Abstract
Background
Decreased efficacy of artemisinin-based combination therapy (ACT) for Plasmodium falciparum malaria has been previously reported in patients with sickle cell disease (SCD). The main purpose of this study was to investigate the in vitro susceptibility of isolates to dihydro-artemisinin (DHA) to provide a hypothesis to explain this treatment failure.
Methods
Isolates were collected from patients attending health centres in Abidjan with uncomplicated P. falciparum malaria. The haemoglobin type has been identified and in vitro drug sensitivity tests were conducted with the ring stage assay and maturation inhibition assay.
Results
134 isolates were obtained. Parasitaemia and haemoglobin levels at inclusion were lower in patients with haemoglobin HbSS and HbSC than in patients with normal HbAA. After ex vivo RSA and drug inhibition assays, the lowest rate of parasitic growth was found with isolates from HbAS red cells. Conversely, a significantly higher survival rate of parasites ranging from 15 to 34% were observed in isolates from HbSS. Isolates with in vitro reduced DHA sensitivity correlate with lower RBC count and haematocrit and higher parasitaemia at inclusion compared to those with isolates with normal DHA sensitivity. However, this decrease of in vitro sensitivity to DHA was not associated with Kelch 13-Propeller gene polymorphism.
Conclusion
This study highlights an in vitro decreased sensitivity to DHA, for isolates collected from HbSS patients, not related to the Pfkelch13 gene mutations. These results are in line with recent studies pointing out the role of the redox context in the efficacy of the drug. Indeed, SCD red cells harbour a highly different ionic and redox context in comparison with normal red cells. This study offers new insights into the understanding of artemisinin selective pressure on the malaria parasite in the context of haemoglobinopathies in Africa.
Keywords: Sickle cell anemia, Artemisinin combination therapy, Plasmodium falciparum, Anti-malarial drugs, Treatment resistance, In vitro drug monitoring
Background
Parasitic infections, such as Plasmodium falciparum malaria, are one of the major causes of morbidity and mortality in patients with HbSS, HbSC and HbCC sickle cell phenotypes [1–3]. Indeed, the development of the parasite in sickle cells [4–6], may cause vaso-occlusive crises and increase haemolytic anaemia through acute haemolysis episodes [7, 8]. Malaria is considered to be one of the main causes of hospitalization for patients with sickle cell disease (SCD) [2, 9]. Studies showed that the sickle cell trait (heterozygous HbAS) does not prevent malaria infection but protects against severe malaria [10, 11]. Despite a lower risk of malaria infection, people with homozygous status (HbSS) are at a higher risk of mortality [12, 13].
Resistance to artemisinin-based combination therapy (ACT) emerged in the Mekong region known to have a very high prevalence of haemoglobin E [14–16]. Recent studies showed that resistance of P. falciparum to artemisinin and its derivatives was based on a quiescence mechanism during the ring stage of the parasite [17, 18]. In Asia, this resistance was attributed to mutations in the propeller region the Kelch13 gene k13 [19]. Polymorphisms in the k13 gene have been recently found in Africa (Y493H, P553L, R561H, M476I, P574L, C580Y and A675V), including Ghana, Rwanda, Uganda, Tanzania [20–23]. Some of the mutations found in African countries are correlated with delayed parasite clearance. However, red blood cells (RBCs) with abnormal haemoglobin differ from normal RBCs in terms of redox potentials and calcium fluxes [24, 25]. They express higher level of reactive oxygen species (ROS) [26] and harbour a defect in antioxidant system [27] associated with externalization of phosphatidylserine [28] associated with capture of the cell by the spleen. ROS and superoxide are also involved in mechanism of action of artemisinin and in the resistance of the parasite [29, 30]. Metabolic changes observed in abnormal red cells could thus induce a high selective pressure on all the ionic regulation pathways of the parasite [4]. They could modulate the metabolic pathways involved in artesunate resistance and selecting resistant parasites, or decreased the efficacy of DHA on its target(s).
In the other side, and despite early description of mutations in the SERCA gene, other genes have been described to correlate with the decrease in artemisinin sensitivity [21, 22]. These genes are ferredoxin (fd), apicoplast ribosomal protein S10 (arps10), Plasmodium falciparum multidrug resistance protein 2 (pfmdr2), Plasmodium falciparum chloroquine resistance transporter (Pfcrt), Plasmodium falciparum adaptor protein complex 2 mu subunit (pfap2mu) and Plasmodium falciparum ubiquitin-specific protease 1 (pfubp1) [31–35]. All these genes are involved in the homeostasis of the cell content and their expression could be highly modulated when facing the very particular cytosol content of the abnormal red cells.
In Côte d’Ivoire, the studies by Tossea et al. showed, a prevalence of the major form (HbSS, HbSC) and the minor form (HbAS) of sickle cell disease in patients with uncomplicated malaria of 2% and 6%, respectively [36]. Since 2005, Côte d’Ivoire has been using artemisinin-based combinations in the treatment of uncomplicated P. falciparum malaria. A high prevalence of polymorphisms in the k13- gene of parasite isolates, was also described [37]. At the same time, Adjei et al. [38] in Ghana, as well as Gbessi et al. in Côte d’Ivoire [39] reported decreased efficacy of ACT in sickle cell patients correlated with a delay in P. falciparum clearance. This suggests a possible resistance of P. falciparum after treatment with artemisinin-based combinations in this population. Gbessi et al. [39] highlighted that a larger phenotypic complexity was found in the parasite populations of patients with SCD than in normal ones. However, the in vivo therapeutic efficacy test for ACT does not allow a direct analysis of parasites response to artemisinin derivatives because the additional effect of the second drug and of patient’s immunity on drug efficacy could mask the detection of chemo-resistant isolates in high transmission area.
Development of in vitro studies of P. falciparum with low sensitivity to artemisinin derivatives in sickle cell patients are urgently needed to discriminate between mechanisms involved i.e., mutation of genes or transcriptomic regulation gene expressions, and/or partial inactivation of the drug in the cytosol of the red cell. The aim of this study was to investigate the in vitro susceptibility to DHA, of parasites inducing malaria in sickle cell patients. For this purpose, phenotypic tests (ring stage assay and schizont maturation tests) and genotypic test (k13 gene sequence analysis) were carried out to evaluate the susceptibility of these parasites to DHA.
Methods
Ethical considerations
Studies were conducted according to the declaration of Helsinki and national legal and regulatory requirements. Protocol, case report form, and informed consent form were approved by the National Ethics and Research Committee of the republic of Côte d’Ivoire. An informed consent was required from each participant and/or parents or legal guardians of children. For children over the age of 9, informed consent was required prior to their inclusion in the study.
Study sites
The study was conducted from May 2017 to February 2020 in Côte d'Ivoire (RCI). In RCI and its neighbouring countries (Ghana, Burkina Faso and Mali) a high rate of sickle cell disease is found with a prevalence between 4 and 25% of the genetic traits [40, 41]. Due to its strategic geographical position between the Golf of Guinea and the Sahel, it is subject to a high migratory flow. Thus, crossbreeding and consanguineous marriages in Côte d'Ivoire are responsible for a sickle cell trait (HbAS, HbAC, HbSS and HbSC) rate of around 14%, with 2% of HbSS and HbSC phenotypes [42, 43]. Data obtained during clinical trials on the efficacy and tolerance of ACT conducted in different regions in CI, highlighted a prevalence of sickle cell disease in malaria patients around 2% for HbSS and HbSC phenotypes and 6% for the sickle cell trait (HbAS) [36].
This prospective study was carried out at the Clinical Hematology department at Yopougon University Hospital (YUH, Abidjan) and at the community health centre of Anonkoua-kouté (ANK, Abidjan). YUH is the reference centre for sickle cell disease in Côte d’Ivoire where about 10,000 patients with HbSS, HbSC and HbCC phenotypes are followed up with free access to medical care. ANK is a secondary level health structure which receives more than 400 patients daily. Patients attending this health centre can benefit from the typing of haemoglobin using acid acetate electrophoresis.
