Abstract
Background:
Deficiency in vitamin D has been shown to increase the risk of injury.
Purpose:
To synthesize current placebo-controlled randomized trials investigating the effect of vitamin D supplementation in elite athletes on (1) aerobic capacity; (2) anaerobic measures, such as strength, speed, and anaerobic power; (3) serum biomarkers of inflammation; and (4) bone health.
Study Design:
Systematic review; Level of evidence, 1.
Methods:
A literature search was conducted on November 30, 2022, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Included were randomized, placebo-controlled studies of longer than 2 weeks on subjects with active participation in organized sport. Excluded were nonrandomized controlled trial study designs, vitamin D administration routes other than oral, studies that did not use vitamin D supplementation as the sole intervention, and studies with nonathletic or military populations.
Results:
Out of 2331 initial studies, 14 studies (482 athletes) were included. Of the 3 studies that assessed aerobic capacity, 2 demonstrated significantly greater improvements in maximal oxygen uptake and physical working capacity-170 (P < .05) in supplemented versus nonsupplemented athletes. Measurements of anaerobic power and strength were consistently increased in supplemented groups compared with nonsupplemented groups in 5 out of the 7 studies that assessed this. Of the 6 studies that assessed sprint speed, 4 found no significant difference between supplemented and nonsupplemented groups. Aside from 1 study that found significantly lower interleukin-6 levels in supplemented athletes, measures of other inflammatory cytokines were not affected consistently by supplementation. The 4 studies that assessed markers of bone health were conflicting regarding benefits of supplementation. One study found demonstrated improvements in bone mineral density in response to supplementation (P = .02) compared with control whereas another found no significant difference between supplemented and nonsupplemented groups. However, in 3 other studies, serum biomarkers of bone turnover such as bone-specific alkaline phosphatase, parathyroid hormone, and N-terminal telopeptide appeared to be higher in subjects with lower serum vitamin D levels (P < .05).
Conclusion:
Results of this systematic review indicated that the greatest benefit of vitamin D supplementation in elite athletes may be improving aerobic endurance, anaerobic power, and strength. More research is needed to determine the effect of vitamin D supplementation on bone health and injury risk in this population.
Keywords: athletics, cholecalciferol, sport, vitamin D, vitamin D deficiency
The prevalence of vitamin D deficiency is increasing worldwide at a rate and distribution consistent with a pandemic.10,17 The percentage of adult Americans with vitamin D deficiency ranges from 36% to 57%,23,40 and as few as 2% of North Americans achieve the recommended vitamin D daily intake. 27 The prevalence of vitamin D deficiency in athletes worldwide follows the prevalence of nonathletic populations and is becoming a growing concern as it pertains to athletic performance, aerobic endurance, and injury risk. § Previous studies have shown that the prevalence of vitamin D deficiency may be up to 26% in professional American football players and 36% of English Premiere League soccer players in Liverpool, England.41,45 Vitamin D has gained popularity in the medical community in recent decades as a hormone-like chemical that influences between 5% and 10% of the total genome. 53 However, determining the precise effects of this important vitamin is not straightforward, and neither is our understanding of its widespread physiological effect.
Vitamin D deficiency, which has been defined differently by multiple authors,51,53 can have detrimental effects on bone, muscle, respiratory, neurological, and respiratory health. 53 Pludowski et al 51 defined vitamin D deficiency as less than 50 nmol/L serum concentration. Athletes who are at highest risk for developing vitamin D deficiency are those who train early in the morning or late at night, those who wear sport kits (ultraviolet B light coverage), and indoor athletes. 47 Previous research has demonstrated that low vitamin D levels are associated with injuries in athletes, 3 particularly bone stress injuries. 3 Serum 25-hydroxyvitamin D (25[OH]D) levels below 30 to 50 nmol/L appear to be associated with increased risk of such injuries.16,57
To our knowledge, no previous systematic review has synthesized multiple placebo-controlled randomized controlled trials investigating the effect of vitamin D supplementation on fitness and sport performance in athletes. The objective of this systematic review was to analyze the current body of literature regarding the effect of vitamin D supplementation on (1) aerobic capacity; (2) anaerobic measures, such as strength, speed, and anaerobic power; (3) serum biomarkers of inflammation; and (4) bone health. We hypothesized that vitamin D supplementation would have a positive effect on all 4 of these outcomes.
Methods
This was a systematic review of randomized, placebo-controlled trials published before November 30, 2022, that investigated the effect of supplementation of vitamin D in athletic populations. This study followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for reporting systematic reviews, 49 and the protocol was registered on PROSPERO (CRD42022381494).
Search Strategy
One author (J.C.) searched the PubMed/Medline, Cochrane, CINAHL, and Embase (OVID) databases on November 30, 2022, using the keywords, “athlete,”“sport,”“athletic performance,”“cholecalciferol,”“vitamin D,” and “ergocalciferol.” A more detailed summary of the search strategy can be found in the Online Appendix.
Screening and Study Inclusion/Exclusion Criteria
Duplicates and papers written in languages other than English were excluded. Two authors (P.B.W. and C.R.R.) independently screened all articles returned from the initial search using this review’s inclusion and exclusion criteria. Each study was first reviewed by title and abstract then by full text if more detail was required to make the decision of inclusion. Conflicts were resolved by a neutral third author (J.R.S.). Inclusion criteria were as follows: randomized, placebo-controlled study design with a duration of longer than 2 weeks and subjects with active participation in organized sport. Exclusion criteria were as follows: nonrandomized controlled trial study designs, vitamin D administration routes other than oral, studies that did not use vitamin D supplementation as the sole intervention in the experimental group, and studies with nonathletic or military populations.
