Abstract
Background
Spatial repellents (SR) may complement current vector control tools and provide additional coverage when people are not under their bednets or are outdoors. Here we assessed the efficacy of a metofluthrin-based SR in reducing exposure to pyrethroid-resistant Anopheles funestus in Siaya County, western Kenya.
Methods
Metofluthrin was vaporized using an emanator configured to a liquid petroleum gas (LPG) canister, placed inside experimental huts (phase 1) or outdoors (phase 2), and evaluated for reductions in human landing rate, density, knockdown and mortality rates of An. funestus, which are present in high density in the area. To demonstrate the mosquito recruiting effect of LPG, a hut with only an LPG cooker but no metofluthrin was added as a comparator and compared with an LPG cooker burning alongside the emanator and a third hut with no LPG cooker as control. Phase 2 evaluated the protective range of the SR product while emanating from the centre of a team of mosquito collectors sitting outdoors in north, south, east and west directions at 5, 10 and 20 feet from the emanating device.
Results
Combustion of LPG with a cook stove increased the density of An. funestus indoors by 51% over controls with no cook stove. In contrast, huts with metofluthrin vaporized with LPG combustion had lower indoor density of An. funestus (99.3% less than controls), with knockdown and mortality rates of 95.5 and 87.7%, respectively, in the mosquitoes collected in the treated huts. In the outdoor study (phase 2), the outdoor landing rate was significantly lower at 5 and 10 feet than at 20 feet from the emanator.
Conclusions
Vaporized metofluthrin almost completely prevented An. funestus landing indoors and led to 10 times lower landing rates within 10 feet of the emanator outdoors, the first product to demonstrate such potential. Cooking with LPG inside the house could increase exposure to Anopheles mosquito bites, but the use of the metofluthrin canister eliminates this risk.
Graphical abstract
Keywords: Anopheles funestus, Emanator, Metofluthrin, Spatial repellent, Western Kenya
Background
In 2021, 247 million cases of malaria and 619,000 deaths were recorded globally [1]. The main tools recommended for public health malaria vector control are long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS), which have led to a massive reduction in cases across endemic countries [2]. Despite the efforts at expansive coverage with these tools, malaria persists, with a disproportionate burden in sub-Saharan Africa (SSA) owing to the mix of a set of efficient Anopheles species and Plasmodium falciparum [1] and the emergence and spread of insecticide resistance, among other factors [3]. In addition, local vector species may exhibit insecticide avoidance, so that mosquitoes actively avoid treated surfaces, adopting a more outdoor biting and resting behaviour and resorting to feeding on animals [4].
In view of these challenges, additional approaches and interventions are required to make further progress in the fight against malaria [5]. Indoor deployment of LLINs and IRS means that they cannot directly protect people outdoors, apart from the benefit derived from a community-wide reduction in mosquito density, and they are also challenging to implement where people do not have permanent homes, such as immigrant or nomad communities. Additionally, IRS is expensive to scale up, hence limiting its coverage to the population at risk [6]. Therefore, vector control measures that can target gaps in protection such as early evening, late morning and outdoor transmission as well as mobile populations are highly desirable. Intra-domiciliary measures are insufficient to reach elimination, and there are calls for new and innovative tools for malaria vector control [7]. Spatial repellents (SR) are a promising vector control paradigm that could add to the existing strategies for malaria prevention [8, 9]. SRs such as mosquito coils have been shown to reduce mosquito biting [10, 11] in studies conducted in Indonesia [11], Peru and China [12]. Additional evidence is currently being generated to show the efficacy of SRs across a range of malaria transmission endemicity characteristics and mosquito vector species and in the context of high LIIN coverage before SRs can be recommended as a tool for malaria control by national malaria control programmes (NMCPs) [13, 14].
SRs have many potential advantages over existing malaria control tools. In contrast to insecticide-treated nets, SRs placed within a house may protect all residents, and in particular at times when they are not under LLINs. SRs with a residual effect would not require daily placement and monitoring like IRS, and would reduce user bias. Unlike IRS, SRs would be effective against mosquitoes that have adapted to avoid landing on treated surfaces within the household. There are very few tools such as attractive targeted sugar bait that have shown efficacy against outdoor biting mosquitoes, especially in rural African settings where malaria is rife [15]. Metofluthrin, as a promising SR, acts through the disruption in orientation towards the host (preventing bites) and knockdown and killing of mosquitoes [16]. SRs have been formulated as coils, paper, gel, liquid and other types of emanators which can be either active (requiring a source of heat) or passive (not requiring a source of heat) [10]. The product used for these evaluations has been assessed for human safety and exposure and is now available in a formulation that is released slowly when heated [17, 18]. Liquefied petroleum gas (LPG)-activated metofluthrin-based SRs have recently been developed by Thermacell Repellents, Inc. (Bedford, MA, USA). LPG is quickly being adopted for cooking in multiple parts of rural and urban Africa [5, 19, 20]. Cooking with LPG in experimental huts in Rwanda increased Anopheles density relative to huts where traditional cooking fuels such as wood or charcoal were burned because burning LPG produces carbon dioxide as a by-product, thus creating an attractant for host-seeking mosquitoes [21].