Patients recruited and samples collection
For patients suffering from fever attending both health structures, a clinical examination was performed before a biological confirmation of malaria. A first screening of malaria was carried out by lateral flow test. Positive results were validated by examination of thick and thin blood Giemsa-stained smears at × 100 with light microscopy. For P. falciparum positive sample, parasite density was calculated. After written informed consent of participants or of their legal representatives, all patients over 6 months of age with a parasite density beyond or equal to 0.1% were included in the study. An electrophoresis of haemoglobin was performed to all the patients registered. For enroled patients, a questionnaire was applied including demographic data, sex, age, place of residence, body temperature and clinical symptoms.
Patients with signs of severe malaria (WHO criteria) and/or requiring intensive medical care for other severe diseases, as well as those already treated with antimalarial drugs or antibiotics within the 30 days prior to medical consultation were not include and directly addressed to physician consultation with their biological results.
In YUH only patients with already known SCD and malaria were recruited. Whereas in Anonkoua-Kouté health centre all the patients with positive thick blood smears for P. falciparum were enroled after informed consent notwithstanding the result of the electrophoresis.
For each patient 3 mL of peripheral venous blood were collected on EDTA tubes for culture, and 2 ml of blood were collected on dry tubes for biochemical tests (CRP). Blood spots were also done with three drops (50 µl each) put on a Whatmann 3MM® filter paper and dried at room temperature for 4 h. For the two sites, samples were kept at 4 °C in an ice chest cooler and sent to the Malaria Unit of the Institut Pasteur of Côte d’Ivoire in less than four hours.
Haemoglobin status
Patients enroled at YUH were already aware of their sickle cell status and were all carriers of major forms (HbSS, HbSC and HbCC). These patients were routinely treated and followed up by the reference centre. Nevertheless, they genetic status was confirmed by PCR/ FRET (Fluorescence Resonance Energy Transfer) method [44]. At the Anonkoua-Kouté Health Centre, screening for SCD diagnosis was done by electrophoretic using an SAIO Electrophoresis instrument (PSE; Italy).
In vitro drug sensitivity test
In vitro tests were performed using RPMI-1640 (Eurobio 479604, 500 ml) medium supplemented with 5% Albumax II, 1% L-glutamine, 2% D-glucose, 0.05% hypoxanthine, 2.5% HEPES (Eurobio 251010) buffer and 0.5% gentamicin (Eurobio 524221). Serum and buffy coat were removed from the whole blood obtained from patients and red blood cells were washed three times in RPMI-1640 medium (centrifugation at 3000 rpm for 10 min) prior to cultivation. Samples were seeded in culture less than 5 h after blood collection. The cultures were conducted in a modular incubator chamber saturated with 5% O2, 5% CO2 and 90% N2 in a humidified atmosphere.
Ring-stage survival assay
The ex vivo RSA test was conducted according to Witkowski et al. [45] with minor modifications. To confirm viability of clinical isolates, two concentrations of dihydro-artemisinin (DHA) were used for each isolate, i.e. 700 nM and 70 nM. Dimethylsulfoxide (DMSO) at 0.1% was used as negative control.
The rest of the procedure did not change. Parasite culture mixture adjusted to 2% haematocrit was prepared. Initial parasitaemia of the isolates was between 0.1 and 1% and no new uninfected RBCs was added. Also, isolates were not synchronized prior to the assay. Briefly the modular incubator chamber was placed in an incubator at 37 °C for six hours. After 6 h of exposure to DHA, the red blood cells were washed three times with a preheated RPMI 1640 medium and suspended in a new complete medium. Cultures were incubated under the same conditions for sixty-six hours. At the end of the culture period (i.e., 72 h), Giemsa-stained thin blood smears were prepared and examined. The number of infected red blood cells containing viable parasites was counted by two independent investigators in a total of at least 10,000 red blood cells. Viable parasites with normal morphologic appearance (either ring stages, trophozoites, or schizonts) were counted to determine the survival rate [45]. The tests were considered to be valid when the parasitaemia at 72 h, in wells without any DHA (nonexposed culture) was higher than the initial parasitaemia [45]. Survival rates were calculated as the ratios of parasitaemia in wells with DHA (exposed) and in wells without (nonexposed) [45]. Parasite isolates demonstrating a survival rate higher than 1% in the RSA were considered to display reduced susceptibility to artemisinin [45, 46]. Based on previous comparative studies of ex vivo RSA and in vivo drug susceptibility tests [45, 46], a high survival rate higher than 10% is likely to have a clearance half-life after artemisinin treatment higher than five hours (cut-off with 89% sensitivity and 91% specificity).To confirm adequate culture conditions, the RSA tests were performed with two P. falciparum reference strains, i.e.K1 (artemisinin sensitive strain) and IPC 3445 (Cambodian strain resistant to artemisinin) as negative and positive control, respectively.
Maturation inhibition assay
The in vitro P. falciparum maturation test was conducted as developed by Jensen and Trager [47], standardized by Le Bras and Durand [48] and modified for fluorescent detection by Smilkstein et al. [49] and Basco [50]. Parasitized red blood cells were seeded in complete medium at haematocrit of 2%. In case of parasitaemia greater than 0.3%, type O positive washed healthy human erythrocytes were added to adjust parasitaemia to 0.3%. Dihydroartemisinin was added in duplicate in 96-well microtiter plate at concentrations ranging from 35.16 nM to 0.55 nM. As previously, incubation was conducted at 37 °C for 72 h in a modular incubator chamber saturated with 5% O2, 5% CO2 and 90% N2 in a humidified atmosphere. After incubation, cultures were frozen for 24 h to stop parasite growth. Cultures were thawed and parasite growth was assessed by SYBR Green I incorporation method using a spectrofluorometer (DELL, FLx800, Biotek) according to Smilkstein et al. [49], Basco [50] and Le Nagard et al. [51]. Drug concentrations inhibiting 50% of the parasite growth (IC50) were determined using IVART (In vitro Analysis and Reporting Tool) software from WWARN's [52]. Validation of each test was also assessed with IVART. Resistance thresholds of DHA (10 nM) was defined according to IVART. As a reference, K1 artemisinin-susceptible strain and MRA-1236 artemisinin-resistant clone (IPC 3445) were used. They were provided by the Malaria Research and Reference Reagent Resource Centre [53].
k13-propeller gene sequencing
Parasitic DNA was extracted from dried blood spots using a Qiagen kit according to the manufacturer's instructions. The fragment 1279–2127 of the coding sequence of k13 gene of P. falciparum was amplified by nested PCR according to Ariey et al. [19]. PCR products were sequenced according to Sanger method by Genewiz compagny. Sequences aligned by Seaview 5 were analysed using BioEdit software version 7.0.9.1 and compared to the k13 sequence (XM_001350122.1).
Statistical analysis
Statistical tests were performed using GraphPad Prism 7.0 version (GraphPad prism software Inc., San Diego, CA, USA), and Statistica v9. The Shapiro–wilk test was used to verify data normality. Medians with interquartile deviations were used for data that do not follow a normal distribution. Mann–Whitney U-test, Kruskal Wallis test and median test were used to compare groups. Correlations were determined using the Spearman test or Kendall Tau test. Comparisons were considered statistically significant when p ≤ 0.05.