Data Extraction
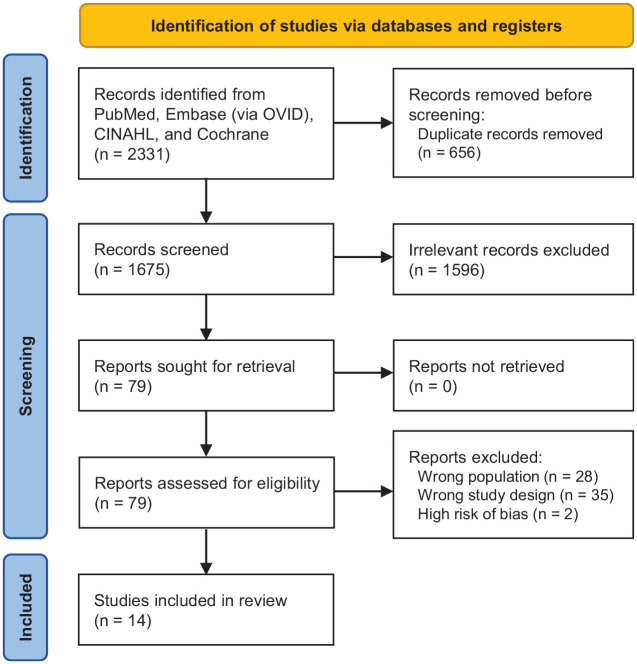
Data were extracted independently by 2 authors (P.B.W. and C.R.R.). The data that were systematically extracted included salient findings pertaining to vitamin D supplementation’s effect on athletic performance, bone health, injury recovery/prevention, biochemical markers, and plasma 25(OH)D levels. The initial search returned 2331 articles. After duplicates were removed (n = 656), 1675 articles were remaining, of which 1596 articles were excluded based on title and abstract screening. This left 79 articles for full-text screening, of which 65 were excluded. A total of 16 studies remained for quality assessment after full-text screening. ‖ The inclusion/exclusion process is depicted in Figure 1.
Figure 1.
PRISMA flow diagram of study inclusion in the review. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Quality Assessment
Two authors (P.B.W. and C.R.R.) assessed each of the 16 studies for risk of bias using the Cochrane Risk of Bias 2 tool for assessing the methodological quality and risk of bias in randomized controlled trials involving human subjects.44,54 Of the included articles, 11 studies were determined to have a low risk of bias, ¶ 3 studies were determined to have “some concerns” regarding risk of bias,11,29,39 and 2 studies were identified as having high concerns for risk of bias and were therefore excluded from the primary analysis.26,50
Outcomes of Interest
All reported outcomes in the included studies were considered of interest in this review.
Results
Characteristics of the Included Studies
After the screening process was completed, 14 studies were selected for inclusion in this systematic review. # The characteristics of the included studies are summarized in Appendix Table A1. There were a total of 482 participants (350 male, 72 female). Two studies did not report participant sex,29,31 leaving a total of 60 subjects of unknown sex. The average age of participants among the studies was 20.87 years (range, 17.20-27.00 years). The most common sport investigated in the included studies was outdoor soccer, totaling 226 subjects across 6 studies.1,7,8,13,29-31 Other sports included were rugby (n = 72),13,21 rowing (n = 36), 43 taekwondo (n = 35), 33 swimming and diving (n = 83),11,39,55 and ultramarathon running (n = 24). 63
Method of Vitamin D Supplementation
All studies employed oral formulations of vitamin D3. Three studies used oral droplets,29-31 whereas the remaining 11 studies used capsules and identically-appearing placebo capsules. ** Dosage ranged from 2000 international units (IU)/day to 7142.86 IU/day,1,21,63 with an average daily dose of 4959.12 IU/day. Eight studies used daily dosing of vitamin D3 (range, 3000-5000 IU/day)11,29-31,33,39,43,55; 1 study used twice daily dosing (1000 IU/dose) 63 ; 2 studies used weekly dosing (range, 20,000-50,000 IU/week)1,13; 2 studies using biweekly dosing (both 20,000 IU twice weekly)7,8; and 1 study used 1 dose of 50,000 IU every 2 weeks. 21 Importantly, only 1 participant among all the studies reported an adverse effect: constipation. 55 More details regarding methods of supplementation can be found in Appendix Table A1.
Geographic Location of Studies
The latitudinal location of the studies in the northern hemisphere ranged from 32.43° N to 54.51° N,1,7,8 with an average latitude of 45.53° N. One study was conducted at 45° S to 46.5° S in New Zealand. 21 The specific latitudinal location for each study is provided in Appendix Table A1.
Aerobic Capacity
Improvements in maximal oxygen uptake (VO2max) were seen in 2 studies.7,31 One study on Polish soccer players demonstrated a significant correlation between increases serum 25(OH)D concentrations and increases in VO2max (r = 0.4192, P = .0024). 7 Jastrzębska et al 31 found an increase in VO2max of 8.65 ± 3.57 mL/kg/min in the vitamin D3-supplemented group compared with 5.03 ± 2.02 mL/kg/min in a nonsupplemented group of soccer players (P = .021). Their study on supplemented Polish soccer athletes demonstrated a significant increase in physical working capacity-170 (PWC-170; a measure of aerobic power) compared with nonsupplemented controls (supplemented group, 5.03 ± 2.02 kGm/kg/min; nonsupplemented group, 3.34 ± 2.28 kgm/kg/min; P = .027). 31 Conversely, a previous study by the same group found no significant difference in PWC-170 in a similar group of Polish soccer players (P > .05). 30
Strength, Speed, and Anaerobic Power
Results regarding anaerobic capacity, strength, and agility are heterogeneous between studies. In the present review, 5 studies demonstrated improvements in anaerobic power/strength or sprint speed,1,21,29,33,55 while 3 studies did not demonstrate a significant difference between supplemented and nonsupplemented groups.7,13,31 Sprint speeds were found to be increased in 2 studies on soccer players compared with nonsupplemented groups (P = .03).1,29 Four studies on soccer and rugby players found no significant difference between groups in sprint speeds (P > .05).7,13,21,30 All sprint distances tested were no longer than 30 m.
