Abstract
Background
Inflammatory bowel disease (IBD) is a chronic, globally‐occurring gastrointestinal disorder and a major cause of illness and disability. It is conventionally classified into Crohn’s disease (CD) and ulcerative colitis (UC). Helminths are parasitic worms with complex life cycles involving tissue‐ or lumen‐dwelling stages in their hosts, and causing long‐lasting or chronic infections that are frequently asymptomatic. Helminths modulate immune responses of their hosts, and many observational and experimental studies support the hypothesis that helminths suppress immune‐mediated chronic inflammation that occurs in asthma, allergy and IBD.
Objectives
The objective was to evaluate the efficacy and safety of helminth treatment for induction of remission in IBD.
Search methods
We searched the following databases from inception to 13 July 2013: MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Inflammatory Bowel Disease Group Specialized Trials Register. We also searched four online trials registries, and abstracts from major meetings. There were no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) where the intervention was any helminth species or combination of helminth species, administered in any dose and by any route and for any duration of exposure to people with active CD or UC, confirmed through any combination of clinical, endoscopic and histological criteria were eligible for inclusion.
Data collection and analysis
Two authors independently extracted data and assessed eligibility using a standardized data collection form. We used the RevMan software for analyses. The primary outcome was induction of remission as defined by the included studies. Secondary outcomes included clinical, histologic, or endoscopic improvement as defined by the authors, endoscopic mucosal healing, change in disease activity index score, change in quality of life score, hospital admissions, requirement for intravenous corticosteroids, surgery, study withdrawal and the incidence of adverse events. We calculated the risk ratio (RR) and corresponding 95% confidence interval (CI) for dichotomous outcomes. We calculated the mean difference (MD) and 95% CI for continuous outcomes. We assessed the methodological quality of included studies using the Cochrane risk of bias tool. The overall quality of the evidence supporting each outcome was assessed using the GRADE criteria.
Main results
Two RCTs (90 participants) were included. One trial assessed the efficacy and safety of Trichuris suis (T. suis) ova in patients with UC (n = 54). The other RCT was a phase one that assessed the safety and tolerability of T. suis ova in patients with CD (n = 36). The risk of bias in both studies was judged to be low. In the UC study, during the 12‐week study period, participants in the active arm received 2‐weekly aliquots of 2500 T. suis eggs, added to 0.8 mL of saline; those in the placebo arm received 0.8 mL saline only. There were sparse data available for the outcomes clinical remission and clinical improvement. Ten per cent (3/30) of patients in the T. suis arm entered remission compared to 4% (1/24) of patients in the placebo arm (RR 2.40, 95% CI 0.27 to 21.63). Forty‐three per cent (13/30) of patients in the T. suis group achieved clinical improvement compared to 17% (4/24) of placebo patients (RR 2.60, 95% CI 0.97 to 6.95). The mean ulcerative colitis disease activity index (UCDAI) score was lower in the T. suis group (6.1 +/‐ 0.61) compared to the placebo group (7.5 +/‐ 0.66) after 12 weeks of treatment (MD ‐1.40, 95% CI ‐1.75 to ‐1.05). There was only limited evidence relating to the proportion of patients who experienced an adverse event. Three per cent (1/30) of patients in the T. suis group experienced at least one adverse event compared to 12% (3/24) of placebo patients (RR 0.27, 95% CI 0.03 to 2.40). None of the adverse events reported in this study were judged to be related to the study treatment. GRADE analyses rated the overall quality of the evidence for the primary and secondary outcomes (i.e. clinical remission and improvement) as low due to serious imprecision. In the CD study, participants received a single treatment of T. suis ova at a dosage of 500 (n = 9), 2500 (n = 9), or 7500 (n = 9) embryonated eggs or matching placebo (n = 9). The CD study did not assess clinical remission or improvement as outcomes. There were sparse data on adverse events at two weeks. Thirty‐seven per cent (10/27) of patients in the T. suis group experienced at least one adverse event compared to 44% (4/9) of placebo patients (RR 0.83, 95% CI 0.35 to 2.01). Only one adverse event (dysgeusia) was judged to be possibly related to treatment in this study. Dysgeusia was reported in one patient in the T. suis group and in one patient in the placebo group.
Authors' conclusions
Currently, there is insufficient evidence to allow any firm conclusions regarding the efficacy and safety of helminths used to treat patients with IBD. The evidence for our primary efficacy outcomes in this review comes from one small study and is of low quality due to serious imprecision. We do not have enough evidence to determine whether helminths are safe when used in patients with UC and CD. Further RCTs are required to assess the efficacy and safety of helminth therapy in IBD.
Plain language summary
Helminth therapy (worms) for induction of remission in inflammatory bowel disease
Inflammatory bowel disease (IBD) is comprised of two disorders: ulcerative colitis and Crohn’s disease. These disorders have both distinct and overlapping symptoms, but the underlying cause remains incompletely understood. Standard therapy for IBD includes sulfasalazine, 5‐ASA drugs, steroids, immunosuppressives such as azathioprine, 6‐mercaptopurine and methotrexate and biological agents such as infliximab. Helminths are worm‐like parasites, that inhabit larger organisms. Helminths cause changes in the immune systems of their hosts including an altered immunological response to antigens and this has implications for the treatment of inflammatory bowel disease which is thought to be caused by immune dysregulation.
The purpose of this systematic review was to examine the effectiveness and safety of helminth therapy for inducing remission in people with IBD. This review identified two randomised controlled trials including a total of 90 participants. One study compared twice weekly treatment with helminths (an 0.8 mL solution containing 2500 live eggs of the helminth Trichuris suis) for 12 weeks to a matching placebo (an 0.8 ml identical looking solution with no Trichuris suis eggs) in 54 patients with active ulcerative colitis. Few remissions occurred during the trial and helminth treatment had no detectable effect on these remissions. Ten per cent (3/30) of patients in the helminth group achieved remission compared to four per cent (1/24) of placebo patients. A higher proportion of patients in the helminth group (43% or 13/30) improved clinically compared to the placebo group (17% or 4/24). However, this difference could be a chance effect. We could not determine whether the proportion of patients who had a side effect was higher in either group. No observed side effects were thought to be related to treatment were reported in this study. The other study compared one treatment with various doses of helminths (a solution of 500, 2500 or 7500 Trichuris suis eggs) to a matching placebo in 36 patients with Crohn's disease. This study was designed to assess side effects and did not measure clinical remission or improvement. There amount of information available on side‐effects at two weeks was limited and the results were uncertain due to the small number of participants in the study. The only side effect that was judged to be possibly related to the study treatment was dysgeusia (a distortion of the sense of taste). This was reported in one patient in the helminth group and in one patient in the placebo group. Currently, there is insufficient evidence to allow any firm conclusions regarding the effectiveness and safety of helminths used to treat patients with IBD. The only information available relating to clinical improvement in patients with active ulcerative colitis comes from one small study. We do not know how safe helminths are when used in patients with ulcerative colitis and Crohn's disease. Further randomised controlled trials are required to assess the efficacy and safety of helminth therapy in IBD.
Summary of findings
Summary of findings for the main comparison. Trichuris suis eggs versus placebo for induction of remission in ulcerative colitis.
| Trichuris suis eggs versus placebo for induction of remission in ulcerative colitis | ||||||
| Patient or population: induction of remission in ulcerative colitis Settings: Outpatient Intervention: Trichuris suis eggs versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Trichuris suis eggs versus placebo | |||||
| Clinical remission Follow‐up: 12 weeks | 42 per 10001 | 100 per 1000 (11 to 533) | RR 2.40 (0.27 to 21.63) | 54 (1 study) | ⊕⊕⊝⊝ Low2 | |
| Clinical improvement at 12 weeks Follow‐up: 12 weeks | 167 per 10001 |
434 per 1000 (162 to 1161) |
RR 2.60 (0.97 to 6.95) | 54 (1 study) | ⊕⊕⊝⊝ Low3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The control group risk estimate comes from the control arm of the included study.
2 There is serious imprecision as there are relatively few events (4 events) and wide confidence interval around estimate of effect.
3 There is serious imprecision as there are relatively few events (17 events) and wide confidence interval around estimate of effect.
Background
Description of the condition
Inflammatory bowel disease (IBD) comprises two major disorders: ulcerative colitis (UC) and Crohn’s disease (CD). These disorders have both distinct and overlapping pathologic and clinical characteristics, but the pathogenesis of these disorders remains incompletely understood.
Symptoms
Ulcerative colitis
The major symptoms of UC are diarrhoea, rectal bleeding, tenesmus, passage of mucus, and crampy abdominal pain with the symptom complex differing according to the extent of colonic involvement. Most (80%) patients have a relapsing remitting course, characterized by intermittent flares interposed between variable periods of remission (Podolsky 2002).