Results
Study population
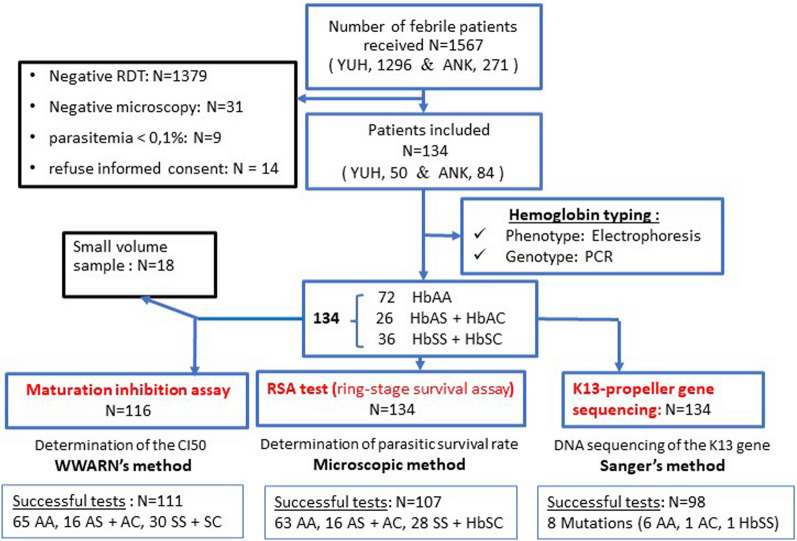
During the study, a total of 1567 patients attended health centres with suspected mild malaria infection. However, most of them (Fig. 1) had either a negative rapid test or a negative thin blood smear or have already undergone treatment, and were not included in the study. A total of 134 patients were enroled and blood sampled. Among them 72 had HbAA phenotypes, 26 were heterozygotes (HbAC or HbAS) and 36 were double mutated (HbSC or HbSS) (Fig. 1). Surprisingly no HbCC was found.
Fig. 1.
Flow chart of the study
In the present study, 70% of the recruited patients were children with ages ranging from 1 to 14 years old (Table 1). Also, there is no age difference between children with normal haemoglobin HbAA, pathological haemoglobin (HbSS, HbSC) or asymptomatic form (HbAS
Table 1.
Basic parameters of the study population
| HbAA (n = 72) | HbAC (n = 7) | HbAS (n = 19) | HbSC (n = 13) | HbSS (n = 23) | |
|---|---|---|---|---|---|
| Sexe ratio (M/F) | 1.1 | 2.5 | 0.4 | 0.5 | 1.6 |
| Age (years), (Mean ± SD) | 12.90 ± 11.08 | 13.47 ± 10.67 | 12.43 ± 4.76 | 10.17 ± 4.42 | 19.15 ± 14.12 |
| Body mass index (mean ± SD) | 20.65 ± 5.77 | 21.67 ± 4.54 | 19.06 ± 5.40 | 18.75 ± 4.22 | 14.41 ± 3.41 |
| Temperature (°C), (mean ± SD) | 39.58 ± 1.16 | 39.87 ± 1.56 | 38.29 ± 0.92 | 38.28 ± 0.71 | 38.22 ± 1 .13 |
| Parasitaemia (/µL of blood), Median (IQR) | 38315 (19515—53543) | 11200 (5610—57100) | 13670 (8220–42600) | 13,250 (4805–21800) | 16,300 (7900—43900) |
| Leukocytes (× 1000/µL), (mean ± SD) | 17.57 ± 15.71 | 13.51 ± 13.07 | 16.80 ± 9.82 | 23.58 ± 22.68 | 17.83 ± 7.80 |
| Erythrocytes (million/µL), (mean ± SD) | 4.65 ± 1.00 | 4.35 ± 0.99 | 3.44 ± 1.75 | 4.03 ± 1.79 | 2.48 ± 1.44 |
| Platelets (× 1000/µL), (mean ± SD) | 233 ± 78.63 | 205.20 ± 88.80 | 282.10 ± 106.21 | 189.37 ± 79.92 | 261.47 ± 114.75 |
| Haemoglobin level (G/100mL), (mean ± SD) | 11.28 ± 2.10 | 13.05 ± 2.53 | 8.66 ± 2.38 | 9.63 ± 1.71 | 6.51 ± 2.24 |
| CRP, (mean ± SD) | 55.31 ± 50.29 | 65.86 ± 61.84 | 68.74 ± 52.39 | 66.31 ± 46.51 | 68. 35 ± 40.60 |
| Haematocrit (%), (mean ± SD) | 38.64 ± 4. 60 | 43.55 ± 8.97 | 26.21 ± 7.88 | 27.15 ± 6.11 | 18.66 ± 6.71 |
| p-Values | HbAA Vs HbAC | HbAA Vs HbAS | HbAA Vs HbSC | HbAA Vs HbSS |
|---|---|---|---|---|
| Age (years) | 0.383 | 0.561 | 0.067 | 0.728 |
| Body mass index | 0.677 | 0.359 | 0.262 | < 0.001 |
| Temperature (°C) | 0.678 | < 0.001 | < 0.001 | < 0.001 |
| Parasitaemia ( /µL of blood) | 0.061 | 0.012 | < 0.001 | 0.014 |
| Leukocytes (× 1000 / µL) | 0.616 | < 0.001 | 0.248 | 0.054 |
| Erythrocytes (million/µL) | 0.576 | < 0.001 | < 0.001 | < 0.001 |
| Platelets (× 1000/µL) | 0.228 | 0.086 | 0.218 | 0.277 |
| Haemoglobin level (G/100mL | 0.083 | < 0.001 | 0.218 | < 0.001 |
| CRP | 0.937 | 0.207 | 0.748 | 0.103 |
| Haematocrit (%) | 0.196 | < 0.001 | < 0.001 | < 0.001 |
Significant p values are given in bold
HbSS homozygous pathological haemoglobin; HbSC double heterozygous pathological haemoglobin; HbAS and HbAC asymptomatic form; HbAA normal haemoglobin; F female; M mal; CRP C Reactiv protein; SD standard deviation; µL microlitre, IQR interquartile range, G gram, mL millilitre
and HbAC) in (Tables 1 and 2). Patients had usually received non-steroidal anti-inflammatory drugs before attending health centres. 81% (29/36) of the participants with a mutated haemoglobin (HbSS and HbSC). The mean temperature and median parasite density at inclusion were lower in patients with HbAS, HbSC and HbSS compared to normal HbAA group (Mann Whitney test, P < 0.001). Likewise, erythrocyte count, haemoglobin and haematocrit were lower at inclusion for HbAS, HbSC and HbSS patients compared to HbAA (Mann Whitney test, P < 0.001). This difference was also found between HbAC and HbSC groups (Mann Whitney test, P < 0.005), and HbSS versus HbAS groups (Mann Whitney test, P < 0.0003) (Table 1). Patients with abnormal HbSS phenotypes had lower haematological parameters than those with HbAS and HbSC forms. Indeed, anaemia (Hb < 11 g/dl blood) was more often found in patients with sickle cell phenotypes HbAS, HbSC and HbSS, than in HbAA (11.28 ± 2.10 g/L) and was more severe for HbSS phenotypes (6.51 ± 2.24 g/L) (Mann Whitney test, p < 0.001).
Table 2.
