Abstract
Progranulin (PGRN) is a growth factor in which mutations are one of the leading causes of frontotemporal dementia (FTD), and has been implicated in an assortment of neurodegenerative diseases. Conversely, higher levels of the protein have shown potential as a general neuronal protective factor. While examining its neuroprotective applications on a broader scale would be unfeasible in mammalian models, we turned to the nematode C. elegans to map the interactions of PGRN across multiple genetic models of neurodegenerative diseases. Our results indicate that while the overexpression of PGRN appears to be protective across all models tested, the loss of PGRN exacerbated the disease phenotypes of all but three of the models tested. Given the ease of genetic analysis in nematodes, we propose this model organism as an efficient tool to build a comprehensive map of PGRN’s genetic interactions.
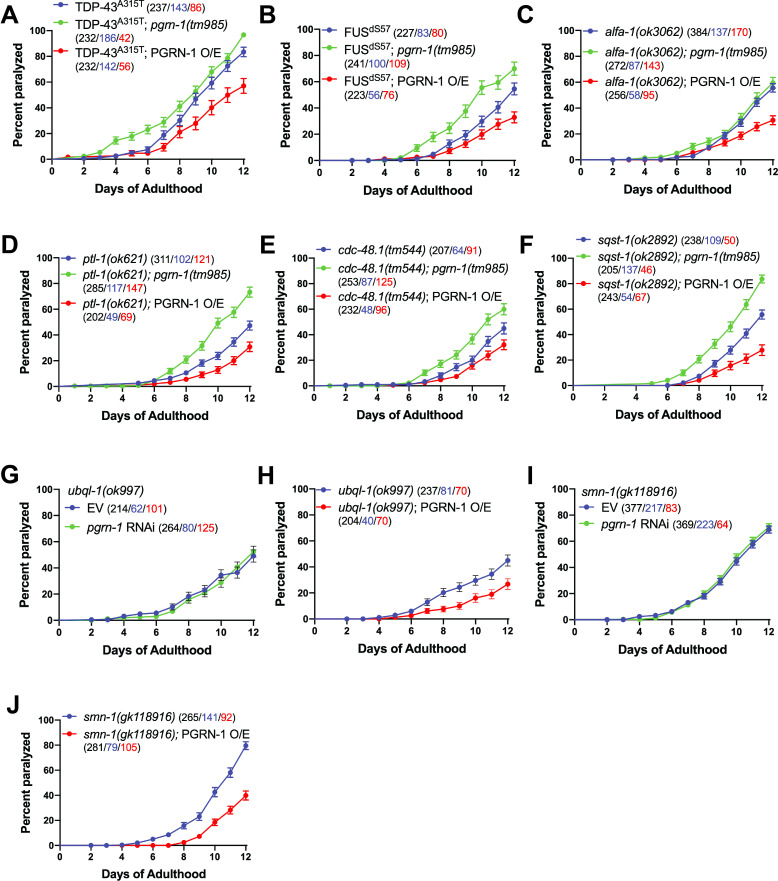
Figure 1. Genetic interactions of pgrn-1 in disease models .
