Abstract
There is a serious worldwide health problem caused by chronic kidney disease (CKD), yet there are few viable therapies. Therapeutic promise in the treatment of chronic kidney disease (CKD) has been shown by the use of the traditional Chinese herbal compound Shengyang Yiwei Decoction (SYD). However, the chemical processes through which SYD exerts its effects are still unknown. The purpose of this network pharmacology research is to better understand the molecular mechanism of action of Shengyang Yiwei Decoction (SYD) in the treatment of chronic kidney disease (CKD). Traditional Chinese Medicine Systems Pharmacology (TCMSP) was first searched for information on the chemical components of Shengyang Yiwei Decoction. The molecular targets of SYD were then predicted using the Pharm Mapper service. After that, we used databases like DIG-SEE, TTD, and OMIM to zero down on the targets most closely linked to CKD. Cytoscape 3.2.1 was used to generate the component-target network representing SYD’s therapy of CKD. In addition, KEGG signal pathways and GO biological processes were analyzed using the DAVID database, and the findings were displayed via OmicShare Tools. Twenty-two active components were isolated from Shengyang Yiwei Decoction, and they were linked to 36 therapeutic targets for CKD in the current investigation. According to the results of the network pharmacology study, 41 signaling pathways are involved in mediating the therapeutic effects of SYD. In addition, SYD’s broad therapeutic impact in CKD therapy was shown to include 29 molecular activities, 14 cell components, and 91 biological processes. This research utilizes a multivariate analysis to provide light on the strategies and outcomes of treating CKD using Shengyang Yiwei Decoction. Clinical therapeutic methods for CKD management may benefit greatly from a thorough knowledge of the underlying processes and material foundation of this disease.
Keywords: Protein-protein interaction network, chronic kidney disease, Shengyang Yiwei Decoction, miRNA-mRNA regulatory network
Introduction
Lower limb edema, weariness, and weakness around the waist and knees are all signs of chronic kidney disease (CKD), a common and complicated chronic illness characterized by renal failure and structural damage. In addition to having negative effects on patients’ health, CKD’s protracted course also places an economic and emotional strain on their loved ones and communities [1]. The major method for treating CKD nowadays is symptomatic management, which aims to reduce protein consumption, manage blood pressure, reduce symptoms, and delay the course of the illness. In spite of their widespread use, these therapies have been shown to be only somewhat successful in treating glomerular basement membrane disease and decreasing urine protein levels [2].
Recent studies have shown that traditional Chinese medicine (TCM) may slow the course of CKD and improve clinical symptoms, therefore it is now considered an essential aspect of CKD therapy. Systemic modulation, multi-target effects, and complicated multi-component formulations are just a few of TCM’s distinguishing features. Research from several angles, not just one, is needed to learn more about the processes and formulation principles of TCM treatments in the development of CKD. Because of this intricacy, improving TCM formulations and understanding their processes has been slowed [3].
Together, the development of network pharmacology and advances in genomics technologies like metabolomics and proteomics provide the groundwork for rigorously and scientifically elucidating the pharmacological processes, material basis of effectiveness, and fundamental principles of traditional Chinese medicine. CKD is classified by TCM based on symptoms such as swelling, weakness, blood in the urine, and cloudy urine, with causes such as moisture, turbidity, blood stasis, and a lack of spleen and stomach qi being blamed. Eliminating turbidity, dissipating moisture, strengthening the spleen, and replenishing qi are common TCM therapeutic concepts for CKD [3].
Poria cocos, codonopsis pilosula, and astragalus are just a few of the medicinal herbs that make up the TCM recipe Shengyang Yiwei (SHY) Decoction, which has the ability to clear the airways, strengthen the spleen, and increase qi. Particularly important for illness prevention and prediction is its capacity to decrease the amount of protein in the urine [4]. Therefore, the purpose of this research is to use network pharmacology technique to investigate the targets and active components of SHY Decoction for the treatment of CKD in order to provide a systematic and thorough explanation of its therapeutic actions. This report details what was learned from the probe.
Materials and methods
Collection and screening of active ingredients in Shengyang Yiwei Decoction
Astragalus, ginseng, codonopsis pilosula, coptis chinensis, pinellia ternata, atractylodes macrocephala, tangerine peel, poria cocos, and fangfeng had their chemical components and ADME (Absorption, Distribution, Metabolism, and Excretion) data culled from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database. Oral bioavailability (OB) ≥ 30% and drug-like index (DL) ≥ 0.18 were used to identify active compounds. For further study, the chosen active compounds were saved in mol2 format.
