Abstract
The family Aoguangviridae includes dsDNA viruses that have been associated with marine archaea. Currently, members of this virus family are known through metagenomics. Virions are predicted to consist of an icosahedral capsid and a helical tail, characteristic of members in the class Caudoviricetes. Aoguangviruses have some of the largest genomes among archaeal viruses and possess most of the components of the DNA replication machinery as well as auxiliary functions. The family Aoguangviridae includes the species Aobingvirus yangshanense. Many unclassified relatives of this virus group, referred to as ‘magroviruses’, have been discovered by metagenomics in globally distributed marine samples. This is a summary of the International Committee on Taxonomy of Viruses (ICTV) Report on the family Aoguangviridae, which is available at ictv.global/report/aoguangviridae.
Keywords: ICTV Report, virus taxonomy, magroviruses, archaeal Caudoviricetes , marine archaea, Magrovirales
Virion
Members of the family Aoguangviridae have been discovered through metagenomics and have yet to be cultured [1–4] (Table 1). However, based on the conservation of the structural module typical of bacterial and archaeal viruses of the class Caudoviricetes [5] and including the HK97-fold major capsid protein, capsid maturation protease, large subunit of the terminase, portal, major tail protein, baseplate, etc., virions are predicted to consist of an icosahedral capsid and a helical tail. The lack of the gene encoding a tail sheath protein suggests that the tails of aoguangvirids are not contractile.
Table 1.
Characteristics of members of the family Aoguangviridae
|
Example |
Poseidoniales virus YSH_150918 (ON649702), species Aobingvirus yangshanense, genus Aobingvirus |
|---|---|
|
Virion |
Predicted to have virions characteristic of members of the class Caudoviricetes, with icosahedral capsids and helical tails |
|
Genome |
Linear, dsDNA genome of 92.2 kbp |
|
Replication |
Virus encoded DNA replisome, including family B DNA polymerase |
|
Translation |
Unknown |
|
Host range |
Marine archaea |
|
Taxonomy |
Realm Duplodnaviria, kingdom Heunggongvirae, phylum Uroviricota, class Caudoviricetes, order Magrovirales: the family includes the genus Aobingvirus and the species Aobingvirus yangshanense |
Genome
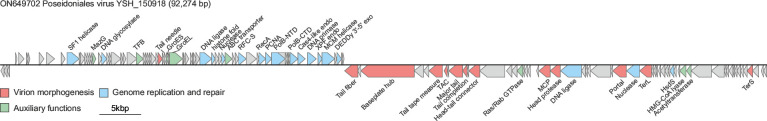
The genome of aoguangvirids is double-stranded DNA of 90–100 kbp, one of the largest described for archaeal viruses. The 92.2 kbp genome of virus YSH_150918 has been assembled as a circular molecule, suggesting terminal redundancy and/or circular permutation. The genome has a G+C content of 31.5 % and encodes 129 predicted proteins [3]. The genes encoding functions related to genome replication and virion morphogenesis are organized into two clusters which are predicted to be transcribed in opposite directions (Fig. 1).
Fig. 1.
Genome organisation of virus YSH_150918. Arrows depict open reading frames indicating the direction of transcription. Abbreviations: SF1, superfamily 1; TFB, transcription factor B; RFC-S, replication factor C, small subunit; PCNA, proliferating cell nuclear antigen/DNA polymerase sliding clamp; PolB-NTD/CTD, family B DNA polymerase, N-/C-terminal domain; endo, endonuclease; MCM, minichromosome maintenance; exo, exonuclease; TAC, tail assembly chaperone; MCP, major capsid protein; TerS/TerL, small/large subunit of the terminase; HsdS, type I restriction modification system specificity (S) subunit; HMG-CoA, β-Hydroxy β-methylglutaryl-CoA.
