Abstract
Older compatible living donor kidney transplant recipients (CLDKT) have higher mortality and death-censored graft failure compared to younger recipients. These risks may be amplified in older incompatible living donor kidney transplant recipients (ILDKT) who undergo desensitization and intense immunosuppression. In a 25-center cohort of ILDKT recipients transplanted between September 24, 1997 and December 15, 2016, we compared mortality, death-censored graft failure (DCGF), delayed graft function (DGF), acute rejection (AR), and length of stay (LOS) between 234 older (age≥60) and 1172 younger (age 18–59) recipients. To investigate whether the impact of age was different for ILDKT recipients compared to 17,542 CLDKT recipients, we used an interaction term to determine whether the relationship between post-transplant outcomes and transplant type (ILDKT vs. CLDKT) was modified by age. Overall, older recipients had higher mortality (HR: 1.632.072.65, p<0.001), lower DCGF (HR: 0.360.530.77, p=0.001) and AR (OR: 0.390.540.74, p<0.001), and similar DGF (OR: 0.461.032.33, p=0.9) and LOS (IRR: 0.880.981.10, p=0.8) compared to younger recipients. The impact of age on mortality (interaction p=0.052), DCGF (interaction p=0.7), AR interaction p=0.2), DGF (interaction p=0.9), and LOS (interaction p=0.5) was similar in ILDKT and CLDKT recipients. Age alone should not preclude eligibility for ILDKT.
Keywords: HLA-incompatible, living donor, kidney transplantation, older, outcomes, clinical research/practice
INTRODUCTION
Over the past decade, there has been a 79% increase in the number of older kidney transplants recipients 1. Like younger transplant candidates, older candidates are also subject to the current organ shortage and therefore, need to pursue all potential avenues to transplantation. For older adults who present with a willing, but incompatible living donor, incompatible living donor kidney transplantation (ILDKT) may be one potential avenue. Given the rigorous desensitization and immunosuppression protocols that accompany ILDKT 2–4, these individuals will have to weigh the potential additional risks associated with ILDKT.
Previous single center studies have demonstrated that ILDKT recipients have a 2.6-fold higher risk of graft loss 5 as well as higher 5-year death-censored graft failure (88.0% vs. 70.7%) 6 compared with CLDKT recipients. A multicenter study by Orandi et al. demonstrated that ILDKT recipients of moderate and high antibody strength had 1.6- to 5-fold higher risk of all-cause graft loss and 2 to 4.6-fold higher risk of mortality compared to CLDKT recipients 7. ILDKT may be especially challenging in older recipients due to age-specific risks, such as a higher prevalence of comorbidities and immunosenescence 8–10, in combination with ILDKT-specific risks.
Although there have been several studies that detail the increased risks that ILDKT recipients face, the risk profile of older ILDKT recipients has not been studied. Quantifying the risk profile of older ILDKT recipients would inform decision-making and patient counseling for older transplant candidates with incompatible living donors. To study this, we used a multi-center cohort linked to national registry data to compare post-ILDKT outcomes in older recipients to those in younger recipients and older CLDKT recipients to determine whether the impact of age is amplified in ILDKT recipients.
METHODS
Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This data has been previously described elsewhere11.
Study Population Definition
We included adults (≥18 years; older recipients ≥60 years) who received a kidney-only transplant from HLA-incompatible living donors at 25 transplant centers across the United States from September 24, 1997 through December 15, 2016. The transplant centers were identified based on a previous survey conducted by the senior author on ILDKT practice 12, which identified and invited all centers that performed at least one ILDKT. Based on this survey, medical and surgical directors were contacted to participate and provide ILDKT data.
We defined ILDKT recipients as those undergoing perioperative desensitization therapy for DSA prior to transplantation, as previously described. Briefly, all participating transplantation centers classified their ILDKT recipients by low, moderate, or high levels of DSA, which corresponded to positive Luminex -negative flow crossmatch (PLNF), positive flow - negative cytotoxic crossmatch (PFNC), or positive cytotoxic crossmatch (PCC), respectively13. Some centers performed actual cell-based crossmatches, whereas others performed virtual crossmatches based on semi-quantitative DSA strength on solid-phase assays. Despite the variation in results of these assays and between each laboratory, each center’s established mean fluorescence intensity benchmarks equated to the three categories evaluated in the present study.
Each center’s MFI benchmarks were equated to the crossmatch categories reported in this study. This categorization was described based on Johns Hopkins mean fluorescence intensity (MFI) categorizations where MFI of ≥1000 have been considered Luminex positive, MFI values 2000–20,000 resulted in a positive flow crossmatch, and ≥10 000 MFI was associated with PCC on phenotype panels 7. Significant variation in the results of solid-phase assays within and especially between laboratories has been previously reported and characterized 14,15. In view of the minimal risk associated with ABO-incompatible transplantation, patients who required both HLA and ABO barriers to be crossed (6.1% of ILDKT recipients) were categorized based on their DSA strength.
To understand how the risks of our outcomes varied in relation to CLDKT, we identified all recipients who received kidney-only transplants from compatible living donors at the same centers and time period (i.e. when each center was performing ILDKT) as their ILDKT counterparts. This study was approved by the Johns Hopkins University Institutional Review Board.