Distribution of different haemoglobin phenotypes by age group
| HbAA (n = 72) | HbAS | HbAC | HbSS | HbSC | ||
|---|---|---|---|---|---|---|
| (n = 19) | (n = 7) | (n = 23) | (n = 13) | |||
| Mean age (years). [± SD] | 12.90 ± 13 | 13.47 ± 10.67 | 12.43 ± 4.76 | 10.17 ± 4.42 | 19.15 ± 14.12 | |
| < 5 yrs | N (%) Patients | 29 (40.28%) | 6 (31.58%) | 0 (0%) | 5 (21.74%) | 2 (15.38%) |
| Mean age [± SD] | 3.3 ± 1.44 | 3.33 ± 1.36 | – | 4.6 ± 0.55 | 4 ± 0.0 | |
| > 5 yrs | N (%) Patients | 43 (59.72%) | 13 (68.42%) | 7 (100%) | 18 (78.26%) | 11 (84.61%) |
| Mean age [± SD] | 19.37 ± 13.33 | 18.15 ± 9.75 | 12.43 ± 4.76 | 11.72 ± 3.68 | 21.91 ± 13.60 | |
| P-Values | HbAA vs HbAS | HbAA vs HbAC | HbAA vs HbSS | HbAA vs HbSC | ||
| > 5 yrs | Mean age | 0.668 | 0.089 | 0.172 | 0.161 | |
N number of patients, HbSS homozygous pathological haemoglobin; HbSC double heterozygous pathological haemoglobin; HbAS and HbAC asymptomatic form; HbAA normal haemoglobin; SD standard deviation; yrs years
Ring-stage survival assay (RSA) and standard maturation test
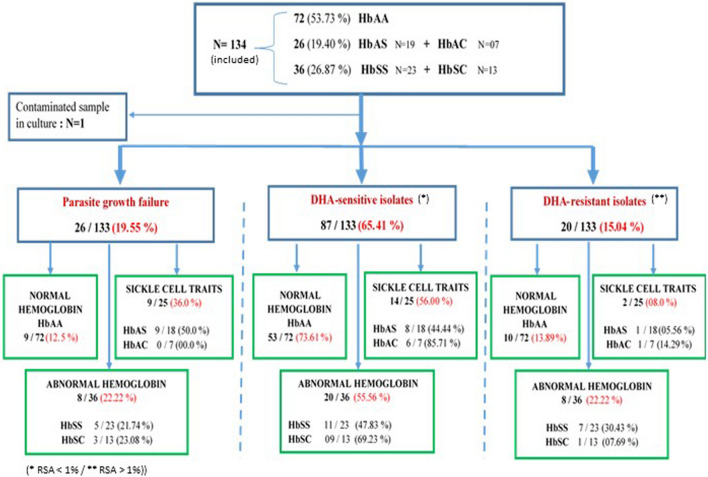
RSA and standard maturation tests were conducted in parallel for 134 and 116 patients respectively. Only 80% (107/134) and 96% (111/116) of RSA and standard maturation test were respectively successful (Fig. 2). Indeed, a low rate of parasitic growth occurred more often with HbAS red cell (Figs. 2, 3A). RSA values for all collected clinical isolates varied from 0 to 33.75% (i.e. ratio of parasitaemia in culture with DHA 700 nM and in culture without DHA). Based on RSA results and according to a threshold of maturation rate of 1%, 65% (87/133) of the clinical isolates were sensitive (< 1%), and 15% (20/133) had a decreased of sensitivity (> 1%) to DHA 700 nM (Fig. 2B).
Fig. 2.
Flow chart of the results of the ex vivo RSA test 700 nM DHA
Fig. 3.
Ex vivo RSA test. A frequency of test failure and low-sensitivity according to the type of haemoglobin. B Sensitivity of isolates to DHA according to the type of haemoglobin. When the proportion of viable parasites in the non-exposed culture (DMSO) at 72 h was higher than the initial parasitaemia at 0 h, the samples were considered to be interpretable. Survival rates is the ratio of parasitaemia in exposed (DHA) and non-exposed cultures (DMSO) calculated as: (parasitaemia at 70 or 700 nM DHA exposed/parasitaemia at 0 nM control) × 100. Geometric means of the isolates is plotted. Isolates with a survival rate of more than 1% were classified as in vitro artemisinin-resistant isolates, and with more than 10% as in vivo resistant
For these twenty isolates, the RSA values varied from 1.28 to 33.75%. All the types of sickle-cell phenotypes were concerned by this decreased sensitivity, but 39% of them were for the HbSS group (Figs. 2, 3B). Indeed, 28% of isolates from HbSS phenotype had survival rates ranging from 14.68% to 33.75% (Figs. 3, 4A).
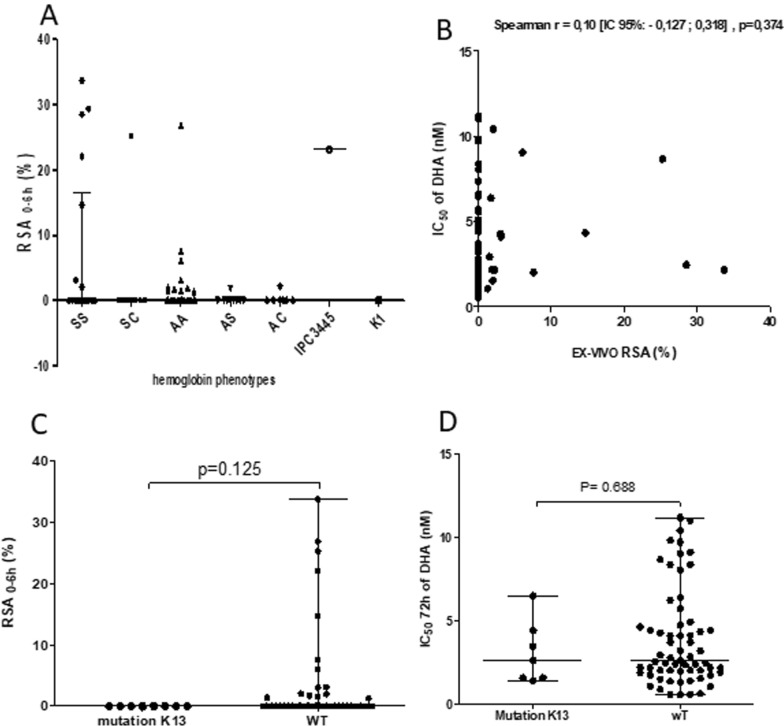
Fig. 4.
In vitro parasites drug sensitivity results. A Distribution of Plasmodium falciparum survival rates in RSA according to the type of haemoglobin. Survival rate was calculated as previously for 700 nM DHA. Geometric means with 95% confidence intervals of the survival rates are shown. B Spearman’s correlation between RSA (%) and IC50 values for DHA for the same isolate. C Correlation of parasite survival rates (Ex-vivo RSA) and k13 polymorphisms. RSA values between parasites with mutations in the kelch propeller domain (> 440 amino acid) and parasites without kelch mutations. D Comparison of CI50 values for DHA between the group of k13 wild-type parasite and the group with mutations in the propeller domain (> 440 amino acid)
In these isolates, proportions of early ring-stage parasites at enrolment were high (70% to 75% of the parasites detected were rings). Parasites developed in HbSS haemoglobin are thus less sensitive in culture to DHA than parasites grown in HbAS, HbAC, HbSC and HbAA haemoglobin (Figs. 2, 3 and 4A). K1 parasite had 0% survival rate whereas IPC 3445 had 23.14%. Three clinical isolates from HbSS patients exhibited higher survival rates (28,53%, 29,40% and 33,75%) at the DHA 700 nM than the artemisinin-resistant IPC 3445 clone (Fig. 4A).
In the same way, 86% (95/111) of the in vitro standard maturation test were successful, with Inhibition Concentration 50% (IC50) ranging between 0.53 and 11.18 nM (geometric mean: 2.71 nM, CI [2.32–3.17], range: [0.53–11.18]) for a threshold of resistance to DHA at 10 nM. However, when comparing RSA and maturation tests with the same isolates (Fig. 4B), some isolates with a DHA-sensitive phenotype in the RSA presented a resistant phenotype in standard maturation test and vice versa. In this study, the pairwise comparison of the two tests was not significant (n = 81, Spearman r = 0.10 [95% CI − 0.127; 0.318], p = 0.374). Maturation test IC50 values were also not correlated with RSA survival rates.
Relations between RSA test and clinical and biologic parameters at enrolment
In order to take into account confounding variables, quantitative parameters of hosts (age, body mass index, CRP, haemoglobin level, haematocrit) were compared firstly with parasite growth rates, and secondly between RSA susceptible or less sensitive (below and above 1%) isolates. These parameters were also compared between RSA sensitivity and partially resistance isolates (with parasite survival rate higher than 10% at 700 nM) (Table 3).
Table 3.
