Abstract
Claudins (CLDNs) are a family of major membrane proteins that form components of tight junctions. In normal tissues, CLDNs seal the intercellular space in the epithelial sheets to regulate tissue permeability, paracellular transport, and signal transduction. Claudin18.2 (CLDN18.2), a member of the CLDN family, is expressed specifically in gastric mucosal cells in normal tissue, and its expression is often retained in gastric cancer cells. CLDN18.2 is ectopically expressed in many cancers other than gastric cancer such as esophageal cancer, pancreatic cancer, biliary tract cancer, non-small-cell lung cancer, and ovarian cancer. Structurally, CLDN18.2 is localized on the apical side of the cell membrane and has extracellular loops capable of binding monoclonal antibodies. Upon malignant transformation, CLDN18.2 is exposed to the cell surface of the whole membrane, which enables the binding of monoclonal antibodies. Based on these characteristics, CLDN18.2 was considered to be optimal for target therapy, and zolbetuximab was developed which is a first-in-class chimeric immunoglobulin G1 monoclonal antibody highly specific for CLDN18.2. It binds to CLDN18.2 on the tumor cell surface and stimulates cellular and soluble immune effectors that activate antibody-dependent cytotoxicity and complement-dependent cytotoxicity. Recently, zolbetuximab combined with chemotherapy demonstrated a survival benefit in patients with CLDN18.2-positive and HER-2-negative gastric or gastroesophageal junction cancers in the global phase III SPOTLIGHT and GLOW trials. From these clinically meaningful results, CLDN18.2-targeting therapy including zolbetuximab has attracted a lot of attention. In this review, we summarize the clinical implications of CLDN18.2-positive gastric or GEJ cancer, and CLDN18.2-targeting therapy, mainly for zolbetuximab.
Keywords: Claudin18.2, gastric cancer, gastroesophageal junction cancer, Zolbetuximab
Introduction
Gastric cancer, including gastroesophageal junction (GEJ) cancer, is the fifth most common type of cancer and the fourth leading cause of cancer-related deaths globally with more than 1 million new cases and an estimated 769,000 deaths in 2020. 1 Platinum-fluoropyrimidine chemotherapy has been a standard first-line treatment for patients with locally advanced unresectable or metastatic gastric or GEJ cancer.2–4 For approximately 15% of patients with human epidermal growth factor receptor 2 (HER2)-positive tumors, trastuzumab can be used in addition to platinum-fluoropyrimidine as first-line treatment. 5 Recently, checkpoint inhibitors such as nivolumab combined with platinum-fluoropyrimidine have been approved as a first-line treatment for HER2-negative patients in some countries.6–8 However, approximately 40% of patients with a programmed death-ligand 1 (PD-L1) combined positive score (CPS) of less than five cannot receive a survival benefit from additional checkpoint inhibitors. 9 While the survival benefit of checkpoint inhibitors is significantly enhanced in patients with microsatellite instability-high (MSI-H) tumors, this subgroup constitutes only about 5% of the population. 7 Therefore, there are still many patients who do not derive benefits from molecular-targeted therapy such as trastuzumab or immunotherapy such as checkpoint inhibitors. Furthermore, despite recent treatment developments, the prognosis remains poor (median overall survival of <18 months), which warrants new molecular-targeted therapy.
Claudin (CLDN) 18.2 is one of the CLDN family of major membrane proteins that form the components of the tight cell junctions. CLDN18.2 is highly expressed specifically in normal gastric mucosa cells and is often retained in gastric cancer cells. Recently, zolbetuximab, a first-in-class chimeric immunoglobulin G1 monoclonal antibody highly specific for CLDN18.2, has been developed. Zolbetuximab combined with chemotherapy improved overall survival (OS) compared with chemotherapy alone in patients with CLDN18.2-positive and HER2-negative gastric or GEJ cancer in global phase III SPOTLIGHT and GLOW trials, which has attracted much attention for CLDN18.2-targeting therapy.10,11 In this review, we delve into the clinical implications of CLDN18.2-positive gastric or GEJ cancer and explore CLDN18.2-targeting therapy mainly for zolbetuximab.
Claudins
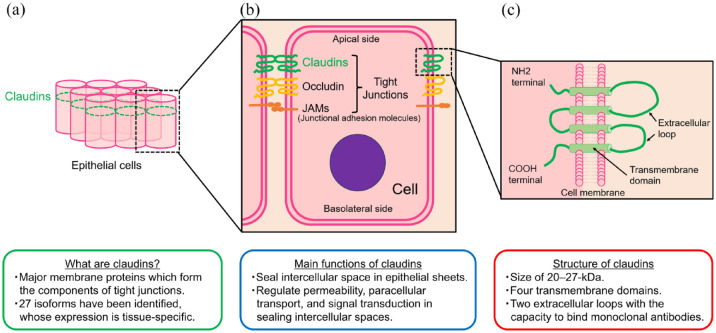
CLDNs are a family of major membrane proteins that form the components of tight junctions and were first reported by Tukita et al. in 1998.12,13 CLDNs close and seal the intercellular space in epithelial sheets and regulate tissue permeability, paracellular transport, and signal transduction 14 [Figure1(a) and (b)]. Their name, ‘claudin’ derives from the Latin word ‘claudere’, meaning ‘close’, reflecting their characteristic function in sealing intercellular spaces. CLDNs are 20- to 27-kDa transmembrane proteins characterized by four transmembrane domains and two extracellular loops13,15,16 [Figure1(c)]. Since their initial discovery, as many as 27 isoforms have been identified. Notably, CLDN expression is tissue specific, and most normal tissues express multiple CLDNs.17,18 For example, normal gastric mucosa expresses CLDN1-5, CLDN7-12, CLDN16, and CLDN18.2. 19
Figure 1.
Main characteristics of claudins: (a) Claudins in epithelial cells, (b) Claudins in tight junctions, (C) Structure of claudins in cell menbrane.
The expression of CLDNs in malignant tissues is altered in several cancer types. 20 For example, the expression pattern of CLDN1, typically observed in breast duct cells, undergoes notable changes. It is nearly lost in invasive ductal carcinoma, whereas partial retention is seen in ductal carcinoma in situ, suggesting a potential association between CLDN1 and cancer invasion and metastasis. 21 Similarly, in breast cancer, CLDN7 expression exhibits a reduction, and lower levels of CLDN7 expression are associated with worse histological grade. 22 In colorectal cancer, some cases also display a decrease in CLDN1 expression, and lower CLDN1 levels are associated with poor survival. 23 These findings indicate that reduced CLDN expression might correlate with worse pathological grade or disease progression, thereby contributing to a worse prognosis. Conversely, there are some reports of CLDN overexpression in several cancer types. For instance, CLDN3 and CLDN4 exhibit marked overexpression in cancers such as ovarian cancer, breast cancer, prostate cancer, and pancreatic cancer.24–27 In preclinical study, knockdown of CLDN3 and CLDN4 expression in ovarian cancer cell lines reduced invasion, while constitutive expression of CLDN3 and CLDN4 in human ovarian cancer cells increased invasion and motility. This suggests that CLDN overexpression may also play a role in promoting invasion or metastasis. 28 Although the precise impact of aberrant expression of each CLDN remains still unclear, it is generally considered that aberrant expression of CLDNs in cancer might lead to the weakening of tight junctions. Consequently, this could contribute to the cancer progression including invasion and metastasis.
Claudin18.2
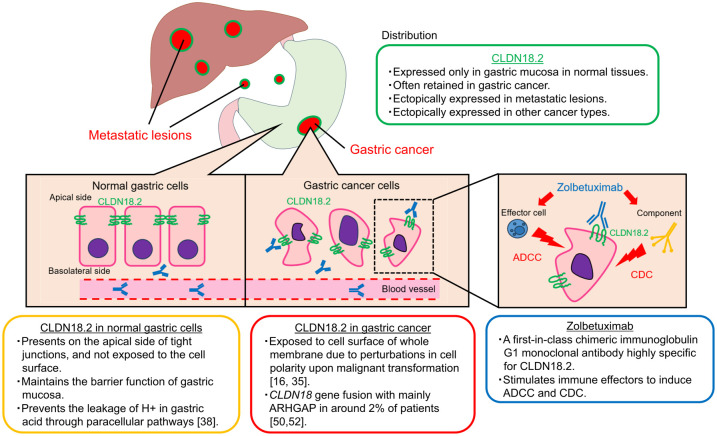
The main features of CLDN18.2 are summarized in Figure 2. Claudin18, one of the claudin family, is divided into two variants, isoforms 1 and 2 due to alternative splicing; CLDN18 isoform 1 (CLDN18.1) and CLDN18 isoform 2 (CLDN18.2) are expressed specifically in lung cells and gastric mucosal cells, respectively. 29 CLDN18.2 is expressed only in the gastric mucosa in normal tissues. In gastric cancer, its expression is often decreased but is retained. The pattern of CLDN18.2 expression in gastric cancer exhibits a distinctive distribution: the proportion of moderate-to-strong CLDN18.2 expression in cancer cells tends to skew toward higher rate (about 70–100%) or lower rate (about 0–10%), with a relatively sparse middle range.30,31 CLDN18.2 is expressed also in metastatic lesions such as lymph nodes and liver metastases of gastric cancer.32–34 In addition to gastric cancer, CLDN18.2 is ectopically expressed in various cancer types including esophageal cancer, pancreatic cancer, biliary tract cancer, non-small-cell lung cancer, and ovarian cancer.16,35
Figure 2.