Prediction and screening of potential targets using a pharmacophore model
Molecular docking using the Pharm Mapper service was used to examine the mol2 files of active component molecules uncovered in the prior screening. Target proteins were identified by searching the UniProt Knowledgebase (UniProt KB) database for Homo sapiens (Human) and matching the anticipated targets. We looked for the genes that coded for the proteins of interest.
GEO data acquisition and difference analysis
The GSE62792 dataset from the Gene Expression Omnibus (GEO) database at the National Centre for Biotechnology Information (NCBI) was downloaded. Data from 18 groups, including both CKD and healthy controls, were included in the mRNA transcriptome dataset. The research made use of the GPL10558 annotation platform. To find genes that were expressed differently in the control and CKD groups and to investigate the underlying molecular mechanisms, a differential gene expression analysis was carried out using the “limma” software, with a screening criteria of P < 0.05.
Collection and screening of CKD-related genes
Genes associated with CKD were compiled from many sources, including the DIG-SEE online database and the Therapeutic Target Database (TTD). Genes involved in the pathophysiology of CKD were identified as active targets and the target genes for Shengyang Yiwei Decoction were made by a comparison and analysis of illness target genes and probable action target genes of the active components.
Network construction and analysis
The software package Cytoscape 3.2.1 was used to create a network diagram showing the connections between the components of Shengyang Yiwei Decoction and the CKD-related target genes that they affect. Topological analysis using intermediate centrality and node degree was made easier by the CytoNCA plugin, which allowed for the identification of key target genes.
Immunocyte infiltration analysis
Gene expression profiles were employed in the CIBERSORT algorithm to characterize and analyse the make-up of immune cells. The CIBERSORT method was used to extract RNS-seq data from several patient subgroups to calculate an approximate percentage of immune infiltrate cells. Immune cell interactions were investigated using the “corrplot” programme, and immune cell relative contents were visualized using the “vioplot” tool. The influence of genes on immunological infiltration was analysed using a Spearman correlation test, with significance set at P < 0.05.
Analysis of KEGG signal pathway and GO biological process
Shengyang Yiwei Decoction was used to treat CKD-related genes, and the DAVID database was used to enrich and analyse the KEGG signal pathways and Gene Ontology (GO) biological processes involved. Mapping was used to provide a visual representation of the enrichment information.
Construction of miRNA-mRNA network
Predicted miRNAs against which important genes were screened for function were compiled from the DIANA TOOLS database. With a miTG score > 0.9 as a screening criterion, mRNA-miRNA interactions were discovered. We used Cytoscape to examine the miRNA-mRNA network we built.
Statistical analysis
The R programming language (version 4.0) was used for all statistical analyses, and all tests were conducted in both directions. The cutoff for significance was determined to be P < 0.05.
Results
Active ingredients of Shengyang Yiwei Decoction
We looked into the contents of Shengyang Yiwei Decoction and found that it contains astragalus, ginseng, pinellia ternata, and coptis chinensis as its active constituents. TCMSP allowed us to get information on 125 ginseng chemical compounds and 87 astragalus species. Using cutoff values of DL ≥ 0.18 and OB ≥ 30%, we isolated chemical components from two varieties of ginseng, twenty types of astragalus, fourteen types of coptis chinensis, and twelve types of pinellia ternata. It is noteworthy that there was no overlap in the components of these compounds (Table 1).
Table 1.
