Abstract
Neutrophils [polymorphonuclear leukocytes (PMNs)] execute important effector functions protecting the host against invading pathogens. However, their activity in tissue can exacerbate inflammation and inflammation-associated tissue injury and tumorigenesis. Until recently, PMNs were considered to be short-lived, terminally differentiated phagocytes. However, this view is rapidly changing with the emerging evidence of increased PMN lifespan in tissues, PMN plasticity, and phenotypic heterogeneity. Specialized PMN subsets have been identified in inflammation and in developing tumors, consistent with both beneficial and detrimental functions of PMNs in these conditions. Because PMN and tumor-associated neutrophil activity and the resulting beneficial/detrimental impacts primarily occur after homing to inflamed tissue/tumors, studying the underlying mechanisms of PMN/tumor-associated neutrophil trafficking is of high interest and clinical relevance. This review summarizes some of the key findings from over a decade of work from my laboratory and others on the regulation of PMN recruitment and identification of phenotypically and functionally diverse PMN subtypes as they pertain to gut inflammation and colon cancer.
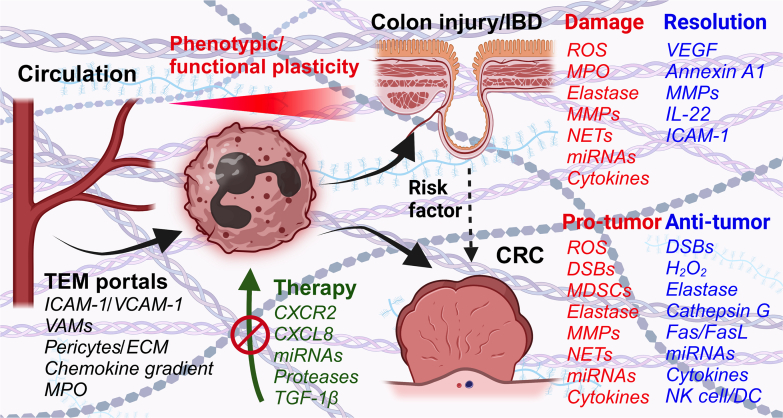
Neutrophils or polymorphonuclear leukocytes (PMNs) account for up to 70% of circulating immune cells in humans and up to 25% in mice.1 PMNs execute most of their effector functions as they enter tissues, and so crossing of the vascular barrier is an important regulatory barrier in health and disease. Until recently, PMNs were considered to be short-lived, terminally differentiated phagocytes providing the first line of host defense during initial stages of inflammation. This view, however, is rapidly changing with the emerging evidence of increased PMN lifespan in tissues, and PMN plasticity and phenotypic heterogeneity. It is now well appreciated that environmental cues, such as cytokines, hypoxia, or pathogen encounters, promote PMN survival both in the circulation and even more so in tissues, supporting the idea of PMN transitional stages and acquisition of new phenotypes. Indeed, recent studies using state-of-the-art single-cell sequencing analyses of mouse and human tissue revealed heterogeneous PMN subsets as early as their development in the bone marrow and later as they are released to the circulation and on entering various tissues.2,3 Given this evidence of PMN phenotypic heterogeneity, it is not surprising that their function in numerous pathologic conditions is similarly highly diverse. For example, in many diseases, including lung and heart disorders, inflammatory bowel diseases (IBDs), and cancer, PMNs have been assigned both beneficial and detrimental roles. The pathologic PMN activity in the conditions mentioned has been associated with poor prognosis and more severe disease symptoms, largely due to PMN-produced proteinases and reactive oxygen species (ROS).4,5 However, PMNs were also shown to play important roles in disease resolution, promoting processes such as angiogenesis, immune modulation, and tissue remodeling/cellular debris removal during wound healing. Thus, PMNs are now being increasingly recognized as instrumental components of homeostatic restoration. Here, I will review some of the key findings from over a decade of research from my laboratory and others on the underlying mechanisms for PMN trafficking and retention in inflamed tissues and tumors and the emerging PMN phenotypic and functional diversity as it pertains to gut inflammation and colon cancer. These data are summarized by the schematic diagram (Figure 1).
Figure 1.
The schematic summarizes polymorphonuclear leukocyte (PMN) recruitment mechanisms in inflamed colon mucosa and colon cancer, highlighting the requirement of transendothelial migration (TEM) portal formation, involving vessel-associated macrophage (VAM) and pericyte signaling, enrichment of adhesion molecules intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), and establishment of chemokine gradients. As PMNs cross the blood vessels and enter tissues, they undergo niche-specific reprogramming, acquiring diverse functional phenotypes, resulting in beneficial or detrimental impacts on disease outcomes. Most protective and detrimental PMN effector functions are enacted via the release of either proresolution or cytotoxic factors, as summarized above. Finally, potential therapeutic approaches currently under investigation or in various testing phases, including inhibition of PMN chemokine receptors or secreted damaging factors, have been discussed. CRC, colorectal cancer; CXCR2, C-X-C chemokine receptor 2; DC, dendritic cell; DSB, double-stranded break; ECM, extracellular matrix; H2O2, hydrogen peroxide; IBD, inflammatory bowel disease; MDSC, myeloid-derived suppressor cell; MMP, matrix metalloproteinase; MPO, myeloperoxidase; NET, neutrophil extracellular trap; NK, natural killer; ROS, reactive oxygen species; TGF-1β, transforming growth factor-1β; VEGF, vascular endothelial growth factor.
Neutrophil Plasticity in Inflammation and Cancer
As omics and sequencing technologies became more user friendly and more commonly used, PMN plasticity and functional diversity began to emerge, rebuking the long-existed dogma that PMNs are transcriptionally not active, committed effector cells. PMN plasticity in inflamed tissue and in tumors is now well appreciated,2,3,6 with evidence emerging of specific tissue niche-driven PMN reprogramming and phenotypic/functional adaptation.7 However, the roles of various PMN subsets within the respective niches are less understood, especially compared with other better studied immune cells, including tissue-resident macrophages or T lymphocytes. This perhaps is due to technical challenges in working with PMNs, given their relatively lower transcriptomes8 and higher turnover. Additionally, to date, PMN classifications into subsets have been primarily based on the identification of phenotypically and/or functionally distinct PMN populations in various tissues. Therefore, whether these are truly committed cell subsets or the same cell population caught at different transitional stages remains to be determined. Recent observation of several transcriptionally distinct PMN subsets in the bone marrow and in the circulation9 perhaps supports the idea of early classification and phenotypic imprinting that could be carried into tissue compartments. Further supporting this notion, transcriptome-guided analyses of PMN granulopoiesis trajectories in pseudo-time revealed a distinct interferon response gene enriched PMN subtype in the circulation that has emerged from the bone marrow while skipping a full maturation program.2 On the other hand, given the robust de novo production and rapid PMN turnover rate in the systemic circulation following initiation of inflammation or tumorigenic cues, the heterogeneity of circulation and tissue PMNs can stem from different aging and activation states. Indeed, both mature and immature neutrophils distinguished respectively by the multilobular versus more banded nuclei morphology and high versus low surface expression of CD16, CD10, and CD66b can be found in peripheral blood in time of immunologic stress in mice and humans.9, 10, 11 The functional properties of the immature CD10-PMN subset are still debatable, with both immunosuppressive activity or stimulation of T-cell survival and proliferation being proposed.12,13 Similarly, activation-driven changes in the expression of surface markers, including increases in CD11b and CD66b and decreases in CD62 ligand and junctional adhesion molecule-like protein, may contribute to phenotypical PMN heterogeneity.14, 15, 16 Other examples of PMN plasticity and functional adaptation in tissue include ability to present antigens via up-regulation of major histocompatibility complex II and other costimulatory molecules,17 acquisition of dendritic cell functionalities and CD11c expression,18,19 as well as expression of what was considered until recently a bonified eosinophilic marker, Siglec-F, to become profibrotic inflammatory cells.20 Murine bone marrow PMNs transdifferentiate into Siglec-F eosinophils with IL-5 treatment and even into monocytes resembling cells in response to macrophage colony-stimulating factor treatment.21 For a detailed summary of markers of PMN maturation, activation, and tissue specialization, please refer to an elegant recent review attempting to standardize PMN nomenclature.22 Finally, although tissue-specific niches clearly impact PMN/tumor-associated neutrophil (TAN) transcriptional programs and protein expression, they could also elicit more dynamic post-transcriptional modifications (ie, protein phosphorylation or glycosylation), contributing to PMN/TAN phenotypic diversity. Although this topic is understudied and requires further investigation, modification of protein glycosylation, for example, has already been shown to impact PMN effector functions (maturation, migration, or phagocytosis),23,24 and as such likely contributes to the establishment of distinct cellular phenotypes.
