Abstract
Riboswitches are regulatory elements found in the untranslated regions (UTRs) of certain mRNA molecules. They typically comprise two distinct domains: an aptamer domain that can bind to specific small molecules, and an expression platform that controls gene expression. Riboswitches work by undergoing a conformational change upon binding to their specific ligand, thus activating or repressing the genes downstream. This mechanism allows gene expression regulation in response to metabolites or small molecules. To systematically summarise riboswitch structures and their related ligand binding functions, we present Ribocentre-switch, a comprehensive database of riboswitches, including the information as follows: sequences, structures, functions, ligand binding pockets and biological applications. It encompasses 56 riboswitches and 26 orphan riboswitches from over 430 references, with a total of 89 591 sequences. It serves as a good resource for comparing different riboswitches and facilitating the identification of potential riboswitch candidates. Therefore, it may facilitate the understanding of RNA structural conformational changes in response to ligand signaling. The database is publicly available at https://riboswitch.ribocentre.org.
Graphical Abstract
Graphical Abstract.
Introduction
Riboswitches are non-coding RNAs of structured RNA domains, which function as switches for metabolic genes (1–3). They provide compelling evidence for the RNA world hypothesis by demonstrating the ability of naturally occurring RNAs to selectively bind small molecules without the forced assistance from protein factors. In order to perform molecular functions of both molecular recognition and conformational switching, riboswitches normally consist of a ligand-sensing aptamer domain and an expression platform that usually regulates gene expression via a molecular mechanism (4). Therefore, riboswitches can be an ideal system to study the ligand binding and regulatory functions in the light of the RNA structures.
Since the initial experimental validation of riboswitches in 2002 (5–7), at least 56 riboswitch classes have been confirmed through experimentation over the past two decades. Riboswitches are categorized according to the ligands they sense and the specific structures they employ to form the aptamer domain. These ligands comprise a diverse list of biologically relevant compounds, including fundamental metabolites derived from RNA nucleotides or their precursors, cofactors, amino acids, sugars and elemental ions (8,9).
Riboswitches can adopt different conformations depending on the presence or absence of specific ligands (3). When a riboswitch binds to its specific metabolite, it induces conformational changes in its aptamer domain and expression platform domain. These conformational changes can lead to the formation of terminators, anti-terminators, or sequestrators of ribosome binding site (RBS) sequences, which in turn regulate downstream gene expression. By optimizing the conformational changes and regulatory mechanisms of riboswitches, researchers can gain better insights into their functions and potential applications in gene expression control (9–12).
The determination of three-dimensional structures has provided valuable insights into riboswitches. To date, over 40 classes of riboswitches have been structurally characterized (9,13,14). These structures exhibit a wide range of shapes and sizes, and the ligand-binding pockets display diverse architectures. The specific three-dimensional structures of these binding pockets are crucial for the selective recognition of ligands by riboswitches (3). Experimental determination of riboswitch structures enables us to gain profound insights into their regulatory mechanisms, which can further advancements in riboswitch related translational research. Additionally, the structure and mechanism of riboswitches can serve as a basis for the design of new antimicrobial agents, making them promising targets for antimicrobial therapy (15–19). Moreover, riboswitches can be engineered and modified for potential applications in synthetic biology (20–22), molecular sensors (23,24) and gene therapy (25,26), offering exciting opportunities for their utilization in various fields.
In recent years, several specialised riboswitch databases have been conceived. RiboD presents a meta information search portal for prokaryotic riboswitches, TBDB provides a data portal exclusively for T-box riboswitch:tRNA pairs, and RSwitch provides various useful tools for sequence analyses of riboswitches in human pathogenic bacteria (27–29). However, the ligand binding mechanisms have not been summarised in these dedicated databases, leaving the ligand-induced conformational changes and gene regulatory functions unclear. In this study, we present Ribocentre-switch, which is a manually curated database summarising a more complete list of all known riboswitches from over 430 publications. The database also summarised different ligand binding models and the related applications in biology. In particular, we elaborate the milestones in the discovery and research of each riboswitch, which may provide additional insights into riboswitch research and facilitate the investigation of new riboswitches. To better capture the sequence and structure patterns related to the ligand binding features of riboswitches, Ribocentre-switch is designed to facilitate the comparisons of riboswitch sequences, structures, binding pockets and regulatory mechanisms, thus shedding light on the molecular mechanism of RNA-ligand interaction.