The genetic loss of pgrn-1 exacerbates the paralysis phenotypes of TDP-43 A315T (A, p =0.0041) and FUS ∆S57 (B, p =0.0007) transgenic animals, and of ptl-1(ok621) (D, p <0.0001), cdc-48.1(tm544) (E, p =0.0016), and sqst-1(ok2892) (F, p <0.0001) mutant animals. Paralysis of alfa-1(ok3062) animals are not affected by the loss of pgrn-1 (C, n.s.), and neither are ubql-1(ok997) (G, n.s.) and smn-1(gk118916) (I, n.s.) animals after depletion of pgrn-1 by RNAi. RNAi experiments were conducted in the uIs60 [unc-119p::YFP + unc-119p::sid-1] background . (A-F, H, J) The overexpression of full-length, wild-type PGRN-1 ameliorates paralysis in all disease models tested (A, p =0.0003; B, p =0.0015; C, p <0.0001; D, p =0.0006; E, p =0.0232; F, p <0.0001; H, p =0.0011; J, p <0.0001). Data on graphs are presented as mean +/- SEM, gathered from multiple biological replicates. (PGRN-1 O/E=PGRN-1 overexpressing strain; black numbers= total worm population; blue numbers= paralyzed animals; red numbers= censored animals)
Description
Progranulin (PGRN) is a highly-conserved protein and widely known to be one of the main genetic causes of frontotemporal dementia (FTD) (Baker et al. 2006; Cruts et al. 2006; Olney et al. 2017; van Swieten and Heutink 2008) , a primary form of early-onset dementia. While first identified as a secreted growth factor (Bandari and Bateman 1992; Bateman et al. 1990; Belcourt et al. 1993) , it has since been found to promote various neuropathologies when depleted or absent (Kao et al. 2017) and, more recently, as having important roles in brain aging (Rhinn and Abeliovich 2017) . PGRN, is known to be an efficient neuroprotective factor being able to delay or offset toxicity of numerous diseases across multiple animal or cellular models (Chitramuthu et al. 2010; Minami et al. 2014; Tauffenberger et al. 2013; Van Kampen et al. 2014) . The inverse is also true, wherein the loss of PGRN has been shown to exacerbate the severity of neurodegenerative diseases (Minami et al. 2014; Salazar et al. 2015) . As a result, many efforts are being put forth to develop PGRN into a general therapeutic for neuronal diseases. However, in order to better understand the potential applications of PGRN as a neurodegenerative therapeutic, its interactions with other neurodegenerative disease-causing genes must be examined in further detail. Furthermore, questions remain as to whether the protective effects of PGRN extend beyond other FTD, and the genetically-related ALS, genes (Ling et al. 2013) . However, performing such studies in mammals is costly and resource-intensive, so, other rapid models are required to perform these broad genetic interaction studies. In this study, we used the nematode C. elegans , to model PGRN’s genetic interactions across the ALS-FTD gene spectrum and beyond.
In order to map PGRN’s genetic interactions and verify its therapeutic potential against a specific genetic mutation, we crossed either pgrn-1 -null animals, pgrn-1(tm985) (Kao et al, 2011; Doyle et al. 2021) , or multi-copy transgenic animals that overexpress wild-type pgrn-1 under the control of its promoter (CF3778, denoted PGRN-1 O/E) (Kao et al. 2011) . We have previously shown that pgrn-1(tm985) animals display an adult-onset paralysis phenotype, which can be rescued by the overexpression of wild-type PGRN-1 (Doyle et al. 2021) , and we selected this phenotype to screen against. We sought to begin to map the interactions by looking at the most common disease-causing genes of the ALS-FTD spectrum. We crossed C. elegans pgrn-1(tm985) animals with transgenic nematode strains expressing ALS-linked, mutant, human TDP-43 and FUS, TDP-43 A315T and FUS ∆S57 (Vaccaro et al. 2012) . We observed that while the loss of pgrn-1 exacerbated the paralysis phenotypes of each ALS model (Figs. 1A and 1B, respectively), the overexpression of PGRN-1 resulted in a suppression of paralysis. In order to test PGRN’s interactions with the most prevalent genetic cause of ALS and FTD, C9orf72 , we turned to a genetic deletion in alfa-1 , the nematode ortholog of C9orf72 (Therrien et al. 2013) . We observed that, while the overexpression of PGRN-1 was able to supress alfa-1 paralysis, the double mutant, pgrn-1(tm985); alfa-1(ok3062) did not have higher levels of paralysis as alfa-1(ok3062) mutants alone ( Fig. 1C ). We next sought to look at how PGRN interacts with MAPT whose ortholog in C. elegans is ptl-1 . We found that the double pgrn-1(tm985); ptl-1(ok621) mutants displayed higher levels of paralysis than ptl-1(ok621) mutants alone, and that the overexpression of PGRN-1 resulted in lower paralysis levels ( Fig. 1D ).