PMN Phenotypes in Human IBD
Macrophages and PMNs are represented by most diverse phenotypes in IBD, as shown by a recent transcriptomic profiling of the colon cellular landscape comparing patients with ulcerative colitis and Crohn disease. These studies identified three unique PMN subtypes with inferred differences in maturity/activation states (based on expression of CXCR4, CCL3, CG63, GBP1, and IRF1), scattered across spatial mucosal compartments, highlighting the potentially important and diverse PMN functions in IBD.25 Another similar transcriptomics analysis of ulcerative colitis and Crohn disease patient colon tissue identified several distinct inflammatory gene modules predicative of nonresponse to anti–tumor necrosis factor (TNF) and corticosteroid therapy. PMNs and fibroblasts encoding genes were primarily enriched within these modules, where fibroblasts in response to IL-1β stimulation were identified as source of neutrophil-targeting C-X-C chemokine receptor (CXCR) 1/CXCR2 ligands, CXCL1-3, CXCL5, CXCL6, and CXCL8.26
PMN Phenotypes in Cancer
The tumor microenvironment (TME) is perhaps the best and most studied example of the ability of PMNs to adapt to environmental cues, not only switching their transcriptional programs but also changing functional phenotypes. TANs in response to interferons were shown to adopt anti-tumorigenic functionalities, characterized by increased ROS generation and secretion of TNF-α and other cytotoxic factors. In contrast, transforming growth factor (TGF)-β polarized neutrophils into the tumor-promoting phenotype, featuring release of arginase, matrix metalloproteinase (MMP)-9, vascular endothelial growth factor (VEGF), and other chemokines supporting immune suppression, tumor vascularization, and growth.27 One example of the TME impact on TAN specialization is the tumor-produced CXCL1 and CXCL12, driving angiogenic VEGF receptor 1+/CD49d+/CXCR4+ TAN specialization and production of VEGF-A/MMP-9 to promote tumor angiogenesis.28,29 Another example is that of immature PMNs released by the bone marrow during emergency granulopoiesis driven by tumor-produced granulocyte-macrophage colony-stimulating factor or granulocyte colony-stimulating factor.30 These cells represent a large portion of TANs; and in response to polarizing cues inducing TGF-β or the IL-17–regulated granulocyte-macrophage colony-stimulating factor/granulocyte colony-stimulating factor signaling, they may be shaped into myeloid-derived suppressor cells.30,31 Mature PMNs can similarly execute both immunosuppressive/protumorigenic and anti-tumoral functions.11,32,33 These include mature TANs expressing immunosuppressive arginase 1 (ARG1), programmed death-ligand 1 (PD-L1), and CCR534 or activated intercellular adhesion molecule 1 (ICAM-1)high/CD62 ligandlow TAN subtypes featuring high ROS and proinflammatory cytokines interferon-γ, IL-12, and TNF-α levels.33
PMN Recruitment and Retention in Inflamed Tissue and the TME
PMN Trafficking in Inflamed Intestines
PMNs execute their effector functions once they exit the blood vessels and enter the underlying tissue. As such, the endothelial layer lining the lumen of blood vessels is the first critical regulatory barrier for this process. PMN transendothelial migration (TEM) involves several well-studied sequential adhesive interactions with vascular endothelial cells (ECs). They start with PMN margination and selectin-mediated rolling, adhesion and intraluminal crawling using β2 integrins and endothelial adhesion molecules, such as ICAM-1/2 and vascular cell adhesion molecule 1, and end with crossing of ECs at designated locations (portals).35, 36, 37 Although PMN-EC adhesive interactions and the molecular cues involved in PMN TEM have been well characterized, mechanisms initiating and terminating PMN TEM are less understood. For example, using murine models, our laboratory and others have noted that PMN TEM in inflamed tissue takes place in designated locations/portals enriched for ICAM-1 expression35,38 and reduced pericyte and collagen density in the perivascular space.39,40 However, mechanisms guiding formation of such portals are not well defined. In a recent work, we identified one such mechanism in inflamed gut mucosa. We found that following colon injury and the resulting inflammation, interstitial mucosal macrophages were recruited to interact with the vessel wall. Vessel-associated macrophages, via TNF-α release and EC TNF receptor 2 signaling, induced localized increases in ICAM-1/vascular cell adhesion molecule 1, forming critical platforms to initiate PMN-EC adhesive interactions, PMN TEM, and subsequent accumulation in the intestinal mucosa.41
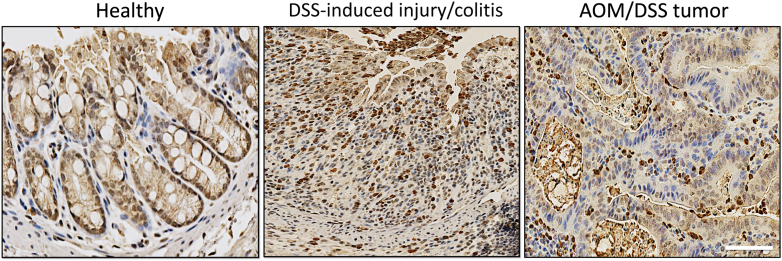
In inflamed gut and pathologies underlining inflammatory bowel conditions (such as IBD), PMN accumulation in the crypt epithelium and the luminal space is a prominent feature, closely associating with disease severity. PMN infiltration of the colon mucosa is similarly a major feature of murine colitis models, such as dextran sulfate sodium (DSS)–induced injury/colitis (Figure 2). Because the crypt mucosa is highly vascularized and blood perfused, we poised that microvessels surrounding intestinal crypts are well positioned to support PMN migration into the epithelial and luminal spaces. Intravital imaging to track PMNs in real time in anesthetized mice indicated distinct trafficking patterns, with initial PMN entry into the inflamed gut mucosa being restricted to larger submucosal vessels. These compartmentalized PMN migration patterns were driven by the elevated CXCL1/2 levels enriched in the submucosal compartment.42 Differences in vascular EC composition and activation may also contribute to distinct PMN migratory patterns; however, this remained to be determined. Entry remote from destination (crypt epithelium or the luminal space) and evidenced increased PMN lifespan in inflamed tissue may allow for transitional stages and PMN adaptation to local environmental cues, consistent with the apparent plasticity of tissue PMNs discussed in Neutrophil Plasticity in Inflammation and Cancer. Interestingly, another recent work has identified mobile and immobile pools of PMNs in the inflamed mucosa.43 Mobile PMNs swarmed to sites of gut injury following leukotriene B4 (LtB4) gradient, whereas immobile fraction exhibited aging/apoptotic markers, indicating perhaps exhausted PMN phenotype. What the function of these exhausted PMNs in gut inflammation is still not defined; however, these observations once more highlight the phenotypic heterogeneity and diverse functions of PMNs in inflamed tissue.
Figure 2.
Murine colitis/colorectal cancer models feature a robust polymorphonuclear leukocyte (PMN) tissue infiltration. This is shown by representative immunohistochemistry images stained for the PMN marker S100A9 (brown) and nuclei stain hematoxylin (blue). Scale bar = 100 μm. AOM, azoxymethane; DSS, dextran sulfate sodium.