Inconsistent findings regarding strength were found among previous studies. The power/strength tests that were found to significantly improve in supplemented groups were predicted 1-repetition maximum weighted reverse-grip chin-ups (increased by 5.5 kg [95% confidence interval (CI), 2.0-8.9 kg]; P = .0002) 21 ; isokinetic knee extension strength (P = .019) 33 ; squat, deadlift, and vertical jump (P < .05) 55 ; and leg press (P = .034). 1 Anerobic power measured by the Wingate test (performed on lower body ergometer) was found to increase significantly in supplemented taekwondo athletes. 33 However, another study found no significant differences in lactate threshold between groups of soccer players (see Appendix Table A1 for more details). 31
Serum Biomarkers and Immune Modulation
All supplemented groups in the included studies demonstrated significantly higher postintervention serum 25(OH)D levels compared with nonsupplemented groups. In 2 studies,31,33 all subjects had baseline serum 25(OH)D concentrations less than 50 nmol/L, which is the proposed as the cut-off for vitamin D “deficiency” by Pludowski et al. 51 All but 1 of the remaining studies had a mix of vitamin D-deficient and -sufficient subjects. 21 The average change in serum 25(OH)D concentration from baseline across the 15 supplemented groups (1 study had 2 supplemented groups) was +38.03 nmol/L. Only 1 study reported a decrease in 25(OH)D serum levels in both the supplemented and nonsupplemented groups. 11 This decrease was somewhat expected as the baseline serum 25(OH)D concentrations were much higher than in other studies (average of 130 nmol/L in the supplemented group). This could be due to the study being conducted at a latitude closer to the equator (38° N) and the sport (swimming and diving) being indoors. Nonetheless, the decrease in this group was significantly smaller in the supplemented group compared with control. 11 Serum levels decreased by only 2.5 nmol/L in a supplemented group compared with 50 nmol/L over 6 months (P < .05) in the placebo group. 11
A significant negative correlation was found between serum 25(OH)D levels and C-reactive protein (CRP) in a group of soccer players (r = -0.459, P = .021). 8 In a subsequent analysis, the magnitude of this effect was greater in supplemented subjects with suboptimal baseline 25(OH)D serum levels (defined as <75 nmol/L). Two studies found no significant correlation between serum 25(OH)D levels and interleukin (IL)-6 levels (an inflammatory cytokine),8,39 whereas another found significantly lower IL-6 levels in a supplemented group versus placebo (P < .01). 63 Other inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and IL-1β appeared to have no significant correlation with serum 25(OH)D levels. 39
One study found a significant increase (20%; P < .001) in parathyroid hormone (PTH) levels after 8 weeks in a nonsupplemented group. 1 No significant increase was observed in the supplemented group in this study. Another study found no significant change in PTH levels throughout the study, 55 whereas another study found changes in PTH were not associated with 25(OH)D serum concentrations. 39
Supplementation appeared to be sufficient to prevent a decline in transferrin (P = .007), hemoglobin (P = .009), and hematocrit (P = .019) in a group of elite male rowers compared with a nonsupplemented group of controls. 43 In addition, higher 25(OH)D serum concentrations were significantly correlated with serum total testosterone levels, but supplementation did not appear to be sufficient to significantly increase total testosterone concentrations.
Bone Health
Serum levels of biomarkers of bone turnover, such as bone-specific alkaline phosphatase (BSAP) and N-terminal telopeptide (NTx), were not significantly different between supplemented and nonsupplemented groups (P > .05).11,39 However, BSAP and NTx appeared to be significantly higher in subjects with larger decreases in serum 25(OH)D over the course of 1 study. 11 Concentrations of BSAP and NTx were not correlated significantly with measurements of bone mineral density (BMD). 11 However, BMD of the proximal femur (P = .02) and mineral-free lean mass (P < .05) were both found to be significantly correlated with serum 25(OH)D levels. 39 Conversely, Cassity et al 11 found that 6-month changes in body composition measures (total mass, lean mass, fat mass, BMD, and body mass index) were not significantly different between groups.
Discussion
This systematic review showed promising, albeit heterogeneous, data supporting the use of vitamin D supplementation in athletes. Athletic measures that appeared to benefit most from vitamin D supplementation were measures of aerobic capacity (VO2max, PWC-170) and some measures of strength and power (vertical jump, various measures of upper body strength).1,7,21,31,33,55 Sprint speed was less affected by supplementation.21,30 Biochemical comparison of supplemented and nonsupplemented athletes demonstrated a possible association between serum 25(OH)D levels and total testosterone. 43 Serum markers of inflammation, such as CRP, ILs, TNF-α, and cortisol were not affected consistently by supplementation.8,39,63 Only 1 of the 2 studies that assessed BMD was able to demonstrate a positive correlation between serum 25(OH)D levels and BMD. 39 However, the biomarkers of bone turnover, such as PTH, BSAP, and NTx, were found to be higher in nonsupplemented groups and correlated negatively with serum 25(OH)D levels.1,11,39
As the prevalence of vitamin D deficiency rises in athletic populations, investigation of the effects of supplementation in this population is warranted. Previous studies have shown that vitamin D deficiency can increase the risk of bone injuries such as stress fracture in military and athletic populations.15,36,39,46,62 Previous systematic reviews and meta-analyses have also shown that vitamin D supplementation can improve skeletal muscle strength12,60 and may even play a role in reducing the risk of acute respiratory infections. 32 This systematic review demonstrates that oral vitamin D supplementation is an effective method of improving serum 25(OH)D levels and that supplementation has a positive effect on aerobic capacity, anaerobic capacity, and strength in athletes.
The Scientific Advisory Committee on Nutrition and the Food Standards Agency of the United Kingdom has defined vitamin D deficiency as a serum 25(OH)D concentration of less than 25 nmol/L. 24 However, more recent guidelines suggest a concentration of less than 50 nmol/L is necessary for identifying vitamin D deficiency. 51 These authors further define vitamin D “insufficiency” as a concentration of 50 to 75 nmol/L, with a target serum concentration of ranging from 75 to 125 nmol/L to 100 to 150 nmol/L. 51 Other authors have defined vitamin D “inadequacy” as a serum concentration of less than 80 nmol/L. 23 The lack of consistent terminology and numerical cut-offs makes it difficult to define an exact serum concentration for vitamin D deficiency. However, most studies, including the included studies in this review, considered values below 50 nmol/L to be “deficient.”
