Abstract
Antibody-drug conjugates (ADCs) are a class of innovative biopharmaceutical drugs, which, via their antibody (mAb) component, deliver and release their potent warhead (a.k.a. payload) at the disease site, thereby simultaneously improving the efficacy of delivered therapy and reducing its off-target toxicity. To design ADCs of promising efficacy, it is crucial to have the critical data of pharma-information and biological activities for each ADC. However, no such database has been constructed yet. In this study, a database named ADCdb focusing on providing ADC information (especially its pharma-information and biological activities) from multiple perspectives was thus developed. Particularly, a total of 6572 ADCs (359 approved by FDA or in clinical trial pipeline, 501 in preclinical test, 819 with in-vivo testing data, 1868 with cell line/target testing data, 3025 without in-vivo/cell line/target testing data) together with their explicit pharma-information was collected and provided. Moreover, a total of 9171 literature-reported activities were discovered, which were identified from diverse clinical trial pipelines, model organisms, patient/cell-derived xenograft models, etc. Due to the significance of ADCs and their relevant data, this new database was expected to attract broad interests from diverse research fields of current biopharmaceutical drug discovery. The ADCdb is now publicly accessible at: https://idrblab.org/adcdb/.
Graphical Abstract
Graphical Abstract.
Introduction
Antibody-drug conjugates (ADCs) are a class of innovative biopharmaceutical drugs, which, via their antibody (mAb) component, deliver and release their potent warhead (a.k.a. payload) at the disease site, thereby simultaneously improving the efficacy of delivered therapy and reducing its off-target toxicity (1). ADCs are recently known as ‘magic biological missiles’ that are expected to open a new era of therapeutic revolution for the targeted anti-cancer therapies (2). As reported, a typical ADC is based on the combination of a mAb that is selective to tumor-associated antigen (with the distinctively good biological activity), a linker that is stable in blood circulation yet is readily cleavable at the target site, and a payload with good biological activity inducing the death of disease cells (3). Moreover, detailed pharma-information of ADCs and their main components (mAb, linker and payload) are of great importance for the modern discovery of biopharmaceutical drugs, which include structures, conjugating type, targeted antigens, therapeutic targets, druglike properties of payload and so on (4). To design the ADC of clinical therapeutic potential, it is key to have those valuable data of biological activity and pharma-information for each ADC.
So far, a variety of knowledgebases have been developed to provide the ADC-related information. Some of them offer the general data of interacting network, disease indication, therapy types for very limited number (<60) of ADCs as part of a broader collection of biological/chemical information, such as Drugs@FDA (5), NCATS Inxights Drugs (6), DrugMAP (7), ChEMBL (8), DrugBank (9) and so on; some others aim at describing the particular components of each ADC, which include (a) those offering mAb moiety, such as: IMGT/mAb-DB (10), Thera-SAbDab (11) and PDB (12), and (b) those showing the physicochemical properties of either linkers or payloads, such as: PubChem (13) and DrugCentral (14). These databases above have attracted considerable attention from the related research communities. However, none of them specializes in providing the biological activity and detailed pharma-information of ADCs, and a knowledgebase that can comprehensively describe such valuable data is therefore highly demanded.
Herein, a database, named ‘ADCdb’, focusing on describing the biological activities and detailed pharma-information of ADC was therefore developed. First, the biological activity of each ADC was collected using a systematic literature review in PubMed, which led to a total of 6572 ADCs (359 approved by FDA or in clinical trial pipelines, 501 in the preclinical tests, 819 with in-vivo testing data, 1868 with only cell line/target testing data and 3025 with no in-vivo/cell line/target testing information). Second, subsequent analyses on the identified ADCs further discovered that, apart from the most popular ADC therapeutic area cancer, the ADCs collected to ADCdb covered many additional therapeutic areas, including arthritis, atherosclerosis, bacteremia, HIV infection, etc. Moreover, the collected ADCs covered a total of 1168 antibodies, 511 linkers, 447 payloads, 327 antigens and 54 targets. Third, a total of 9171 literature-published biological activities were discovered. Particularly, 739 biological activities were identified from 447 clinical trial pipelines; 587 activities were reported based on 270 patient-derived xenograft models; 2013 activities were acquired from 311 cell line-derived xenograft models; 5725 activities were revealed by 737 cell line data; and 107 activities from diverse model organisms. Finally, all the ADC data were fully cross-linked to a variety of well-established molecular biology databases, such as DrugMAP (10), ChEMBL (8), DrugBank (9), PubChem (13), TTD (15) and so on. To the best of our knowledge, ADCdb is the first ADC knowledgebase that had ever been developed. Due to the importance of the biological activity and pharma-information of ADC and the severe lack of such valuable data in existing databases, this new database is highly expected to attract broad research interests from diverse cutting-edge directions of current biopharmaceutical drug discovery. ADCdb is now free and open to all users without login requirement at: https://idrblab.org/adcdb/
Factual content and data retrieval
Systematic collection of antibody–drug conjugate (ADC) information
Explicit pharma-information of antibody–drug conjugates (ADCs) together with their biological activity data were collected by the following procedures. First, a comprehensive literature review in diverse official patent organizations (such as World Intellectual Property Organization, United States Patent and Trademark Office, European Patent Office and so on), various R&D pipelines of hundreds of pharmaceutical companies and many literature databases (such as PubMed) was conducted by searching such keywords as ‘antibody–drug conjugate’, ‘ADC’, etc. This literature review identified a total of 6572 ADCs (which comprised 359 approved by FDA/in clinical trials, 501 in preclinical tests and 5712 investigative agents). Particularly, a full list of approved ADCs was explicitly described in Table 1. Second, the pharma-information of the ADCs, together with their three main components (mAb, linker, payload), were systematically extracted from diverse publications identified based on literature reviews. Such information contained therapeutic class & disease indication (together with the corresponding clinical status), structure, conjugating type, targeted antigen, drug-like properties of payload, etc. Third, the biological activity of these ADCs was further retrieved using comprehensive literature review in diverse literature knowledgebases (such as PubMed) based on such keyword combinations as ‘antibody–drug conjugate + biological activity’, ‘ADC name + activity’, etc. Furthermore, for patent-protected ADCs, their bioactivity data were collected from both literature review and the corresponding patents. As a result, a total of 9171 biological activities were collected, including 739 from clinical trial, 2707 from in-vivo model and 5725 from cell line data. ADCdb covered the most diverse set of pharma-information and biological activities for the largest number of ADCs among all existing databases. Moreover, the disease classes of these collected data were very diverse, which included not only cancer but also many other diseases (such as arthritis, atherosclerosis, bacteremia, HIV infection, etc.).
Table 1.
