Key Points
Question
Can the combination of central nervous system (CNS) radiotherapy with pyrotinib and capecitabine improve CNS progression-free survival (PFS) in patients with ERBB2-positive breast cancer with brain metastases?
Findings
In this phase 2 nonrandomized trial of 40 patients with ERBB2-positive breast cancer, the combination of CNS radiotherapy and pyrotinib plus capecitabine was associated with a 1-year CNS PFS rate of 74.9% and a median CNS PFS of 18.0 months, with an acceptable radiation necrosis rate.
Meaning
The results of this trial suggest that there are potential benefits of combining radiotherapy with pyrotinib and capecitabine for patients with ERBB2-positive breast cancer with brain metastases, suggesting a promising novel treatment approach for this challenging clinical scenario.
Abstract
Importance
The potential benefit of combining intracranial effective systemic therapy with radiotherapy for patients with breast cancer with brain metastases remains unclear.
Objective
To assess the activity and safety of combining radiotherapy with pyrotinib and capecitabine in patients with ERBB2-positive breast cancer and brain metastases.
Design, Setting, and Participants
This was a single-arm, single-center, phase 2 nonrandomized clinical trial with a safety run-in phase. Between January 2020 and August 2022, patients with ERBB2-positive breast cancer and brain metastases were enrolled. The data cutoff date was February 1, 2023.
Interventions
Patients received either fractionated stereotactic radiotherapy or whole-brain radiotherapy. Treatment with pyrotinib (400 mg, once daily) and capecitabine (1000 mg/m2, twice daily, on days 1-14 of each 21-day cycle) was initiated from the first day of radiotherapy to the seventh day after the completion of radiotherapy and continued until disease progression or unacceptable toxic effects.
Main Outcomes and Measures
The primary end point was 1-year central nervous system (CNS) progression-free survival (PFS) rate. Secondary end points included CNS objective response rate (ORR), PFS, overall survival (OS), safety, and changes in neurocognitive function.
Results
A total of 40 female patients (median age, 50.5 years [IQR, 46-59 years]) were enrolled and received treatment, including 3 patients in safety run-in phase. With a median follow-up of 17.3 months (IQR, 10.3-26.9), the 1-year CNS PFS rate was 74.9% (95% CI, 61.9%-90.7%), and the median CNS PFS was 18.0 months (95% CI, 15.5 to not reached). The 1-year PFS rate was 66.9% (95% CI, 53.1%-84.2%), and the median PFS was 17.6 months (95% CI, 12.8-34.1). The CNS objective response rate was 85% (34 of 40). Median overall survival was not reached. The most common grade 3 or 4 treatment-related adverse event was diarrhea (7.5%). Asymptomatic radiation necrosis was identified in 4 of 67 lesions (6.0%) treated with fractionated stereotactic radiotherapy. Most patients maintained neurocognitive function, as evaluated by the Mini-Mental State Examination at different points.
Conclusions and Relevance
The results of this trial suggest that radiotherapy combined with pyrotinib and capecitabine is associated with long intracranial survival benefit in patients with ERBB2-positive advanced breast cancer and brain metastases with an acceptable safety profile. This combination deserves further validation.
Trial Registration
ClinicalTrials.gov Identifier: NCT04582968
This nonrandomized trial examines the activity and safety of combining radiotherapy with pyrotinib and capecitabine in patients with ERBB2-positive breast cancer and brain metastases.
Introduction
Despite substantial improvements in clinical outcomes with ERBB2 (formerly HER2)–directed therapies, approximately half of patients with ERBB2-positive advanced breast cancer will develop brain metastases (BMs) over time.1,2,3 Local radiotherapy or surgery is the standard of care for treating BMs among patients with breast cancer.4 However, more than 50% of patients will still develop local recurrence or new brain lesions within 1 year after radiotherapy.5,6 Several retrospective studies have demonstrated that the intracranial progression rate is higher in ERBB2-positive subtype with BMs after brain radiotherapy.7,8
Pyrotinib is an oral irreversible ErbB receptor small-molecule tyrosine-kinase inhibitor (TKI) that targets HER1, ERBB2, and HER4. Recently, the PERMEATE study has shown intracranial efficacy of pyrotinib plus capecitabine among patients with radiotherapy-naive BMs, with a median progression-free survival (PFS) of 11.3 months.9 However, intracranial progression remains a substantial challenge, as demonstrated by the 1-year central nervous system (CNS) PFS rate of 42% in the HER2CLIMB study and the fact that 58% of patients in PERMEATE discontinued treatment due to intracranial progression.9,10,11
We hypothesized that combining CNS radiotherapy with systemic therapy may be associated with further reduced intracranial progression risk. Therefore, this study aimed to investigate the combination of radiotherapy with pyrotinib and capecitabine in patients with ERBB2-positive breast cancer and BMs.