In mouse models of gut inflammation, CXCL1/2, through interactions with PMN receptors CXCR1/2, seem to be major drivers of PMN accumulation in inflamed mucosa. Gut resident T cells, macrophages, and mast cells produce CXCL1/2 to mobilize PMNs into inflamed gut,44,45 whereas CXCR2 inhibition alleviated DSS–acute colitis symptoms, by reducing excessive neutrophil recruitment to the colonic lamina propria.46 Similarly, CXCR1/2 high PMNs were noted in patients with active IBD, as well as high expression of another CXCR1/2 ligand, CXCL8 (IL-8).47 Other molecules that have been implicated in PMN gut infiltration are the complement proteins C5a48 and eicosanoid hepoxilin A3 synthesized by epithelial cells and secreted from their apical surface via multidrug resistance protein 2 to promote PMN migration across epithelial cells.49 Indeed, based on multidrug resistance protein 2/hepoxilin A3 roles in mediating PMN transepithelial migration, a small molecule inhibitor, ADS051, has been designed, to efficiently suppress PMN trafficking across epithelial layers.50 Glycans coating PMN integrins, including sialic acid, which is present in abundance on PMN CD11b/CD18, have also emerged as important regulators of PMN intestinal trafficking; and their removal suppressed PMN accumulation and activity in inflamed mucosa.51 Interestingly, an unexpected role of myeloperoxidase (MPO) was recently identified in negative regulation of PMN trafficking in inflamed mucosa. MPO is an azurophilic granule enzyme, which, together with hydrogen peroxide, forms a powerful antimicrobial system designed to kill ingested bacteria. However, in activated PMNs, it is mobilized to the cell surface to antagonize PMN binding to endothelial cells suppressing their ability to extravasate.52
Mechanisms of TAN Recruitment
Many molecular events in TAN recruitment and retention within the tumor niche have been identified; however, this is still an active area of investigation. This investigation particularly considers the emerging TAN plasticity and still debated bona fide TAN subtypes versus adaptation within the TME. PMN recruitment and homing to tumors involves cytokines, growth factors, and chemokines produced by stromal, immune, and tumor cells. As with tissue inflammation, carcinomas up-regulate CXCL8 expression to facilitate PMN recruitment.53 CXCL1 and its receptor, CXCR2, are elevated in human sporadic colorectal cancer (CRC).54 CXCR2 deletion in murine azoxymethane (AOM)/DSS-induced CRC model (dominated by TAN infiltration, as shown by the representative image) (Figure 2) attenuates tumor growth via reduction of tumor burden.55 CXCR2-dependent TAN accumulation within the premetastatic niche has been shown to be driven by epithelial neurogenic locus notch homolog protein 1 (NOTCH1)–dependent induction in TGF-β signaling to generate an immunosuppressive environment in murine KrasG12D/+-driven CRC model.56
CXCL8 in human CRC has also been shown to be released by apoptotic tumor cells, preferentially attracting PMNs to these regions, and via interactions with tumor macrophages promoting immunosuppressive TME.57 Similarly, CD4+ type 17 helper T cells in CRC facilitated recruitment of neutrophils through CXCL8 secretion.58 CXCL8 expression is not limited to CRC and has been shown to mediate PMN recruitment via CXCR2 in various tumor types.59 Chemokine (C-C motif) ligand (CCL) 15 is another chemokine that was induced by SMAD4 in CRC cells to recruit both immature and mature PMNs, impacting tumor development.60
PMN Beneficial versus Detrimental Activity in Inflamed Gut Mucosa
PMN Pathology in Gut Inflammation
PMN presence in the tissue is often viewed as detrimental and is associated with exacerbated inflammation and injury.61 The pathologic effects of PMNs in gut inflammation are driven by excessive ROS production and release of MPO, elastase, proteinases, neutrophil extracellular traps (NETs), inflammatory cytokines, and miRNAs. These factors cause DNA damage and epithelial cell death and drive disruption of epithelial junctions and epithelial monolayer integrity, resulting in epithelial barrier dysfunction and impaired wound healing.
PMNs are known to produce ROS aimed at bacterial killing; however, in tissue, high ROS levels leads to lipid peroxidation and DNA oxidation, resulting in shifts in cell metabolism, cytotoxic stress, and cell cycle arrest.62,63 An additional and perhaps more prominent mechanism of PMN-mediated DNA damage to epithelial cells was recently identified, resulting in significant impairments of inflammation resolution and colon wound healing.64 Mucosa-infiltrating PMNs were found to release extracellular vesicles (or microparticles) shuttling inflammatory miRNAs, miR-23a and miR-155, which were deposited to intestinal epithelial cells (IECs) to down-regulate nuclear envelope protein lamin B1 and homologous recombination regulator RAD51. Depletion of both proteins in IECs resulted in an induction of double-stranded breaks (DSBs) and inability to resolve these cytotoxic DNA lesion due to potent suppression of DSB repair by homologous recombination.64 Extracellular vesicles released by PMNs infiltrating the epithelial layers can similarly transport MMP-9 and MPO to damage IECs. MMP-9 shuttled by PMN extracellular vesicles and deposited to IECs induced cleavage of desmoglein-2, leading to disruption of IEC intercellular adhesions and barrier dysfunction.65 MPO released in association with PMN extracellular vesicles was similarly found to inhibit IEC wound closure in vitro and in vivo by suppressing IEC migration and proliferation.66 Whether these observed impacts of MPO were due to its enzymatic activity and ROS generation or via another more direct mechanism remains to be determined. Junctional adhesion molecule-like protein is another neutrophil-derived factor that has been shown to compromise IEC barrier and wound healing. Junctional adhesion molecule-like protein is shed from the PMN surface during transepithelial migration; and in its soluble form, it binds epithelial tight junction protein coxsackie-adenovirus receptor to exacerbate epithelial damage.16 Finally, PMN NETs correlate with disease severity and intestinal damage in patients with IBD and in murine models of colitis.67,68 NETs have been suggested to impair epithelial barrier function and promote IEC cell apoptosis; however, the exact mechanisms remain to be elucidated.
PMN Contributions to Resolution of Gut Inflammation
As highlighted in PMN Pathology in Gut Inflammation, PMN pathologic impacts mostly associate with conditions featuring heightened numbers of tissue-infiltrating PMNs. However, the view that PMNs are the bad guys is changing with the emerging evidence of PMN homeostatic and proresolution functions. PMNs can contribute to tissue repair by multiple mechanisms, including clearance of cellular debris, immune regulation/suppression, resolution of inflammation, and tissue remodeling. Specific examples include the release of MMP-9, which degrades damage-associated molecular pattern molecules to dampen recruitment of other immune cells. Via the release of VEGF and dynamic interactions with macrophages, PMNs can promote vascular growth and neovascularization.69 Similarly, clearance of apoptotic PMNs by tissue-resident macrophages in a process termed efferocytosis shifts the macrophage phenotype from inflammatory to proresolution, facilitating tissue repair.70 PMNs can also serve as sources of proresolving soluble mediators, such as annexin A1, which has been shown to reduce inflammatory cartilage damage71 and promote intestinal wound repair,72 or IL-22 produced by the specialized CD177+ PMN subtype, which has been suggested to play protective roles in human IBD and mouse models of colitis.73 Finally, we showed that PMNs migrating toward the intestinal lumen can engage epithelial apical surface adhesion receptors, such as ICAM-1, to initiate reparative signaling to promote reepithelization and wound healing.74
PMN Pro-Tumor Activity versus Anti-Tumor Activity
Tumor-Promoting Functions of TANs
As with acute or chronic inflammatory conditions, functional diversity of TANs is well recognized. TANs can promote tumorigenesis or suppress tumor growth, depending on the tumor type and tumor grade/stage. TANs have been recognized for their pathologic significance in several cancers, including gastric cancers,75 cholangiocarcinoma,76 melanoma,77 renal cell carcinoma,78 and hepatocellular carcinoma.79 Particularly in CRC, inflammation and TANs play prominent roles in tumor progression and dissemination. Patients with IBD with heightened PMN activity and an underlying chronic inflammation are at a significantly higher risk of developing CRC80; however, sporadic CRCs similarly feature prominent inflammatory responses and an abundance of TANs. Systemic inflammation and the important roles PMNs/TANs play in CRC are evidenced by the prognostic ability of neutrophil/lymphocyte ratio to predict inferior disease outcomes and overall survival in patients with CRC.81
