezx207. [DOI] [PubMed] [Google Scholar]
- 2.Gosvig K, Jensen SS, Qvist N, Agnus V, Jensen TS, Lindner V, Marescaux J, Diana M, Ellebæk MB. Remote computer-assisted analysis of ICG fluorescence signal for evaluation of small intestinal anastomotic perfusion: a blinded, randomized, experimental trial. Surg Endosc. 2020;34:2095–2102. doi: 10.1007/s00464-019-06990-w. [DOI] [PubMed] [Google Scholar]
- 3.Pichi F, Aggarwal K, Neri P, Salvetti P, Lembo A, Nucci P, Gemmy Cheung CM, Gupta V. Choroidal biomarkers. Indian J Ophthalmol. 2018;66:1716–1726. doi: 10.4103/ijo.IJO_893_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forcione M, Chiarelli AM, Davies DJ, Perpetuini D, Sawosz P, Merla A, Belli A. Cerebral perfusion and blood-brain barrier assessment in brain trauma using contrast-enhanced near-infrared spectroscopy with indocyanine green: a review. J Cereb Blood Flow Metab. 2020;40:1586–1598. doi: 10.1177/0271678X20921973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng HC, Hu JL, Bai JW, Zhang GJ. Detection of sentinel lymph nodes with near-infrared imaging in malignancies. Mol Imaging Biol. 2019;21:219–227. doi: 10.1007/s11307-018-1237-4. [DOI] [PubMed] [Google Scholar]
- 6.Ito A, Takao M, Shimamoto A, Shimpo H. Prolonged intravenous indocyanine green visualization by temporary pulmonary vein clamping: real-time intraoperative fluorescence image guide for thoracoscopic anatomical segmentectomy. Eur J Cardiothorac Surg. 2017;52:1225–1226. doi: 10.1093/ejcts/ezx233. [DOI] [PubMed] [Google Scholar]
- 7.Mehta M, Patel YS, Yasufuku K, Waddell TK, Shargall Y, Fahim C, Hanna WC. Near-infrared mapping with indocyanine green is associated with an increase in oncological margin length in minimally invasive segmentectomy. J Thorac Cardiovasc Surg. 2019;157:2029–2035. doi: 10.1016/j.jtcvs.2018.12.099. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, Yang R, Cao H. Near-infrared intraoperative imaging with indocyanine green is beneficial in video-assisted thoracoscopic segmentectomy for patients with chronic lung diseases: a retrospective single-center propensity-score matched analysis. J Cardiothorac Surg. 2020;15:303. doi: 10.1186/s13019-020-01310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu YJ, Shi QT, Zhang Y, Wang YL. Thoracoscopic segmentectomy and lobectomy assisted by three-dimensional computed-tomography bronchography and angiography for the treatment of primary lung cancer. World J Clin Cases. 2021;9:10494–10506. doi: 10.12998/wjcc.v9.i34.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Dai K, Chen C, Liang Z. Subdivision and presentation of the pulmonary vasculature of the right upper lobe for anatomical segmentectomy with three-dimensional computed tomography reconstruction. Asian J Surg. 2021;44:1023–1025. doi: 10.1016/j.asjsur.2021.04.045. [DOI] [PubMed] [Google Scholar]
- 11.Kim KS. Single-staged uniportal VATS in the supine position for simultaneous bilateral primary spontaneous pneumothorax. J Cardiothorac Surg. 2017;12:25. doi: 10.1186/s13019-017-0591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Fu F, Chen H. Management of ground-glass opacities in the lung cancer spectrum. Ann Thorac Surg. 2020;110:1796–1804. doi: 10.1016/j.athoracsur.2020.04.094. [DOI] [PubMed] [Google Scholar]
- 13.Jiang L, He J, Shi X, Shen J, Liang W, Yang C, He J. Prognosis of synchronous and metachronous multiple primary lung cancers: systematic review and meta-analysis. Lung Cancer. 2015;87:303–310. doi: 10.1016/j.lungcan.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Nakata M, Sawada S, Yamashita M, Saeki H, Kurita A, Takashima S, Tanemoto K. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg. 2004;78:1194–1199. doi: 10.1016/j.athoracsur.2004.03.102. [DOI] [PubMed] [Google Scholar]
- 15.Yao F, Yang H, Zhao H. Single-stage bilateral pulmonary resections by video-assisted thoracic surgery for multiple small nodules. J Thorac Dis. 2016;8:469–475. doi: 10.21037/jtd.2016.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Ma L, Lin F, Mei J, Pu Q, Liao H, Guo C, Liu L. Single-staged uniportal VATS major pulmonary resection for bilateral synchronous multiple primary lung cancers. J Thorac Dis. 2014;6:1315–1318. doi: 10.3978/j.issn.2072-1439.2014.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S, Yang C, Guo X, Xu Y, Wang L, Wang Z, Yu X, Wang C, Yu Z. Simultaneous Uniportal video-assisted thoracic surgery of bilateral pulmonary nodules. J Cardiothorac Surg. 2021;16:42. doi: 10.1186/s13019-021-01423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang R, Huang J, Sun X, Chu X, Wang F, Zhou J, Fan Q, Pang L. Construction of in vitro 3-D model for lung cancer-cell metastasis study. BMC Cancer. 2022;22:438. doi: 10.1186/s12885-022-09546-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K, Aoki T, Okami J, Yoshino I, Ito H, Okumura N, Yamaguchi M, Ikeda N, Wakabayashi M, Nakamura K, Fukuda H, Nakamura S, Mitsudomi T, Watanabe SI, Asamura H West Japan Oncology Group and Japan Clinical Oncology Group. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399:1607–1617. doi: 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- 20.Altorki NK, Wang X, Wigle D, Gu L, Darling G, Ashrafi AS, Landrenau R, Miller D, Liberman M, Jones DR, Keenan R, Conti M, Wright G, Veit LJ, Ramalingam SS, Kamel M, Pass HI, Mitchell JD, Stinchcombe T, Vokes E, Kohman LJ. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503) Lancet Respir Med. 2018;6:915–924. doi: 10.1016/S2213-2600(18)30411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SX, Liu Y. Computer-based three-dimensional reconstruction of lung cancer using 64-slice CT scanning data and virtual surgery. Nan Fang Yi Ke Da Xue Xue Bao. 2016;36:562–566. [PubMed] [Google Scholar]
- 22.Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–255. [PMC free article] [PubMed] [Google Scholar]
- 23.Nusrath S, Thammineedi SR, Saksena AR, Patnaik SC, Reddy P, Usofi Z, Kumar S. Thoracic duct lymphography by near-infrared indocyanine green fluorescence imaging in thoracic surgery. A review. Indian J Surg Oncol. 2022;13:415–420. doi: 10.1007/s13193-022-01493-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