Given the length of the study period, which includes a period of time when ILDKT was not routinely performed (1997–2001), we have quantified the number of ILDKT’s performed over time and conducted a sensitivity analysis excluding these first few years to ensure our findings were robust (Supplemental Figure). The inferences of our analyses were unchanged.
Data Linkage
Data on ILDKT recipients provided by the participating transplant centers were linked to the SRTR for reliable ascertainment of outcomes, such as graft loss and death. The SRTR supplements death ascertainment through linkage to the Social Security Death Master File and death and graft loss ascertainment through linkage to data from the Centers for Medicare & Medicaid Services (CMS).
Outcomes After ILDKT
Mortality and Death-Censored Graft Failure
Patients were followed from date of transplant until date of death (or graft failure), or date of administrative censorship on September 1, 2019, whichever came first. Death-censored graft failure (DCGF) was defined as re-transplantation or return to dialysis. We compared the cumulative incidence of mortality and DCGF in older ILDKT to that of younger ILDKT recipients with Kaplan-Meier methodology using log-rank tests and Cox regression. Outcomes were also stratified by age and DSA strength (Supplemental Table, Table S1).
Delayed Graft Function, Acute Rejection, and Length of Stay
Delayed graft function (DGF) was defined as the need for postoperative dialysis within 7 days after transplantation, as collected by the OPTN. Acute rejection (AR) was defined as an AR event that occurred within the first year of transplant as reported by individual centers to the OPTN, which have been previously described elsewhere 12. Length of stay (LOS) was defined as transplant date until date of discharge. We used logistic regression to evaluate DGF and AR, and negative binomial regression to evaluate LOS in older ILDKT versus younger ILDKT recipients. Models were adjusted for sex and race. Outcomes were also stratified by age and DSA strength (Table S1).
DSA Strength and Interactions for DCGF, Mortality, DGF, Acute Rejection, and LOS
Among ILDKT recipients, we tested for effect modification by DSA strength to determine if the association between age and post-ILDKT outcomes varied by DSA strength. We repeated the analyses above including an interaction term between DSA strength and age. Outcomes were also stratified by age and DSA strength (Table S1).
Interaction between Age and ILDKT
Using an interaction term, we tested whether ILDKT modified the association between age and post-transplant outcomes (mortality, DCGF, DGF, AR, and LOS) when compared to CLDKT. That is, we tested whether older ILDKT recipients were at even higher risk of poor post-transplant outcomes compared to older CLDKT recipients. Models were adjusted for recipient (sex, race, body mass index [BMI], cause of ESRD, peak c/panel reactive antibody [c/PRA], number of previous transplants, and years on dialysis), donor (LD KDPI), and transplant characteristics (human leukocyte antigen [HLA] mismatch and cold ischemia time [CIT]). Multiple imputation by chained equations with 10 imputations over 100 iterations was used to handle missing data 16; missingness ranged from 0.7%-25.5%. As a sensitivity analysis, we limited our definition of ILDKT to include PFNC and PCC recipients to ensure robustness of our findings; inferences were consistent with our main analyses.
Statistical Analysis
Patients for whom outcomes data could not be ascertained were excluded from analyses (DGF: N=1 [0.005%], AR: N=205 [1.1%], LOS: N=23 [0.1%]). To account for within center clustering of outcomes, a Huber-White sandwich estimator was used for all models. Confidence intervals were reported as per the method of Louis and Zeger 17. All analyses were performed using Stata 16.0/MP 18.
RESULTS
Study Population
We identified 1172 younger and 234 older ILDKT recipients from 25 transplant centers. Among older ILDKT recipients, 34.2% (vs. 25.3% for younger) were PLNF, 44.9% (vs. 49.7%) were PFNC, and 20.9% (vs. 25.1%) were PCC (p=0.02) (Table 1). The median age of older ILDKT was 64 years (interquartile range, IQR: 61–69) compared to 42 years (IQR: 33–50) for younger ILDKT. Compared to younger ILDKT, older ILDKT were more likely to be female (73.9% vs 62.1%, p<0.001) and less likely to be Black (9.0% vs 18.7%, p<0.001). Older ILDKT were less likely to have ESRD due to glomerular diseases (19.0% vs 39.0%), 100% peak cPRA (6.4% vs. 10.7%), ≥2 previous transplants (1.3% vs. 7.3%), and more likely to be preemptively transplanted (32.5% vs 20.5%) compared to their younger counterparts (p for all<0.001) (Table 1). Compared to younger ILDKT, those who were older were more likely to receive transplants from older donors (median age, [IQR]; 44 [37–54] vs. 40 [30–49] years, p<0.001) and less likely to be related to their donor (42.3% vs. 50.7%, p=0.02)
Table 1.