Materials and methods
Riboswitch data collection and curation
The Ribocentre-switch primarily gathered information from PubMed, utilizing various dimensions of riboswitch features that are organized into distinct pages including Riboswitches, Tandem RS (Riboswitches), Orphan RS, Structures, Binding pockets, Applications and Publications. The information was collected by initially searching PubMed (30) for relevant reviews and articles on riboswitches, which were summarised. Subsequently, searches were conducted on the Rfam database (31), Google search (https://www.google.com), and the RCSB PDB (32) using the keyword ‘riboswitch’ to review and supplement the riboswitch list. The final list of riboswitches was compiled through an extensive literature review. Data from various sources were cross-validated and thoroughly examined to ensure the accuracy of the information. The table search function is powered by various open-source JavaScript libraries within the DataTables library (http://datatables.net). These libraries enable the implementation of interactive data tables on the Applications, Structures and Publications pages, allowing users to download and print the data as needed. Additionally, the Help page serves as a gateway for users to provide feedback. User feedback is collected using Google Sheets, facilitating simple and efficient data collection for valuable user inputs and suggestions.
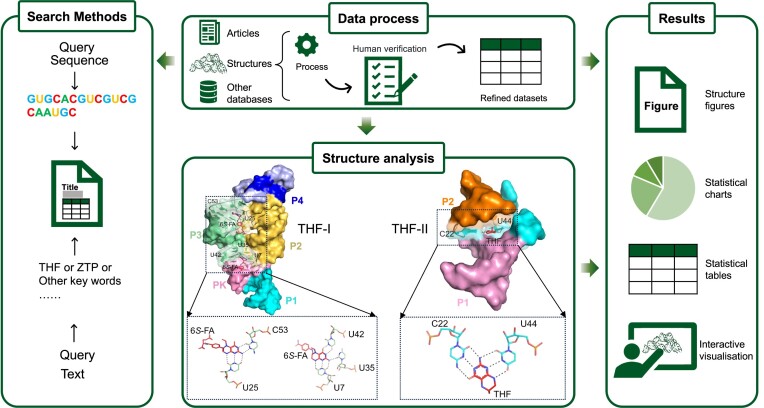
The 2D structures in the database are classified into two types. The first type is obtained from the Rfam database, which provides conserved motif information of riboswitches. The second type is to generate 2D structure annotations using tools like RiboDraw (33), which primarily utilizes MC-annotate (34) to extract structural characteristics, such as base pairs, stems and stacking interactions, from 3D structures. By comparing these two approaches, the database showcases specific structural information, allowing users to explore and analyse the riboswitches from both 2D and 3D perspectives. To generate specific static structure maps, images are created using software tools such as Adobe Illustrator (https://www.adobe.com/products/illustrator.html), PyMOL (https://pymol.org/) and KingDraw (http://www.kingdraw.cn/). These tools provide capabilities for rendering and annotating the 3D structures of riboswitches. Additionally, the pdbe-molstar plugin (35) is utilized as a molecular 3D data viewer, enabling interactive 3D rendering of ribonucleic acid structures within the database. These tools and plugins enhance the visualization and interactivity of the riboswitch structures for users (Figure 1).
Figure 1.
The data processing workflow of THF riboswitch as an example. The data processing workflow in Ribocentre-switch includes four main steps: data processing (top centre), which includes collection, processing, organisation and validation; data search (left) offering sequence search and keyword search function; structural analysis (bottom centre), exemplified by THF riboswitch binding pockets, where all the riboswitches in the THF family are listed, and their binding pockets and ligands are compared and analysed; and data results (right) presented in the form of figures, charts, tables and interactive visualisation.
Web portal development
Ribocentre-switch database was developed using Python 3.10.6 (https://www.python.org/) and Jekyll (https://jekyllrb.com/) which is a nimble website builder based on the Ruby programming language combined with Bootstrap (http://www.getbootstrap.com/) and jQuery (https://jquery.com/). We designed a search interface that is easy to use and allows for quick retrieval of relevant data. The database can be searched using various parameters such as ‘Riboswitches’, ‘THF’ and ‘Structures’. Additionally, the database can be queried using sequence as search criteria, which is currently being improved. Riboswitch sequences can be easily downloaded by users using the ‘Download’ option. The database is accessible across all platforms, such as mobile phones, tablets and PCs, without registration. It is user-friendly and we recommend using a PC for optimal browsing experience. Furthermore, the website supports HTTPS, guaranteeing the secure transmission of data between users and the site, thereby enhancing the overall security of data interaction.
Database contents
Ribocentre-switch is a comprehensive database of natural riboswitches, including 56 riboswitches and 26 orphan riboswitches. It comprises 89591 sequences, over 430 references, consisting of 78 in the classical review category and 352 in the experimental category. Currently, the database includes a total of 356 riboswitch structures sourced from the PDB database (32). Furthermore, it classifies 35 riboswitch-related applications into four distinct categories. The web pages are organized as follows:
The Home page of the Ribocentre-switch provides a concise overview of the definition, classification, binding pocket structures and gene regulatory mechanisms of riboswitches. Notably, it offers convenient sequence search and site-wide search functions directly on the homepage. Users can search for riboswitches by inputting sequence information or explore riboswitch families of interest using relevant keywords. Multiple examples of searches are provided, each accompanied by separate search boxes to facilitate efficient navigation and exploration of the database.