A list of worldwide antibody–drug conjugates that have been approved for clinical use (as of August 2023, in a reverse chronological order of their approval dates)
| ADC Name (developing company) | Antigen | Linker | Payload | Target | Approval office (approved date) | Approved disease indications |
|---|---|---|---|---|---|---|
| Mirvetuximab soravtansine (ImmunoGen) | FRα | Sulfo-SPDB (cleavable) | DM4 | DNA (human) | U.S. FDA (Nov, 2022) | FRα-positive epithelial ovarian cancer |
| Tisotumab vedotin (Seagen) | TF | Mc-Val-Cit-PABC (cleavable) | MMAE | Microtubule (human) | U.S. FDA (Sep, 2021) | Recurrent or metastatic cervical cancer |
| Disitamab vedotin (RemeGen) | HER2 | Mc-Val-Cit-PABC (cleavable) | MMAE | Microtubule (human) | China NMPA (Jun, 2021) | Advanced or metastatic gastric cancer |
| Loncastuximab tesirine (ADC Therapeutics) | CD19 | Mal-PEG8-Val-Ala- PABC (cleavable) | SG3199 | DNA (human) | U.S. FDA (Apr, 2021) | Relapsed or refractory large B-cell lymphoma |
| Cetuximab sarotalocan (Rakuten Medical) | EGFR | Linear alkyl/alkoxy linker (uncleavable) | IRDye 700DX | not available | Japan PMDA (Sep, 2020) | Advanced or recurrent head and neck cancer |
| Belantamab mafodotin (GlaxoSmithKline) | BCMA | Maleimido-caproyl (uncleavable) | MMAF | Microtubule (human) | U.S. FDA (Aug, 2020) | Relapsed or refractory multiple myeloma |
| Sacituzumab govitecan (Gilead Sciences) | TROP2 | CL2A (cleavable) | SN38 | TOP1 (human) | U.S. FDA (Apr, 2020) | Metastatic triple-negative breast cancer |
| Enfortumab vedotin (Astellas) | Nectin-4 | Mc-Val-Cit-PABC (cleavable) | MMAE | Microtubule (human) | U.S. FDA (Dec, 2019) | Advanced or metastatic urothelial cancer |
| Trastuzumab deruxtecan (Daiichi Sankyo) | HER2 | Mc-Gly-Gly-Phe- Gly (cleavable) | DXd | TOP1 (human) | U.S. FDA (Dec, 2019) | Unresectable HER2-positive breast cancer |
| Polatuzumab vedotin (Roche) | CD79b | Mc-Val-Cit-PABC (cleavable) | MMAE | Microtubule (human) | U.S. FDA (Jun, 2019) | Diffuse large B-cell lymphoma |
| Moxetumomab pasudotox (AstraZeneca) | CD22 | Mc-Val-Cit-PABC (cleavable) | PE38 | EEF2K (human) | U.S. FDA (Sep, 2018) | Relapsed or refractory hairy cell leukemia |
| Inotuzumab ozogamicin (Pfizer) | CD22 | AcButDMH (cleavable) | N-acetyl-γ-calicheamicin | DNA (human) | U.S. FDA (Aug, 2017) | B-cell precursor acute lymphoblastic leukemia |
| Trastuzumab emtansine (Roche) | HER2 | SMCC (uncleavable) | DM1 | Microtubule (human) | U.S. FDA (Feb, 2013) | HER2-positive metastatic breast cancer |
| Brentuximab vedotin (Seagen) | CD30 | Mc-Val-Cit-PABC (cleavable) | MMAE | Microtubule (human) | U.S. FDA (Aug, 2011) | Hodgkin lymphoma; Large-cell lymphoma |
| Gemtuzumab ozogamicin (Pfizer) | CD33 | AcButDMH (cleavable) | N-acetyl-γ-calicheamicin | DNA (human) | U.S. FDA (Mar, 2000) | CD33-positive acute myeloid leukemia |
AcButDMH: AcBut acyl hydrazonedisulfide; BCMA: B-cell maturation antigen; CD19: B-lymphocyte antigen CD19; CD22: B-cell receptor CD22; CD30: lymphocyte activation antigen CD30; CD33: myeloid cell surface antigen CD33; CD79b: immunoglobulin-associated beta; DM1: derivative of maytansine 1; DM4: derivative of maytansine 4; DXd: DX-8951 derivative; EEF2K: eukaryotic elongation factor 2 kinase; EGFR: epidermal growth factor receptor; TF: tissue factor; FRα: folate receptor alpha; HER2: human epidermal growth factor receptor 2; Mc-Gly-Gly-Phe-Gly: maleimidocaproyl-glycine-phenylalanine-glycine- glycine; Mc-Val-Cit-PABC: maleimidocaproyl-valine-citrulline-p-aminobenzoyloxycarbonyl; MMAE: monomethyl auristatin-E; MMAF: monomethyl auristatin-F; Nectin-4: Nectin cell adhesion molecule 4; PE38: 38kD fragment of Pseudomonas exotoxin A; SMCC: succinimidyl‐4‐(N‐maleimidomethyl)cyclohexane‐1‐carboxylate; SN38: active metabolite of irinotecan; Sulfo-SPDB: sulfonyl-N-succinimidyl 4-(2-pyridyldithio) butyrate; TOP1: DNA topoisomerase 1; TROP2: tumor-associated calcium signal transducer 2.
The approved administrations/offices included: U.S. Food and Drug Administration (U.S. FDA), National Medical Products Administration of China (China NMPA) and Pharmaceuticals and Medical Devices Agency of Japan (Japan PMDA).
Explicit description on the pharma-information of each studied ADC
The development of novel ADC was frequently inspired by the valuable pharma-information of the pre-existed ADCs (16–18). Such information included: the aimed disease indications together with their corresponding clinical status, ADC’s structure describing antibody, linker and payload, diverse clinical response data (such as the clinical details of both tested ADC and enrolled patient, the reported ADMET properties of ADC, administration dosage, reported adverse drug reactions and clinical primary endpoints), drug-antibody ratio of each studied ADC (19–24). Moreover, the pharma-information of three components (mAb, linker and payload) of each studied ADC should also be explicitly described in any ADC-related pharmaceutical knowledgebase, since these data were discovered to be valuable for the clinical evaluations of ADC’s therapeutic window (25,26), the differentiation of ADCs’ sensitivities among various indications (27–29), the identification of ADC’s drug-like characteristics (30) and the determination of conjugation strategy and payload release mechanism of studied ADCs (31–35). All in all, such comprehensive pharma-information was crucial for the design and iterative development of ADCs (3), which were thus systematically described in our newly developed online database ADCdb (https://idrblab.org/adcdb/).
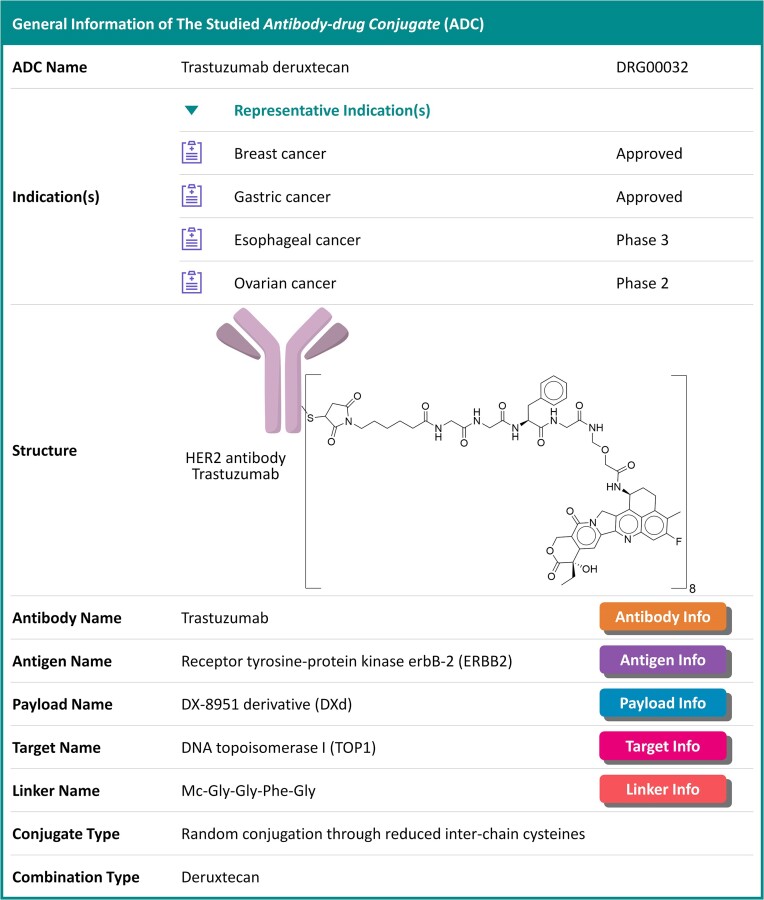
In ADCdb, detailed pharma-information of each ADC were explicitly provided. As illustrated in Figure 1 (the webpage of a well-known ADC trastuzumab deruxtecan), the pharma-information provided on this page included: ADC name, ADC synonyms, aimed disease & the corresponding clinical status, ADC structure (downloadable in 2D & 3D formats), ADC components (antibody, linker & payload), the antigen of antibody, the target of payload, antibody-linker conjugate type, linker-payload combination type and various external links to many other established molecular biological databases including PubChem (13), DrugMAP (10), TTD (15), Chembl (8), DrugBank (9) and so on (36–39). To have a detailed description on the pharma-information provided in the ADCdb, explicit illustration on such important information was given. As illustrated in the upper section of Figure 2, detailed data of ADC were described: the aimed disease indications together with their corresponding clinical status, ADC’s structure describing antibody, linker and payload, diverse clinical response data (such as the clinical details of both tested ADC and enrolled patient, the reported ADMET properties of ADC, administration dosage, reported adverse drug reactions and clinical primary endpoints), drug-antibody ratio of each studied ADC, etc. Such information could be an indispensable complement to currently available pharmaceutical databases.