Crohn’s disease
Although CD usually presents as right lower quadrant pain, weight loss and diarrhoea due to acute or chronic bowel inflammation, the inflammatory process can lead to complications such as stricturing and penetrating disease. The site of disease influences clinical manifestations (Podolsky 2002):
ileocaecal disease can presents as partial small bowel obstruction;
colonic disease may present with bloody diarrhoea similar to UC (though pain may be more prominent); and
perianal disease may present with anorectal fistulae, perirectal abscesses and anal strictures.
Management
The diagnosis of both UC and CD is based on a combination of clinical, endoscopic and histologic findings. The primary goals of therapy in UC and CD are to induce and maintain remission. A number of therapeutic agents are commonly used, as follows.
Glucocorticoids are used for induction of remission in UC and CD (Benchimol 2008; Seow 2008), but are not generally used for long‐term treatment of IBD.
Oral and topical (i.e. per rectum) 5‐aminosalicylates and sulphasalazine are used for induction and maintenance of remission in UC (Marshall 2010; Feagan 2012a; Feagan 2012b). Sulphasalazine may provide a modest benefit for active CD (Lim 2010).
The purine analogues azathioprine and 6‐mercaptopurine are used for maintenance of remission in CD (Prefontaine 2009) and UC (Timmer 2012) and generally are used in steroid dependant and steroid refractory disease. Azathioprine does not appear to provide any benefit for the treatment of active CD (Chande 2013). A single trial of methotrexate showed no benefit over placebo for induction of remission in active ulcerative colitis (Chande 2007) but there is evidence methotrexate provides a benefit for induction of remission and complete withdrawal from steroids in patients with refractory Crohn's disease (McDonald 2012).
Biological agents, particularly TNF‐alpha antagonists, are used for the induction and maintenance of remission in steroid and immunosuppressant refractory disease in both UC and CD, and in fistulising Crohn’s disease (Akobeng 2003; Lawson 2006; Behm 2008).
There is evidence that antibiotic therapy may improve clinical outcomes in patients with active Crohn's disease (Wang 2012).
The surgical therapy commonly advocated for UC is restorative proctocolectomy with permanent ileostomy or ileal pouch‐anal anastomosis. Surgery for UC is usually advised for indications such as medically refractory disease, intolerance of drug adverse effects, development of colonic dysplasia or carcinoma and complications such as toxic megacolon. Surgery is recommended for CD when medical therapy has failed or when complications (e.g. strictures causing obstruction, fulminant colitis and medically refractory anorectal disease) compel this.
Description of the intervention
Helminths
‘Helminth’ is derived from the Greek word helmins, meaning worm. The helminths of humans include species from the following four groups:
annelids (segmented worms);
nematodes (roundworms);
trematodes (flukes) and
cestodes (tapeworms).
The commonly encountered helminths of humans are listed in Appendix 1. The important biological characteristics of helminths are listed in Appendix 2.
Effectiveness of the intervention – experimental evidence
The use of helminths as a therapeutic intervention is an entirely novel concept in disease treatment. Despite its novelty, the concept is already at an advanced stage of testing for several diseases, through randomised controlled trials (RCTs) involving two different helminth species. Trichuris suis (T. suis) eggs (i.e. porcine whipworm) have been used as oral therapy in RCTs involving participants with allergic rhinitis (Bager 2010), ulcerative colitis (Summers 2005a) and Crohn's disease (Sandborn 2013). Necator americanus larvae, applied to the skin, have been used in RCTs involving participants with allergic rhinitis (Blount 2009), allergic rhinitis and subclinical asthma (Feary 2009), clinical asthma (Feary 2010) and coeliac disease (Daveson 2011). These studies have generally supported the concept of using helminths to treat allergic or immune‐mediated diseases, although the effect sizes in these trials have been very different. The two helminth species have been found to produce subclinical stimulation of the participants’ immune systems, after about two months of ingestion or application of the intervention.
Safety of the intervention – experimental evidence
The RCTs carried out to date have consistently reported no serious adverse effects (i.e. hospitalisation or death) associated with helminth therapy. Bager 2011 reported that a single oral dose of T. suis eggs caused a three‐ to 19‐fold increase in episodes of flatulence, diarrhoea and abdominal pain compared to placebo patients. However, the frequency and intensity of these symptoms abated, with subsequent doses (Bager 2011). Blount 2009, Feary 2009, Feary 2010 and Daveson 2011 reported that small numbers of Necator americanus larvae applied to the skin produced a transient and localised skin redness or itching or both. Other adverse events experienced by some participants who received Necator americanus include abdominal pain, loss of appetite and nausea (Blount 2009; Feary 2009; Feary 2010; Daveson 2011).
How the intervention might work
Modulation of the immune response
Humans and mice infested with helminths have blunted immune responses to antigens that do not derive from these parasites (Sabin 1996; Borkow 2000; Elliott 2000; Wammes 2010). These changes in immune responsiveness can persist long after the helminth clears from its host. This has implications of possible relevance for the prevention and treatment of IBD and other diseases caused by immune dysregulation.
Evidence from animal models of human IBD suggests that helminths act through cellular components of the innate immune system to prevent disease. Helminths do not necessarily require direct interactions with T or B cells (i.e. the components of adaptive immunity) to render their hosts disease‐resistant. However in the adaptive immune system helminth exposure can, for example, alter intestinal dendritic cell function, rendering these cells highly regulatory (Hang 2010). Dendritic cells are the major antigen‐presenting cells in the body and play a pivotal role in promoting, modulating and suppressing many aspects of the adaptive immune response.
Macrophages, which are plentiful in the intestines, are versatile phagocytic cells. Helminth induction of regulatory macrophages could have a role in protecting against IBD activity, as shown in dextran sodium sulphate induced enteritis (Smith 2007) and an IL10 KO model of IBD (Schnoeller 2008).
Helminths induce various regulatory T cell subsets, some of which express the transcription factor Foxp3 in the gut mucosa of their hosts. During helminth infestation, there occurs an expansion of IL10 and TGFb, as well as of Foxp3 positive T cells in the intestines. Helminths, in part through the induction of IL10 secretion and regulatory T cells, help limit Th1 responses (Elliott 2007) in various models of immunological diseases (Elliott 2003; Mangan 2004; Hunter 2005; Kitagaki 2006). TGFb also participates in this protective role (Ince 2009). Helminths induce the expression of Th2 cells that make IL4. Helminths protect mice from TNBS‐induced, Th1‐type colitis by restraining the IFNg/IL12 p40 response in the colon. IL4 can block colitis in this IBD model. IL4 working in conjunction with IL10 also has a role in limiting the secretion of IL17 (Elliott 2008), which is another colitogenic cytokine.
Changes in the lumen of intestine
Two physical changes in the lumen of the intestine have been postulated to explain how helminths might protect against IBD. These include an increase in the number of enteric goblet cells, resulting in increased mucus production (McKay 1990); and an increase in rostral‐caudal peristalsis of the intestine, resulting in decreased duration of contact between luminal contents and the epithelium (Dwinell 1997).
Why it is important to do this review
Current treatments have been effective for many patients with UC or CD, but have numerous limitations for patients with moderate to severe disease. These limitations include the following.
Long‐term glucocorticoid usage is often associated with prolonged and debilitating, systemic adverse effects such as osteoporosis, muscle weakness, cataracts, osteonecrosis, cushingoid appearance and weight gain (Podolsky 2002).
The slow onset of action of purine analogues generally precludes their use for induction of remission in clinical settings; purine analogues are also associated with infectious complications and development of lymphoma (Podolsky 2002).
Biological agents are costly and are associated with infectious complications (Rutgeerts 2005).
The limitations of current therapies for IBD indicate a significant need for safer and more effective therapies. This systematic review will summarize the current evidence regarding the use of helminths for induction of remission in IBD.
The intervention to be assessed in this review is the deliberate exposure of a person with confirmed IBD to one or more helminth species. The routes of exposure are likely to be:
oral (the human participant swallows helminth eggs or cysts); or
percutaneous (helminth larvae or cercariae are applied to the skin of the human participant, and penetrate the epidermis to reach their preferred end‐stage body structures).
The intervention may or may not be terminated through participants in the active arm taking an appropriate anthelmintic drug.
Objectives
To systematically evaluate the efficacy and safety of helminths for induction of remission in IBD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials using adequate or quasi methods of randomisation were considered for inclusion. Single‐blind, double‐blind, triple‐blind or open label studies were all eligible for inclusion.
Types of participants
Participants included patients with active UC or CD, confirmed by a combination of clinical, endoscopic and histological criteria.
Types of interventions
We considered studies for inclusion where human participants were exposed to a helminth species or combination of species:
at any developmental stage of the parasite (eggs, cysts, larvae, cercariae, adult worms);
in any dose;
by any route (oral, percutaneous, other); and
for any duration of exposure (hours, days, weeks, months).
