Abstract
A physical and genetic map of the Pasteurella multocida A:1 genome was generated by using the restriction enzymes ApaI, CeuI, and NotI. The positions of 23 restriction sites and 32 genes, including 5 rrn operons, were localized on the 2.35-Mbp single circular chromosome. This report presents the first genetic and physical map for this genus.
The gram-negative, facultative bacterium Pasteurella multocida is an important veterinary pathogen with worldwide distribution. It is a member of the Pasteurellaceae family (16), which includes the genera Haemophilus, Actinobacillus, and Lonepinella (15). Certain serotypes are the etiological agents of a number of severe pasteurelloses, such as fowl cholera in poultry, atrophic rhinitis in swine, and hemorrhagic septicemia in cattle and buffalo. Despite much research into the various diseases caused by P. multocida, there has been little characterization of this organism at the molecular level, and few of its genes have been characterized.
Here we describe the construction of a physical and genetic map of P. multocida serotype A:1, an Australian fowl cholera isolate, strain PBA100 (7). Restriction enzymes with GC-rich recognition sequences were chosen, as the G+C content of P. multocida is between 40 and 43% (14). The intron-encoded endonuclease CeuI, which recognizes a 26-bp sequence found exclusively in the 23S rRNA gene (12, 23), was used for restriction mapping and the analysis of the P. multocida rRNA (rrn) operons.
Restriction fragment analysis and estimation of genome size.
High-molecular-weight DNA was purified by the method of Smith and Cantor (21), except that the detergent Brij 58 was omitted from the lysis solution. The enzymes ApaI (Boehringer Mannheim), NotI, and CeuI (both from New England Biolabs) were used in physical mapping of the P. multocida genome according to the manufacturers’ instructions. Double digestions were performed as two consecutive 16-h digests, with CeuI digestion always performed second.
The resulting restriction fragments were separated by pulsed-field gel electrophoresis (PFGE) in 1% agarose gels in 0.5× Tris-borate-EDTA buffer (20) at 14°C with a contour-clamped homogeneous electric field apparatus (CHEF DRII; Bio-Rad). A range of conditions for electrophoresis was used to visualize and size the macrorestriction fragments. Typically, fragments between 50 and 500 kb were separated by using a linear ramp time of 10 to 60 s for 24 h at 200 V. Larger restriction fragments were observed by using longer pulse times of 10 to 150 s and 180 V for 24 h.
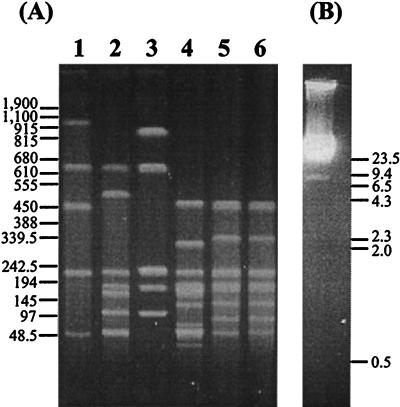
PFGE profiles resulting from single and double restriction digestions are shown in Fig. 1A. The sizes of the resulting macrorestriction fragments estimated from a number of pulsed-field gels run under different conditions to optimize the separation of bands are listed in Table 1. When standard agarose gel electrophoresis was used to visualize restriction fragments under 20 kb, only the 9-kb NF band was detected (Fig. 1B). The ApaI and ApaI/CeuI restriction profiles appeared identical (Fig. 1A, lanes 5 and 6), indicating the close linkage of the five CeuI sites to five ApaI sites. No evidence of extrachromosomal elements was found in the strain studied by using either a standard alkaline lysis plasmid preparation or the method of Kado and Liu (8) for the extraction of large plasmids. Based on these data, the P. multocida A:1 genome was estimated to be 2.35 Mbp.
FIG. 1.
(A) PFGE of P. multocida DNA fragments produced after digestion with CeuI (lane 1), NotI/CeuI (lane 2), NotI (lane 3), NotI/ApaI (lane 4), ApaI (lane 5), and ApaI/CeuI (lane 6). The positions of standard DNA size markers (in kilobases) are shown on the left. Electrophoresis was performed in 1% agarose with a pulse time of 10 to 80 s at 200 V for 24 h. In this figure, a single band represents each of the ApaI fragment pairs, AA/AB, AD/AE, and AF/AG (lane 5). (B) Standard agarose gel electrophoresis, showing the migration of P. multocida genomic DNA after NotI digestion, to visualize the 9-kb NF restriction fragment. The positions of HindIII-digested lambda DNA size markers (in kilobases) are shown on the right. The originals were scanned with a Hewlett-Packard ScanJet 4cse by using the Adobe Photoshop 2.5.1 LE program and exported into Deneba Canvas 3.5.4 for presentation.