Relationship between Ex vivo RSA test results and patients clinical and biologic parameters at enrolment
| Count of P. f isolates | Failure of RSA test | In vitro RSA resistance test (RSA > 1%) | Significant in vivo resistance (RSA > 10%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure of RSA test | Successful RSA test | p value | Sensitive (RSA survival < 1%) | Resistant (RSA survival ≥ 1%) | p value | Sensitive (RSA survival < 10%) | Resistant (RSA survival ≥ 10%) | p value | |
| N = 26 | N = 107 | N = 87 | N = 20 | N = 107 | N = 07 | ||||
| mean (N) | mean (N) | mean (N) | mean (N) | mean (N) | mean (N) | ||||
| Age | 13.00 (26) | 11.00 (107) | 0.892 | 11.00 (87) | 12.00 (20) | 0.740 | 11.00 (102) | 13.00 (7) | 0.492 |
| ParaT0 | 21130 (26) | 24070 (107) | 0.454 | 30795 (87) | 44778 (20) | 0.006 | 22730 (102) | 43900 (7) | 0.076 |
| CRP | 96.00 (26) | 44.00 (106) | 0.151 | 42.00 (86) | 67.50 (20) | 0.843 | 41.00 (101) | 96.00 (7) | 0.524 |
| GB | 14.77 (25) | 13.94 (100) | 0.410 | 13.80 (80) | 16.19 (20) | 0.110 | 13.90 (95) | 21.80 (7) | 0.118 |
| GR | 03.07 (25) | 04.32 (100) | 0.016 | 04.37 (80) | 3.81 (20) | 0.013 | 04.37 (95) | 01.63 (7) | 0.002 |
| HB | 08.37 (26) | 10.40 (105) | 0.005 | 10.60 (85) | 10.30 (20) | 0.220 | 10.82 (100) | 05.67 (7) | 0.003 |
| PLAQ | 271.1 (25) | 231.0 (100) | 0.541 | 222.5 (80) | 236.0 (20) | 0.403 | 230.0 (95) | 319.0 (7) | 0.314 |
| HCT | 28.10 (25) | 37.08 (100) | 0.006 | 37.40 (80) | 35.40 (20) | 0.009 | 37.40 (95) | 14.50 (7) | 0.001 |
| BMI | 18.85 (26) | 18.30 (105) | 0.940 | 19.00 (87) | 16.80 (20) | 0.118 | 18.60 (100) | 15.50 (7) | 0.100 |
| Temp | 38.45 (26) | 39.10 (107) | 0.060 | 39.10 (87) | 38.40 (20) | 0.076 | 39.10 (100) | 37.90 (7) | 0.064 |
Significant p values are given in bold
N represents the number of isolates, the numbers in brackets represent the mean; RSA, ring-stage survival assay
ParaT0, parasitaemia at inclusion (per µL); CRP GB Leukocytes level (× 1000/µL); GR Erythrocytes level (million/µL); HB Haemoglobin level (G/100 mL); PLAQ Platelets level (× 1000/µL); HCT Haematocrit (%); BMI Body Mass Index; Temp temperature (°C)
Firstly red blood cell count, haemoglobin level and haematocrit level at inclusion were significantly lower in patients carrying isolates with failure of the test, compared to isolates with growth rate > 1% (Mann Whitney test, P = 0.016, P = 0.005, and P = 0.006 respectively) (Table 3). In the same way, patients with DHA- less sensitive isolates (RSA > 1% at DHA 700 nM) had a lower red blood cell count and haematocrit level compared to DHA-sensitive isolates. DHA-partially resistant isolates (RSA > 10%) had also a significantly higher parasitaemia at inclusion than DHA-sensitive isolates (44778 ± 22347 Vs 30795 ± 26671; Mann Whitney test, P = 0.006).
For HbSS patients, RSA DHA- less sensitive isolates had a significantly higher parasite growth rate compared to DHA-sensitive isolates (5.04 ± 4.92% Vs 1.57 ± 0.44%; Mann Whitney test, P = 0.0002). For the isolates with very low sensitivity (partial resistance RSA > 10%) low red blood cell count, haemoglobin level and haematocrit at inclusion were observed only for HbSS patients (P-value: 0.002, 0.003, and 0.001, respectively). Overall, parasites collected in patients with HbSS phenotype harbored more often isolates with less sensitivity to DHA 700 nM (Table 3).
Survival rate and point mutations in the PfKelch 13 gene
Genomic DNA was obtained from 134 isolates and an 849 bp PCR fragment corresponding to the Kelch13 Propeller region was amplified and sequenced. Polymorphism analysis was possible for 74% (99/134) of these PCR products. Overall, 16 SNPs (Single Nucleotide Polymorphisms) were detected for only 7% of the sequences (i.e., 7/99). Among these SNPs, 94.44% were non-synonymous mutations (15/16). The synonymous mutation (G287G) was located before the propeller region of the k13 gene (< 442 amino acids), while the 15 non-synonyms were located in this propeller region. Mutations were all different (Table 4). No key mutations already identified in the Kelch 13 propeller domain by other authors (such as C580Y, R539T, Y493H, P574L, I543T, F446I, R561H, A675V) and associated with a delay in parasite clearance was found. Only one mutation was found for patients with HbSS or HbAC phenotypes. No mutation was associated with a decreased drug susceptibility both for RSA and standard maturation test. No difference in IC50 values for DHA 700 nM was found between isolates with and without mutations (Mann Whitney U test, P = 0.688) (Fig. 4C, D).
Table 4.
Mutations identified in kelch13 propeller gene of Plasmodium falciparum in abnormal haemoglobin (sickle cell disease) patients
| Type of mutation | clinical isolates | Type of Haemoglobin | Sensitivity to DHA | Nucleic acid | Amino-Acid | HbAA | HbAS | HbAC | HbSS | HbSC |
|---|---|---|---|---|---|---|---|---|---|---|
| Synonymous | ANK-074 | HbAA | Sensitive | GGC→GGT | G287G | 1 | – | – | – | – |
| Non-synonymous | ANK-030 | HbAC | Sensitive | TTA→TTT | L462F | – | – | 1 | – | – |
| ANK-035 | HbAA | Sensitive | TGG→GGG | W565G | 1 | – | – | – | – | |
| AAT→TAT | N585Y | 1 | – | – | – | – | ||||
| GGT→GCT | G595A | 1 | – | – | – | – | ||||
| TAT→TCT | Y635S | 1 | – | – | – | – | ||||
| GGA→AGG | G450R | 1 | – | – | – | – | ||||
| AAT→AAA | N498K | 1 | – | – | – | – | ||||
| ANK-054 | HbAA | Sensitive | GTG→GCG | V568A | 1 | – | – | – | – | |
| ANK-060 | HbAA | Sensitive | AAT→CAT | N554H | 1 | – | – | – | – | |
| TAT→CAC | Y588H | 1 | – | – | – | – | ||||
| TTT→GTT | F451V | 1 | – | – | – | – | ||||
| GTG→GGT | V520G | 1 | – | – | – | – | ||||
| ANK-034 | HbAA | Parasite growth failure | TGT→AGT | C447S | 1 | – | – | – | – | |
| TCT→TTT | S549F | 1 | – | – | – | – | ||||
| YOP-042 | HbSS | Sensitive | TAT→GAT | Y519D | – | – | – | 1 | ||
| Mutations observed | 7 | – | 16 | 16 | 14 | 0 | 1 | 1 | 0 | |
| Total sequenced 99 | – | – | – | 57 | 13 | 3 | 17 | 9 | ||
Sensitivity to DHA (RSA survival ≥ 1% = ‘low sensitivity” and RSA survival < 1% = ‘Sensitive’)
Discussion
In vitro tests and gene polymorphism analysis combined with in vivo clinical studies, can serve as predictive markers for epidemiological surveillance of artemisinin resistance [19, 52, 54]. However, very few studies address sensitivity of isolates from patients with abnormal haemoglobin. This question is of importance, as in Côte d’Ivoire almost 20% of the population is carrying at least an abnormal haemoglobin gene. Homozygote patients are at risk of severe occlusive crisis when infected with malaria. In the same time, cytosolic content of the abnormal red blood cells [55–57] can provide a specific biochemical environment susceptible to select or promote ACT decreased sensitivity in field isolates.
