Abstract
Hepatitis E virus (HEV) is a small non-enveloped virus that is transmitted via the fecal-oral route. It is a highly common cause of acute hepatitis, particularly in low to middle income regions of Asia, Africa, and Central America. Most cases are self-limited, and symptomatic patients usually present with acute icteric hepatitis. A subset of patients including pregnant women, older men, those with pre-existing liver disease and immunocompromised patients however, may develop severe disease and hepatic failure. Immunocompromised patients are also at risk for chronic infection, and their immunosuppression should be decreased in order to facilitate viral clearance. HEV can also present with a variety of extra-intestinal manifestations including neurological, renal, hematological, and pancreatic derangements. The gold standard of diagnosis is HEV ribonucleic acid detection via nucleic acid amplification testing. Currently, there are no approved treatments for Hepatitis E, though ribavirin is the most commonly used agent to reduce viral load. Studies assessing the safety and efficacy of other antiviral agents for HEV are currently underway. HEV vaccination has been approved in China, and is currently being investigated in other regions as well. This review article aims to discuss the epidemiology, pathogenesis, presentation, diagnosis, complications, and treatment of Hepatitis E infection.
Keywords: Hepatitis E, Acute hepatitis, Chronic hepatitis, Viral hepatitis, Vaccination
Core Tip: Hepatitis E is a common viral infection that has been increasing in developed nations. It usually causes a self-resolving acute hepatitis. It can sometimes lead to chronic hepatitis, and even cirrhosis/hepatic failure. Several subtypes exist, however the types responsible for infections in humans are generally spread via pork consumption or contaminated water. Treatment is usually supportive, however, ribavirin has shown efficacy in those with severe or chronic infection. Immunocompromised and pregnant patients should be evaluated with particular caution. Vaccination is currently licensed in China, and many studies are underway assessing vaccination efficacy in other nations as well.
INTRODUCTION
Hepatitis E virus (HEV) is a small non-enveloped virus in the Hepeviridae family. It was first discovered in the 1980s in a military camp in Afghanistan and was identified via electron microscopy in an individual who had symptoms of acute viral hepatitis[1]. Globally, HEV accounts for a significant proportion of liver disease, and is responsible for up to 70% of adult sporadic hepatitis cases in endemic regions. It is thought to be the most common etiology of acute viral hepatitis with an estimated incidence of 20 million cases yearly[2]. HEV is primarily transmitted via the fecal-oral route, and is responsible for multiple epidemics in developing countries within Asia and Africa[2]. However, it has become increasingly prevalent as a zoonotic viral infection in developed countries as well. Though HEV infection is self-limited in many cases, mortality rates and the incidence of fulminant hepatic failure (FHF) are significant in older male patients (6.5-10% mortality), pregnant patients (25%-30% mortality), and those with chronic liver disease (22%-43% mortality)[3]. Management is usually supportive, however, immunocompromised patients with chronic infection as well as high-risk populations may require antiviral treatment in order to prevent progression of liver disease and associated morbidity and mortality. HEV vaccination is currently approved in China, and multiple randomized control trials are underway in other endemic regions including Pakistan and Bangladesh.
EPIDEMIOLOGY
Hepatitis E is a hepatic infection caused by the HEV, a positive sense ribonucleic (RNA) virus, and is considered a global health issue. According to the World Health Organization, an estimated 20 million cases of HEV infection occur yearly resulting in 70000 deaths[4]. Particularly endemic to developing countries, HEV can be found in Asia, Africa, and Central America, and is especially prevalent in low to middle income regions of those areas[5]. The primary route in endemic areas of infection is fecal-oral, making areas with poor water sanitation particularly susceptible[6]. Four genotypes (1-4) are largely implicated in cases of HEV infection. In the above mentioned endemic regions, genotype 1 and 2 are predominantly the causative strains[5]. Sporadic cases and outbreaks can also occur, both in developed and under-developed regions, for which genotypes 3-4 are largely responsible and are most often secondary to zoonotic transmission, primarily from domestic pigs and wild boars[7]. Additionally, contaminated water can lead to viral transmission through shellfish, fruit, and salads[8,9]. In the United States (US), HEV is largely considered a travel-associated disease, usually brought into the country by travelers returning from endemic areas. However, a retrospective study of nationwide hospitalizations from 2010-2017 found that the incidence of HEV in the US has increased nearly two-fold[10]. In autochthonous (locally acquired) cases, HEV is thought to be predominantly caused by zoonotic transmission, usually originating from undercooked pork[4,7].
PATHOGENESIS
HEV is primarily spread fecal-orally via contaminated water or food (e.g. undercooked pork). The virus is a single-stranded, positive-sense RNA virus and is divided into two genera: Piscihepevirus and Orthohepevirus, the latter of which is divided further into 4 species (A-D). Interestingly, HEV-C is primarily spread by rats, and only shares 50%-60% identity with HEV-A. Some case reports describe HEV-C infection in transplant recipients, however, its infectious potential in humans remains unclear[11]. HEV-A has seven genotypes, of which 1, 2, 3, 4 and 7 infect humans[12]. HEV primarily targets hepatocytes however, until recently, the route of HEV reaching the hepatocytes was poorly understood. It is now thought that the virus first replicates enterically, with studies finding HEV RNA and ORF2 antigens in intestinal crypts of chronically infected patients. From here, the virus is thought to then enter the portal circulation and infect hepatocytes causing inflammation. The mechanism of viral entry into the hepatocyte is still poorly understood[13]. After entering the hepatocyte, the HEV genome is released into the cytoplasm where the virus hijacks intracellular machinery to replicate vital proteins and the RNA genome. ORF4 is critical for the replication process, and ORF3 is necessary for viral release from infected cells[12]. The HEV virion is shown in Figures 1 and 2.
Figure 1.
Naked hepatitis E virus virion structure. HEV: Hepatitis E virus.
Figure 2.
Quasi-enveloped hepatitis E virus virion structure. HEV: Hepatitis E virus.