The National Academy of Medicine (formerly known as the Institute of Medicine) has defined the tolerable upper limit of vitamin D supplementation as 4000 IU/day; the risk of adverse events increases at serum 25(OH)D levels greater than 125 nmol/L. However, the no-observed-adverse-effects level has been found to be 10,000 IU/day, established by the same group. 56 Doses higher than 70,000 IU/week have been shown to induce 24-hydroxylase after 12 weeks of supplementation. This enzyme converts 1,25(OH)D (the bioactive form of vitamin D) to 24,25(OH)D, which provides negative feedback on the activity of 1,25(OH)D. This produces a paradoxically decreased effect of 1,25(OH)D with high-dose vitamin D supplementation. 48 Therefore, more research is needed to determine an appropriate dose that will produce desired effects in athletic populations.
It is worth noting that all but 3 of the included studies were conducted at latitudes greater than 35° N.1,21,33 It has been shown that ultraviolet B (UVB) radiation is nonexistent at latitudes greater than 35° N.9,28,47 This reflects the higher prevalence of vitamin D inadequacy in athletes found by Farrokhyar et al 23 in a meta-analysis of 2313 international subjects. The authors calculated a risk ratio of 1.85 (95% CI, 1.25-2.53) for vitamin D inadequacy (<80 nmol/L) in athletes who lived above 40° N when outliers from studies in the Middle East were excluded. Supplementation should be considered for athletes living at UVB-deficient latitudes, especially in the winter months.
The gold standard for measuring aerobic capacity is VO2max, a measurement of maximal oxygen consumption.31,35 VO2max is extrapolated most commonly from graded exercise testing on a leg cycle ergometer or treadmill. 35 The mechanism behind the positive correlation of serum 25(OH)D levels on VO2max is poorly understood but may be related to increased erythropoiesis. 58 This hypothesis is supported by the findings of this review that hemoglobin, hematocrit, and transferrin all were found to increase in a supplemented group of rowers. 43 The current review agrees with earlier literature suggesting that VO2max is correlated positively with serum 25(OH)D levels in vitamin D-deficient athletes as well as in nonathletic adult populations.4,61 On the other hand, some studies have reported no correlation in serum 25(OH)D status and VO2max.25,34 Variability in VO2max between studies is expected as VO2max is heavily influenced by a multitude of factors such as age, sex, body composition, heredity, cardiac output, pulmonary blood flow, lung diffusion capacity, and lung ventilation. 6
The effect of vitamin D supplementation on strength and power appears to be inconsistent and is influenced heavily by the type of training that is undertaken in each study. The inconsistency in the effect of vitamin D on anaerobic strength/power and sprint speed among the included studies is likely owed to the variability in training modality and sport-specific metabolic demands.
A previous systematic review and meta-analysis of 310 young and active participants concluded that vitamin D supplementation was associated with increased upper (P = .005) and lower (P = .04) body strength. 60 Strength gains associated with vitamin D supplementation have been estimated to range from 1.37% to 18.75%. 12 These effects have been hypothesized to be due to promotion of skeletal muscle regeneration and maintenance of mitochondrial health, according to stem cell models. 38 Furthermore, vitamin D receptor expression in skeletal muscle is highly expressed and focused within regenerating muscle fibers after injury (ie, high-intensity exercise). 59 Activation of the vitamin D receptor by 1,25-hydroxyvitamin D appears to have many roles in the regeneration of skeletal muscle, including increased satellite (stem) cell regeneration and increasing mitochondrial turnover by promoting mitophagy and mitochondrial biogenesis and fusion signaling. 38
Immune modulation is a function of vitamin D that is poorly understood. Previous studies have shown that vitamin D supplementation may safely reduce risk of acute respiratory infections. 32 Others have shown that supplementation may also inhibit the expression of proinflammatory cytokines such as IL-1, -6, -8, and -122 and even decrease serum levels of these substances.18,19 The current review similarly demonstrates that serum IL-6 and CRP concentrations may be correlated negatively with serum 25(OH)D levels and improve with supplementation - especially in 25(OH)D-deficient subjects.8,63 However, 1 included study reported no significant effect of supplementation on inflammatory cytokines. 39 This difference may be due to different training intensities and variability in the duration of study.
Limitations
Our study is not without limitations. This study is limited primarily by the high heterogeneity of training protocol, geographical location, and inclusion of both indoor and outdoor sports. In addition, it is difficult to determine how the outcomes were affected by the variable serum 25(OH)D concentrations among subjects at baseline. Further, this study was limited due to a lack of a meta-analysis, which we determined was impractical considering the variability in protocols and outcome reporting methods between studies. Despite these limitations, findings from this review successfully identified several positive, albeit inconsistent, effects of vitamin D supplementation that can direct further randomized controlled trials on this topic.
Conclusion
The benefits of vitamin D supplementation in athletes remain unclear and inconsistent. However, its greatest benefit in this population may be improving aerobic endurance, anaerobic power, and strength. Given the high prevalence of vitamin D deficiency in this population, supplementation in the winter months should be considered, especially for those in geographic areas that receive less sunlight. Future studies should investigate the benefits of long-term supplementation towards outcomes such as injury prevention.
Appendix
Appendix Table A1.