Methods
Study Design and Participants
This was a nonrandomized phase 2 trial with a safety run-in phase conducted at a tertiary hospital in China. The study protocol was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice and was approved by the ethics committee of Fudan University Shanghai Cancer Center. Written informed consent was obtained from all patients. This trial followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
Eligible patients were 18 years or older with histologically proven ERBB2-positive breast cancer and BMs confirmed by contrast-enhanced brain magnetic resonance imaging. Patients who had previously received CNS local therapy and presented with new lesions were included. Previous systemic therapy for breast cancer, except for pyrotinib and capecitabine, was allowed. Patients were excluded if they had leptomeningeal or hemorrhagic metastases. The full eligibility criteria are listed in the protocol (Supplement 1).
Procedures
The safety run-in phase followed a modified 3 + 3 design to evaluate the safety of the study treatment, as detailed in the protocol (Supplement 1). Radiotherapy was administered after enrollment based on the size and number of BMs as well as the location of parenchymal brain lesions (Supplement 1). For patients with 1 to 4 intact lesions, fractionated stereotactic radiotherapy (FSRT) with a total dose of 24 to 40 Gy in 3 to 5 fractions (8 Gy per fraction) was delivered. For patients with 5 to 10 lesions, either FSRT or whole-brain radiotherapy (WBRT) was administered. For patients with more than 10 lesions, WBRT was recommended, with a prescribed dose of 30 Gy in 10 fractions. Patients were allowed to receive dexamethasone (up to 16 mg orally per day) for the management of neurological symptoms during and after radiotherapy.
Treatment with pyrotinib and capecitabine was allowed to be initiated from the first day of brain radiotherapy to the seventh day after the completion of brain radiotherapy. Patients received pyrotinib (400 mg orally, once daily) and capecitabine (1000 mg/m2 orally, twice daily, on days 1-14 of each 21-day cycle) until disease progression, unacceptable toxic effects, or death. Treatment interruptions and dose reductions were allowed to manage adverse events (AEs), as detailed in the protocol (Supplement 1). Dose reductions of pyrotinib were permitted stepwise from 400 mg to 320 mg to 240 mg if necessary. Dose reductions of capecitabine were permitted stepwise by 25%.
AEs were documented according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Intracranial response was assessed according to the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) criteria.12 Evaluation of extracranial lesions was performed following the Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1. Follow-up was conducted every 2 months during the first year and every 3 months thereafter. Mini-Mental State Examination (MMSE) scores were collected from patients who were able and willing to undergo the examination at baseline, 2 months, 8 months, and 12 months after treatment, and annually thereafter.
Outcomes
In the safety run-in phase, the safety was assessed based on the occurrence of dose-limiting toxic effects within 8 weeks of study treatment. The primary end point of the study was the 1-year CNS PFS rate. Secondary end points included CNS objective response rate (ORR), PFS, overall survival (OS), safety, and change in neurocognitive function. CNS PFS was defined as the time from the initiation of study treatment to disease progression in the brain or any-cause death or last follow-up, whichever occurred first. CNS ORR was defined as the proportion of patients with a confirmed complete or partial response in intracranial lesions (per RANO-BM criteria). PFS was defined as the time from the initiation of the study treatment to any disease progression or any-cause death or last follow-up, whichever occurred first. OS was defined as the time from the initiation of the study treatment to any-cause death or last follow-up, whichever occurred first.
Statistical Analysis
Based on historical data,5,6 CNS radiotherapy showed a 1-year CNS PFS rate of approximately 48%. A sample size of 39 patients was required to detect a significant increase in 1-year CNS PFS rate to 70%, with a 1-sided α of .025 and power of 80%.
Activity and safety were analyzed in patients who received at least 1 study treatment. Continuous data were presented as median (IQR or range), while categorical data were presented as frequency (percentage). Survival was estimated using the Kaplan-Meier method, and the corresponding 95% CIs were estimated using the Brookmeyer-Crowley method. Maintained neurocognitive function was defined as reduction in MMSE score by no more than 2 points from baseline. Post hoc comparisons of the proportion of patients with maintained neurocognitive function at different points were performed between the FSRT and WBRT subgroups using the Fisher exact test. No data imputation method was used for missing data of MMSE score. A 2-sided P < .05 was considered statistically significant. All statistical analyses were performed using R (version 4.0.2; R Foundation).