The humoral immune response in conjunction with cellular immunity limits viral replication and allows the host to clear the infection, which is largely responsible for the self-limited nature of majority of cases[14]. In acutely infected patients, anti-HEV immunoglobulin M (IgM) antibodies peak in 6 wk, followed by anti-HEV IgG antibodies for long-term (years to decades) protection[13]. Acute infections are also associated with elevated T cells, with increases seen in both CD4+ and CD8+ populations, and subsequent release of pro and anti-inflammatory cytokines interferon (IFN) gamma and interleukin (IL)-10[15]. Further immune protection is provided by the innate lymphoid cell response, with natural killer (NK) cells combating viral infection with both cell-mediated cytotoxicity and by producing IFN gamma[12]. The same immune response responsible for limiting HEV infection is also largely the cause of hepatocellular damage and liver inflammation[12]. In their efforts to clear HEV from the host, CD8+ and NK cells along with the production of interferons, cause intrinsic damage to hepatocytes, leading to hepatitis[16].
CLINICAL PRESENTATION
Hepatitis E infection can present with a wide range of clinical manifestations. Most commonly, infected hosts remain asymptomatic. Symptomatic cases present as acute icteric hepatitis, which occurs in 5%-30% of infected hosts. This presentation includes a prodromal phase that lasts up to one week, manifested as fever, nausea, vomiting, and malaise[17]. Following the prodromal phase, dark urine and jaundice signal the onset of the icteric phase. During this time, mortality rates range from 0.5%-4%, however symptoms usually resolve spontaneously within a week[18]. More severe presentations can occur, such as fulminant HEV infection and/or progression to chronic HEV infection (sustained HEV replication for more than 3 mo). The populations most susceptible to these outcomes are pregnant and immunocompromised patients, such as solid organ transplant recipients and those with human immunodeficiency virus (HIV). Extra-hepatic complications can occur in both acute and chronic HEV infection, ranging from renal impairment to neurological symptoms (see section on complications). Extra-hepatic manifestations seem to be driven both directly by HEV replication and indirectly by immune system mediated effects[19].
DIAGNOSIS
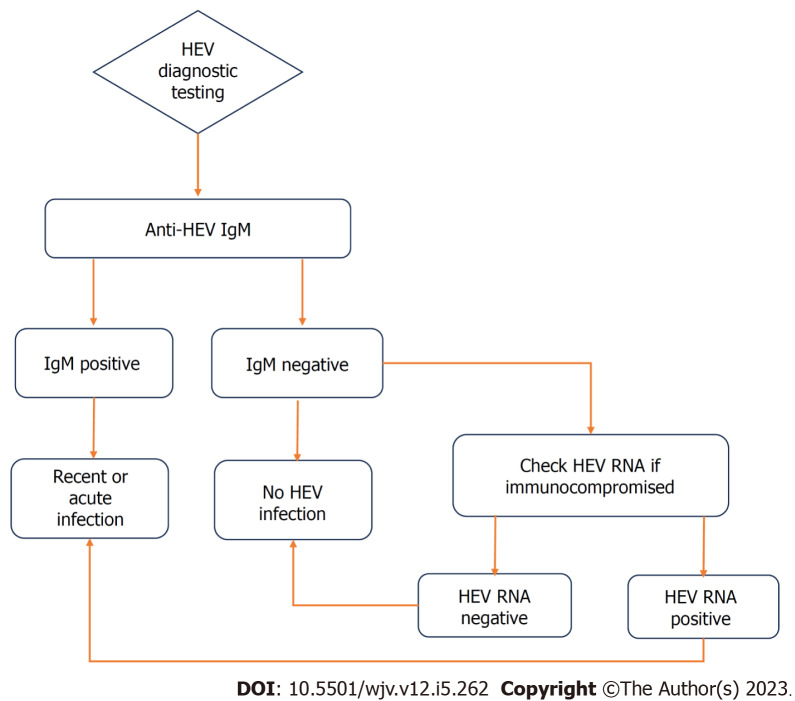
Diagnosis of acute HEV infection initially involves the detection of anti-HEV antibodies (IgM). IgM antibodies appear in the acute phase of infection, and are detectable approximately 4 d after onset of jaundice. They may remain detectable for up to 5 mo. Anti-HEV IgG antibodies develop shortly after IgM, and remain in the serum for up to 14 years post-infection[20]. Sensitivity of traditional immunoassays range from 90-97%, with false positives up to 2.5%[21]. Immunocompromised patients may have delayed or absent seroconversion to anti-HEV antibodies, rendering this diagnostic modality insufficient in which case nucleic acid amplification testing (NAT) to detect HEV RNA from stool, serum, or liver biopsy can be used[21].
The gold standard test for confirming acute HEV hepatitis is detection of HEV RNA via NAT from serum, stool or on liver biopsy. However, there are several factors that make RNA detection a faulty method. Firstly, detection of HEV RNA is dependent on time of patient presentation. Following onset of illness, RNA is detectable in the serum up to 4 wk later, and up to 6 wk in the stool. Secondly, viral load can remain low and therefore even during the detectable periods, may not be captured by NAT[20]. The availability of HEV RNA testing in commercial labs remains limited, further restricting its use. Furthermore, the methodology of HEV NAT has not been standardized and large variability in sensitivities has been noted in the various techniques. Greater sensitivity has been noted in real-time reverse transcription polymerase chain reaction (RT-PCR) compared to nested RT-PCR assays[22]. A flowchart of HEV diagnosis is outlined in Figure 3.
Figure 3.
Flowchart of diagnostic testing for acute hepatitis E virus infection. HEV: Hepatitis E virus; IgM: Immunoglobulin M.
COMPLICATIONS/SPECIAL POPULATIONS
Hepatic complications
Chronic HEV infection is primarily seen in immunocompromised hosts, and is exceedingly rare in immunocompetent patients. These patients largely consist of solid organ transplant patients, however other cohorts, such as HIV patients and chemotherapy patients, have also been described. These patients largely remain asymptomatic, and usually only have mild to moderate derangement in liver enzymes[23]. HEV-induced liver cirrhosis is a complication only seen in immunocompromised patients, often seen in HIV patients with low CD4 (< 200) counts or recent organ transplantation. Patients who have undergone solid organ transplant and are infected with HEV have a 50% chance of progressing to liver cirrhosis over several years, with 10% of patients reaching that point within 5 years[23-25].