TANs can promote tumor growth through immunosuppression, modulation of the DNA repair landscape, tumor cell proliferation, and angiogenesis. As discussed in PMN Phenotypes in Cancer, immature TAN populations have a substantial footprint in developing CRC. In mice, these cells were identified by CD11b+/Ly6G+/Ly6Clow expression; and in humans, they were identified by Lin−/human leukocyte antigen-DR−/CD11b+/CD14−/CD15+/CD33+.82 These cells suppress the adaptive immunity aimed at recognition and killing of tumor cells via heightened production of immunosuppressive cytokines, such as TGF-β and IL-10 or arginase I, which depletes l-arginine to halt T-cell proliferation and CD8+ T cell antitumor responses (previously reviewed in Sieminska and Baran82). Heightened ROS and hydrogen peroxide production similarly potently suppress T-cell proliferation.83,84
TANs are potent modulators of the tumor cell DNA damage repair landscape. TANs, via miR-155 activity in progressive CRC, suppress DSB repair by homologous recombination, pushing tumor DNA damage repair adaptation and use of highly error-prone nonhomologous end joining. With highly active nonhomologous end joining, tumor cells overcome significant DSB burden, which is caused by TANs themselves, oxidative stress,85 or hypoxia,86 and survive with unstable genomes. Through crosstalk with mesenchymal stem cells, TANs can promote cancer-associated fibroblast trans-differentiation to drive tumor cell proliferation.87 TANs can similarly promote tumor growth by facilitating tumor vascularization by supplying angiogenic factors, such as matrix remodeling MMP-9 and VEGF, in the tumor microenvironment.88
Finally, TANs can promote tumor cell dissemination to distant organs by facilitating their escape from primary tumors, survival, and guidance in the circulation and seeding of the metastatic niche.89,90 Via the release of proteinases, such as MMP-9 and neutrophil elastase, TANs can cleave interepithelial junctions and degrade the extracellular matrix, facilitating tumor cell release into the circulation. Through NET release, PMNs/TANs can reawaken dormant metastatic cells re-engage their proliferative capacity, promoting pulmonary metastasis,91 or trap circulating tumor cells via β1-integrin binding to help escort tumor cells into the metastatic niche. PMNs, through direct interactions with circulating tumor cells, forming tumor-immune clusters, promote their survival in the circulation92 or, via ROS/NET release, compromise the endothelial barrier facilitating transendothelial migration of breast cancer cells, promoting successful metastatic seeding in a distant organ.93
Protective Roles of TANs in Cancer
As with inflammation, TANs can also provide protection against tumor development via various cytotoxic mechanisms. In the early stages of CRC establishment, TANs can promote tumor cytotoxicity via suppression of DSB repair by homologous recombination and an induction of replicative stress and cell cycle arrest.94 TANs can kill tumor cells via elastase-mediated liberation of the CD95 death domain95 or cathepsin-G–mediated killing following TAN recognition of the cancer cell receptor for advanced glycation end products.96 TANs recognize monoclonal antibody–opsonized tumor cells and kill them via activation of Fas/FasL pathway.97 TANs can suppress tumor cell proliferation via release of hydrogen peroxide acting on cancer cell hydrogen peroxide–dependent Ca2+ channel transient receptor potential cation channel 2 (TRPM2) expression.98 TANs can exert their anti-tumorigenic effects by modulating tumor immune responses and enhancing tumor cell recognition and clearance. TANs can recruit cytotoxic CD8+ T cells via the release of CCL3, CXCL10, or IL-12 or cross-present tumor antigens to CD8+ T cells to stimulate tumor-specific effector T-cell responses.19,99 TANs can also activate dendritic cells, increasing antitumor T-cell priming,100 or activate natural killer cells to promote tumor killing.101
Neutrophil-Aimed Therapies in Inflammation and Cancer
Given the roles of PMNs in inflammation and cancer, targeting their activity in tissues could offer potential new therapeutic opportunities. However, although the evaluation of PMNs as therapeutic targets is still ongoing, given the many potentially beneficial functions of PMNs in host defense and resolution of inflammation and tumorigenesis, approaches targeting specific PMN/TAN subtypes and activities should be considered.
Although the field of specialized PMN phenotypes and functions for therapeutics is still evolving, inhibition of PMN trafficking is perhaps the best studied and closest to being used in the clinic.
Several small-molecule inhibitors directed at PMN trafficking are currently in phase 2/3 clinical trials. Reparixin and ladarixin, both CXCR2 inhibitors, are trialed for treatment of metastatic triple-negative breast cancer (NCT02370238,102 https://clinicaltrials.gov/study/NCT02370238, last accessed September 16, 2022) and type 1 diabetes (NCT04628481,103,104 https://clinicaltrials.gov/study/NCT04628481, last accessed October 5, 2023). In preclinical animal models, CXCR2 inhibition has been shown to improve immune checkpoint blockade efficacy105 and reduce tumor-associated NETs in models of melanoma and breast and colorectal cancers.106 Dual blockade of CXCR2+ TANs and CCR2+ TAMs disrupts myeloid cell recruitment, improving anti-tumor immunity in a mouse model of pancreatic cancer.107 AZD5069, another CXCR2 small-molecule inhibitor, has been successfully used to block TAN accumulation and suppressed metastasis of genetically induced CRC.56
Other approaches to target PMN/TAN activity may include chemokine antagonism, inhibition of PMN proteinases, miRNAs, and NET release. As such, inhibition or genetic deletion of MMP-9 shows a reduction in the severity of DSS experimental colitis due to reduced neutrophil infiltration in the colon.108 Inhibition of MPO activity by the synthetic inhibitor, AZD3241, or use of the antimalarial drug atovaquone (repurposing this US Food and Drug Administration–approved drug for the use in IBD), improves DSS-induced colitis outcomes.109,110 Disruption of the 12-lipoxygenase pathway reduces PMN-mediated tissue trauma in colitis in mice models.49 Similarly, in DSS-colitis and in AOM/DSS-driven CRC models, specific inhibition of PMN-derived miR-23a/miR-155 improves mucosal healing and limited tumor growth. miRNA therapy has a significant potential given its high targeting efficacy, with the caveat of miRNAs acting on multiple targets. Intratumoral delivery of neutrophil elastase or neutralization of serine proteases in the TME, including α-1-antitrypsin, secretory leukocyte peptidase inhibitor, and serpin family B member 1, improves TAN cancer cell killing capability.95 Given the ability of TGF-β to modulate and polarize TANs into tumor-supporting phenotypes, it is also an attractive target for anti-tumor therapies, of course with a caveat of it playing central roles in many other processes and cell types. A TGF-β receptor II antibody (IMC-TR1) has been developed, showing potent tumor growth inhibition in murine CRC and breast cancer by acting on tumor immune cells.111
Finally, another important aspect of mucosal injury, tumor immunity, and therapeutic response, particularly in CRC, is the gut microbiome. The current review does not touch upon this, but the field of microbiome interactions with the immune system, impacting their cell trafficking and activity and likely phenotypic specialization, is evolving and should be further explored and considered in the design of new proresolution or anti-tumor therapeutic strategies.
Disclosure Statement
None declared.
Acknowledgments
I thank the American Society for Investigative Pathology for providing a scientific home for me for over 15 years now, for honoring me with the Cotran Early Career Investigator Award, and for encouraging me to write this report; and all the members of the Sumagin laboratory, past and present, for contributions to deepening understanding of neutrophil biology and making the exciting discoveries that I had the pleasure to discuss in the current review.
Footnotes
Supported by The Crohn's & Colitis Foundation grant 624450 and NIHNational Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Allergy and Infectious Diseases grants R01 DK124199 and R01 AI153568.
The American Society for Investigative Pathology (ASIP) Cotran Early Career Investigator Award recognizes early career investigators with demonstrated excellence as an investigator with recently established or emerging independence and with a research focus leading to an improved understanding of the conceptual basis of disease. Ronen Sumagin is the recipient of the ASIP 2023 Cotran Early Career Investigator Award, and delivered a lecture entitled “Neutrophil Phenotypic and Functional Plasticity in Gut Inflammation and Colon Cancer” on October 24, 2023, at the 2023 Joint Meeting of the American Society for Matrix Biology (ASMB), The Histochemical Society (HCS), and ASIP held in Salt Lake City, Utah.