We further sought to look at PGRN’s interactions with the less prevalent ALS-FTD causing genes, VCP, SQSTM1, and UBQLN2. When pgrn-1 -null animals were crossed with cdc-48.1 mutants, the worm ortholog of the human VCP, we observed an exacerbation of paralysis, whereas the overexpression resulted in a reduction of paralysis ( Fig. 1E ). We observed a similar pattern with sqst-1(ok2892 ) animals, orthologous to the human SQSTM1 ( Fig. 1F ). For UBQLN2/ ubql-1 , we could not cross ubql-1(ok997) animals with pgrn-1(tm985) animals since both genes are located on the same chromosome. We therefore turned to RNAi and fed ubql-1(ok997) animals RNAi against pgrn-1 to deplete it, which we have previously shown induces a phenotype in wild-type animals (Doyle et al. 2021) . In doing so, we did not observe any exacerbation of mutant ubql-1 paralysis ( Fig. 1G ). We were, however, able to cross PGRN-1 overexpressing animals with ubql-1 mutants, which resulted in a reduction of ubql-1 paralysis ( Fig. 1H ). Finally, looking beyond genes involved in the ALS-FTD spectrum, we looked at the effect of PGRN loss and overexpression on smn-1 , the worm ortholog of SMN1 known to cause spinal muscular atrophy (SMA) in humans. Much like ubql-1 , smn-1 is located on the same chromosome as pgrn-1 so we had to turn to RNAi to deplete pgrn-1 transcripts. We used here a newly-characterized nematode smn-1 model, harboring the gk118916 allele (Doyle et al. 2021) . In doing so, we observed that worms fed pgrn-1 RNAi did not have any change in paralysis levels ( Fig. 1I ), whereas the overexpression of PGRN-1 resulted in a decrease of smn-1 paralysis ( Fig. 1J ).
We observed that the loss of C. elegans pgrn-1 resulted in an exacerbation of toxicity in all but three genetic backgrounds, alfa-1 , ubql-1 , and smn-1 . In the case of C9orf72/alfa-1 , this finding was of interest since previous studies have identified patients with both GRN and C9orf72 mutations had pathology consistent with C9orf72 mutations alone (Lashley et al. 2014) . However, another study has observed mixed pathology in patients carrying both C9orf72 and GRN mutations therefore drawing uncertainty on this (van Blitterswijk et al. 2013) . Nonetheless, the C9orf72 / alfa-1 case is supported by recent findings suggesting that like PGRN, the C9ORF72 protein is involved in lysosomal function, and it has been hypothesized that its loss results in lysosomal function and trafficking defects which could be responsible for its pathology (Amick and Ferguson 2017; Amick et al. 2016; Shi et al. 2018) . Therefore, our finding is not surprising since it reinforces the suggestion that both GRN / pgrn-1 and C9orf72 / alfa-1 may act through overlapping pathogenic mechanisms.
Unlike all the other genetic backgrounds we looked at, in the case of ubql-1 and smn-1 we could not introduce a pgrn-1 -null allele through genetic crossing, so we turned to an RNAi approach to deplete pgrn-1 transcript levels in the animals (Calixto et al. 2010; Fire et al. 1998; Kamath et al. 2001; Rocheleau 2012) . Therefore, as with the alfa-1 results, our results suggest that pgrn-1 contributes to pathology through similar pathways as ubql-1 or smn-1 . While there have been studies showing that UBQLN2 mutations can affect lysosomal-autophagic pathways, its primary role is in the ubiquitin-proteasome system (Renaud et al. 2019) . Furthermore, there is little evidence SMA pathology acts through lysosomal disruption (Chaytow et al. 2018) , so therefore, we believe that these results should be validated using genetically-edited mutants in pgrn-1 in ubql-1 and smn-1 mutant backgrounds.
When looking at the effect that the overexpression of PGRN-1 had, we notice that it was able to reduce paralysis levels across all disease models tested, suggesting PGRN is, in fact, an effective and broad protective factor against neurodegenerative diseases; however, at this stage we cannot rule out that the tagged PGRN-1 is acting as a modifier of the phenotypes we are studying. Interestingly, it can rescue genetic disease models that do not have a known link to lysosomes, one of PGRN’s primary cellular functions, such as smn-1 . This could suggest that one of PGRN’s mechanisms of action is on a pathway with broad-acting neuroprotective outcomes. Alternatively, PGRN has been shown to be a regulator of normal brain aging through its interaction with TMEM106b, a regulator of lysosomal function, whereby lower PGRN levels were associated with its accelerated aging, even in the absence of any other neuropathology (Rhinn and Abeliovich 2017) . Therefore, PGRN may promote neuronal health by maintaining normal lysosomal function.
Together, our results suggest that PGRN is, in fact, a broad-acting protective factor against a variety of neurodegenerative diseases. While we acknowledge this model system's limits to testing genetic interactions related to human diseases, our results provide a first stepping stone to elucidate the larger map of PGRN’s interactions.