Characteristics of ILDKT and CLDKT transplant recipients, by age.
| ILDKT | CLDKT | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | ≥60 years (n=234) | <60 years (n=1172) | p-value | ≥60 years (n=4267) | <60 years (n=13275) | p-value |
|
| ||||||
| Recipient |
||||||
| DSA strength, no. (%) | 0.02 | |||||
| PLNF | 80 (34.2) | 296 (25.3) | ||||
| PFNC | 105 (44.9) | 582 (49.7) | ||||
| PCC | 49 (20.9) | 294 (25.1) | ||||
| Age (years), median (IQR) | 64.0 (61.0, 69.0) | 42.0 (33.0, 50.0) | <0.001 | 65.0 (62.0, 69.0) | 44.0 (34.0, 52.0) | <0.001 |
| Female sex, no. (%) | 173 (73.9) | 728 (62.1) | <0.001 | 1449 (34.0) | 5282 (39.8) | <0.001 |
| Race/ethnicity, no. (%) | <0.001 | |||||
| White | 184 (78.6) | 783 (66.8) | 3248 (76.1) | 8582 (64.6) | <0.001 | |
| Black | 21 (9.0) | 219 (18.7) | 470 (11.0) | 2120 (16.0) | ||
| Other | 29 (12.4) | 170 (14.5) | 549 (12.9) | 2573 (19.4) | ||
| BMI (kg/m2), median (IQR) | 27.5 (23.4, 31.9) | 25.6 (22.2, 30.2) | <0.001 | 27.9 (24.6, 31.8) | 26.5 (22.9, 31.0) | |
| Cause of ESRD, no. (%) | <0.001 | <0.001 | ||||
| Glomerular diseases | 44 (19.0) | 441 (39.0) | 808 (19.0) | 4321 (32.9) | ||
| Diabetes | 48 (20.7) | 153 (13.5) | 1291 (30.4) | 2644 (20.1) | ||
| Hypertension | 54 (23.3) | 131 (11.6) | 906 (21.3) | 2035 (15.5) | ||
| Polycystic kidney disease | 26 (11.2) | 116 (10.2) | 385 (9.1) | 1566 (11.9) | ||
| Other | 60 (25.9) | 291 (25.7) | 854 (20.1) | 2560 (19.5) | ||
| Years on dialysis, no. (%) | <0.001 | <0.001 | ||||
| Preemptive | 76 (32.5) | 240 (20.5) | 1608 (37.7) | 4634 (34.9) | ||
| <2 years | 84 (35.9) | 428 (36.5) | 1845 (43.2) | 6029 (45.4) | ||
| 2–6 years | 60 (25.6) | 321 (27.4) | 733 (17.2) | 2156 (16.2) | ||
| >6 years | 14 (6.0) | 183 (15.6) | 81 (1.9) | 456 (3.4) | ||
| Peak c/PRA (%), no. (%) | 0.06 | <0.001 | ||||
| 0 | 41 (17.5) | 179 (15.3) | 2938 (71.2) | 8707 (66.7) | ||
| 1–20 | 33 (14.1) | 130 (11.1) | 562 (13.6) | 1994 (15.3) | ||
| 21–79 | 90 (38.5) | 395 (33.7) | 481 (11.7) | 1665 (12.8) | ||
| 80–97 | 46 (19.7) | 267 (22.8) | 105 (2.5) | 470 (3.6) | ||
| 98 | 7 (3.0) | 36 (3.1) | 10 (0.2) | 48 (0.4) | ||
| 99 | 2 (0.9) | 40 (3.4) | 12 (0.3) | 61 (0.5) | ||
| 100 | 15 (6.4) | 125 (10.7) | 17 (0.4) | 103 (0.8) | ||
| No. of previous transplants, no. (%) | <0.001 | <0.001 | ||||
| 0 | 202 (86.3) | 664 (56.7) | 4060 (95.1) | 11659 (87.8) | ||
| 1 | 29 (12.4) | 423 (36.1) | 185 (4.3) | 1414 (10.7) | ||
| ≥2 | 3 (1.3) | 85 (7.3) | 22 (0.5) | 202 (1.5) | ||
| ≥3 HLA mismatches, no. (%) | 179 (76.8) | 930 (79.9) | 0.04 | 3088 (73.0) | 9488 (72.0) | <0.001 |
| Immunosuppression, no. (%) | 0.3 | <0.001 | ||||
| None | 11 (5.1) | 68 (6.4) | 218 (5.5) | 876 (7.2) | ||
| Non-Depleting | 14 (6.5) | 96 (9.0) | 1130 (28.6) | 2620 (21.5) | ||
| Depleting | 192 (88.5) | 901 (84.6) | 2598 (65.8) | 8677 (71.3) | ||
| Early Steroid Withdrawal | 62 (27.0) | 232 (20.0) | 0.02 | 1827 (43.1) | 5420 (41.1) | 0.03 |
| Donor | ||||||
|
| ||||||
| Age (years), median (IQR) | 44.0 (37.0, 54.0) | 40.0 (30.0, 49.0) | <0.001 | 45.0 (37.0, 57.0) | 42.0 (32.0, 50.0) | <0.001 |
| Female sex, no. (%) | 141 (60.3) | 652 (55.6) | 0.2 | 2640 (61.9) | 7996 (60.2) | 0.06 |
| Race/ethnicity, no. (%) | 0.08 | <0.001 | ||||
| White | 179 (76.5) | 817 (69.7) | 3286 (77.0) | 9068 (68.3) | ||
| Black | 25 (10.7) | 187 (16.0) | 436 (10.2) | 1823 (13.7) | ||
| Other | 30 (12.8) | 168 (14.3) | 545 (12.8) | 2383 (18.0) | ||
| Related donor, no. (%) | 99 (42.3) | 594 (50.7) | 0.02 | 1986 (46.5) | 6124 (46.1) | 0.7 |
| ABOi, no. (%) | 13 (5.6) | 72 (6.1) | 0.7 | 137 (3.2) | 402 (3.0) | 0.6 |
| Transplant |
||||||
| CIT <8 hours, no. (%) | 194 (82.9) | 858 (73.2) | <0.01 | 3362 (78.8) | 9691 (73.0) | <0.001 |
ABOi, ABO incompatible; BMI, body mass index; CIT, cold ischemia time; CLDKT, compatible living donor kidney transplant recipients; c/PRA, calculated/panel reactive antibody; ESRD, end stage renal disease; HLA, human leukocyte antigen; ILDKT, incompatible living donor kidney transplant recipients; IQR, interquartile range; no., number; PCC, positive cytotoxic crossmatch; PFNC, positive flow, negative cytotoxic crossmatch; PLNF, positive Luminex, negative flow crossmatch.
