Abstract
The par region of the stably maintained broad-host-range plasmid RK2 is organized as two divergent operons, parCBA and parDE, and a cis-acting site. parDE encodes a postsegregational killing system, and parCBA encodes a resolvase (ParA), a nuclease (ParB), and a protein of unknown function (ParC). The present study was undertaken to further delineate the role of the parCBA region in the stable maintenance of RK2 by first introducing precise deletions in the three genes and then assessing the abilities of the different constructs to stabilize RK2 in three strains of Escherichia coli and two strains of Pseudomonas aeruginosa. The intact parCBA operon was effective in stabilizing a conjugation-defective RK2 derivative in E. coli MC1061K and RR1 but was relatively ineffective in E. coli MV10Δlac. In the two strains in which the parCBA operon was effective, deletions in parB, parC, or both parB and parC caused an approximately twofold reduction in the stabilizing ability of the operon, while a deletion in the parA gene resulted in a much greater loss of parCBA activity. For P. aeruginosa PAO1161Rifr, the parCBA operon provided little if any plasmid stability, but for P. aeruginosa PAC452Rifr, the RK2 plasmid was stabilized to a substantial extent by parCBA. With this latter strain, parA and res alone were sufficient for stabilization. The cer resolvase system of plasmid ColE1 and the loxP/Cre system of plasmid P1 were tested in comparison with the parCBA operon. We found that, not unlike what was previously observed with MC1061K, cer failed to stabilize the RK2 plasmid with par deletions in strain MV10Δlac, but this multimer resolution system was effective in stabilizing the plasmid in strain RR1. The loxP/Cre system, on the other hand, was very effective in stabilizing the plasmid in all three E. coli strains. These observations indicate that the parA gene, along with its res site, exhibits a significant level of plasmid stabilization in the absence of the parC and parB genes but that in at least one E. coli strain, all three genes are required for maximum stabilization. It cannot be determined from these results whether or not the stabilization effects seen with parCBA or the cer and loxP/Cre systems are strictly due to a reduction in the level of RK2 dimers and an increase in the number of plasmid monomer units or if these systems play a role in a more complex process of plasmid stabilization that requires as an essential step the resolution of plasmid dimers.
Plasmids use a variety of mechanisms to maintain themselves within bacterial populations. Plasmid-encoded systems for stable maintenance include copy number control, active partitioning systems, multimer resolution, and postsegregational killing (18). RK2, identical to RP4, is a 60-kb broad-host-range plasmid that exists in Escherichia coli at five to eight copies/chromosome (40). It is a member of the IncPα family of plasmids and can be stably maintained in a wide range of gram-negative bacteria (12, 33, 37, 38, 40). A stability region of RK2 designated par is comprised of five genes in two divergently oriented autoregulated operons, parCBA and parDE (15, 33, 38). The coding frames in each of the two operons are translationally coupled. parDE encodes a postsegregational killing system and comprises a 0.7-kb region. The ParD protein acts as an antitoxin to neutralize the toxic effects of the ParE protein (22). This system has a function similar to that of the postsegregational killing systems of plasmids F (ccd), R1 (kis/kid), R100 (pemI/pemK), and P1 (phd/doc) in ensuring that plasmid-free cells that may arise after cell division do not survive (7, 14, 20, 21, 24, 25, 28).
The parCBA operon comprises a 2.3-kb region and encodes three proteins, ParA, ParB, and ParC. The ParA protein, along with its res site, located between promoters PparCBA and PparDE, functions as a site-specific recombination system homologous to the Tn3 family of resolvases (13). parB encodes a nuclease with homology to a Staphylococcus nuclease (9, 17a). The function of ParC has yet to be determined. The role of these three proteins in stabilizing RK2, other than multimer resolution by ParA, is not known. It has been proposed that these three proteins together make up or contribute to an active partitioning complex (15, 17a, 33, 37, 38).
Recently, it was shown that the parCBA region is capable of stabilizing both minireplicons of RK2 and the intact RK2 plasmid in E. coli and several other gram-negative bacteria (12c, 37). These studies involved the use of an RK2 plasmid with deletions of the par operons (37) and reinsertion of the intact parCBA and parDE operons together or separately into the intact RK2 plasmid with par deletions (12c). It was found that the parCBA region could stabilize RK2 but that the level of stability provided was dependent on the strain and the growth conditions under which the assays were carried out. In order to further characterize the parCBA operon and its role in the stabilization of RK2, precise deletions which maintained the translational coupling arrays were made in each of the three structural genes, and the resulting deletion derivatives were cloned into RK2 with par deletions. The stability of these various constructs in three strains of E. coli and two strains of Pseudomonas aeruginosa was then determined. Furthermore, the ability of two multimer resolution systems, cer of the ColE1 plasmid and loxP/Cre of the P1 plasmid, to replace the parCBA operon for plasmid stabilization was assessed. It is clear from the results obtained that parA, along with its res site, can in some circumstances provide a significant level of plasmid stabilization in the absence of parB and parC in both E. coli and P. aeruginosa. In addition, the loxP/Cre multimer resolution system provides a substantial level of stability in the absence of the parCBA operon in all three E. coli strains. It is not clear from the results whether these different resolution systems act solely to increase the number of plasmid monomer units for segregation or whether they play a role in a more complex process of stabilization of plasmid RK2 during cell growth and division.
MATERIALS AND METHODS
Materials.
Restriction endonucleases, the Klenow fragment of E. coli DNA polymerase, T4 DNA ligase, linkers, and shrimp alkaline phosphatase were obtained from commercial suppliers and used as recommended by the manufacturers. Antibiotics were obtained from Sigma Chemical Co. (St. Louis, Mo.).
Strains and media.