Figure 1.
A typical webpage showing the general information of ADC (trastuzumab deruxtecan). Diverse pharma-information were provided, which included: ADC name, ADC synonym, aimed disease and the corresponding clinical status, ADC structures (downloadable in 2D & 3D format), key ADC components (antibody, linker & payload), the antigen of antibody, the target of payload, antibody-linker conjugate type, linker-payload combination type and a variety of external links to established molecular biological databases (PubChem, DrugMAP, TTD, DrugBank, etc.).
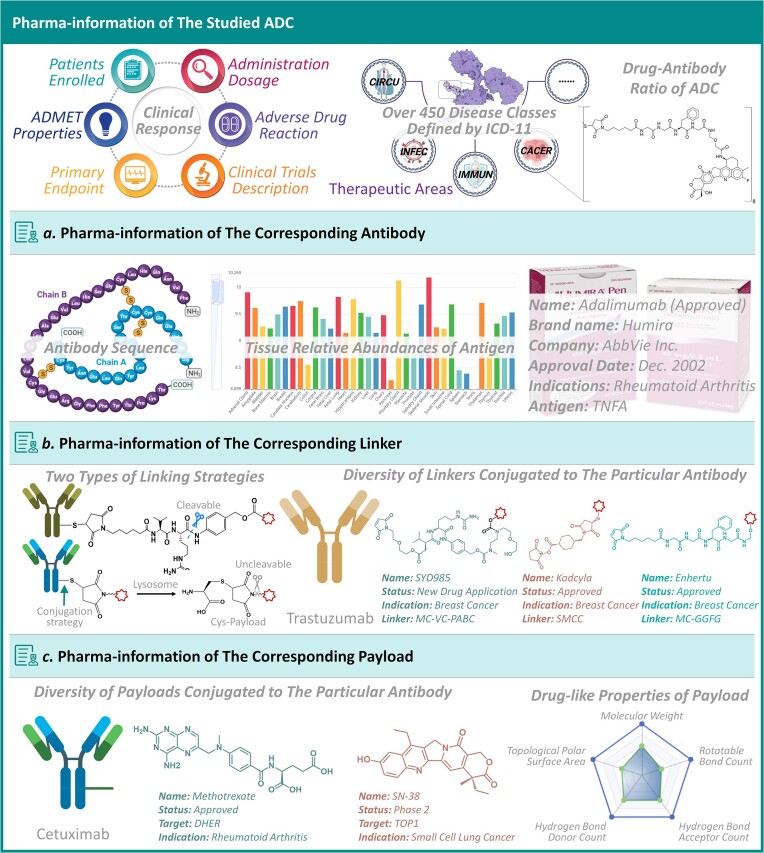
Figure 2.
A comprehensive set of pharma-information for studied ADC. First, the detailed ADC data were described, which included: aimed disease indications together with their corresponding clinical status, ADC’s structure describing antibody, linker and payload, diverse clinical response data (such as the clinical detail of both ADC and enrolled patient, the reported ADMET properties of ADC, administration dosages, reported adverse drug reactions and clinical primary endpoints), drug-antibody ratio of ADC, etc. Second, the pharma-information of the key components of each ADC was explicitly illustrated. (a) the pharma-information of corresponding antibody included: antibody sequence, targeted antigen, tissue-specific antigen abundances, various pharmaceutical data of this antibody (disease, clinical status, brand name, developing company); (b) the pharma-information of corresponding linker contained: linking strategies (cleavable/uncleavable), those linkers conjugated to antibody; (c) the pharma-information of payload covered: the target of any studied payload, diverse payloads conjugated to studied antibody and the drug-like properties of studied payloads (such as: molecular weight, topological polar surface area, rotatable bond count, hydrogen bond donor count and hydrogen bond acceptor count).
The pharma-information of those three key components of each studied ADC was also explicitly shown in ADCdb. As illustrated in Figure 2, the pharma-information of corresponding antibody included: antibody sequence, targeted antigen, tissue-specific abundances of antigen and various pharmaceutical data of this antibody (such as disease, clinical status, brand name and developing company); the pharma-information of corresponding linker contained: diverse linking strategies (cleavable (40–45) & uncleavable (46–48)), and those diverse linkers conjugated to each antibody, which kept witnessing advancement in linker chemistry, thereby enhancing the safety & efficacy of a studied ADC; the pharma-information of payload covered: the target of any studied payload, diverse payloads conjugated to studied antibody and the drug-like properties of studied payloads (such as: molecular weight, topological polar surface area, rotatable bond count, hydrogen bond donor count and hydrogen bond acceptor count). To the best of our knowledge, ADCdb was the first database that describing the comprehensive pharma-information for not only ADCs but also their components, which should be carefully considered in the development of new ADC.
Diverse biological activities of each studied ADC and its components
The extensive and reliable biological activity data were key for developing and optimizing ADCs (49). These valuable data were essential for evaluating ADC’s functional property and assessing its efficacy, selectivity, metabolic properties and safety (50,51). The efficacy of any studied ADC could be determined by different perspectives: whether the antibody could accurately bind to the antigen in lesion site (52–58) and whether the payload could exert effective therapeutic effects in disease tissue (59,60). Moreover, during the discovery process of ADCs, the biological activities of various related ADCs were reported to be informative in determining the optimal combination of maximum efficacy and minimum off-target effects (61). Such activities included: the objective response rates (ORRs) and complete remissions (CRs) of ADC in different stages of clinical trial, the inhibition level and growth delay of ADC in diverse disease PDX models/cell lines, and the half maximal inhibitory concentration (IC50) of ADC across different disease cell lines. Because these activities were found capable of enabling the selection of suitable candidates for preclinical and clinical studies, advancing therapeutic strategies in oncology and others (49–66), such critical data were systematically collected to and provided by the newly constructed ADCdb.