This study evaluated the efficacy of metofluthrin cartridges attached to LPG-based cookers against malaria vectors in reducing mosquito entry into experimental huts in western Kenya, with major outcomes being reductions in landing rates, deterrence and induced exophily. The study also evaluated the minimum optimal duration of emanation of the SRs required to achieve overnight efficacy in experimental huts as well as the distance of protection realized from outdoor placement of the SRs in the rural village compounds. In addition, the study evaluated the role of cooking with LPG in recruiting Anopheles mosquitoes indoors relative to huts without LPG cooking, and whether volatilization of metofluthrin indoors, where LPG cooking takes place, obviated the attractive effect of combusted LPG to Anopheles mosquitoes.
Methods
Study site
The study was conducted in the Dala Suna experimental hut site on the shores of Lake Kanyaboli (0° 02′ 08.5″ N, 34° 11′ 05.0″ E) and in villages close to the hut site in Alego-Usonga sub-County, Siaya County, western Kenya. It is situated close to the swamps that provide conducive breeding habitats for malaria vectors, and characterized by high year-round abundance of Anopheles funestus and seasonal peaks of Anopheles arabiensis, with average household density > 300 and > 20 per night, respectively (Ochomo et al., unpublished). The area experiences two rainy seasons, one from March to May and the other from October to November, with high malaria transmission occurring throughout the year [22]. The primary economic activities of the local population are subsistence farming, livestock keeping, fishing and small-scale trading.
Experimental huts
There are seven experimental huts, each measuring 6 m long, 3 m wide and 2 m high. The experimental huts are designed to resemble a typical Kenyan household in terms of structure and mosquito exit/entry points (eaves, windows and doors) (Fig. 1a). Mosquito exit traps were fitted to all four windows of the experimental huts—two windows on the front face and two on the backside of the huts. The walls are made of blocks and lined with mud on the inside. The huts have corrugated iron roofs and a 10-cm eave gap on all sides. To prevent mosquitoes from exiting the huts, baffles are installed at the eave gaps, allowing easy entry for mosquitoes (Fig. 1b). In addition, the floors are tiled with white tiles for ease of collection of knocked-down and dead mosquitoes (Fig. 1c). The huts are elevated above the ground on a concrete base surrounded by a water-filled moat to keep ants away [23].
Fig. 1.

Experimental hut design: A front view of the hut fitted with window exit traps, B showing the wood baffles, and C showing the tiled floor and the hut interior walls
Experimental design and set-up
The trial was conducted in two phases, with phase 1 evaluations being carried out indoors in experimental huts and phase 2 evaluations conducted outdoors in compounds in the nearby village. The study used two collection methods to assess the effectiveness of the emanator in preventing mosquitoes from landing on study participants and entering the huts. Human landing catch (HLC) was used to determine the landing rates of mosquitoes on humans [23], both indoors and outdoors, while mouth aspiration was used to determine indoor resting density as a measure of the deterrence and induction of exophily by the emanator. Volunteers aged between 18 and 45 years from neighbouring villages who consented to participate were recruited, trained and tested for malaria. Those who tested positive were treated with antimalarial medication. All the study participants were placed on prophylaxis with a weekly dose of Mefloquine® once clear of infection or if they had a negative test at the time of consenting. The SR device manufactured by Thermacell Repellents, Inc. (Bedford, MA, USA) vaporizes metofluthrin as its active ingredient. It functions like a diffuser by using heat to evaporate a small amount of insecticide into the air. The canister containing metofluthrin is attached to the LPG cylinder. Once the gas burner is turned on, it produces heat that vaporizes the metofluthrin SR into the air. The complete set of Thermacell technology, along with the 6-kg cylinder gas used during emanation is shown in Fig. 2, where the Thermacell emanator consists of three main parts. Part A is the Thermacell technology that attaches to the gas cylinder. Part B is where the metofluthrin SR container/cartridge is held in position when in use. Part C shows the metofluthrin cartridge fixed into a 6-kg gas cylinder during emanation.
Fig. 2.

A Thermacell emanator, B metofluthrin cartridge attached to the Thermacell emanator–metofluthrin SR container, C Thermacell emanator attached to 6-kg gas cylinders
Human landing catch
HLC was conducted for 12 h (18:00–06:00) every night for five nights inside the experimental huts as shown in Table 1. Each study participant collected mosquitoes throughout a 6-h shift (18:00–00:00 or 00:00–06:00) with a 15-min break within every hour of collection and were rotated between the shifts. During the mosquito collection process, the collectors sat in a chair wearing shorts and long-sleeved shirts. They used a mouth aspirator (Model 412, John W. Hock Company, Gainesville, FL, USA) to collect the mosquitoes that landed on their lower legs. Any mosquitoes that were collected were placed in a clean paper cup. These cups were changed every hour and for each location. The mosquitoes were provided access to 10% sugar solution. At the end of each night collection, the mosquitoes were transported in a cooler box to the field laboratory for processing. The paper cups were sorted, and those with mosquitoes were placed in the killing jar with chloroform soaked in cotton wool to knock down mosquitoes. The mosquitoes were sorted, the culicines were counted, and the number of males and females recorded and then discarded. The Anopheles mosquitoes were separated by species, sex and abdominal status (blood-fed, non-blood-fed or gravid) for females, and numbers collected per hour were recorded. Morphological identification was performed on the mosquitoes using taxonomic keys [24] to differentiate between An. funestus sensu lato (s.l.) and Anopheles gambiae s.l. and other secondary malaria vectors.