Mortality and Death-Censored Graft Failure
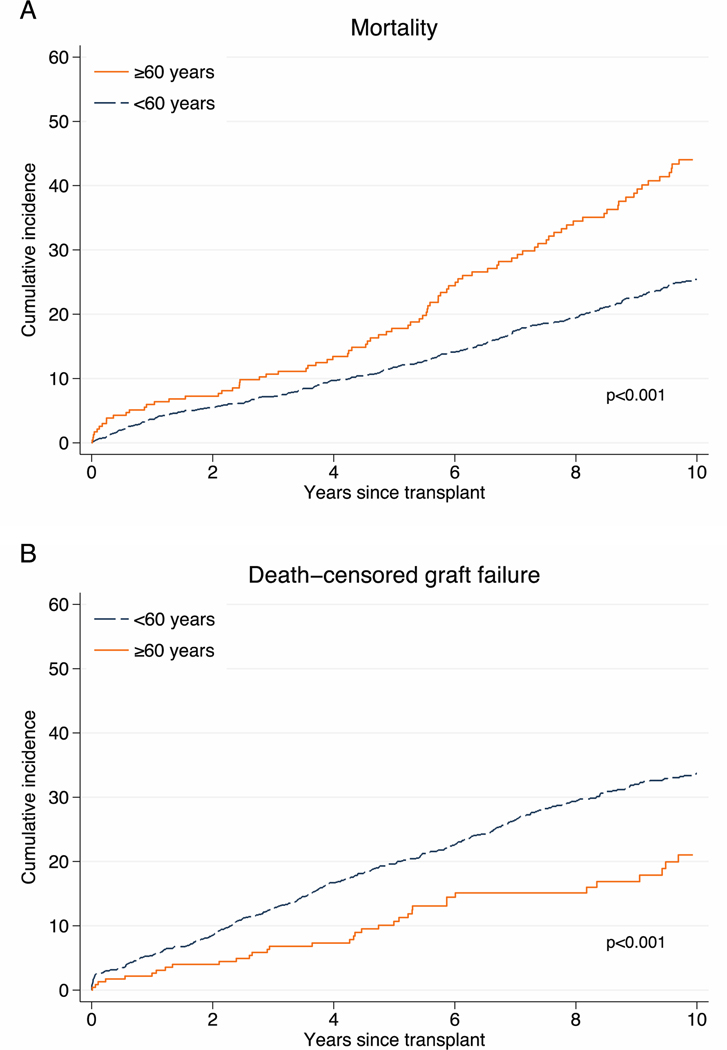
Older ILDKT had higher 1-year (6.0% vs. 3.7%), 5-year (17.8% vs. 11.8%), and 10-year (44.0% vs. 25.4%) mortality compared to younger ILDKT recipients (p<0.001) Figure 1A). This translated into a 2.07-fold higher mortality risk (hazard ratio [HR]: 1.632.072.65, p<0.001) for older compared to younger ILDKT (Table 2). There was no statistically significant interaction between age and DSA strength (PFNC: p=0.8; PCC: p=0.1), indicating that the mortality risk does not differ across levels of DSA strength.
Figure 1.
Mortality (A) and death-censored graft failure (B) among older and younger ILDKT recipients.
Older ILDKT recipients have higher mortality (A), but lower death-censored graft failure (B) compared to their younger counterparts (p for all <0.001).
Table 2.
Post-transplant outcomes for older and younger ILDKT recipients.
| Outcome | Older vs younger ILDKT | Interaction terma | p for interactiona |
|---|---|---|---|
|
| |||
| Mortality | 1.63 2.07 2.65 | 0.570.751.00 | 0.052b |
| DCGF | 0.36 0.53 0.77 | 0.640.931.34 | 0.7 |
| DGF | 0.531.031.96 | 0.410.952.15 | 0.9 |
| AR | 0.39 0.54 0.74 | 0.510.781.19 | 0.2 |
| LOS | 0.880.981.10 | 0.820.951.10 | 0.5 |
Bold indicates p<0.05
Interaction term and p-value evaluating whether age modified the relationship between ILDKT and post-transplant outcomes
Sensitivity analysis excluding PLNF: interaction term=0.570.761.01, p=0.06
AR, Acute rejection; DCGF, death-censored graft failure; DGF, delayed graft function; LOS, length of stay.