TABLE 1.
Sizes of restriction fragments observed by PFGE after digestion of P. multocida A:1 (PBA100) genomic DNA with restriction enzymes as indicated
|
ApaI
|
CeuI
|
NotI
|
ApaI-CeuI
|
NotI-CeuI
|
|||
|---|---|---|---|---|---|---|---|
| Fragment | Size (kb) | Fragment | Size (kb) | Fragment | Size (kb) | Size (kb) | Size (kb) |
| AA | 460 | CA | 980 | NA | 850 | 460 | 630 |
| AB | 450 | CB | 630 | NB | 630 | 330 | 520 |
| AC | 340 | CC | 450 | NC1 | 230 | 230 | 230 |
| AD | 230 | CD | 230 | NC2 | 230 | 185 | 180 |
| AE | 228 | CE | 55 | ND | 180 | 180 | 175a |
| AF | 185 | NE1 | 110 | 175a | 110a | ||
| AG | 180 | NE2 | 110 | 125 | 55b | ||
| AH | 130 | NF | 9 | 75 | 9 | ||
| AI | 95 | 55c | |||||
| AJ | 55 | 53 | |||||
| 33 | |||||||
| 9 | |||||||
| Totald | 2,353 | 2,345 | 2,349 | 2,360 | 2,359 | ||
Band contains two comigrating fragments.
Band contains four comigrating fragments.
Band contains six comigrating fragments.
The genome size of 2.35 Mbp was derived from the mean of the five total genomic size estimations from each restriction digestion shown above.
Construction of the physical and genetic map.
Single and double restriction digestion, partial ApaI digestion, Southern hybridization using homologous and heterologous gene probes, the generation of linking clones, and reciprocal hybridization using macrorestriction fragments as probes were used to generate a physical and genetic map of the P. multocida A:1 chromosome. All hybridization, probe labeling, and chemiluminescence detection steps were performed according to the Dig System User’s Guide (Boehringer Mannheim). Hybridizations with homologous probes were performed under conditions of high stringency at 68°C, whereas those with heterologous probes were performed at 55°C under medium-stringency conditions consisting of two 5-min washes at room temperature in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) and two 15-min washes at 37°C in 1× SSC–0.1% SDS.
A small number of NotI and ApaI linking clones were constructed by digesting 1 μg of genomic DNA to completion with BamHI, EcoRI, or HindIII. Restriction digestion products were self-ligated and digested with either NotI or ApaI. The resulting linear fragments were then cloned into NotI- or ApaI-digested pBluescript II KS (Stratagene) and used as probes in Southern hybridization experiments. The linkages NA-NE1, NB-NC2, and NC1-ND were established by using the isolated NotI linking clones. These linkages were found to occur in the AE and CC fragments, the AD and CD fragments, and the AC and CA fragments, respectively.
A number of the ApaI linking clones isolated were of limited use in linkage analysis, as they hybridized to multiple ApaI fragments, all CeuI fragments, and NotI fragments NA, NB, and NC. The remaining ApaI linking clone linked AA with AG, and this linkage was found to lie within the CB and NA fragments. This approach thus definitively identified overlapping groups of restriction fragments between the three restriction enzymes.
Reciprocal hybridization experiments using macrorestriction fragments as probes extended the linkage data and allowed for the placement of fragments into a circular genomic map. However, because AA and AG occurred entirely within NA and CB, their order could not be determined by reciprocal hybridization or linker probe data alone. Partial ApaI restriction digestion was used to determine the orientations of these fragments. ApaI partial fragments of 790 and 415 kb, which may comprise AA-AC (800 kb) and AG-AE (408 kb), respectively, were observed. If the ApaI fragments were present in the order AC-AG-AA-AE, a 688-kb fragment representing the linkage AA-AE and a 520-kb fragment representing AC-AG would be expected. Although a 680-kb fragment was seen, no partial fragment in the vicinity of 520 kb was observed. This suggested that the most likely arrangement of these fragments was AC-AA-AG-AE.