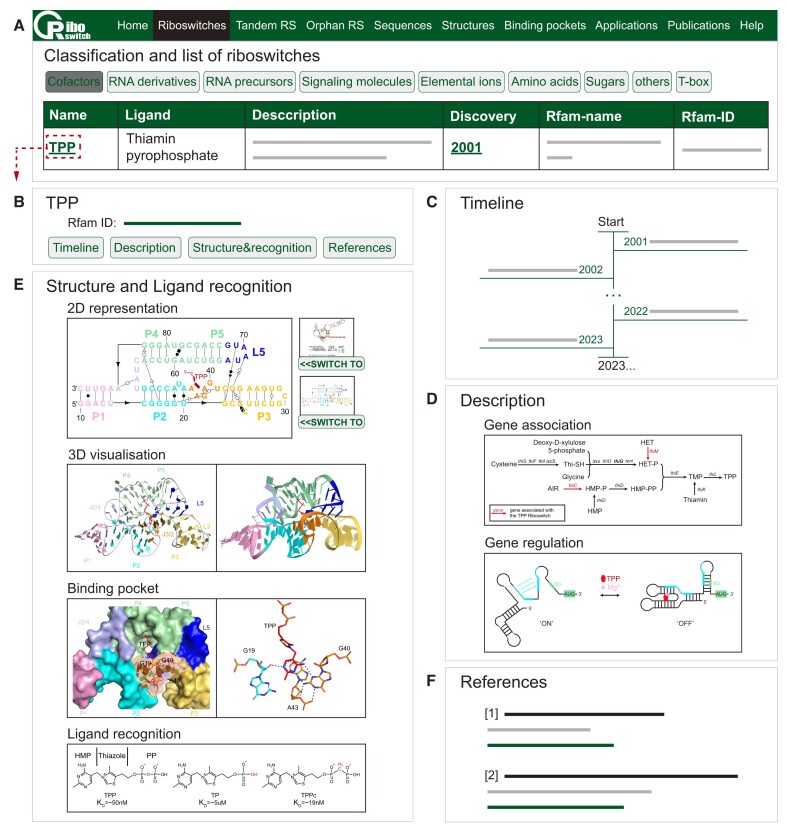
On the Riboswitches page, we classify all the riboswitches by biochemical category of their cognate ligands (8,13), namely: Cofactors, RNA derivatives, RNA precursors, Signaling molecules, Elemental ions, Amino acids, Sugars, Others and T-box (Figures 2 and 3A). As the users choose the appropriate category, there will be a list of riboswitches showing the riboswitch name, associated ligand, a brief description, discovery date, Rfam name and Rfam ID (Figure 2A). Clicking on the riboswitch's name (e.g. TPP), users will be redirected to the single entry page for the corresponding riboswitch (Figure 2B). Within the page of each riboswitch, users can utilize the navigation buttons to browse four content sections: a timeline showcasing research results (Figure 2C), a comprehensive description of each riboswitch (Figure 2D), details about structure and ligand recognition (Figure 2E) and a list of references (Figure 2F). We make a timeline for each riboswitch mostly consisting of the discovery of conserved RNA structure as well as gene association, validation of supposed secondary structure and function, significant nuclear magnetic resonance spectroscopy (NMR) or crystallographic studies of the 3D structure and determinants of ligand specificity. In the description section, we provide a brief introduction, detailed information on the gene association and the gene regulation mechanism of the riboswitch. In the structure and ligand recognition section, we present consensus sequence and secondary structure model, tertiary structures, the structural detail of the binding pocket and the chemical structures as well as apparent KD of ligand and its analogs. In addition, users can click the green fonts on the timeline section or references section to link to the corresponding literature pages on Pubmed.
Figure 2.
The Riboswitches page at Ribocentre-switch. (A) A list of 20 riboswitch classes sensing cofactors is shown on the page. (B) Clicking on the riboswitch's name will bring it to the corresponding information page. (C–F) Four content sections, composed of timeline, description, structure and ligand recognition, and references for the TPP riboswitch, are displayed on its page in turn.