Summary of Included Studies a
| Lead Author (Year) | N | Sport (Indoor/Outdoor) | Latitudinal Location | Vitamin D Administration Protocol | Salient Findings |
|---|---|---|---|---|---|
| Jastrzębska 31 (2018) | 36 | Soccer (outdoor) | 52° N | • Experimental group: vitamin D3 oral drops, 5000 IU/day for 8 weeks • Control group: placebo oral drops |
Vitamin D supplementation augmented the training response of VO2max (P = .021) and PWC-170 to an 8-week sport-specific training program (P = .027) |
| Jastrzębska 29 (2022) | 24 | Soccer (outdoor) | 52° N | • Experimental group: vitamin D3 oral drops, 5000 IU/day for 8 weeks • Control group: placebo oral drops |
Higher 25(OH)D plasma levels were negatively correlated with sprint times at 10 m (r = -0.32, P = .006) and 30 m (r = -0.36, P = .002) |
| Jung 33 (2018) | 35 | Taekwondo (indoor) | 33° N | • Experimental group: vitamin D3 oral capsule, 5000 IU/day for 4 weeks • Control group: placebo capsule |
• Peak anaerobic power improved in both groups, but significantly more in the experimental group, as measured by the Wingate anaerobic test (F = 7.486, P = .010). • Changes in plasma 25(OH)D concentration were correlated positively with changes in peak power (r = 0.443, P = .009) • Isokinetic knee extension strength at 180° improved significantly in the experimental group, but not in the control group (F = 6.078, P = .019) |
| Brzeziański 7 (2022) | 25 | Soccer (outdoor) | 54.51° N | • Experimental group: vitamin D3 oral capsule, 20,000 IU, given twice weekly for 8 weeks • Control group: placebo capsule |
Positive correlation between serum 25(OH)D levels and VO2max (r = 0.4192, P = .0024) |
| Jastrzębska 30 (2016) | 36 | Soccer (outdoor) | 52° N | • Experimental group: vitamin D3 oral drops, 5000 IU/day for 8 weeks • Control group: placebo capsule |
No significant differences in sprint speed, jump distance, or peak power/total work in Wingate test or PWC-170. |
| Brzeziański 8 (2022) | 25 | Soccer (outdoor) | 54.51° N | • Experimental group: vitamin D3 oral capsule, 20,000 IU, given twice weekly for 8 weeks • Control group: placebo capsule |
Negative correlation between serum 25(OH)D levels and C-reactive protein levels in both groups (r = -0.459, P = .021) |
| Rockwell 55 (2020) | 19 | Swimming (outdoor and indoor) | 37.23° N | • Experimental group: vitamin D3 oral capsule, 5000 IU/day for 12 weeks | • Squat, deadlift, and vertical jump performance improved significantly in the experimental group, but not the control group (P < .05). • Fat-free body mass increased by 13.6% in the supplemental group (P < .05) but did not change in the control group. • Significant decrease in total testosterone in males and females in the placebo group over the course of the intervention period (P < .05). Experimental group also decreased, but not at a statistically significant rate (P > .05). • Regression analysis showed that serum 25(OH)D levels predicted free testosterone (R2 = 0.4906, P < .05) |
| Mielgo-Ayuso 43 (2018) | 36 | Rowing (outdoor) | 43.16° N | • Experimental group: vitamin D3 oral capsule, 3000 IU/day for 8 weeks • Control group: placebo capsule |
• Placebo group demonstrated a decrease in both plasma hemoglobin and hematocrit levels (group-time interaction was -1.57% ± 2.29%, P = .009; -1.57% ± 2.49% for hemoglobin and hematocrit, respectively). Experimental group demonstrated no such decline in either measurement • Transferrin levels increased in experimental group compared with the control group (group-time interaction was 6.51% ± 4.36% in the experimental group versus 0.67% ± 4.88% in the control group (P = .007) • There was a positive association between 25(OH)D levels and testosterone and cortisol; however, supplementation was not sufficient to change these hormone levels significantly |
| Alimoradi 1 (2019) | 69 | Soccer (outdoor) | 32.43° N | • Experimental group: vitamin D3 oral capsule 50,000 IU, once weekly for 8 weeks • Control group: placebo capsule |
• Significantly greater increase in leg press strength seen in the experimental group (P = .034), but no difference in ergo jump, vertical jump, or agility tests. • Experimental group showed a significant improvement in maximal sprint speed (P = .03) while the control group did not; however, no between group significant differences (P > .05). • Placebo group demonstrated a 20% increase in PTH (P < .001). No significant increase in the experimental group |
| Cassity 11 (2016) | 32 | Swimming and diving (indoor) | 38° N | • Experimental group: vitamin D3 oral capsule, 4000 IU/day for 6 months • Control group: placebo capsule |
• No significant difference between groups in bone turnover markers. • However, athletes with higher bone turnover markers showed significantly greater decreases in serum 25(OH)D (P = .03). • In the experimental group, there was a significant negative correlation between BMI and 6-month increase in serum 25(OH)D measurements (r = -0.496, P = .03). |
| Żebrowska63 (2020) | 24 | Ultramarathon running (outdoor) | 52° N | • Experimental group: vitamin D3 oral capsule, 1000 IU, twice daily for 3 weeks • Control group: placebo capsule |
• Experimental group demonstrated significantly decreased levels of max troponin (P = .004), 1-hour postexercise troponin (P = .03), 1-hour postexercise myoglobin concentration (P = .01), 24-hour postexercise creatine kinase (P < .05), and TNF-α (P < .03) at the end of the 3 weeks when compared with the control group • Significant negative correlation between postexercise 25(OH)D levels and myoglobin levels (r = -0.57, P = .05) and TNF-α (r = -0.58, P = .05) in the experimental group |
| Lewis 39 (2013) | 32 | Swimming/ diving (indoor) | 38° N | • Experimental group: vitamin D3 oral capsule, 4000 IU/day for 6 months • Control group: placebo capsule |
Changes in 25(OH)D serum concentrations did not correlate significantly with changes in bone turnover markers (BSAP or NTx) at any timepoint • Serum 25(OH)D was not positively correlated with BMD in the proximal femur or lumbar spine. • In men, total body (r = 0.48, P = .03) and trunk (P = .04) mineral-free lean mass correlated positively with serum 25(OH)D levels • In women, right femoral neck BMD (P = .02) was correlated positively with serum 25(OH)D • There was no correlation between serum 25(OH)D levels and inflammatory cytokine levels any time point in either group (TNF-α, IL-6, or IL-1β) |
| Close 13 (2013) | 30 | Rugby and soccer (outdoor) | 53° N | • Experimental group 1: vitamin D3 oral capsule, 40,000 IU once per week for 12 weeks • Experimental group 2: vitamin D3 oral capsule, 20,000 IU once per week for 12 weeks • Control group: placebo capsule |
• Significantly higher serum 25(OH)D levels in the 40,000 IU group at 6 weeks when compared with the 20,000 IU group (P = .016); however, there was no significant difference between these 2 groups at 12 weeks • The control group saw a progressive decline in serum 25(OH)D throughout the course of the study (P < .01) • No significant differences in 1-repetition maximum bench press, leg press, vertical jump height, or 20-m sprint (P > .1 for all) |
| Fairbairn 21 (2018) | 57 | Rugby (outdoor) | 45°-46.5° S | • Experimental group: vitamin D3 oral capsule, 50,000 IU once every 2 weeks for 11-12 weeks • Control group: placebo capsule once daily |
• Significant increase in predicted 1-repetition maximum chin-up performance in the experimental group when compared with the control group (5.5 kg, 95% CI, 2.0-8.9 kg, P = .0002). • No significant difference in 30-m sprint, 10-m sprint, yoyo intermittent exercise recovery test, or predicted 1-repetition maximum bench pull and bench press between groups. |
25(OH)D, 25-hydroxyvitamin D; BMD, bone mineral density; BMI, body mass index; BSAP, bone-specific alkaline phosphatase; CI, confidence interval; IL-1β, interleukin-1β; IL-6, interleukin-6; IU, international units; NTx, N-terminal telopeptide; PTH, parathyroid hormone; PWC-170, power working capacity-170; TNF-α, tumor necrosis factor-alpha; vitamin D3, cholecalciferol; VO2max, maximal rate of oxygen consumption by working tissue.