The bacteria used and their sources are listed in Table 1. E. coli strains were grown in Luria-Bertani (LB) medium (29) (GIBCO BRL Scientific, Grand Island, N.Y., or Sigma). Antibiotics used for plasmid selection with E. coli were added to final concentrations of 250 μg/ml for penicillin, 50 μg/ml for kanamycin, 50 μg/ml for spectinomycin, 25 μg/ml for chloramphenicol, 10 μg/ml for tetracycline, 15 μg/ml for gentamicin, and 10 μg/ml for nalidixic acid. P. aeruginosa strains were grown in nutrient broth (NB) (Difco Laboratories, Detroit, Mich.). Antibiotics used for plasmid selection with P. aeruginosa were added to final concentrations of 500 μg/ml for spectinomycin, 100 μg/ml for carbenicillin, and 75 μg/ml for rifampin.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype and other relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| MV10 | thr-1 leuB6 lacY1 thi-1 tonA21 supE44 rfbD1 ΔtrpE5 λ− | 37 |
| MV10Δlac | MV10 Δ(argF-lac) deoC1::Tn10 (Tetr) | 37 |
| MC1061K | F−araD139 Δ(ara-leu)7696 galE15 galK16 Δ(lac) X74 rpsL (Strsr) hsdR2 rK mK+mcrA mcrB1 Kmr | 32 |
| RR1 | F− Δ(gpt-proA)62 leuB6 supE44 ara-14 galK2 lacY1 Δ(mcrC-mrr) rpsL20 (Strr) xyl-5 mtl-1 | 27 |
| DH5 α | F−endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZY -argF)U169 deoR [80dlacΔ(lacZ)M15] | 23 |
| DPB635 | Wild type | 6 |
| DBP636 | DPB635 topA66 | 6 |
| Pseudomonas aeruginosa | ||
| PAO1161Rif | Spontaneous Nalr Rifr derivative of PAO1161 | 5a |
| PAC452Rif | Spontaneous Rifr derivative of PAC452 | 31 |
| Plasmids | ||
| pCE61 | Derivative of RK21382 with parCBA and parDE deletions | 12a |
| pCE61-2.3 | pCE61 containing parCBA | 12a |
| pCE61-91 | pCE61 containing parA | This work |
| pCE61-92 | pCE61 containing parAB | This work |
| pCE61-94 | pCE61 containing parAC | This work |
| pCE61-95 | pCE61 containing parBC | This work |
| pVW8703 | pUC8Cm containing traFG | 42 |
| pAL4000 | RSF1010 derivative with promotorless luciferase gene | 16 |
| pTD10 | pAL4000 with parCBA promoter | 12 |
| pEKA28 | IncQ (RSF1010) pMHL3 derivative | 36b |
| pEKA30 | Derivative of pEKA28 containing Cre | 36b |
| pEKA28G | Gentamicin-resistant derivative of pEKA28 | This work |
| pEKA30G | Gentamicin-resistant derivative of pEKA30 | This work |
Plasmid constructions.
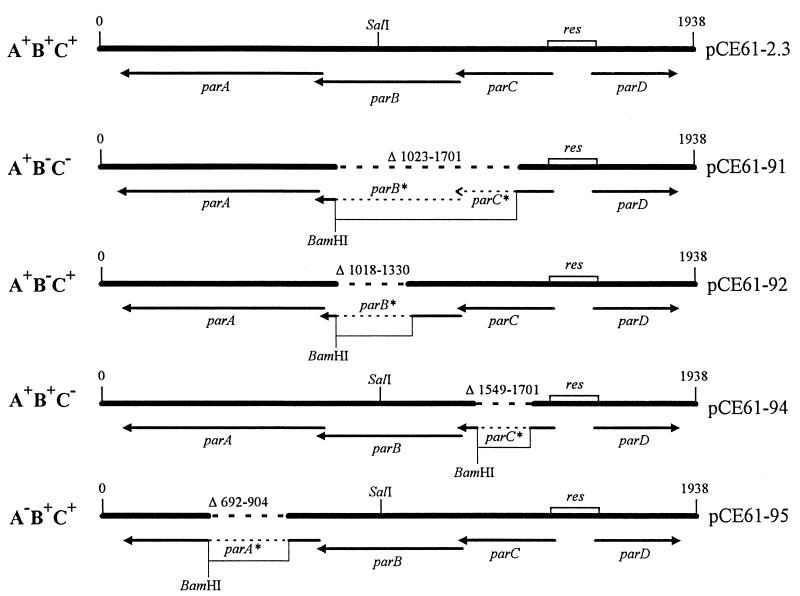
All DNA manipulations, such as restriction enzyme digestion, filling in of 5′ overhangs with the Klenow fragment, shrimp alkaline phosphatase treatment, DNA ligation, agarose gel electrophoresis, and transformation of E. coli, have been described elsewhere (27). Plasmid DNA was isolated by either the boiling lysis (19) or the alkaline lysis (27) procedure. Construction of the pCE61 and pCE61-2.3 plasmids has been described elsewhere (12c). Several deletion constructs in which different Par proteins were truncated while retaining translational coupling were constructed. A derivative of pCE61 carrying the parCBA operon with deletions in both parC and parB was constructed as follows. An SphI-BamHI fragment carrying the parA gene was isolated from plasmid pGMA7 (15) and ligated to pUC18 digested with SphI and BamHI, resulting in plasmid pUCGMA7. The BamHI fragment from pGMA41 (15) containing the promoter PparCBA and the parD gene was isolated and ligated to BamHI-digested pUCGMA7. The resulting plasmid, containing the BamHI fragment from pGMA41 in the correct orientation, was designated pCESH201. In order to delete the parD gene, pCESH201 was digested with AflIII and filled in, and then HindIII linkers were added. The resulting plasmid, pCE91, was digested with HindIII, and the HindIII fragment carrying parA plus the deletions in parB and parC (bp 1023 to 1701) (15) was ligated to HindIII-digested pCE61. The resulting plasmid was designated pCE61-91.
Construction of a plasmid carrying parCBA with a deletion in the parB gene (bp 1018 to 1380) (15) was carried out by first isolating a BamHI-SphI fragment containing parA and part of the parB gene from pGMA45 (15) and ligating that fragment to pUC18 digested with BamHI and SphI to produce plasmid pUCGMA45. pUCGMA45 was digested with BamHI and ligated with the BamHI fragment from pGMA45 (15) which contained the parC and parD genes. The orientation of the BamHI fragment was checked by clonal analysis, and the clone with the correct orientation was designated pCESH202. pCESH202 was digested with AflII, filled in, and linked to HindIII to remove parD. The resulting plasmid, designated pCE92, was used for the cloning of pCE61-92. pCE61-92 was produced by insertion of the HindIII fragment from pCE92 carrying the parCBA region with a deletion in parB into HindIII-digested pCE61.