The participants included in the study were divided into four groups according to their sickle cell phenotypes (HbSS, HbSC, HbAS, HbAC) and HbAA as control. No significant difference in mean age was found, underlining the efficacy of the clinical management of children with SCD at the YUH [9, 58, 59]. Likewise, patients with abnormal HbSS phenotypes had lower haemoglobin content than those with HbAS, HbAC, HbSC and HbAA forms. As already reported, parasitaemia and haemoglobin levels at inclusion were lower in sickle cell patients with HbSS and HbSC than in patients with normal phenotype [60–62]. This low parasite density could be an element explaining a protective effect against severe malaria. It could be due (i) to dehydration of red blood cells which could inhibit the invasion and growth of P. falciparum parasites [62–64]; or (ii) to inhibition of osmotic shock in HbSS phenotype erythrocytes [65] resulting in reduced merozoite release [66, 67]. The short lifespan of sickle cell erythrocytes and the clearance of erythrocytes infected by P. falciparum can reduce also the parasite density. Malaria alters the red blood cells during the endo-erythrocytic phase of the development of P. falciparum and the phenomenon of sequestration of parasitized red blood cells affects the circulation and consequently provokes a vaso-occlusive crisis. Due to their particular intra-erythrocyte microenvironment [56, 57] attention must be paid to confirm viability of the parasites during the in vitro culture. A lower dose of drug i.e., 70 nM of DHA was introduced as control during the RSA test as this low dose can be tolerated by most of the parasites and give an internal control of viability of the parasites.
Witkowski et al. showed a strong correlation between ex vivo RSA survival rates at DHA 700 nM and in vivo parasite clearance half-lives in Cambodia [45], with a 89% sensitivity and 91% specificity [45]. Overall, in this study, the survival rates obtained during RSA test at DHA 700 nM, showed higher values for isolates from patients with HbSS phenotype than others. These data suggest that these isolates have a decreased sensitivity to DHA in vitro (survival rate > 1%), and potentially in vivo (survival rate > 10%). These results could be due to a higher density of ring stages (70–75%) before culture which are known to enter quiescence in the presence of DHA 700 nM [17, 68, 69]. Because many factors such as levels of host immunity and pharmacokinetics could modulate drug clinical effectiveness, further correlation of ex vivo RSA and in vivo studies is strongly required in the various malaria endemic regions with different population ethnicities and malaria ecologies.
Nevertheless, mutations in the k13 gene associated with decrease in sensitivity to DHA (in particular the WHO-validated C580Y, R539T, Y493H, P574L, I543T, F446I, R561H, A675V, N458Y [70]) have not be found during this study. This absence of link between k13 mutations and DHA sensitivity was already described elsewhere in Africa, as in Cameroon [71], Uganda [72] and even in Cambodia [73]. However, several other genes could be involved in the resistant phenotype as falcipain 2a (FP2a) a cysteine protease and haemoglobinase. Mutations in this enzyme (FP2a) reduce enzymatic activity and haemoglobin digestion, and increase the survival rate in the ring stage of P. falciparum [74, 75]. Mutations outside the k13 gene could also induce compensation effect as already reported with the Pfcrt gene in French Guiana [76–78]. However, this study supports overall the idea that isolates from HbSS sickle cell patients, can express DHA resistant phenotype without k13 gene polymorphism [70, 71, 79].
However, a recent work in Abidjan underlined as well a higher genetic complexity of the parasite isolates in patients with SCD or trait compared with control ones [39]. This can point out selection of a specific set of parasites entering abnormal red cells. To test this hypothesis, sequencing of the full genome of parasites collected during this study is in process. In these strains, low sensitivity could be the result of an adaptation of the parasite to novel micro-environmental or biochemical conditions [24, 57, 80] with a different transcriptome regulation and activation of specific genes [81]. Gene expression analysis studies should than be conducted. Abnormal RBCs contain could also simply inhibited artemisinin efficacy by inactivation or decrease interaction of the molecule with its target(s). Resistance could at last be explained by activation of alternative metabolic pathways as the “unfolded protein pathways” which seem up-regulated to attenuate artemisinin-induced protein damage [81]. One of the last hypotheses is a higher capacity of resistant parasite to tackle with high level of oxidative radical as these are particularly high in SCD. Resistant parasites have an undoubtable advantage to develop in these cells.
Conclusion
This work demonstrates that malaria isolates can exhibit low DHA-sensitivity when HbSS RBCs in vitro, which is not related to polymorphism in the propeller region of the Pfkelch 13 gene. The decreased sensitivity of P. falciparum to anti-malarial drugs will challenge malaria control. This study also provides evidence of an absence of relationship between Pfkech13 polymorphism and survival rate in RSA test in sickle cell patients living in Abidjan. Taken together, these results highlight the need for appropriate and effective treatment in these subjects to protect them from severe attacks and to avoid the emergence of truly resistant strains.
Acknowledgements
We thank all the patients who agreed to participate, and the staff of YUH and ANK for their help. The authors would also like to acknowledge the National Malaria Control Program (PNLP-CI).
Abbreviations
- ACT
Artemisinin-based combination therapy
- SCD
Sickle cell disease
- DHA
Dihydro-artemisinin
- ROS
Reactive oxygen species
- YUH
Yopougon University Hospital
- ANK
Community health centre of Anonkoua-kouté
- PCR
Polymerase chain reaction
- FRET
Fluorescence Resonance Energy Transfer
- RSA
Ring-stage survival assay
- IC50
Inhibition Concentration 50%
- CI
Confidence interval
- WHO
World Health Organization
- Hb
Haemoglobin
Author contributions
AAG collected the samples, practiced culture tests, analyzed data and wrote the first draft; OAT coordinated the study, contributed to the writing; BAA analyzed genomic data, TSK practiced SS molecular typing, SEA analyzed genomic data, EAG collected the samples, practiced culture tests, LTN practiced culture tests; KT practiced culture tests, SB practiced culture tests, SBA supervised patients recruitment, FAY coordinated the study, contributed to the writing; IS coordinated patients’ management; RJ supervised the study both at the clinical and laboratory stages, obtained funds, analyzed data and proposed the final version of the manuscript.
Funding
This programme and AG were supported by a grant from the Rotary Foundation.
Availability of data and materials
The data that support the findings of this study are available from Andre Toure but restrictions apply to the availability of these data, as they are not publicly available. Data are however available from the authors upon reasonable request and with permission of Ministry of Health of Ivory Coast.
Declarations
Ethics approval and consent to participate
This project received agreement from the National Ethic Committee of Ivory Coast. Patients gave their written consent to participate.