We considered studies where the intervention was exposure to a helminth species or combination of species not normally found in humans. The control group received placebo (i.e. sham helminth exposure), no treatment or any other active intervention. Helminth‐derived molecular products were outside the scope of this review and were not included.
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of patients who achieved clinical remission as defined by primary studies and expressed as a percentage of those participants randomised to the intervention (i.e. intention‐to‐treat analysis).
Secondary outcomes
Secondary outcomes included the following.
Clinical, histologic, endoscopic improvement as defined by the authors.
Endoscopic mucosal healing (endoscopic remission).
Change in disease activity index score.
Quality of life.
Hospital admissions.
Requirement for intravenous corticosteroids.
Surgery.
Adverse events.
Study withdrawal.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no restrictions on language, publication year, or publication status. If trial reports were unclear, we attempted to contact original authors for clarification and for further data. We were to arrange translations of papers where necessary.
Electronic searches
We identified published, unpublished and ongoing studies by searching the following databases from inception to July 2013: Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders (IBD/FBD) Group Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library); PubMed; EMBASE; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; CNKI; ISCTRN; ClinicalTrials.gov; ICTRP; and Google.
Search strategies for databases were modelled on a search strategy designed for CENTRAL for this systematic review (Appendix 3). Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials, as described in The Cochrane Handbook for Systematic Reviews of Interventions Version 5.1, Box 6.4.b (Lefebvre 2011).
Searching other resources
We scanned the reference lists of identified publications for additional trials, and contacted trial authors. In addition, we searched PubMed, TRIPdatabase, and Google Scholar to retrieve existing systematic reviews relevant to this systematic review, and scanned their reference lists for additional trials. We searched for conference abstracts using the Cochrane Inflammatory Bowel Disease and Functional Bowel Disorders (IBD/FBD) Group Specialised Trials Register. In addition, we manually searched conference abstract databases including Digestive Disease Week (DDW), the United European Gastroenterology Week (UEGW) and the European Crohn’s and Colitis Organisation (ECCO).
Data collection and analysis
Selection of studies
Phase one
Two authors (AC and SG) independently inspected all abstracts of studies identified by the literature search to determine potentially relevant studies. Disagreement, if any, as to the potential relevance of a particular study was resolved through discussion. Where doubt persisted, the full text of the trial was retrieved for inspection.
Phase two
We retrieved for further assessment, and for a final decision on inclusion, the full text of all those reports judged to be potentially relevant (see Criteria for considering studies for this review). Once the full texts were obtained, two authors (AC and SG) independently inspected the full reports and decided whether they met the inclusion criteria. AC and SG were not blinded to the names of the authors, source institutions, or journal of publication. Where difficulties or disputes on study eligibility arose, we asked PB for help.
PRISMA flow diagram
We included a PRISMA flow diagram to illustrate the results of our literature search and the process of screening and selecting studies for inclusion in the review (Moher 2009).
Data extraction and management
We designed a data extraction form to record data from five key domains of each included study, as follows:
Study characteristics (study design, date of study, total study duration, number of study centres and location, study withdrawals);
Participants (N, mean age, age range, gender distribution, sociodemographic characteristics, ethnicity, inclusion criteria, exclusion criteria);
Interventions (for each intervention: total number in intervention arm, helminth species used, developmental stage of the helminth, dose of exposure, route of exposure, duration of exposure);
Controls (for each control: total number in control arm; where control was an active pharmacological intervention: nature, dose and route of administration); and
Outcomes (outcomes specified and collected, time points reported).
For eligible studies, AC and SG independently extracted the data using the data extraction form. AC and SG resolved discrepancies through discussion; failing resolution, we consulted PB. We entered data into the Review Manager (RevMan) software version 5.2 (RevMan 2012) and checked data for accuracy. When information regarding any data item was unclear, we contacted the authors of the original reports to provide further details.
Assessment of risk of bias in included studies
AC and SK independently assessed the methodological quality of each included study using the Cochrane Collaboration’s ‘Risk of bias’ tool (Higgins 2011a).
The study features assessed included:
Random sequence generation;
Allocation concealment;
Blinding of participants, personnel, and outcome assessors;
Incomplete outcome data;
Selective reporting; and
Other bias.
We rated each of these factors as ‘low risk’, ‘high risk’ or ‘unclear risk’, with a brief overview provided in table format. If an item was rated as ‘unclear’, we attempted to seek clarification from trial authors. Appendix 4 gives more information about the assessment scheme.
Measures of treatment effect
We performed statistical analysis using Review Manager 5.2.
Dichotomous data
We calculated a risk ratio (RR) with corresponding 95% confidence intervals (95% CI) for dichotomous outcomes. Where appropriate, we expressed the estimated effects as NNTB (number needed to treat, to benefit). The NNTB corresponds mathematically to the inverse of the risk difference, and clinically to the number of patients to be treated to achieve one desirable event.
Continuous data
For continuous outcomes, we calculated the mean difference (MD) along with the corresponding 95% CI. We planned to calculate the standardized mean difference (SMD) and corresponding 95% CI for continuous outcomes measured on different scales.
Summary data
For those RCTs where the only data available is a summary measure of effect (e.g. crossover studies), along with a precision estimate, we were to use the generic inverse variance method to analyse that data.
Unit of analysis issues
If any trials had multiple treatment groups, the ‘shared’ comparison group was to be divided into the number of treatment groups, and comparisons between each treatment group and the split comparison group were to be treated as independent comparisons.
Dealing with missing data
Where data were missing, we were to contact the study authors directly to obtain this missing information.
For all outcomes, in all studies, we carried out analyses on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and we analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
For continuous data that were missing, we were to estimate the standard deviations from other available data (e.g. standard errors), or to impute them using methods suggested by Higgins 2011b. We made no assumptions about loss to follow‐up for continuous data, and we based our analyses on those participants completing the trial. We intended to perform a sensitivity analysis by calculating the treatment effect of including and excluding the imputed data, to see whether this altered the outcome of the analysis. We planned to investigate the effect of study withdrawals and exclusions by conducting worst‐ versus best‐case scenario analyses.
If there was discrepancy between the number randomised and the number analysed in each treatment group, we were to calculate and report the percentage lost to follow‐up in each group. If study withdrawals exceeded 10% for any trial, we were to assign the worst outcome to those lost to follow‐up for dichotomous outcomes, and to assess the impact of this sensitivity analysis against the results for those completing the study. Where it was not possible to obtain missing data, we planned to record in the data extraction form and report in the ‘Risk of bias’ table. For included studies, we noted the levels of attrition. We were to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect, by using sensitivity analyses.
Assessment of heterogeneity
We planned to assess heterogeneity between pooled trials using:
the Chi2 test; in conjunction with
the I2 statistic, which describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error, or chance (Higgins 2003).
A P value of < 0.10 was to be considered statistically significant.
If enough trials were identified, we were to explore the sources of heterogeneity using subgroup analyses. We displayed results graphically using forest plots, with a summary statistic presented if there is no major statistical heterogeneity (i.e. no overlap of confidence intervals in the forest plots). We intended to use a I2 value of:
< 25% to denote low heterogeneity;
≥ 50% to denote significant heterogeneity; and
≥ 75% to denote substantial and major heterogeneity.
Assessment of reporting biases
If sufficient trials and data were available (e.g. 10 or more studies), we were to assess publication bias by preparing a funnel plot. We also planned to perform a visual assessment of funnel plot asymmetry, and to carry out exploratory analyses to investigate any suggestion of visual asymmetry in the funnel plots. Our searches for trials and trial protocols listed in clinical trial registries would help to avoid publication bias, and assist in assessing outcome selection bias. Where necessary, we planned to contact study authors in an attempt to either establish a full dataset or obtain reasons for the non‐reporting of certain outcomes.
Data synthesis
If sufficient clinically similar studies were available, and substantial heterogeneity between the studies was not identified, we planned to pool their results in meta‐analyses. We planned to use adjusted summary statistics, if available; otherwise unadjusted results were to be used. Pooling of data was planned as follows:
for dichotomous outcomes a pooled RR and corresponding 95% CI was to be calculated;
for continuous outcomes measured on the same scale, the pooled MD and corresponding 95% CI was to be calculated;
for continuous outcomes measured on different scales, the pooled SMD and corresponding 95% CI was to be calculated; and
for time‐to‐event data, hazard ratios (HRs) were to be pooled using the generic inverse variance facility of RevMan (Deeks 2011).
We planned to use a fixed‐effect model to pool data in the absence of heterogeneity. If statistically significant heterogeneity was identified we planned to use a random‐effects model to pool data.
We used the GRADE approach (Schünemann 2011) to assess and summarise the quality of the evidence for the primary outcome and secondary outcomes of interest. Domains that may decrease the quality of the evidence include:
The study design;
Risk of bias;
Inconsistency of results;
Indirectness (i.e. non‐generalisability);
Imprecision (i.e. insufficient data); and
Other factors (e.g. reporting bias).