Effective ingredients screened from Shengyang Yiwei Decoction
| Medicinal | Ingredient ID | Ingredients | OB/% | DL |
|---|---|---|---|---|
| Astragalus | MOL000098 | Quercetin | 46.43 | 0.28 |
| MOL000442 | 1,7-Dihydroxy-3,9-dimethoxy ptero-carpene | 39.05 | 0.48 | |
| MOL000439 | Isomucronulatol-7,2’-di-O-glucosiole | 49.28 | 0.62 | |
| MOL000438 | (3R)-3-(2-hydroxy-3,4-dimethoxy-phenyl)chroman-7-ol | 67.67 | 0.26 | |
| MOL000433 | FA | 68.96 | 0.71 | |
| MOL000422 | Kaempferol | 41.88 | 0.24 | |
| MOL000417 | Calycosin | 47.75 | 0.24 | |
| MOL000398 | Isoflavanone | 109.99 | 0.3 | |
| MOL000392 | Formononetin | 69.67 | 0.21 | |
| MOL000387 | Bifendate | 31.1 | 0.67 | |
| MOL000380 | (6aR,11aR)-9,10-dimethoxy-6a,11a-dihydro-6H-benzofurano[3,2-c]chromen-3-ol | 64.26 | 0.42 | |
| MOL000378 | 7-O-dimethoxypterocarpan-3-O-β-D-glucoside | 36.74 | 0.92 | |
| MOL000374 | 5’-hydroxyiso-muronulatol-2’,5’-di-O-glucoside | 41.72 | 0.69 | |
| MOL000371 | 3,9-di-O-methylnissolin | 53.74 | 0.48 | |
| MOL000354 | Isorhamnetin | 49.6 | 0.31 | |
| MOL00033 | (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R,5S)-5-propan-2-yloctan-2-y]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopernta phenanthren-3-ol | 36.23 | 0.78 | |
| MOL000296 | Hederagenin | 36.91 | 0.75 | |
| MOL000239 | Jaranol | 50.83 | 0.29 | |
| MOL000211 | Mairin | 55.38 | 0.78 | |
| Ginseng | MOL000449 | Stigmasterol | 43.83 | 0.76 |
| MOL000358 | Beta-sitosterol | 36.91 | 0.76 | |
| Pinellia ternata | 24-Ethylcholest-4-en-3-one | 36.08 | 0.76 | |
| Cavidine | 35.64 | 0.81 | ||
| Baicalein | 33.52 | 0.21 | ||
| Baicalin | 40.12 | 0.75 | ||
| Beta-sitosterol | 36.91 | 0.75 | ||
| Stigmasterol | 43.83 | 0.76 | ||
| Gondoicacid | 30.70 | 0.20 | ||
| Coniferin | 31.11 | 0.32 | ||
| 10,13-eicosadienoic | 39.99 | 0.20 | ||
| 12,13-epoxy-9-hydroxynonadeca-7,10-dienoic | 42.15 | 0.24 | ||
| Beta-D-Ribofuranoside,xanthine-9 | 44.72 | 0.21 | ||
| Cycloartenol | 38.69 | 0.78 | ||
| Coptis chinensis | Coptisine | 30.67 | 0.86 | |
| Palmidin A | 35.74 | 0.65 | ||
| Berberrubine | 35.74 | 0.73 | ||
| Berlambine | 36.68 | 0.82 | ||
| Berberine | 36.86 | 0.78 | ||
| Epiberberine | 43.09 | 0.78 | ||
| Obacunone | 43.29 | 0.77 | ||
| Worenine | 45.83 | 0.87 | ||
| Quercetin | 46.43 | 0.28 | ||
| (R)-Canadine | 55.37 | 0.77 | ||
| Magnogradiolide | 63.71 | 0.19 | ||
| Palmatine | 64.60 | 0.65 | ||
| Moupinamide | 86.71 | 0.26 | ||
| Corchoroside A-qt | 104.95 | 0.78 |
Screening of “drug disease” intersection targets and construction of protein-protein interaction network
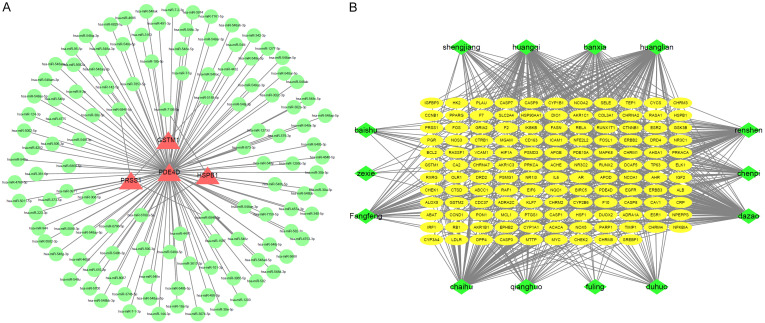
We gathered 249 potential targets for CKD and the Jianpi Yishen prescription in order to better understand the relationship between Shengyang Yiwei Decoction and CKD. Using the String database, we reduced the number of unrelated targets by 40 by setting the minimum needed interaction score to 0.900. We used Cytoscape 3.7.1 to display the resulting 209 protein-protein interactions in Figure 1A.