Consent for publication
NA No personal data is presented in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis. 2010;14:e2–e12. doi: 10.1016/j.ijid.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Makani J, Komba AN, Cox SE, Oruo J, Mwamtemi K, Kitundu J, et al. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115:215–220. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 4.Naumann KM, Jones GL, Saul A, Ross S. Parasite-induced changes to localized erythrocyte membrane deformability in Plasmodium falciparum cultures. Immunol Cell Biol. 1992;70:267–275. doi: 10.1038/icb.1992.34. [DOI] [PubMed] [Google Scholar]
- 5.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 6.Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ. 2001;79:704–712. [PMC free article] [PubMed] [Google Scholar]
- 7.Nebor D, Bowers A, Hardy-Dessources M-D, Knight-Madden J, Romana M, Reid H, et al. Frequency of pain crises in sickle cell anemia and its relationship with the sympatho-vagal balance, blood viscosity and inflammation. Haematologica. 2011;96:1589–1594. doi: 10.3324/haematol.2011.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stettler N, McKiernan CM, Melin CQ, Adejoro OO, Walczak NB. Proportion of adults with sickle cell anemia and pain crises receiving hydroxyurea. JAMA. 2015;313:1671–1672. doi: 10.1001/jama.2015.3075. [DOI] [PubMed] [Google Scholar]
- 9.Serjeant GR. Mortality from sickle cell disease in Africa. BMJ. 2005;330:432–433. doi: 10.1136/bmj.330.7489.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams TN, Mwangi TW, Wambua S, Alexander ND, Kortok M, Snow RW, et al. Sickle celle trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–186. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams TN. Human red blood cell polymorphisms and malaria. Curr Opin Microbiol. 2006;9:388–394. doi: 10.1016/j.mib.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS ONE. 2011;6:e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams TN, Obaro SK. Sickle cell disease and malaria morbidity: a tale with two tails. Trends Parasitol. 2011;27:315–320. doi: 10.1016/j.pt.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Thriemer K, Van Hong N, Rosanas-Urgell A, Phuc BQ, Ha DM, Pockele E, et al. Delayed parasite clearance after treatment with dihydroartemisinin-piperaquine in Plasmodium falciparum malaria patients in central Vietnam. Antimicrob Agents Chemother. 2014;58:7049–7055. doi: 10.1128/AAC.02746-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang B, Deng C, Yang T, Xue L, Wang Q, Huang S, et al. Polymorphisms of the artemisinin resistant marker (K13) in Plasmodium falciparum parasite populations of Grande Comore Island 10 years after artemisinin combination therapy. Parasit Vectors. 2015;8:634. doi: 10.1186/s13071-015-1253-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohashi J, Naka I, Patarapotikul J, Hananantachai H, Brittenham G, Looareesuwan S, et al. Extended linkage disequilibrium surrounding the haemoglobin E variant due to malarial selection. Am J Hum Genet. 2004;74:1198–1208. doi: 10.1086/421330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witkowski B, Lelievre J, Lopez Barragan MJ, Laurent V, Su XZ, Berry A, et al. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–7. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyser T, Paloque L, Ouji M, Nguyen M, Ménard S, Witkowski B, et al. Identification of compounds active against quiescent artemisinin-resistant Plasmodium falciparum parasites via the quiescent-stage survival assay (QSA) J Antimicrob Chemother. 2020;75:2826–2834. doi: 10.1093/jac/dkaa250. [DOI] [PubMed] [Google Scholar]
- 19.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois A-C, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bwire GM, Ngasala B, Mikomangwa WP, Kilonzi M, Kamuhabwa AAR. Detection of mutations associated with artemisinin resistance at k13-propeller gene and a near complete return of chloroquine susceptible falciparum malaria in Southeast of Tanzania. Sci Rep. 2020;10:3500. doi: 10.1038/s41598-020-60549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uwimana A, Umulisa N, Venkatesan M, Svigel SS, Zhou Z, Munyaneza T, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis. 2021;21:1120–1128. doi: 10.1016/S1473-3099(21)00142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asua V, Conrad MD, Aydemir O, Duvalsaint M, Legac J, Duarte E, et al. Changing prevalence of potential mediators of aminoquinoline, antifolate, and artemisinin resistance across Uganda. J Infect Dis. 2021;223:985–994. doi: 10.1093/infdis/jiaa687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndwiga L, Kimenyi KM, Wamae K, Osoti V, Akinyi M, Omedo I, et al. A review of the frequencies of Plasmodium falciparum Kelch 13 artemisinin resistance mutations in Africa. Int J Parasitol Drugs Drug Resist. 2021;16:155–161. doi: 10.1016/j.ijpddr.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiley JS, McCulloch KE. Calcium ions, drug action and the red cell membrane. Pharmacol Ther Dent. 1982;18:271–292. doi: 10.1016/0163-7258(82)90070-5. [DOI] [PubMed] [Google Scholar]
- 25.Cheemadan S, Ramadoss R, Bozdech Z. Role of calcium signaling in the transcriptional regulation of the apicoplast genome of Plasmodium falciparum. Biomed Res Int. 2014;2014:869401. doi: 10.1155/2014/869401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gizi A, Papassotiriou I, Apostolakou F, Lazaropoulou C, Papastamataki M, Kanavaki I, et al. Assessment of oxidative stress in patients with sickle cell disease: the glutathione system and the oxidant–antioxidant status. Blood Cells Mol Dis. 2011;46:220–225. doi: 10.1016/j.bcmd.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Zwieten RV, Vherhoeven AJ, Roos D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic Biol Med. 2014;67:377–386. doi: 10.1016/j.freeradbiomed.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Tinku B, Kuypers FA. Reactive oxygen species and phosphatidylserine externalization in murine sickle red cells. Br J Haematol. 2004;124:391–402. doi: 10.1046/j.1365-2141.2003.04781.x. [DOI] [PubMed] [Google Scholar]
- 29.Egwu CO, Augereau JM, Reybier K, Benoit-Vical F. Reactive oxygen species as the brainbox in malaria treatment. Antioxidants. 2021;10:1872. doi: 10.3390/antiox10121872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egwu CO, Ioannis T, Pério P, Augereau JM, Benoit-Vical F, Reybier K. Superoxide: a major role in the mechanism of action of essential antimalarial drugs. Free Radic Biol Med. 2021;167:271–275. doi: 10.1016/j.freeradbiomed.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Adams T, Ennuson NAA, Quashie NB, Futagbi G, Matrevi S, Hagan OCK, et al. Prevalence of Plasmodium falciparum delayed clearance associated polymorphisms in adaptor protein complex 2 mu subunit (pfap2mu) and ubiquitin specific protease 1 (pfubp1) genes in Ghanaian isolates. Parasit Vectors. 2018;11:175. doi: 10.1186/s13071-018-2762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miotto O, Amato R, Ashley EA, Macinnis B, Almagro-Garcia J, Amaratunga C, et al. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet. 2015;47:226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henrici RC, Van-Schalkwyk DA, Sutherland CJ. Modification of pfap2μ and pfubp1 markedly reduces ring-stage susceptibility of Plasmodium falciparum to artemisinin in vitro. Antimicrob Agents Chemother. 2020;64:e01542–e1619. doi: 10.1128/AAC.01542-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amato R, Campino S, Mead D, Drury E, Kekre M, Sanders M, et al. Genomic epidemiology of artemisinin resistant malaria. Elife. 2016;5:e08714. doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henriques G, Hallett RL, Beshir KB, Gadalla NB, Johnson RE, Burrow R, et al. Directional selection at the pfmdr1, pfcrt, pfubp1, and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J Infect Dis. 2014;210:2001–2008. doi: 10.1093/infdis/jiu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tossea SK, Adji EG, Coulibaly B, Ako BA, Coulibaly DN, Joly P, et al. Cross sectional study on prevalence of sickle cell alleles S and C among patients with mild malaria in Ivory Coast. BMC Res Notes. 2018;11:215. doi: 10.1186/s13104-018-3296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ménard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adjei GO, Goka BQ, Enweronu-Laryea CC, Rodrigues OP, Renner L, Sulley AM, et al. A randomized trial of artesunate-amodiaquine versus artemether-lumefantrine in Ghanaian paediatric sickle cell and non-sickle cell disease patients with acute uncomplicated malaria. Malar J. 2014;13:369. doi: 10.1186/1475-2875-13-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gbessi EA, Toure OA, Gnondjui A, Koui TS, Coulibaly B, Ako BA, et al. Artemisinin derivative-containing therapies and abnormal hemoglobin: do we need to adapt the treatment? Parasite. 2021;28:67–77. doi: 10.1051/parasite/2021063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lainé A, Diallo D, Traoré B. De Koloci à la drépanocytose. Anthropologie et Santé. 2012 doi: 10.4000/anthropologiesante.884. [DOI] [Google Scholar]