We reduced the quality of the evidence by one level for each domain where poor quality was encountered. We assessed all plausible confounding factors and considered their effects as a reason to reduce any claimed effect and dose response gradient. We defined levels of evidence as below.
High quality evidence
The following statement applies to all of the domains: Further research is very unlikely to change our confidence in the estimate of effect. There are consistent findings, that are generalisable to the population of interest, in 75% of RCTs with low risk of bias. There are sufficient data, with narrow confidence intervals. There are no known or suspected reporting biases.
Moderate quality evidence
The following statement applies to one of the domains: Further research is likely to have an important impact on our confidence in the estimate of effect, and may change the estimate.
Low quality evidence
The following statement applies to two of the domains: Further research is very likely to have an important impact on our confidence in the estimate of effect, and is likely to change the estimate.
Very low quality evidence
The following statement applies to three of the domains: We are very uncertain about the estimate.
No evidence
The following statement applies: No RCTs were identified that measured the outcome of interest.
We also considered a number of other factors to place the results into a wider clinical context: temporality, plausibility, strength of association, and adverse events.
We generated a Summary of Findings (SoF) table to report the results of the GRADE analysis for the following outcomes:
Clinical remission; and
Clinical improvement.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we intended to perform subgroup analyses to explore the effects of:
different helminth species or combination of helminth species;
different developmental stages of the administered helminths;
different exposure doses;
different routes of administration of the helminths; and
different durations of exposure to the helminths.
Sensitivity analysis
Where possible, we planned to perform sensitivity analyses to explore the effects of various aspects of trial and review methodology, including the effects of missing data and whether or not allocation was concealed.
If sufficient data were available, we were to perform sensitivity analyses to determine the impact of excluding those studies with lower methodological quality, for example:
trials at high or unclear risk of bias;
unpublished studies (since these may not have been subjected to the peer review process and may have intrinsic biases);
industry‐sponsored studies; and
trials that have not assessed compliance.
Results
Description of studies
Results of the search
A literature search conducted on July 13, 2013 identified 164 records. After excluding duplicates, two authors (SG and AC) independently examined the abstracts of 139 citations to identify eligible studies. After exclusion of non‐applicable studies, we retrieved the full‐text articles of five studies for assessment. Three of these studies were excluded. We identified two randomised controlled trials as eligible for inclusion in the review (Figure 1). We identified two ongoing studies from Clinicaltrials.gov website (http://www.clinicaltrials.gov/).
1.
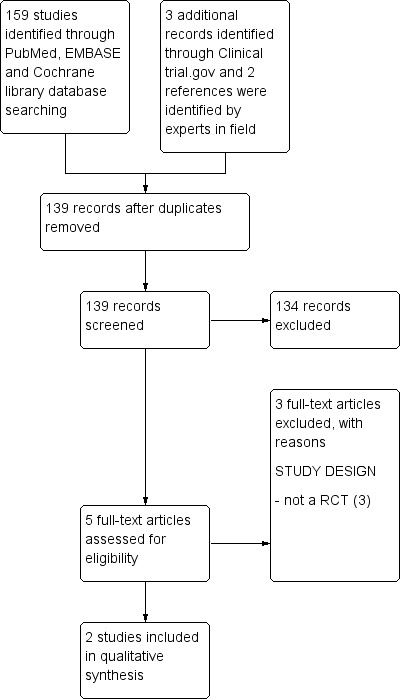
Study flow diagram.
Included studies
Summers 2005a was a randomised, double blind, placebo‐controlled trial conducted at the University of Iowa and at select US private practices. The participants were treated for six months with a median length of follow‐up of three months. The study duration was 12 weeks. This study randomised 54 adult participants (18 to 72 years) with a diagnosis of active ulcerative colitis, defined as an Ulcerative Colitis Disease Activity Index (UCDAI) of ≥ 4. Participants received 2500 T. suis eggs (n = 30) or placebo (n = 24) orally at 2‐week intervals for 12 weeks. The eggs of T. suis were obtained from the US Department of Agriculture. Eggs were treated with antibiotics and 0.2% K2Cr2O7 to render them bacteria‐free, and were also tested for viral and bacterial pathogens. The primary study outcome was clinical improvement at 12 weeks, defined as a decrease in the UCDAI of ≥ 4. Secondary outcomes included clinical remission at 12 weeks, defined as UCDAI of ≤ 2, and adverse events. Medications which were permitted and continued at the same dose throughout the study were: oral sulphasalazine, mesalamine, mesalamine derivatives, or oral prednisone at a dose up to 25 mg/day (if received for > 8 weeks and if received at the same dose for at least 4 weeks prior to entry); and azathioprine or 6‐mercaptopurine (if received for > 6 months and if received at the same dose for at least 8 weeks prior to entry).
Sandborn 2013 was a phase one, randomised, double‐blind, placebo‐controlled, multi‐centre, sequential dose‐escalation study designed to evaluate the safety of a single dose of oral suspension T. suis ova in patients with Crohn’s disease. This study enrolled 36 adult patients (18 to 55 years) participants with a confirmed diagnosis of Crohn's disease by established criteria with minimum disease duration of 3 months. Participants received sequential dose‐escalation (500, 2500 and 7500 viable embryonated T. suis ova or placebo) in three cohorts. Within each cohort nine patients were randomised to T. suis ova and three to placebo. A total of 27 patients received T. suis ova and nine received placebo. The primary study outcome was assessment of the safety and tolerability of single escalating doses of T. suis ova.
Excluded studies
Croese 2006 was a 20‐week safety trial in Crohn’s disease. Five of the nine participants enrolled in this trial had active Crohn’s disease, while the other four were in long‐standing remission. Participants were inoculated percutaneously with 25 to 100 Necator americanus larvae. Some participants developed abdominal discomfort and other symptoms in the first few weeks of the trial, due to the magnitude of the helminth infection. Four of the five participants with active disease had improvement in their Crohn’s Disease Activity Index (CDAI) and also in the Inflammatory Bowel Disease Quality of Life index (IBDQ), and four participants achieved remission. The four participants with initial long‐standing remission remained in remission at week 20. Five of these patients (i.e. overall, 5/9) received a second inoculation and were followed‐up for an additional 25 weeks. Their disease activity remained low during this second treatment interval.
Summers 2003 studied four patients with active CD and three with UC. In an initial treatment and observation period, a single dose of 2500 live T. suis eggs was given orally, and patients were followed up every 2 weeks, for 12 weeks in total. Baseline medications were continued at the same dose throughout the study. Safety was monitored by assessing the patients’ clinical status at regular intervals and performing laboratory investigations. Patients were also monitored regularly using the Crohn’s Disease Activity Index (CDAI), the Simple Clinical Colitis Activity Index (SCCAI), and the Inflammatory Bowel Disease Quality of Life index (IBDQ). To assess safety and efficacy with repeat doses, two patients with CD and two with UC were given 2500 T. suis eggs at 3‐week intervals as maintenance treatment, for a period of > 28 weeks, and using the same evaluation parameters.
During the treatment and observation period, all patients improved clinically, without any adverse events or laboratory abnormalities. Three of the four patients with CD entered remission. The fourth patient experienced a clinical response (CDAI reduction of 151) but did not achieve remission. Patients with UC experienced a reduction of the SCCAI to 57% of baseline. Assessed by the IBDQ index, six of seven patients (86%) achieved remission. The benefit derived from the initial dose was temporary. In the maintenance period, multiple doses again caused no adverse effects and there was sustained clinical improvement in all patients treated every 3 weeks for > 28 weeks.
Summers 2005b enrolled 29 participants with active CD, defined by a Crohn’s disease activity index (CDAI) ≥ 220. All the study participants ingested 2500 live T. suis eggs every three weeks for 24 weeks. The primary outcomes were remission (defined as a decrease in CDAI to < 150) or response (defined as a decrease in CDAI of > 100 compared to baseline). At Week 12, 76% of participants achieved clinical response, and 66% achieved clinical remission. By week 24, 79% responded and 72% were in remission. There were no adverse effects or complications attributable to T. suis egg administration. This was an uncontrolled open‐label study and so participants knew they were ingesting T. suis eggs. The results of the study suggest that patients with CD may improve with T. suis exposure, and that porcine whipworm may be safe in people with CD, some of whom were on prednisone or azathioprine or both. At week 24, 23 participants (79.3%) achieved clinical response, and 21 (72.4%) participants achieved remission. The mean CDAI of responders decreased by 177.1 points compared to baseline.