Construction of the parC deletion derivative (bp 1549 to 1701) (15) was carried out in the following manner. First, a plasmid carrying a truncated parC was constructed by insertion of a SalI-RsaI fragment from pGMA30 (15) containing portions of parB and parC into SalI-EcoRV-digested pBSkII (cloning vector from Stratagene). The resulting plasmid, pBS-Sal/Rsa, was digested with EcoRI, and the unique site was filled in to produce plasmid pBS-Sal/RsaE*. Plasmid pUCGMA16 was constructed by insertion of an SphI-BamHI fragment from pGMA16 (15) containing the parCBA operon into pUC18 digested with SphI and BamHI. The SalI-BamHI fragment from pBS-Sal/RsaE* was ligated with SalI-BamHI-digested pUCGMA16. The resulting plasmid was designated pUCGMA16R. The BamHI fragment from pGMA41 was isolated and ligated to BamHI-digested pUCGMA16R. The resulting plasmid, pCESH204, was digested with AflII, filled in, and linked with HindIII to remove parD and to add a HindIII site. The resulting HindIII-linked plasmid was designated pCE94 and was used for the subsequent insertion of the parCBA operon with a parC deletion into the intact RK2 plasmid pCE61. The HindIII fragment from pCE94 was isolated and inserted into HindIII-digested pCE61. The resulting plasmid was named pCE61-94.
In order to construct a parCBA operon with deletions of segments of the parA gene (bp 692 to 940) (15), several intermediate plasmids were constructed. An SphI-BamHI fragment from plasmid pGMA49 (15) carrying parB and a portion of parA was isolated and inserted into pUC18 digested with SphI and BamHI. The resulting plasmid was designated pUCGMA49. Plasmid pGEM5-parB* was used for the next step. To construct pGEM5-parB,* pGEM5-parB, which carries the parB gene (36a), was digested with SacII and AvaI, and oligonucleotides containing a BamHI site and SacII and AvaI ends (GGATCCCGACTTCACCAGGTTGCAGGTCGGCGGGATCGCGC) (GCGATCCCGCCGACCTGCAACCTGGTGAAGTCGGGATCCGGCC) were inserted into the compatible ends. The resulting plasmid, pGEM5-parB*, was digested with BamHI and SacI and inserted into the BamHI-SacI sites of pUCGMA49 to produce plasmid pUCGMA49A−. A SalI-EcoRI fragment from plasmid pGMA27SB (this work), which is a pUC18 derivative carrying the parB, parC, and parD genes on the SalI-BamHI fragment from pGMA27 (15), was isolated and inserted into SalI-EcoRI-digested pUCGMA49A−. We designated the resulting plasmid pCESH205. pCESH205 was digested with AflIII, filled in, and linked with HindIII to remove parD and to add a HindIII site (pCE95). The HindIII fragment carrying the par region with the deletion in parA was isolated and inserted into pCE61 digested with HindIII. The resulting construct was designated pCE61-95. The pCESH series of pUC18 derivatives was checked for the integrity of the downstream genes by an in vitro assay for ParB nuclease activity (17a). The integrity of the deletion derivatives of the RK2 plasmid was examined by an in vivo assay for ParA resolvase activity (33).
Plasmid pEKA28 (36c) is a tetracycline-resistant derivative of plasmid pMHL3 (a derivative of plasmid RSF1010) and was constructed by inserting the tetracycline resistance gene into the unique HindIII site of pMHL3. pEKA30 (5) is a derivative of pEKA28 which contains the cre gene of P1 inserted into the unique EcoRI site of pEKA28. pEKA28 and pEKA30 were kindly provided by E. Sia and D. Figurski. Gentamicin-resistant plasmids were constructed by insertion of the gentamicin resistance gene carried on a HindIII fragment from plasmid pUC19-G/S (41). Plasmid pUC19-G/S is a derivative of pUC19 which contains the gentamicin resistance gene (36) cloned into the multiple cloning region of pUC19. Plasmids pEKA28G and pEKA30G, gentamicin-resistant derivatives of plasmids pEKA28 and pEKA30, respectively (5), were constructed by digestion with HindIII and insertion of the gentamicin resistance gene carried on a HindIII fragment from pUC19-G/S.
Conjugal plasmid transfer.
Plasmid pCE61 and its derivatives were established in P. aeruginosa PAC452Rifr and PAO1161Rifr by conjugal mating with E. coli DH5α carrying both the specific pCE61 derivative and pVW8703 (pUC8-Cm derivative and source of the TraG protein) (42). pCE61 and its derivatives carry a mutation in traG and are therefore unable to transfer unless TraG is provided in trans. Exconjugants were selected with NB agar containing rifampin, spectinomycin, and carbenicillin.
Plasmid stabilization assays.
Stabilization assays were previously described (34). Overnight liquid cultures of E. coli or P. aeruginosa containing the various RK2 derivatives were grown under antibiotic selection at 30 or 37°C. A portion of each culture was diluted 104- to 106-fold in prewarmed LB broth for E. coli or NB for P. aeruginosa and grown under antibiotic selection to the mid-log phase at 30 or 37°C. The remaining portion of each overnight culture was used to isolate plasmid DNA to confirm the presence and integrity of the particular plasmid. At time zero, cells were diluted into antibiotic-free LB broth or NB and maintained without selection for 50 to 200 generations of log-phase growth. Portions of the cell cultures were plated onto antibiotic-free LB agar for E. coli strains or antibiotic-free NB agar for P. aeruginosa strains and then replica picked onto LB or NB agar with and without the appropriate antibiotic(s) to determine the percentage of cells maintaining the plasmid. The percent plasmid loss per generation was calculated as described previously (12c).
Luciferase assay.
E. coli MV10 (the tetracycline-sensitive parent of MV10Δlac) and RR1 carrying the various RK2 plasmids were transformed with plasmids pAL4000 and pTD10 (12, 16). Strains were grown to the mid-log phase at 30°C, and luciferase assays were carried out with a luciferase assay system from Promega (product no. E1500) and following manufacturer recommendations. Activity was measured with a Monolight 2001 luminometer (Analytical Luminescence Laboratories, San Diego, Calif.).
Analysis of plasmids carrying the loxP/Cre system of P1.