Patients with pre-existing liver disease are at increased risk for severe HEV infection and liver failure, and should be evaluated with caution. A recent meta-analysis of 18 studies by Qiu et al[26] found a 35.8% rate of liver failure and 14.3% mortality rate in patients with chronic liver disease and superimposed HEV infection. Patients with cirrhosis had a two-fold increase in risk of liver failure and four-fold increase in risk of death compared to patients without cirrhosis. Similarly, a retrospective study by Tseng et al[27] demonstrated that HEV infection increases the rate of liver disease progression in patients with chronic hepatitis B (HBV) infection. The study also found an increased risk of mortality in patients in HBV-cirrhosis compared to non-cirrhotic patients (30% vs 0%, P < 0.001). Other studies have shown similar results regarding the effects of HEV superinfection in patients with pre-existing liver disease, prompting a discussion on vaccination for HEV in all patients with chronic liver disease in endemic regions (see section on vaccination)[28-30].
Pregnancy
HEV infection in pregnancy can be life-threatening with mortality rate up to 30% with HEV genotype 1 infection largely due to the development of HEV-induced FHF[12]. Studies have shown that pregnant patients with a progesterone receptor gene mutation, PROGINS, had reduced NK cell activity along with altered humoral and cellular immune responses[31]. Other studies have shown that pregnant patients have higher levels of tumor necrosis factor alpha, IL-6, and IFN-gamma, and that these cytokines had a significant positive correlation with HEV viral load, serum bilirubin, and prothrombin time. This raises the possibility that increased severity of HEV infection in pregnant patients may be mediated by increased levels of cytokines in the serum[12,32]. Non-host complications of HEV infection in pregnancy include vertical transmission of the disease, increased preterm births, stillbirths and neonatal mortality[33].
Extrahepatic manifestations
HEV infection can be complicated by extrahepatic manifestations ranging from neurological to renal complications. These manifestations can occur in both acute and chronic infection and are thought to arise from a combination of HEV replication in involved tissues and immune system related effects. Neurological pathologies have been widely reported and are seen largely in HEV genotypes 1 and 3. Reported disorders included Guillain-Barre syndrome, Bell’s palsy, polyradiculopathy, neuralgic amyotrophy, acute transverse myelitis, and acute meningoencephalitis[18]. The patho-physiology behind neurological symptoms in HEV infection remains unclear, however it is hypothesized that the host immune response plays a large role, with studies showing that immunocompetent patients are more likely to have neurological complications than immunocompromised ones[18].
Renal injury is seen in both acute and chronic HEV infection, again with HEV 1 and 3 genotypes. Renal biopsies in affected patients show histological patterns of membranoproliferative glomerulonephritis and membranous glomerulonephritis. These complications are seen in both immunocompetent and immunocompromised patients. The pathophysiology is poorly understood, though it is possible that immune complex deposition, such as that seen in hepatitis C infection, could be the cause[18,34].
Hematological complications such as aplastic anemia and thrombocytopenia have been reported, though the mechanism behind these complications is not well understood. Monoclonal gammopathy of unknown significance (MGUS), was found to have the prevalence of 26.2% according to a study by Woolson et al[35]. Studies have yet to determine whether the inflammatory state of HEV leads to increased prevalence of MGUS or if it is immunosuppression caused by MGUS that predisposes to HEV infection. Brown et al[36] found a significantly elevated risk of MGUS and multiple myeloma with infectious hepatitis (RR 1.82; 95%CI 1.25-2.65), though the study did not identify specific etiologies of the infectious hepatitis. Severe thrombocytopenia (platelet count < 20000) has been reported in rare cases of HEV infection, and as of 2010 there were only 6 known reported cases. Though the cause remains unclear, one theory is that the diminished platelet count is secondary to an immune-mediated response. This is supported by several of the patients having anti-platelet antibodies in their serum, response to immunosuppressive therapy, and increase in cell counts as the HEV infection resolved[37-39]. However, transient thrombocytopenia can be seen in a variety of inflammatory and infectious conditions, and further studies are needed to explore the underlying etiology given the rarity of this presentation.
Acute pancreatitis is another rare and poorly understood complication of HEV infection affecting only 6.2% of patients with acute HEV infection according to a study in Nepal[40]. Pancreatitis is more common, however, in patients with FHF, with one autopsy study finding pancreatitis in 44% of patients afflicted with FHF. It should be noted, however, that the study did not differentiate between different pathogens of viral hepatitis[41]. Interestingly, the few cases of HEV-related acute pancreatitis have almost exclusively been reported in India, where the virus is still endemic[42]. A single-center study in India by Raj et al[43] found that 2.1% of all patients with acute pancreatitis had acute HEV infection.
The pathophysiology of pancreatitis in HEV infection is poorly described in literature, though several theories have been postulated. One of the prevailing theories is that HEV virus is directly cytotoxic to pancreatic cells[44]. Other studies have hypothesized that swelling at the ampulla of Vater is caused by inflammation which inhibits secretion of pancreatic fluids[41]. One proposed cause is release of lysosomal enzymes from the liver which activate trypsin from trypsinogen and cause inflammation of the pancreas[40]. HEV-related pancreatitis is poorly understood, and further studies are warranted to elucidate the relationship between HEV infection and acute pancreatitis, as well as patient outcomes.