Risk of bias in included studies
Both included studies used adequate methods of randomisation, blinding, and allocation concealment and were rated as low risk of bias for these items (See Figure 2). Both studies were rated as low risk of bias for incomplete outcome data, selective reporting and other potential sources of bias.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Helminth therapy for UC
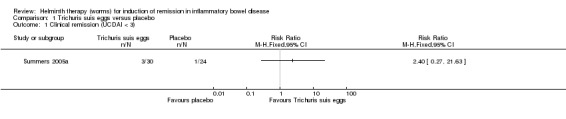
Induction of remission at 12 weeks. There was no statistically significant difference in the proportion of participants who achieved clinical remission at 12 weeks. Ten per cent (3/30) of patients in the T. suis arm achieved clinical remission compared to 4.2% (1/24) of patients in the placebo arm (RR 2.40, 95% CI 0.27 to 21.63; Figure 3).
3.

Forest plot of comparison: 1 Trichuris suis eggs versus placebo, outcome: 1.1 Clinical remission (UCDAI < 3).
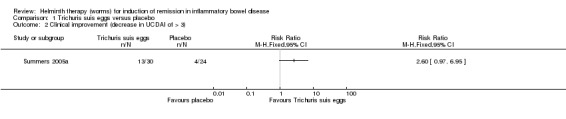
Clinical improvement at 12 weeks. There was no statistically significant difference in the proportion of participants who achieved clinical improvement. Forty‐three per cent (13/30) of patients in the T. suis group achieved clinical improvement compared to 17% (4/24) of placebo patients (RR: 2.60, 95% CI 0.97 to 6.95; Figure 4).
4.

Forest plot of comparison: 1 Trichuris suis eggs versus placebo, outcome: 1.2 Clinical improvement (decrease in UCDAI of > 3).
Histologic and endoscopic improvement.Summers 2005a did not report on histologic or endoscopic improvement as outcomes.
Endoscopic mucosal healing.Summers 2005a did not report on endoscopic remission as an outcome.
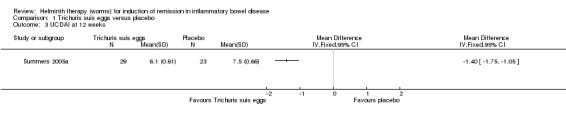
Ulcerative Colitis Disease Activity Index (UCDAI) at 12 weeks. The mean UCDAI score was lower in the T. suis egg group compared to the placebo group after 12 weeks of treatment (MD ‐1.40, 95% CI ‐1.75 to ‐1.05; Figure 5).
5.

Forest plot of comparison: 1 Trichuris suis eggs versus placebo, outcome: 1.3 UCDAI at 12 weeks.
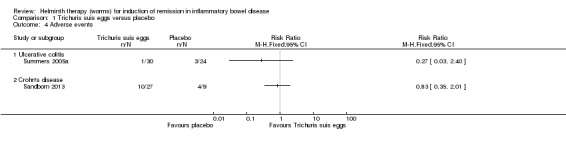
Frequency and nature of adverse events. There was no statistically significant difference in the proportion of patients who experienced at least one adverse event. Three per cent (1/30) of patients in the T. suis group experienced an adverse event compared to 12% (3/24) of placebo patients (RR 0.27, 95% CI 0.03 to 2.40). None of the adverse events were attributed to the therapeutic agent. No serious adverse events were reported. One patient in the T. suis group had mild hydrochlorothiazide‐induced pancreatitis that resolved after the drug was stopped. Adverse events in the placebo group included pneumonia and exacerbation of chronic obstructive pulmonary disease (in one patient), pain due to rib fracture, and hyperglycemia.There were no significant differences between groups in their haematologic, hepatic, or renal profile during the 12‐week study period. No worms or eggs were identified in stools.
Quality of life.Summers 2005a did not report on quality of life.
Hospital admissions.Summers 2005a study did not report information on hospital admission rates.
Surgery.Summers 2005a did not report on the effect of the intervention on the need for surgery.
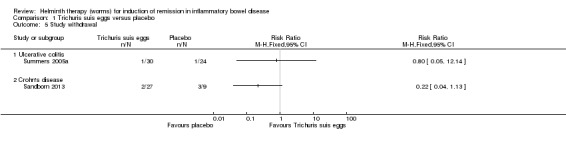
Study withdrawal. There was no statistically significant difference in the proportion of patients who withdrew before study completion. One patient in each group discontinued treatment (RR 0.80, 95% CI 0.05 to 12.14).
Requirement for intravenous steroids. No information was available on the effect of the intervention on the requirement for intravenous steroids.
Helminth therapy for CD
The purpose of the Sandborn 2013 study was to assess the safety and tolerability of T. suis ova in CD. This study didn't report on efficacy outcomes such as clinical remission or response.
Frequency and nature of adverse events. There was no statistically significant difference in the proportion of patients who experienced at least one adverse event. Thirty‐seven per cent (10/27) of patients in the T. suis group experienced an adverse event compared to 44% (4/9) of placebo patients (RR 0.83, 95% CI 0.35 to 2.01).
In the first 2 weeks 14 patients experienced treatment emergent adverse events including 6 (66.7%) patients in the T. suis ova 7500 group, 3 (33.3%) T. suis 500 patients, 1 (11.1%) T. suis 2500 patient and 4 (44.4%) placebo patients. Adverse events included nausea, vomiting, abdominal pain, flatulence, decreased appetite, mucus stools, sinus congestion, headache, rectal haemorrhage, oropharyngeal pain, laryngeal oedema and nasopharyngitis. All adverse events were in mild in severity and most of them were judged to be not related to treatment. There was no dose dependent increase in adverse events. The only adverse event that was judged by the investigators to be possibly related to study treatment was dysgeusia. Dysgeusia was reported in one patient in the T. suis 7500 group and in one patient in the placebo group. Adverse events reported during months two through six were consistent with that expected in patients with CD. Five serious adverse events were reported in three patients during the follow‐up period. These events included small intestinal resection and serious abdominal pain in two placebo patients and Crohn's disease flare, anovaginal fistula and Clostridium difficile infection in a patient treated with T. suis 7500. None of these serious adverse events were judged to be related to study treatment.
Study withdrawal. There was no statistically significant difference in the proportion of patients who withdrew before study completion. Seven per cent of T. suis patients withdrew before study completion compared to 33% (3/9) placebo patients (RR 0.22, 95% CI 0.04 to 1.13).
Discussion
This is the first systematic review on the use of helminths for treatment of IBD, and the second Cochrane review to investigate the therapeutic uses of helminths. Our previous review was published in 2012 (Croft 2012).
Summary of main results
This systematic review on the use of helminths for induction of remission in IBD identified two trials which met the inclusion criteria. Summers 2005a enrolled 54 participants with chronic active ulcerative colitis, and compared 2500 Trichuris suis eggs ingested every 2 weeks with placebo. Trichuris suis eggs did not provide any statistically significant benefit over placebo for induction of remission or clinical improvement. Although there was a statistically significant difference in the mean UCDAI score favouringTrichuris suis eggs over placebo this difference is unlikely to be of any clinical significance as the mean scores indicated that most patients in both groups still had active disease and the actual mean difference was quite small (i.e. 1.4 points) and was not likely to be detectable by patients or physicians (Brant 1999). There was no statistically significant difference in the proportion of patients who experienced an adverse event. No serious adverse effects from T. suis egg therapy were reported. Another included study enrolled 36 patients with Crohn's disease (Sandborn 2013). Patients were randomised to receive either placebo or three different doses of T. suis ova (i.e. 500, 2500 or 7500 viable embryonated T. suis ova). Patients were followed for a two month period. This study was done to assess the safety and tolerability of a single dose of T. suis ova. There was no statistically significant difference in the proportion of patients who experienced an adverse event. All adverse events were in mild in severity and most of these events were judged to be not related to treatment. The only adverse event that was judged by the investigators to be possibly related to study treatment was dysgeusia and this was reported in one patient in the T. suis 7500 group and in one placebo patient.
Overall completeness and applicability of evidence
The Summers 2005a study was small, with a power of only 43% to detect a 20% increase in the proportion of participants with clinical improvement after helminth therapy, assuming alpha error of 0.05. The results of this study should be interpreted with caution given the imprecise estimate of effect. The Sandborn 2013 study was a small phase one study performed to assess the safety and tolerability of a single dose of T. suis therapy in patients with Crohn's disease. Thus no conclusions can be made about the efficacy of T. suis therapy for induction of remission or clinical improvement in patients with Crohn's disease.
Quality of the evidence
The overall quality of the evidence as assessed by the GRADE approach was low for the primary outcome (i.e. remission at three months) due to serious imprecision. This indicates that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. The overall quality of the evidence for clinical improvement at three months was also judged to be low, indicating low confidence in this effect estimate.
Potential biases in the review process
We did not identify any potential biases in the review process. A comprehensive search was performed and all studies were independently assessed and data extracted by at least two review authors. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
Agreements and disagreements with other studies or reviews
No other similar review has been conducted.
Authors' conclusions
Authors' conclusions
Implications for practice.