Figure 1.
A. Protein interaction network diagram of common target of Shengyang Yiwei Decoction and CKD. B. Gene network diagram of target components of Shengyang Yiwei Decoction.
Analysis of interaction network between active ingredients and CKD targets
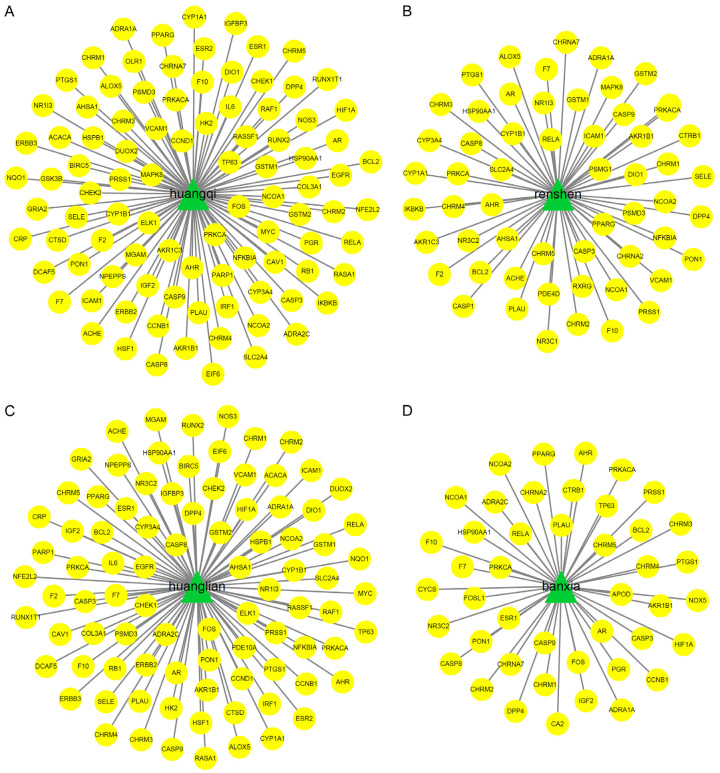
We used the Pharm Mapper system to convert the anticipated target proteins to gene names and then compared those names to a database of CKD-related genes. Thirty-six matching genes were found as a result of this study. To create a network pharmacological diagram (Figure 1B), we then imported the components of Shengyang Yiwei Decoction and the CKD genes to which they are linked into Cytoscape 3.2.1. In addition, separate target gene networks (Figure 2A-D) were constructed for astragalus, ginseng, codonopsis pilosula, and coptis chinensis.
Figure 2.
A. Network diagram of astragalus target genes. B. Ginseng target gene network. C. Gene network of coptis chinensis target. D. Pinellia ternata target gene network.
Biological function analysis of targets
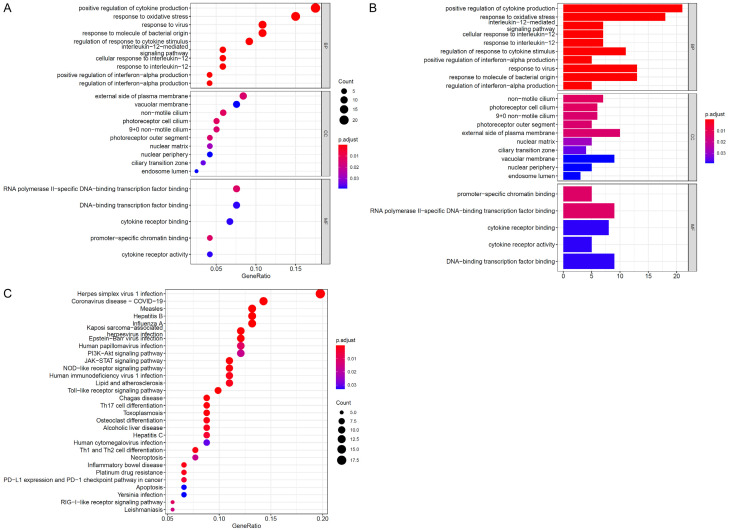
Shengyang Yiwei Decoction targets 36 genes and is engaged in 41 signaling pathways, including the HIF-1, Jak-STAT, and PI3K-Akt pathways, as shown by a GO function enrichment study. Involvement with 29 molecular activities, 14 cellular components, and 91 biological processes allows this medication to exert its therapeutic benefits. Important cellular processes affected by the intervention include the inhibition of apoptosis, the promotion of DNA transcription and cell proliferation, the enhancement of signal transmission, and the enhancement of transcription from the promoter of RNA polymerase II. Cell fluid, the plasma membrane, the outer plasma membrane, and the cytoplasm are most impacted (Figure 3A). Binding to zinc ions, ATP, enzymes, proteins, and even to other copies of the same protein are the most common molecular activities (Figure 3B).