The ability of the P1 multimer resolution system loxP/Cre to stabilize pCE61 and its derivatives was tested as follows. Plasmid pCE61 contains the loxP site of P1 (5, 37). pCE61 was established in E. coli RR1 along with the RSF1010 plasmids pEKA28 (vector) and pEKA30 (vector with Cre protein expressed from its native promoter). pEKA28G and pEKA30G (constructed as described above) were established in E. coli MV10Δlac and MC1061K along with pCE61. E. coli MV10Δlac and MC1061K containing plasmids pCE61-2.3 and pCE61-91, carrying the parCBA and parA genes, respectively, along with either pEKA28 or pEKA28G were also constructed. Stability assays were carried out as described above with LB medium at 30°C, except that selection was maintained on the pEKA28 and pEKA30 plasmids or their gentamicin-resistant derivatives. The stability of the pCE61 plasmid and its derivatives in the presence of the pEKA28 and pEKA30 plasmids or their gentamicin-resistant derivatives was assessed by replica plating onto LB agar plates containing spectinomycin and calculating the percentage of cells retaining the various pCE61 plasmids.
RESULTS
Effects of deletions within the three structural genes of the parCBA operon on RK2 maintenance in three E. coli strains.
Previous studies that used the intact RK2 plasmid examined the contributions to stability of the intact par region and the parCBA and parDE operons separately (12c, 37). These earlier studies showed that the intact par region provided a high level of stability to RK2 under all growth conditions and in all strains tested. The abilities of the two individual par operons to stabilize RK2 were dependent on the strain and growth conditions. To determine the requirement of each of the three genes within the parCBA operon for the stabilization of RK2 maintenance by this operon, deletion derivatives of the parCBA operon were constructed and inserted into plasmid pCE61 (Fig. 1). Since the three genes in the parCBA operon are translationally coupled (15), the deletions were constructed in a manner that maintained the translational reading frame of each gene containing a deletion. Plasmid pCE61 itself is a derivative of RK2 with a deletion of the entire par region and containing defective traG, so that it is incapable of conjugal transfer. The stability of pCE61 with the various inserts was tested in three E. coli strains, MC1061K, RR1, and MV10Δlac. Previously, it was shown that the intact parCBA operon by itself was very effective in stabilizing pCE61 in MC1061K but relatively ineffective in MV10Δlac. When various deletion derivatives of the parCBA operon were tested in MC1061K, there was a substantial reduction of stabilization as a result of a deletion in parC or parB and a total loss of stabilization with a parA deletion (Table 2). For E. coli RR1, the intact parCBA operon by itself, as in the case of MC1061K, provided a high level of stable maintenance of plasmid pCE61. In this strain, however, a deletion within the parC or parB gene had relatively little effect on the stabilization activity of the parCBA operon (Table 2). However, deletions in both the parB and the parC genes significantly reduced the effectiveness of the parCBA operon. Finally, the stability of pCE61 carrying intact parCBA and deletion derivatives of parCBA was tested with E. coli MV10Δlac. As shown before (37), the parCBA operon was found to be relatively ineffective in stabilizing pCE61 in this E. coli strain. A deletion in parC, parB, or both had no effect on the low level of stabilization seen with the parCBA operon, and a deletion in parA resulted in a complete loss of parCBA stabilization activity. Thus, in two strains in which the parCBA operon was effective, MC1061K and RR1, deletions in parB, parC, or both somewhat reduced (approximately twofold) the stabilization activity of the operon, while a deletion in parA resulted in a much greater loss of stabilization activity.
FIG. 1.
RK2 derivatives carrying different deletions in the par region. The site of insertion of the fragments carrying deletions in the par region was at the HindIII sequence within the kanamycin resistance gene, as described in Materials and Methods. Insertions at this site result in inactivation of the kanamycin resistance gene. The orientation of the insert in each case is not known. The positions of the various deletions have been described by Gerlitz et al. (15).
TABLE 2.
Stability of RK2 plasmids carrying deletion derivatives in three E. coli strains
| Strain | Rate of loss (% per generation)a of:
|
|||||
|---|---|---|---|---|---|---|
| pCE61 (parCBA) | pCE91-2.3 (parC+B+A+) | pCE61-91 (parCBA+) | pCE61-92 (parC+BA+) | pCE61-94 (parCB+A+) | pCE61-95 (parC+B+A) | |
| MC1061K | 0.65 | 0.04 | 0.27 | 0.23 | 0.22 | 0.57 |
| RR1 | 0.52 | 0.07 | 0.19 | 0.03 | 0.04 | 0.35 |
| MV10Δlac | 0.85 | 0.45 | 0.33 | 0.35 | 0.33 | 0.88 |
Stability assays were carried out at 30°C for an average of 50 to 100 generations. The results are from a single experiment; however, essentially similar results were obtained when the experiment was repeated two more times.
Contributions of the parCBA region to stability in two strains of P. aeruginosa.
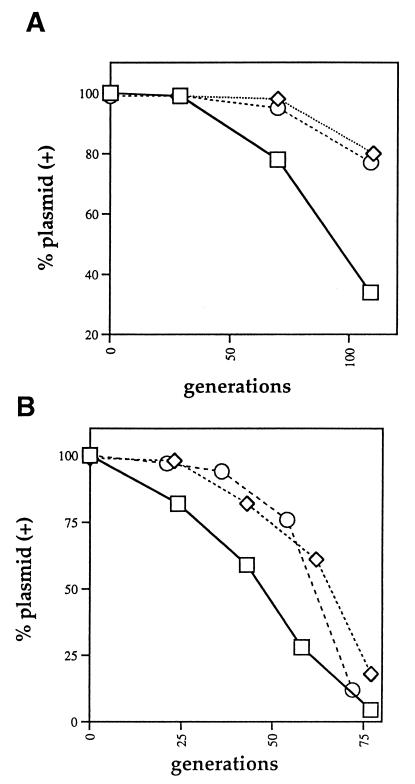
Since RK2 is a broad-host-range plasmid with the ability to be stably maintained in a wide range of gram-negative bacteria, we decided to test the effect of deletions in both the parB and the parC genes on parCBA activity in two strains of P. aeruginosa, PAC452Rifr and PAO1161Rifr. pCE61 and its derivatives were established in each strain, and the stability of the plasmid was assessed over 75 to 100 generations. For strain PAC452Rifr, the parCBA operon or a parC parB parA+ deletion derivative provided a significant level of stabilization (Fig. 2A). After 100 generations, greater than 75% of the cells retained the plasmid carrying parC+ parB+ parA+ (pCE61-2.3) or parC parB parA+ (pCE61-91). For strain PAO1161Rifr, neither parCBA nor parC parB parA+ was able to provide a substantial level of stabilization (Fig. 2B). Less than 25% of the cells contained the plasmid after approximately 75 generations of growth. It is of interest that in both of these strains, the intact par region (parCBA and parDE) functioned very well in stabilizing the pCE61 plasmid in that greater than 95% of the cells retained the plasmid after 100 generations of growth (data not shown).