Footnotes
Final revision submitted May 27, 2023; accepted July 31, 2023.
One or more of the authors has declared the following potential conflict of interest or source of funding: J.R.S. has received education payments from Fortis Surgical. C.N.O. has received education payments from Fortis Surgical. R.S.O. has received a grant from Arthrex and education payments from Arthrex, Fortis Surgical, Smith & Nephew, and Alon Medical Technology. A.R.V. has received education payments from Supreme Orthopedic Systems and hospitality payments from Smith & Nephew and Arthrex. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Alimoradi K, Nikooyeh B, Ravasi AA, et al. Efficacy of vitamin D supplementation in physical performance of Iranian elite athletes. Int J Prev Med. 2019;10:100. doi: 10.4103/ijpvm.IJPVM_227_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almerighi C, Sinistro A, Cavazza A, Ciaprini C, Rocchi G, Bergamini A. 1Alpha,25-dihydroxyvitamin D3 inhibits CD40L-induced pro-inflammatory and immunomodulatory activity in human monocytes. Cytokine. 2009;45(3):190-197. doi: 10.1016/j.cyto.2008.12.009 [DOI] [PubMed] [Google Scholar]
- 3. Ammerman BM, Ling D, Callahan LR, Hannafin JA, Goolsby MA. Prevalence of Vitamin D insufficiency and deficiency in young, female patients with lower extremity musculoskeletal complaints. Sports Health. 2021;13(2):173-180. doi: 10.1177/1941738120953414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ardestani A, Parker B, Mathur S, et al. Relation of vitamin D level to maximal oxygen uptake in adults. Am J Cardiol. 2011;107(8):1246-1249. doi: 10.1016/j.amjcard.2010.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Backx EMP, Tieland M, Maase K, et al. The impact of 1-year vitamin D supplementation on vitamin D status in athletes: a dose-response study. Eur J Clin Nutr. 2016;70(9):1009-1014. doi: 10.1038/ejcn.2016.133 [DOI] [PubMed] [Google Scholar]
- 6. Beneke R, Böning D. The limits of human performance. Essays Biochem. 2008;44:11-25. doi: 10.1042/BSE0440011 [DOI] [PubMed] [Google Scholar]
- 7. Brzeziański M, Migdalska-Sęk M, Czechowska A, et al. Correlation between the positive effect of vitamin D supplementation and physical performance in young male soccer players. Int J Environ Res Public Health. 2022;19(9):5138. doi: 10.3390/ijerph19095138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brzeziański M, Pastuszak-Lewandoska D, Migdalska-Sęk M, et al. Effect of vitamin D3 supplementation on interleukin 6 and C-reactive protein profile in athletes. J Nutr Sci Vitaminol (Tokyo). 2022;68(5):359-367. doi: 10.3177/jnsv.68.359 [DOI] [PubMed] [Google Scholar]
- 9. Cannell JJ, Hollis BW, Sorenson MB, Taft TN, Anderson JJB. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41(5):1102-1110. doi: 10.1249/MSS.0b013e3181930c2b [DOI] [PubMed] [Google Scholar]
- 10. Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033-1044. doi: 10.3945/ajcn.115.120873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cassity EP, Redzic M, Teager CR, Thomas DT. The effect of body composition and BMI on 25(OH)D response in vitamin D-supplemented athletes. Eur J Sport Sci. 2016;16(7):773-779. doi: 10.1080/17461391.2015.1125952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiang C-M, Ismaeel A, Griffis RB, Weems S. Effects of vitamin D supplementation on muscle strength in athletes: a systematic review. J Strength Cond Res. 2017;31(2):566-574. doi: 10.1519/JSC.0000000000001518 [DOI] [PubMed] [Google Scholar]
- 13. Close GL, Leckey J, Patterson M, et al. The effects of vitamin D(3) supplementation on serum total 25[OH]D concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013;47(11):692-696. [DOI] [PubMed] [Google Scholar]
- 14. Close GL, Russell J, Cobley JN, et al. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013;31(4):344-353. doi: 10.1080/02640414.2012.733822 [DOI] [PubMed] [Google Scholar]
- 15. Dao D, Sodhi S, Tabasinejad R, et al. Serum 25-hydroxyvitamin D levels and stress fractures in military personnel: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2064-2072. doi: 10.1177/0363546514555971 [DOI] [PubMed] [Google Scholar]
- 16. Davey T, Lanham-New SA, Shaw AM, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of stress fracture during Royal Marine recruit training. Osteoporos Int. 2016;27(1):171-179. doi: 10.1007/s00198-015-3228-5 [DOI] [PubMed] [Google Scholar]
- 17. de la Puente Yagüe M, Collado Yurrita L, Ciudad Cabañas MJ, Cuadrado Cenzual MA. Role of vitamin D in athletes and their performance: current concepts and new trends. Nutrients. 2020;12(2):579. doi: 10.3390/nu12020579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Della Gatta PA, Cameron-Smith D, Peake JM. Acute resistance exercise increases the expression of chemotactic factors within skeletal muscle. Eur J Appl Physiol. 2014;114(10):2157-2167. doi: 10.1007/s00421-014-2936-4 [DOI] [PubMed] [Google Scholar]