Figure 3.
A. Bubble diagram of go cell composition pathway enrichment analysis. B. Bubble diagram of go molecular functional pathway enrichment analysis. C. Bubble diagram of KEGG signal pathway enrichment analysis.
Pathway enrichment analysis of targets
According to the results of a KEGG pathway enrichment study, Shengyang Yiwei Decoction affects 41 significantly enriched pathways (P < 0.05) out of a total of 50 signaling pathways. More enriched genes were found in the Jak-STAT signaling pathway, PI3K-Akt signaling route, and cancer signaling pathway, respectively (Figure 3C).
Discussion
Non-infectious CKD causes gradual decline in kidney function and may eventually cause uremia and renal failure. CKD is a leading cause of death and presents considerable therapeutic hurdles. According to the 2012 diagnostic criteria established by the Global Organization for Improving the Prognosis of Kidney Disease (KDIGO), CKD is diagnosed when aberrant renal function or structure persists for more than three months. Modern methods of therapy concentrate on symptom management, renal fibrosis improvement, hypertension management, and protein restriction. Kidney transplantation, peritoneal dialysis, or hemodialysis may be used to increase survival in patients with chronic renal failure [5].
Based on the clinical symptoms of proteinuria, hematuria, and edema, CKD is classified into categories such as “clearance”, “hematuria”, “lumbago”, “asthenic fatigue”, and “edema” in traditional Chinese medicine (TCM). Blood stasis, wind evil, damp-heat, qi and blood deficit, spleen and kidney deficiencies, and so on are all implicated in CKD according to TCM [6]. The Yellow Emperor’s Classic of Internal Medicine, a seminal TCM text, identifies moisture and heat as two of the most widespread aetiologias of disease. Traditional Chinese Medicine (TCM) approaches the treatment of CKD by focusing on eliminating toxicity and dampness, then fortifying the spleen and restoring qi [6]. Due to its ability to eliminate heat, dehumidify, warm the kidneys, induce diuresis, strengthen the spleen, and replenish qi, Shengyang Yiwei Decoction has been used to treat CKD. Astragalus, licorice, atractylodes macrocephala, ginseng, Alisma orientalis, Poria cocos, tangerine peel, pinellia ternate, Duhuo, Qianghuo, Fangfeng, and Bupleurum are all used in the decoction. The active constituents of Shengyang Yiwei Decoction, especially Astragalus, have been shown to have anti-inflammatory and antioxidant effects in scientific studies. Preventing glomerular mesangial hyperplasia, enhancing renal interstitial fibrosis, and decreasing serum creatinine and uric acid levels are all possible outcomes of taking astragalu [7].
Shengyang Yiwei Decoction has been shown to be effective in treating CKD, however the mechanism of action is unclear. However, network pharmacology provides a holistic method to investigate its multifaceted activity. EGFR, AR, and REN were identified as the primary targets of Shengyang Yiwei Decoction for the treatment of CKD in this investigation. Patients with CKD often have a mutation in the REN gene, which leads to renal tubular dysplasia, anemia, and hyperuricemia. Increased generation of reactive oxygen species (ROS) and worsening of capillary endothelial damage may result from high uric acid and glucose levels [8]. Perhaps ferroptosis, which is associated with such oxidative stress and was previously thought to be related to cancer [9], is the cause of regular cell death in all patients with CKD, which is a hot topic of research recently. Renal interstitial fibrosis may be made worse by EGFR abnormalities, which upregulate the production of fibrosis-promoting molecules. Further, IL-2 may limit the generation of pro-inflammatory cytokines, and NOS2 activation can improve antioxidant capacity and preserve renal function [10]. Shengyang Yiwei Decoction has been shown to be effective in treating CKD, with the enrichment analysis of target pathways revealing the participation of the HIF-1, Jak-STAT, and PI3K-Akt signaling pathways. Renal fibrosis and podocyte damage caused by insulin resistance involve the PI3K-Akt pathway. Similar to Atractylenolides, which in addition to anti-tumor effects [11], especially the Atractylenolide III can prevent muscle wasting in CKD patients through the Oxidative Stress-Mediated PI3K/AKT/mTOR signaling pathway [12]. Renal fibrosis may be improved and early renal damage in diabetes can be alleviated by blocking activation of the Jak-STAT signaling pathway or by fighting glomerulosclerosis [13]. Ursolic acid (UA), a pentacyclic triterpenoid, has been shown to have beneficial effects on a rat model of renal fibrosis [14]. It has also been discovered to have anti-cancer properties, which it does via inhibiting the Wnt/β-Catenin signaling pathway [15,16]. Because of the similarities in their pathogenic mechanisms, histone deacetylase inhibitors are being examined not only for the treatment of malignancies but also for the prevention and treatment of kidney lesions and renal fibrosis [17].