TREATMENT
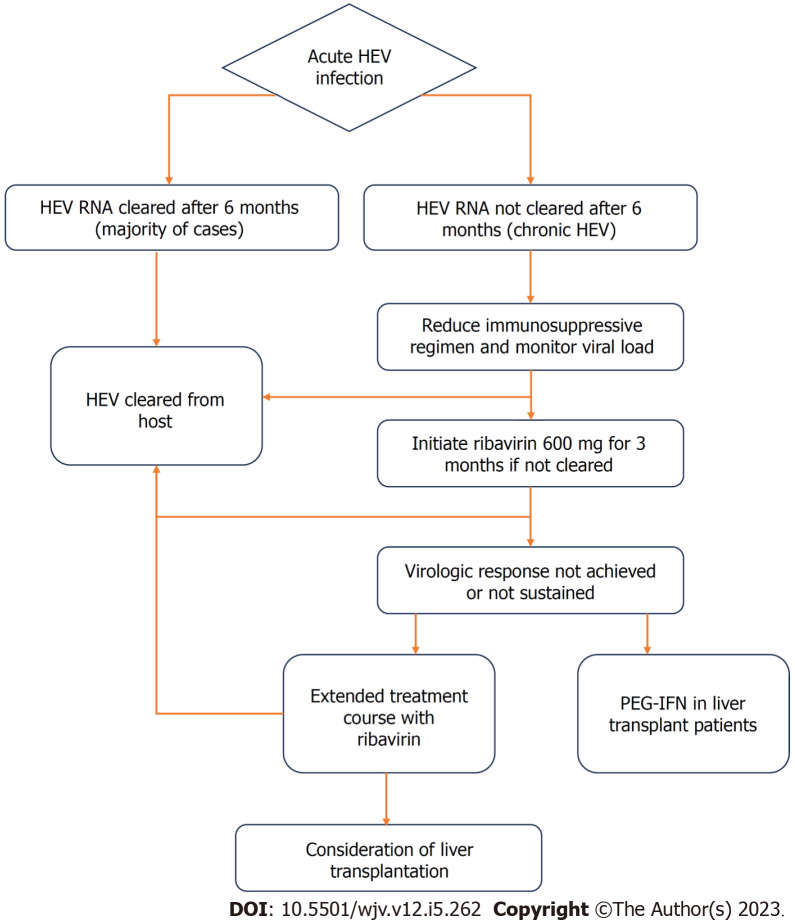
Acute HEV infection usually does not require antiviral treatment, however, it should be considered in high risk patients or those with chronic infection. A small proportion of patients with acute HEV may progress to FHF or acute-on-chronic liver failure, particularly older men, pregnant women, and patients with underlying chronic liver disease. The most commonly used treatment in such cases is ribavirin, a guanosine analog[45]. Ribavirin has been shown to help clear the HEV virus and normalize liver enzymes[46,47]. The mechanism is not well understood, but is thought to deplete guanosine triphosphate pools, thus inhibiting HEV RNA replication. Though rare, ribavirin has been associated with hemolytic anemia, which can be severe in patients with underlying liver disease or chronic kidney disease[48].
Chronic HEV is most commonly seen in solid-organ transplant recipients, and the first step in management of these patients is to decrease the dose of immunosuppressive agents that target T-lymphocytes. Studies have shown that this step alone can lead to HEV clearance in 25%-33% of patients[49,50]. Pegylated interferon-alpha (PEG-IFN) should be avoided in patients with a history of solid organ transplants including heart, lung, pancreas, or kidney due to an increased risk of rejection. However, PEG-IFN can cautiously be used in patients with a history of liver transplantation, since the risk of rejection is lower[51,52]. Ribavirin is thought to be safe for use in the transplant population and therefore is the preferred agent. Retrospective studies have shown that 78-81% of patients with chronic HEV had undetectable HEV RNA in the serum 6 mo after completion of the ribavirin treatment course. This proportion increased to as high as 90% when treatment duration was prolonged in those who did not achieve sustained virologic response[53]. The regimen of choice is ribavirin 600 milligrams daily for 3 mo (unless longer course desired due to lack of sustained response). Similar to in hepatitis C (HCV) infection, some associations including the Grupo de Estudio de Hepatitis Virales (GeHEP) de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) recommend a weight-adjusted dosing of ribavirin for treatment of HEV[54,55].
One of the limitations of ribavirin in the management of HEV is its potential for teratogenicity, given that pregnant patients are at increased risk of developing severe infection. However, a study by Sinclair et al[56] found no evidence for teratogenic effects in pregnant patients with HCV. The mortality rate of HEV infection in the third trimester of pregnancy is nearly 20%, so ribavirin should be considered in this population as organogenesis is generally complete prior to this phase of pregnancy. Additionally, severe hemolytic anemia is a potential complication of ribavirin, and patients should be monitored closely. Liver transplantation should be considered in patients that are progressing to liver failure despite appropriate management[56].
Sofosbuvir, the NS5B polymerase inhibitor used to treat HCV, has been a subject of investigation for the treatment of HEV. It has questionable efficacy as monotherapy for HEV, given the high relapse rates and incomplete initial response[57]. Studies have found mixed results regarding sofosbuvir/ribavirin combination therapy, with some showing efficacy in acute HEV infection and other showing inadequate response in solid-organ transplant patients with chronic infection[58-60]. Further studies and randomized clinical trials are needed to determine the proper treatment regimen and patient population best suited for these agents. A treatment flowchart for HEV infection is outlined in Figure 4.
Figure 4.
Flowchart of treatment for hepatitis E virus infection.
VACCINATION
There is only one currently approved vaccine for HEV, which was first licensed in China in 2011[61]. The HEV vaccine has been found to be effective in establishing long-lasting immunity against HEV genotypes 1 and 4[62,63]. In the study, 48420 healthy subjects received three doses (given at 0, 1 and 6 mo) of the vaccine and 48420 received placebo. No patients in the vaccine group developed HEV infection after 12 mo, compared to 15 patients in the placebo group, giving the vaccine 100% efficacy[64]. A clinical trial is currently recruiting in China to assess the long term effectiveness of the vaccine (NCT05976594)[65]. Li et al[66] found that the HEV vaccine is effective against genotype 3 in rabbit models, however its efficacy in humans remains unclear. Additionally, Sridhar et al[67] demonstrated that the HEV vaccine is not effective in HEV-C due to antigenic divergence, however, identified HEV-C1 p241 peptides as a potential vaccine candidate against HEV-C infection.