Methods
Paralysis assays: This assay quantifies the presence of the paralysis phenotype within a worm population. 25-30 L4 larval animals were placed onto NGM plates and scored daily starting the following day, at day 1 of adulthood. Worms were counted as paralyzed if they failed to move their body when prodded with a platinum worm pick. Worms were considered dead if they failed to respond to heat stimuli or if exhibited no pharyngeal pumping; dead worms were censored from statistical analyses. For each assay, a minimum of 200 animals were scored per genotype and per condition across 3 biological replicates. Animals were transferred to fresh plates every second day. Paralysis assays with RNAi were performed in the same way, and RNAi treatment was administered through feeding using standard protocols (Conte et al. 2015; Timmons et al. 2001) .
Statistical analyses: Paralysis curves were compared using Mantel-Cox tests calculated in GraphPad Prism software.
Reagents
|
Strain Name |
Genotype |
Short Name in Article |
Source |
|
XQ561 |
pgrn-1(tm985) |
n/a |
NBRP |
|
CF3778 |
pgrn-1(tm985); muIs213[pgrn-1p::pgrn-1::rfp] |
PGRN-1 O/E |
|
|
TU3311 |
uIs60 [unc-119p::YFP + unc-119p::sid-1] |
n/a |
CGC |
|
XQ207 |
xqIs133[unc- 47p::TDP-43[A315T];unc-119(+)] |
TDP-43 A315T |
|
|
XQ209 |
xqIs98[unc- 47p::FUS[delS57];unc-119(+)] |
FUS ∆S57 |
|
|
XQ236 |
alfa-1(ok3062) |
n/a |
CGC |
|
RB809 |
ptl-1(ok621) |
n/a |
CGC |
|
RB1050 |
ubql-1(ok997) |
n/a |
CGC |
|
FX544 |
cdc-48.1(tm544) |
n/a |
CGC |
|
VC2196 |
sqst-1(ok2892) |
n/a |
CGC |
|
XQ545 |
smn-1(gk118916) |
n/a |
MMP* |
The strain resulting from the MMP (Million Mutation Project) was backcrossed to wild-type N2 animals 6 times. Other mutations were backcrossed 4 times.
Acknowledgments
Acknowledgments
Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), as well as the Japanese National BioResource Project. We thank A. Kao for the PGRN-1 O/E strain, and S. Peyrard and C. Maios for technical support.
Funding Statement
<p>Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, ALS Canada, Brain Canada</p>
References
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006 Jul 16;442(7105):916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Alexander AG, Marfil V, Li C. Use of Caenorhabditis elegans as a model to study Alzheimer's disease and other neurodegenerative diseases. Front Genet. 2014 Sep 5;5:279–279. doi: 10.3389/fgene.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amick J, Ferguson SM. C9orf72: At the intersection of lysosome cell biology and neurodegenerative disease. Traffic. 2017 Mar 23;18(5):267–276. doi: 10.1111/tra.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amick J, Roczniak-Ferguson A, Ferguson SM. C9orf72 binds SMCR8, localizes to lysosomes, and regulates mTORC1 signaling. Mol Biol Cell. 2016 Aug 24;27(20):3040–3051. doi: 10.1091/mbc.E16-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, Alper S. What Can We Learn About Human Disease from the Nematode C. elegans? Methods Mol Biol. 2018;1706:53–75. doi: 10.1007/978-1-4939-7471-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V, Bateman A. Structure and chromosomal location of the human granulin gene. Biochem Biophys Res Commun. 1992 Oct 15;188(1):57–63. doi: 10.1016/0006-291x(92)92349-3. [DOI] [PubMed] [Google Scholar]
- Bateman A, Belcourt D, Bennett H, Lazure C, Solomon S. Granulins, a novel class of peptide from leukocytes. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1161–1168. doi: 10.1016/s0006-291x(05)80908-8. [DOI] [PubMed] [Google Scholar]
- Bateman A, Bennett HP. The granulin gene family: from cancer to dementia. Bioessays. 2009 Nov 1;31(11):1245–1254. doi: 10.1002/bies.200900086. [DOI] [PubMed] [Google Scholar]