Table 1.
Schedule of experiments conducted in the experimental huts including the rotation of the sleepers and HLC volunteers
| Date | Activity | Hut 1 | Hut 2 | Hut 3 |
|---|---|---|---|---|
| 23-Aug-22 | Sleepers | 3 | 2 | 6 |
| 24-Aug-22 | HLC | 2 and 3 | 1 and 4 | 5 and 6 |
| 25-Aug-22 | HLC | 4 and 1 | 6 and 5 | 3 and 2 |
| 26-Aug-22 | Sleepers | 2 | 6 | 3 |
| 28-Aug-22 | Sleepers | 6 | 3 | 2 |
| 29-Aug-22 | HLC | 5 and 6 | 2 and 3 | 1 and 4 |
| 30-Aug-22 | HLC | 3 and 2 | 4 and 1 | 6 and 5 |
| 31-Aug-22 | HLC | 1 and 4 | 5 and 6 | 2 and 3 |
| 01-Sept-22 | Sleepers | 3 | 2 | 6 |
| 02-Sept-22 | Sleepers | 2 | 6 | 3 |
Aspiration collections
Aspiration collections were carried out in the morning following overnight sleeping in the experimental huts. The participants reported sleeping in the huts under an untreated bednet from 20:30 until 06:30. Following World Health Organization (WHO) guidelines on simulating wear and tear, each net was intentionally holed with six 16-cm2 holes (two holes on each long side and one hole on each short side) [25]. Trained field assistants conducted mosquito collections using mouth aspirators every morning from 06:30 to 07:30 after overnight sleeping activity. Mosquitoes were scored by location—net and under bed, roof, floor, wall and exit traps—and transported in a cooler box to the field laboratory for sorting as dead or alive, unfed, fed, gravid or half-gravid. Knockdown and dead mosquitoes were recorded 1 h post-collection. Live mosquitoes were held at 27 ± 2 °C and provided access to 10% sugar solution for up to 24 h to assess delayed mortality. Females were identified at the species level using morphological keys [24].
Experimental hut evaluation to assess the impact of LPG use on mosquito activity
Phase 1 of the study was conducted for 10 nights from 23 August to 2 September 2022 to investigate whether cooking with LPG gas impacted mosquito activity in three experimental huts: Hut 1 was allocated an LPG cooker burning from 18:00 to 20:00 to simulate local cooking practices. Hut 2 had an LPG cooker burning for 2 h (18:00–20:00), and a metofluthrin emanator attached to a separate LPG cylinder ran for 12 h from 18:00 to 06:00. The third hut was a negative control with neither a metofluthrin emanator nor an LPG cooker. Aspiration and HLC were used for mosquito collections for five alternate nights each (Table 1).
Evaluation of the residual efficacy of the SR emanator to inform combination deployment with LLINs
Between January and February 2023, the efficacy of the SR product deployed for limited durations was evaluated in comparison with an all-night deployment to evaluate any residual efficacy and to inform possible deployment in local households with optimal coverage and use of LLINs. Four different durations of metofluthrin emanation—0 h (control), 2 h (18:00–20:00), 4 h (18:00–22:00) and 12 h (18:00–06:00)—were evaluated. To understand the efficacy in reducing morning biting, additional emanation for 1 h was added to the 2-h and 4-h arms between 05:00 and 06:00.
Small-scale field trial to determine outdoor protective efficacy
The field trial was conducted to test the effectiveness of the metofluthrin emanator outdoors. The trial involved 10 compounds for each of the emanation distances of 5, 10 and 20 feet. In each compound, we placed the SR emanator attached to the LPG cylinder at the centre and had four HLC volunteers stationed equidistantly in the north, south, west and east directions from the emanator. Volunteers were paired up and rotated every night, each working a 6-h shift from either 18:00 to 00:00 or 00:00 to 06:00, and each volunteer pair rotated through the four directions. To prevent exhaustion, volunteers took a break after three consecutive nights of HLC. The emanators were run continuously from 18:00 to 06:00.
Statistical analysis
All the data collected were entered into the Commcare® version 2.53.1 platform and parameters such as vector abundance assessed using descriptive statistics (means, proportions and 95% confidence intervals [CI]). Generalized linear mixed models (GLMM) using Template Model Builder (package glmmTMB) were fitted using negative binomial distribution for analysis of mosquito numbers at different emanation periods. Models were adjusted for repeated measures using the hut or compound ID and hour as random effects. All data analyses were performed using R statistical software version 4.1.2, and the significance level was set at α = 0.05.