Main characteristics of claudin18.2 and zolbetuximab.
ADCC, activate antibody-dependent cytotoxicity; CDC, complement-dependent cytotoxicity; CLDN18.2, claudin18.2.
Structurally, CLDN18.2 has extracellular loops with the capacity to bind monoclonal antibodies. In normal gastric tissue, CLDN18.2 is located in the apical side of tight junctions of differentiated epithelial cells, while it is absent from the gastric stem cell zone. 16 CLDN18.2 in normal tissue is strictly confined, which makes it difficult for zolbetuximab to bind. By contrast, in the context of gastric cancer, CLDN18.2 is considered to be exposed to the cell surface due to perturbations in cell polarity resulting from malignant transformation. In addition, CLDN18.2 tends to be located on the basolateral side, potentially rendering it more accessible for zolbetuximab to bind.19,26
Functionally, CLDN18.2 has been associated with anion permeability and barrier function. The loss of CLDN18.2 has been implicated in conditions such as atrophic gastritis and tumor development.36–39 A preclinical study conducted on CLDN18-knockout mice revealed the onset of atrophic gastritis, coinciding with paracellular H (+) leak, upregulation of proinflammatory cytokines including IL-1β and recrement of neutrophils. This evidence suggests that CLDN18 normally plays a crucial role in forming a paracellular barrier against H (+). 37 Furthermore, another preclinical study demonstrated that Helicobacter pylori (H. pylori) infection in mice led to a decrease in CLDN18 expression early in gastric cancer progression. In addition, CLDN18-knockout mice demonstrated low levels of inflammation, increased cell proliferation, and downregulation of signal transduction pathways such as p53 and STAT signaling, and subsequently developed dysplastic polypoid tumors without H. pylori infection. 38 Similarly, it was observed that CLDN18-knockout mice developed chronic active gastritis, and a notable subset (20–30%) of these mice developed gastric tumors without H. pylori infection. 39 Based on these findings, the downregulation of CLDN18, whether triggered by H. pylori infection or other factors, appears to play a pivotal role in initiating atrophic gastritis, which might cause tumor development. After gastric cancer development, downregulation of CLDN18.2 also might be associated with cancer progression. In a clinical study of early-stage gastric cancer resected by endoscopy, CLDN18 expression at the invasive front was inversely associated with the Ki-67 index which is an indicator of cancer proliferation, and downregulation of CLDN18 was associated with proliferation. This correlation suggested that the downregulation of CLDN18 might play a role in cancer proliferation of gastric cancer. 40 Conversely, the behavior of CLDN18 appears to differ when it is ectopically expressed in cancers. For instance, in bile duct cancer cell lines, downregulation of CLDN18 led to suppression of cell growth, invasiveness, and tumorigenicity in vivo. These findings suggest that ectopic CLDN18 expression might play a role in promoting cancer progression. 41 However, it is important to note that these preclinical insights are preliminary and have not been clinically substantiated, which warrants further investigations.
At the transcriptional level, CLDN18 expression is known to be regulated by multiple pathways including the methylation of CpG islands, the protein kinase C (PKC) pathway, the extracellular signal-related kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway, the HER2/HER3 signaling pathway, and microRNA.41–48 Intriguingly, activator protein (AP)-1, an intracellular transcriptional activator, plays a pivotal role in this network. AP-1 binds to the cis-regulatory elements (CREs) of the CLDN18.2 promotor and induces the transcription of CLDN18.2. The PKC and ERK/MAPK pathway regulate AP-1, subsequently influencing CLDN18.2 expression. 42 In addition, the HER2/HER3 signaling pathway has been associated with CLDN18 expression in preclinical studies of acute respiratory distress syndrome (ARDS), although this pertains CLDN18.1.47,48 In these studies, IL-1β, identified as a core gene of ARDS, downregulated CLDN18 through the activation of HER2/HER3 signaling, which promoted lung barrier dysfunction and induced ARDS in vitro and animal models. Notably, HER2 blocker lapatinib was observed to block the effect of IL-1β on CLDN18 downregulation. 47 Furthermore, regarding other signaling pathways, ectopic CLDN18 expression was found to induce growth and invasiveness in bile duct cancer cells via activation of epidermal growth factor receptor (EGFR)/ERK signaling. 41 These researches exploring the signaling pathways regarding CLDN18 might provide new insights into CLDN18.2-targeting therapy, which warrants further examination.
CLDN18-ARHGAP fusion
Genetic alteration of CLDN18 is a rare event in gastric cancer and the main alteration is gene fusion with ARHGAP. 49 ARHGAP is a member of the Rho-GAP family, which encodes a Rho GTPase-activating protein that switches RHOA inactive. The key roles of RHOA are the reorganization of the actin cytoskeleton and the regulation of cell shape, attachment, and motility. 50 The CLDN18-ARHGAP fusion was first detected by the Cancer Genome Atlas (TCGA) group in 2014. 51 CLDN18-ARHGAP fusion was most frequent (15%) in the genomic stable type, and mutually exclusive with alterations related to the diffuse type such as RHOA and CDH1 mutations. The CLDN18-ARHGAP fusion lacks the PDZ-domain binding motif in CLDN18 which is required to maintain epithelial integrity but retains Rho-GAP in ARHGAP which inhibits RHOA activity. Both of these result in the loss of epithelial integrity and an increase in invasiveness, which was demonstrated in a preclinical study. 52 In a cohort study, gastric cancer with CLDN18-ARHGAP fusion retained CLDN18 expression compared with fusion-negative gastric cancer. In addition, CLDN18-ARHGAP fusion was associated with diffuse type and a higher incidence of distant metastasis. 53 Other studies have demonstrated that CLDN18-ARHGAP fusion was associated with younger age at diagnosis, lymph node metastases, signet-ring cell content, female sex, advanced stage, and worse prognosis.54,55 These data suggest that gastric cancer with CLDN18-ARHGAP fusion retains CLDN18.2 expression but the functionality is compromised, potentially contributing to disease progression, metastasis, and a poor prognosis. However, data on gastric cancer harboring the CLDN18-ARHGAP fusion are limited, and further investigations are needed.
Clinicopathological features and clinical impact of CLDN18.2-positive gastric or GEJ cancer
The prevalence of CLDN18.2 positive assessed by immunohistochemistry (IHC) in gastric or GEJ cancer has been reported as ranging from 24.0% to 33.4% in recent cohort studies,34,56 and it was found to be 38.4% in screening examinations of the phase III SPOTLIGHT and GLOW trials which demonstrated a survival benefit of zolbetuximab combined with chemotherapy10,11,31 (Table 1). In recent cohort studies, CLDN18.2 positivity was not associated with HER2 or PD-L1 CPS status.34,57 However, in the screening evaluation in the SPOTLIGHT and GLOW, CLDN18.2 positivity was numerically higher in HER2-negative patients compared with HER2-positive patients (20.2% in HER2-negative and 42.3% in HER2-positive patients). In addition, only 17.4% exhibited a PD-L1 CPS ⩾ 5 among CLDN18.2-positive patients with available PD-L1 CPS status in the screening examinations of SPOTLIGHT and GLOW, although the incidence might be affected by the retrospective nature of the exploratory analysis. In summary, there appears to be a relatively limited overlap between HER2 positive and CLDN18.2 positive, or between PD-L1 CPS ⩾ 5 and CLDN18.2 positive, which warrants further examination.
Table 1.