Older ILDKT recipients have a 2.07-fold higher mortality risk compared to younger ILDKT recipients. When compared to younger CLDKT recipients, younger ILDKT have a 51% increased mortality risk (aHR: 1.311.511.75, p<0.001). Among older recipients, this risk is attenuated and not statistically significant (aHR: 0.891.141.45, p=0.3); the interaction between age and incompatibility status was not statistically significant (p interaction=0.052).
Conversely, older ILDKT had a lower 1-year (2.6% vs. 5.4%), 5-year (10.7% vs. 19.6%), and 10-year (21.0% vs. 33.7%) DCGF compared to younger ILDKT (p<0.001) (Figure 1B). This translated into a 47% lower risk of DCGF (HR: 0.360.530.77, p=0.001) for older compared to younger ILDKT (Table 2). There was no statistically significant interaction between age and DSA (PFNC: p=0.5; PCC: p=0.7), indicating that the risk of DCGF does not differ across levels of DSA strength.
Delayed Graft Function, Acute Rejection, and Length of Stay
DGF occurred in 5.2% of older ILDKT and 5.6% of younger ILDKT. There was no difference in the odds of developing DGF (odds ratio [OR]: 0.461.032.33, p=0.9) between older and younger ILDKT (Table 2). There was no statistically significant interaction between age and DSA (PFNC: p=0.2, PCC: p=0.9), indicating that the odds of developing DGF does not differ across levels of DSA strength.
AR occurred in 12.8% of older ILDKT and 22.1% of younger ILDKT. This translated into 46% lower odds of acute rejection (OR: 0.390.540.74, p<0.001) for older compared to younger ILDKT. There was no statistically significant interaction between age and DSA (PFNC: p=0.4, PCC: p=0.3), indicating that the odds of developing AR do not differ across levels of DSA strength.
Median LOS was 7 days (IQR: 5–12) for older ILDKT and 6 days (IQR: 4–11) for younger ILDKT. There was no evidence of difference in LOS (incidence rate ratio [IRR]: 0.880.981.10, p=0.8) between older and younger ILDKT (Table 2). There was no statistically significant interaction between age and DSA (PFNC: p=0.8, PCC: p=0.5), indicating that LOS does not differ across levels of DSA strength.
Risk Modification by Age
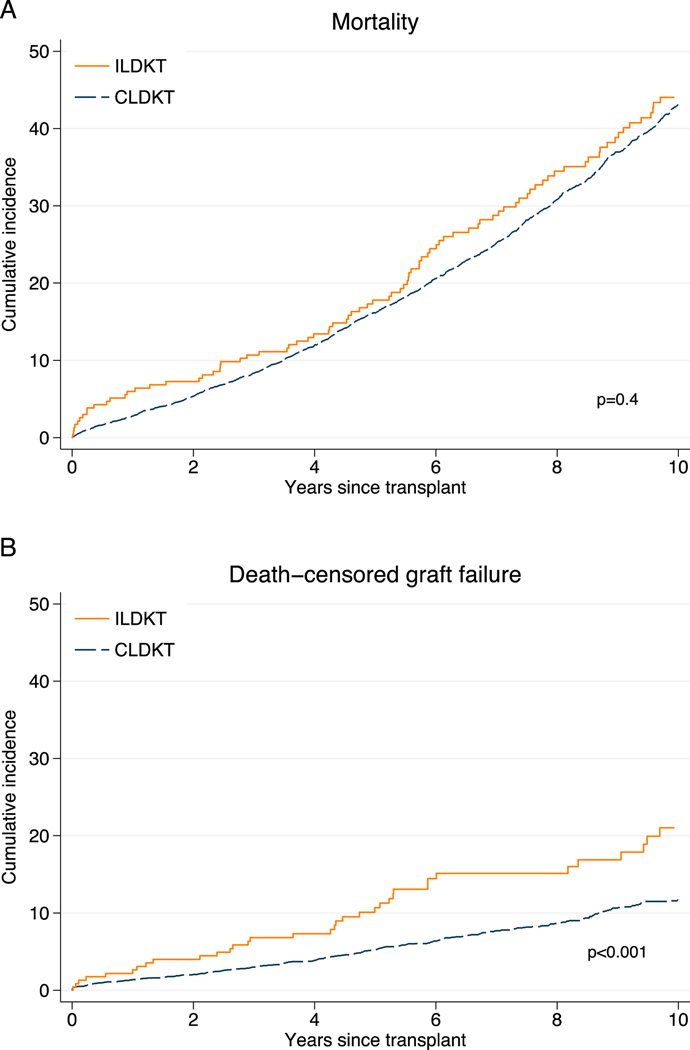
We then compared post-transplant outcomes of 1,406 ILDKT recipients to that of 17,542 CLDKT recipients to determine whether age was associated with additional risks for post-transplant outcomes beyond that conferred by ILDKT. Older ILDKT recipients have similar overall mortality (p=0.4), but higher death-censored graft failure compared to older CLDKT recipients (p<0.001) (Figure 2). There was no statistically significant interaction between age and ILDKT status (p for interaction >0.05), indicating that the risks for mortality, DCGF, DGF, AR, or LOS did not differ with age (Table 2).
Figure 2.
Mortality (A) and death-censored graft failure (B) among older ILDKT recipients.