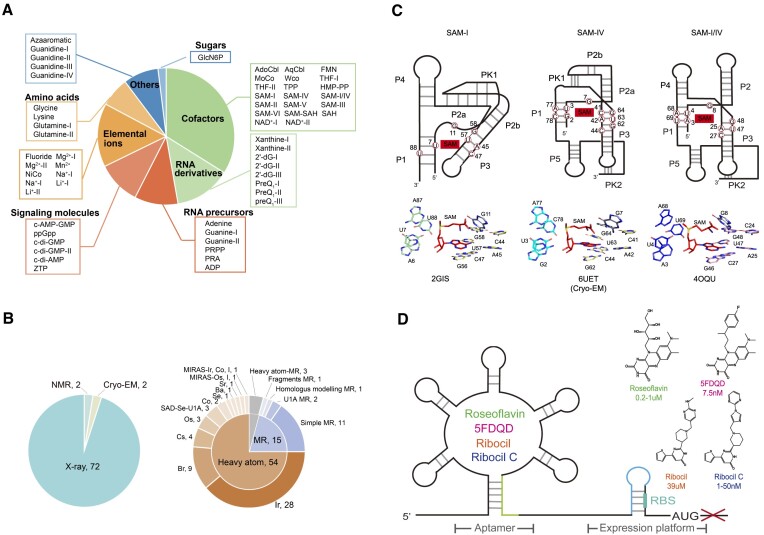
Figure 3.
Several summaries at Ribocentre-switch. (A) Summary and classification of experimentally validated riboswitches. The plotted data corresponds to the classification of the riboswitches according to the biochemical category of their cognate ligands. This panel was adapted from Ron Breaker (8). (B) Statistics of structure determination and phasing methods for riboswitches. Structure determination for riboswitches (left). The inner circle shows cases of molecular replacement (MR) and de novo phasing based on heavy atom derivatives. The outer circle further indicates the heavy atoms used for de novo phasing and the searching models for MR (right). (cryogenic electron microscopy, Cryo-EM; nuclear magnetic resonance spectroscopy, NMR; single-wavelength anomalous dispersion, SAD; multiple isomorphous replacement with anomalous scattering, MIRAS). (C) An example of the comparison of binding pockets of the riboswitches on the Binding pockets page. Top: The cartoon format secondary structures of the three members of the SAM-I clan riboswitches. The binding site regions and peripheral subdomains of the three riboswitches are shown, along with the nucleic acids (in red circle) that interact with the S-adenosylmethionine (SAM) (in red shade) (56). Bottom: The binding sites of the three riboswitches are shown. The nucleic acids and the SAM ligand are shown in sticks (56–58). (D) Schematic representation of the FMN riboswitch as a new antibacterial target. Different antibacterial compounds bind to the FMN riboswitch and abolish the expression of the downstream gene (left). The structural formulas and KD values of the antibacterial compounds are additionally provided (right) (16,53–55).
Although the majority of riboswitches employ a single aptamer domain followed by a solitary expression platform, many researches have unveiled that certain riboswitches adopt a tandem arrangement of aptamer domains or entire riboswitches, resulting in intricate regulatory behaviours, such as independent function at distinct stages of the gene expression pathway or the creation of two-input logic gates (36–38). The Tandem Riboswitch (Tandem RS) page offers a comprehensive summary of studies on the regulatory mechanisms of tandem riboswitches and unique riboswitch variants. Each study includes information on the riboswitch's name, species, a brief synopsis, its authors, title, publication year and journal.
Furthermore, we provide a brief summary of orphan riboswitch candidates on the Orphan Riboswitch (Orphan RS) page. Orphan riboswitches are structured noncoding RNA motifs which likely function as riboswitches, but whose cognate ligands have yet to be established (39,40). In addition to the name and the Rfam ID, the consensus sequence and secondary structure model are provided, both of which are available for download.
On the Structures page, we summarise the biophysical approaches used for structure determination and phase problem solution in riboswitch research (Figure 3B). Molecular replacement (MR) and experimental phasing are two main methods utilized for determining phase. Iridium is the most popular choice and has been utilized to phase nearly half of the determined riboswitch structures solved by heavy atoms. In addition, we provide extensive details about the solved structures of the different riboswitch classes in this collection, along with their corresponding entries in the Protein Data Bank (PDB) as well as the publication years, all of which are hyperlinked to provide more information. By clicking on a specific riboswitch's name, users will be taken to a dedicated entry page for a more thorough exploration.
To have a broader view of the similarities and differences between the riboswitches family, we prepare the Binding pockets page for users, where the comparisons of the binding pockets from different riboswitches can be accessed. We sorted out comparisons respectively among ‘Purine riboswitches family’ (41–44), ‘SAM riboswitches family’ (45,46) and ‘Guanidine riboswitches family’ (47–51). Each comparison includes a detailed description, careful contrasts of secondary structures and tertiary structures within different binding pockets. The users can click the ‘Download’ button below the Image to download the content of interest from Ribocentre-switch.