Currently, there is insufficient evidence to allow any firm conclusions regarding the efficacy and safety of helminths used to treat patients with IBD. The results for our primary efficacy outcomes in this review come from a single trial in patients with active ulcerative colitis. The findings of this study need to be interpreted with caution as they are based on a small number of patients and the overall quality of the evidence was low. We do not have enough evidence to determine whether helminths are safe when used in patients with ulcerative colitis and Crohn's disease.
Implications for research.
Further randomised controlled trials are needed to assess the efficacy and safety of T. suis ova therapy in ulcerative colitis and Crohn's disease. Trials should be adequately powered and should include clinically relevant outcomes including mucosal healing. These studies should investigate different doses and duration of T. suis treatment. From the study on ulcerative colitis patients, and based on the clinical improvement rates achieved in the placebo group at 12 weeks, we estimate that at least 91 participants per study arm would need to be enrolled in any future study, to detect a 20% increase in the proportion of participants with clinical improvement from helminth therapy (assuming alpha error of 0.05 and power of 80%). To satisfy safety concerns, there is a need for research on helminths with little pathogenic potential, which would not be able multiply in their human host or be spread easily to other people.
What's new
| Date | Event | Description |
|---|---|---|
| 14 January 2014 | Amended | Correction of typo |
Acknowledgements
We thank the Editorial Board of the IBD/FBD Review Group for their support and assistance.
We thank Gemma Sandberg from the Cochrane ENT Disorders Group for her help in designing the CENTRAL search strategy.
We thank Dr Joel Weinstock for his helpful comments on an earlier draft of this review.
Funding for the IBD/FBD Review Group (September 1, 2010 ‐ August 31, 2015) has been provided by the Canadian Institutes of Health Research (CIHR) Knowledge Translation Branch (CON ‐ 105529) and the CIHR Institutes of Nutrition, Metabolism and Diabetes (INMD); and Infection and Immunity (III) and the Ontario Ministry of Health and Long Term Care (HLTC3968FL‐2010‐2235).
Miss Ila Stewart has provided support for the IBD/FBD Review Group through the Olive Stewart Fund.
Appendices
Appendix 1. Commonly encountered helminths of humans
| Phylum | Common species / definitive anatomical sites as adult worms |
| Annelids (segmented worms) | Class: Hirudinea (leeches) |
| Nematodes (roundworms) |
Class: Nematoda (roundworms) The dermis: Mansonella streptocercac The gut – small intestine: Ancylostoma duodenale (hookworm)a, Ascaris lumbricoides (roundworm)a, Capillaria philippinensisd, Necator americanus (hookworm)a, Strongyloides stercoralis (threadworm)a,Trichostrongylus orientalisd The gut – large intestine: Trichuris trichiura (whipworm)d The gut – caecum: Enterobius vermicularis (pinworm)d The lymphatic system: Brugia malayia, Brugia timoria, Wuchereria bancroftia The pericardial, peritoneal and pleural cavities: Dracunculus medinensis (Guinea worm)b,Mansonella perstansa The subcutaneous tissues: Loa loac, Mansonella ozzardic, Onchocerca volvulusc |
| Platyhelminths (flatworms) |
Class: Trematoda (flukes) The bronchi: Paragonimus sppa The gut – small intestine: Echinostoma sppd, Fasciolopsis sppd, Gastrodiscoides sppd, Heterophyes sppd, Metagonimus sppd The hepatobiliary system: Clonorchis sinensisd, Fasciola giganticaa, Fasciola hepaticaa,Opisthorchis felineusd, Opisthorchis viverrinid The venous system – mesenteric veins: Schistosoma intercalatuma, Schistosoma japonicuma, Schistosoma mansonia, Schistosoma mekongia The venous system – vesical plexus: Schistosoma haematobiuma |
|
Class: Cestoda (tapeworms) The gut – small intestine: Diphyllobothrium latum (fish tapeworm)d, Diphyllobothrium pacificumd, Dipylidium caninumd,Hymenolepsis diminuta (rat tapeworm)d,Hymenolepsis nana (dwarf tapeworm)d, Taenia saginata (beef tapeworm)d, Taenia solium (pork tapeworm)d |
aMigrates through host tissues, in larval forms.
bMigrates through host tissues, as adult worm.
cMigrates through host tissues in both larval forms and as adult worm.
dNo significant tissue migration through host tissues.
Appendix 2. Biological characteristics of helminths
Helminths are complex multicellular organisms. When mature, they range in length from 2 mm (Strongyloides stercoralis adults) to 8 m (Taenia saginata adults) (Weller 2008).
Most helminth species are free‐living, and inhabit either bodies of fresh water, or else warm, moist soil. The latter group of helminths are known collectively as soil‐transmitted helminths (or ‘geohelminths’).
Helminths have highly developed internal structures, including alimentary and reproductive tracts.
Helminths have complex and highly varied life cycles, with multiple developmental stages. Some developmental stages may take place in an intermediate host. Some helminth species require two distinct, successive intermediate hosts.
Helminths are highly species‐specific, in most cases with a biological dependence on a single definitive host; where they have one or more intermediate hosts, they are highly species‐specific for these also (Strickland 1999).
A very few helminth species (e.g. Enterobius vermicularis and Strongyloides stercoralis) can be transmitted directly from person to person. Generally, however, person‐to‐person transmission is not possible (and hence helminths meet minimum safety criteria as therapeutic interventions).
With the exception of leeches, which are solely ectoparasites, helminths enter their definitive, human hosts either orally (as eggs or cysts) or percutaneously (as larvae or cercariae). A specific arthropod vector such as a specific mosquito species (in lymphatic filariasis) or a specific species of biting fly or midge (in loiasis, onchocerciasis and mansonellosis) may be necessary for the helminth to achieve successful percutaneous penetration of the host.
Multiple infestations with different helminth species are common in endemic areas (Finch 2009).
The larval and adult forms of helminths are always motile. Helminths often have a larval migratory phase in their human hosts, before taking up residence as adult worms in their definitive anatomical site (see Appendix 1). Eosinophilia and elevated serum IgE levels are features of many helminthic infestations (Weller 2008).
Once established in their definitive anatomical site, adult helminths may be very long‐lived (up to 30 years in the case of the schistosomes) (Finch 2009). However because most helminth parasites do not self‐replicate, the acquisition of a heavy burden of adult worms requires repeated exposure to the parasite in its infectious stage, whether egg or larva. Hence clinical disease, as opposed to asymptomatic infestation, generally develops only with prolonged residence in an endemic area (Weller 2008).
On account of their large size, helminths are solely extracellular; hence they are sometimes referred to as ‘macroparasites’ (Olano 2006).
Because of their size, and their prolonged life cycles and generation times, helminths have limited capacity for genetic alteration, compared to smaller, simpler microbes, or ‘microparasites’ (Olano 2006).
Appendix 3. CENTRAL search strategy
#1 ulcerative colitis #2 ulcerative colitis [MeSH] #3 colitis #4 colitis [MeSH] #5 Crohn* disease #6 Crohn disease (MeSH) #7 Regional enteritis #8 Ileitis #9 Ileitis (MeSH) #10 inflammatory bowel disease #11 inflammatory bowel disease [MeSH] #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 MeSH descriptor Helminths explode all trees #14 MeSH descriptor Antigens, Helminth explode all trees #15 MeSH descriptor Antibodies, Helminth explode all trees #16 MeSH descriptor Parasitology explode all trees #17 (helminth* OR anti‐helminth* OR antihelminth* OR anthelmint* OR aschelminth* OR soil‐transmitted helminth* OR geohelminth* OR parasit*) #18 (annelid* OR hirudine* OR leech*) #19 (nematod* OR roundworm* OR hookworm* OR pinworm* OR threadworm* OR whipworm* OR ancylostom* OR ascari* OR brugia OR enterobi* OR loa OR mansonell* OR onchocerc* necator OR strongyl* OR toxocar* OR trichin* OR trichur* OR wuchereria) #20 ((filarial AND worm*) OR filariasis OR onchocerca OR onchocerciasis OR loa‐loa OR loiasis OR wuchereria OR brugia OR mansonella OR mansonellosis) #21 (dracuncul* OR (guinea AND worm*)) #22 (platyhelminth* OR flatworm* OR trematod* OR fluke* OR clonorchis OR echinostom* fasciol* OR gastrodiscoid* OR heterophy* OR metagonim* OR opisthorch* OR paragonim* OR schistosom*) #23 (cestod* OR tapeworm* OR diphyllobothrium OR hymenolepis OR taenia* OR tenia* OR cysticerc*) #24 #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 #25 #12 AND #24
Appendix 4. Strategy to assess risk of bias
Each of the following study features will be assessed and recorded as representing a ‘Low risk’, ‘High risk’ or ‘Unclear risk’ of bias.
1. Random sequence generation
Was the allocation sequence adequately generated: e.g. coin toss, random number tables, computer generated, other?