A variety of biological activities of each antibody–drug conjugate
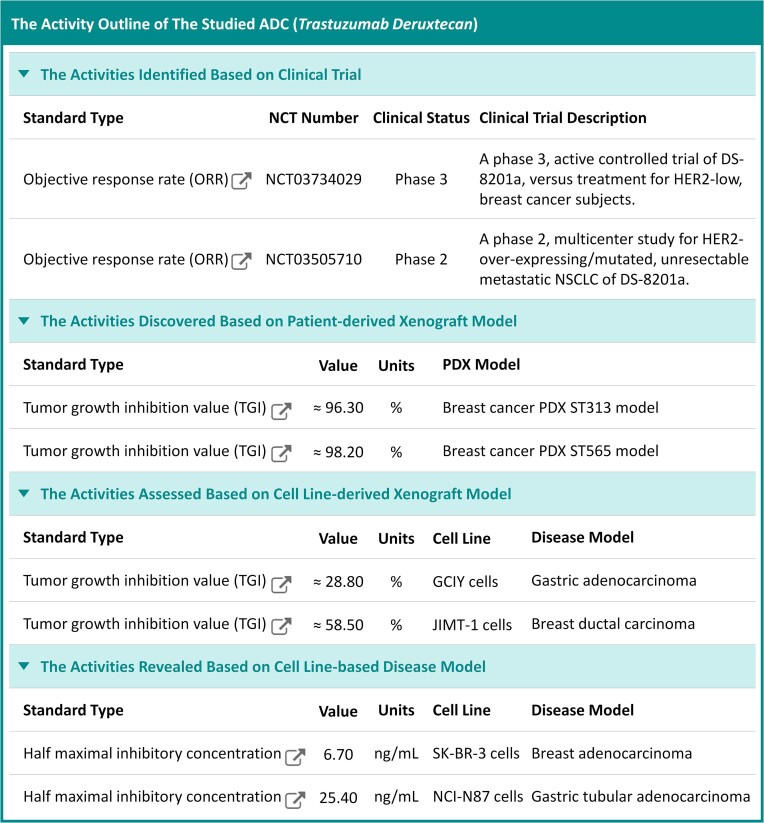
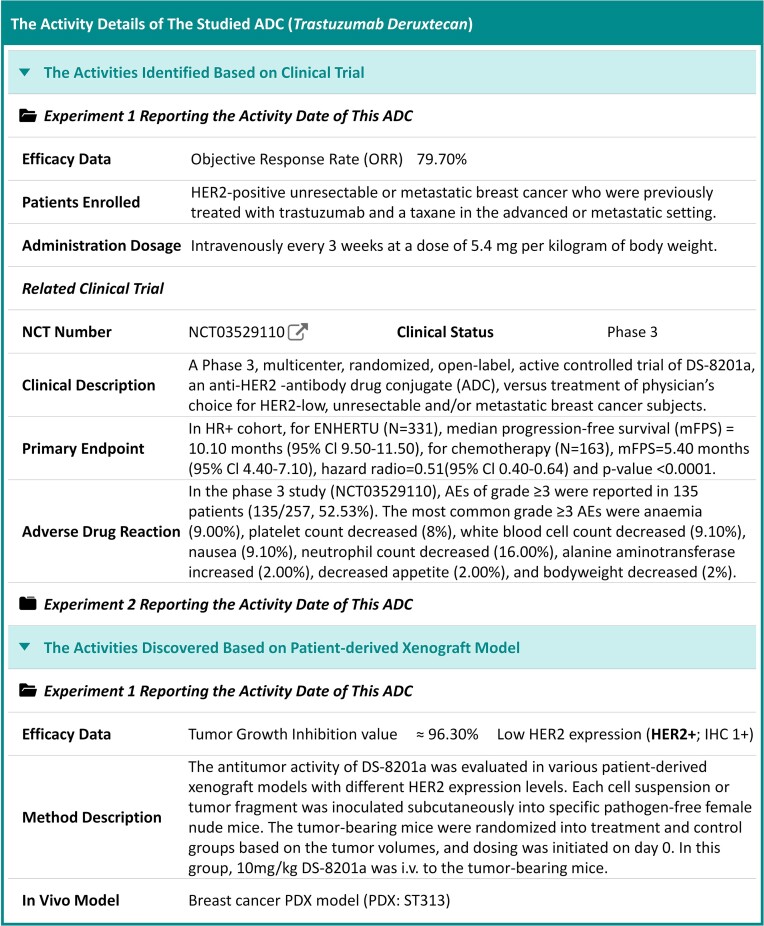
As illustrated in Figure 3, the Activity Outline of a typical ADC (named: trastuzumab deruxtecan) was provided, which offered comprehensive description on multiple types of biological activities. As discussed above, those ADC activity data that were covered in ADCdb included: the objective response rates (ORRs) and complete remissions (CRs) of ADC in different stages of clinical trial, the inhibition level and growth delay of ADC in diverse disease PDX models/cell lines, and the half maximal inhibitory concentration (IC50) of ADC in various disease cells. Taking the activity data of ‘trastuzumab deruxtecan’ as an example (shown in Figure 3), the specific activity values were provided, together with the corresponding activity type, unit and experimental labels (such as NCT number, PDX name, cell line & disease model) and the detailed information underlying these activities could be further accessed by simply clicking on the provided hyperlinks.
Figure 3.
A typical page describing the activity outline of ADC (trastuzumab deruxtecan). Such activity data included: objective response rates (ORRs) and complete remissions (CRs) of ADCs in different stages of clinical trial, inhibition levels and growth delays of ADCs in diverse disease PDX models/cell lines and half maximal inhibitory concentrations of ADCs in different disease cells. The specific activity values of ‘trastuzumab deruxtecan’ were provided, together with the corresponding activity type, unit and experimental labels (such as NCT number, PDX name, cell line and disease model), and the detailed information underlying these activities could be further accessed by simply clicking on those provided hyperlinks.
Detailed biological activities were also explicitly described in ADCdb, which could significantly expand the knowledge in ADC’s ‘Activity Outline’ section. Taking the ‘trastuzumab deruxtecan’ as an example (shown in Figure 4), its detailed biological activity data were also provided in our newly constructed database. As illustrated, such valuable data included: the efficacy information of ADC, the clinical status, primary endpoint, patient enrollment information of the clinical study of ADC, the reported adverse drug reaction of ADC, etc. In ADCdb, the details of a total of 9171 biological activity data were collected. Moreover, a variety of experimental details were included: clinical response information (patient enrollment data, administration dosage, primary endpoint, antigen expression level, etc.), analyzed in-vivo model (breast cancer PDX ST313 model, gastric cancer PDX A11068 model, acute contact hypersensitivity model, etc.), disease cell lines (GCIY, JIMT-1, SK-BR-3, NCI-N87 and SNU1, etc.) and so on. ADC’s ADMET property together with its reported adverse drug reaction were also identified and provided in ADCdb.
Figure 4.
A typical webpage offering the activity details of ADC (trastuzumab deruxtecan). Such activity data included: efficacy information of ADC, the clinical status, primary endpoint, patient enrollment information of the clinical study of ADC, the reported adverse drug reactions of ADC, etc. Various experimental details were also provided based on the original publications.
The biological activities of the antibody & payload in each ADC
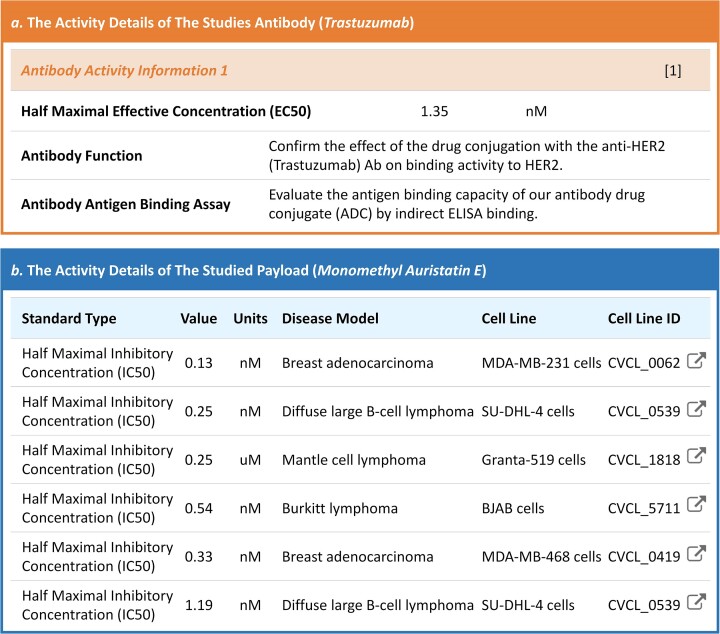
Apart from the activities of a studied ADC, the activity information of its key components (mAb and payload) was also reported to be critical in determining ADC’s overall effectiveness (67,68). Therefore, the activity details of both antibody & payload in any studied ADC were also covered by ADCdb. As illustrated in Figure 5a, the activity details of an exemplar antibody (trastuzumab) were described (which quantitatively provided the binding affinity of this antibody on its antigen), and its therapeutic mechanism together with the experimental method used to measure its binding affinity to antigen were also systematically provided. Meanwhile, activity details of the exemplar payload (monomethyl auristatin E) were also covered by ADCdb (as demonstrated in Figure 5b), under each activity datum, the corresponding experimental validation method (including various cell line, diseased model and experimental detail) were fully collected and described. According on such valuable information, the users of our ADCdb could readily retrieve a list of activity data that might be capable of facilitating the improvement of their studied ADCs, including increasing ADC efficacy, reducing adverse drug reaction and reversing ADC resistance (69–73).
Figure 5.
The activity information of ADC’s key components (antibody & payload). The activity detail of an exemplar antibody (trastuzumab) was described (the binding affinity of this antibody on its antigen), and its therapeutic mechanism together with the experimental method applied to measure its antigen affinity were also systematically provided. Meanwhile, activity details of the exemplar payload (monomethyl auristatin E) were also provided. Under each activity datum, the corresponding experimental validation method (including various cell lines, diseased models and experimental details) were described. The detailed information underlying these activities could be further accessed by simply clicking on those provided hyperlinks.