A topic of interest in recent years has been vaccination of at-risk populations. Ji et al[68] established a proof of concept demonstrating that administering the HEV vaccine to a German population with high levels of pork consumption would result in an 80% reduction in human HEV cases. Immunosuppressed patients (i.e. organ transplant recipients) are at increased risk for developing chronic infection, and therefore may warrant extra consideration for vaccination. However, rabbit models have demonstrated that HEV vaccination following initiation of immunosuppressive agents only conferred partial immunity, which did not improve with additional or increased vaccine doses[69]. Pregnant women are also considered high risk, and a randomized control trial is currently underway assessing the efficacy of HEV vaccination in pregnant women in rural Bangladesh[70]. A phase II randomized clinical trial assessing the safety and immunogenicity of the HEV vaccine in pregnant patients in Pakistan is expected to reach completion in 2025 (NCT05808166)[71]. Given the evidence showing worse outcomes and accelerated progression of liver damage in patients with pre-existing liver disease, HEV vaccination should be considered in these individuals. A major limitation of the HEV vaccine trials is the exclusion of patients with chronic liver disease, necessitating further studies to assess vaccine efficacy in this group[26].
CONCLUSION
HEV infection is a common cause of acute hepatitis worldwide that is usually characterized by an acute, self-limited course of symptoms including anorexia, nausea and jaundice. It has been the causative agent of many outbreaks in developing nations in Africa, Asia, and Central America, but has also been increasing in prevalence in developed countries. Risk factors such as pregnancy and chronic liver disease have been associated with a more severe disease course and immunosuppression with chronic HEV infection. Though there is currently no Food and Drug Administration approved treatment for HEV, ribavirin has shown efficacy in many studies and is the most commonly recommended treatment. The recombinant HEV vaccination licensed in China is the only vaccine currently available for HEV, and its long-term efficacy as well as its safety in various populations is being studied. Further studies are needed to establish a guideline-based treatment regimen for HEV in order to decrease global morbidity, mortality, and healthcare burden.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest associated with any of the authors of this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 11, 2023
First decision: November 21, 2023
Article in press: December 5, 2023
Specialty type: Virology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghazy A, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Zhang YL
Contributor Information
Humzah Iqbal, Department of Internal Medicine, University of California San Francisco, Fresno, CA 93701, United States.
Bilal Fazal Mehmood, Department of Internal Medicine, University of California San Francisco, Fresno, CA 93701, United States.
Aalam Sohal, Department of Hepatology, Liver Institute Northwest, Seattle, WA 98105, United States. asohal@liverinstitutenw.org.
Marina Roytman, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93701, United States.
References
- 1.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 2.Petrik J, Lozano M, Seed CR, Faddy HM, Keller AJ, Prado Scuracchio PS, Wendel S, Andonov A, Fearon M, Delage G, Zhang J, Shih JW, Gallian P, Djoudi R, Tiberghien P, Izopet J, Dreier J, Vollmer T, Knabbe C, Aggarwal R, Goel A, Ciccaglione AR, Matsubayashi K, Satake M, Tadokoro K, Jeong SH, Zaaijer HL, Zhiburt E, Chay J, Teo D, Chua SS, Piron M, Sauleda S, Echevarría JM, Dalton H, Stramer SL. Hepatitis E. Vox Sang. 2016;110:93–130. doi: 10.1111/vox.12285. [DOI] [PubMed] [Google Scholar]
- 3.Meng XJ. Hepatitis E virus. Viral Infections of Humans: Epidemiology and Control 2023; 1-37. [Google Scholar]
- 4."Hepatitis E. " World Health Organization. Accessed 19 July 2023. Available from: www.who.int/news-room/fact-sheets/detail/hepatitis-e .
- 5.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 6.Boxman ILA, Verhoef L, Dop PY, Vennema H, Dirks RAM, Opsteegh M. High prevalence of acute hepatitis E virus infection in pigs in Dutch slaughterhouses. Int J Food Microbiol. 2022;379:109830. doi: 10.1016/j.ijfoodmicro.2022.109830. [DOI] [PubMed] [Google Scholar]
- 7.Crotta M, Pellicioli L, Gaffuri A, Trogu T, Formenti N, Tranquillo V, Luzzago C, Ferrari N, Lanfranchi P. Analysis of seroprevalence data on Hepatitis E virus and Toxoplasma gondii in wild ungulates for the assessment of human exposure to zoonotic meat-borne pathogens. Food Microbiol. 2022;101:103890. doi: 10.1016/j.fm.2021.103890. [DOI] [PubMed] [Google Scholar]
- 8.Prpić J, Baymakova M. Hepatitis E Virus (HEV) Infection among Humans and Animals: Epidemiology, Clinical Characteristics, Treatment, and Prevention. Pathogens. 2023;12 doi: 10.3390/pathogens12070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terio V, Bottaro M, Pavoni E, Losio MN, Serraino A, Giacometti F, Martella V, Mottola A, Di Pinto A, Tantillo G. Occurrence of hepatitis A and E and norovirus GI and GII in ready-to-eat vegetables in Italy. Int J Food Microbiol. 2017;249:61–65. doi: 10.1016/j.ijfoodmicro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Wasuwanich P, Ingviya T, Thawillarp S, Teshale EH, Kamili S, Crino JP, Scheimann AO, Argani C, Karnsakul W. Hepatitis E-Associated Hospitalizations in the United States: 2010-2015 and 2015-2017. J Viral Hepat. 2021;28:672–681. doi: 10.1111/jvh.13458. [DOI] [PubMed] [Google Scholar]