Results
Patient Characteristics
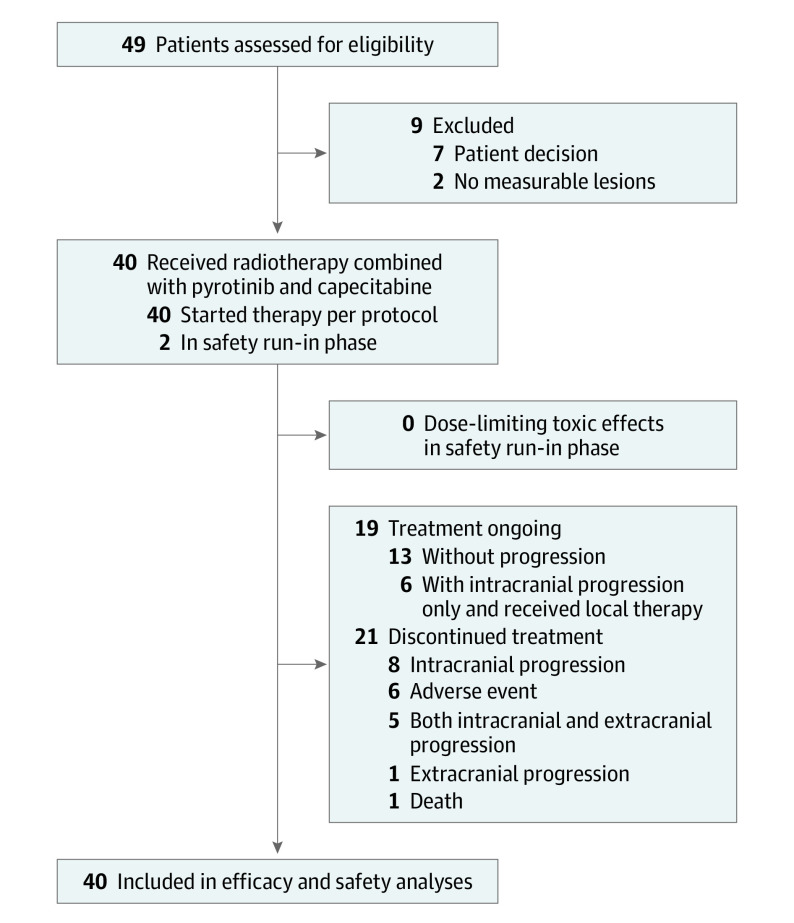
Between January 2020 and August 2022, 49 female patients were screened for eligibility and 40 patients were enrolled. The first 3 patients were evaluated in safety run-in phase, while all the 40 patients were included for efficacy and safety analysis (Figure 1). Baseline characteristics are provided in Table 1. Median age was 50.5 years (IQR, 46-59 years). Thirty patients (75%) had neurological symptoms at baseline. Twenty-one patients (52.5%) had received previous systemic therapy for metastatic disease, and the median number of previous systemic therapy lines for these 21 patients was 2 (range, 1-4). Five patients (12.5%) had previously received CNS local therapy, including 2 with previous surgery, 2 with previous FSRT, and 1 with previous WBRT. The median time from the last local therapy to enrollment for these 5 patients was 9.3 months (range, 1.5-13.3).
Figure 1. Patient Flowchart.
Table 1. Baseline Characteristics.