Natural riboswitches exhibit remarkable versatility, offering numerous opportunities for practical applications. The applications of riboswitches in developing new antibacterial, building biosensors, synthetic biology and gene therapy have been recognized. Riboswitches have the ability to impact bacterial homeostasis, development, pathogenicity and antibiotic resistance at the transcriptional or translational level. Moreover, they can form intricate 3D structures that possess specific binding sites for small molecules. Consequently, these characteristics render them attractive targets for antimicrobial therapy. For example, the Gram-positive bacterium Bacillus subtilis utilizes the FMN riboswitch to regulate genes involved in riboflavin biosynthesis (7,26,52). Roseoflavin, a chemical analog of FMN, was firstly validated that can directly bind to FMN riboswitch and has antimicrobial activity (53,54). And then, 5FDQD, ribocil and ribocil C were found to target the FMN riboswitch and inhibit gene expression (Figure 3D) (16,55). The database provides an Applications page that summarises researches on the application of riboswitches. Each item includes a brief summary, the authors, reference, publication year and journal. The search box and download button allow the users to search and download the content of interest.
The Publications page in the Ribocentre-switch presents a summary of literature information related to riboswitches. The page is divided into two parts: the search area and the download area. Users have multiple options for accessing specific literature. Firstly, they can directly type in the string of interest in the search bar. Alternatively, they can click on different buttons to explore literature categorized based on their interests. Moreover, users can sort the table's content by clicking on column headings, enabling them to narrow down their search. For a detailed view of specific literature, users can click on the journal link or the riboswitch name, which can lead them to the corresponding page. Finally, users can select and download the desired category of interest from the page to obtain a complete list of journals.
The Help page of the Ribocentre-switch offers a comprehensive overview of its features, encompassing the search function, download function and interactive interface function. Users can access tutorials containing visual images and explanatory text to facilitate easy navigation and efficient utilization of the database. To keep pace with the latest developments in riboswitch research, the database will undergo regular updates. Users are actively encouraged to contribute by submitting new riboswitch cases through the feedback portal on the Help page or via direct email, and their valuable input will play a crucial role in enhancing and improving the database, ensuring that it better serves the research community.
Discussion
Ribocentre-switch is a specialised database dedicated to riboswitches, providing detailed and summary information on natural riboswitches and related publications over the past 20 years. The database covers a total number of more than 56 experimentally validated riboswitches, classified based on the ligands they sense (Figure 3A) and offers the single entry page for each riboswitch on the Riboswitches page with exhaustive data meticulously gathered from various sources of published papers. Furthermore, the database offers a summary of the methods used for structure determination and phasing of riboswitches on the basis of collecting and analysing all the solved structures of the riboswitches, which denotes that Iridium is the most popular choice utilized for phase determination (Figure 3B). And then, the database facilitates a comparison of the binding pockets of these regulatory elements, shedding light on the similarities and differences within the riboswitch family (Figure 3C). Lastly but not least, the database features an Applications page that summarises the potential uses and practical applications of riboswitches (Figure 3D). This information can be beneficial for researchers seeking to explore the various possibilities of employing riboswitches in different contexts.
To ensure the continuous improvement and user-friendliness of Ribocentre-switch, the database remains open to feedback and updated information from its users. It encourages users to report any errors or malfunctions they encounter while utilizing the database. The team behind Ribocentre-switch actively considers and implements user feedback and ideas, ensuring that the data remains updated, errors are rectified promptly and links to external databases are kept current.
The Ribocentre-switch database provides an invaluable and comprehensive resource regarding the experimentally validated riboswitches, facilitating an enhanced comprehension of these crucial biological elements. Users can conveniently get access to a wealth of information encompassing the research history, structural organisation, ligand recognition, potential biological applications and more. Consequently, this comprehensive database serves as a catalyst for advancing riboswitch research, fostering novel insights in the field. Overall, Ribocentre-switch strives to be a reliable and dynamic resource in the field of riboswitch research.
Acknowledgements
We thank Yaohuang Shi, Ziliang Huang, Ke Fang and Ying Ao for their suggestions for website design.
Contributor Information
Fan Bu, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230027, China; GMU-GIBH Joint School of Life Sciences, The Guangdong-Hong Kong-Macau Joint Laboratory for Cell Fate Regulation and Diseases,Guangzhou National Laboratory, Medical University, Guangzhou 510180, China.
Xiaowei Lin, Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China; Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Wenjian Liao, Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China; Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Zhizhong Lu, School of Life Sciences and Biopharmaceutics, Guangdong Pharmaceutical University, Guangzhou 510006, China.
Yuanlin He, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Yuhang Luo, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Xuemei Peng, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Mengxiao Li, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Yuanyin Huang, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Xiaoxue Chen, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Bowen Xiao, GMU-GIBH Joint School of Life Sciences, The Guangdong-Hong Kong-Macau Joint Laboratory for Cell Fate Regulation and Diseases,Guangzhou National Laboratory, Medical University, Guangzhou 510180, China.