- Belcourt DR, Lazure C, Bennett HP. Isolation and primary structure of the three major forms of granulin-like peptides from hematopoietic tissues of a teleost fish (Cyprinus carpio). J Biol Chem. 1993 May 5;268(13):9230–9237. [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May 1;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat Methods. 2010 May 30;7(7):554–559. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytow H, Huang YT, Gillingwater TH, Faller KME. 2018. The role of survival motor neuron protein (smn) in protein homeostasis. Cell Mol Life Sci. 75(21):3877-3894. [DOI] [PMC free article] [PubMed]
- Chaytow H, Huang YT, Gillingwater TH, Faller KME. The role of survival motor neuron protein (SMN) in protein homeostasis. Cell Mol Life Sci. 2018 Jun 5;75(21):3877–3894. doi: 10.1007/s00018-018-2849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte D Jr, MacNeil LT, Walhout AJM, Mello CC. RNA Interference in Caenorhabditis elegans. Curr Protoc Mol Biol. 2015 Jan 5;109:26.3.1–26.3.30. doi: 10.1002/0471142727.mb2603s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Paulson EA, Grana TM, Harris MA, Batzli JM. Studying human disease genes in Caenorhabditis elegans: a molecular genetics laboratory project. CBE Life Sci Educ. NaN NaN NaN;11(2):165–179. doi: 10.1187/cbe-11-06-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van Duijn C, Peeters K, Sciot R, Santens P, De Pooter T, Mattheijssens M, Van den Broeck M, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van Broeckhoven C. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006 Jul 16;442(7105):920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998 Feb 19;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2000 Dec 20;2(1):RESEARCH0002–RESEARCH0002. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao AW, McKay A, Singh PP, Brunet A, Huang EJ. Progranulin, lysosomal regulation and neurodegenerative disease. Nat Rev Neurosci. 2017 Apr 24;18(6):325–333. doi: 10.1038/nrn.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley T, Rohrer JD, Mahoney C, Gordon E, Beck J, Mead S, Warren J, Rossor M, Revesz T. A pathogenic progranulin mutation and C9orf72 repeat expansion in a family with frontotemporal dementia. Neuropathol Appl Neurobiol. 2014 Jun 1;40(4):502–513. doi: 10.1111/nan.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013 Aug 7;79(3):416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami SS, Min SW, Krabbe G, Wang C, Zhou Y, Asgarov R, Li Y, Martens LH, Elia LP, Ward ME, Mucke L, Farese RV Jr, Gan L. Progranulin protects against amyloid β deposition and toxicity in Alzheimer's disease mouse models. Nat Med. 2014 Sep 28;20(10):1157–1164. doi: 10.1038/nm.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney NT, Spina S, Miller BL. 2017. Frontotemporal dementia. Neurol Clin. 35(2):339-374. [DOI] [PMC free article] [PubMed]
- Olney NT, Spina S, Miller BL. Frontotemporal Dementia. Neurol Clin. 2017 May 1;35(2):339–374. doi: 10.1016/j.ncl.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn H, Abeliovich A. Differential Aging Analysis in Human Cerebral Cortex Identifies Variants in TMEM106B and GRN that Regulate Aging Phenotypes. Cell Syst. 2017 Mar 18;4(4):404–415.e5. doi: 10.1016/j.cels.2017.02.009. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE. RNA interference: Systemic RNAi SIDes with endosomes. Curr Biol. 2012 Oct 23;22(20):R873–R875. doi: 10.1016/j.cub.2012.08.039. [DOI] [PubMed] [Google Scholar]