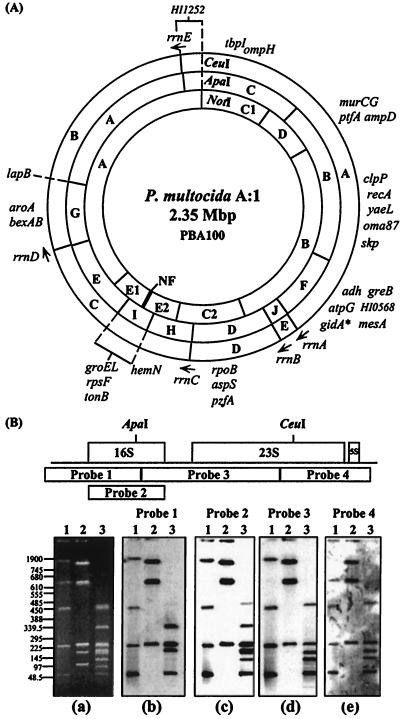
Gene probes (Table 2) were hybridized to single and double digests of P. multocida genomic DNA. This led to the positioning of genes which covered much of the genome onto the physical map and provided further evidence for the overlapping linkage groups of restriction fragments, hence permitting a more precise alignment of the three restriction maps with respect to each other. The combined physical and genetic map is shown in Fig. 2A.
TABLE 2.
Gene probes used for physical and genetic mapping
| Probe | Gene | Function or description | Sourceb |
|---|---|---|---|
| Homologous | |||
| pMEC100 | aroA | Aromatic amino acid biosynthesis | 6 |
| pPBA822 | gidAa | Glucose-inhibited cell division protein | A |
| pPBA826 | atpGa | ATP synthetase gamma subunit | A |
| pPBA838 | hemNa | Heme biosynthesis | A |
| pPBA1039 | rpsFa | Ribosomal protein | A |
| pPBA1137 | yaeLa | Hypothetical E. coli protein | 18 |
| pPBA1137 | oma87 | 87-kDa outer membrane protein | 19 |
| pPBA1137 | skpa | Chaperone protein | 18 |
| pPBA1155 | clpPa | Protease | A |
| pPBA1166 | murCGa | Peptidoglycan synthesis | A |
| pPBA1169 | groELa | Heat shock protein | A |
| pPBA1211 | adha | Alcohol dehydrogenase | A |
| pPBA1211 | mesA | Esterase | A |
| pPBA1211 | HI0568a | Orthologue of HI0568, H. influenzae Rd | A |
| pPBA1211 | greBa | Transcriptional elongation factor | A |
| pPBA1217 | recA | Homologous recombination | A |
| pPBA1245 | lapBa | Membrane protein | A |
| pPBA1248 | aspSa | Aspartyl-tRNA synthetase | A |
| pPBA1267 | tonBa | Iron uptake | A |
| pPBA1268 | tbpIa | Transferrin binding protein | A |
| pPBA1271 | ampDa | β-Lactamase regulation | 18 |
| pPBA1271 | ptfAa | Type IV fimbrial subunit gene | 18 |
| pPBA1285 | HI1252a | Orthologue of HI1252, H. influenzae Rd | A |
| pPBA1458 | pzfAa | Putative zinc finger; regulation | A |
| pPBAYZ1 | rpoBa | RNA polymerase beta subunit | A |
| PCR amplicon | ompH | Major outer membrane porin | B |
| PCR amplicon (probe 2) | rrs | 16S rRNA gene 1.5-kb PCR product | A |
| Heterologous | |||
| pJSK3 | bexAB | Polysaccharide export; H. influenzae type b | 10 |
| pJIR632 (probe 1) | rrs | 5′ region of D. nodosus rrs gene | 11 |
| pJIR697 (probe 3) | rrs-rrl | 3′ region of rrs and 5′ region of rrl gene of D. nodosus | 11 |
| pJIR873 (probe 4) | rrl-rrf | 3′ region of rrl and complete rrf gene of D. nodosus | 11 |
FIG. 2.