Jiuhong Jiang, GMU-GIBH Joint School of Life Sciences, The Guangdong-Hong Kong-Macau Joint Laboratory for Cell Fate Regulation and Diseases,Guangzhou National Laboratory, Medical University, Guangzhou 510180, China.
Jie Deng, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Jian Huang, Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China; Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Tianxin Lin, Department of Urology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China; Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Zhichao Miao, Shanghai Key Laboratory of Anesthesiology and Brain Functional Modulation, Clinical Research Center for Anesthesiology and Perioperative Medicine, Translational Research Institute of Brain and Brain-Like Intelligence, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, Shanghai 200434, China; GMU-GIBH Joint School of Life Sciences, The Guangdong-Hong Kong-Macau Joint Laboratory for Cell Fate Regulation and Diseases,Guangzhou National Laboratory, Medical University, Guangzhou 510180, China.
Lin Huang, Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Guangdong-Hong Kong Joint Laboratory for RNA Medicine, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China.
Data availability
The web interface to the database is available at https://riboswitch.ribocentre.org. This website is free, open to all users and no login or password is required.
Funding
Z.M. is supported by the Natural Science Foundation of China [32270707]; National Key R&D Program of China [2021YFF1200900, 2021YFF1200903]; R&D Programs of Guangzhou Laboratory [SRPG22-003, SRPG22-006, SRPG22-007, YW-YFYJ0102]; L.H. is supported by National Natural Science Foundation of China [32171191]; Guangdong Science and Technology Department [2022A1515010328, 2023B1212060013, 2020B1212030004]; Fundamental Research Funds for the Central Universities, Sun Yat-sen University [23ptpy41]. Funding for open access charge: National Natural Science Foundation.
Conflict of interest statement. None declared.
References
- 1. Mandal M., Breaker R.R.. Gene regulation by riboswitches. Nat. Rev. Mol. Cell Biol. 2004; 5:451–463. [DOI] [PubMed] [Google Scholar]
- 2. Roth A., Breaker R.R.. The structural and functional diversity of metabolite-binding riboswitches. Annu. Rev. Biochem. 2009; 78:305–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serganov A., Patel D.J.. Metabolite recognition principles and molecular mechanisms underlying riboswitch function. Annu. Rev. Biophys. 2012; 41:343–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breaker R.R. Riboswitches and the RNA world. Cold Spring Harb. Perspect. Biol. 2012; 4:a003566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nahvi A., Sudarsan N., Ebert M.S., Zou X., Brown K.L., Breaker R.R.. Genetic control by a metabolite binding mRNA. Chem. Biol. 2002; 9:1043. [DOI] [PubMed] [Google Scholar]
- 6. Winkler W., Nahvi A., Breaker R.R.. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002; 419:952–956. [DOI] [PubMed] [Google Scholar]
- 7. Mironov A.S., Gusarov I., Rafikov R., Lopez L.E., Shatalin K., Kreneva R.A., Perumov D.A., Nudler E.. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. Cell. 2002; 111:747–756. [DOI] [PubMed] [Google Scholar]
- 8. Salvail H., Breaker R.R.. Riboswitches. Curr. Biol. 2023; 33:R343–R348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kavita K., Breaker R.R.. Discovering riboswitches: the past and the future. Trends Biochem. Sci. 2023; 48:119–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nudler E., Mironov A.S.. The riboswitch control of bacterial metabolism. Trends Biochem. Sci. 2004; 29:11–17. [DOI] [PubMed] [Google Scholar]
- 11. Peselis A., Serganov A.. Themes and variations in riboswitch structure and function. Biochim. Biophys. Acta. 2014; 1839:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serganov A., Nudler E.. A decade of riboswitches. Cell. 2013; 152:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCown P.J., Corbino K.A., Stav S., Sherlock M.E., Breaker R.R.. Riboswitch diversity and distribution. RNA. 2017; 23:995–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breaker R.R. The biochemical landscape of riboswitch ligands. Biochemistry. 2022; 61:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sudarsan N., Cohen-Chalamish S., Nakamura S., Emilsson G.M., Breaker R.R.. Thiamine pyrophosphate riboswitches are targets for the antimicrobial compound pyrithiamine. Chem. Biol. 2005; 12:1325–1335. [DOI] [PubMed] [Google Scholar]
- 16. Howe J.A., Wang H., Fischmann T.O., Balibar C.J., Xiao L., Galgoci A.M., Malinverni J.C., Mayhood T., Villafania A., Nahvi A.et al.. Selective small-molecule inhibition of an RNA structural element. Nature. 2015; 526:672–677. [DOI] [PubMed] [Google Scholar]