- Salazar DA, Butler VJ, Argouarch AR, Hsu TY, Mason A, Nakamura A, McCurdy H, Cox D, Ng R, Pan G, Seeley WW, Miller BL, Kao AW. The Progranulin Cleavage Products, Granulins, Exacerbate TDP-43 Toxicity and Increase TDP-43 Levels. J Neurosci. 2015 Jun 24;35(25):9315–9328. doi: 10.1523/JNEUROSCI.4808-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lin S, Staats KA, Li Y, Chang WH, Hung ST, Hendricks E, Linares GR, Wang Y, Son EY, Wen X, Kisler K, Wilkinson B, Menendez L, Sugawara T, Woolwine P, Huang M, Cowan MJ, Ge B, Koutsodendris N, Sandor KP, Komberg J, Vangoor VR, Senthilkumar K, Hennes V, Seah C, Nelson AR, Cheng TY, Lee SJ, August PR, Chen JA, Wisniewski N, Hanson-Smith V, Belgard TG, Zhang A, Coba M, Grunseich C, Ward ME, van den Berg LH, Pasterkamp RJ, Trotti D, Zlokovic BV, Ichida JK. Haploinsufficiency leads to neurodegeneration in C9ORF72 ALS/FTD human induced motor neurons. Nat Med. 2018 Feb 5;24(3):313–325. doi: 10.1038/nm.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006 Feb 11;:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Suzuki G, Matsuwaki T, Hosokawa M, Serrano G, Beach TG, Yamanouchi K, Hasegawa M, Nishihara M. Progranulin regulates lysosomal function and biogenesis through acidification of lysosomes. Hum Mol Genet. 2017 Mar 1;26(5):969–988. doi: 10.1093/hmg/ddx011. [DOI] [PubMed] [Google Scholar]
- Tauffenberger A, Chitramuthu BP, Bateman A, Bennett HP, Parker JA. Reduction of polyglutamine toxicity by TDP-43, FUS and progranulin in Huntington's disease models. Hum Mol Genet. 2012 Nov 19;22(4):782–794. doi: 10.1093/hmg/dds485. [DOI] [PubMed] [Google Scholar]
- Therrien M, Rouleau GA, Dion PA, Parker JA. Deletion of C9ORF72 results in motor neuron degeneration and stress sensitivity in C. elegans. PLoS One. 2013 Dec 12;8(12):e83450–e83450. doi: 10.1371/journal.pone.0083450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001 Jan 24;263(1-2):103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Vaccaro A, Tauffenberger A, Aggad D, Rouleau G, Drapeau P, Parker JA. Mutant TDP-43 and FUS cause age-dependent paralysis and neurodegeneration in C. elegans. PLoS One. 2012 Feb 21;7(2):e31321–e31321. doi: 10.1371/journal.pone.0031321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk M, Baker MC, DeJesus-Hernandez M, Ghidoni R, Benussi L, Finger E, Hsiung GY, Kelley BJ, Murray ME, Rutherford NJ, Brown PE, Ravenscroft T, Mullen B, Ash PE, Bieniek KF, Hatanpaa KJ, Karydas A, Wood EM, Coppola G, Bigio EH, Lippa C, Strong MJ, Beach TG, Knopman DS, Huey ED, Mesulam M, Bird T, White CL 3rd, Kertesz A, Geschwind DH, Van Deerlin VM, Petersen RC, Binetti G, Miller BL, Petrucelli L, Wszolek ZK, Boylan KB, Graff-Radford NR, Mackenzie IR, Boeve BF, Dickson DW, Rademakers R. C9ORF72 repeat expansions in cases with previously identified pathogenic mutations. Neurology. 2013 Sep 11;81(15):1332–1341. doi: 10.1212/WNL.0b013e3182a8250c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kampen JM, Baranowski D, Kay DG. Progranulin gene delivery protects dopaminergic neurons in a mouse model of Parkinson's disease. PLoS One. 2014 May 7;9(5):e97032–e97032. doi: 10.1371/journal.pone.0097032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swieten JC, Heutink P. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol. 2008 Sep 2;7(10):965–974. doi: 10.1016/S1474-4422(08)70194-7. [DOI] [PubMed] [Google Scholar]
- Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, Zhou P, de Luis A, Neukomm LJ, Cabello J, Farese RV Jr, Kenyon C. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci U S A. 2011 Feb 28;108(11):4441–4446. doi: 10.1073/pnas.1100650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Vrancx C, Maios C, Labarre A, Patten SA, Parker JA. Modulating the endoplasmic reticulum stress response attenuates neurodegeneration in a Caenorhabditiselegans model of spinal muscular atrophy. Dis Model Mech. 2020 Dec 22;13(12) doi: 10.1242/dmm.041350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Maios C, Vrancx C, Duhaime S, Chitramuthu B, Bennett HPJ, Bateman A, Parker JA. Chemical and genetic rescue of in vivo progranulin-deficient lysosomal and autophagic defects. Proc Natl Acad Sci U S A. 2021 Jun 22;118(25) doi: 10.1073/pnas.2022115118. [DOI] [PMC free article] [PubMed] [Google Scholar]