Constructing antigen-centric panorama for promoting ADC discovery
The random combinations among the antibodies, linkers and payloads provided in ADCdb could result in numerous potential ADCs, which were found to have different therapeutic effectiveness (most were ineffective) on diseases (74). Taking three marketed ADCs as examples, trastuzumab deruxtecan, trastuzumab emtansine and disitamab vedotin had been approved for the treatments of HER2-positive breast cancer, uroepithelial carcinoma, gastric cancer and non-small cell lung cancer, since the antigens of the antibodies (trastuzumab & disitamab) of these three ADCs were identical (HER2). However, due to their distinct combinations among different mAbs, linkers & payloads, their resulting biological activities and targeted disease indications varied significantly (75–79). Particularly, the approved indications (including HER2-positive uroepithelial carcinoma) of ‘disitamab vedotin’ were substantially different from that of the other two ADCs; ‘trastuzumab deruxtecan’ exhibited the greatly enhanced clinical efficacy compared to ‘trastuzumab emtansine’ owing to their distinct strategies in selecting linkers and payloads (78–84). In other words, it was possible that a given payload might be poorly active in a specific indication and that a change of payload while conserving the same antibody might result in promising clinical outcome (85–87); it was crucial to have the diverse combination data of ADCs (with discovered clinical importance and targeted the same antigen), since such combinations were found to be critical for discovering new ADC (88–91). Therefore, an antigen-centric panorama was provided in our newly developed ADCdb to establish the correlations between diverse ADCs & different component combinations, which might facilitate the future discovery of ADC analogous based on QSAR analysis (92–96).
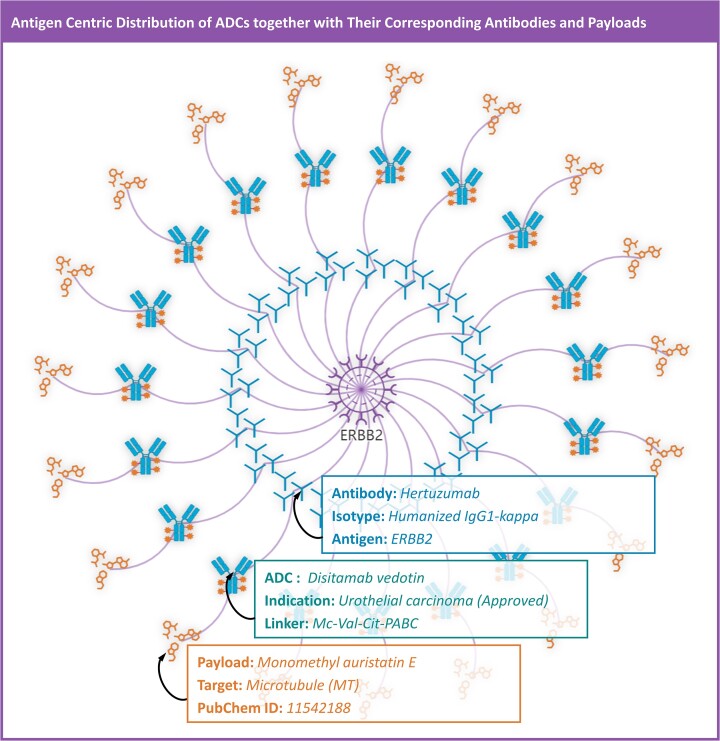
Taking the epidermal growth factor receptor ErbB2 (HER2) as an example, it had been frequently found overexpressed in the patients of various cancer types, and therefore attracted considerable attentions from the community of ADC development (2,49). Therefore, in ADCdb, HER2-centric panorama was provided (illustrated in Figure 6) for establishing the correlations between diverse ADCs and different components combinations. As shown, antigen ERBB2 was first linked to a list of its binding antibodies (indicated by the ‘Y’ shape in blue color), and these linked antibodies were then connected to their resulting ADCs (denoted by the ADC icon colored by both blue and orange; which were further linked to the corresponding payload highlighted in orange and placed in the outermost layer). By placing the mouse on any of the antibodies, ADCs and payloads, their brief introduction would be retrieved (including the name of the selected antibody/ADC/payload, the isotype & antigen of studied antibody, the indication & linker of selected ADC, the targets & structure of studied payload, etc.). By clicking any of the antibody/ADC/payload, the user would be redirected to the corresponding page of the selected antibody/ADC/payload. All in all, ADCdb was unique in providing an antigen-centric panorama that enabled visualization of all antibodies and payloads in the development of ADCs targeting specific antigens, which was useful for users in understanding the component combination profile of any clinically-important ADCs.
Figure 6.
A typical antigen-centric panorama (using ERBB2 as an example) for establishing the correlations between diverse ADCs and different components combinations. The antigen ERBB2 was first linked to a list of its binding antibodies (indicated by ‘Y’ shape in blue color), and these linked antibodies were then connected to their resulting ADCs (denoted by the ADC icon colored by both blue and orange; which were further linked to the corresponding payload highlighted in orange and placed in the outermost layer). By placing the mouse on any of the antibodies, ADCs and payloads, their introduction would be retrieved (the name of selected antibody/ADC/payload, the isotype & antigen of studied antibody, the indication & linker of selected ADC, the targets & structure of studied payload, etc.).
Standardization, access & download of ADCs and their activity data
To make the access and analysis of ADCdb data convenient for all readers, the collected raw data were carefully cleaned up and then systematically standardized. These standardizations included: (a) all those ADCs, antigens, targets, linkers, payloads and cell lines in ADCdb were cross-linked to established databases; (b) all the disease indications were standardized by the last International Classification of Disease (ICD-11) that was officially released by the World Health Organization (97). Moreover, a user-friendly interface was provided by ADCdb to enable a convenient browse and search of data. ADC-relevant data could be viewed, accessed and downloaded from ADCdb, which could be freely accessed without any login requirement at: https://idrblab.org/adcdb/.
Conclusion and perspectives
In this study, the first database, named ADCdb, specialized in describing the biological activities and pharma-information for thousands of antibody–drug conjugates (ADCs) was developed. Due to the importance of these newly collected data and severe lack of these data in existing databases, this newly constructed database is highly expected to attract broad research interests from diverse cutting-edge directions of current biopharmaceutical drug discovery.
Contributor Information
Liteng Shen, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China; Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China; Postgraduate Training Base Alliance of Wenzhou Medical University (Zhejiang Cancer Hospital), Hangzhou 310022, China; College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, China.
Xiuna Sun, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China; Innovation Institute for Artificial Intelligence in Medicine of Zhejiang University, Alibaba-Zhejiang University Joint Research Center of Future Digital Healthcare, Hangzhou 330110, China.
Zhen Chen, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China.
Yu Guo, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China.
Zheyuan Shen, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China.
Yi Song, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China.
Wenxiu Xin, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China.
Haiying Ding, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China.
Xinyue Ma, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China; Postgraduate Training Base Alliance of Wenzhou Medical University (Zhejiang Cancer Hospital), Hangzhou 310022, China.
Weiben Xu, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China; College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, China.
Wanying Zhou, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China; Postgraduate Training Base Alliance of Wenzhou Medical University (Zhejiang Cancer Hospital), Hangzhou 310022, China.
Jinxin Che, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China.
Lili Tan, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China; Postgraduate Training Base Alliance of Wenzhou Medical University (Zhejiang Cancer Hospital), Hangzhou 310022, China.
Liangsheng Chen, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China; Postgraduate Training Base Alliance of Wenzhou Medical University (Zhejiang Cancer Hospital), Hangzhou 310022, China.
Siqi Chen, School of Pharmaceutical Science, Zhejiang Chinese Medical University, Hangzhou 310053, China.
Xiaowu Dong, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China; College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, China.
Luo Fang, Department of Pharmacy, Zhejiang Cancer Hospital, Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou 310005, China; Postgraduate Training Base Alliance of Wenzhou Medical University (Zhejiang Cancer Hospital), Hangzhou 310022, China; College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, China; School of Pharmaceutical Science, Zhejiang Chinese Medical University, Hangzhou 310053, China.
Feng Zhu, College of Pharmaceutical Sciences, The Second Affiliated Hospital, Zhejiang University School of Medicine, Zhejiang University, Hangzhou 310058, China; Innovation Institute for Artificial Intelligence in Medicine of Zhejiang University, Alibaba-Zhejiang University Joint Research Center of Future Digital Healthcare, Hangzhou 330110, China.