References
- 1.Eruslanov E.B., Singhal S., Albelda S.M. Mouse versus human neutrophils in cancer: a major knowledge gap. Trends Cancer. 2017;3:149–160. doi: 10.1016/j.trecan.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie X., Shi Q., Wu P., Zhang X., Kambara H., Su J., Yu H., Park S.Y., Guo R., Ren Q., Zhang S., Xu Y., Silberstein L.E., Cheng T., Ma F., Li C., Luo H.R. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol. 2020;21:1119–1133. doi: 10.1038/s41590-020-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaillon S., Ponzetta A., Di Mitri D., Santoni A., Bonecchi R., Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485–503. doi: 10.1038/s41568-020-0281-y. [DOI] [PubMed] [Google Scholar]
- 4.Fournier B.M., Parkos C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 5.Barry R., Ruano-Gallego D., Radhakrishnan S.T., Lovell S., Yu L., Kotik O., Glegola-Madejska I., Tate E.W., Choudhary J.S., Williams H.R.T., Frankel G. Faecal neutrophil elastase-antiprotease balance reflects colitis severity. Mucosal Immunol. 2020;13:322–333. doi: 10.1038/s41385-019-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palomino-Segura M., Sicilia J., Ballesteros I., Hidalgo A. Strategies of neutrophil diversification. Nat Immunol. 2023;24:575–584. doi: 10.1038/s41590-023-01452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maas R.R., Soukup K., Fournier N., Massara M., Galland S., Kornete M., Wischnewski V., Lourenco J., Croci D., Álvarez-Prado Á F., Marie D.N., Lilja J., Marcone R., Calvo G.F., Santalla Mendez R., Aubel P., Bejarano L., Wirapati P., Ballesteros I., Hidalgo A., Hottinger A.F., Brouland J.P., Daniel R.T., Hegi M.E., Joyce J.A. The local microenvironment drives activation of neutrophils in human brain tumors. Cell. 2023;186:4546–4566.e27. doi: 10.1016/j.cell.2023.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Tak T., Tesselaar K., Pillay J., Borghans J.A., Koenderman L. What's your age again? determination of human neutrophil half-lives revisited. J Leukoc Biol. 2013;94:595–601. doi: 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- 9.Evrard M., Kwok I.W.H., Chong S.Z., Teng K.W.W., Becht E., Chen J., Sieow J.L., Penny H.L., Ching G.C., Devi S., Adrover J.M., Li J.L.Y., Liong K.H., Tan L., Poon Z., Foo S., Chua J.W., Su I.H., Balabanian K., Bachelerie F., Biswas S.K., Larbi A., Hwang W.Y.K., Madan V., Koeffler H.P., Wong S.C., Newell E.W., Hidalgo A., Ginhoux F., Ng L.G. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity. 2018;48:364–379.e8. doi: 10.1016/j.immuni.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Adrover J.M., Nicolás-Ávila J.A., Hidalgo A. Aging: a temporal dimension for neutrophils. Trends Immunol. 2016;37:334–345. doi: 10.1016/j.it.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Marini O., Costa S., Bevilacqua D., Calzetti F., Tamassia N., Spina C., De Sabata D., Tinazzi E., Lunardi C., Scupoli M.T., Cavallini C., Zoratti E., Tinazzi I., Marchetta A., Vassanelli A., Cantini M., Gandini G., Ruzzenente A., Guglielmi A., Missale F., Vermi W., Tecchio C., Cassatella M.A., Scapini P. Mature CD10(+) and immature CD10(−) neutrophils present in G-CSF-treated donors display opposite effects on T cells. Blood. 2017;129:1343–1356. doi: 10.1182/blood-2016-04-713206. [DOI] [PubMed] [Google Scholar]
- 12.Tuzcu E.M., Simpfendorfer C., Gossman D., Badwhar K. Long-term follow-up of PTCA of the right coronary artery with shepherd's crook morphology. Cleve Clin J Med. 1989;56:577–579. doi: 10.3949/ccjm.56.6.577. [DOI] [PubMed] [Google Scholar]
- 13.Pillay J., Kamp V.M., van Hoffen E., Visser T., Tak T., Lammers J.W., Ulfman L.H., Leenen L.P., Pickkers P., Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortunati E., Kazemier K.M., Grutters J.C., Koenderman L., Van den Bosch v.J. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhns D.B., Long Priel D.A., Gallin J.I. Loss of L-selectin (CD62L) on human neutrophils following exudation in vivo. Cell Immunol. 1995;164:306–310. doi: 10.1006/cimm.1995.1174. [DOI] [PubMed] [Google Scholar]
- 16.Weber D.A., Sumagin R., McCall I.C., Leoni G., Neumann P.A., Andargachew R., Brazil J.C., Medina-Contreras O., Denning T.L., Nusrat A., Parkos C.A. Neutrophil-derived JAML inhibits repair of intestinal epithelial injury during acute inflammation. Mucosal Immunol. 2014;7:1221–1232. doi: 10.1038/mi.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vono M., Lin A., Norrby-Teglund A., Koup R.A., Liang F., Loré K. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood. 2017;129:1991–2001. doi: 10.1182/blood-2016-10-744441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushima H., Geng S., Lu R., Okamoto T., Yao Y., Mayuzumi N., Kotol P.F., Chojnacki B.J., Miyazaki T., Gallo R.L., Takashima A. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;121:1677–1689. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mysore V., Cullere X., Mears J., Rosetti F., Okubo K., Liew P.X., Zhang F., Madera-Salcedo I., Rosenbauer F., Stone R.M., Aster J.C., von Andrian U.H., Lichtman A.H., Raychaudhuri S., Mayadas T.N. Fc[gamma]R engagement reprograms neutrophils into antigen cross-presenting cells that elicit acquired anti-tumor immunity. Nat Commun. 2021;12:4791. doi: 10.1038/s41467-021-24591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu S., Shin J.W., Kwon S., Lee J., Kim Y.C., Bae Y.S., Bae Y.S., Kim D.K., Kim Y.S., Yang S.H., Kim H.Y. Siglec-F-expressing neutrophils are essential for creating a profibrotic microenvironment in renal fibrosis. J Clin Invest. 2022;132 doi: 10.1172/JCI156876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong B.M., Walker M.T., Rodriguez R., Coden M.E., Nagasaka R., Doan T.C., Politanska Y., Abdala-Valencia H., Berdnikovs S. More than neutrophils: Lin(+)Ly6G(+)IL-5R[alpha](+) multipotent myeloid cells (MMCs) are dominant in normal murine bone marrow and retain capacity to differentiate into eosinophils and monocytes. J Leukoc Biol. 2022;111:113–122. doi: 10.1002/JLB.1AB0519-170RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenna E., Mhaonaigh A.U., Wubben R., Dwivedi A., Hurley T., Kelly L.A., Stevenson N.J., Little M.A., Molloy E.J. Neutrophils: need for standardized nomenclature. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.602963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelm M., Lehoux S., Azcutia V., Cummings R.D., Nusrat A., Parkos C.A., Brazil J.C. Regulation of neutrophil function by selective targeting of glycan epitopes expressed on the integrin CD11b/CD18. FASEB J. 2020;34:2326–2343. doi: 10.1096/fj.201902542R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawahara R., Ugonotti J., Chatterjee S., Tjondro H.C., Loke I., Parker B.L., Venkatakrishnan V., Dieckmann R., Sumer-Bayraktar Z., Karlsson-Bengtsson A., Bylund J., Thaysen-Andersen M. Glycoproteome remodeling and organelle-specific N-glycosylation accompany neutrophil granulopoiesis. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2303867120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garrido-Trigo A., Corraliza A.M., Veny M., Dotti I., Melón-Ardanaz E., Rill A., Crowell H.L., Corbí Á., Gudiño V., Esteller M., Álvarez-Teubel I., Aguilar D., Masamunt M.C., Killingbeck E., Kim Y., Leon M., Visvanathan S., Marchese D., Caratù G., Martin-Cardona A., Esteve M., Panés J., Ricart E., Mereu E., Heyn H., Salas A. Macrophage and neutrophil heterogeneity at single-cell spatial resolution in human inflammatory bowel disease. Nat Commun. 2023;14:4506. doi: 10.1038/s41467-023-40156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich M., Pohin M., Jackson M.A., Korsunsky I., Bullers S.J., Rue-Albrecht K., Christoforidou Z., Sathananthan D., Thomas T., Ravindran R., Tandon R., Peres R.S., Sharpe H., Wei K., Watts G.F.M., Mann E.H., Geremia A., Attar M., McCuaig S., Thomas L., Collantes E., Uhlig H.H., Sansom S.N., Easton A., Raychaudhuri S., Travis S.P., Powrie F.M. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat Med. 2021;27:1970–1981. doi: 10.1038/s41591-021-01520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridlender Z.G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G.S., Albelda S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christoffersson G., Vågesjö E., Vandooren J., Lidén M., Massena S., Reinert R.B., Brissova M., Powers A.C., Opdenakker G., Phillipson M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–4662. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massena S., Christoffersson G., Vågesjö E., Seignez C., Gustafsson K., Binet F., Herrera Hidalgo C., Giraud A., Lomei J., Weström S., Shibuya M., Claesson-Welsh L., Gerwins P., Welsh M., Kreuger J., Phillipson M. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood. 2015;126:2016–2026. doi: 10.1182/blood-2015-03-631572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casbon A.J., Reynaud D., Park C., Khuc E., Gan D.D., Schepers K., Passegué E., Werb Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci U S A. 2015;112:E566–E575. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfirschke C., Engblom C., Gungabeesoon J., Lin Y., Rickelt S., Zilionis R., Messemaker M., Siwicki M., Gerhard G.M., Kohl A., Meylan E., Weissleder R., Klein A.M., Pittet M.J. Tumor-promoting Ly-6G(+) SiglecF(high) cells are mature and long-lived neutrophils. Cell Rep. 2020;32 doi: 10.1016/j.celrep.2020.108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eruslanov E.B., Bhojnagarwala P.S., Quatromoni J.G., Stephen T.L., Ranganathan A., Deshpande C., Akimova T., Vachani A., Litzky L., Hancock W.W., Conejo-Garcia J.R., Feldman M., Albelda S.M., Singhal S. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blattner C., Fleming V., Weber R., Himmelhan B., Altevogt P., Gebhardt C., Schulze T.J., Razon H., Hawila E., Wildbaum G., Utikal J., Karin N., Umansky V. CCR5(+) myeloid-derived suppressor cells are enriched and activated in melanoma lesions. Cancer Res. 2018;78:157–167. doi: 10.1158/0008-5472.CAN-17-0348. [DOI] [PubMed] [Google Scholar]
- 35.Sumagin R., Sarelius I.H. Intercellular adhesion molecule-1 enrichment near tricellular endothelial junctions is preferentially associated with leukocyte transmigration and signals for reorganization of these junctions to accommodate leukocyte passage. J Immunol. 2010;184:5242–5252. doi: 10.4049/jimmunol.0903319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grönloh M.L.B., Arts J.J.G., van Buul J.D. Neutrophil transendothelial migration hotspots - mechanisms and implications. J Cell Sci. 2021;134 doi: 10.1242/jcs.255653. [DOI] [PubMed] [Google Scholar]
- 37.Grönloh M.L.B., Tebbens M.E., Kotsi M., Arts J.J.G., van Buul J.D. Intercellular adhesion molecule 2 regulates diapedesis hotspots by allowing neutrophil crawling against the direction of flow. Vasc Biol. 2023;5 doi: 10.1530/VB-23-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arts J.J., Mahlandt E.K., Grönloh M.L., Schimmel L., Noordstra I., Gordon E., van Steen A.C., Tol S., Walzog B., van Rijssel J., Nolte M.A., Postma M., Khuon S., Heddleston J.M., Wait E., Chew T.L., Winter M., Montanez E., Goedhart J., van Buul J.D. Endothelial junctional membrane protrusions serve as hotspots for neutrophil transmigration. Elife. 2021;10 doi: 10.7554/eLife.66074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Proebstl D., Voisin M.B., Woodfin A., Whiteford J., D'Acquisto F., Jones G.E., Rowe D., Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voisin M.B., Pröbstl D., Nourshargh S. Venular basement membranes ubiquitously express matrix protein low-expression regions: characterization in multiple tissues and remodeling during inflammation. Am J Pathol. 2010;176:482–495. doi: 10.2353/ajpath.2010.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren X., Manzanares L.D., Piccolo E.B., Urbanczyk J.M., Sullivan D.P., Yalom L.K., Bui T.M., Lantz C., Najem H., Dulai P.S., Heimberger A.B., Thorp E.B., Sumagin R. Macrophage-endothelial cell crosstalk orchestrates neutrophil recruitment in inflamed mucosa. J Clin Invest. 2023;133 doi: 10.1172/JCI170733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sullivan D.P., Bui T., Muller W.A., Butin-Israeli V., Sumagin R. In vivo imaging reveals unique neutrophil transendothelial migration patterns in inflamed intestines. Mucosal Immunol. 2018;11:1571–1581. doi: 10.1038/s41385-018-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azcutia V., Kelm M., Kim S., Luissint A.C., Flemming S., Abernathy-Close L., Young V.B., Nusrat A., Miller M.J., Parkos C.A. Distinct stimulus-dependent neutrophil dynamics revealed by real-time imaging of intestinal mucosa after acute injury. PNAS Nexus. 2022;1:pgac249. doi: 10.1093/pnasnexus/pgac249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Filippo K., Dudeck A., Hasenberg M., Nye E., van Rooijen N., Hartmann K., Gunzer M., Roers A., Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 45.van Lierop P.P., de Haar C., Lindenbergh-Kortleve D.J., Simons-Oosterhuis Y., van Rijt L.S., Lambrecht B.N., Escher J.C., Samsom J.N., Nieuwenhuis E.E. T-cell regulation of neutrophil infiltrate at the early stages of a murine colitis model. Inflamm Bowel Dis. 2010;16:442–451. doi: 10.1002/ibd.21073. [DOI] [PubMed] [Google Scholar]
- 46.Zhu F., He H., Fan L., Ma C., Xu Z., Xue Y., Wang Y., Zhang C., Zhou G. Blockade of CXCR2 suppresses proinflammatory activities of neutrophils in ulcerative colitis. Am J Transl Res. 2020;12:5237–5251. [PMC free article] [PubMed] [Google Scholar]
- 47.Kvedaraite E., Lourda M., Ideström M., Chen P., Olsson-Åkefeldt S., Forkel M., Gavhed D., Lindforss U., Mjösberg J., Henter J.I., Svensson M. Tissue-infiltrating neutrophils represent the main source of IL-23 in the colon of patients with IBD. Gut. 2016;65:1632–1641. doi: 10.1136/gutjnl-2014-309014. [DOI] [PubMed] [Google Scholar]
- 48.Johswich K., Martin M., Bleich A., Kracht M., Dittrich-Breiholz O., Gessner J.E., Suerbaum S., Wende E., Rheinheimer C., Klos A. Role of the C5a receptor (C5aR) in acute and chronic dextran sulfate-induced models of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1812–1823. doi: 10.1002/ibd.21012. [DOI] [PubMed] [Google Scholar]
- 49.Mrsny R.J., Gewirtz A.T., Siccardi D., Savidge T., Hurley B.P., Madara J.L., McCormick B.A. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci U S A. 2004;101:7421–7426. doi: 10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy C.K., Dixit B., Oleson F.B., Dolle R.E., Farquhar R., McCormick B.A. Development of ADS051, an oral, gut-restricted, small molecule neutrophil modulator for the treatment of neutrophil-mediated inflammatory diseases. FEBS Open Bio. 2023;13:1434–1446. doi: 10.1002/2211-5463.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azcutia V., Kelm M., Fink D., Cummings R.D., Nusrat A., Parkos C.A., Brazil J.C. Sialylation regulates neutrophil transepithelial migration, CD11b/CD18 activation, and intestinal mucosal inflammatory function. JCI Insight. 2023;8 doi: 10.1172/jci.insight.167151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rehring J.F., Bui T.M., Galán-Enríquez C.S., Urbanczyk J.M., Ren X., Wiesolek H.L., Sullivan D.P., Sumagin R. Released myeloperoxidase attenuates neutrophil migration and accumulation in inflamed tissue. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.654259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bates R.C., DeLeo M.J., 3rd, Mercurio A.M. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299:315–324. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 54.Rubie C., Frick V.O., Wagner M., Schuld J., Gräber S., Brittner B., Bohle R.M., Schilling M.K. ELR+ CXC chemokine expression in benign and malignant colorectal conditions. BMC Cancer. 2008;8:178. doi: 10.1186/1471-2407-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katoh H., Wang D., Daikoku T., Sun H., Dey S.K., Dubois R.N. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackstadt R., van Hooff S.R., Leach J.D., Cortes-Lavaud X., Lohuis J.O., Ridgway R.A., Wouters V.M., Roper J., Kendall T.J., Roxburgh C.S., Horgan P.G., Nixon C., Nourse C., Gunzer M., Clark W., Hedley A., Yilmaz O.H., Rashid M., Bailey P., Biankin A.V., Campbell A.D., Adams D.J., Barry S.T., Steele C.W., Medema J.P., Sansom O.J. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell. 2019;36:319–336.e7. doi: 10.1016/j.ccell.