Thyroid hormone levels, oxidative stress, inflammation, angiogenesis, and local tissue blood supply may all be affected by therapy with Shengyang Yiwei Decoction. Ras, toxoplasmosis, and cancer are only a few examples of disorders whose signaling pathways may be affected by TCM’s “treating different diseases with the same treatment” approach [18]. Reduced antioxidant capacity, elevated oxidative stress, and insulin resistance have all been linked to CKD. Increased expression of certain RNAs, such the Long noncoding RNA p21, has been linked to the induction of oxidative stress, lipotoxicity and CKD in tubular cells [19]. Despite the paradoxical function shown by stimulating the SESN2/AMPK/TSC2 pathway in repairing endothelial damage due to hypertension [20]. Shengyang Yiwei Decoction is able to reduce oxidative stress and renal interstitial fibrosis because it stimulates the FOXO signaling pathway. Reducing the risk of cardiovascular death in CKD patients is a primary goal of therapy [18].
In conclusion, this research integrated and analyzed substantial data to provide a thorough review of the methods and goals of using Shengyang Yiwei Decoction to treat CKD. The findings are consistent with the comprehensive approach of TCM and provide useful insights for therapeutic practice. There is a need for further experimental validation of predicted signal pathways and targets, and the research did not conduct a systematic or exhaustive screening of active components. To further our knowledge of the mechanism of action of Shengyang Yiwei Decoction in treating CKD, future studies should strive to resolve these limitations and corroborate the results.
Disclosure of conflict of interest
None.
References
- 1.Takase R, Nakata T, Aoki K, Okamoto M, Fukuda A, Fukunaga N, Goto K, Masaki T, Shibata H. The relationship between edema and body functions in patients with chronic kidney disease: a preliminary study. Cureus. 2022;14:e27118. doi: 10.7759/cureus.27118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Feng Y, Li M, Yang M, Shi G, Xuan Z, Yin D, Xu F. Traditional Chinese medicine in the treatment of chronic kidney diseases: theories, applications, and mechanisms. Front Pharmacol. 2022;13:917975. doi: 10.3389/fphar.2022.917975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee DYW, Li QY, Liu J, Efferth T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: clinical experience and scientific basis. Phytomedicine. 2021;80:153337. doi: 10.1016/j.phymed.2020.153337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laster ML, Rowan B, Chen HC, Schwantes-An TH, Sheng X, Friedman PA, Ikizler TA, Sinshiemer JS, Ix JH, Susztak K, de Boer IH, Kestenbaum B, Hung A, Moe SM, Perwad F, Robinson-Cohen C. Genetic variants associated with mineral metabolism traits in chronic kidney disease. J Clin Endocrinol Metab. 2022;107:e3866–e3876. doi: 10.1210/clinem/dgac318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace AS, Chang AR, Shin JI, Reider J, Echouffo-Tcheugui JB, Grams ME, Selvin E. Obesity and chronic kidney disease in US adults with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2022;107:1247–1256. doi: 10.1210/clinem/dgab927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal D, Varade D, Shah H, Nazar A, Krishnan J, Shukla V, Ramakrishna C, Bandara Galahitiyawa MC, Mavani SB, Rajanna S, Jikki P, De Silva S, Ruhela V, Koradia P, Kansagra K, Kanani P, Sharma N, Zala K, Parmar D Study Investigator Group. Desidustat in anemia due to non-dialysis-dependent chronic kidney disease: a phase 3 study (DREAM-ND) Am J Nephrol. 2022;53:352–360. doi: 10.1159/000523961. [DOI] [PubMed] [Google Scholar]