- 17. Stamatopoulou V., Apostolidi M., Li S., Lamprinou K., Papakyriakou A., Zhang J., Stathopoulos C.. Direct modulation of T-box riboswitch-controlled transcription by protein synthesis inhibitors. Nucleic Acids Res. 2017; 45:10242–10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Motika S.E., Ulrich R.J., Geddes E.J., Lee H.Y., Lau G.W., Hergenrother P.J.. Gram-negative antibiotic active through inhibition of an essential riboswitch. J. Am. Chem. Soc. 2020; 142:10856–10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Panchal V., Brenk R.. Riboswitches as drug targets for antibiotics. Antibiotics (Basel). 2021; 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robinson C.J., Vincent H.A., Wu M.-C., Lowe P.T., Dunstan M.S., Leys D., Micklefield J.. Modular riboswitch toolsets for synthetic genetic control in diverse bacterial species. J. Am. Chem. Soc. 2014; 136:10615–10624. [DOI] [PubMed] [Google Scholar]
- 21. Van Vlack E.R., Topp S., Seeliger J.C.. Characterization of engineered PreQ1 riboswitches for inducible gene regulation in Mycobacteria. J. Bacteriol. 2017; 199:e00656-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villa J.K., Su Y., Contreras L.M., Hammond M.C.. Synthetic biology of small RNAs and riboswitches. Microbiol. Spectr. 2018; 6:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perkins K.R., Atilho R.M., Moon M.H., Breaker R.R.. Employing a ZTP riboswitch to detect bacterial folate biosynthesis inhibitors in a small molecule high-throughput screen. ACS Chem. Biol. 2019; 14:2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma Y., Mou Q., Yan P., Yang Z., Xiong Y., Yan D., Zhang C., Zhu X., Lu Y.. A highly sensitive and selective fluoride sensor based on a riboswitch-regulated transcription coupled with CRISPR-Cas13a tandem reaction. Chem. Sci. 2021; 12:11740–11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takahashi K., Yokobayashi Y.. Reversible gene regulation in mammalian cells using riboswitch-engineered vesicular stomatitis virus vector. ACS Synth. Biol. 2019; 8:1976–1982. [DOI] [PubMed] [Google Scholar]
- 26. Vikram Mishra, V., Rana A., Ahire J.J.. Riboswitch-mediated regulation of riboflavin biosynthesis genes in prokaryotes. 3 Biotech. 2022; 12:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukherjee S., Das Mandal S., Gupta N., Drory-Retwitzer M., Barash D., Sengupta S.. RiboD: a comprehensive database for prokaryotic riboswitches. Bioinformatics. 2019; 35:3541–3543. [DOI] [PubMed] [Google Scholar]
- 28. Marchand J.A., Pierson Smela M.D., Jordan T.H.H., Narasimhan K., Church G.M.. TBDB: a database of structurally annotated T-box riboswitch:tRNA pairs. Nucleic Acids Res. 2021; 49:D229–D235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Penchovsky R., Pavlova N., Kaloudas D.. RSwitch: a novel bioinformatics database on riboswitches as antibacterial drug targets. IEEE/ACM Trans. Comput. Biol. Bioinform. 2021; 18:804–808. [DOI] [PubMed] [Google Scholar]
- 30. Sayers E.W., Bolton E.E., Brister J.R., Canese K., Chan J., Comeau D.C., Connor R., Funk K., Kelly C., Kim S.et al.. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022; 50:D20–D26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalvari I., Nawrocki E.P., Ontiveros-Palacios N., Argasinska J., Lamkiewicz K., Marz M., Griffiths-Jones S., Toffano-Nioche C., Gautheret D., Weinberg Z.et al.. Rfam 14: expanded coverage of metagenomic, viral and microRNA families. Nucleic Acids Res. 2021; 49:D192–D200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burley S.K., Bhikadiya C., Bi C., Bittrich S., Chen L., Crichlow G.V., Christie C.H., Dalenberg K., Di Costanzo L., Duarte J.M.et al.. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021; 49:D437–D451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Das R., Watkins A.M.. RiboDraw: semiautomated two-dimensional drawing of RNA tertiary structure diagrams. NAR Genom Bioinform. 2021; 3:lqab091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gendron P., Lemieux S., Major F.. Quantitative analysis of nucleic acid three-dimensional structures. J. Mol. Biol. 2001; 308:919–936. [DOI] [PubMed] [Google Scholar]