| Characteristic | Patients, No. (%) (N = 40) |
|---|---|
| Age at development of brain metastases, median (IQR), y | 50.5 (46-59) |
| Stage at initial diagnosis of breast cancera | |
| 0 | 4 (10) |
| I | 2 (5) |
| II | 13 (32.5) |
| III | 12 (30) |
| IV | 9 (22.5) |
| Hormone receptor status | |
| ER+ and/or PgR+ | 9 (22.5) |
| ER− and PgR− | 31 (77.5) |
| Karnofsky performance status | |
| <70 | 6 (15) |
| 70-80 | 20 (50) |
| 90-100 | 14 (35) |
| Diagnosis-specific graded prognostic assessment score | |
| 1.5-2.0 | 8 (20) |
| 2.5-3.0 | 23 (57.5) |
| 3.5-4.0 | 9 (22.5) |
| Symptomatic brain metastases | |
| Yes | 30 (75) |
| No | 10 (25) |
| No. of brain metastases | |
| 1-4 | 28 (70) |
| 5-9 | 6 (15) |
| ≥ 10 | 6 (15) |
| Size of the largest brain lesion, cm | |
| 1-2 | 15 (37.5) |
| 2.1-3 | 12 (30) |
| 3.1-4 | 8 (20) |
| > 4 | 5 (12.5) |
| No. of previous systemic therapy lines in metastatic setting | |
| 0 | 19 (47.5) |
| 1 | 10 (25) |
| 2 | 7 (17.5) |
| ≥ 3 | 4 (10) |
| Previous ERBB2-directed systemic therapy | |
| Trastuzumab | |
| For advanced disease | 5 (12.5) |
| As adjuvant or neoadjuvant therapy | 21 (52.5) |
| Both | 14 (35) |
| Pertuzumab | |
| For advanced disease | 5 (12.5) |
| As adjuvant or neoadjuvant therapy | 5 (12.5) |
| Both | 1 (2.5) |
| Neratinib | 1 (2.5) |
| Lapatinib | 3 (7.5) |
| ARX788 | 2 (5) |
| Previous CNS local therapy | |
| Surgery | 2 (5) |
| Fractionated stereotactic radiotherapy | 2 (5) |
| Whole-brain radiotherapy | 1 (2.5) |
| None | 35 (87.5) |
| Extracranial metastases | |
| Yes | 22 (55) |
| No | 18 (45) |
| Site of metastasesb | |
| Bone | 16 (40) |
| Breast or chest wall | 5 (12.5) |
| Liver | 7 (17.5) |
| Lung | 10 (25) |
| Lymph nodes | 12 (30) |
| Parenchymal CNS disease | 40 (100) |
| Parotid | 1 (2.5) |
Abbreviations: CNS, central nervous system; ER, estrogen receptor; PgR, progesterone receptor.
Pathologic or yield pathologic stage for nonstage IV disease.
Categories are not mutually exclusive.
Eleven patients (27.5%) received WBRT (30 Gy in 10 fractions), and 29 patients (72.5%) received FSRT (32 Gy in 4 fractions for 20 patients, 24 Gy in 3 fractions for 6 patients, and 40 Gy in 5 fractions for 3 patients). Twenty-four patients (60%) had concomitant corticosteroid use during the study treatment.
Efficacy
As of February 1, 2023, the median follow-up duration was 17.3 months (IQR, 10.3-26.9). The 1-year CNS PFS rate was 74.9% (95% CI, 61.9%-90.7%), and the median CNS-PFS was 18.0 months (95% CI, 15.5 to not reached; Figure 2A). Among the 40 patients, 21 (52.5%) developed intracranial progression. Nine patients experienced intracranial progression within 1 year, including 3 with progression within 6 months. The details of intracranial progression sites are shown in eTable 1 in Supplement 2.
Figure 2. Kaplan-Meier Curves for Central Nervous System (CNS) Progression-Free Survival (PFS) and PFS.
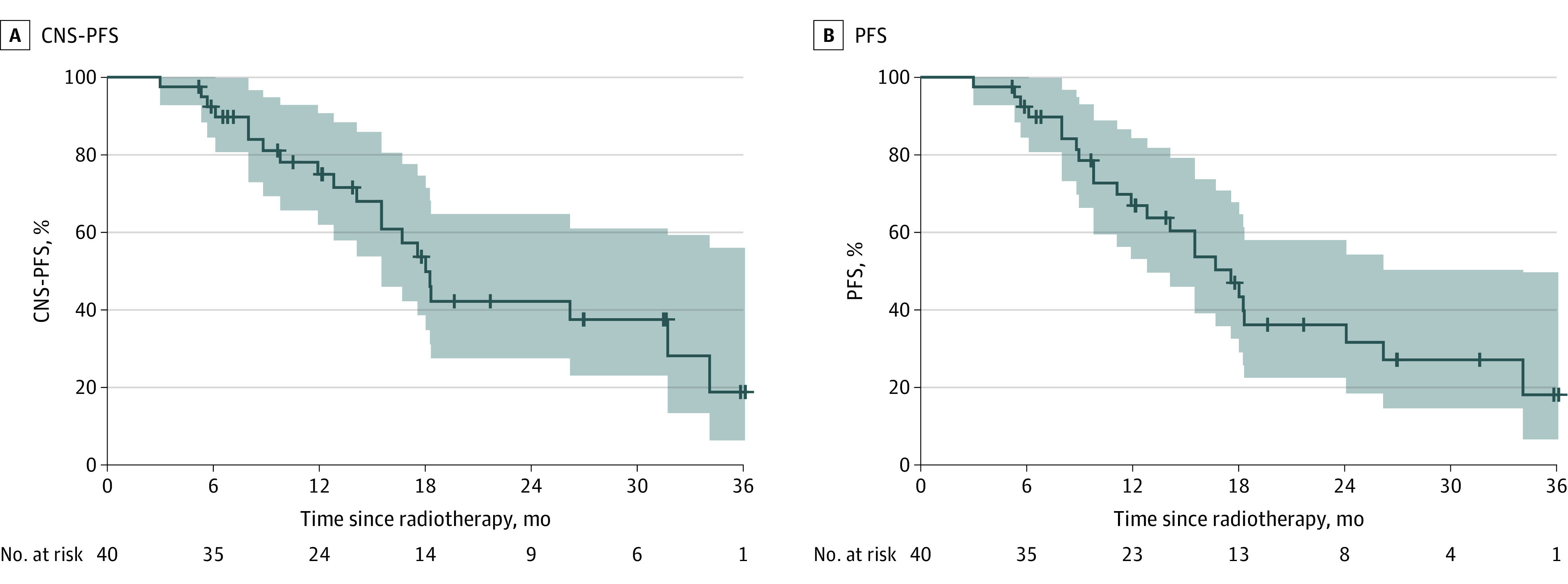
95% CI bands are shown.