(A) Physical and genetic map of the 2.35-Mbp circular chromosome of P. multocida A:1 PBA100. The positions of the ApaI, CeuI, and NotI restriction sites are shown, and fragment names are indicated. The genes are positioned on the map to the minimum region localized by hybridization. The order of genes given in a particular region is arbitrary and does not necessarily represent the actual order of genes in that area. The genes groEL, rpsF, and tonB can be localized only to the 95-kb AI fragment, as it is not known to which NE fragment these genes belong. Asterisk, putative location of the origin of replication, linked to gidA. The arrows indicate the presumed 5′→3′ direction of transcription of the rrn operons given the gene order rrs-rrl-rrf. (B) Determination of rrn gene position and orientation on the P. multocida chromosome. (Top) Diagrammatic representation of a ribosomal operon and the positions of heterologous D. nodosus probes 1, 3, and 4 and homologous rrs gene probe 2. The approximate positions of ApaI and CeuI sites in the P. multocida rrn operons are indicated. (a) PFGE-resolved restriction fragments of P. multocida DNA obtained by using running conditions of 20 to 60 s at 200 V for 24 h. The positions of standard DNA size markers (in kilobases) are shown on the left. (b through e) Resulting Southern hybridization profiles obtained by using the probes indicated above. In all panels, lanes 1, 2, and 3 represent CeuI, NotI, and ApaI restriction digestion fragments, respectively. The originals were scanned with a Hewlett-Packard ScanJet 4cse by using the Adobe Photoshop 2.5.1 LE program and exported into Deneba Canvas 3.5.4 for presentation.
Location, orientation and operon structure of the P. multocida A:1 rrn genes.
The intron-encoded endonuclease CeuI has been found to cut exclusively in the rrl genes of many bacteria (12). Thus, the five restriction fragments generated indicated that PBA100 has five copies of the rrl gene. To determine if rRNA genes in P. multocida were organized into operons, heterologous rrn probes from Dichelobacter nodosus, probe 1, probe 3, and probe 4 (11), and a homologous PCR-derived 16S rRNA probe, probe 2, with the primers 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-GGTTACCTTGTTACGACTTC-3′, which bind to conserved regions of the 16S rRNA gene, were used in Southern hybridization analysis (Fig. 2B). With all rrn probes used, an invariant NotI hybridization profile of NA, NB, and NC was seen, indicating that rrn genes resided only on these NotI restriction fragments. The linkage of the five CeuI fragments to five ApaI fragments was apparent from the similarity of the ApaI and ApaI/CeuI digests (Fig. 1) and the resulting physical map. Hybridization results, taken in conjunction with the physical mapping data, indicated that the rrs genes in P. multocida traverse ApaI sites. This was confirmed by the isolation of ApaI linking clones that hybridized to the same set of NotI fragments (NA, NB, and NC), many ApaI restriction fragments, and all CeuI fragments. One such clone, a 5.5-kb EcoRI-generated ApaI linking clone, was partially sequenced, revealing the presence of an rrs gene containing a single ApaI site and both rrl and rrf genes. Considering the sequencing data and the rrn hybridization profiles, P. multocida rRNA genes appear to be organized into five operons, with the gene order of these operons, like that of many other eubacteria, being rrs-rrl-rrf. CeuI and ApaI/CeuI digestion of these linking clones indicated the presence of a unique CeuI site and demonstrated an ApaI/CeuI fragment of either 2.9 or 3.1 kb, thus indicating the presence of two classes of rrn loci with different spacing of the ApaI and CeuI restriction sites. The direction of transcription of each operon, designated rrnA through rrnE, was ascertained from hybridization analysis (Fig. 2).
Genetic organization.
This study, which positions 23 restriction sites and 32 genetic markers onto a 2.35-Mbp circular chromosome, details the first physical and genetic map for the species P. multocida and the genus Pasteurella. Key genetic markers such as gidA, the five rrn operons, recA, and groEL have been positioned onto the chromosome. The genome size derived in this study places P. multocida in bacterial genome group 2 as defined by Cole and Saint Girons (2), which includes genomes between 1.5 and 3 Mbp. This group includes a range of human and animal pathogens, such as the gram-positive bacteria Streptococcus pneumoniae and Staphylococcus aureus and the gram-negative bacteria Campylobacter jejuni, Neisseria gonorrhoeae, and Haemophilus influenzae (2).
Several features of genome organization common among many bacteria appear to be conserved in P. multocida. The gidA gene, encoding the glucose-inhibited division protein, is adjacent to the origin of replication, oriC, in Pseudomonas putida, Pseudomonas aeruginosa, Escherichia coli, Bacillus subtilis, and the closely related strain H. influenzae Rd (3, 22). If gidA is also linked to the origin of replication in P. multocida, then genes involved in transcription, as in many other bacteria, map close to the origin: greB and atpG are both located on the same fragment as gidA, and rpoB and aspS occur within approximately 285 kb of this site (Fig. 2A).