Results
Species composition
During the study period, a total of 3995 mosquitoes were collected. Among all the Anopheles collected, the most abundant species was An. funestus, with a total of 2547, constituting 100% of the Anopheles population, followed by An. arabiensis with only 11 mosquitoes, representing 0%. In addition, a total of 1437 Culex spp. mosquitoes were collected. It is worth noting that only An. funestus is reported in these results, as it was the most abundant species of Anopheles collected.
Hut entry
Higher average numbers of An. funestus mosquitoes were recorded in the hut with LPG (N = 302.3) than in the control hut (N = 199.6) and the hut with metofluthrin and LPG (N = 36) (Table 2). Of mosquitoes that entered the hut, the metofluthrin SR knocked down 95.5% of the mosquitoes collected in the treated hut, resulting in 87.7% mortality after 24 h. The use of LPG increased An. funestus mosquito entry by 51%; however, indoor use of metofluthrin SR deterred An. funestus mosquito entry by 82%.
Table 2.
Mean density of Anopheles mosquitoes caught in the experimental huts during the evaluation of whether cooking with LPG gas has an impact on mosquito activity inside a house
| Mosquitoes collected | Collection location/point | Hut 1 (LPG only) | Hut 2 (metofluthrin + LPG) | Hut 3 (control) |
|---|---|---|---|---|
| An. funestus (female) | Floor | 0 | 19.4 | 0 |
| Net | 33.4 | 0 | 16.8 | |
| Roof | 93.4 | 0 | 53.4 | |
| Wall | 18.2 | 0 | 14.4 | |
| An. funestus (male) | Floor | 0 | 13 | 0 |
| Net | 42.2 | 0 | 17.6 | |
| Roof | 82.6 | 0 | 62.2 | |
| Wall | 15.8 | 0 | 20.4 | |
| An. gambiae (female) | Floor | 0 | 1.2 | 0.2 |
| Net | 3 | 0 | 4 | |
| Roof | 4.8 | 0 | 2.8 | |
| Wall | 1.4 | 0 | 1 | |
| An. gambiae (male) | Floor | 0 | 2.2 | 0.8 |
| Net | 3.2 | 0 | 2.8 | |
| Roof | 3 | 0 | 1.8 | |
| Wall | 1.2 | 0 | 1.4 | |
| Average An. funestus entry per hut | 302.2 | 35.8 | 199.6 | |
| Percentage of total collected per hut | 56.2 | 6.7 | 37.1 | |
Metofluthrin efficacy in the experimental hut
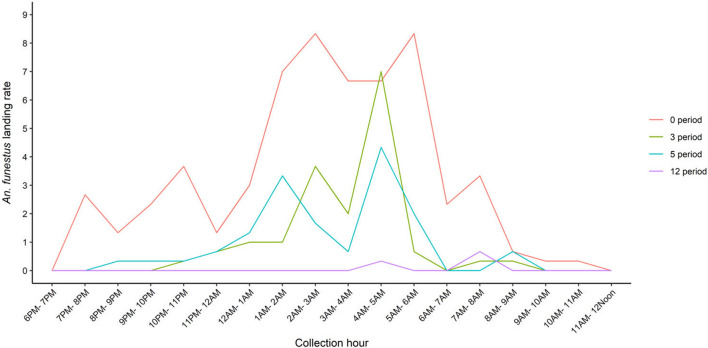
The landing rate of An. funestus was observed to be significantly lower in hut with a 12-h emanation period (odds ratio [OR] = 0.14; 95% CI [0.004–0.050]; P < 0.0001) when compared to control. However, there was no significant difference in landing rates in the hut with a 2-h emanation period (OR = 0.941; 95% CI [0.518–1.709]; P = 0.8418) compared with the control hut (Table 3). Adding an extra hour of emanation between 05:00 and 06:00 to the 2- and 4-h emanation periods resulted in a significant decrease in the landing rate of An. funestus in experimental huts. This reduction was observed in huts with a 3-h emanation period (RR = 0.195; 95% CI [0.104–0.364]; P < 0.0001) and 5-h emanation period (RR = 0.208; 95% CI [0.112–0.384]; P < 0.0001) compared with the 2-h and 4-h emanation periods, respectively (Table 3). The host-seeking trend of An. funestus in the experimental huts demonstrated a distinct bimodal pattern, with the first increase in mosquito activity occurring between 02:00 and 03:00, followed by a second peak between 05:00 and 06:00 (Fig. 3).
Table 3.
Mean landing rates of An. funestus in treated and untreated huts under different emanation periods
| Emanation period (h) | Mean | Relative risk | P-value |
|---|---|---|---|
| 12 | 0.056 | 0.014 (0.004–0.050) | < 0.0001 |
| 5 | 0.870 | 0.208 (0.112–0.384) | < 0.0001 |
| 4 | 3.775 | 0.566 (0.319–1.002) | 0.0507 |
| 3 | 0.944 | 0.195 (0.104–0.364) | < 0.0001 |
| 2 | 6.281 | 0.941 (0.518–1.709) | 0.8418 |
| 0 | 6.675 | Ref. |
Odds ratio, 95% confidence intervals and P-values were obtained from statistical analysis using generalized linear models. Data from the emanation periods were aggregated; all emanation periods were compared with the negative control
Fig. 3.