FIG. 2.
Stabilization of RK2 by different par regions in two strains of P. aeruginosa. (A) Stability in P. aeruginosa PAC452Rifr at 37°C in NB. (B) Stability in P. aeruginosa PAO1161Rifr at 37°C in NB. Symbols: □, pCE61; ◊, pCE61-2.3 (parC+B+A+); ○, pCE61-91 (parCBA+).
Assessing ParA levels in vivo for the various deletion constructs.
Various constructs that contained a deletion in either parB or parC or both were designed to minimize the effects of the deletions on the downstream expression of ParA levels. This fact is of importance to ensure that any effect of a deletion in the upstream parC and parB genes is not the result of a change in the amount of ParA provided by the translationally coupled operon. To test the activity and relative amounts of ParA produced by the various deletion derivatives, we used a luciferase assay. Plasmid pTD10 is an RSF1010 derivative that carries the luciferase gene fused to the parCBA promoter (12, 16). It has been shown that the amount of luciferase activity expressed from the promoter of this fusion construct is a function of the level in vivo of the ParA protein. The ParA protein represses the activity of this promoter by binding to the operator region (12). pTD10 was established in E. coli MV10 and RR1 along with various pCE61 plasmid derivatives (E. coli MV10Δlac is tetracycline resistant; therefore, its tetracycline-sensitive parent, MV10, was used). As shown in Table 3, the level of ParA produced by the intact parCBA operon substantially reduced the level of expression of luciferase in both E. coli RR1 and MV10 (compare pCE61 and pCE61-2.3). Constructs having a deletion in parC (pCE61-94), parB (pCE61-92), or both parB and parC (pCE61-91) similarly resulted in a substantial reduction of luciferase expression by plasmid pTD10, indicating that these deletion constructs produced levels of ParA comparable to that produced by the intact parCBA construct. Not surprisingly, the construct with a deletion in parA failed to repress luciferase expression. Based upon the results obtained with these two E. coli strains, it appears that the functional parC and parB genes are directly responsible for the loss of the stabilization activity of the parCBA operon for plasmid RK2 in strain RR1, as deletions in the upstream genes do not result in a reduction of ParA levels.
TABLE 3.
Use of a parCBA promoter and a luciferase fusion construct to measure ParA levels provided by various deletion constructs
| Plasmids | Stabilization region | Luciferase unitsa of E. coli
|
|
|---|---|---|---|
| RR1 | MV10 | ||
| pCE61 + pAL4000 | None | 251 | 131 |
| pCE61 + pTD10 | None | 2,024 | 1,443 |
| pCE61-2.3 + pTD10 | parCBA | 619 | 383 |
| pCE61-91 + pTD10 | parA | 652 | 422 |
| pCE61-92 + pTD10 | parBA | 491 | 473 |
| pCE61-94 + pTD10 | parCA | 164 | 950 |
| pCE61-95 + pTD10 | parCB | 2,092 | 2,693 |
Luciferase units were calculated as (reading 1 + reading 2)/200(optical density at 600 nm).
Stability of the plasmid RK2 in a topA E. coli strain.
Previous studies with minireplicons of the pSC101, F, and P1 plasmids with deletions of a stabilization region revealed increased levels of plasmid stability when the plasmids were carried in an E. coli topA mutant (4, 6, 10, 11). It has been proposed that the increased negative superhelicity of plasmid DNA as a result of a deficiency in the type I topoisomerase (TopA) enzyme in a topA mutant results in improved distribution of the plasmid to daughter cells during cell division (4, 6, 10, 11). It was previously demonstrated that ParA binds to the res site at three distinguishable positions (13). To assess whether or not the increased stabilization of RK2 by the parCBA operon, including its res site, in E. coli is due to a superhelical change in the DNA as a result of binding of the ParA protein and/or the ParB and ParC proteins to the plasmid, the effect of a topA mutation on RK2 stabilization was tested. The par deletion plasmid pCE61 was established in the E. coli topA strain DPB636 and the isogenic topA+ parent DPB635, and the rates of plasmid loss were compared. There was no increase in the stability of the pCE61 plasmid in strain DPB636 (0.32% loss per generation in pDB635 versus 0.46% loss in DB636). Since, unlike the case with pSC101, F, and P1, the presumed increase in the negative superhelicity of RK2 in the topA mutant did not increase the stability of the plasmid, it is unlikely that the stabilization seen with parA and its res site is due to a change in RK2 superhelicity as a result of binding of the ParA protein and/or the other Par proteins.
Resolvase activity contributes to RK2 stabilization.
The resolvase activity of ParA suggests that the stabilization of RK2 observed when ParA and its res site are inserted into pCE61 is due to the resolution of dimers and/or higher-multimer forms of the plasmid. It was previously observed that the cer multimer resolution system, when inserted into pCE61, did not increase the stability of the plasmid in E. coli MC1061K (12c). To explore further the role of resolvase activity in plasmid stabilization, we took advantage of the fact that plasmid pCE61 was constructed with a vector excision system which necessitated replacing the par region with a spectinomycin resistance gene cassette and the loxP site of prophage P1 (5). Thus, by supplying the Cre recombinase protein in trans from a compatible plasmid, it was possible to determine if multimer resolution via this system was able to stabilize pCE61. pCE61 was established in E. coli RR1, MC1061K, and MV10Δlac along with plasmid pEKA30G, which is a derivative of the compatible plasmid RSF1010 containing the cre gene. In addition, pCE61 and the RSF1010 vector without the cre gene were established in all three strains as a control. In all three strains, the rate of loss of pCE61 was higher than previously observed for this plasmid in the absence of the RSF1010 vector (Tables 1 and 4). This finding may have been due to a low level of incompatibility between the two plasmids. Nevertheless, it is clear that the loxP/Cre system provided a high level of stability for pCE61 in all three E. coli strains (Table 4). Because of this surprising result with the loxP/Cre system and in view of the earlier observation that the cer system did not stabilize pCE61 in MC1061K, the effect of inserting the cer system into RK2 was reinvestigated with MC1061K and examined with E. coli MV10Δlac and RR1. As shown in Table 4, the cer system once again failed to stabilize pCE61 in MC1061K; however, this multimer resolution system was effective in stabilizing the plasmid in RR1. As in the case of MC1061K, insertion of the cer site into RK2 did not improve its stability in MV10Δlac. That the cer system was capable of resolving multimers in all three strains was shown in an earlier study (12c).