2. Allocation concealment
Was allocation adequately concealed in a way that would not allow both the investigators and the participants to know or influence the intervention group before an eligible participant is entered into the study: e.g. central randomisation, or sequentially numbered, opaque, sealed envelopes?
3. Blinding of participants and personnel
Were participants blinded to the helminth interventions they were receiving?
Were investigators blinded to the helminth interventions they were administering?
For each of these groups, blinding will be recorded as: Yes, No, Not Possible or Unclear. The study will be recorded as double‐blind if both the participants and the personnel were blinded.
4. Blinding of outcome assessment
Were assessors blinded to the effects they were assessing?
Blinding of assessors will be recorded as: Yes, No, Not Possible or Unclear. The study will be recorded as double‐blind if both the investigators and participants were blinded, and as triple‐blind if all three groups were blinded.
5. Incomplete outcome data
Were incomplete outcome data adequately addressed?
If any withdrawals occurred, were these withdrawals described and reported by treatment group?
Were clear explanations recorded for withdrawals and dropouts in treatment groups?
Incomplete outcomes data essentially include attrition, exclusions and missing data. An example of an adequate method to address incomplete outcome data is the use of intention‐to‐treat analysis (ITT).
6. Selective reporting
Are reports of the study free from any suggestion of selective outcome reporting?
If reports are free of this suggestion, this will be interpreted as representative of no evidence that statistically non‐significant results have been selectively withheld from publication (e.g. through selective under‐reporting of data, or through selective reporting of a subset of the data).
7. Other bias
Was the study apparently free of other defects that could put it at a high risk of bias (e.g. baseline imbalance, or the use of an insensitive instrument to measure outcomes)?
Data and analyses
Comparison 1. Trichuris suis eggs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical remission (UCDAI < 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical improvement (decrease in UCDAI of > 3) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 UCDAI at 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ulcerative colitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Crohn's disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Study withdrawal | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ulcerative colitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Crohn's disease | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
Comparison 1 Trichuris suis eggs versus placebo, Outcome 1 Clinical remission (UCDAI < 3).
1.2. Analysis.
Comparison 1 Trichuris suis eggs versus placebo, Outcome 2 Clinical improvement (decrease in UCDAI of > 3).
1.3. Analysis.
Comparison 1 Trichuris suis eggs versus placebo, Outcome 3 UCDAI at 12 weeks.
1.4. Analysis.
Comparison 1 Trichuris suis eggs versus placebo, Outcome 4 Adverse events.
1.5. Analysis.
Comparison 1 Trichuris suis eggs versus placebo, Outcome 5 Study withdrawal.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Sandborn 2013.
| Methods | Randomised, double blind, placebo‐controlled, parallel group trial Clinical and laboratory examinations were performed at entry, 2 weeks and 6 months |
|
| Participants | Adult patients (18 to 55 years) with a confirmed diagnosis of Crohn's disease by established criteria with a minimum duration of 3 months were recruited from were recruited from 6 investigational centres in US | |
| Interventions | A single treatment of Trichuris suis ova at doses of 500 (n = 9), 2500 (n = 9) or 7500 (n = 9) embryonated viable T. suis ova or matching placebo (n = 9) | |
| Outcomes | Adverse events Patient diary card |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation was generated by the drug packaging vendor using a software algorithm that allocated patients to each treatment according to the study design specifications" |
| Allocation concealment (selection bias) | Low risk | Centralized allocation by the drug packaging vendor |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient withdrawal was equal in all groups |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Summers 2005a.
| Methods | Randomised, double blind, placebo‐controlled, parallel group trial Clinical and laboratory examinations were performed at recruitment, and weeks 2, 4 and 8 |
|
| Participants | Adult patients (18 to 72 years) with active ulcerative colitis (defined by a UCDAI score of > 4) were recruited from the University of Iowa's Center for Digestive Diseases and select gastroenterology practices in the State of Iowa (N = 54) | |
| Interventions | All patients received 2500 Trichuris suis ova (n = 30) or placebo (n = 24) orally at 2 week intervals for 12 weeks | |
| Outcomes | Clinical improvement at 12 weeks (defined as a decrease in the UCDAI of more than 3 from baseline) was the primary measure of efficacy Clinical remission, as defined by UCDAI of less than 3, was a secondary end point |
|
| Notes | The sponsors did not take part in and in no way influenced the research design,data collection, data analyses, interpretation of the data, or preparation and approval of the manuscript | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "This individual, an experienced nurse investigator, assigned participants to receive ova or placebo by using a set of random numbers that was selected at the time of enrolment". |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation by nurse investigator "An individual not involved in the study and who had no patient contact prepared and coded all vials" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo and active treatment vials were indistinguishable" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although blinding of outcomes assessment was not clearly described we believe outcome assessors were blind due to the indistinguishable placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient in each group discontinued treatment |
| Selective reporting (reporting bias) | Low risk | The published report included all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Croese 2006 | Not a RCT |
| Summers 2003 | Not a RCT |
| Summers 2005b | Not a RCT |
Characteristics of ongoing studies [ordered by study ID]
NCT01279577.
| Trial name or title | Trichuris suis ova (TSO) suspension versus placebo in active Crohn's disease |
| Methods | Randomised, double‐blind, placebo‐controlled, multi‐centre phase II study |
| Participants | Adult patients (18 to 75 years) with an established diagnosis of Crohn's disease of at least 3 months duration prior to screening |
| Interventions | Different doses of Trichuris suis ova (defined as low, medium and high dose) or placebo |
| Outcomes | Primary Outcome: rate of clinical remission at week 12 (LOCF) defined as a CDAI < 150 Secondary Outcomes: reduction of > 100 points in CDAI from baseline at week 12 and adverse events |
| Starting date | November 2010 |
| Contact information | Dr Ralph Müller |
| Notes |
NCT01434693.
| Trial name or title | A sequential dose‐escalation, double‐blind, placebo‐controlled, phase I study to evaluate the safety and tolerability of single doses of 3 different doses of oral CNDO‐201 Trichuris suis ova suspension (Tso) in patients with Crohn's disease |
| Methods | Randomised, double‐blind, placebo‐controlled, multi‐centre phase I study |
| Participants | Adult patients (18 to 55 years) with a confirmed diagnosis of Crohn's disease with a minimum disease duration of 3 months |
| Interventions | TSO 500 TSO 2500 TSO 7500 Placebo |
| Outcomes | Incidence of adverse events with a specific focus on reported gastrointestinal signs and symptoms (6 month follow‐up) |
| Starting date | September 2011 |
| Contact information | Coronado Biosciences, Inc |
| Notes |
LOCF: Last observation carried forward
Differences between protocol and review
None
Contributions of authors
SG helped develop the protocol, for studies, carried out initial screening and quality assessment, wrote to authors, performed data extraction, data entry and data analysis, and wrote the review text.
AC conceived and co‐ordinated the review, registered the title, wrote the protocol, helped design the CENTRAL search strategy, searched for and screened studies, checked data and helped write the review text.
PB helped develop the protocol, checked data and helped write the review text.
Sources of support
Internal sources
No sources of support supplied
External sources
Commander Regional Forces, UK.
Declarations of interest
PB is a scientific consultant for Coronado Biosciences Inc, a company involved in the development of Trichuris suis egg therapy.
The other authors have no financial conflicts of interest and declare that they do not have any association with any manufacturers or promoters of pharmaceutical or helminth products, or with any parties who may have vested interests in the results of this review.
New
References
References to studies included in this review
Sandborn 2013 {published data only}
- Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry‐Wheeler A, Silver N, et al. Randomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn's disease. Alimentary Pharmacology and Therapeutics 2013;38(3):255‐63. [DOI] [PubMed] [Google Scholar]
Summers 2005a {published data only}
- Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 2005;128(4):825‐32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Croese 2006 {published data only}
- Croese J, O'neil J, Masson J, Cooke S, Melrose W, Pritchard D, et al. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut 2006;55(1):136‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Summers 2003 {published data only}
- Summers RW, Elliott DE, Qadir K, Urban JFJ, Thompson R, Weinstock JV. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. American Journal of Gastroenterology 2003;98(9):2034‐41. [DOI] [PubMed] [Google Scholar]
Summers 2005b {published data only}
- Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut 2005;54(1):87‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01279577 {published data only}
- NCT01279577. Trichuris suis ova (TSO) suspension versus placebo in active Crohn's disease (TRUST‐2). http://clinicaltrials.gov/ct2/show/NCT01279577 accessed 13 July 2013.
NCT01434693 {published data only}
- NCT01434693. Safety and tolerability of single doses oral CNDO 201 Trichuris suis ova in patients with Crohn's disease. http://clinicaltrials.gov/ct2/show/NCT01434693 accessed 13 July 2013.