Host-seeking pattern of An. funestus in the experimental huts under different emanation periods. Emanation of 3 h indicates emanation between 18:00 and 20:00 and between 05:00 and 06:00, 5 h indicates emanation between 18:00 and 22:00 and between 05:00 and 06:00, and 12 h indicates emanation between 18:00 and 06:00 non-stop
Outdoor evaluation of metofluthrin SR
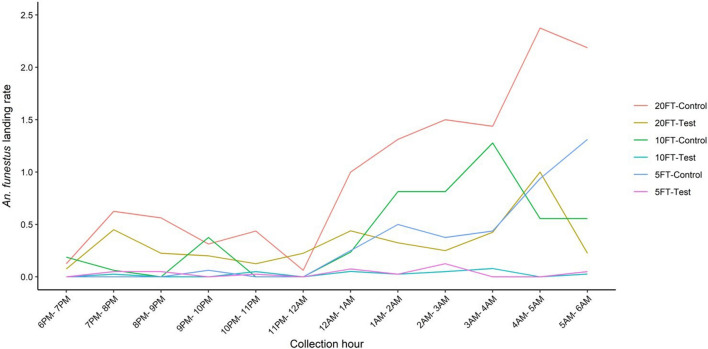
The mean landing rate of An. funestus mosquitoes per emanation distance is presented in Table 4. The mean landing rate of An. funestus in the presence of metofluthrin SR was significantly lower at all emanation distances than when metofluthrin SR was absent: 5 feet (RR = 0.151; 95% CI [0.070–0.327]; P < 0.001), 10 feet (RR = 0.063; 95% CI [0.021–0.192]; P < 0.001) and 20 feet (RR = 0.547; 95% CI [0.331–0.905]; P = 0.019). The outdoor host-seeking pattern exhibited two peaks in landing activity as shown in Fig. 4.
Table 4.
Comparison of mean landing rate of An. funestus outdoors in the presence and absence of metofluthrin SR at varying emanation distances
| Emanation distance | Metofluthrin SR | Mean | Relative risk | P-value |
|---|---|---|---|---|
| 5 feet | Yes | 0.034 | 0.151 (0.070–0.327) | < 0.001 |
| No | 0.323 | Ref. | ||
| 10 feet | Yes | 0.025 | 0.063 (0.021–0.192) | < 0.001 |
| No | 0.417 | Ref. | ||
| 20 feet | Yes | 0.331 | 0.547 (0.331–0.905) | 0.019 |
| No | 0.995 | Ref. |
Odds ratio, 95% confidence intervals and P-values were obtained from statistical analysis using generalized linear models. Data from the emanation distances were aggregated; all treatments were compared with the negative control
Fig. 4.
Outdoor host-seeking pattern of An. funestus in the villages at emanation distances of 5, 10 and 20 feet
Discussion
The deployment of metofluthrin SR in the experimental huts resulted in reduced entry, reduced biting and high knockdown and mortality of An. funestus within 24 h. Our study showed over 99% reduction in landing rates of An. funestus when metofluthrin emanators were placed indoors for 12 h. However, marginal variations were witnessed at reduced emanation periods (2-h and 4-h), and mosquito numbers increased within the next hour, indicating that the SR had no residual effect and thus may need to be paired with LLINs as a complementary tool when people go to bed [26]. In this experiment, additional coverage was considered by the addition of a 1-h period of emanation in the morning between 05:00 and 06:00 when mosquitoes have been shown to be most active and as people wake up and leave, their bednets [27]. The 2–4-h emanation at night would protect individuals inside the house before they go to sleep, where they would be under their bednets, and the additional hour in the morning would prevent biting when people are likely to be waking up and around the house before they leave for the day. This study, therefore, proposes targeted emanation periods for this product given the proposal to attach it to LPG cookers where it would passively emanate as people cooked their dinner and breakfast, respectively. Metofluthrin SR has shown potential for reducing human–mosquito contact [17, 28, 29], and here we demonstrate that an LPG-dependent emanator could be an effective complement to ensure reduced human–vector contact indoors when people are not under their LLINs.
In comparison with An. arabiensis, An. funestus demonstrated a distinct bimodal pattern in host-seeking both indoors and outdoors, with the first increase in mosquito activity occurring between 02:00 and 03:00, which is a time when the majority of people would be asleep under their LLINs, as reported previously [30, 31], followed by a second peak between 05:00 and 06:00. There was still a high level of mosquito activity even after the collections had stopped, suggesting that it may be necessary to conduct HLC collections in the late morning hours to monitor the behaviour of vectors. The second peak of mosquito activity occurred when most people were out of the protection of LLINs, and hence the need for additional vector control strategies like SRs. The high density of An. funestus reported in this study and others [32–34] in western Kenya indicates that An. funestus is the dominant malaria vector both indoors and outdoors, with an early morning peak in biting indicating potential biting when people are just stepping away from the cover of their LLINs. Thus, LLINs alone will not be sufficient for malaria vector control. The decline in the An. gambiae s.l. population in the study area could be because of LLIN use, as indicated in previous works [33, 35], or attributable to differences in their breeding preference, especially given that this study was conducted in the dry season when abundant An. funestus density was due to the swamps on the edge of Lake Kanyaboli.