The 1-year PFS rate was 66.9% (95% CI, 53.1%-84.2%), and the median PFS was 17.6 months (95% CI, 12.8-34.1; Figure 2B). Of 40 patients, 24 (60%) had disease progression or died, including 15 patients (37.5%) with intracranial progression only, 2 (5%) with extracranial progression only, and 6 (15%) with intracranial and extracranial progression. The extracranial progression sites are shown in eTable 2 in Supplement 2.
The CNS ORR was 85% (34 of 40), including 17 patients (42.5%) with a confirmed complete response and 17 (42.5%) with a confirmed partial response (Figure 3A). The median time to CNS response was 3.6 months (IQR, 2.5-6.8).
Figure 3. Best Central Nervous System (CNS) Response by Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) Criteria and Treatment Duration.
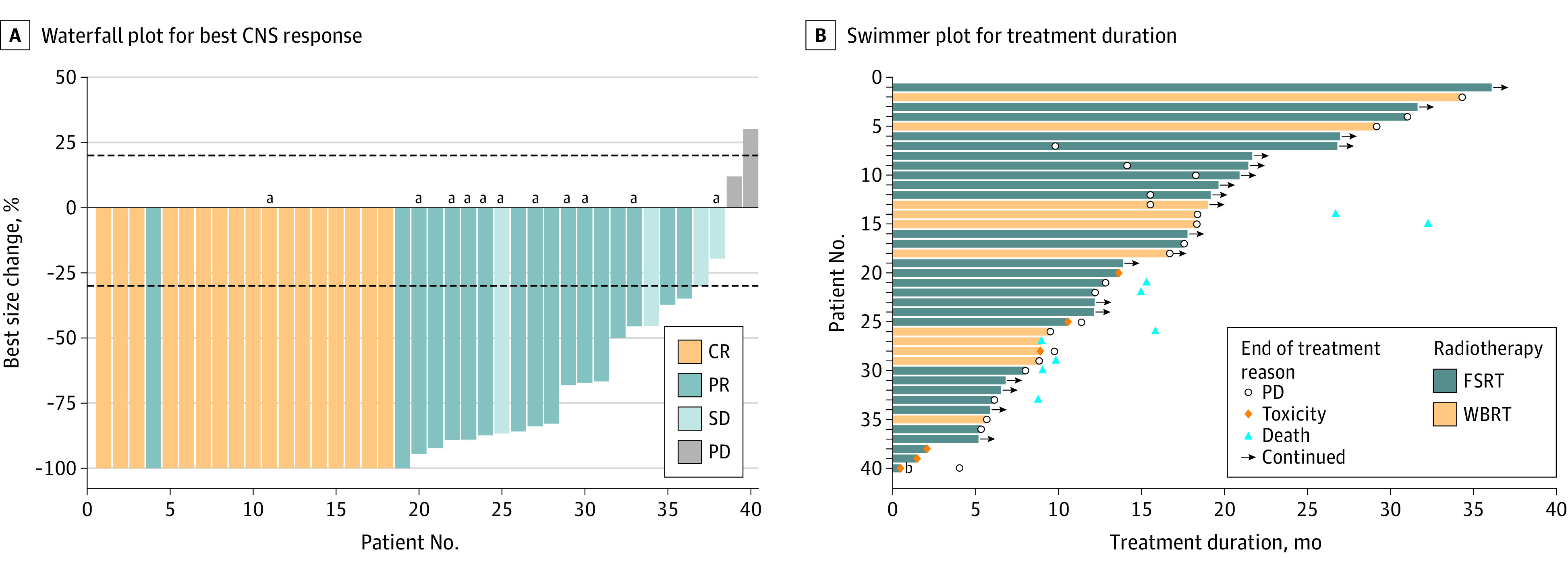
A, Dashed lines at 20% and –30% denote thresholds for progressive disease (PD) and partial response (PR), respectively, according to the RANO-BM criteria. CR indicates complete response; FSRT, fractionated stereotactic radiotherapy; SD, stable disease; WBRT, whole-brain radiotherapy
aPatients for whom WBRT was delivered.