Data availability
All antibody–drug conjugate data can be viewed, accessed and downloaded from ADCdb, which is freely accessible without any login requirement by all users at: https://idrblab.org/adcdb/.
Funding
Natural Science Foundation of China [82173660, 81973172, 82373790, 82274020, 81973396, 82373790, U1909208, 22220102001]; Natural Science Fund for Distinguished Young Scholars of Zhejiang [LR21H300003, LR21H300001]; Key R&D Program of Zhejiang Province [2023C03111]; Fund for the open access charge: Natural Science Funds for Distinguished Young Scholars of Zhejiang Province [LR21H300003, LR21H300001].
Conflict of interest statement. None declared.
References
- 1. Mullard A. First-in-class tissue factor-targeted antibody–drug conjugate secures FDA approval. Nat. Rev. Drug Discov. 2021; 20:806. [DOI] [PubMed] [Google Scholar]
- 2. Fu Z., Li S., Han S., Shi C., Zhang Y.. Antibody drug conjugate: the biological missile for targeted cancer therapy. Signal. Transduct. Target Ther. 2022; 7:93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin Y., Schladetsch M., Huang X., Balunas M., Wiemer A.. Stepping forward in antibody–drug conjugate development. Pharmacol. Ther. 2022; 229:107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mair M., Bartsch R., Le Rhun E., Berghoff A., Brastianos P., Cortes J., Gan H., Lin N., Lassman A., Wen P.et al.. Understanding the activity of antibody–drug conjugates in primary and secondary brain tumours. Nat. Rev. Clin. Oncol. 2023; 20:372–389. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz L., Woloshin S., Zheng E., Tse T., Zarin D. ClinicalTrials.gov and Drugs@FDA: a comparison of results reporting for new drug approval trials. Ann. Intern. Med. 2016; 165:421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siramshetty V.B., Grishagin I., Nguyen Eth T., Peryea T., Skovpen Y., Stroganov O., Katzel D., Sheils T., Jadhav A., Mathe E.A.et al.. NCATS inxight drugs: a comprehensive and curated portal for translational research. Nucleic Acids Res. 2022; 50:D1307–D1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li F., Yin J., Lu M., Mou M., Li Z., Zeng Z., Tan Y., Wang S., Chu X., Dai H.et al.. DrugMAP: molecular atlas and pharma-information of all drugs. Nucleic Acids Res. 2023; 51:D1288–D1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendez D., Gaulton A., Bento A., Chambers J., De Veij M., Felix E., Magarinos M., Mosquera J., Mutowo P., Nowotka M.et al.. ChEMBL: towards direct deposition of bioassay data. Nucleic Acids Res. 2019; 47:D930–D940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wishart D., Feunang Y., Guo A., Lo E., Marcu A., Grant J., Sajed T., Johnson D., Li C., Sayeeda Z.et al.. DrugBank 5.0: a major update to the drugbank database for 2018. Nucleic Acids Res. 2018; 46:D1074–D1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manso T., Kushwaha A., Abdollahi N., Duroux P., Giudicelli V., Kossida S.. Mechanisms of action of monoclonal antibodies in oncology integrated in IMGT/mAb-DB. Front. Immunol. 2023; 14:1129323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raybould M., Marks C., Lewis A., Shi J., Bujotzek A., Taddese B., Deane C.. Thera-SAbDab: the therapeutic structural antibody database. Nucleic Acids Res. 2020; 48:D383–D388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burley S., Bhikadiya C., Bi C., Bittrich S., Chao H., Chen L., Craig P., Crichlow G., Dalenberg K., Duarte J.et al.. RCSB protein data bank: delivery of experimentally-determined PDB structures alongside one million computed structure models of proteins from artificial intelligence/machine learning. Nucleic Acids Res. 2023; 51:D488–D508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim S., Chen J., Cheng T., Gindulyte A., He J., He S., Li Q., Shoemaker B., Thiessen P., Yu B.et al.. PubChem 2023 update. Nucleic Acids Res. 2023; 51:D1373–D1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Avram S., Wilson T., Curpan R., Halip L., Borota A., Bora A., Bologa C., Holmes J., Knockel J., Yang J.et al.. DrugCentral 2023 extends human clinical data and integrates veterinary drugs. Nucleic Acids Res. 2023; 51:D1276–D1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Y., Zhang Y., Lian X., Li F., Wang C., Zhu F., Qiu Y., Chen Y.. Therapeutic target database update 2022: facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Res. 2022; 50:D1398–D1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beck A., Goetsch L., Dumontet C., Corvaia N.. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017; 16:315–337. [DOI] [PubMed] [Google Scholar]
- 17. Baah S., Laws M., Rahman K.M.. Antibody-drug conjugates-a tutorial reviewew. Molecules. 2021; 26:2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walsh S.J., Bargh J.D., Dannheim F.M., Hanby A.R., Seki H., Counsell A.J., Ou X., Fowler E., Ashman N., Takada Y.et al.. Site-selective modification strategies in antibody–drug conjugates. Chem. Soc. Rev. 2021; 50:1305–1353. [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann R.M., Coumbe B.G.T., Josephs D.H., Mele S., Ilieva K.M., Cheung A., Tutt A.N., Spicer J.F., Thurston D.E., Crescioli S.et al.. Antibody structure and engineering considerations for the design and function of antibody drug conjugates (ADCs). Oncoimmunology. 2018; 7:e1395127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dean A.Q., Luo S., Twomey J.D., Zhang B.. Targeting cancer with antibody–drug conjugates: promises and challenges. MAbs. 2021; 13:1951427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perrotti V., Caponio V.C.A., Mascitti M., Lo Muzio L., Piattelli A., Rubini C., Capone E., Sala G.. Therapeutic potential of antibody–drug conjugate-based therapy in head and neck cancer: a systematic review. Cancers. 2021; 13:3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swain S.M., Shastry M., Hamilton E.. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov. 2023; 22:101–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anami Y., Yamazaki C.M., Xiong W., Gui X., Zhang N., An Z., Tsuchikama K.. Glutamic acid-valine-citrulline linkers ensure stability and efficacy of antibody–drug conjugates in mice. Nat. Commun. 2018; 9:2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cang Z., Munch E., Wei G.W.. Evolutionary homology on coupled dynamical systems with applications to protein flexibility analysis. J. Appl. Comput. Topol. 2020; 4:481–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Colombo R., Rich J.R.. The therapeutic window of antibody drug conjugates: a dogma in need of revision. Cancer Cell. 2022; 40:1255–1263. [DOI] [PubMed] [Google Scholar]
- 26. Ashman N., Bargh J.D., Spring D.R.. Non-internalising antibody–drug conjugates. Chem. Soc. Rev. 2022; 51:9182–9202. [DOI] [PubMed] [Google Scholar]
- 27. Conilh L., Sadilkova L., Viricel W., Dumontet C.. Payload diversification: a key step in the development of antibody–drug conjugates. J. Hematol. Oncol. 2023; 16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giugliano F., Corti C., Tarantino P., Michelini F., Curigliano G.. Bystander effect of antibody–drug conjugates: fact or fiction?. Curr. Oncol. Rep. 2022; 24:809–817. [DOI] [PubMed] [Google Scholar]
- 29. Anami Y., Deng M., Gui X., Yamaguchi A., Yamazaki C.M., Zhang N., Zhang C.C., An Z., Tsuchikama K.. LILRB4-targeting antibody–drug conjugates for the treatment of acute myeloid leukemia. Mol. Cancer Ther. 2020; 19:2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khera E., Dong S., Huang H., de Bever L., van Delft F.L., Thurber G.M.. Cellular-resolution imaging of bystander payload tissue penetration from antibody–drug conjugates. Mol. Cancer Ther. 2022; 21:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsuchikama K., An Z.. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018; 9:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nicolaou K.C., Chen Q., Li R., Anami Y., Tsuchikama K.. Total synthesis of the monomeric unit of lomaiviticin A. J. Am. Chem. Soc. 2020; 142:20201–20207. [DOI] [PubMed] [Google Scholar]