Additional references
Akobeng 2003
- Akobeng AK, Zachos M. Tumor necrosis factor‐alpha antibody for induction of remission in Crohn’s disease. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003574.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bager 2010
- Bager P, Arnved J, Rønborg S, Wohlfahrt J, Poulsen LK, Westergaard T, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double‐blind, placebo‐controlled clinical trial. Journal of Allergy and Clinical Immunology 2010;125(1):123‐30. [DOI] [PubMed] [Google Scholar]
Bager 2011
- Bager P, Kapel C, Roepstorff A, Thmaborg S, Arnved J, Rønborg S, et al. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo‐controlled double‐blind clinical trial. PLoS One 2011;8(6):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Behm 2008
- Behm BW, Bickston SJ. Tumor necrosis factor‐alpha antibody for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006893] [DOI] [PubMed] [Google Scholar]
Benchimol 2008
- Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006792.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blount 2009
- Blount D, Hooi D, Feary J, Venn A, Telford G, Brown A, et al. Immunologic profiles of persons recruited for a randomized, placebo‐controlled clinical trial of hookworm infection. American Journal of Tropical Medicine and Hygiene 2009;81(5):911‐6. [DOI] [PubMed] [Google Scholar]
Borkow 2000
- Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. Journal of Clinical Investigation 2000;106(8):1053‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brant 1999
- Brant R, Sutherland L, Hilsden R. Examining the minimum important difference. Statistics in Medicine 1999;18(19):2593‐603. [DOI] [PubMed] [Google Scholar]
Chande 2007
- ChandeN, MacDonald JK, McDonald JWD. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006618.pub2] [DOI] [PubMed] [Google Scholar]
Chande 2013
- Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6‐mercaptopurine for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD000545.pub4] [DOI] [PubMed] [Google Scholar]
Croft 2012
- Croft AM, Bager P, Kumar S. Helminth therapy (worms) for allergic rhinitis. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009238.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daveson 2011
- Daveson JA, Jones DM, Gaze S, McSorley H, Clouston A, Pascoe A, et al. Effect of hookworm infection on wheat challenge in celiac disease – a randomised double‐blind placebo controlled trial. PLoS One 2011;6(3):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Dwinell 1997
- Dwinell MB, Bass P, Schaefer DM, Oaks JA. Tapeworm infection decreases intestinal transit and enteric aerobic bacterial populations. American Journal of Physiology 1997;273(2 Pt 1):G480‐5. [DOI] [PubMed] [Google Scholar]
Elliott 2000
- Elliott DE, Urban JF Jr, Argo CK, Weinstock JV. Does the failure to acquire helminthic parasites predispose to Crohn's disease?. FASEB Journal 2000;14(12):1848‐55. [DOI] [PubMed] [Google Scholar]
Elliott 2003
- Elliott DE, Li J, Blum A, Metwali A, Qadir K, Urban JF Jr, et al. Exposure to schistosome eggs protects mice from TNBS‐induced colitis. American Journal of Physiology. Gastrointestinal and Liver Physiology 2003;284(3):G385‐91. [DOI] [PubMed] [Google Scholar]
Elliott 2007
- Elliott DE, Summers RW, Weinstock JV. Helminths as governors of immune‐mediated inflammation. International Journal for Parasitology 2007;37(5):457‐64. [DOI] [PubMed] [Google Scholar]
Elliott 2008
- Elliott DE, Metwali A, Leung J, Setiawan T, Blum AM, Ince MN, et al. Colonization with Heligmosomoides polygyrus suppresses mucosal IL‐17 production. Journal of Immunology 2008;181(4):2414‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feagan 2012a
- Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000543.pub3] [DOI] [PubMed] [Google Scholar]
Feagan 2012b
- Feagan BG, MacDonald JK. Oral 5‐aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000544.pub3] [DOI] [PubMed] [Google Scholar]
Feary 2009
- Feary J, Venn A, Brown A, Hooi D, Falcone FH, Mortimer K, et al. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo‐controlled feasibility study. Clinical and Experimental Allergy 2009;39(7):1060‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feary 2010
- Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, Falcone FH, et al. Experimental hookworm infection: a randomized placebo‐controlled trial in asthma. Clinical and Experimental Allergy 2009;40(2):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 2009
- Finch RG, Irving WL, Moss P, AndersonJ. Infectious diseases, tropical medicine and sexually transmitted infection. In: Kumar P, Clark M editor(s). Kumar and Clark’s Clinical Medicine. 7th Edition. London: Saunders, 2009. [Google Scholar]
Hang 2010
- Hang L, Setiawan T, Blum AM, Urban J, Stoyanoff K, Arihiro S, et al. Heligmosomoides polygyrus infection can inhibit colitis through direct interaction with innate immunity. Journal of Immunology 2010;185(6):3184‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Hunter 2005
- Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti‐IL‐10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. Journal of Immunology 2005;174(11):7368‐75. [DOI] [PubMed] [Google Scholar]
Ince 2009
- Ince MN, Elliott DE, Setiawan T, Metwali A, Blum A, Chen HL, et al. Role of T cell TGF‐beta signaling in intestinal cytokine responses and helminthic immune modulation. European Journal of Immunology 2009;39(7):1870‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitagaki 2006
- Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. Journal of Immunology 2006;177(3):1628‐35. [DOI] [PubMed] [Google Scholar]
Lawson 2006
- Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005112.pub2] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Lim 2010
- Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn's disease. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008870] [DOI] [PubMed] [Google Scholar]
Mangan 2004
- Mangan NE, Fallon RE, Smith P, Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL‐10‐producing B cells. Journal of Immunology 2004;173(10):6346‐56. [DOI] [PubMed] [Google Scholar]
Marshall 2010
- Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5‐aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD004115.pub2] [DOI] [PubMed] [Google Scholar]
McDonald 2012
- McDonald JW, Tsoulis DJ, MacDonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn's disease. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD003459.pub3] [DOI] [PubMed] [Google Scholar]
McKay 1990
- McKay DM, Halton DW, McCaigue MD, Johnston CF, Fairweather I, Shaw C. Hymenolepis diminuta: intestinal goblet cell response to infection in male C57 mice. Experimental Parasitology 1990;71(1):9‐20. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olano 2006
- Olano JP, Weller PF, Guerrant RL, Walker DH. Principles and general considerations. In: Guerrant RL, Walker DH, Weller PF editor(s). Tropical Infectious Diseases – Principles, Pathogens and Practice. 2nd Edition. London: Churchill Livingstone, 2006. [Google Scholar]
Podolsky 2002
- Podolsky DK. Inflammatory bowel disease. New England Journal of Medicine 2002;347(6):417‐29. [DOI] [PubMed] [Google Scholar]
Prefontaine 2009
- Prefontaine E, Sutherland LR, MacDonald JK, Cepoiu M. Azathioprine or 6‐mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000067.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rutgeerts 2005
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine 2005;353(23):2462‐76. [DOI] [PubMed] [Google Scholar]
Sabin 1996
- Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid‐specific Th1‐like immune responses in humans infected with Schistosoma mansoni. Journal of Infectious Diseases 1996;173(1):269‐72. [DOI] [PubMed] [Google Scholar]
Schnoeller 2008
- Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, et al. A helminth immunomodulator reduces allergic and inflammatory response by induction of IL‐10‐producing macrophages. Journal of Immunology 2008;180(6):4265‐72. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Seow 2008
- Seow CH, Benchimol EI, Griffiths AM, Otley AR, Steinhart AH. Budesonide for induction of remission in Crohn's disease. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD000296.pub3] [DOI] [PubMed] [Google Scholar]
Smith 2007
- Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, Rooijen N, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage‐mediated mechanism. Journal of Immunology 2007;178(7):4557‐66. [DOI] [PubMed] [Google Scholar]
Strickland 1999
- Strickland GT. Helminthic infections. In: Strickland GT editor(s). Hunter's Tropical Medicine and Emerging Infectious Diseases. 9th Edition. Philadelphia, Pa: Saunders, 1999. [Google Scholar]
Timmer 2012
- Timmer A, McDonald JWD, Tsoulis DJ, MacDonald JK. Azathioprine and 6‐mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD000478.pub3] [DOI] [PubMed] [Google Scholar]
Wammes 2010
- Wammes LJ, Hamid F, Wiria AE, Gier B, Sartono E, Maizels RM, et al. Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. European Journal of Immunology 2010;40(2):437‐42. [DOI] [PubMed] [Google Scholar]
Wang 2012
- Wang SL, Wang ZR, Yang CQ. Meta‐analysis of broad‐spectrum antibiotic therapy in patients with active inflammatory bowel disease. Experimental and Therapeutic Medicine 2012;4(6):1051‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weller 2008
- Weller PF, Nutman TB. Intestinal nematodes. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al. editor(s). Harrison’s Principles of Internal Medicine. 17th Edition. New York: McGraw‐Hill, 2008. [Google Scholar]