- 19. Della Gatta PA, Garnham AP, Peake JM, Cameron-Smith D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav Immun. 2014;39:80-86. doi: 10.1016/j.bbi.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 20. Dubnov-Raz G, Livne N, Raz R, Cohen AH, Constantini NW. Vitamin D supplementation and physical performance in adolescent swimmers. Int J Sport Nutr Exerc Metab. 2015;25(4):317-325. doi: 10.1123/ijsnem.2014-0180 [DOI] [PubMed] [Google Scholar]
- 21. Fairbairn KA, Ceelen IJM, Skeaff CM, Cameron CM, Perry TL. Vitamin D3 supplementation does not improve sprint performance in professional rugby players: a randomized, placebo-controlled, double-blind intervention study. Int J Sport Nutr Exerc Metab. 2018;28(1):1-9. doi: 10.1123/ijsnem.2017-0157 [DOI] [PubMed] [Google Scholar]
- 22. Farrokhyar F, Sivakumar G, Savage K, et al. Effects of vitamin D supplementation on serum 25-hydroxyvitamin D concentrations and physical performance in athletes: a systematic review and meta-analysis of randomized controlled trials. Sports Med Auckl NZ. 2017;47(11):2323-2339. doi: 10.1007/s40279-017-0749-4 [DOI] [PubMed] [Google Scholar]
- 23. Farrokhyar F, Tabasinejad R, Dao D, et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45(3):365-378. doi: 10.1007/s40279-014-0267-6 [DOI] [PubMed] [Google Scholar]
- 24. Gillie O. Sunlight robbery: a critique of public health policy on vitamin D in the UK. Mol Nutr Food Res. 2010;54(8):1148-1163. doi: 10.1002/mnfr.200900589 [DOI] [PubMed] [Google Scholar]
- 25. Gregory SM, Parker BA, Capizzi JA, et al. Changes in vitamin D are not associated with changes in cardiorespiratory fitness. Clin Med Res. 2013;2(4):68-72. doi: 10.11648/j.cmr.20130204.16 [DOI] [Google Scholar]
- 26. Hew-Butler T, Aprik C, Byrd B, et al. Vitamin D supplementation and body composition changes in collegiate basketball players: a 12-week randomized control trial. J Int Soc Sports Nutr. 2022;19(1):34-48. doi: 10.1080/15502783.2022.2046444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hill KM, Jonnalagadda SS, Albertson AM, Joshi NA, Weaver CM. Top food sources contributing to vitamin D intake and the association of ready-to-eat cereal and breakfast consumption habits to vitamin D intake in Canadians and United States Americans. J Food Sci. 2012;77(8):H170-H175. doi: 10.1111/j.1750-3841.2012.02787.x [DOI] [PubMed] [Google Scholar]
- 28. Holick MF. Vitamin D: a D-lightful health perspective. Nutr Rev. 2008;66(10)(suppl 2):S182-S194. doi: 10.1111/j.1753-4887.2008.00104.x [DOI] [PubMed] [Google Scholar]
- 29. Jastrzębska J, Skalska M, Radzimiński L, et al. Changes of 25(OH)D concentration, bone resorption markers and physical performance as an effect of sun exposure, supplementation of vitamin D and lockdown among young soccer players during a one-year training season. Nutrients. 2022;14(3):521. doi: 10.3390/nu14030521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jastrzębska M, Kaczmarczyk M, Jastrzebski Z. Effect of vitamin D supplementation on training adaptation in well-trained soccer players. J Strength Cond Res. 2016;30(9):2648-2655. doi: 10.1519/JSC.0000000000001337 [DOI] [PubMed] [Google Scholar]
- 31. Jastrzębska M, Kaczmarczyk M, Michalczyk M, et al. Can supplementation of vitamin D improve aerobic capacity in well trained youth soccer players? J Hum Kinet. 2018;61:63-72. doi: 10.2478/hukin-2018-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jolliffe DA, Camargo CA, Sluyter JD, et al. Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021;9(5):276-292. doi: 10.1016/S2213-8587(21)00051-6 [DOI] [PubMed] [Google Scholar]
- 33. Jung HC, Seo MW, Lee S, Jung SW, Song JK. Correcting vitamin D insufficiency improves some but not all aspects of physical performance during winter training in taekwondo athletes. Int J Sport Nutr Exerc Metab. 2018;28(6):635-643. doi: 10.1123/ijsnem.2017-0412 [DOI] [PubMed] [Google Scholar]
- 34. Książek A, Zagrodna A, Dziubek W, Pietraszewski B, Ochmann B, Słowińska-Lisowska M. 25(OH)D3 levels relative to muscle strength and maximum oxygen uptake in athletes. J Hum Kinet. 2016;50:71-77. doi: 10.1515/hukin-2015-0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langeskov-Christensen M, Langeskov-Christensen D, Overgaard K, Møller AB, Dalgas U. Validity and reliability of VO2-max measurements in persons with multiple sclerosis. J Neurol Sci. 2014;342(1-2):79-87. doi: 10.1016/j.jns.2014.04.028 [DOI] [PubMed] [Google Scholar]
- 36. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23(5):741-749. doi: 10.1359/jbmr.080102 [DOI] [PubMed] [Google Scholar]
- 37. Larson-Meyer DE, Willis KS. Vitamin D and athletes. Curr Sports Med Rep. 2010;9(4):220-226. doi: 10.1249/JSR.0b013e3181e7dd45 [DOI] [PubMed] [Google Scholar]
- 38. Latham CM, Brightwell CR, Keeble AR, et al. Vitamin D promotes skeletal muscle regeneration and mitochondrial health. Front Physiol. 2021;12:660498. doi: 10.3389/fphys.2021.660498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lewis RM, Redzic M, Thomas DT. The effects of season-long vitamin D supplementation on collegiate swimmers and divers. Int J Sport Nutr Exerc Metab. 2013;23(5):431-440. doi: 10.1123/ijsnem.23.5.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D deficiency - is there really a pandemic? N Engl J Med. 2016;375(19):1817-1820. doi: 10.1056/NEJMp1608005 [DOI] [PubMed] [Google Scholar]