Prevalence of CLDN18.2-positive gastric or GEJ cancer and the clinicopathological features.
| Design/cohort | Screening test in SPOTLIGHT and GLOW | Kubota et al. 57 | Pellino et al. 34 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Design | Screening test | Retrospective study | Retrospective study | |||||||||
| Race (country) | Various | Asian (Japan) | Caucasian (Italy) | |||||||||
| Stage | IV | IV | I–IV | |||||||||
| N | 4507 | 408 | 350 | |||||||||
| Feature/biomarker | CLDN18.2+ | CLDN18.2− | p | CLDN18.2+ | CLDN18.2− | p | CLDN18.2+ | CLDN18.2− | p | |||
| Overall | 1730 (38.4%) | 2777 (61.6%) | 98 (24.0%) | 310 (76.0%) | 117 (33.4%) | 223 (66.6%) | ||||||
| Male | 1093 (36.2%) | 1924 (63.8%) | <0.0001 | 66 (23.5%) | 215 (76.5%) | 0.709 | 80 (25.2%) | 238 (74.8%) | 0.1214 | |||
| Female | 637 (42.8%) | 853 (57.2%) | 32 (25.2%) | 95 (74.8%) | 37 (28.0%) | 95 (72.0%) | ||||||
| Age | ⩽65 | 1136 (40.9%) | 1639 (59.1%) | <0.0001 | <65 | 45 (26.6%) | 124 (73.4%) | 0.347 | <70 | 83 (39.7%) | 126 (60.3%) | 0.0035 |
| >65 | 594 (34.3%) | 1138 (34.3%) | ⩾65 | 53 (22.2%) | 186 (77.8%) | >70 | 34 (24.1%) | 107 (75.9%) | ||||
| Gastric | 1341 (39.9%) | 2016 (60.1%) | 0.1846 | 85 (23.4%) | 278 (76.6%) | 0.459 | 90 (32.1%) | 190 (67.9%) | 0.3799 | |||
| GEJ | 338 (37.5%) | 563 (62.5%) | 13 (28.9%) | 32 (71.1%) | 27 (38.6%) | 43 (61.4%) | ||||||
| Lauren classification | ||||||||||||
| Diffuse | 553 (48.3%) | 592 (51.7%) | 0.0002 | 47 (25.5%) | 137 (74.5%) | 0.561 | 47 (40.2%) | 70 (59.8%) | 0.076 | |||
| Intestinal | 308 (38.8%) | 486 (61.2%) | 51 (22.8%) | 173 (77.2%) | 54 (29.0%) | 132 (71.0%) | 0.0813 | |||||
| Mixed | 134 (42.9%) | 178 (57.1%) | 14 (35.9%) | 25 (64.1%) | 0.8676 | |||||||
| Other | 252 (37.2%) | 425 (62.8%) | ||||||||||
| Unknown | 412 (35.0%) | 765 (65.0%) | ||||||||||
| Macroscopic classification | 0.019 | |||||||||||
| Type4 | NA | NA | NA | 28 (34.6%) | 53 (65.4%) | 0.019 | NA | NA | ||||
| Non-Type4 | NA | NA | 70 (21.4%) | 257 (78.6%) | NA | NA | ||||||
| HER2+ | 162 (20.2%) | 640 (79.8%) | NE | 15 (25.9%) | 43 (74.1%) | 0.741 | 17 (32.7%) | 35 (67.3%) | 1 | |||
| HER2− | 1568 (42.3%) | 2137 (57.7%) | 83 (23.7%) | 267 (76.3%) | 100 (33.6%) | 198 (66.4%) | ||||||
| dMMR/MSI-H | NA | NA | NA | 5 (20.8%) | 19 (79.2%) | 0.81 | 15 (23.1%) | 50 (76.9%) | 0.2424 | |||
| EBV+ | NA | NA | NA | 4 (26.7%) | 11 (73.3%) | 0.763 | 7 (87.5%) | 1 (12.5%) | 0.0024 | |||
| CPS < 5 | 495/599 (82.6%*) | NA | NA | 54/93 (58.1%*) | 142/293 (48.5%*) | 0.122 | 21 (10.3%*) | 183 (89.7%*) | 0.529 | |||
| CPS ⩾ 5 | 104/599 (17.4%*) | 39/93 (41.9%*) | 151/293 (51.5%*) | 96 (65.8%*) | 50 (34.2%*) | |||||||
| Clinical features of CLDN18.2+ GC/GEJC | Female, younger age, diffuse type | Type 4 | Younger age, higher stage (III–IV), peritoneal meta, fewer liver meta | |||||||||
| Survival impact on CLDN18.2+ | NA | Not associated | Not associated | |||||||||
| Reference | Shitara et al. 31 | Kubota et al. 57 | Pellino et al. 34 | |||||||||
Percentage in CLDN18.2 positive or CLDN18.2-negative group.
Abbreviations: meta, metastasis; GC, gastric cancer; GEJC, Gastro-Esophageal Junction Cancer ; NA, not available; NE, not evaluated.
In previous cohort studies of patients with gastric or GEJ cancer including those with resectable stage, CLDN18.2 expression was associated with some clinical features such as diffuse type, Epstein-Barr virus (EBV) positive.32,57–61 However, a meta-analysis including these studies revealed that CLDN18.2 expression was not significantly associated with TNM stage, Lauren classification, HER2, or overall survival. 62 It is important to note that these studies had limitations including differences in the definitions of CLDN18.2-positive assessed by IHC which was moderate-to-strong staining in ⩾40% or ⩾50% of tumor cells, and inclusion of resectable stage disease. Thus, the clinicopathological features and clinical implications including overall survival of CLDN18.2-positive gastric or GEJ cancer using the recent definition (moderate-to-strong expression in ⩾75% of tumor cells) remained unclear.
In a cohort study using the recent definition, CLDN18.2 positive was identified in 33.4% of patients diagnosed with stage I–IV gastric cancer. 34 CLDN18.2 positive was significantly associated with age younger than 70 years, EBV positive, higher-stage disease (stages III and IV) at diagnosis, peritoneal involvement, and lower incidence of liver metastases. However, CLDN18.2 positive did not show significant associations with overall survival and molecular markers such as HER2 status, MMR status, or PD-L1 CPS. While these findings are clinically important, this cohort study also had limitations including the inclusion of patients with earlier-stage gastric cancer and a lack of associations with treatment outcomes or genomic information. Therefore, we conducted a cohort study to analyze comprehensive clinical and molecular characteristics of CLDN18.2-positive gastric or GEJ cancer. 56 We analyzed 408 Japanese patients with advanced gastric or GEJ cancer who received systemic chemotherapy. Among the 408 patients, CLDN18.2 positive was identified in 98 patients, accounting for 24.0% of the cohort. Remarkably, CLDN18.2-positive patients were almost equally distributed among the four molecular subtypes of mismatch repair-deficient (MMR-D), EBV positive, HER2 positive, and all negative. CLDN18.2 positive was significantly associated with Borrmann type4, KRAS amplification. Progression-free survival and objective response rates of standard first-line (platinum-fluoropyrimidine), second-line chemotherapy (taxane with or without ramucirumab), and anti-PD-1 therapy showed no significant differences according to CLDN18.2 status. Furthermore, overall survival with standard first-line chemotherapy was not significantly different between CLDN18.2-positive and -negative groups with the median OS of 18.4 months versus 20.1 months [HR (hazard ratio) 1.26; 95% CI (confidence interval) 0.89–1.78; p = 0.191].
In this study, we also evaluated tumor microenvironment (TME) in patients who received anti-PD1 antibodies. Notably, the CLDN18.2-positive group exhibits a significantly lower number of CD16-positive cells (NK cells; p = 0.028), while the number of CD68-positive cells was significantly higher. Our study showed no significant difference in the number of CD8-positive cells between CLDN18.2 positive and CLDN18.2 negative. However, another study using multiplex IHC demonstrated that the proportion of CD8-positive cells lacking checkpoint markers such as PD-1, LAG3, or TIM3 was significantly higher in CLDN18.2-positive tumors. 63 These observations, including the reduced level of NK cells and the downregulation of checkpoint markers in CD8-positive cells in CLDN18.2-positive tumors, might potentially impact the efficacy of checkpoint inhibitors, which warrants further examination.