- 33. Zhang S., Sun X., Mou M., Amahong K., Sun H., Zhang W., Shi S., Li Z., Gao J., Zhu F.. REGLIV: molecular regulation data of diverse living systems facilitating current multiomics research. Comput. Biol. Med. 2022; 148:105825. [DOI] [PubMed] [Google Scholar]
- 34. Yang Q., Wang Y., Zhang Y., Li F., Xia W., Zhou Y., Qiu Y., Li H., Zhu F.. NOREVA: enhanced normalization and evaluation of time-course and multi-class metabolomic data. Nucleic Acids Res. 2020; 48:W436–W448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang J., Fu J., Wang Y., Li B., Li Y., Yang Q., Cui X., Hong J., Li X., Chen Y.et al.. ANPELA: analysis and performance assessment of the label-free quantification workflow for metaproteomic studies. Brief Bioinform. 2020; 21:621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun X., Zhang Y., Li H., Zhou Y., Shi S., Chen Z., He X., Zhang H., Li F., Yin J.et al.. DRESIS: the first comprehensive landscape of drug resistance information. Nucleic Acids Res. 2023; 51:D1263–D1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fu T., Li F., Zhang Y., Yin J., Qiu W., Li X., Liu X., Xin W., Wang C., Yu L.et al.. VARIDT 2.0: structural variability of drug transporter. Nucleic Acids Res. 2022; 50:D1417–D1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin J., Li F., Zhou Y., Mou M., Lu Y., Chen K., Xue J., Luo Y., Fu J., He X.et al.. INTEDE: interactome of drug-metabolizing enzymes. Nucleic Acids Res. 2021; 49:D1233–D1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yin J., Sun W., Li F., Hong J., Li X., Zhou Y., Lu Y., Liu M., Zhang X., Chen N.et al.. VARIDT 1.0: variability of drug transporter database. Nucleic Acids Res. 2020; 48:D1042–D1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bargh J.D., Isidro-Llobet A., Parker J.S., Spring D.R.. Cleavable linkers in antibody–drug conjugates. Chem. Soc. Rev. 2019; 48:4361–4374. [DOI] [PubMed] [Google Scholar]
- 41. Bargh J.D., Walsh S.J., Ashman N., Isidro-Llobet A., Carroll J.S., Spring D.R.. A dual-enzyme cleavable linker for antibody–drug conjugates. Chem. Commun. 2021; 57:3457–3460. [DOI] [PubMed] [Google Scholar]
- 42. Song J., Wang Y., Li F., Akutsu T., Rawlings N.D., Webb G.I., Chou K.C.. iProt-Sub: a comprehensive package for accurately mapping and predicting protease-specific substrates and cleavage sites. Brief. Bioinform. 2019; 20:638–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Song J., Li F., Leier A., Marquez-Lago T.T., Akutsu T., Haffari G., Chou K.C., Webb G.I., Pike R.N., Hancock J.. PROSPERous: high-throughput prediction of substrate cleavage sites for 90 proteases with improved accuracy. Bioinformatics. 2018; 34:684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li F., Leier A., Liu Q., Wang Y., Xiang D., Akutsu T., Webb G.I., Smith A.I., Marquez-Lago T., Li J.et al.. Procleave: predicting protease-specific substrate cleavage sites by combining sequence and structural information. Genomics Proteomics Bioinform. 2020; 18:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li F., Wang Y., Li C., Marquez-Lago T.T., Leier A., Rawlings N.D., Haffari G., Revote J., Akutsu T., Chou K.C.et al.. Twenty years of bioinformatics research for protease-specific substrate and cleavage site prediction: a comprehensive revisit and benchmarking of existing methods. Brief Bioinform. 2019; 20:2150–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ogitani Y., Hagihara K., Oitate M., Naito H., Agatsuma T.. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016; 107:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Su Z., Xiao D., Xie F., Liu L., Wang Y., Fan S., Zhou X., Li S.. Antibody-drug conjugates: recent advances in linker chemistry. Acta Pharm Sin B. 2021; 11:3889–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lambert J.M., Chari R.V.. Ado-trastuzumab emtansine (T-DM1): an antibody–drug conjugate (ADC) for HER2-positive breast cancer. J. Med. Chem. 2014; 57:6949–6964. [DOI] [PubMed] [Google Scholar]
- 49. Drago J.Z., Modi S., Chandarlapaty S.. Unlocking the potential of antibody–drug conjugates for cancer therapy. Nat. Rev. Clin. Oncol. 2021; 18:327–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Walles M., Connor A., Hainzl D. ADME and safety aspects of non-cleavable linkers in drug discovery and development. Curr. Top. Med. Chem. 2017; 17:3463–3475. [DOI] [PubMed] [Google Scholar]
- 51. Mou S., Huang Y., Rosenbaum A.I.. ADME considerations and bioanalytical strategies for pharmacokinetic assessments of antibody–drug conjugates. Antibodies. 2018; 7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hasan M.M., Laws M., Jin P., Rahman K.M.. Factors influencing the choice of monoclonal antibodies for antibody–drug conjugates. Drug Discov. Today. 2022; 27:354–361. [DOI] [PubMed] [Google Scholar]
- 53. Tsumura R., Manabe S., Takashima H., Koga Y., Yasunaga M., Matsumura Y.. Influence of the dissociation rate constant on the intra-tumor distribution of antibody–drug conjugate against tissue factor. J. Control Release. 2018; 284:49–56. [DOI] [PubMed] [Google Scholar]
- 54. Cilliers C., Menezes B., Nessler I., Linderman J., Thurber G.M.. Improved tumor penetration and single-cell targeting of antibody–drug conjugates increases anticancer efficacy and host survival. Cancer Res. 2018; 78:758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walsh S.J., Omarjee S., Galloway W., Kwan T.T., Sore H.F., Parker J.S., Hyvonen M., Carroll J.S., Spring D.R.. A general approach for the site-selective modification of native proteins, enabling the generation of stable and functional antibody–drug conjugates. Chem. Sci. 2019; 10:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Murer P., Neri D. Antibody-cytokine fusion proteins: a novel class of biopharmaceuticals for the therapy of cancer and of chronic inflammation. N. Biotechnol. 2019; 52:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pluss L., Peissert F., Elsayed A., Rotta G., Romer J., Dakhel Plaza S., Villa A., Puca E., De Luca R., Oxenius A.et al.. Generation and in vivo characterization of a novel high-affinity human antibody targeting carcinoembryonic antigen. MAbs. 2023; 15:2217964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang M., Cang Z., Wei G.W.. A topology-based network tree for the prediction of protein-protein binding affinity changes following mutation. Nat. Mach. Intell. 2020; 2:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheng-Sanchez I., Moya-Utrera F., Porras-Alcala C., Lopez-Romero J.M., Sarabia F.. Antibody-drug conjugates containing payloads from marine origin. Mar Drugs. 2022; 20:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Casi G., Neri D. Antibody-drug conjugates and small molecule-drug conjugates: opportunities and challenges for the development of selective anticancer cytotoxic agents. J. Med. Chem. 2015; 58:8751–8761. [DOI] [PubMed] [Google Scholar]
- 61. Khongorzul P., Ling C.J., Khan F.U., Ihsan A.U., Zhang J.. Antibody-drug conjugates: a comprehensive review. Mol. Cancer Res. 2020; 18:3–19. [DOI] [PubMed] [Google Scholar]
- 62. De Cecco M., Galbraith D.N., McDermott L.L.. What makes a good antibody–drug conjugate?. Expert Opin. Biol. Ther. 2021; 21:841–847. [DOI] [PubMed] [Google Scholar]
- 63. Hobson A.D., McPherson M.J., Hayes M.E., Goess C., Li X., Zhou J., Wang Z., Yu Y., Yang J., Sun L.et al.. Discovery of ABBV-3373, an anti-TNF glucocorticoid receptor modulator immunology antibody drug conjugate. J. Med. Chem. 2022; 65:15893–15934. [DOI] [PubMed] [Google Scholar]
- 64. Tarantino P., Ricciuti B., Pradhan S.M., Tolaney S.M.. Optimizing the safety of antibody–drug conjugates for patients with solid tumours. Nat. Rev. Clin. Oncol. 2023; 20:558–576. [DOI] [PubMed] [Google Scholar]