Metofluthrin significantly reduced the An. funestus landing rate. However, its effectiveness decreased with increasing distance from the emanator (85% at 5 feet, 94% at 10 feet, and 45% at 20 feet). This variation in outdoor protective efficacy could be due to the effect of wind, as reported previously [36]. In addition, the technology lacks a residual effect, has high operational costs and requires a configuration that combines an emanator and cooker. This study confirms the protective efficacy of metofluthrin SR against mosquito bites, as reported in other studies [37], and in this case shows an additional benefit in outdoor use, indicating that SRs could play a role in both indoor and outdoor control of mosquitoes and could therefore be deployed in response to changing mosquito behaviour [38].
This study revealed increased entry of An. funestus mosquitoes into the huts with LPG cookers relative to the control hut, indicating the added attraction of mosquitoes to houses where LPG cookers are used. Previous experimental and observational studies assessing the combustion effects of LPG on mosquito behaviour in houses have reported similar effects of LPG on Anopheles mosquito behaviour [21, 39]. As LPG is projected to become the dominant fuel in many malaria-endemic countries [40], we must consider the fact that indoor LPG use is likely to increase exposure to Anopheles through increased household entry and host-seeking [41–43] due to increased production of carbon dioxide (CO2) per kilogram of fuel than commonly used fuels such as charcoal and wood [44, 45]. An increase as small as 0.01% in ambient CO2 levels above baseline levels can stimulate female mosquitoes to search for blood meals [20, 46] and could explain the increased abundance. Vector control tools that can be coupled with LPG stoves therefore could provide the additional benefit of reduced malaria transmission in Africa.
Conclusions
The deployment of the metofluthrin-based SR indoors almost completely prevented An. funestus landing indoors and led to 10 times lower biting rates within 10 feet of the emanator outdoors. The effectiveness of Thermacell®-based metofluthrin SRs certainly warrants their inclusion in the package of vector control tools aimed at reducing human–mosquito contact both indoors and outdoors. The observation of higher mosquito numbers with the use of LPG gas indoors suggests that cooking with LPG can potentially increase human–mosquito exposure to Anopheles mosquitoes, hence increasing malaria transmission.
Acknowledgements
We would like to thank the study volunteers who helped with the experiment both at the experimental huts and in the community.
Author contributions
EO and BA conceived the study. SA and EO designed the study and executed the trial. VM and MM analysed the data. SA wrote the manuscript. JK, SO, JG, NW, EO and NA revised the manuscript. All authors reviewed and approved the final manuscript for publication.
Funding
This study was supported with funds from Thermacell Repellents, Inc. The funder had no role in the design and implementation of the trial.
Data availability
All the raw data are available and were submitted alongside this manuscript.
Declarations
Ethics approval and consent to participate
This study was approved by the Kenya Medical Research Institute/Scientific and Ethics Review Unit 4536. Written consent forms were obtained from all study volunteers who participated in both the huts and the community.
Consent for publication
Individuals whose faces are shown in the images in this document consented to the publication of their pictures.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Silas Agumba, Email: s_agumba@yahoo.com.
Eric Ochomo, Email: ericochomo@yahoo.com.
References
- 1.WHO. World malaria report 2022. Geneva: World Health Organization; 2022.