bThe patient discontinued treatment with pyrotinib plus capecitabine on day 14 of the first cycle due to serious pulmonary infection and lung abscess and subsequently received multiple antibiotic therapies and thoracoscopic empyema resection. After enrollment, the patient received FSRT for 2 large lesions in the cerebella. At 3 months, magnetic resonance imaging found a shrunk tumor in the cerebella but with multiple new lesions in the frontal and parietal lobes. The patient then received treatment with pyrotinib plus capecitabine again and had SD by the end of follow-up.
Nine patients (22.5%) died by the end of follow-up, and the median OS was not reached. Six patients died of uncontrolled intracranial progression, 2 died of extracranial progression (eTable 2 in Supplement 2), and 1 died due to an unknown reason.
Treatment Duration
All 40 patients completed radiotherapy, and 39 received at least 1 cycle of pyrotinib plus capecitabine. The median time from CNS radiotherapy to the start of pyrotinib plus capecitabine was 4 days (IQR, 0-13.25). Fifteen of 40 patients (37.5%) were treated with FSRT and pyrotinib plus capecitabine concurrently. As of February 1, 2023, 13 patients (32.5%) were still receiving pyrotinib plus capecitabine without progression. Six patients who developed intracranial progression only continued to receive pyrotinib plus capecitabine after local therapy. The median treatment duration was 13.2 months (IQR, 8.6-20.0). Reasons for treatment discontinuation were disease progression (14 [35%]), AEs (6 [15%]), and death (1 [2.5%]). The swimmer plot for treatment duration is shown in Figure 3B.
Safety
None of the three patients in the safety run-in phase experienced a dose-limiting toxic effect. Treatment-related AEs (TRAEs) are summarized in Table 2. The most common grade 3 or higher TRAE was diarrhea (3 [7.5%]). One patient experienced serious TRAEs (grade 4 hypokalemia and grade 3 acute kidney injury) that led to hospitalization. No treatment-related deaths occurred.
Table 2. Treatment-Related Adverse Events for All 40 Patients.
| Event | No. (%) | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Diarrhea | 20 (50) | 10 (25) | 3 (7.5) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 11 (27.5) | 8 (20) | 1 (2.5) | 0 |
| Nausea | 17 (42.5) | 0 | 1 (2.5) | 0 |
| Anorexia | 15 (37.5) | 1 (2.5) | 0 | 0 |
| Vomiting | 13 (32.5) | 2 (5) | 1 (2.5) | 0 |
| Fatigue | 12 (30) | 2 (5) | 0 | 0 |
| Anemia | 12 (30) | 1 (2.5) | 1 (2.5) | 0 |
| Blood bilirubin conjugated level increased | 11 (27.5) | 0 | 0 | 0 |
| Blood bilirubin unconjugated level increased | 11 (27.5) | 0 | 0 | 0 |
| Lymphocyte cell count decreased | 9 (22.5) | 0 | 0 | 0 |
| Alopecia | 8 (20) | 0 | 0 | 0 |
| Weight loss | 6 (15) | 2 (5) | 0 | 0 |
| Headache | 6 (15) | 1 (2.5) | 0 | 0 |
| White blood cell count decreased | 4 (10) | 1 (2.5) | 1 (2.5) | 0 |
| Alanine aminotransferase level increased | 4 (10) | 2 (5) | 0 | 0 |
| Creatinine level increased | 5 (12.5) | 1 (2.5) | 0 | 0 |
| Aspartate aminotransferase level increased | 5 (12.5) | 1 (2.5) | 0 | 0 |
| Dizziness | 4 (10) | 1 (2.5) | 0 | 0 |
| Hypertriglyceridemia | 5 (12.5) | 0 | 0 | 0 |
| Hypokalemia | 4 (10) | 0 | 0 | 1 (2.5) |
| Intracranial radiation necrosis | 4 (10) | 0 | 0 | 0 |
| Radiation dermatitis | 3 (7.5) | 1 (2.5) | 0 | 0 |
| Neutrophil count decreased | 3 (7.5) | 1 (2.5) | 0 | 0 |
| Gamma-glutamyl transpeptidase increased | 4 (10) | 0 | 0 | 0 |
| Pruritus | 3 (7.5) | 0 | 0 | 0 |
| Rash | 3 (7.5) | 0 | 0 | 0 |
| Pigmentation disorder | 1 (2.5) | 1 (2.5) | 0 | 0 |
| Platelet count decreased | 2 (5) | 0 | 0 | 0 |
| Dyspepsia | 1 (2.5) | 1 (2.5) | 0 | 0 |
| Mucositis oral | 2 (5) | 0 | 0 | 0 |
| Acute kidney injury | 0 | 0 | 1 (2.5) | 0 |