Older ILDKT recipients have similar overall patient survival (p=0.4), but higher death-censored graft failure compared to older CLDKT recipients (p<0.001).
DISCUSSION
In this 25-center cohort study on post-ILDKT outcomes and age, older ILDKT recipients had an increased mortality risk, a lower risk of DCGF, lower odds of developing AR, and similar DGF and LOS compared to younger recipients post-ILDKT (Table 2). However, the risk associated with older age did not differ among compatible or incompatible recipients. Furthermore, the risks of mortality, DCGF, DGF, AR, and LOS among older ILDKT and younger ILDKT recipients did not differ across levels of DSA. Our findings showed that despite having increased mortality risk, older ILDKT recipients have comparable outcomes in terms of DGF and LOS, and better outcomes in terms of risk of DCGF and AR compared to younger ILDKT recipients and that this risk profile of ILDKT for older recipients is comparable to that of older CLDKT recipients.
The increased mortality risk we observed in older ILDKT recipients compared to younger ILDKT recipients is consistent with prior studies in older recipients 19–24. For example, a study using OPTN data has shown that older recipients have lower 5-year patient survival following living donor kidney transplantation (80.7% vs 94%) and deceased donor kidney transplantation (DDKT) (67.2% vs 89.6%) compared to younger recipients (35–49 years old) 25. Our findings demonstrated that although older ILDKT recipients have a higher mortality risk than that of younger ILDKT recipients, in appropriately selected older recipients, there is no additional risk conferred by age.
Previous studies in CLDKT recipients have also demonstrated that DCGF in older kidney transplant recipients is lower than younger recipients, after adjusting for patient and donor characteristics 22,26. Our finding of decreased death censored graft failure of older ILDKT recipients compared to younger ILDKT recipients is consistent with these studies. An analysis of OPTN data showed that older recipients had a lower risk of DCGF compared to that of younger recipients post DDKT 22. These results support our findings of 51% decreased risk of DCGF in older ILDKT recipients compared to younger ILDKT recipients. Death-censored graft failure allows us to focus on only graft related outcomes by excluding older recipients who died with a functioning graft. Our findings suggest that overall graft failure in older recipients is likely influenced by the increased risk of mortality, not necessarily inherent graft function.
Our finding that older recipients have 43% decreased risk of AR is consistent with previous studies that showed decreased rates of AR in older recipients 26–28. This decreased rate of AR could be due to immunosenescence and progressive decline in immune function due to changes including decreased circulating lymphocytes 29–32 and changes in cytokine profiles 32–34. This unique characteristic of older recipients may make ILDKT more favorable. We also showed that there was no difference in risk of developing DGF in older ILDKT recipients compared to younger ILDKT recipients, which may explain their comparable lengths of stay. This finding was also observed by a previous study where older recipients had similar rates of DGF and length of initial hospital stay compared to that of younger recipients after compatible DDKT and LDKT 28. These findings support ILDKT in appropriately selected individuals.
There are some limitations to our study worth discussing. Our cohort consisted of 25 participating transplant centers that may be uniquely different than the national sample of transplant centers performing ILDKT. Though our study relies on data from 25 centers, these centers are likely to be broadly representative of ILDKT programs in the country 7. Given the long follow up period of our study, there may be considerable variation in immunosuppression protocols as the landscape of ILDKT management has changed from 1997–2016. We also acknowledge that our findings are limited by sample size of older ILDKT patients and that since 2016, there have been ongoing developing changes in immunosuppression, clinical care, and growth of kidney paired donation (KPD) that are not captured by our data. Though there may be variability in treatment and reporting between centers that this study did not assess, our study’s strength is that it represents the largest and only study of older recipients compared to younger recipients who received ILDKT. Another notable point, although we conclude that mortality risk is not impacted by age given the borderline P value, this should be taken with consideration with respect to our sample size and limitations to power. Although we leveraged SRTR data for reliable ascertainment of post-transplant outcomes, the study is also limited by known limitations of retrospective registry analysis such as granularity of data captured. Information such as cardiac comorbidities prior to KT and infectious complications following KT are more granular outcomes limited by registry analysis that can be ascertained in studies moving forward.
Incompatible living donor kidney transplant requires resources and experience to manage complex coordination of care. Although the utilization of ILDKT has decreased significantly due to the ability to identify a compatible donor through KPD and changes to organ allocation for highly sensitized recipients, the practice of performing ILDKT is still relevant for centers, both for centers not participating in exchange programs and recipients who cannot be referred to programs that can offer more extensive exchange options or are unlikely to identify a compatible donor via exchange due to prohibitive longer waiting times 35. Therefore, understanding the impact of recipient age on patient and graft outcomes of ILDKT remains relevant. Findings of this study may help physicians and patients better understand the favorable outcomes associated with ILDKT in older recipients, which may increase access to transplant.