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schimek V., Strasser K., Beer A., Göber S., Walterskirchen N., Brostjan C., Müller C., Bachleitner-Hofmann T., Bergmann M., Dolznig H., Oehler R. Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment. Cell Death Dis. 2022;13:113. doi: 10.1038/s41419-022-04585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amicarella F., Muraro M.G., Hirt C., Cremonesi E., Padovan E., Mele V., Governa V., Han J., Huber X., Droeser R.A., Zuber M., Adamina M., Bolli M., Rosso R., Lugli A., Zlobec I., Terracciano L., Tornillo L., Zajac P., Eppenberger-Castori S., Trapani F., Oertli D., Iezzi G. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut. 2017;66:692–704. doi: 10.1136/gutjnl-2015-310016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanmamed M.F., Carranza-Rua O., Alfaro C., Oñate C., Martín-Algarra S., Perez G., Landazuri S.F., Gonzalez A., Gross S., Rodriguez I., Muñoz-Calleja C., Rodríguez-Ruiz M., Sangro B., López-Picazo J.M., Rizzo M., Mazzolini G., Pascual J.I., Andueza M.P., Perez-Gracia J.L., Melero I. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin Cancer Res. 2014;20:5697–5707. doi: 10.1158/1078-0432.CCR-13-3203. [DOI] [PubMed] [Google Scholar]
- 60.Inamoto S., Itatani Y., Yamamoto T., Minamiguchi S., Hirai H., Iwamoto M., Hasegawa S., Taketo M.M., Sakai Y., Kawada K. Loss of SMAD4 promotes colorectal cancer progression by accumulation of myeloid-derived suppressor cells through the CCL15-CCR1 chemokine axis. Clin Cancer Res. 2016;22:492–501. doi: 10.1158/1078-0432.CCR-15-0726. [DOI] [PubMed] [Google Scholar]
- 61.Silvestre-Roig C., Hidalgo A., Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood. 2016;127:2173–2181. doi: 10.1182/blood-2016-01-688887. [DOI] [PubMed] [Google Scholar]
- 62.Maynard S., Schurman S.H., Harboe C., de Souza-Pinto N.C., Bohr V.A. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winterbourn C.C., Kettle A.J., Hampton M.B. Reactive oxygen species and neutrophil function. Annu Rev Biochem. 2016;85:765–792. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 64.Butin-Israeli V., Bui T.M., Wiesolek H.L., Mascarenhas L., Lee J.J., Mehl L.C., Knutson K.R., Adam S.A., Goldman R.D., Beyder A., Wiesmuller L., Hanauer S.B., Sumagin R. Neutrophil-induced genomic instability impedes resolution of inflammation and wound healing. J Clin Invest. 2019;129:712–726. doi: 10.1172/JCI122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butin-Israeli V., Houser M.C., Feng M., Thorp E.B., Nusrat A., Parkos C.A., Sumagin R. Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. 2016;30:4007–4020. doi: 10.1096/fj.201600734R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slater T.W., Finkielsztein A., Mascarenhas L.A., Mehl L.C., Butin-Israeli V., Sumagin R. Neutrophil microparticles deliver active myeloperoxidase to injured mucosa to inhibit epithelial wound healing. J Immunol. 2017;198:2886–2897. doi: 10.4049/jimmunol.1601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li T., Wang C., Liu Y., Li B., Zhang W., Wang L., Yu M., Zhao X., Du J., Zhang J., Dong Z., Jiang T., Xie R., Ma R., Fang S., Zhou J., Shi J. Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease. J Crohns Colitis. 2020;14:240–253. doi: 10.1093/ecco-jcc/jjz132. [DOI] [PubMed] [Google Scholar]
- 68.Dinallo V., Marafini I., Di Fusco D., Laudisi F., Franzè E., Di Grazia A., Figliuzzi M.M., Caprioli F., Stolfi C., Monteleone I., Monteleone G. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019;13:772–784. doi: 10.1093/ecco-jcc/jjy215. [DOI] [PubMed] [Google Scholar]
- 69.Peiseler M., Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129:2629–2639. doi: 10.1172/JCI124616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiesolek H.L., Bui T.M., Lee J.J., Dalal P., Finkielsztein A., Batra A., Thorp E.B., Sumagin R. Intercellular adhesion molecule 1 functions as an efferocytosis receptor in inflammatory macrophages. Am J Pathol. 2020;190:874–885. doi: 10.1016/j.ajpath.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Headland S.E., Jones H.R., Norling L.V., Kim A., Souza P.R., Corsiero E., Gil C.D., Nerviani A., Dell'Accio F., Pitzalis C., Oliani S.M., Jan L.Y., Perretti M. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci Transl Med. 2015;7:315ra190. doi: 10.1126/scitranslmed.aac5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leoni G., Neumann P.A., Kamaly N., Quiros M., Nishio H., Jones H.R., Sumagin R., Hilgarth R.S., Alam A., Fredman G., Argyris I., Rijcken E., Kusters D., Reutelingsperger C., Perretti M., Parkos C.A., Farokhzad O.C., Neish A.S., Nusrat A. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. 2015;125:1215–1227. doi: 10.1172/JCI76693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou G., Yu L., Fang L., Yang W., Yu T., Miao Y., Chen M., Wu K., Chen F., Cong Y., Liu Z. CD177(+) neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67:1052–1063. doi: 10.1136/gutjnl-2016-313535. [DOI] [PubMed] [Google Scholar]
- 74.Sumagin R., Brazil J.C., Nava P., Nishio H., Alam A., Luissint A.C., Weber D.A., Neish A.S., Nusrat A., Parkos C.A. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 2016;9:1151–1162. doi: 10.1038/mi.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jung M.R., Park Y.K., Jeong O., Seon J.W., Ryu S.Y., Kim D.Y., Kim Y.J. Elevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancer. J Surg Oncol. 2011;104:504–510. doi: 10.1002/jso.21986. [DOI] [PubMed] [Google Scholar]
- 76.Mao Z.Y., Zhu G.Q., Xiong M., Ren L., Bai L. Prognostic value of neutrophil distribution in cholangiocarcinoma. World J Gastroenterol. 2015;21:4961–4968. doi: 10.3748/wjg.v21.i16.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paesmans M., Sculier J.P., Lecomte J., Thiriaux J., Libert P., Sergysels R., Bureau G., Dabouis G., Van Cutsem O., Mommen P., Ninane V., Klastersky J. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer. 2000;89:523–533. doi: 10.1002/1097-0142(20000801)89:3<523::aid-cncr7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 78.Jensen H.K., Donskov F., Marcussen N., Nordsmark M., Lundbeck F., von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 79.Li Y.W., Qiu S.J., Fan J., Zhou J., Gao Q., Xiao Y.S., Xu Y.F. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011;54:497–505. doi: 10.1016/j.jhep.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 80.Dulai P.S., Sandborn W.J., Gupta S. Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, pathophysiology, and management. Cancer Prev Res (Phila) 2016;9:887–894. doi: 10.1158/1940-6207.CAPR-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazaki J., Katsumata K., Kasahara K., Tago T., Wada T., Kuwabara H., Enomoto M., Ishizaki T., Nagakawa Y., Tsuchida A. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20:922. doi: 10.1186/s12885-020-07429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sieminska I., Baran J. Myeloid-derived suppressor cells in colorectal cancer. Front Immunol. 2020;11:1526. doi: 10.3389/fimmu.2020.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corzo C.A., Cotter M.J., Cheng P., Cheng F., Kusmartsev S., Sotomayor E., Padhya T., McCaffrey T.V., McCaffrey J.C., Gabrilovich D.I. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabrilovich D.I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Venkatachalam G., Surana U., Clement M.V. Replication stress-induced endogenous DNA damage drives cellular senescence induced by a sub-lethal oxidative stress. Nucleic Acids Res. 2017;45:10564–10582. doi: 10.1093/nar/gkx684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ng N., Purshouse K., Foskolou I.P., Olcina M.M., Hammond E.M. Challenges to DNA replication in hypoxic conditions. FEBS J. 2018;285:1563–1571. doi: 10.1111/febs.14377. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J., Ji C., Li W., Mao Z., Shi Y., Shi H., Ji R., Qian H., Xu W., Zhang X. Tumor-educated neutrophils activate mesenchymal stem cells to promote gastric cancer growth and metastasis. Front Cell Dev Biol. 2020;8:788. doi: 10.3389/fcell.2020.