- 11.Sridhar S, Yip CCY, Wu S, Cai J, Zhang AJ, Leung KH, Chung TWH, Chan JFW, Chan WM, Teng JLL, Au-Yeung RKH, Cheng VCC, Chen H, Lau SKP, Woo PCY, Xia NS, Lo CM, Yuen KY. Rat Hepatitis E Virus as Cause of Persistent Hepatitis after Liver Transplant. Emerg Infect Dis. 2018;24:2241–2250. doi: 10.3201/eid2412.180937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schemmerer M, Wenzel JJ, Stark K, Faber M. Molecular epidemiology and genotype-specific disease severity of hepatitis E virus infections in Germany, 2010-2019. Emerg Microbes Infect. 2022;11:1754–1763. doi: 10.1080/22221751.2022.2091479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yadav KK, Kenney SP. Hepatitis E Virus Immunopathogenesis. Pathogens. 2021;10 doi: 10.3390/pathogens10091180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kupke P, Werner JM. Hepatitis E Virus Infection-Immune Responses to an Underestimated Global Threat. Cells. 2021;10 doi: 10.3390/cells10092281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tripathy AS, Das R, Rathod SB, Gurav YK, Arankalle VA. Peripheral T regulatory cells and cytokines in hepatitis E infection. Eur J Clin Microbiol Infect Dis. 2012;31:179–184. doi: 10.1007/s10096-011-1291-1. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu SB, Gupta P, Durgapal H, Rath S, Gupta SD, Acharya SK, Panda SK. Study of cellular immune response against Hepatitis E virus (HEV) J Viral Hepat. 2011;18:587–594. doi: 10.1111/j.1365-2893.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- 17.Kovvuru K, Carbajal N, Pakanati AR, Thongprayoon C, Hansrivijit P, Boonpheng B, Pattharanitima P, Nissaisorakarn V, Cheungpasitporn W, Kanduri SR. Renal manifestations of hepatitis E among immunocompetent and solid organ transplant recipients. World J Hepatol. 2022;14:516–524. doi: 10.4254/wjh.v14.i3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lhomme S, Marion O, Abravanel F, Izopet J, Kamar N. Clinical Manifestations, Pathogenesis and Treatment of Hepatitis E Virus Infections. J Clin Med. 2020;9 doi: 10.3390/jcm9020331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aslan AT, Balaban HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020;26:5543–5560. doi: 10.3748/wjg.v26.i37.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirazo S, Ramos N, Mainardi V, Gerona S, Arbiza J. Transmission, diagnosis, and management of hepatitis E: an update. Hepat Med. 2014;6:45–59. doi: 10.2147/HMER.S63417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson GJ, Mushahwar IK, Chau KH, Gitnick GL. Detection of long-lasting antibody to hepatitis E virus in a US traveller to Pakistan. Lancet. 1992;340:426–427. doi: 10.1016/0140-6736(92)91507-5. [DOI] [PubMed] [Google Scholar]
- 22.Baylis SA, Hanschmann KM, Blümel J, Nübling CM HEV Collaborative Study Group. Standardization of hepatitis E virus (HEV) nucleic acid amplification technique-based assays: an initial study to evaluate a panel of HEV strains and investigate laboratory performance. J Clin Microbiol. 2011;49:1234–1239. doi: 10.1128/JCM.02578-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb GW, Dalton HR. Hepatitis E: an underestimated emerging threat. Ther Adv Infect Dis. 2019;6:2049936119837162. doi: 10.1177/2049936119837162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Péron JM. Hepatitis E Virus Infection and Cirrhosis of the Liver. Gastroenterol Hepatol (N Y) 2016;12:565–567. [PMC free article] [PubMed] [Google Scholar]
- 25.Thandassery RB, Kaur R, Sharma S. Journal Summary: Hepatitis E Virus Infection after Liver Transplantation. JGI. 2023;13:48–51. [Google Scholar]
- 26.Qiu LX, Huang Y, Quan JL, Bi ZF, Zhong GH, Wang JY, Huang SJ, Su YY, Wu T, Zhang J, Lu GY, Zhang GM, Xia NS. Prognosis of hepatitis E infection in patients with chronic liver disease: A meta-analysis. J Viral Hepat. 2023;30:101–107. doi: 10.1111/jvh.13754. [DOI] [PubMed] [Google Scholar]
- 27.Tseng TC, Liu CJ, Chang CT, Su TH, Yang WT, Tsai CH, Chen CL, Yang HC, Liu CH, Chen PJ, Chen DS, Kao JH. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J Hepatol. 2020;72:1105–1111. doi: 10.1016/j.jhep.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Hoan NX, Tong HV, Hecht N, Sy BT, Marcinek P, Meyer CG, Song le H, Toan NL, Kurreck J, Kremsner PG, Bock CT, Velavan TP. Hepatitis E Virus Superinfection and Clinical Progression in Hepatitis B Patients. EBioMedicine. 2015;2:2080–2086. doi: 10.1016/j.ebiom.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilonzo SB, Wang YL, Jiang QQ, Wu WY, Wang P, Ning Q, Han MF. Superinfective Hepatitis E Virus Infection Aggravates Hepatocytes Injury in Chronic Hepatitis B. Curr Med Sci. 2019;39:719–726. doi: 10.1007/s11596-019-2097-0. [DOI] [PubMed] [Google Scholar]