- 41.Douamba S, Nagalo K, Tamini L, Traoré I, Kam M, Kouéta F, et al. [Major sickle cell syndromes and infections associated with this condition in children in Burkina Faso.](in French) Pan Afr Med J. 2017;26:7. doi: 10.11604/pamj.2017.26.7.9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolo-Diebkilé A, Koffi KG, Nanho DC, Sawadogo D, Kouakou B, Siransy-Bogui L, et al. Drépanocytose homozygote chez l’adulte ivoirien de plus de 21 ans. Sante. 2010;20:63–67. doi: 10.1684/san.2010.0184. [DOI] [PubMed] [Google Scholar]
- 43.Sawadogo D, Tolo-Dilkébié A, Sangaré M, Aguéhoundé N, Kassi H, Latte T. Influence of the clinical status on stress reticulocytes, CD 36 and CD 49d of SSFA 2 homozygous sickle cell patients followed in Abidjan. Adv Hematol. 2014;2014:273860. doi: 10.1155/2014/273860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esposito A, Tiffert T, Mauritz JMA, Schlachter S, Bannister LH, Kaminski CF, et al. FRET imaging of hemoglobin concentration in Plasmodium falciparum-infected red cells. PLoS One. 2008;3:e3780. doi: 10.1371/journal.pone.0003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–1049. doi: 10.1016/S1473-3099(13)70252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amaratunga C, Witkowski B, Dek D, Try V, Khim N, Miotto O, et al. Plasmodium falciparum founder populations in Western Cambodia have reduced artemisinin sensitivity in vitro. Antimicrob Agents Chemother. 2014;58:4935–4937. doi: 10.1128/AAC.03055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen JB, Trager W. Plasmodium falciparum in culture : use of outdated erythrocytes and description of the candle jar method. J Parasitol. 1976;6:883–886. [PubMed] [Google Scholar]
- 48.Le Bras J, Durand R. The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum. Fundam Clin Pharmacol. 2003;17:147–153. doi: 10.1046/j.1472-8206.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- 49.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basco LK. Field application of in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. 2007. https://apps.who.int/iris/bitstream/handle/10665/ 43610/9789241595155_eng.pdf
- 51.Le Nagard H, Vincent C, Mentré F, Le Bras J. Online analysis of in vitro resistance to antimalarial drugs through nonlinear regression. Comput Methods Programs Biomed. 2011;104:10–18. doi: 10.1016/j.cmpb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Wang Z, Wang Y, Cabrera M, Zhang Y, Gupta B, Wu Y, et al. Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrob Agents Chemother. 2015;59:6952–6959. doi: 10.1128/AAC.01255-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y, Fairfield AS, Oduola A, Cypess RH, MR4 Scientific Advisory Committee The malaria research and reference reagent resource (MR4) center-creating African opportunities. Afr J Med Medic Sci. 2001;30(Suppl):52–54. [PubMed] [Google Scholar]
- 54.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galactéros F. Physiopathologie de la drépanocytose, de la théorie aux aspects pratiques. Rev Prat. 2004;54:1534–1542. [PubMed] [Google Scholar]
- 56.Labie D, Elion J. Bases moléculaires et physiopathologiques des maladies de l’hémoglobine. Encyclopedie Medico-Chirurgicale Hématolgie. 2005;2:220–239. [Google Scholar]
- 57.Huynh-Moynot S, Moynot JC, Commandeur D, Des Deserts MD, Montelescaut É, Kenane N, et al. Drépanocytose: des aspects moĺculaires à la pratique clinique. À propos d’un cas et revue de la litt́rature. Ann Biol Clin. 2011;69:679–84. doi: 10.1684/abc.2011.0633. [DOI] [PubMed] [Google Scholar]
- 58.Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41:S398–S405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wastnedge E, Waters D, Patel S, Morrison K, Goh MY, Adeloye D, et al. The global burden of sickle cell disease in children under five years of age: a systematic review and meta-analysis. J Glob Health. 2018;8:021103. doi: 10.7189/jogh.08.021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kosiyo P, Otieno W, Gitaka J, Munde EO, Ouma C. Association between haematological parameters and sickle cell genotypes in children with Plasmodium falciparum malaria resident in Kisumu County in Western Kenya. BMC Infect Dis. 2020;20:887. doi: 10.1186/s12879-020-05625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kosiyo P, Otieno W, Gitaka J, Munde EO, Ouma C. Haematological abnormalities in children with sickle cell disease and non-severe malaria infection in western Kenya. BMC Infect Dis. 2021;21:329. doi: 10.1186/s12879-021-06025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao H, Antonopoulos A, Henderson S, Wassall H, Brewin J, Masson A, et al. Red blood cell mannoses as phagocytic ligands mediating both sickle cell anaemia and malaria resistance. Nat Commun. 2021;12:1792. doi: 10.1038/s41467-021-21814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lew VL, Bookchin RM. Ion transport pathology in the mechanism of sickle cell dehydration. Physiol Rev. 2005;85:179–200. doi: 10.1152/physrev.00052.2003. [DOI] [PubMed] [Google Scholar]
- 64.Archer NM, Petersen N, Clark MA, Buckee CO, Childs LM, Duraisingh MT. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc Natl Acad Sci USA. 2018;115:7350–7355. doi: 10.1073/pnas.1804388115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glushakova S, Humphrey G, Leikina E, Balaban A, Miller J, Zimmerberg J. New stages in the program of malaria parasite egress imaged in normal and sickle erythrocytes. Curr Biol. 2010;20:1117–1121. doi: 10.1016/j.cub.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olson JA, Nagel RL. Synchronized cultures of P falciparum in abnormal red cells: the mechanism of the inhibition of growth in HbCC cells. Blood. 1986;67:997–1001. doi: 10.1182/blood.V67.4.997.997. [DOI] [PubMed] [Google Scholar]
- 67.Rangachari K, Dluzewski A, Wilson RJM, Gratzer WB. Control of malarial invasion by phosphorylation of the host cell membrane cytoskeleton. Nature. 1986;324:364–365. doi: 10.1038/324364a0. [DOI] [PubMed] [Google Scholar]
- 68.Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis. 2010;202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ménard S, Ben Haddou T, Ramadani AP, Ariey F, Iriart X, Beghain J, et al. Induction of multidrug tolerance in Plasmodium falciparum by extended artemisinin pressure. Emerg Infect Dis. 2015;21:1733–1741. doi: 10.3201/eid2110.150682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO. Artemisinin and artemisinin-based combination therapy resistance: status report 2016. Geneva, World Health Organization, 2016. https://apps.who.int/iris/bitstream/handle/10665/250294/WHO-HTM-GMP-2016.11-eng.pdf
- 71.Menard S, Tchoufack JN, Maffo CN, Nsango SE, Iriart X, Abate L, et al. Insight into k13-propeller gene polymorphism and ex vivo DHA-response profiles from Cameroonian isolates. Malar J. 2016;15:572. doi: 10.1186/s12936-016-1622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ikeda M, Kaneko M, Tachibana S-I, Balikagala B, Sakurai-Yatsushiro M, Yatsushiro S, et al. Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis. 2018;24:718–726. doi: 10.3201/eid2404.170141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukherjee A, Bopp S, Magistrado P, Wong W, Daniels R, Demas A, et al. Artemisinin resistance without pfkelch13 mutations in Plasmodium falciparum isolates from Cambodia. Malar J. 2017;16:195. doi: 10.1186/s12936-017-1845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klonis N, Crespo-Ortiz MP, Bottova I, Abu-Bakar N, Kenny S, Rosenthal PJ, et al. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. P Proc Natl Acad Sci USA. 2011;108:11405–11410. doi: 10.1073/pnas.1104063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Siddiqui FA, Cabrera M, Wang M, Brashear A, Kemirembe K, Wang Z, et al. Plasmodium falciparum falcipain-2a polymorphisms in Southeast Asia and their association with artemisinin resistance. J Infect Dis. 2018;218:434–442. doi: 10.1093/infdis/jiy188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leang R, Taylor WRJ, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: dihydroartemisinin-piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miotto O, Almagro-Garcia J, Manske M, MacInnis B, Campino S, Rockett KA, et al. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat Genet. 2013;45:648–655. doi: 10.1038/ng.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pelleau S, Moss EL, Dhingra SK, Volney B, Casteras J, Gabryszewski SJ, et al. Adaptive evolution of malaria parasites in French Guiana: Reversal of chloroquine resistance by acquisition of a mutation in pfcrt. Proc Natl Acad Sci USA. 2015;112:11672–11677. doi: 10.1073/pnas.1507142112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis. 2014;211:1352–1355. doi: 10.1093/infdis/jiu608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sutherland CJ, Henrici RC, Artavanis-Tsakonas K. Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing. FEMS Microbiol Revi. 2021;45:fuaa056. doi: 10.1093/femsre/fuaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mok S, Ashley EA, Ferreira PE, Zhu L, Lin Z, Yeo T, et al. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2015;347:431–435. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from Andre Toure but restrictions apply to the availability of these data, as they are not publicly available. Data are however available from the authors upon reasonable request and with permission of Ministry of Health of Ivory Coast.