TABLE 4.
Effect of insertion of the multimer resolution systems of plasmids P1 and ColE1 on the stable maintenance of RK2 with deletions of the par region
| Plasmid(s) | Resolvase system | Rate of loss (% per generation)a with strain
|
||
|---|---|---|---|---|
| MC1061K | MV10Δlac | RR1b | ||
| pCE61 + pEKA28G | None | 1.50 | 0.45 | 2.71 |
| pCE61 + pEKA30G | loxP/Cre | 0.13 | 0.02 | 0.14 |
| pCE61 cer | cer | 1.24 | 0.83 | 0.05 |
The number of generations ranged from 75 to 90 in LB medium at 30°C. Similar results were obtained when the experiment was repeated.
Plasmids pEKA28 and pEKA30 were used in trans in this strain instead of plasmids pEKA28G and pEKA30G.
DISCUSSION
The par region of plasmid RK2, consisting of two divergently oriented operons, parCBA and parDE, clearly plays a major role in the stabilization of this broad-host-range plasmid in a growing population of bacteria. While the mechanism of stabilization by parDE is well documented, the role of the parCBA operon in stabilizing this plasmid is still not fully understood. In addition, although the stabilization seen with the complete par region can be accounted for largely by the summation of the activities of the parCBA and parDE operons acting individually, we cannot rule out the possibility that the two operons act synergistically in providing virtually complete stabilization when the par region is intact.
In an attempt to obtain some insight into the mechanism by which parCBA stabilizes RK2, a series of precise deletions were constructed to specifically remove each of the three genes in the operon. Since the three genes are translationally coupled, care was exercised to maintain the reading frames in each of the various deletions. Results from the stability assays were somewhat equivocal with respect to the necessity of the parB and parC genes. In the two E. coli strains in which the parCBA operon is effective in stabilizing RK2, MC1061K and RR1, the inactivation of parB, parC, or both parB and parC caused an approximately twofold reduction in the activity of the operon. However, a deletion in the parA gene caused a much greater loss of parCBA activity. In P. aeruginosa strains, in which parCBA shows a high level of stabilization activity, the inactivation of parB and parC did not diminish the activity observed. These results indicate a key role for the ParA- and res site-specific recombination system in the stabilization activity that is observed for the parCBA operon. However, when the level of ParA produced by the deletion constructs was approximated in two of the E. coli strains by determining the level of repression of the par promoter by ParA, it appeared that the reduced stabilization activity seen when parB, parC, or both genes were inactivated was not due to an effect of the deletions on ParA production.
These various observations do not, of course, provide any insight into the role of parB and parC in the stabilization seen for the intact parCBA operon in E. coli strains. If the stabilization activity of the parCBA operon is due solely to site-specific recombination, ParB and ParC may be accessory proteins that enhance the activity of ParA acting on its res site in resolving dimers and/or higher-multimer forms. The biochemical product of ParA acting on res sites in a dimeric plasmid is a catenate of two monomers (1–3). Additional enzymatic steps are required to separate the monomers. ParB, a calcium-dependent nuclease (17a), and ParC conceivably could play a role in resolving these catenates. Furthermore, differences in the requirements for parB and parC for full activity of the parCBA operon in different hosts may reflect differences in the abilities of different strains to compensate for the loss of parB and/or parC by providing host proteins with comparable activities. Alternatively, ParB and ParC could play an adaptive role in directing the parA-res system to an appropriate site in the bacterium where the multimer resolution system can facilitate segregation of the plasmid to daughter cells. The translational coupling of the three genes in the parCBA operon argues, albeit weakly, for the formation of a specific complex by the three proteins. If this were the case, then, of course, other proteins and even other multimer resolution systems might supplant the components of the parCBA system in a host-dependent manner in facilitating partitioning of the plasmid during cell division.
The high level of effectiveness of the loxP/Cre system in stabilizing plasmid RK2 was surprising in view of the failure in earlier studies of the cer multimer resolution system to stabilize the plasmid in one E. coli strain tested (12c) . In addition, a significant level of RK2 dimers was not observed in the E. coli strains used in this study despite an effort to detect higher forms of RK2 with a procedure which involves direct lysis in agarose gels and which detects high-molecular-weight forms of supercoiled DNA (unpublished observations). Similar results have been reported for pBR322 derivatives containing intact or deletion RP4 par fragments (38). It has been argued, however, that even very low amounts of the dimeric form of a plasmid under certain circumstances can substantially increase the rate of loss of the plasmid (39). It is also worth pointing out that the plasmid RK2 derivative, pCE61, used in this study carries a functional Tn1 transposon that includes an active resolvase system (8, 12b, 17, 30). Grinter et al. (17) showed that deletion of the Tn1 transposon in RK2 had little effect on the stability of the plasmid in comparison with deletion of the par region. The Tn1 system obviously cannot compensate for the loss of the parA-res system in providing for stabilization of the RK2 plasmid. Again, this idea may not be surprising, since even the highly effective ColE1 plasmid cer system was found, unlike the loxP/Cre multimer resolution system, to be active in only one of three E. coli strains in substituting for the loss of the parCBA operon. This finding suggests that the actual level of resolvase activity in a particular strain, the nucleotide sequence context, or perhaps the topological position of the multimer resolution site within the supercoiled plasmid DNA influences the effectiveness of the system in functioning as a resolvase system for stabilizing the plasmid. Studies involving the dif locus of the E. coli chromosome, the site of multimer resolution of the chromosome within the terminus region, demonstrated a position requirement for the functionality of this sequence (26). In addition, the Dif phenotype of an E. coli strain caused by deletion of the dif locus could be suppressed only by replacement with a functional multimer resolution site such as psi (pSC101) or loxP (and Cre) in the same terminus region and not at another site on the chromosome (26). Finally, it is worth emphasizing that studies on the effectiveness of the various multimer resolution systems and the contribution of the parB and parC genes to the effectiveness of the parCBA operon in stabilizing the broad-host-range plasmid RK2 have been limited to relatively few bacterial hosts. A more substantial role for all three genes in stabilizing the RK2 plasmid may be revealed by extension of the analysis to a more diverse set of bacteria.