- 30.Nasir M, Wu GY. HEV and HBV Dual Infection: A Review. J Clin Transl Hepatol. 2020;8:313–321. doi: 10.14218/JCTH.2020.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurz C, Tempfer CB, Boecskoer S, Unfried G, Nagele F, Hefler LA. The PROGINS progesterone receptor gene polymorphism and idiopathic recurrent miscarriage. J Soc Gynecol Investig. 2001;8:295–298. doi: 10.1016/s1071-5576(01)00123-x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar A, Devi SG, Kar P, Agarwal S, Husain SA, Gupta RK, Sharma S. Association of cytokines in hepatitis E with pregnancy outcome. Cytokine. 2014;65:95–104. doi: 10.1016/j.cyto.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 33.Shalimar. Acharya SK. Hepatitis e and acute liver failure in pregnancy. J Clin Exp Hepatol. 2013;3:213–224. doi: 10.1016/j.jceh.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erratum for the Research Article: "Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs" by R. Sharma, V. Khristov, A. Rising, B. S. Jha, R. Dejene, N. Hotaling, Y. Li, J. Stoddard, C. Stankewicz, Q. Wan, C. Zhang, M. M. Campos, K. J. Miyagishima, D. McGaughey, R. Villasmil, M. Mattapallil, B. Stanzel, H. Qian, W. Wong, L. Chase, S. Charles, T. McGill, S. Miller, A. Maminishkis, J. Amaral, K. Bharti. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolson KL, Forbes A, Vine L, Beynon L, McElhinney L, Panayi V, Hunter JG, Madden RG, Glasgow T, Kotecha A, Dalton HC, Mihailescu L, Warshow U, Hussaini HS, Palmer J, Mclean BN, Haywood B, Bendall RP, Dalton HR. Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther. 2014;40:1282–1291. doi: 10.1111/apt.12986. [DOI] [PubMed] [Google Scholar]
- 36.Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 2008;111:3388–3394. doi: 10.1182/blood-2007-10-121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fourquet E, Mansuy JM, Bureau C, Recher C, Vinel JP, Izopet J, Péron JM. Severe thrombocytopenia associated with acute autochthonous hepatitis E. J Clin Virol. 2010;48:73–74. doi: 10.1016/j.jcv.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Singh NK, Gangappa M. Acute immune thrombocytopenia associated with hepatitis E in an adult. Am J Hematol. 2007;82:942–943. doi: 10.1002/ajh.20960. [DOI] [PubMed] [Google Scholar]
- 39.Colson P, Payraudeau E, Leonnet C, De Montigny S, Villeneuve L, Motte A, Tamalet C. Severe thrombocytopenia associated with acute hepatitis E virus infection. J Clin Microbiol. 2008;46:2450–2452. doi: 10.1128/JCM.02295-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thapa R, Biswas B, Mallick D, Ghosh A. Acute pancreatitis--complicating hepatitis E virus infection in a 7-year-old boy with glucose 6 phosphate dehydrogenase deficiency. Clin Pediatr (Phila) 2009;48:199–201. doi: 10.1177/0009922808327107. [DOI] [PubMed] [Google Scholar]
- 41.Jain P, Nijhawan S, Rai RR, Nepalia S, Mathur A. Acute pancreatitis in acute viral hepatitis. World J Gastroenterol. 2007;13:5741–5744. doi: 10.3748/wjg.v13.i43.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhagat S, Wadhawan M, Sud R, Arora A. Hepatitis viruses causing pancreatitis and hepatitis: a case series and review of literature. Pancreas. 2008;36:424–427. doi: 10.1097/MPA.0b013e31815d9d53. [DOI] [PubMed] [Google Scholar]
- 43.Raj M, Kumar K, Ghoshal UC, Saraswat VA, Aggarwal R, Mohindra S. Acute Hepatitis E-Associated Acute Pancreatitis: A Single Center Experience and Literature Review. Pancreas. 2015;44:1320–1322. doi: 10.1097/MPA.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 44.Haffar S, Bazerbachi F, Garg S, Lake JR, Freeman ML. Frequency and prognosis of acute pancreatitis associated with acute hepatitis E: A systematic review. Pancreatology. 2015;15:321–326. doi: 10.1016/j.pan.2015.05.460. [DOI] [PubMed] [Google Scholar]
- 45.Loustaud-Ratti V, Debette-Gratien M, Jacques J, Alain S, Marquet P, Sautereau D, Rousseau A, Carrier P. Ribavirin: Past, present and future. World J Hepatol. 2016;8:123–130. doi: 10.4254/wjh.v8.i2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Péron JM, Dalton H, Izopet J, Kamar N. Acute autochthonous hepatitis E in western patients with underlying chronic liver disease: a role for ribavirin? J Hepatol. 2011;54:1323–4; author reply 1324. doi: 10.1016/j.jhep.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Pischke S, Hardtke S, Bode U, Birkner S, Chatzikyrkou C, Kauffmann W, Bara CL, Gottlieb J, Wenzel J, Manns MP, Wedemeyer H. Ribavirin treatment of acute and chronic hepatitis E: a single-centre experience. Liver Int. 2013;33:722–726. doi: 10.1111/liv.12114. [DOI] [PubMed] [Google Scholar]
- 48.Odenwald MA, Paul S. Viral hepatitis: Past, present, and future. World J Gastroenterol. 2022;28:1405–1429. doi: 10.3748/wjg.v28.i14.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, Dumortier J, Cannesson A, Cassuto-Viguier E, Thervet E, Conti F, Lebray P, Dalton HR, Santella R, Kanaan N, Essig M, Mousson C, Radenne S, Roque-Afonso AM, Izopet J, Rostaing L. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140:1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 50.Debing Y, Emerson SU, Wang Y, Pan Q, Balzarini J, Dallmeier K, Neyts J. Ribavirin inhibits in vitro hepatitis E virus replication through depletion of cellular GTP pools and is moderately synergistic with alpha interferon. Antimicrob Agents Chemother. 2014;58:267–273. doi: 10.1128/AAC.01795-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D'Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 52.Haagsma EB, Riezebos-Brilman A, van den Berg AP, Porte RJ, Niesters HG. Treatment of chronic hepatitis E in liver transplant recipients with pegylated interferon alpha-2b. Liver Transpl. 2010;16:474–477. doi: 10.1002/lt.22014. [DOI] [PubMed] [Google Scholar]