In conclusion, we found that older ILDKT recipients had an increased mortality risk, a lower risk of DCGF, and lower odds of developing acute rejection compared to younger ILDKT recipients. Although older ILDKT recipients have higher mortality, their risk profile post-ILDKT is comparable or better than that of younger ILDKT recipients. Furthermore, the risk profile of older ILDKT recipients is equivalent to that of older CLDKT recipients, therefore age alone should not preclude eligibility for ILDKT. These findings should be taken into consideration among various patient characteristics when counseling on ILDKT risks.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all the transplant centers that generously provided data on their ILDKT patients. We would also like to thank the co-investigators, coordinators, and research staff who contributed to this study. Thank you to Madeleine Waldram for contributions with data collection. This work was supported by grant numbers R01DK98431 (PI: Segev), R01DK120518 and R01DK114074 (PI: McAdams-DeMarco), and F32DK113719 (PI: Jackson) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), R01AG055781, R01AG077888, and K02AG076883 (PI: McAdams-DeMarco) from the National Institute on Aging, as well as the Doris Duke Charitable Research Foundation Clinician Scientist Development Award (PI: Garonzik-Wang). Dr. Segev is supported by grant number K24AI144954 from the National Institute of Allergy and Infectious Diseases (NIAID).
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Abbreviations:
- aHR
adjusted hazard ratio
- aIRR
adjusted incidence ratio
- aOR
adjusted odds ratio
- BMI
body mass index
- CIT
cold ischemia time
- CLDKT
compatible living donor kidney transplantation
- DCGF
death censored graft failure
- LDKTT
living donor kidney transplantation
- DDKT
deceased donor kidney transplantation
- DGF
delayed graft function
- DSA
donor-specific antibody
- ESRD
end stage renal disease
- HLA
human leukocyte antigen
- HRSA
Health Resources and Services Administration
- IQR
interquartile range
- ILDKT
incompatible living donor kidney transplantation
- KPD
kidney paired donation
- LD KDPI
living donor kidney donor profile index
- LOS
length of stay
- MFI
mean fluorescence intensity
- OPTN
Organ Procurement Transplantation Network
- PCC
positive cytotoxic crossmatch
- PLNF
positive Luminex, negative flow crossmatch
- PFNC
positive flow, negative cytotoxic crossmatch
- PRA
panel reactive antibody
- SD
standard deviation
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
Dr. Orandi has received grant support from Hansa biopharma. Dr. McAdams-DeMarco has received support from Chiesi. All other authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.OPTN. Transplants in the U.S. by Recipient Age. National Data. [Google Scholar]
- 2.Montgomery R, Lonze B. Desensitization in HLA-incompatible kidney recipients and survival. … England Journal of …. Published online 2011. [DOI] [PubMed] [Google Scholar]
- 3.Pham TA, Lee JI, Melcher ML. Kidney paired exchange and desensitization: Strategies to transplant the difficult to match kidney patients with living donors. Transplantation Reviews. 2017;31(1):29–34. doi: 10.1016/j.trre.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 4.Malvezzi P, Jouve T, Noble J, Rostaing L. Desensitization in the Setting of HLA-Incompatible Kidney Transplant. Experimental and Clinical Transplantation. 2018;4:367–375. doi: 10.6002/ect.2017.0355 [DOI] [PubMed] [Google Scholar]
- 5.Haririan A, Nogueira J, Kukuruga D, et al. Positive Cross-Match Living Donor Kidney Transplantation: Longer-Term Outcomes. American Journal of Transplantation. 2009;9(3):536–542. doi: 10.1111/j.1600-6143.2008.02524.x [DOI] [PubMed] [Google Scholar]
- 6.Bentall A, Cornell LD, Gloor JM, et al. Five-Year Outcomes in Living Donor Kidney Transplants With a Positive Crossmatch. American Journal of Transplantation. 2013;13(1):76–85. doi: 10.1111/j.1600-6143.2012.04291.x [DOI] [PubMed] [Google Scholar]
- 7.Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: A multicenter study. American Journal of Transplantation. 2014;14(7):1573–1580. doi: 10.1111/ajt.12786 [DOI] [PubMed] [Google Scholar]
- 8.Huang E, Segev D, Rabb H. Kidney Transplant in the Elderly. Semin Nephrol. 2009;29(6):621–635. doi: 10.1016/j.semnephrol.2009.07.011.Kidney [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faravardeh A, Eickhoff M, Jackson S, et al. Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation. 2013;96(12):1089–1096. doi: 10.1097/TP.0b013e3182a688e5 [DOI] [PubMed] [Google Scholar]
- 10.Yango A, Gohh R, Monaco A, et al. Excess Risk of Renal Allograft Loss and Early Mortality Among Elderly Transplant Recipients Associated with Poor Exercise Capacity. Transplantation. 2004;78(2). [DOI] [PubMed] [Google Scholar]