- 2.Steketee RW, Campbell CC. Impact of national malaria control scale-up programmes in Africa: magnitude and attribution of effects. Malar J. 2010;9:299. doi: 10.1186/1475-2875-9-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killeen GF, Seyoum A, Gimnig JE, Stevenson JC, Drakeley CJ, Chitnis N. Made-to-measure malaria vector control strategies: rational design based on insecticide properties and coverage of blood resources for mosquitoes. Malar J. 2014;13:146. doi: 10.1186/1475-2875-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kypridemos C, Puzzolo E, Aamaas B, Hyseni L, Shupler M, Aunan K, et al. Health and climate impacts of scaling adoption of liquefied petroleum gas (LPG) for clean household cooking in Cameroon: a modeling study. Environ Health Perspect. 2020;128:47001. doi: 10.1289/EHP4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feachem RGA, Chen I, Akbari O, Bertozzi-Villa A, Bhatt S, Binka F, et al. Malaria eradication within a generation: ambitious, achievable, and necessary. Lancet. 2019;394:1056–1112. doi: 10.1016/S0140-6736(19)31139-0. [DOI] [PubMed] [Google Scholar]
- 7.White MT, Conteh L, Cibulskis R, Ghani AC. Costs and cost-effectiveness of malaria control interventions—a systematic review. Malar J. 2011;10:337. doi: 10.1186/1475-2875-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.malERA Consultative Group on Vector Control A research agenda for malaria eradication: vector control. PLoS Med. 2011;8:e1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Achee NL, Bangs MJ, Farlow R, Killeen GF, Lindsay S, Logan JG, et al. Spatial repellents: from discovery and development to evidence-based validation. Malar J. 2012;11:164. doi: 10.1186/1475-2875-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogoma SB, Ngonyani H, Simfukwe ET, Mseka A, Moore J, Killeen GF. Spatial repellency of transfluthrin-treated hessian strips against laboratory-reared Anopheles arabiensis mosquitoes in a semi-field tunnel cage. Parasit Vectors. 2012;5:54. doi: 10.1186/1756-3305-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawada H, Temu EA, Minjas JN, Matsumoto O, Iwasaki T, Takagi M. Field evaluation of spatial repellency of metofluthrin-impregnated plastic strips against Anopheles gambiae complex in Bagamoyo, coastal Tanzania. J Am Mosq Control Assoc. 2008;24:404–409. doi: 10.2987/5743.1. [DOI] [PubMed] [Google Scholar]
- 12.Lucas JR, Shono Y, Iwasaki T, Ishiwatari T, Spero N, Benzon GUS. laboratory and field trials of metofluthrin (SumiOne) emanators for reducing mosquito biting outdoors. J Am Mosq Control Assoc. 2007;23:47–54. doi: 10.2987/8756-971X(2007)23[47:ULAFTO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Hill N, Zhou HN, Wang P, Guo X, Carneiro I, Moore SJ. A household randomized, controlled trial of the efficacy of 0.03% transfluthrin coils alone and in combination with long-lasting insecticidal nets on the incidence of Plasmodium falciparum and Plasmodium vivax malaria in Western Yunnan Province, China. Malar J. 2014;13:208. doi: 10.1186/1475-2875-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Hulle S, Sagara I, Mbodji M, Nana GI, Coulibaly M, Dicko A, et al. Evaluation of the protective efficacy of a spatial repellent to reduce malaria incidence in children in Mali compared to placebo: study protocol for a cluster-randomized double-blinded control trial (the AEGIS program) Trials. 2022;23:259. doi: 10.1186/s13063-022-06197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochomo EO, Gimnig JE, Bhattarai A, Samuels AM, Kariuki S, Okello G, et al. Evaluation of the protective efficacy of a spatial repellent to reduce malaria incidence in children in western Kenya compared to placebo: study protocol for a cluster-randomized double-blinded control trial (the AEGIS program) Trials. 2022;23:260. doi: 10.1186/s13063-022-06196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traore MM, Junnila A, Traore SF, Doumbia S, Revay EE, Kravchenko VD, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali. West Africa Malar J. 2020;19:72. doi: 10.1186/s12936-020-3132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie SA, Devine GJ. Confusion, knock-down and kill of Aedes aegypti using metofluthrin in domestic settings: a powerful tool to prevent dengue transmission? Parasit Vectors. 2013;6:262. doi: 10.1186/1756-3305-6-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bibbs CS, Xue RD. OFF! clip-on repellent device with metofluthrin tested on Aedes aegypti (Diptera: Culicidae) for mortality at different time intervals and distances. J Med Entomol. 2016;53:480–483. doi: 10.1093/jme/tjv200. [DOI] [PubMed] [Google Scholar]
- 19.Dame DA, Meisch MV, Lewis CN, Kline DL, Clark GG. Field evaluation of four spatial repellent devices against Arkansas rice-land mosquitoes. J Am Mosq Control Assoc. 2014;30:31–36. doi: 10.2987/13-6379.1. [DOI] [PubMed] [Google Scholar]
- 20.Healy TP, Copland MJ. Activation of Anopheles gambiae mosquitoes by carbon dioxide and human breath. Med Vet Entomol. 1995;9:331–336. doi: 10.1111/j.1365-2915.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 21.Pope D, Bruce N, Higgerson J, Hyseni L, Ronzi S, Stanistreet D, MBatchou B, Puzzolo E. Household determinants of liquified petroleum gas (LPG) as a cooking fuel in south west Cameroon. Ecohealth. 2018;15:729–743. [DOI] [PMC free article] [PubMed]
- 22.Steketee RW, Brandling-Bennett AD, Kaseje DC, Schwartz IK, Churchill FC. In vivo response of Plasmodium falciparum to chloroquine in pregnant and non-pregnant women in Siaya District. Kenya Bull World Health Organ. 1987;65:885–890. [PMC free article] [PubMed] [Google Scholar]
- 23.Oumbouke WA, Fongnikin A, Soukou KB, Moore SJ, N'Guessan R. Relative performance of indoor vector control interventions in the Ifakara and the West African experimental huts. Parasit Vectors. 2017;10:432. doi: 10.1186/s13071-017-2365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Guidelines for laboratory and field-testing of long-lasting insecticidal nets. No. WHO/HTM/NTD/WHOPES/2013.1. Geneva: World Health Organization; 2013.