Zolbetuximab
Zolbetuximab, previously known as IMAB362 or claudiximab, is a first-in-class chimeric immunoglobulin G1 monoclonal antibody highly specific for CLDN18.2. This agent is derived from a mouse monoclonal antibody and has been chimerized to present the human IgG1 constant region for clinical application.64,65 Zolbetuximab binds to CLDN18.2 exposed on the tumor cell surface and stimulates immune effectors to induce antibody-dependent cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Zolbetuximab is also considered to induce apoptosis and inhibit cell proliferation. 64 In a preclinical study, zolbetuximab was highly selective for CLDN18.2 both in vivo and in vitro. Zolbetuximab not only stimulated target-selective ADCC against gastric cancer cell lines expressing CLDN18.2 but also induced CDC-mediated lysis of tumor cells expressing CLDN18.2. Importantly, the presence of CLDN18.2-negative cancer cells did not diminish these effects. 66 When used in combination with cytotoxic agents, zolbetuximab enhanced ADCC and CDC by upregulating CLDN18.2 expression, leading to enhanced antitumor activity observed in xenografted mice treated with zolbetuximab + chemotherapy compared to those treated with chemotherapy alone. Moreover, in another preclinical study using pancreatic cancer models, zolbetuximab demonstrated the ability to induce ADCC and CDC against human pancreatic cancer cells expressing CLDN18.2, and the magnitude of ADCC and CDC correlated with cell surface CLDN18.2 levels. 67 Notably, gemcitabine upregulated CLDN18.2 expression and enhanced zolbetuximab-induced ADCC in cultured human pancreatic cancer cells. These preclinical findings are consistent with the clinical improvements observed when cytotoxic agents are combined with zolbetuximab. Furthermore, zolbetuximab combined with chemotherapy is considered to activate T-cell infiltration and stimulate the production of pro-inflammatory cytokines. 66 While this combination therapy with checkpoint inhibitors holds promise, the synergistic effect of zolbetuximab combined with checkpoint inhibitor has not been shown clinically, as shown in the ILUSTRO trial below.
Clinical trials of zolbetuximab
Clinical trials of zolbetuximab are summarized in Table 2. In a phase I study of zolbetuximab in patients with advanced gastric and GEJ cancer, 15 patients were enrolled into five sequential single dose-escalation cohorts (33, 100, 300, 600, and 1000 mg/m2) following 3 + 3 design. 65 The most common treatment-related adverse events (TRAEs) were gastrointestinal toxicities. However, zolbetuximab was generally well tolerated, and no dose-limiting toxicity was observed in any of the cohorts. The pharmacokinetic (PK) profile of zolbetuximab was dose-proportional with a mean half-life (t1/2) of 17.2 days. Based on PK data and estimation of biological activity through preclinical pharmacology, a dose range of 300–600 mg/m2 every 2 weeks was determined to be appropriate for further exploration in clinical trials.
Table 2.
Summary of phase II/III trials of zolbetuximab.
| Design/result | MONO | FAST* | ILUSTRO | SPOTLIGHT | GLOW | ||||
|---|---|---|---|---|---|---|---|---|---|
| Trial design | Single arm phase IIa | Randomized phase IIb | Multi-cohort single phase II | Randomized, double-blinded phase III | Randomized, double-blinded phase III | ||||
| Primary endpoint | ORR | PFS | ORR (Cohort1A) | PFS | PFS | ||||
| Cohort | Main | Subgroup | Main | Subgroup | 1A | 2 | 3A | – | – |
| CLDN IHC 2+/3+** | ⩾50% a | ⩾70% a | ⩾40% b | ⩾70% b | ⩾75% c | ⩾75% c | ⩾50% c | ⩾ 75% c | ⩾ 75% c |
| Treatment line | ⩾2nd | ⩾2nd | 1st | 1st | ⩾3rd | 1st | ⩾3rd | 1st | 1st |
| Asian/non-Asian | – | – | – | – | – | – | – | 31.3%/68.7% | 62.1%/37.9% |
| Experimental arm (versus control arm) | Zmab | Zmab | EOX + Zmab vs EOX |
EOX + Zmab versus EOX |
Zmab | FOLFOX + Zmab | Zmab + Pembro | FOLFOX + Zmab vs FOLFOX | CAPOX + Zmab vs CapeOX |
| N | N = 43 | N = 29 | N = 161 | N = 116 | N = 30 | N = 21 | N = 3 | N = 565 | N = 507 |
| ORR | 9% | 14% | 39.0% vs 25.0% +14.0% |
NA | 0% | 71.4% | 0% | 60.7% vs 62.1% −1.4% |
53.8% vs 48.8% +5.0% |
| DCR | 23% | 31% | 83.1% vs 76.2% +7.9% |
NA | 44.4% | 100% | 66.7% | 82.0% vs 86.7% −4.7% |
77.5% vs 76.6% +0.9% |
| Median PFS | 3.2m | NA | 7.5 m vs 5.3 m +2.2 m HR 0.44 p < 0.0005 |
9.0 m vs 5.7 m +3.3 m HR 0.38 p < 0.0005 |
1.54 m | 17.8 m | 2.96 m | 10.61 m vs 8.67 m +1.94 m HR 0.751 p = 0.0066 |
8.21 m vs 6.80 m +1.41 m HR 0.687 p = 0.0007 |
| Median OS | NA | NA | 13.0 m vs 8.3 m +5.7 m HR 0.55 p < 0.0005 |
16.5 m vs 8.9 m +7.6 m HR 0.50 p < 0.0005 |
5.62 m | NA | NA | 18.23 m vs 15.54 m +2.69 m HR 0.750 p = 0.0053 |
14.39 m vs 12.16 m +2.23 m HR 0.771 p = 0.0118 |
| Reference | Türeci et al. 68 | Sahin et al. 69 | Klempner et al. 70 | Shitara et al. 10 | Shah et al. 11 | ||||
CAPOX, Capecitabine + Oxaliplatin; CLDN, Claudins; DCR, disease control rate; EOX, Epirubicin + Oxaliplatin + Capecitabine; FOLFOX, Fluoropyrimidine + Oxaliplatin; m, months; HR, Hazard Ratio; NA, not available; ORR, objective response rate; OS, overall survival; Pembro, Pembrolizumab; PFS, progression-free survival; vs, versus; Zmab, zolbetuximab.
Data of exploratory arm is omitted in this table.
CLDN assay is different among the trials: a. InvitrogenTM , b.CLAUDETECT TM 18.2, c. VENTANA CLDN18 (43-14A).
The MONO study, a phase IIa study, evaluated the efficacy and safety of zolbetuximab monotherapy in patients with CLDN18.2-positive advanced gastric, EGJ, and esophageal cancer. 68 CLDN18.2 positive was defined as moderate-to-strong staining of CLDN18.2 in ⩾50% of tumor cells in the Invitrogen™ IHC assay (Thermo Fisher Scientific; Waltham, MA, USA). A total of 268 patients were screened, and 54 patients were enrolled in this study. Initially, four patients received a safety lead-in dose of 300 mg/m2 (every 2 weeks), followed by 50 patients who received the targeted dose of 600 mg/m2 (every 2 weeks). Among the 43 patients with available antitumor activity data, 4 patients achieved a partial response (PR), resulting in an objective response rate (ORR) of 9%, and 6 patients exhibited stable disease (SD), resulting in a disease control rate (DCR) of 23%. Notably, the antitumor activity was enhanced in patients with moderate-to-strong staining of CLDN18.2 in greater than equal to 70% of tumor cells with an ORR of 14%. The most frequent TRAEs of grade ⩾3 were vomiting (22%) and nausea (15%). Based on the PK data, it was deemed appropriate to administer doses every 3 weeks.