No radiation necrosis was recorded during the first 8 weeks of treatment. As of February 1, 2023, 4 patients (14%) had radiation necrosis among 29 patients (4 of 67 brain lesions [6%]) with FSRT. No patients had headache or neurological symptoms. The median time to radiation necrosis was 17.4 months (IQR, 13.4-20.9). Of the 4 patients, 1 had pathologically confirmed necrosis in the right cerebellum, while the other 3 were confirmed through O-(2-[18F]fluoroethyl)-L-tyrosine positron emission tomography or functional magnetic resonance imaging. All 4 patients maintained a stable condition after short-term use of dexamethasone or bevacizumab.
Four of 40 patients (10%) needed a pyrotinib dose reduction to 320 mg, and 1 (2.5%) had an additional dose reduction to 240 mg. Five patients (12.5%) required capecitabine dose reductions. Five patients discontinued both pyrotinib and capecitabine due to TRAEs (diarrhea [n = 4] and hand-foot syndrome [n = 1]), and 1 discontinued both drugs due to treatment-unrelated severe pulmonary infection and lung abscess.
Neurocognitive Function
Among the 40 patients, 28 (70%) were able to complete the MMSE at baseline, 10 (25%) could not complete the MMSE due to severe neurological symptoms associated with brain edema (including severe headache, visual and auditory problems, and hemiparesis), and 2 (5%) had difficulty in reading or understanding due to low educational levels.
The median score at baseline was 27 (IQR, 25.8-29.0), and 17 of 28 patients (61%) had a score of 27 or higher. The WBRT subgroup showed a significant deterioration in neurocognitive function at 2 months (P = .04). At 8 months and 12 months, patients in the WBRT subgroup showed a lower proportion, with unchanged MMSE scores compared with the FSRT subgroup, although without statistical significance. However, both of the long-term survivors in the WBRT group showed maintained neurocognitive function at 24 months (eTable 3 in Supplement 2).
Discussion
To our knowledge, this is the first prospective study evaluating the combination of local therapy with pyrotinib and capecitabine in patients with ERBB2-positive breast cancer and BMs. The results suggested that the CNS radiotherapy combined with pyrotinib plus capecitabine was associated with favorable outcomes without an increase in radiation-induced injury.
TKIs are commonly considered for treating ERBB2-positive metastatic breast cancer that has spread to the CNS due to their ability to cross the blood-brain barrier.9,10,11,13,14 A recent retrospective study showed a lower number of CNS progression events in the lapatinib plus stereotactic radiosurgery (SRS) group compared with SRS alone group, suggesting a potential synergistic association of combining local therapy and TKI with the control of intracranial lesions.15 However, there is concern about the potential risk of radiation necrosis with the combination of CNS radiotherapy and systemic therapy. In the current study, radiation necrosis occurred for 4 of 29 patients (67 lesions) who were treated with FSRT, and the median time to necrosis was 17.4 months (IQR, 13.4-20.9). The radiation necrosis rate did not exceed expectation compared with previous studies of SRS or FSRT alone.16,17,18,19
The PERMEATE study has suggested promising antitumor activity of pyrotinib plus capecitabine in patients with ERBB2-positive metastatic breast cancer and BMs, with a CNS ORR of 74.6% and a median PFS of 11.3 months in the radiotherapy-naive population.9 In the current study, the combination of radiotherapy and pyrotinib plus capecitabine showed better intracranial response and survival benefit. However, fewer patients in this study had extracranial metastases (55% vs 88%) compared with PERMEATE.9 The patients were enrolled from the radiation oncology department, in which patients mostly presented with symptomatic brain metastases (75% vs 32% in PERMEATE), and some of them had large brain lesions (32.5% with lesion size >3 cm). The different clinical settings between studies and the small sample size of our study should be considered when interpreting these results.