- 41. Maroon JC, Mathyssek CM, Bost JW, et al. Vitamin D profile in National Football League players. Am J Sports Med. 2015;43(5):1241-1245. doi: 10.1177/0363546514567297 [DOI] [PubMed] [Google Scholar]
- 42. Mehran N, Schulz BM, Neri BR, Robertson WJ, Limpisvasti O. Prevalence of vitamin D insufficiency in professional hockey players. Orthop J Sports Med. 2016;4(12):2325967116677512. doi: 10.1177/2325967116677512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mielgo-Ayuso J, Calleja-Gonzalez J, Urdampilleta A, et al. Effects of vitamin D supplementation on haematological values and muscle recovery in elite male traditional rowers. Nutrients. 2018;10(12):1968. doi: 10.3390/nu10121968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Minozzi S, Dwan K, Borrelli F, Filippini G. Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB2) improved with the use of implementation instruction. J Clin Epidemiol. 2022;141:99-105. doi: 10.1016/j.jclinepi.2021.09.021 [DOI] [PubMed] [Google Scholar]
- 45. Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD. Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab. 2012;37(4):798-802. doi: 10.1139/h2012-037 [DOI] [PubMed] [Google Scholar]
- 46. Nieves JW, Melsop K, Curtis M, et al. Nutritional factors that influence change in bone density and stress fracture risk among young female cross-country runners. PM R. 2010;2(8):740-750; quiz 794. doi: 10.1016/j.pmrj.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 47. Ogan D, Pritchett K. Vitamin D and the athlete: risks, recommendations, and benefits. Nutrients. 2013;5(6):1856-1868. doi: 10.3390/nu5061856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Owens DJ, Tang JC, Bradley WJ, et al. Efficacy of high-dose vitamin D supplements for elite athletes. Med Sci Sports Exerc. 2017;49(2):349-356. doi: 10.1249/MSS.0000000000001105 [DOI] [PubMed] [Google Scholar]
- 49. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pancar Z, Cinar V, Aydemir I, Bulguru B. The effect of vitamin D3 supplement with football training on glucose, insulin, cortisol and ACTH levels: vitamin supplement study. Pak J Med Health Sci. 2021;15(2):830-833. [Google Scholar]
- 51. Pludowski P, Holick MF, Grant WB, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018;175:125-135. doi: 10.1016/j.jsbmb.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 52. Rankinen T, Lyytikäinen S, Vanninen E, Penttilä I, Rauramaa R, Uusitupa M. Nutritional status of the Finnish elite ski jumpers. Med Sci Sports Exerc. 1998;30(11):1592-1597. [DOI] [PubMed] [Google Scholar]
- 53. Ribbans WJ, Aujla R, Dalton S, Nunley JA. Vitamin D and the athlete-patient: state of the art. J ISAKOS. 2021;6(1):46-60. doi: 10.1136/jisakos-2020-000435 [DOI] [PubMed] [Google Scholar]
- 54. RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. Accessed February 20, 2023. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 55. Rockwell MS, Frisard MI, Rankin JW, et al. Effects of seasonal vitamin D3 supplementation on strength, power, and body composition in college swimmers. Int J Sport Nutr Exerc Metab. 2020;30(2):165-173. doi: 10.1123/IJSNEM.2019-0250 [DOI] [PubMed] [Google Scholar]
- 56. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. doi: 10.1210/jc.2010-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shimasaki Y, Nagao M, Miyamori T, et al. Evaluating the risk of a fifth metatarsal stress fracture by measuring the serum 25-hydroxyvitamin D levels. Foot Ankle Int. 2016;37(3):307-311. doi: 10.1177/1071100715617042 [DOI] [PubMed] [Google Scholar]
- 58. Smith EM, Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):432-438. doi: 10.1097/MED.0000000000000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Srikuea R, Zhang X, Park-Sarge OK, Esser KA. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: potential role in suppression of myoblast proliferation. Am J Physiol Cell Physiol. 2012;303(4):C396-C405. doi: 10.1152/ajpcell.00014.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tomlinson PB, Joseph C, Angioi M. Effects of vitamin D supplementation on upper and lower body muscle strength levels in healthy individuals. A systematic review with meta-analysis. J Sci Med Sport. 2015;18(5):575-580. doi: 10.1016/j.jsams.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 61. von Hurst PR, Beck KL. Vitamin D and skeletal muscle function in athletes. Curr Opin Clin Nutr Metab Care. 2014;17(6):539-545. doi: 10.1097/MCO.0000000000000105 [DOI] [PubMed] [Google Scholar]
- 62. Williams K, Askew C, Mazoue C, Guy J, Torres-McGehee TM, Jackson JB, III. Vitamin D3 supplementation and stress fractures in high-risk collegiate athletes - a pilot study. Orthop Res Rev. 2020;12:9-17. doi: 10.2147/ORR.S233387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Żebrowska A, Sadowska-Krępa E, Stanula A, et al. The effect of vitamin D supplementation on serum total 25(OH) levels and biochemical markers of skeletal muscles in runners. J Int Soc Sports Nutr. 2020;17:18. doi: 10.1186/s12970-020-00347-8 [DOI] [PMC free article] [PubMed] [Google Scholar]