- 26.Kawada H, Nakazawa S, Shimabukuro K, Ohashi K, Kambewa EA, Foster PD. Effect of metofluthrin-impregnated spatial repellent devices combined with new long-lasting insecticidal nets (olyset® plus) on pyrethroid-resistant malaria vectors and malaria prevalence: field trial in south-eastern Malawi. Jpn J Infect Dis. 2020;73:124–131. doi: 10.7883/yoken.JJID.2019.311. [DOI] [PubMed] [Google Scholar]
- 27.Odero JI, Abong’o B, Moshi V, et al. Early morning anopheline mosquito biting, a potential driver of malaria transmission in Busia County, western Kenya Investigators. Res Sq. 2023 doi: 10.21203/rs.3.rs-3443261/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarella O, Ekwomadu U, Romer Y, Kirstein OD, Che-Mendoza A, González-Olvera G, et al. Experimental evaluation of a metofluthrin passive emanator against Aedes albopictus. PLoS ONE. 2022;17:e0267278. doi: 10.1371/journal.pone.0267278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bibbs CS, Kaufman PE, Xue RD. Adulticidal efficacy and sub lethal effects of metofluthrin in residual insecticide blends against wild Aedes albopictus (Diptera: Culicidae) J Econ Entomol. 2021;114:928–936. doi: 10.1093/jee/toaa321. [DOI] [PubMed] [Google Scholar]
- 30.Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, Githeko AK, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors. 2014;7:380. doi: 10.1186/1756-3305-7-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province. Kenya Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259. doi: 10.1186/s12936-015-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCann RS, Ochomo E, Bayoh MN, Vulule JM, Hamel MJ, Gimnig JE, Hawley WA, Walker ED. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am J Trop Med Hyg. 2014;90:597–604. doi: 10.4269/ajtmh.13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinya F, Mutero CM, Sang R, Owino EA, Rotich G, Ogola EO, et al. Outdoor malaria vector species profile in dryland ecosystems of Kenya. Sci Rep. 2022;12:7131. doi: 10.1038/s41598-022-11333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takken W. Do insecticide-treated bednets have an effect on malaria vectors? Trop Med Int Health. 2002;7:1022–1030. doi: 10.1046/j.1365-3156.2002.00983.x. [DOI] [PubMed] [Google Scholar]
- 36.Stevenson JC, Simubali L, Mudenda T, Cardol E, Bernier UR, Vazquez AA, et al. Controlled release spatial repellent devices (CRDs) as novel tools against malaria transmission: a semi-field study in Macha, Zambia. Malar J. 2018;17:437. doi: 10.1186/s12936-018-2558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darbro JM, Muzari MO, Giblin A, Adamczyk RM, Ritchie SA, Devine GJ. Reducing biting rates of Aedes aegypti with metofluthrin: investigations in time and space. Parasit Vectors. 2017;10:69. doi: 10.1186/s13071-017-2004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sukkanon C, Tisgratog R, Muenworn V, Bangs MJ, Hii J, Chareonviriyaphap T. Field evaluation of a spatial repellent emanation vest for personal protection against outdoor biting mosquitoes. J Med Entomol. 2021;58:756–766. doi: 10.1093/jme/tjaa213. [DOI] [PubMed] [Google Scholar]
- 39.Haines A, Bruce N, Cairncross S, Davies M, Greenland K, Hiscox A, et al. Promoting health and advancing development through improved housing in low-income settings. J Urban Health. 2013;90:810–831. doi: 10.1007/s11524-012-9773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van L, Richenda, Alex E, Besnik H. Increasing the use of liquefied petroleum gas in cooking in developing countries. 2017. https://documents.worldbank.org/curated/en/707321494347176314/pdf/114846-REVISED-LW74-LJ-fin-logo-OKR.pdf. Accessed 17 Sept. 2023.
- 41.Killeen GF, McKenzie FE, Foy BD, Schieffelin C, Billingsley PF, Beier JC. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am J Trop Med Hyg. 2000;62:535–544. doi: 10.4269/ajtmh.2000.62.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churcher TS, Trape JF, Cohuet A. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nat Commun. 2015;6:6054. doi: 10.1038/ncomms7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trape JF, Lefebvre-Zante E, Legros F, Ndiaye G, Bouganali H, Druilhe P, Salem G. Vector density gradients and the epidemiology of urban malaria in Dakar. Senegal Am J Trop Med Hyg. 1992;47:181–189. doi: 10.4269/ajtmh.1992.47.181. [DOI] [PubMed] [Google Scholar]
- 44.Edwards JH, Langpap C. Fuel choice, indoor air pollution and children's health. Environ Dev Econ. 2012;4:379–406. doi: 10.1017/S1355770X12000010. [DOI] [Google Scholar]
- 45.WHO indoor air quality guidelines. Household fuel combustion. Geneva: World Health Organization; 2014. [PubMed]
- 46.Webster B, Lacey ES, Cardé RT. Waiting with bated breath: opportunistic orientation to human odor in the malaria mosquito, Anopheles gambiae, is modulated by minute changes in carbon dioxide concentration. J Chem Ecol. 2015;41:59–66. doi: 10.1007/s10886-014-0542-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the raw data are available and were submitted alongside this manuscript.