- 53.Gorris M, van der Lecq BM, van Erpecum KJ, de Bruijne J. Treatment for chronic hepatitis E virus infection: A systematic review and meta-analysis. J Viral Hepat. 2021;28:454–463. doi: 10.1111/jvh.13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivero-Juárez A, Aguilera A, Avellón A, García-Deltoro M, García F, Gortazar C, Granados R, Macías J, Merchante N, Oteo JA, Pérez-Gracia MT, Pineda JA, Rivero A, Rodriguez-Lazaro D, Téllez F, Morano-Amado LE Grupo redactor de GeHEP SEIMC. Executive summary: Consensus document of the diagnosis, management and prevention of infection with the hepatitis E virus: Study Group for Viral Hepatitis (GEHEP) of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) Enferm Infecc Microbiol Clin (Engl Ed) 2020;38:28–32. doi: 10.1016/j.eimc.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Kamar N, Abravanel F, Behrendt P, Hofmann J, Pageaux GP, Barbet C, Moal V, Couzi L, Horvatits T, De Man RA, Cassuto E, Elsharkawy AM, Riezebos-Brilman A, Scemla A, Hillaire S, Donnelly MC, Radenne S, Sayegh J, Garrouste C, Dumortier J, Glowaki F, Matignon M, Coilly A, Figueres L, Mousson C, Minello A, Dharancy S, Rerolle JP, Lebray P, Etienne I, Perrin P, Choi M, Marion O, Izopet J Hepatitis E Virus Ribavirin Study Group. Ribavirin for Hepatitis E Virus Infection After Organ Transplantation: A Large European Retrospective Multicenter Study. Clin Infect Dis. 2020;71:1204–1211. doi: 10.1093/cid/ciz953. [DOI] [PubMed] [Google Scholar]
- 56.Sinclair SM, Jones JK, Miller RK, Greene MF, Kwo PY, Maddrey WC. The Ribavirin Pregnancy Registry: An Interim Analysis of Potential Teratogenicity at the Mid-Point of Enrollment. Drug Saf. 2017;40:1205–1218. doi: 10.1007/s40264-017-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kar P, Sengupta A. A guide to the management of hepatitis E infection during pregnancy. Expert Rev Gastroenterol Hepatol. 2019;13:205–211. doi: 10.1080/17474124.2019.1568869. [DOI] [PubMed] [Google Scholar]
- 58.Horvatits T, Schulze Zur Wiesch J, Lütgehetmann M, Lohse AW, Pischke S. The Clinical Perspective on Hepatitis E. Viruses. 2019;11 doi: 10.3390/v11070617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biliotti E, Franchi C, Spaziante M, Garbuglia AR, Volpicelli L, Palazzo D, De Angelis M, Esvan R, Taliani G. Autochthonous acute hepatitis E: treatment with sofosbuvir and ribavirin. Infection. 2018;46:725–727. doi: 10.1007/s15010-018-1168-7. [DOI] [PubMed] [Google Scholar]
- 60.Donnelly MC, Imlach SN, Abravanel F, Ramalingam S, Johannessen I, Petrik J, Fraser AR, Campbell JD, Bramley P, Dalton HR, Hayes PC, Kamar N, Simpson KJ. Sofosbuvir and Daclatasvir Anti-Viral Therapy Fails to Clear HEV Viremia and Restore Reactive T Cells in a HEV/HCV Co-Infected Liver Transplant Recipient. Gastroenterology. 2017;152:300–301. doi: 10.1053/j.gastro.2016.05.060. [DOI] [PubMed] [Google Scholar]
- 61.Schulz M, Papp CP, Bock CT, Hofmann J, Gerlach UA, Maurer MM, Eurich D, Mueller T. Combination therapy of sofosbuvir and ribavirin fails to clear chronic hepatitis E infection in a multivisceral transplanted patient. J Hepatol. 2019;71:225–227. doi: 10.1016/j.jhep.2019.03.029. [DOI] [PubMed] [Google Scholar]
- 62.Ma Z, de Man RA, Kamar N, Pan Q. Chronic hepatitis E: Advancing research and patient care. J Hepatol. 2022;77:1109–1123. doi: 10.1016/j.jhep.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Zhang XF, Huang SJ, Wu T, Hu YM, Wang ZZ, Wang H, Jiang HM, Wang YJ, Yan Q, Guo M, Liu XH, Li JX, Yang CL, Tang Q, Jiang RJ, Pan HR, Li YM, Shih JW, Ng MH, Zhu FC, Xia NS. Long-term efficacy of a hepatitis E vaccine. N Engl J Med. 2015;372:914–922. doi: 10.1056/NEJMoa1406011. [DOI] [PubMed] [Google Scholar]
- 64.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, Wang H, Yang CL, Jiang HM, Cai JP, Wang YJ, Ai X, Hu YM, Tang Q, Yao X, Yan Q, Xian YL, Wu T, Li YM, Miao J, Ng MH, Shih JW, Xia NS. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 65.Jun Z. Long-term effectiveness of a Recombinant Hepatitis E Vaccine - Full Text View - ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. ClinicalTrials.gov Identifier: NCT05976594. Available from: https://clinicaltrials.gov/ct2/show/NCT05976594 .
- 66.Li M, Li S, He Q, Liang Z, Wang L, Wang Q. Hepatitis E-related adverse pregnancy outcomes and their prevention by hepatitis E vaccine in a rabbit model. Emerg Microbes Infect. 2019;8:1066–1075. doi: 10.1080/22221751.2019.1643260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sridhar S, Situ J, Cai JP, Yip CC, Wu S, Zhang AJ, Wen L, Chew NF, Chan WM, Poon RW, Chan JF, Tsang DN, Chen H, Xia NS, Yuen KY. Multimodal investigation of rat hepatitis E virus antigenicity: Implications for infection, diagnostics, and vaccine efficacy. J Hepatol. 2021;74:1315–1324. doi: 10.1016/j.jhep.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 68.Ji Y, Li P, Jia Y, Wang X, Zheng Q, Peppelenbosch MP, Ma Z, Pan Q. Estimating the burden and modeling mitigation strategies of pork-related hepatitis E virus foodborne transmission in representative European countries. One Health. 2021;13:100350. doi: 10.1016/j.onehlt.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He Q, Zhang F, Shu J, Li S, Liang Z, Du M, Liu X, Liu T, Li M, Yin X, Pan Q, Lu F, Wang L. Immunocompromised rabbit model of chronic HEV reveals liver fibrosis and distinct efficacy of different vaccination strategies. Hepatology. 2022;76:788–802. doi: 10.1002/hep.32455. [DOI] [PubMed] [Google Scholar]
- 70.Zaman K, Dudman S, Stene-Johansen K, Qadri F, Yunus M, Sandbu S, Gurley ES, Overbo J, Julin CH, Dembinski JL, Nahar Q, Rahman A, Bhuiyan TR, Rahman M, Haque W, Khan J, Aziz A, Khanam M, Streatfield PK, Clemens JD. HEV study protocol: design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open. 2020;10:e033702. doi: 10.1136/bmjopen-2019-033702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song K. Safety and Immunogenicity of Hecolin in Healthy Pregnant Women - Full Text View - ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. NCT05808166. ClinicalTrials.gov Identifier: NCT05808166. Available from: https://clinicaltrials.gov/ct2/show/