Replication
No member of the family Aoguangviridae has yet been cultured and so insights into their biology can be only gleaned from computational analysis of the virus genome. Aoguangvirids do not encode an identifiable integrase or transposase and are likely to lead a lytic lifestyle. A characteristic feature of aoguangvirids, also observed in other, unclassified members of the order Magrovirales, is the extensive set of genes required for genome replication and repair [1–3]. In particular, virus YSH_150918 encodes a family B DNA polymerase, archaeo-eukaryotic DNA primase, DNA polymerase sliding clamp, clamp loader, replicative minichromosome maintenance helicase, ATP-dependent DNA ligase, and other proteins [3]. Thus, it is likely that aoguangvirids replicate their genome with little, if any, requirement for host replication factors. Besides the genes required for genome replication and repair, as well as virion morphogenesis, aoguangvirids encode auxiliary functions implicated in protein folding (e.g., chaperonin GroEL and cochaperonin GroES) and modulation of the host metabolism (e.g., β-Hydroxy β-methylglutaryl-CoA lyase) (Fig. 1).
Taxonomy
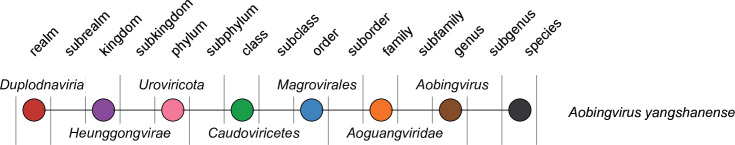
Current taxonomy: ictv.global/taxonomy. The family Aoguangviridae includes the genus Aobingvirus and the species Aobingvirus yangshanense (Fig. 2). Viruses in the family Aoguangviridae are most closely related to unclassified archaeal tailed viruses associated with marine archaea of the order Poseidoniales , formerly known as Marine Group II Euryarchaeota [1, 2], and more distantly related to viruses of the family Druskaviridae infecting hyperhalophilic archaea [6].
Fig. 2.
Relationships of the taxa connected to the family Aoguangviridae.
Resources
Full ICTV Report on the family Aoguangviridae: ictv.global/report/aoguangviridae.
Funding information
Production of this Profile, the ICTV Report, and associated resources was supported by the Microbiology Society.
Acknowledgements
We thank Stuart G. Siddell, Elliot J. Lefkowitz, Sead Sabanadzovic, Peter Simmonds, F. Murilo Zerbini, Evelien Adriaenssens, Jens H. Kuhn, Luisa Rubino, Arvind Varsani (ICTV Report Editors), and Donald B. Smith (Managing Editor, ICTV Report).
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Nishimura Y, Watai H, Honda T, Mihara T, Omae K, et al. Environmental viral genomes shed new light on virus-host interactions in the ocean. mSphere. 2017;2:e00359-16. doi: 10.1128/mSphere.00359-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philosof A, Yutin N, Flores-Uribe J, Sharon I, Koonin EV, et al. Novel abundant oceanic viruses of uncultured marine group II euryarchaeota. Curr Biol. 2017;27:1362–1368. doi: 10.1016/j.cub.2017.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Zhou L, Yan S, Chen L, Krupovic M, et al. Diverse viruses of marine archaea discovered using metagenomics. Environ Microbiol. 2023;25:367–382. doi: 10.1111/1462-2920.16287. [DOI] [PubMed] [Google Scholar]
- 4.Xu B, Fan L, Wang W, Zhu Y, Zhang C. Diversity, distribution, and functional potentials of magroviruses from marine and brackish waters. Front Microbiol. 2023;14:1151034. doi: 10.3389/fmicb.2023.1151034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baquero DP, Liu Y, Wang F, Egelman EH, Prangishvili D, et al. Structure and assembly of archaeal viruses. Adv Virus Res. 2020;108:127–164. doi: 10.1016/bs.aivir.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Demina TA, Roux S, Aiewsakun P, Kazlauskas D, et al. Diversity, taxonomy, and evolution of archaeal viruses of the class Caudoviricetes . PLoS Biol. 2021;19:e3001442. doi: 10.1371/journal.pbio.3001442. [DOI] [PMC free article] [PubMed] [Google Scholar]