The FAST study, a randomized phase II study, examined the clinical outcomes of zolbetuximab plus EOX (Epirubicin + Oxaliplatin + Capecitabine) versus EOX alone in patients with CLDN18.2-positive advanced gastric, EGJ, and esophageal cancer. 69 CLDN18.2 positive was defined as moderate-to-strong staining of CLDN18.2 in ⩾ 40% of tumor cells in the CLAUDETECT™ 18.2 IHC assay (Ganymed Pharmaceuticals; Mainz, Germany). A total of 730 patients were screened, and 252 patients were randomized to three arms: control arm (arm1) receiving EOX (every 3 weeks), experimental arm (arm2) receiving EOX plus zolbetuximab (loading dose of 800 mg/m2 followed by 600 mg/m2, every 3 weeks), and exploratory arm (arm3) receiving EOX plus zolbetuximab (1000 mg/m2, every 3 weeks). EOX plus zolbetuximab (arm2) significantly improved both progression-free survival (PFS) and OS compared to EOX alone (arm1): The median PFS were 7.5 months and 5.3 months (HR 0.44; 95% CI, 0.29–0.67, p < 0.0005), and the median OS were13.0 months and 8.3 months (HR 0.55; 95% CI, 0.39–0.77; p < 0.0005), respectively. In the subgroup analysis stratified by CLDN18.2 status, the clinical benefits of PFS and OS were observed exclusively in patients with moderate-to-strong staining of CLDN18.2 in ⩾70% of tumor cells, while no such benefits were noted in patients with that in 40–69% of tumor cells. EOX plus zolbetuximab (arm3) also showed significant improvement in PFS compared to EOX alone (arm1) in the overall population but it was not seen in the subgroup with CLDN18.2 expression in ⩾70% of tumor cells. Furthermore, significant OS benefit was not observed in arm3, neither the overall population nor the subgroup of patients with CLDN18.2 expression in greater than or equal to 70% of tumors. The most common TRAEs of grade ⩾3 in arm2 and arm1 were neutropenia (32.5% versus 21.4%), anemia (11.7% versus 7.1%), weight loss (11.7% versus 3.6%), vomiting (10.4% versus 3.6%), and nausea (6.5% versus 4.8%). Importantly, patient-reported outcomes demonstrated that EOX plus zolbetuximab maintained a good quality of life and low symptom burden for an extended duration compared to EOX alone. 71 In summary, the addition of zolbetuximab, administered as a loading dose of 800 mg/m2 followed by 600 mg/m2 every 3 weeks, demonstrated improvements in both PFS and OS compared to EOX alone in patients with CLDN18.2 positive, especially CLDN18.2 expression in greater than or equal to 70% tumor cells, advanced gastric, EGJ, and esophageal cancer while maintaining a manageable safety profile. These findings led to the following phase III studies.
The SPOTLIGHT trial is a global, randomized, placebo-controlled, double-blind, phase III trial designed to evaluate the efficacy and safety of first-line zolbetuximab plus mFOLFOX6 (modified folinic acid or levofolinate, fluorouracil, and oxaliplatin) in patients with CLDN18.2-positive, HER2-negative, untreated, advanced gastric, or GEJ adenocarcinoma. 10 CLDN18.2 positive was defined as moderate-to-strong staining of CLDN18.2 in greater than or equal to 75% of tumor cells by central IHC using the VENTANA CLDN18 [43-14A] RxDx Assay (Roche Diagnostic Solutions; Tucson, AZ, USA). This automatic CLDN18.2 IHC assay was changed from the manual CLAUDETECT™ assay used in FAST, which led to the adjustment of CLDN18.2 positivity cutoff from greater than or equal to 70% in FAST to greater than or equal to 75% in SPOTLIGHT to identify a similar patient population. Of the 2403 patients who were successfully assessed for CLDN18.2 status, 922 patients (38.4%) were found to be CLDN18.2 positive. Among these, 565 patients were randomly assigned to either receive zolbetuximab plus mFOLFOX6 or placebo plus mFOLFOX6. The combination of zolbetuximab plus mFOLFOX6 demonstrated a significant improvement in both PFS and OS with 25% risk reduction compared to placebo plus mFOLFOX6: The median PFS was 10.61 months and 8.67 months (HR 0.75, 95% CI 0.60–0.94, p = 0.0066), and the median OS was 18.23 months and 15.54 months (HR 0.75, 95% CI 0.60–0.94, p = 0.0053). The most frequent TRAEs of grade greater than or equal to 3 were neutropenia (28% versus 23%), nausea (16% versus 6%), and vomiting (16% versus 6%). Importantly, this trial met the primary endpoint of PFS assessed by an independent review committee for all randomly assigned patients and also demonstrated a significant benefit in terms of OS.
The GLOW trial is a global, randomized, placebo-controlled, double-blind, phase III trial that was conducted to evaluate the efficacy and safety of first-line zolbetuximab plus CAPOX (capecitabine and oxaliplatin) in patients with CLDN18.2-positive, HER2-negative, untreated, advanced gastric, or GEJ adenocarcinoma. 11 The assessment method of CLDN18.2 IHC and the definition of CLDN18.2 positive were the same as those in the SPOTLIGHT trial. Notably, more patients were enrolled from China than SPOTLIGHT study. Of the 2104 patients successfully assessed for CLDN18.2 status, 808 patients (38.4%) were identified as CLDN18.2 positive. Among these, 507 patients were randomized to receive either zolbetuximab plus CAPOX or placebo plus CAPOX. Zolbetuximab plus CAPOX achieved a significant improvement in both PFS and OS compared with placebo plus CAPOX: The median PFS were 8.21 months and 6.80 months (HR 0.687, 95% CI 0.544–0.866, p = 0.0007), and the median OS were 14.39 months and 12.16 months (HR 0.771, 95% CI 0.615–0.965, p = 0.0118). The most frequent TRAEs of grade greater than or equal to 3 were nausea (8.7% versus 2.4%), vomiting (12.2% versus 3.6%), and decreased appetite (6.7% versus 1.6%), and there were no new safety signals.
The benefits of zolbetuximab on OS and PFS were consistently observed between the SPOTLIGHT and GLOW. However, it is worth noting that the median PFS and OS in the control arm were numerically longer in the SPOTLIGHT compared with those in the GLOW. It is unlikely that the difference in the backbone chemotherapy could explain these variations, considering that treatment outcomes of FOLFOX and CAPOX were generally comparable in a subgroup analysis in CheckMate649. 7 An important distinction between the two trials is the higher number of patients enrolled from China in the GLOW than in the SPOTLIGHT. Previous studies have suggested that survival outcomes of Chinese patients were closer to those of the non-Asian population than Japanese or Korean patients.72,73 This different distribution of regions might have resulted in different outcomes between the two trials, although the obvious reasons remain unclear.
The ILUSTRO trial is a global multi-cohort phase II trial that evaluates the efficacy and safety of zolbetuximab, alone or with mFOLFOX6 or with pembrolizumab in patients with CLDN18.2-positive, HER2-negative, advanced gastric, or GEJ adenocarcinoma. 70 The eligibility for CLDN18.2 expression was a moderate-to-strong expression in greater than or equal to 75% of tumor cells in Cohorts 1A and 2, and greater than or equal to 50% in Cohort 3A. Eligible patients received were allocated to one of three cohorts: Cohort 1A (n = 30) received zolbetuximab as monotherapy in the third- or later-line treatment, Cohort 2 (n = 21) received zolbetuximab combined with mFOLFOX6 as a first-line treatment, and Cohort 3 (n = 3) received zolbetuximab in combined with pembrolizumab in the third- or later-line treatment (Cohort 3A, n = 3). The primary endpoint of ORR in Cohort1A was 0%. The results of secondary endpoints were as follows: ORR in Cohorts 2 and 3A were 71.4% and 0%, respectively; the median PFS was 1.54 months (95% CI, 1.31–2.56) in Cohort 1A, 17.8 months (95% CI, 8.05–25.69) in Cohort 2, and 2.96 months (95% CI, 1.48–4.44) in Cohort 3A, respectively; the median OS in Cohort 1A was 5.62 months (95% CI, 2.27–11.53). The safety profile was consistent with previous trials, and zolbetuximab treatment was tolerable with no new safety signals. The results of mFOLFOX6 plus zolbetuximab were compatible with the results of the SPOTLIGHT trial. However, the efficacy of zolbetuximab alone or combined with pembrolizumab in the third line or later line was limited.
Based on the promising results obtained from the SPOTLIGHT and GLOW, the combination of zolbetuximab with chemotherapy (mFOLFOX6 or CAPOX) is expected to become a novel first-line treatment option for patients with CLDN18.2-positive, HER2-negative, untreated, advanced gastric, or GEJ adenocarcinoma. Notably, CLDN18.2 positivity was identified in 38.4% of patients in the screening tests of SPOTLIGHT and GLOW trials, which accounts for about twice as many as HER2 positive. Consequently, the landscape of first-line treatment for gastric or GEJ cancer is anticipated to change dramatically in the near future. Currently, platinum-fluoropyrimidine combined with or without checkpoint inhibition is the gold standard first-line treatment for HER2-negative gastric or GEJ cancer in many countries. With zolbetuximab as an emerging first-line treatment option, it is necessary to consider whether a checkpoint inhibitor or zolbetuximab should be combined with chemotherapy for the initial treatment of HER2-negative and CLDN 18.2-positive tumors. It is important to note that no definitive conclusion can be drawn at this stage due to the absence of direct comparisons. However, for patients with PD-L1 negative or low (i.e. CPS < 5) and CLDN18.2 positive, zolbetuximab might be preferred because the subgroup of PD-L1 CPS < 5 has limited survival benefit from additional checkpoint inhibitors. 9 By contrast, the effect of nivolumab was markedly enriched in patients with MSI-H in CheckMate649. 7 Therefore, nivolumab might be preferred for these patients. There is no exact answer for the choice from these two regiments in patients with overlapping CPS > 5 and CLDN 18.2 positive and either could be a reasonable treatment option. Several other aspects such as differences in enhancement of tumor response, different toxicity profiles, and availability in later-line settings should be taken into account for treatment selection. Therefore, a shared decision-making process with patients will become more important in determining the most suitable treatment approach after the approval of zolbetuximab.