The rrn operons of P. multocida A:1 strain PBA100 occur in two unequal groups, transcribed divergently from the putative site of oriC, which may itself be linked to the rrnA operon. This is a common feature of many bacterial chromosomes, in which the rrn genes are often located within one-third to one-half of the chromosome (17). The operons rrnA, rrnB, rrnC, and rrnD, which are transcribed in the same direction away from the presumed location of oriC, lie within a 735-kb region (31% of the chromosome). In many bacteria, a single rrn operon is transcribed divergently from the other rrn loci. However, in PBA100 the single divergently transcribed rrnE operon is located at an unusually large distance of almost 980 kb away from the putative origin of replication, with the result that the five rrn operons of P. multocida are spread over a much greater proportion of the chromosome than is common in other bacteria.
Varying degrees of genome structure conservation are seen within and between bacterial genera and species (4). A comparison of the genetic maps of the Pasteurellaceae members P. multocida and H. influenzae Rd revealed both similarities and differences, but no long-range colinearity of gene order was found. The conservation of gene order among some bacteria is often most marked close to the origin (9). Local gene order and similar genome locations were apparent for the orthologous genes greB and HI0568. An order and spacing of these two genes in P. multocida similar to those in H. influenzae Rd were determined from sequence analysis of pPBA1211 (Table 2). These genes mapped to the macrorestriction fragment AF, which contained the putative location of oriC.
The yaeL-oma87-skp-firA region of P. multocida (18, 19) was identified as another region of local conservation of gene order, aligning with the H. influenzae Rd genes yaeL-D15-skp-firA (3). However, these orthologues have different chromosomal locations. Differences in gene positions are also apparent when the clustering together of murCG and ptfA within a 125-kb region, the clustering of aroA and lapB within a 180-kb region, and the close proximity of tonB to groEL and rpsF in the P. multocida genome are taken into consideration. In H. influenzae Rd, these groups of genes are separated by much greater distances. Despite the fact that the genome of P. multocida is 520 kb larger, there was no evidence for the occurrence of multiple lapB and tbpI loci, such as are found in H. influenzae Rd. Also, P. multocida has only five rrn operons, which are arranged differently from the six rrn operons of H. influenzae Rd.
Differences between these Pasteurellaceae members are not unexpected when the different growth requirements of these two pathogens and the different diseases they cause are considered. Additionally, different NotI profiles have been reported previously for other P. multocida isolates (24). We have also observed differing CeuI profiles in other serotype A:1 isolates and some isolates of heterologous serotypes (data not shown), indicating that P. multocida exhibits significant intraspecies heterogeneity. The marked differences between restriction fragment profiles may be the result of a heterogeneous group of organisms being grouped together as a single species. Alternatively, while no extrachromosomal elements were found in PBA100, a variety of bacteriophages and plasmids have been characterized in other P. multocida isolates (1, 5). Hence, the tools for genome plasticity are present in the P. multocida species.
This genomic map provides a basis for the study of genomic organization in P. multocida. Comparative analysis of the ApaI and CeuI profiles of P. multocida isolates will be useful in tracking the number and arrangement of the rrn loci. The placement on the map of more virulence-associated genes and the future use of this map in comparative studies with other P. multocida isolates of the same or different serotypes will provide greater insights into the genomic architecture of P. multocida and may begin to answer questions about the molecular basis for the differences in host predilection and pathogenicity in this diverse species.
Acknowledgments
We are grateful to Ian McPherson and Vicki Vallance for their excellent technical assistance. We also thank Dario Diberardino for the 16S rRNA primers and Sharon La Fontaine for helpful discussions.
This work was supported by research grants from the Chicken Meat Research and Development Council of Australia and the Australian Research Council.