- 8.Kelly DM, Pendlebury ST, Rothwell PM. Associations of chronic kidney disease with dementia before and after TIA and stroke: population-based cohort study. Neurology. 2022;98:e711–e720. doi: 10.1212/WNL.0000000000013205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, Han X, Xiang Y, Huang X, Lin H, Xie T. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelmaguid A, Roberts LN, Tugores L, Joslin JR, Hunt BJ, Parmar K, Nebres D, Naga SS, Khalil ES, Bramham K. Evaluation of novel coagulation and platelet function assays in patients with chronic kidney disease. J Thromb Haemost. 2022;20:845–856. doi: 10.1111/jth.15653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Van der Jeught K, Zhou Z, Zhang L, Yu T, Sun Y, Li Y, Wan C, So KM, Liu D, Frieden M, Fang Y, Mosley AL, He X, Zhang X, Sandusky GE, Liu Y, Meroueh SO, Zhang C, Wijeratne AB, Huang C, Ji G, Lu X. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J Clin Invest. 2021;131:e146832. doi: 10.1172/JCI146832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Hu R, Wang Y, Liu L, You H, Zhang J, Wu X, Pei T, Wang F, Lu L, Xiao W, Wei L. Atractylenolide III attenuates muscle wasting in chronic kidney disease via the oxidative stress-mediated PI3K/AKT/mTOR pathway. Oxid Med Cell Longev. 2019;2019:1875471. doi: 10.1155/2019/1875471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otobe Y, Rhee CM, Nguyen M, Kalantar-Zadeh K, Kopple JD. Current status of the assessment of sarcopenia, frailty, physical performance and functional status in chronic kidney disease patients. Curr Opin Nephrol Hypertens. 2022;31:109–128. doi: 10.1097/MNH.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakur R, Sharma A, Lingaraju MC, Begum J, Kumar D, Mathesh K, Kumar P, Singh TU, Kumar D. Ameliorative effect of ursolic acid on renal fibrosis in adenine-induced chronic kidney disease in rats. Biomed Pharmacother. 2018;101:972–980. doi: 10.1016/j.biopha.2018.02.143. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Tang S, Tao Q, Ming T, Lei J, Liang Y, Peng Y, Wang M, Liu M, Yang H, Ren S, Xu H. Ursolic acid suppresses colorectal cancer by down-regulation of Wnt/β-catenin signaling pathway activity. J Agric Food Chem. 2023;71:3981–3993. doi: 10.1021/acs.jafc.2c06775. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Kwon HY, Sohn EJ, Kim KA, Kim B, Jeong SJ, Song JH, Koo JS, Kim SH. Inhibition of Wnt/β-catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharmacol Rep. 2013;65:1366–1374. doi: 10.1016/s1734-1140(13)71495-6. [DOI] [PubMed] [Google Scholar]
- 17.Gao Y, Zhang H, Lirussi F, Garrido C, Ye XY, Xie T. Dual inhibitors of histone deacetylases and other cancer-related targets: a pharmacological perspective. Biochem Pharmacol. 2020;182:114224. doi: 10.1016/j.bcp.2020.114224. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Lü Z, Tian C, Ouyang W, Xiong Y, You Y, Chen L, Deng Y, Zhao X, Sun X. Mechanism of Shenbing decoction III in the treatment of proteinuria in chronic kidney disease: a network pharmacology-based study. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:227–234. doi: 10.12122/j.issn.1673-4254.2019.02.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li B, Leung JCK, Chan LYY, Li HY, Yiu WH, Lok SWY, Xue R, Zou YX, Chen W, Lai KN, Tang SCW. Tubule-specific deletion of LincRNA-p21ameliorates lipotoxic kidney injury. Mol Ther Nucleic Acids. 2021;26:1280–1290. doi: 10.1016/j.omtn.2021.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Lin L, Zhang L, Xu R, Chen X, Ji J, Li Y. Long noncoding RNA p21 enhances autophagy to alleviate endothelial progenitor cells damage and promote endothelial repair in hypertension through SESN2/AMPK/TSC2 pathway. Pharmacol Res. 2021;173:105920. doi: 10.1016/j.phrs.2021.105920. [DOI] [PubMed] [Google Scholar]