Further perspectives of CLDN18.2-targeting therapy
In light of the promising results of SPOTLIHGT and GLOW, CLDN18.2-targeting therapy has attracted increasing attention. Beyond zolbetuximab, several agents targeting CLDN18.2 have been developed including monoclonal antibodies, antibody–drug conjugates (ADC), chimeric antigen receptor cells (CAR-T), and bispecific T-cell engager (BiTE). Major studies of CLDN18.2-targeting therapy are summarized in Table 3.
Table 3.
Major studies of CLDN18.2-targeting therapy.
| Agent type | Trial number (NCT) | Phase | Cancer type | Agent | Experimental arm | Estimated enrollment |
|---|---|---|---|---|---|---|
| mAb | 3505320 | II | GC/EGJC | Zolbetuximab | 1. Monotherapy 2. +FOLFOX 3. +Pembrolizumab 4. +FOLFOX + nivolumab |
116 |
| 3816163 | II | PC | Zolbetuximab | +Gemcitabine + nab-paclitaxel | 369 | |
| 4400383 | I | Solid tumor | AB011 | Monotherapy | 228 | |
| 4671875 | I | Solid tumor | MIL93 | Monotherapy | 197 | |
| 4683939 | I/II | Solid tumor | BNT141 | 1. Monotherapy 2. +Gemcitabine + nab-paclitaxel |
96 | |
| 5008445 | I/II | Solid tumor | LM-102 | 1. Monotherapy 2. +Standard of care |
265 | |
| 5065710 | I/II | Solid tumor | ZL-1211 | Monotherapy | 162 | |
| BsAb | 5482893 | I | GC/GEJC, PC | PT886 | Monotherapy | 58 |
| 4900818 | I | Solid tumor | TJ033721 (TJ-CD4B) | Monotherapy | 102 | |
| ADC | 5043987 | I | GC/GEJC, PC | CPO102 | Monotherapy | 72 |
| 5001516 | I/II | Solid tumor | LM302 | Monotherapy | 142 | |
| 5161390 | I/II | Solid tumor | LM302 | Monotherapy | 128 | |
| 5994001 | I/II | BTC | LM302 | +Cardonilizumab | 96 | |
| 5205850 | I/IIa | Solid tumor | RC118 | Monotherapy | 135 | |
| 5867563 | I | Solid tumor | TQB2103 | Monotherapy | 71 | |
| 4805307 | I | Solid tumor | CMG901 | Monotherapy | 162 | |
| CAR-T cell | 4404595 | Ib/II | GC/GEJC, PC | CT041 | Monotherapy | 110 |
| 4581473 | Ib/II | GC/GEJC, PC | CT041 | Monotherapy | 192 | |
| 4467853 | Ib/II | GC/GEJC | LCAR-C18S | Monotherapy | 64 | |
| 4966143 | Ib/II | PC | LY011 | Monotherapy | 30 | |
| 5472857 | I | GC/GEJC, PC, OC | IMC002 | Monotherapy | 30 | |
| 5539430 | I | EC, GC/GEJC, PC | LB1908 | Monotherapy | 56 | |
| 5620732 | NA | GC, PC | CLDN18.2CAR-T | Monotherapy | 20 | |
| 5952375 | I | GC | XKDCT086 | Monotherapy | 9 | |
| BiTE | 4260191 | I | GC/GEJC | AMG 910 | Monotherapy | 70 |
| 4856150 | I | Solid tumor | Q-1802 | Monotherapy | 66 | |
| 6005493 | I/II | EC, GC/GEJC, PC | AZD5863 | Monotherapy | 200 | |
| 5365581 | I | GC/GEJC, PC | ASP2138 | Monotherapy | 240 | |
| Other | 5009966 | I | Solid tumor | LB4330 (specific bifunctional molecule) | Monotherapy | 66 |
Regarding combination therapy, the ILUSTRO trial has expanded an additional cohort to evaluate zolbetuximab combined with mFOLFOX6 and nivolumab (NCT03505320). Furthermore, several ongoing clinical trials are exploring the potential of combining anti-CLDN18.2 therapies with cytotoxic agents and/or checkpoint inhibitors in patients with several cancer types including gastric or GEJ cancer and pancreatic cancer (NCT04396821, NCT04495296, NCT04683939, and NCT05008445).
Most recently, several results of phase I/II trials of novel anti-CLDN18.2 therapies have been reported (Table 4). ASKB589, a humanized IgG1 monoclonal antibody-targeting CLDN18.2, achieved favorable antitumor activity in patients with gastric cancer. ASKB589 achieved an ORR of 9.5% as a monotherapy, and combination therapy with CAPOX (capecitabine plus oxaliplatin) resulted in an impressive ORR of 75%. 74 Osemitamab (TST001), the second most advanced CLDN18.2-targeting antibody, exhibits high affinity, leading to enhanced ADCC and CDC. Osemitamab combined with CAPOX demonstrated favorable anti-tumor activity with an ORR of 66.7% and a median PFS of 9.5 months.75,76 SYSA1801 and CMG901, two other ADCs targeting CLDN18.2, demonstrated promising efficacy in patients with gastric cancer, achieving ORRs of 47.1% and 75.0%, respectively.77,78 In addition, CT041, a claudin18.2-specific CAR-T therapy, achieved hopeful efficacy in patients with several cancer types including gastric cancer, with an ORR of 57.1% in gastric cancer and an ORR of 22.2% in other cancer types. 79 In these trials, nausea and vomiting, which were common adverse events of zolbetuximab, are also observed. However, overall, these novel therapies generally showed acceptable and manageable safety profiles. In addition, results of the ongoing phase I trials exploring LB1908, an autologous CAR-T cell therapy of LB1908, and a native IgG-like BiTE-targeting CD47 and CLDN18.2 of PT886, are awaited.80,81
Table 4.
Summary of phase I/II trials of CLDN18.2-targeting therapy.
| Design/result/adverse event | NCT04632108 | TranStar102 trial (NCT04495296) |
NCT05009966 | NCT04805307 |
NCT03874897 (Interim results) |
|||
|---|---|---|---|---|---|---|---|---|
| Phase | I/II | I/IIa | I | I | I | |||
| Treatment | ASKB589 | CAPOX+ASKB589 | CAPOX + Osemitamab (TST001) | SYSA1801 | CMG901 | CT041 | ||
| Agent type | mAb | mAb | ADC | ADC | CAR-T cells | |||
| Cancer type (cohort) | GC (A) | GC (B) | GC | GC | PC | GC/PC | GC | Others |
| N (all) | N = 40 | N = 45 | N = 64 | N = 26 | N = 7 | N = 27 | N = 28 | N = 9 |
| N (efficacy analyzed) | N = 21 | N = 24 |
N = 42 (ORR) N = 64 (PFS) |
N = 17 | N = 4 |
N = 8 (CLDN18.2-positive GC) |
N = 28 | N = 9 |
| ORR | 9.5% | 75% | 66.7% | 47.1% | 0% | 75.0% | 57.1% | 22.2% |
| DCR | 47.6% | 100% | 97.6% | 64.7% | 25% | 100% | 75.0% | 66.7% |
| mPFS | Not released | Not released | 9.5 m | Not released | Not reached | 4.2 m | 2.6 m | |
| OS | Not released | Not released | Not released | Not released | Not reached | 6 m OS: 81.2% | 6 m OS: 77.8% | |
| TRAEs ⩾ Gr1 | 80% | 81% | 100.0% | 75.8% | 96.3% | 100.0% | ||
| Nausea ⩾ Gr1 | 53% | 76% | 70.3% | 42.4% | 40.7% | 48.6% | ||
| Vomiting ⩾ Gr1 | 43% | 66% | 53.1% | 36.4% | 70.4% | 35.1% | ||
| Other common TRAEs ⩾ Gr1 |
Hypoalbuminemia (40%) Loss of appetite (30%) |
Hypoalbuminemia (52%) Granulocytopenia (38%) | Anemia (82.8%) Hypoalbuminemia (65.4%) |
Dry eye syndrome (21.2%) Anemia (21.2%) | Decreased appetite (59.3%) Hypoalbuminemia (44.4%) | CRS Gr1, 2: 94.6% (CRS Gr⩾3 CRS: 0%) |
||
| TRAEs ⩾ Gr3 | 10% | 38% | 65.6% | 24.2% | 11.1% | 100% (almost hematology) | ||
| DLT | None | None | None | Two DLTs at the 3 mg/kg dose (Gr3 nausea and vomiting) |
One DLT at 2.2 mg/kg | None | ||
| MTD | Not reached | Not reached | Not reached | Reached | Not reached | Not reached | ||
| TRD | None | None | None | None | 1 (3.7%) | None | ||
| Reference | Zhang et al. 74 | Shen et al., 75 Guo et al. 76 | Wang et al. 77 | Xu et al. 78 | Qi et al. 79 | |||
ADC, Antibody-Drug Conjugate; CAPOX, Capecitabine + Oxaliplatin; CAR-T, Chimeric Antigen Receptor-T cell; CRS, Cytokine Release Syndrome; DCR, Disease Control Rate; DLT, Dose-Limiting Toxicity; GC, Gastric Cancer; Gr, Grade; m, months; mAb, Monoclonal Antibody; mPFS, median Progression-Free Survival; MTD, Maximum Tolerated Dose; ORR, Objective Response Rate; OS, Overall Survival; PC, Pancreatic Cancer; TRAEs, Treatment Related Adverse Events; TRD, Treatment Related Death.