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hawinkels L.J., Zuidwijk K., Verspaget H.W., de Jonge-Muller E.S., van Duijn W., Ferreira V., Fontijn R.D., David G., Hommes D.W., Lamers C.B., Sier C.F. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 2008;44:1904–1913. doi: 10.1016/j.ejca.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 89.Xiong S., Dong L., Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14:173. doi: 10.1186/s13045-021-01187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mizuno R., Kawada K., Itatani Y., Ogawa R., Kiyasu Y., Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. 2019;20:529. doi: 10.3390/ijms20030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albrengues J., Shields M.A., Ng D., Park C.G., Ambrico A., Poindexter M.E., Upadhyay P., Uyeminami D.L., Pommier A., Küttner V., Bružas E., Maiorino L., Bautista C., Carmona E.M., Gimotty P.A., Fearon D.T., Chang K., Lyons S.K., Pinkerton K.E., Trotman L.C., Goldberg M.S., Yeh J.T., Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361 doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szczerba B.M., Castro-Giner F., Vetter M., Krol I., Gkountela S., Landin J., Scheidmann M.C., Donato C., Scherrer R., Singer J., Beisel C., Kurzeder C., Heinzelmann-Schwarz V., Rochlitz C., Weber W.P., Beerenwinkel N., Aceto N. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553–557. doi: 10.1038/s41586-019-0915-y. [DOI] [PubMed] [Google Scholar]
- 93.McDowell S.A.C., Luo R.B.E., Arabzadeh A., Doré S., Bennett N.C., Breton V., Karimi E., Rezanejad M., Yang R.R., Lach K.D., Issac M.S.M., Samborska B., Perus L.J.M., Moldoveanu D., Wei Y., Fiset B., Rayes R.F., Watson I.R., Kazak L., Guiot M.C., Fiset P.O., Spicer J.D., Dannenberg A.J., Walsh L.A., Quail D.F. Neutrophil oxidative stress mediates obesity-associated vascular dysfunction and metastatic transmigration. Nat Cancer. 2021;2:545–562. doi: 10.1038/s43018-021-00194-9. [DOI] [PubMed] [Google Scholar]
- 94.Bui T.M., Butin-Israeli V., Wiesolek H.L., Zhou M., Rehring J.F., Wiesmüller L., Wu J.D., Yang G.Y., Hanauer S.B., Sebag J.A., Sumagin R. Neutrophils alter DNA repair landscape to impact survival and shape distinct therapeutic phenotypes of colorectal cancer. Gastroenterology. 2021;161:225–238.e15. doi: 10.1053/j.gastro.2021.03.027. [DOI] [PubMed] [Google Scholar]
- 95.Cui C., Chakraborty K., Tang X.A., Zhou G., Schoenfelt K.Q., Becker K.M., Hoffman A., Chang Y.F., Blank A., Reardon C.A., Kenny H.A., Vaisar T., Lengyel E., Greene G., Becker L. Neutrophil elastase selectively kills cancer cells and attenuates tumorigenesis. Cell. 2021;184:3163–3177.e21. doi: 10.1016/j.cell.2021.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sionov R.V., Fainsod-Levi T., Zelter T., Polyansky L., Pham C.T., Granot Z. Neutrophil cathepsin G and tumor cell RAGE facilitate neutrophil anti-tumor cytotoxicity. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1624129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Egmond M., Bakema J.E. Neutrophils as effector cells for antibody-based immunotherapy of cancer. Semin Cancer Biol. 2013;23:190–199. doi: 10.1016/j.semcancer.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 98.Gershkovitz M., Caspi Y., Fainsod-Levi T., Katz B., Michaeli J., Khawaled S., Lev S., Polyansky L., Shaul M.E., Sionov R.V., Cohen-Daniel L., Aqeilan R.I., Shaul Y.D., Mori Y., Karni R., Fridlender Z.G., Binshtok A.M., Granot Z. TRPM2 mediates neutrophil killing of disseminated tumor cells. Cancer Res. 2018;78:2680–2690. doi: 10.1158/0008-5472.CAN-17-3614. [DOI] [PubMed] [Google Scholar]
- 99.Singhal S., Bhojnagarwala P.S., O'Brien S., Moon E.K., Garfall A.L., Rao A.S., Quatromoni J.G., Stephen T.L., Litzky L., Deshpande C., Feldman M.D., Hancock W.W., Conejo-Garcia J.R., Albelda S.M., Eruslanov E.B. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell. 2016;30:120–135. doi: 10.1016/j.ccell.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Humbert M., Guery L., Brighouse D., Lemeille S., Hugues S. Intratumoral CpG-B promotes antitumoral neutrophil, cDC, and T-cell cooperation without reprograming tolerogenic pDC. Cancer Res. 2018;78:3280–3292. doi: 10.1158/0008-5472.CAN-17-2549. [DOI] [PubMed] [Google Scholar]
- 101.Sun R., Luo J., Li D., Shu Y., Luo C., Wang S.S., Qin J., Zhang G.M., Feng Z.H. Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal NK cells. Oncotarget. 2014;5:12621–12634. doi: 10.18632/oncotarget.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldstein L.J., Mansutti M., Levy C., Chang J.C., Henry S., Fernandez-Perez I., Prausova J., Staroslawska E., Viale G., Butler B., McCanna S., Ruffini P.A., Wicha M.S., Schott A.F., fRida Trial Investigators A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida) Breast Cancer Res Treat. 2021;190:265–275. doi: 10.1007/s10549-021-06367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kemp D.M., Pidich A., Larijani M., Jonas R., Lash E., Sato T., Terai M., De Pizzol M., Allegretti M., Igoucheva O., Alexeev V. Ladarixin, a dual CXCR1/2 inhibitor, attenuates experimental melanomas harboring different molecular defects by affecting malignant cells and tumor microenvironment. Oncotarget. 2017;8:14428–14442. doi: 10.18632/oncotarget.14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mattos M.S., Ferrero M.R., Kraemer L., Lopes G.A.O., Reis D.C., Cassali G.D., Oliveira F.M.S., Brandolini L., Allegretti M., Garcia C.C., Martins M.A., Teixeira M.M., Russo R.C. CXCR1 and CXCR2 inhibition by ladarixin improves neutrophil-dependent airway inflammation in mice. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.566953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Highfill S.L., Cui Y., Giles A.J., Smith J.P., Zhang H., Morse E., Kaplan R.N., Mackall C.L. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra67. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teijeira Á., Garasa S., Gato M., Alfaro C., Migueliz I., Cirella A., de Andrea C., Ochoa M.C., Otano I., Etxeberria I., Andueza M.P., Nieto C.P., Resano L., Azpilikueta A., Allegretti M., de Pizzol M., Ponz-Sarvisé M., Rouzaut A., Sanmamed M.F., Schalper K., Carleton M., Mellado M., Rodriguez-Ruiz M.E., Berraondo P., Perez-Gracia J.L., Melero I. CXCR1 and CXCR2 chemokine receptor agonists produced by tumors induce neutrophil extracellular traps that interfere with immune cytotoxicity. Immunity. 2020;52:856–871.e8. doi: 10.1016/j.immuni.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 107.Nywening T.M., Belt B.A., Cullinan D.R., Panni R.Z., Han B.J., Sanford D.E., Jacobs R.C., Ye J., Patel A.A., Gillanders W.E., Fields R.C., DeNardo D.G., Hawkins W.G., Goedegebuure P., Linehan D.C. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67:1112–1123. doi: 10.1136/gutjnl-2017-313738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castaneda F.E., Walia B., Vijay-Kumar M., Patel N.R., Roser S., Kolachala V.L., Rojas M., Wang L., Oprea G., Garg P., Gewirtz A.T., Roman J., Merlin D., Sitaraman S.V. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 109.Ahmad G., Chami B., Liu Y., Schroder A.L., San Gabriel P.T., Gao A., Fong G., Wang X., Witting P.K. The synthetic myeloperoxidase inhibitor AZD3241 ameliorates dextran sodium sulfate stimulated experimental colitis. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.556020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manzanares L.D., David J., Ren X., Yalom L.K., Piccolo E.B., Dehghan Y., David A.J., Hanauer S.B., Sumagin R. Atovaquone attenuates experimental colitis by reducing neutrophil infiltration of colonic mucosa. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1011115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhong Z., Carroll K.D., Policarpio D., Osborn C., Gregory M., Bassi R., Jimenez X., Prewett M., Liebisch G., Persaud K., Burtrum D., Wang S., Surguladze D., Ng S., Griffith H., Balderes P., Doody J., Schwartz J.D., Youssoufian H., Rowinsky E.K., Ludwig D.L., Witte L., Zhu Z., Wu Y. Anti-transforming growth factor beta receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res. 2010;16:1191–1205. doi: 10.1158/1078-0432.CCR-09-1634. [DOI] [PubMed] [Google Scholar]