Limitations
Several limitations need to be acknowledged in this study. First, as a single-arm, single-center study, the small sample size and potential selection bias in the recruited participants might limit the generalizability of the results. Second, MMSE, which is commonly used to assess cognitive function, is considered less sensitive compared with the Hopkins Verbal Learning Test-Revised. In addition, patients who were able to complete the MMSE might be those who were less affected by the neurotoxic effects of radiotherapy plus systemic therapy, as reflected by the low compliance rate at baseline and high attrition rate in the continued participation with MMSE over time. A large-scale randomized clinical trial including careful assessment of neurocognitive function is warranted in the future.
Conclusions
The results of this nonrandomized trial suggest that radiotherapy combined with pyrotinib and capecitabine was well tolerated and associated with long intracranial survival benefit in patients with ERBB2-positive breast cancer and BMs. This combination deserves further validation in randomized clinical trials.
Trial protocol
eTable 1. Intracranial progression sites
eTable 2. Extracranial progression sites
eTable 3. Maintenance of neurocognitive function evaluated by MMSE
Data sharing statement
References
- 1.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278-5286. doi: 10.1200/JCO.2008.19.8481 [DOI] [PubMed] [Google Scholar]
- 2.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244-248. doi: 10.1016/S1470-2045(13)70017-2 [DOI] [PubMed] [Google Scholar]
- 3.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972-2977. doi: 10.1002/cncr.11436 [DOI] [PubMed] [Google Scholar]
- 4.Ramakrishna N, Anders CK, Lin NU, et al. Management of advanced human epidermal growth factor receptor 2–positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022;40(23):2636-2655. doi: 10.1200/JCO.22.00520 [DOI] [PubMed] [Google Scholar]
- 5.Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1040-1048. doi: 10.1016/S1470-2045(17)30414-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141. doi: 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JA, Kotecha R, Ahluwalia MS, et al. Overall survival and the response to radiotherapy among molecular subtypes of breast cancer brain metastases treated with targeted therapies. Cancer. 2017;123(12):2283-2293. doi: 10.1002/cncr.30616 [DOI] [PubMed] [Google Scholar]
- 8.Cagney DN, Lamba N, Montoya S, et al. Breast cancer subtype and intracranial recurrence patterns after brain-directed radiation for brain metastases. Breast Cancer Res Treat. 2019;176(1):171-179. doi: 10.1007/s10549-019-05236-6 [DOI] [PubMed] [Google Scholar]
- 9.Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353-361. doi: 10.1016/S1470-2045(21)00716-6 [DOI] [PubMed] [Google Scholar]
- 10.Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. doi: 10.1056/NEJMoa1914609 [DOI] [PubMed] [Google Scholar]
- 11.Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610-2619. doi: 10.1200/JCO.20.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin NU, Lee EQ, Aoyama H, et al. ; Response Assessment in Neuro-Oncology Group . Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-e278. doi: 10.1016/S1470-2045(15)70057-4 [DOI] [PubMed] [Google Scholar]
- 13.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64-71. doi: 10.1016/S1470-2045(12)70432-1 [DOI] [PubMed] [Google Scholar]
- 14.Freedman RA, Gelman RS, Anders CK, et al. ; Translational Breast Cancer Research Consortium . TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2–positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081-1089. doi: 10.1200/JCO.18.01511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JM, Miller JA, Kotecha R, et al. Stereotactic radiosurgery with concurrent HER2-directed therapy is associated with improved objective response for breast cancer brain metastasis. Neuro Oncol. 2019;21(5):659-668. doi: 10.1093/neuonc/noz006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049-1060. doi: 10.1016/S1470-2045(17)30441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401-409. doi: 10.1001/jama.2016.9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minniti G, Scaringi C, Paolini S, et al. Single-fraction versus multifraction (3 × 9 Gy) stereotactic radiosurgery for large (>2 cm) brain metastases: a comparative analysis of local control and risk of radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys. 2016;95(4):1142-1148. doi: 10.1016/j.ijrobp.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 19.Milano MT, Grimm J, Niemierko A, et al. Single- and multifraction stereotactic radiosurgery dose/volume tolerances of the brain. Int J Radiat Oncol Biol Phys. 2021;110(1):68-86. doi: 10.1016/j.ijrobp.2020.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Intracranial progression sites
eTable 2. Extracranial progression sites
eTable 3. Maintenance of neurocognitive function evaluated by MMSE
Data sharing statement