- 65. Thomas A., Teicher B.A., Hassan R.. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016; 17:e254–e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neri D. Antibody-cytokine fusions: versatile products for the modulation of anticancer immunity. Cancer Immunol. Res. 2019; 7:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Birrer M.J., Moore K.N., Betella I., Bates R.C.. Antibody-drug conjugate-based therapeutics: state of the science. J. Natl. Cancer Inst. 2019; 111:538–549. [DOI] [PubMed] [Google Scholar]
- 68. Joubert N., Beck A., Dumontet C., Denevault-Sabourin C.. Antibody-drug conjugates: the last decade. Pharmaceuticals. 2020; 13:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhu Y., Liu K., Wang K., Zhu H.. Treatment-related adverse events of antibody–drug conjugates in clinical trials: a systematic review and meta-analysis. Cancer. 2023; 129:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Subhan M.A., Torchilin V.P.. Advances in targeted therapy of breast cancer with antibody–drug conjugate. Pharmaceutics. 2023; 15:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shin S.H., Park Y.H., Park S.S., Ju E.J., Park J., Ko E.J., Bae D.J., Kim S.Y., Chung C.W., Song H.Y.et al.. An elaborate new linker system significantly enhances the efficacy of an HER2-antibody–drug conjugate against refractory HER2-positive cancers. Adv. Sci. 2021; 8:e2102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen J., Wang R., Gilby N.B., Wei G.W.. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J. Chem. Inf. Model. 2022; 62:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang Q., Gong Y., Zhu F.. Critical assessment of the biomarker discovery and classification methods for multiclass metabolomics. Anal. Chem. 2023; 95:5542–5552. [DOI] [PubMed] [Google Scholar]
- 74. Samantasinghar A., Sunildutt N.P., Ahmed F., Soomro A.M., Salih A.R.C., Parihar P., Memon F.H., Kim K.H., Kang I.S., Choi K.H.. A comprehensive review of key factors affecting the efficacy of antibody drug conjugate. Biomed. Pharmacother. 2023; 161:114408. [DOI] [PubMed] [Google Scholar]
- 75. Rinnerthaler G., Gampenrieder S.P., Greil R.. HER2 directed antibody–drug-conjugates beyond T-DM1 in breast cancer. Int. J. Mol. Sci. 2019; 20:1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shitara K., Bang Y.J., Iwasa S., Sugimoto N., Ryu M.H., Sakai D., Chung H.C., Kawakami H., Yabusaki H., Lee J.et al.. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020; 382:2419–2430. [DOI] [PubMed] [Google Scholar]
- 77. Deeks E.D. Disitamab vedotin: first approval. Drugs. 2021; 81:1929–1935. [DOI] [PubMed] [Google Scholar]
- 78. Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., Tsurutani J., Ueno N.T., Prat A., Chae Y.S.et al.. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022; 387:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hurvitz S.A., Hegg R., Chung W.P., Im S.A., Jacot W., Ganju V., Chiu J.W.Y., Xu B., Hamilton E., Madhusudan S.et al.. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023; 401:105–117. [DOI] [PubMed] [Google Scholar]
- 80. Cortes J., Kim S.B., Chung W.P., Im S.A., Park Y.H., Hegg R., Kim M.H., Tseng L.M., Petry V., Chung C.F.et al.. Trastuzumab Deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 2022; 386:1143–1154. [DOI] [PubMed] [Google Scholar]
- 81. Takegawa N., Nonagase Y., Yonesaka K., Sakai K., Maenishi O., Ogitani Y., Tamura T., Nishio K., Nakagawa K., Tsurutani J.. DS-8201a, a new HER2-targeting antibody–drug conjugate incorporating a novel DNA topoisomerase I inhibitor, overcomes HER2-positive gastric cancer T-DM1 resistance. Int. J. Cancer. 2017; 141:1682–1689. [DOI] [PubMed] [Google Scholar]
- 82. Cancer D. T-DXd: new standard for HER2-low breast cancer. Cancer Discov. 2022; 12:1828. [DOI] [PubMed] [Google Scholar]
- 83. Ogitani Y., Aida T., Hagihara K., Yamaguchi J., Ishii C., Harada N., Soma M., Okamoto H., Oitate M., Arakawa S.et al.. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I Inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 2016; 22:5097–5108. [DOI] [PubMed] [Google Scholar]
- 84. Li Y.H., Li X.X., Hong J.J., Wang Y.X., Fu J.B., Yang H., Yu C.Y., Li F.C., Hu J., Xue W.W.et al.. Clinical trials, progression-speed differentiating features and swiftness rule of the innovative targets of first-in-class drugs. Brief Bioinform. 2020; 21:649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dumontet C., Reichert J.M., Senter P.D., Lambert J.M., Beck A.. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023; 22:641–661. [DOI] [PubMed] [Google Scholar]
- 86. Lai W., Zhao S., Lai Q., Zhou W., Wu M., Jiang X., Wang X., Peng Y., Wei X., Ouyang L.et al.. Design, synthesis, and bioevaluation of a novel hybrid molecular pyrrolobenzodiazepine-anthracenecarboxyimide as a payload for antibody–drug conjugate. J. Med. Chem. 2022; 65:11679–11702. [DOI] [PubMed] [Google Scholar]
- 87. Yamazaki C.M., Yamaguchi A., Anami Y., Xiong W., Otani Y., Lee J., Ueno N.T., Zhang N., An Z., Tsuchikama K.. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 2021; 12:3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kostova V., Desos P., Starck J.B., Kotschy A.. The chemistry behind ADCs. Pharmaceuticals. 2021; 14:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cang Z., Wei G.W.. Analysis and prediction of protein folding energy changes upon mutation by element specific persistent homology. Bioinformatics. 2017; 33:3549–3557. [DOI] [PubMed] [Google Scholar]
- 90. Xue W., Yang F., Wang P., Zheng G., Chen Y., Yao X., Zhu F.. What contributes to serotonin-norepinephrine reuptake inhibitors' dual-targeting mechanism? The key role of transmembrane domain 6 in human serotonin and norepinephrine transporters revealed by molecular dynamics simulation. ACS Chem. Neurosci. 2018; 9:1128–1140. [DOI] [PubMed] [Google Scholar]
- 91. Wang X., Li F., Qiu W., Xu B., Li Y., Lian X., Yu H., Zhang Z., Wang J., Li Z.et al.. SYNBIP: synthetic binding proteins for research, diagnosis and therapy. Nucleic Acids Res. 2022; 50:D560–D570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Matikonda S.S., McLaughlin R., Shrestha P., Lipshultz C., Schnermann M.J.. Structure-activity relationships of antibody–drug conjugates: a systematic review of chemistry on the trastuzumab scaffold. Bioconjug. Chem. 2022; 33:1241–1253. [DOI] [PubMed] [Google Scholar]
- 93. Xia W., Zheng L., Fang J., Li F., Zhou Y., Zeng Z., Zhang B., Li Z., Li H., Zhu F.. PFmulDL: a novel strategy enabling multi-class and multi-label protein function annotation by integrating diverse deep learning methods. Comput. Biol. Med. 2022; 145:105465. [DOI] [PubMed] [Google Scholar]
- 94. Li F., Zhou Y., Zhang Y., Yin J., Qiu Y., Gao J., Zhu F.. POSREG: proteomic signature discovered by simultaneously optimizing its reproducibility and generalizability. Brief. Bioinform. 2022; 23:bbac040. [DOI] [PubMed] [Google Scholar]
- 95. Yang Q., Li B., Tang J., Cui X., Wang Y., Li X., Hu J., Chen Y., Xue W., Lou Y.et al.. Consistent gene signature of schizophrenia identified by a novel feature selection strategy from comprehensive sets of transcriptomic data. Brief. Bioinform. 2020; 21:1058–1068. [DOI] [PubMed] [Google Scholar]
- 96. Zhang Y., Sun H., Lian X., Tang J., Zhu F.. ANPELA: significantly enhanced quantification tool for cytometry-based single-cell proteomics. Adv. Sci. (Weinh.). 2023; 10:e2207061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lancet T. ICD-11. Lancet. 2019; 393:2275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All antibody–drug conjugate data can be viewed, accessed and downloaded from ADCdb, which is freely accessible without any login requirement by all users at: https://idrblab.org/adcdb/.