A much larger question is whether or not the juxtapositioning of the parCBA and parDE operons in the RK2 plasmid is fortuitous or whether it reflects an interaction between the five proteins and their DNA in providing virtually complete stabilization of the plasmid in a wide range of bacterial hosts. One study carried out with whole RK2 and E. coli MV10Δlac failed to show enhancement of parCBA stabilization activity on RK2 when the parDE products were provided in trans from a compatible plasmid (unpublished observations). Again, it will be of interest to extend the analysis to other bacteria to determine if the two operons act synergistically. Finally, all five proteins from these two operons have been purified (13, 17a, 21a, 22, 35) and are now available for determining if the proteins from the two operons interact with each other at the biochemical level. These additional approaches should provide some clarification of whether or not the parCBA products or multimer resolution systems such as cer and loxP/Cre stabilize RK2 only by increasing monomer units (or decreasing the number of potentially destabilizing dimeric forms of the plasmid) or if these systems play a role in a complex plasmid stabilization mechanism that requires as one essential step the resolution of dimeric forms of the plasmid. The view of a more complex function of the RK2 par region is supported by the recent finding that the resolution of dimer chromosomes at the dif locus of E. coli is coupled to cell division (38a). In this hypothetical stabilization system, parDE may work in concert with the parCBA products in providing for the stable maintenance of RK2 in a wide range of bacterial hosts.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-07194 from the National Institutes of Health and by a minority predoctoral fellowship (5 F31 GM 17230-02) from the National Institute of General Medical Sciences to C.L.E. H.S. was supported by a Fulbright fellowship during the course of this study.
We thank Eva Garrett for providing technical assistance and Regina Neves for assistance in the preparation of the manuscript. We also thank Aresa Toukdarian and Rick Roberts for helpful suggestions.
REFERENCES
- 1.Abremski K, Hoess R. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein. J Biol Chem. 1984;259:1509–1514. [PubMed] [Google Scholar]
- 2.Adams D E, Bliska J B, Cozzarelli N R. Cre-lox recombination in Escherichia coli cells. Mechanistic differences from the in vitro reaction. J Mol Biol. 1992;226:661–673. doi: 10.1016/0022-2836(92)90623-r. [DOI] [PubMed] [Google Scholar]
- 3.Adams E A, Shekhtman E M, Zechiedrich E L, Schmid M B, Cozzarelli N R. The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell. 1992;71:277–288. doi: 10.1016/0092-8674(92)90356-h. [DOI] [PubMed] [Google Scholar]
- 4.Austin S J, Ziese M, Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981;25:729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
- 5.Ayres E K, Thompson V J, Merino G, Balders D, Figurski D H. Precise deletions in large bacterial genomes by vector-mediated excision (VEX). The trfA gene of promiscuous plasmid RK2 is essential for replication in several gram-negative hosts. J Mol Biol. 1993;230:174–185. doi: 10.1006/jmbi.1993.1134. [DOI] [PubMed] [Google Scholar]
- 5a.Bagdasarian, M. Unpublished data.
- 6.Biek D P, Cohen S N. Involvement of integration host factor (IHF) in maintenance of plasmid pSC101 replication in Escherichia coli: mutations in the topA gene allow pSC101 replication in the absence of IHF. J Bacteriol. 1989;171:2066–2074. doi: 10.1128/jb.171.4.2066-2074.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bravo A, Sagrario O, deTorrontegoi G, Diaz-Orejas R. Killing of Escherichia coli cells modulated by components of the stability system of ParD of plasmid R1. Mol Gen Genet. 1988;215:146–151. doi: 10.1007/BF00331316. [DOI] [PubMed] [Google Scholar]
- 8.Chen S T, Clowes R C. Variations between the nucleotide sequences of Tn1, Tn2, and Tn3 and expression of β-lactamase in Pseudomonas aeruginosa and Escherichia coli. J Bacteriol. 1987;169:913–916. doi: 10.1128/jb.169.2.913-916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Close S M, Kado C I. A gene near the plasmid pSa origin of replication encodes a nuclease. Mol Microbiol. 1992;6:521–527. doi: 10.1111/j.1365-2958.1992.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 10.Conley D, Cohen S N. Isolation and characterization of plasmid mutations that enable partitioning of pSC101 replicons lacking the partition (par) locus. J Bacteriol. 1995;177:1086–1089. doi: 10.1128/jb.177.4.1086-1089.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conley D L, Cohen S N. Effects of the pSC101 partition (par) locus on in vivo DNA supercoiling near the plasmid replication origin. Nucleic Acids Res. 1995;23:701–707. doi: 10.1093/nar/23.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis T L, Helinski D R, Roberts R C. Transcription and autoregulation of the stabilizing functions of broad-host-range plasmid RK2 in Escherichia coli, Agrobacterium tumefaciens, and Pseudomonas aeruginosa. Mol Microbiol. 1992;6:1981–1994. doi: 10.1111/j.1365-2958.1992.tb01371.x. [DOI] [PubMed] [Google Scholar]
- 12a.Easter C. Ph.D. thesis. University of California, San Diego; 1997. [Google Scholar]
- 12b.Easter, C. Unpublished observations.