REFERENCES
- 1.Ackermann H W, Karaivanov L. Morphology of Pasteurella multocida bacteriophages. Can J Microbiol. 1984;30:1141–1148. doi: 10.1139/m84-179. [DOI] [PubMed] [Google Scholar]
- 2.Cole S T, Saint Girons I. Bacterial genomics. FEMS Microbiol Rev. 1994;14:139–160. doi: 10.1111/j.1574-6976.1994.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 4.Fonstein M, Haselkorn R. Physical mapping of bacterial genomes. J Bacteriol. 1995;177:3361–3369. doi: 10.1128/jb.177.12.3361-3369.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haghour R, Hellmann E, Schmidt J. Plasmids and resistance to 9 chemotherapeutic agents of Pasteurella multocida and Pasteurella haemolytica. Epidemiological aspects. J Vet Med. 1987;34:509–518. doi: 10.1111/j.1439-0450.1987.tb00427.x. [DOI] [PubMed] [Google Scholar]
- 6.Homchampa P, Strugnell R A, Adler B. Molecular analysis of the aroA gene of Pasteurella multocida and vaccine potential of a constructed aroA mutant. Mol Microbiol. 1992;6:3585–3593. doi: 10.1111/j.1365-2958.1992.tb01794.x. [DOI] [PubMed] [Google Scholar]
- 7.Ireland L, Adler B, Milner A R. Proteins and antigens of Pasteurella multocida serotype 1 from fowl cholera. Vet Microbiol. 1991;27:175–185. doi: 10.1016/0378-1135(91)90009-5. [DOI] [PubMed] [Google Scholar]
- 8.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolsto A B. Dynamic bacterial genome organization. Mol Microbiol. 1997;24:241–248. doi: 10.1046/j.1365-2958.1997.3501715.x. [DOI] [PubMed] [Google Scholar]
- 10.Kroll J S, Hopkins I, Moxon E R. Capsule loss in Haemophilus influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell. 1988;53:347–356. doi: 10.1016/0092-8674(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 11.La Fontaine S. Ph.D. thesis. Clayton, Victoria, Australia: Monash University; 1994. [Google Scholar]
- 12.Liu S L, Hessel A, Sanderson K E. Genomic mapping with I-CeuI, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc Natl Acad Sci USA. 1993;90:6874–6878. doi: 10.1073/pnas.90.14.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo Y, Glisson J R, Jackwood M W, Hancock R E, Bains M, Cheng I H, Wang C. Cloning and characterization of the major outer membrane protein gene (ompH) of Pasteurella multocida X-73. J Bacteriol. 1997;179:7856–7864. doi: 10.1128/jb.179.24.7856-7864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutters R, Ihm P, Pohl S, Frederiksen W, Mannheim W. Reclassification of the genus Pasteurella Trevisan 1887 on the basis of deoxyribonucleic acid homology, with proposals for the new species Pasteurella dagmatis, Pasteurella canis, Pasteurella stomatis, Pasteurella anatis, and Pasteurella langaa. Int J Syst Bacteriol. 1985;35:309–322. [Google Scholar]
- 15.Osawa R, Rainey F, Fujisawa T, Lang E, Busse H J, Walsh T P, Stackebrandt E. Lonepinella koalarum gen. nov., sp. nov., a new tannin-protein complex degrading bacterium. Syst Appl Microbiol. 1995;18:368–373. [Google Scholar]
- 16.Phol S. DNA relatedness among members of Haemophilus, Pasteurella and Actinobacillus. In: Kilian M, Frederiksen W, Biberstein E L, editors. Haemophilus, Pasteurella and Actinobacillus. London, United Kingdom: Academic Press, Inc.; 1981. pp. 245–253. [Google Scholar]
- 17.Roussel Y, Pebay M, Guedon G, Simonet J M, Decaris B. Physical and genetic map of Streptococcus thermophilus A054. J Bacteriol. 1994;176:7413–7422. doi: 10.1128/jb.176.24.7413-7422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruffolo C. Ph.D. thesis. Clayton, Victoria, Australia: Monash University; 1997. [Google Scholar]
- 19.Ruffolo C G, Adler B. Cloning, sequencing, expression, and protective capacity of the oma87 gene encoding the Pasteurella multocida 87-kilodalton outer membrane antigen. Infect Immun. 1996;64:3161–3167. doi: 10.1128/iai.64.8.3161-3167.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Smith C L, Cantor C R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- 22.Smith D W, Yee T W, Baird C, Krishnapillai V. Pseudomonad replication origins: a paradigm for bacterial origins? Mol Microbiol. 1991;5:2581–2587. doi: 10.1111/j.1365-2958.1991.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 23.Toda T, Itaya M. I-CeuI recognition sites in the rrn operons of the Bacillus subtilis 168 chromosome: inherent landmarks for genome analysis. Microbiology. 1995;141:1937–1945. doi: 10.1099/13500872-141-8-1937. [DOI] [PubMed] [Google Scholar]
- 24.Townsend K M, Dawkins H J S, Papadimitriou J M. Analysis of haemorrhagic septicaemia-causing isolates of Pasteurella multocida by ribotyping and field alternation gel electrophoresis (FAGE) Vet Microbiol. 1997;57:383–395. doi: 10.1016/s0378-1135(97)00121-1. [DOI] [PubMed] [Google Scholar]