Summary
CLDN18.2 has emerged as a new target molecule not only for gastric or GEJ cancer but also for various other cancer types. Zolbetuximab stands out as a pioneering agent directed against CLDN18.2, and it has demonstrated a significant improvement of OS in patients with gastric or GEJ cancer. In the near future, the combination of zolbetuximab and platinum-fluoropyrimidine may become a new standard treatment option for patients with CLDN18.2-positive and HER2-negative gastric or GEJ cancer. Moreover, there is a wave of new agents targeting CLDN18.2, including monoclonal antibodies, ADC, CAR-T cells, and BiTE, which are currently under development. The results from ongoing trials exploring these agents are anticipated.
Acknowledgments
None.
Footnotes
ORCID iD: Yohei Kubota  https://orcid.org/0000-0002-6578-688X
https://orcid.org/0000-0002-6578-688X
Contributor Information
Yohei Kubota, Department of Clinical Oncology, St. Marianna University School of Medicine, Kanagawa, Japan.
Kohei Shitara, Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, 6-5-1 Kashiwanoha, Kashiwa, Chiba 277-8577, Japan.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Yohei Kubota: Investigation; Writing – original draft.
Kohei Shitara: Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: YK reports receiving research funding from Astellas Pharma. KS reports receiving personal fees for advisory roles from Lilly, Bristol Myers Squibb, Takeda, Pfizer, Ono Pharmaceutical, Merck Pharmaceutical, Taiho Pharmaceutical, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Amgen, Boehringer Ingelheim, and Janssen; receiving honoraria (lecture fee) from Takeda and Bristol-Myers Squibb; and receiving research funding from Astellas, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Chugai, MSD, Medi Science, Eisai and Amgen, outside the submitted work.
Availability of data and materials: Not applicable.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022; 20: 167–192. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358: 36–46. [DOI] [PubMed] [Google Scholar]
- 4. Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008; 9: 215–221. [DOI] [PubMed] [Google Scholar]
- 5. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastrooesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet 2010; 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 6. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022; 603: 942–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022; 23: 234–247. [DOI] [PubMed] [Google Scholar]
- 9. Zhao JJ, Yap DWT, Chan YH, et al. Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol 2022; 40: 392–402. [DOI] [PubMed] [Google Scholar]
- 10. Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2023; 401: 1655–1668. [DOI] [PubMed] [Google Scholar]
- 11. Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med 2023; 29: 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furuse M, Fujita K, Hiiragi T, et al. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141: 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsukita S, Tanaka H, Tamura A. The Claudins: from tight junctions to biological systems. Trends Biochem Sci 2019; 44: 141–152. [DOI] [PubMed] [Google Scholar]
- 14. Zihni C, Mills C, Matter K, et al. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 2016; 17: 564–580. [DOI] [PubMed] [Google Scholar]
- 15. Morita K, Furuse M, Fujimoto K, et al. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 1999; 96: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sahin U, Koslowski M, Dhaene K, et al. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res 2008; 14: 7624–7634. [DOI] [PubMed] [Google Scholar]
- 17. Tsukita S, Furuse M. Occludin and claudins in tight junction strands: leading or supporting players? Trend. Cell Biol 1999; 9: 268–273. [DOI] [PubMed] [Google Scholar]
- 18. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2: 285–93. [DOI] [PubMed] [Google Scholar]
- 19. Kyuno D, Takasawa A, Takasawa K, et al. Claudin-18.2 as a therapeutic target in cancers: cumulative findings from basic research and clinical trials. Tissue Barriers 2022; 2: 10: 1967080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 2005; 65: 9603–9606. [DOI] [PubMed] [Google Scholar]
- 21. Tokés AM, Kulka J, Paku S, et al. Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res 2005; 7: R296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kominsky SL, Argani P, Korz D, et al. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene 2003; 22: 2021–2033. [DOI] [PubMed] [Google Scholar]
- 23. Resnick MB, Konkin T, Routhier J, et al. Claudin-1 is a strong prognostic indicator in stage II colonic cancer: a tissue microarray study. Mod Pathol 2005; 18: 511–518. [DOI] [PubMed] [Google Scholar]
- 24. Hough CD, Sherman-Baust CA, Pizer ES, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res 2000; 60: 6281–6287. [PubMed] [Google Scholar]
- 25. Kominsky SL, Vali M, Korz D, et al. Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin 3 and 4. Am J Pathol 2004; 164: 1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long H, Crean CD, Lee WH, et al. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res 2001; 61: 7878–7881. [PubMed] [Google Scholar]
- 27. Gress TM, Muller-Pillasch F, Geng M, et al. A pancreatic cancer-specific expression profile. Oncogene 1996; 13: 1819–1830. [PubMed] [Google Scholar]
- 28. Agarwal R, D’Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res 2005; 65: 7378–85. [DOI] [PubMed] [Google Scholar]
- 29. Niimi T, Nagashima K, Ward JM, et al. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 2001; 21: 7380–7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moran D, Maurus D, Rohde C, et al. Prevalence of CLDN18.2, HER2, and PD-L1 in gastric cancer samples. ESMO 2018. Congress. Abstract 2171. [Google Scholar]
- 31. Shitara K, Xu RH, Moran DM, et al. Global prevalence of CLDN18.2 in patients with locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Biomarker analysis of two zolbetuximab phase 3 studies (SPOTLIGHT and GLOW). J Clin Oncol 2023; 41(Suppl 16): 4035.37315297 [Google Scholar]
- 32. Rohde C, Yamaguchi R, Mukhina S, et al. Comparison of claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol 2019; 49: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coati I, Lotz G, Fanelli GN, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer 2019; 121: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pellino A, Brignola S, Riello E, et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J Pers Med 2021; 11: 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shinozaki A, Shibahara J, Noda N, et al. Claudin-18 in biliary neoplasms. Its significance in the classification of intrahepatic cholangiocarcinoma. Virchows Arch 2011; 459: 73–80. [DOI] [PubMed] [Google Scholar]
- 36. Caron TJ, Scott KE, Sinha N, Muthupalani S, et al. Claudin-18 loss alters transcellular chloride flux but not tight junction ion selectivity in gastric epithelial cells. Cell Mol Gastroenterol Hepatol 2021; 11: 783–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayashi D, Tamura A, Tanaka H, et al. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1β, and atrophic gastritis in mice. Gastroenterology 2012; 142: 292–304. [DOI] [PubMed] [Google Scholar]
- 38. Hagen SJ, Ang LH, Zheng Y, et al. Loss of tight junction protein claudin 18 promotes progressive neoplasia development in mouse stomach. Gastroenterology 2018; 155: 1852–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suzuki K, Sentani K, Tanaka H, et al. Deficiency of stomach-type Claudin-18 in mice induces gastric tumor formation independent of H pylori infection. Cell Mol Gastroenterol Hepatol 2019; 8: 119–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oshima T, Shan J, Okugawa T, et al. Down-regulation of claudin-18 is associated with the proliferative and invasive potential of gastric cancer at the invasive front. PloS One 2013; 8: e74757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takasawa K, Takasawa A, Osanai M, et al. Claudin-18 coupled with EGFR/ERK signaling contributes to the malignant potentials of bile duct cancer. Cancer Lett 2017; 403: 66–73. [DOI] [PubMed] [Google Scholar]