- 11.Massie AB, Kuricka LM, Segev DL. Big data in organ transplantation: Registries and administrative claims. American Journal of Transplantation. 2014;14(8):1723–1730. doi: 10.1111/ajt.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garonzik Wang JM, Montgomery RA, Kucirka LM, Berger JC, Warren DS, Segev DL. Incompatible Live-Donor Kidney Transplantation in the United States: Results of a National Survey. Clinical Journal of the American Society of Nephrology. 2011;6(8):2041–2046. doi: 10.2215/CJN.02940311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motter JD, Jackson KR, Long JJ, et al. Delayed graft function and acute rejection following HLA-incompatible living donor kidney transplantation. American Journal of Transplantation. 2020;n/a(n/a). doi: 10.1111/ajt.16471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zachary AA, Vega RM, Lucas DP, Leffell MS. HLA Antibody Detection and Characterization by Solid Phase Immunoassays: Methods and Pitfalls. In: Christiansen FT, Tait BD, eds. Immunogenetics. Vol 882. Methods in Molecular Biology. Humana Press; 2012:289–308. doi: 10.1007/978-1-61779-842-9_17 [DOI] [PubMed] [Google Scholar]
- 15.Ellis TM. Interpretation of HLA single antigen bead assays. Transplantation Reviews. 2013;27(4):108–111. doi: 10.1016/j.trre.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 16.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine. 2011;30(4):377–399. doi: 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- 17.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10(1):1–2. doi: 10.1093/biostatistics/kxn014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.StataCorp. Stata Statistical Software: Release 15. Published online 2017. [Google Scholar]
- 19.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. New England Journal of Medicine. 1999;341(23):1725–1730. doi: 10.1056/NEJM199912023412303 [DOI] [PubMed] [Google Scholar]
- 20.Humar A, Denny R, Matas AJ, Najarian JS. Graft and quality of life outcomes in older recipients of a kidney transplant. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2003;1(2):69–72. doi: 10.1080/10454438.2016.1274705 [DOI] [PubMed] [Google Scholar]
- 21.Otero-Raviña F, Rodríguez-Martínez M, Gude F, González-Juanatey JR, Valdés F, Sánchez-Guisande D. Renal transplantation in the elderly: does patient age determine the results? Age and Ageing. 2005;34(6):583–587. doi: 10.1093/ageing/afi200 [DOI] [PubMed] [Google Scholar]
- 22.Keith DS, Cantarovich M, Paraskevas S, Tchervenkov J. Recipient age and risk of chronic allograft nephropathy in primary deceased donor kidney transplant. Transplant International. 2006;19(8):649–656. doi: 10.1111/j.1432-2277.2006.00333.x [DOI] [PubMed] [Google Scholar]
- 23.Shah T, Bunnapradist S, Hutchinson I, et al. The evolving notion of “senior” kidney transplant recipients. Clinical Transplantation. 2008;22(6):794–802. doi: 10.1111/j.1399-0012.2008.00881.x [DOI] [PubMed] [Google Scholar]
- 24.Frei U, Noeldeke J, Machold-Fabrizii V, et al. Prospective Age-Matching in Elderly Kidney Transplant Recipients—A 5-Year Analysis of the Eurotransplant Senior Program. American Journal of Transplantation. 2008;8(1):50–57. doi: 10.1111/j.1600-6143.2007.02014.x [DOI] [PubMed] [Google Scholar]
- 25.Knoll GA. Kidney Transplantation in the Older Adult. American Journal of Kidney Diseases. 2013;61(5):790–797. doi: 10.1053/j.ajkd.2012.08.049 [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Shapiro R, Tan H, et al. Kidney transplantation in elderly people: The influence of recipient comorbidity and living kidney donors. Journal of the American Geriatrics Society. 2008;56(2):231–238. doi: 10.1111/j.1532-5415.2007.01542.x [DOI] [PubMed] [Google Scholar]
- 27.Meier-Kriesche HU, Srinivas TR, Kaplan B. Interaction between acute rejection and recipient age on long-term renal allograft survival. Transplantation Proceedings. 2001;33(7–8):3425–3426. doi: 10.1016/S0041-1345(01)02477-0 [DOI] [PubMed] [Google Scholar]
- 28.Dempster NJ, Ceresa CDL, Aitken E, Kingsmore D. Outcomes following renal transplantation in older people: A retrospective cohort study. BMC Geriatrics. 2013;13(1):1. doi: 10.1186/1471-2318-13-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunology. 2004;5(2):133–139. doi: 10.1038/ni1033 [DOI] [PubMed] [Google Scholar]
- 30.Naylor K, Li G, Vallejo AN, et al. The Influence of Age on T Cell Generation and TCR Diversity. The Journal of Immunology. 2005;174(11):7446 LP - 7452. doi: 10.4049/jimmunol.174.11.7446 [DOI] [PubMed] [Google Scholar]
- 31.Yan J, Greer JM, Hull R, et al. The effect of ageing on human lymphocyte subsets: Comparison of males and females. Immunity and Ageing. 2010;7:1–10. doi: 10.1186/1742-4933-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valiathan R, Ashman M, Asthana D. Effects of Ageing on the Immune System: Infants to Elderly. Scandinavian Journal of Immunology. 2016;83(4):255–266. doi: 10.1111/sji.12413 [DOI] [PubMed] [Google Scholar]
- 33.Yen CJ, Lin SL, Huang KT, Lin RH. Age-associated changes in interferon-γ and interleukin-4 secretion by purified human CD4+ and CD8+ T cells. Journal of Biomedical Science. Published online 2000. doi: 10.1007/bf02253251 [DOI] [PubMed] [Google Scholar]
- 34.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. Published online 2000. doi: 10.1146/annurev.med.51.1.245 [DOI] [PubMed] [Google Scholar]
- 35.Holscher CM, Jackson K, Chow EKH, et al. Kidney exchange match rates in a large multicenter clearinghouse. American Journal of Transplantation. 2018;18(6):1510–1517. doi: 10.1111/ajt.14689 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.