- 12c.Easter C L, Sobecky P A, Helinski D R. Contribution of different segments of the par region to stable maintenance of the broad-host-range plasmid RK2. J Bacteriol. 1997;179:6472–6479. doi: 10.1128/jb.179.20.6472-6479.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberl L, Kristensen C S, Givskov M, Grohmann E, Gerlitz M, Schwab H. Analysis of the multimer resolution system encoded by the parCBA operon of broad-host-range plasmid RP4. Mol Microbiol. 1994;12:131–141. doi: 10.1111/j.1365-2958.1994.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerdes K, Poulson L K, Thisted T, Nielsen A K, Martinussen J, Andreasen P H. The hok killer gene family in Gram-negative bacteria. New Biol. 1990;2:946–956. [PubMed] [Google Scholar]
- 15.Gerlitz M, Hrabak O, Schwab H. Partitioning of broad-host-range plasmid RP4 is a complex system involving site-specific recombination. J Bacteriol. 1990;172:6194–6203. doi: 10.1128/jb.172.11.6194-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greener A, Lehman S M, Helinski D R. Promoters of the broad host range plasmid RK2: analysis of transcription (initiation) in five species of Gram-negative bacteria. Genetics. 1992;130:27–36. doi: 10.1093/genetics/130.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grinter N J, Brewster G, Barth P T. Two mechanisms necessary for the stable inheritance of plasmid RP4. Plasmid. 1989;22:203–214. doi: 10.1016/0147-619x(89)90003-6. [DOI] [PubMed] [Google Scholar]
- 17a.Grohmann E, Stanzer T, Schwab H. The parB protein encoded by the RP4 par region is a Ca2+-dependent nuclease linearizing circular DNA substrates. Microbiology. 1997;143:3889–3898. doi: 10.1099/00221287-143-12-3889. [DOI] [PubMed] [Google Scholar]
- 18.Helinski D R, Toukdarian A E, Novick R P. Replication control and other stable maintenance mechanisms of plasmids. In: Niedhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: American Society for Microbiology; 1996. pp. 2295–2324. [Google Scholar]
- 19.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe A, Ogura T, Hiraga S. Effects of the ccd function of the F plasmid on bacterial growth. J Bacteriol. 1985;163:841–849. doi: 10.1128/jb.163.3.841-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen R B, Grohmann E, Schwab H, Diaz-Orejas R, Gerdes K. Comparison of ccd of F, parDE of RP4, and parD of R1 using novel conditional replication control system of plasmid R1. Mol Microbiol. 1995;17:211–221. doi: 10.1111/j.1365-2958.1995.mmi_17020211.x. [DOI] [PubMed] [Google Scholar]
- 21a.Johnson, E. Unpublished data.
- 22.Johnson E P, Strom A, Helinski D R. Plasmid RK2 toxin protein ParE: purification and interaction with the ParD protein. J Bacteriol. 1996;178:1420–1429. doi: 10.1128/jb.178.5.1420-1429.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn M, Ow D, Sauer R, Rabinowitz A, Calendar R. Genetic analysis of bacteriophage P4 using P4-plasmid ColE1 hybrids. Mol Gen Genet. 1980;177:399–412. doi: 10.1007/BF00271478. [DOI] [PubMed] [Google Scholar]
- 24.Lehnerr H, Maguin E, Jafri S, Yarmolinsky M B. Plasmid addiction genes of bacteriophage P1: doc, which causes cell death on curing of prophage, and phd, which prevents host death when prophage is retained. J Mol Biol. 1993;233:414–423. doi: 10.1006/jmbi.1993.1521. [DOI] [PubMed] [Google Scholar]
- 25.Lehnerr L, Yarmolinsky M. Addiction protein Phd of plasmid prophage P1 is substrate of the ClpXP serine protease of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:3274–3277. doi: 10.1073/pnas.92.8.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leslie N R, Sheratt D J. Site-specific recombination in the replication terminus region of Escherichia coli: functional replacement of dif. EMBO J. 1995;14:1561–1570. doi: 10.1002/j.1460-2075.1995.tb07142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 28.Miki T, Park J A, Nagao K, Murayama N, Horiuchi T. Control of segregation of chromosomal DNA by sex factor F in Escherichia coli. Mutants of DNA gyrase subunit A suppress letD(ccdB) product growth inhibition. J Mol Biol. 1992;225:39–52. doi: 10.1016/0022-2836(92)91024-j. [DOI] [PubMed] [Google Scholar]
- 29.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 30.Pansegrau W, Lanka E, Barth P T, Figurski D H, Guiney D G, Haas D, Helinski D R, Schwab H, Stanisich V A, Thomas C M. Complete nucleotide sequence of Birmingham IncPalpha plasmids. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 31.Prince A S, Barlam T. Isolation of a DNA fragment containing replication functions from IncP2 megaplasmid pMG2. J Bacteriol. 1985;161:792–794. doi: 10.1128/jb.161.2.792-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raleigh E A, Lech K, Brent R. In: Current protocols in molecular biology. Ausubel F M, et al., editors. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1989. pp. 1190–1219. [Google Scholar]
- 33.Roberts R C, Burioni R, Helinski D R. Genetic characterization of the stability functions of a region of broad-host-range plasmid RK2. J Bacteriol. 1990;172:6204–6216. doi: 10.1128/jb.172.11.6204-6216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts R C, Helinski D R. Definition of a minimal plasmid stabilization system from the broad-host-range plasmid RK2. J Bacteriol. 1992;174:8119–8132. doi: 10.1128/jb.174.24.8119-8132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts R C, Strom A R, Helinski D R. The parDE operon of the broad-host-range plasmid RK2 specifies growth inhibition associated with plasmid loss. J Mol Biol. 1994;237:35–51. doi: 10.1006/jmbi.1994.1207. [DOI] [PubMed] [Google Scholar]
- 36.Rubin R A. Genetic analysis of the gentamicin resistance region of pPH1JI incorporation into a wide host range cloning vehicle. Plasmid. 1987;18:84–88. doi: 10.1016/0147-619x(87)90081-3. [DOI] [PubMed] [Google Scholar]
- 36a.Schwab, H. Unpublished data.
- 36b.Sia, E. Unpublished data.
- 36c.Sia, E., and D. Figurski. Unpublished data.
- 37.Sia E A, Roberts R C, Easter C E, Helinski D R, Figurski D H. Different relative importances of the par operons and the effect of conjugal transfer on the maintenance of intact promiscuous plasmid RK2. J Bacteriol. 1995;177:2789–2797. doi: 10.1128/jb.177.10.2789-2797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sobecky P A, Easter C L, Bear P D, Helinski D R. Characterization of the stable maintenance properties of the par region of broad-host-range plasmid RK2. J Bacteriol. 1996;178:2086–2093. doi: 10.1128/jb.178.7.2086-2093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Steiner W W, Kuempel P L. Cell division is required for resolution of dimer chromosomes at the dif locus of Escherichia coli. Mol Microbiol. 1998;27:257–268. doi: 10.1046/j.1365-2958.1998.00651.x. [DOI] [PubMed] [Google Scholar]
- 39.Summers D K, Beton C W H, Withers H L. Multicopy plasmid instability: the dimer catastrophe hypothesis. Mol Microbiol. 1993;8:1031–1038. doi: 10.1111/j.1365-2958.1993.tb01648.x. [DOI] [PubMed] [Google Scholar]
- 40.Thomas C M. Recent studies on control of plasmid replication. Biochim Biophys Acta. 1988;949:253–263. doi: 10.1016/0167-4781(88)90150-9. [DOI] [PubMed] [Google Scholar]
- 41.Toukdarian, A. Unpublished data.
- 42.Waters, G. Unpublished data.