- 42. Chen J, Xu Z, Hu C, et al. Targeting CLDN18.2 in cancers of the gastrointestinal tract: New drugs and new indications. Front Oncol 2023; 13: 1132319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yano K, Imaeda T, Niimi T. Transcriptional activation of the human claudin-18 gene promoter through two AP-1 motifs in PMA-stimulated MKN45 gastric cancer cells. Am J Physiol Gastrointest Liver Physiol 2008; 294: 336–343. [DOI] [PubMed] [Google Scholar]
- 44. Ito T, Kojima T, Yamaguchi H, et al. Transcriptional regulation of claudin-18 via specific protein kinase C signaling pathways and modification of DNA methylation in human pancreatic cancer cells. J Cell Biochem 2011; 112: 1761–72. [DOI] [PubMed] [Google Scholar]
- 45. Li T, Liu X, Riederer B, et al. Genetic ablation of carbonic anhydrase IX disrupts gastric barrier function via claudin-18 downregulation and acid backflux. Acta Physiol 2018; 222: e12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shen CH, Lin JY, Lu CY, et al. SPAK-p38 MAPK signal pathway modulates claudin-18 and barrier function of alveolar epithelium after hyperoxic exposure. BMC Pulm Med 2021; 21: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma X, Yu X, Zhou Q. The IL1beta-HER2-CLDN18/CLDN4 axis mediates lung barrier damage in ARDS. Aging (Albany NY) 2020; 12: 3249–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Finigan JH, Faress JA, Wilkinson E, et al. Neuregulin-1-human epidermal receptor-2 signaling is a central regulator of pulmonary epithelial permeability and acute lung injury. J Biol Chem 2011; 286: 10660–100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li J, Zhang Y, Hu D, et al. Analysis of the expression and genetic alteration of CLDN18 in gastric cancer. Aging (Albany NY) 2020; 12: 14271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ikari N, Serizawa A, Tanji E, et al. Analysis of RHOA mutations and their significance in the proliferation and transcriptome of digestive tract cancer cells. Oncol Lett 2021; 22: 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yao F, Kausalya JP, Sia YY, et al. Recurrent fusion genes in gastric cancer: CLDN18-ARHGAP26 induces loss of epithelial integrity. Cell Rep 2015; 12:272–285. [DOI] [PubMed] [Google Scholar]
- 53. Tanaka A, Ishikawa A, Ushiku T, et al. Frequent CLDN18-ARHGAP fusion in highly metastatic diffuse-type gastric cancer with relatively early onset. Oncotarget 2018; 9: 29336–29350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nakayama I, Shinozaki E, Sakata, et al. Enrichment of CLDN18-ARHGAP fusion gene in gastric cancers in young adults. Cancer Sci 2019; 110: 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shu Y, Zhang W, Hou Q, et al. Prognostic significance of frequent CLDN18-ARHGAP26/6 fusion in gastric signet-ring cell cancer. Nat Commun 2018; 9: 2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kubota Y, Kawazoe A, Mishima S, et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 2023; 8: 100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baek JH, Park DJ, Kim GY, et al. Clinical implications of Claudin18.2 expression in patients with gastric cancer. Anticancer Res 2019; 39: 6973–6979. [DOI] [PubMed] [Google Scholar]
- 58. Coati I, Lotz G, Fanelli GN, et al. Claudin-18 expression in oesophagogastric adenocarcinomas: a tissue microarray study of 523 molecularly profiled cases. Br J Cancer 2019; 121: 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dottermusch M, Krüger S, Behrens HM, et al. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch 2019; 475: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sanada Y, Oue N, Mitani Y, et al. Down–regulation of the claudin–18 gene, identified through serial analysis of gene expression data analysis, in gastric cancer with an intestinal phenotype. J Pathol 2006; 208: 633–42. [DOI] [PubMed] [Google Scholar]
- 61. Jun KH, Kim JH, Jung JH, et al. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg 2014; 12: 156–162. [DOI] [PubMed] [Google Scholar]
- 62. Ungureanu BS, Lungulescu CV, Pirici D, et al. Clinicopathologic relevance of claudin 18.2 expression in gastric cancer: a meta-analysis. Front Oncol 2021; 11: 643872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jia K, Chen Y, Sun Y, et al. Multiplex immunohistochemistry defines the tumor immune microenvironment and immunotherapeutic outcome in CLDN18.2-positive gastric cancer. BMC Med 2022; 20: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol 2017; 10: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sahin U, Schuler M, Richly H, et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur J Cancer 2018; 100: 17–26. [DOI] [PubMed] [Google Scholar]
- 66. Minacht-Kraus R, Kreuzberg M, Utsch M, et al. Preclinical characterization of IMAB362 for the treatment of gastric carcinoma. Annals Oncol 2017; 28: 378P. [Google Scholar]
- 67. Türeci Ö, Mitnacht-Kraus R, Wöll S, et al. Characterization of zolbetuximab in pancreatic cancer models. OncoImmunology 2018; 8: e1523096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol 2019; 30: 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sahin U, Türeci Ö, Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol 2021; 32: 609–619. [DOI] [PubMed] [Google Scholar]
- 70. Klempner SJ, Lee KW, Shitara K, et al. ILUSTRO: Phase 2 multicohort trial of zolbetuximab in patients with advanced or metastatic claudin 18.2-positive gastric or gastroesophageal junction adenocarcinoma. Clin Cancer Res 2023; 29: 3882–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lordick F, Al-Batran SE, Ganguli A, et al. Patient-reported outcomes from the phase II FAST trial of zolbetuximab plus EOX compared to EOX alone as first-line treatment of patients with metastatic CLDN18.2+ gastroesophageal adenocarcinoma. Gastric Cancer 2021; 24: 721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu T, Bai Y, Lin X, et al. First-line nivolumab plus chemotherapy versus chemotherapy in patients with advanced gastric, gastroesophageal junction and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int J Cancer 2023; 152: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer 2015; 18: 168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang M, Gong J, Wang J, et al. A phase I/II study of ASKB589 (anti-claudin 18.2 [CLDN18.2] monoclonal antibody) in patients with solid tumors. J Clin Oncol 2023; 41(Suppl 4): 397. [Google Scholar]
- 75. Shen L, Liu D, Li N, et al. Osemitamab in combination with capecitabine and oxaliplatin (CAPOX) as a first line treatment of advanced G/GEJ cancer: updated data of cohort C from a phase I/IIa, multi-center study (TranStar102/TST001-1002). J Clin Oncol 2023; 41(Suppl 16): 4046. [Google Scholar]
- 76. Guo W, Germa C, Qi C, et al. TST001 (a high affinity humanized anti-claudin18.2 monoclonal antibody) in combination with nivolumab plus capecitabine and oxaliplatin as first-line or with nivolumab as late-line treatment in locally advanced and metastatic gastric/gastroesophageal junction (G/GEJ) cancer: design of cohorts from a phase I/IIa study (TST001-1002). J Clin Oncol 2023; 41(Suppl 4): TPS476. [Google Scholar]
- 77. Wang Y, Gong J, Lin R, et al. First-in-human dose escalation and expansion study of SYSA1801, an antibody-drug conjugate targeting claudin 18.2 in patients with resistant/refractory solid tumors. J Clin Oncol 2023; 41(Suppl 16): 3016. [Google Scholar]
- 78. Xu RH, Wei X, Zhang D, et al. A phase 1a dose escalation, multicenter trial of anti-claudin 18.2 antibody drug conjugate CMG901 in patients with resistant/refractory solid tumors. J Clin Oncol 2023; 41(Suppl 4): 352. [Google Scholar]
- 79. Qi C, Gong J, Li J, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med 2022; 28: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhen DB, Thota R, del Corral C, et al. A phase 1, open-label, dose escalation and expansion, multicenter study of claudin 18.2-targeted chimeric antigen receptor T-cells in patients with unresectable, locally advanced, or metastatic gastric, gastroesophageal junction, esophageal, or pancreatic adenocarcinoma. J Clin Oncol 2023; 41(Suppl 4): TPS480. [Google Scholar]
- 81. Overman MJ, Melhem R, Blum-Murphy MA, et al. A phase I, first-in-human, open-label, dose escalation and expansion study of PT886 in adult patients with advanced gastric, gastroesophageal junction, and pancreatic adenocarcinomas. J Clin Oncol 2023; 41(Suppl 4): TPS765. [Google Scholar]