- 35. Sehnal D., Bittrich S., Deshpande M., Svobodová R., Berka K., Bazgier V., Velankar S., Burley S.K., Koča J., Rose A.S.. Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021; 49:W431–W437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roßmanith J., Narberhaus F.. Modular arrangement of regulatory RNA elements. RNA Biol. 2017; 14:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ariza-Mateos A., Nuthanakanti A., Serganov A.. Riboswitch mechanisms: new tricks for an old dog. Biochemistry. 2021; 86:962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sherlock M.E., Higgs G., Yu D., Widner D.L., White N.A., Sudarsan N., Sadeeshkumar H., Perkins K.R., Mirihana Arachchilage G., Malkowski S.N.et al.. Architectures and complex functions of tandem riboswitches. RNA Biol. 2022; 19:1059–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greenlee E.B., Stav S., Atilho R.M., Brewer K.I., Harris K.A., Malkowski S.N., Mirihana Arachchilage G., Perkins K.R., Sherlock M.E., Breaker R.R.. Challenges of ligand identification for the second wave of orphan riboswitch candidates. RNA Biol. 2018; 15:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barrick J.E., Corbino K.A., Winkler W.C., Nahvi A., Mandal M., Collins J., Lee M., Roth A., Sudarsan N., Jona I.et al.. New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc. Natl. Acad. Sci. U.S.A. 2004; 101:6421–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Serganov A., Yuan Y.-R., Pikovskaya O., Polonskaia A., Malinina L., Phan A.T., Hobartner C., Micura R., Breaker R.R., Patel D.J.. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem. Biol. 2004; 11:1729–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pikovskaya O., Polonskaia A., Patel D.J., Serganov A.. Structural principles of nucleoside selectivity in a 2’-deoxyguanosine riboswitch. Nat. Chem. Biol. 2011; 7:748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matyjasik M.M., Batey R.T.. Structural basis for 2’-deoxyguanosine recognition by the 2'-dG-II class of riboswitches. Nucleic Acids Res. 2019; 47:10931–10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hamal Dhakal S., Panchapakesan S.S.S., Slattery P., Roth A., Breaker R.R.. Variants of the guanine riboswitch class exhibit altered ligand specificities for xanthine, guanine, or 2’-deoxyguanosine. Proc. Natl. Acad. Sci. U.S.A. 2022; 119:e2120246119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price I.R., Grigg J.C., Ke A.. Common themes and differences in SAM recognition among SAM riboswitches. Biochim. Biophys. Acta. 2014; 1839:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zheng L., Song Q., Xu X., Shen X., Li C., Li H., Chen H., Ren A.. Structure-based insights into recognition and regulation of SAM-sensing riboswitches. Sci. China Life Sci. 2023; 66:31–50. [DOI] [PubMed] [Google Scholar]
- 47. Huang L., Wang J., Wilson T.J., Lilley D.M.J.. Structure of the guanidine III riboswitch. Cell Chem. Biol. 2017; 24:1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reiss C.W., Strobel S.A.. Structural basis for ligand binding to the guanidine-II riboswitch. RNA. 2017; 23:1338–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Battaglia R.A., Price I.R., Ke A.. Structural basis for guanidine sensing by the family of riboswitches. RNA. 2017; 23:578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang L., Wang J., Lilley D.M.J.. The Structure of the Guanidine-II Riboswitch. Cell Chem. Biol. 2017; 24:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reiss C.W., Xiong Y., Strobel S.A.. Structural basis for ligand binding to the guanidine-I riboswitch. Structure. 2017; 25:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Winkler W.C., Cohen-Chalamish S., Breaker R.R.. An mRNA structure that controls gene expression by binding FMN. Proc. Natl. Acad. Sci. U.S.A. 2002; 99:15908–15913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serganov A., Huang L., Patel D.J.. Coenzyme recognition and gene regulation by a flavin mononucleotide riboswitch. Nature. 2009; 458:233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee E.R., Blount K.F., Breaker R.R.. Roseoflavin is a natural antibacterial compound that binds to FMN riboswitches and regulates gene expression. RNA Biol. 2009; 6:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Blount K.F., Megyola C., Plummer M., Osterman D., O’Connell T., Aristoff P., Quinn C., Chrusciel R.A., Poel T.J., Schostarez H.J.et al.. Novel riboswitch-binding flavin analog that protects mice against Clostridium difficile infection without inhibiting cecal flora. Antimicrob. Agents Chemother. 2015; 59:5736–5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trausch J.J., Xu Z., Edwards A.L., Reyes F.E., Ross P.E., Knight R., Batey R.T.. Structural basis for diversity in the SAM clan of riboswitches. Proc. Natl. Acad. Sci. U.S.A. 2014; 111:6624–6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Montange R.K., Batey R.T.. Structure of the S-adenosylmethionine riboswitch regulatory mRNA element. Nature. 2006; 441:1172–1175. [DOI] [PubMed] [Google Scholar]
- 58. Zhang K., Li S., Kappel K., Pintilie G., Su Z., Mou T.-C., Schmid M.F., Das R., Chiu W.. Cryo-EM structure of a 40 kDa SAM-IV riboswitch RNA at 3.7 Å resolution. Nat. Commun. 2019; 10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The web interface to the database is available at https://riboswitch.ribocentre.org. This website is free, open to all users and no login or password is required.