Abstract
Objective
HbA1c is an insensitive marker for assessing real‐time dysglycemia in obesity. This study investigated whether 1‐h plasma glucose level (1‐h PG) ≥155 mg/dL (8.6 mmol/L) during an oral glucose tolerance test (OGTT) and continuous glucose monitoring (CGM) measurement of glucose variability (GV) better reflected dysglycemia than HbA1c after weight loss from metabolic and bariatric surgery.
Methods
This was a prospective cohort study of 10 participants with type 2 diabetes compared with 11 participants with non‐diabetes undergoing sleeve gastrectomy (SG). At each research visit; before SG, and 6 weeks and 6 months post‐SG, body weight, fasting lipid levels, and PG and insulin concentrations during an OGTT were analyzed. Mean amplitude of glycemic excursions (MAGE), a CGM‐derived GV index, was analyzed.
Results
The 1‐h PG correlated with insulin resistance markers, triglyceride/HDL ratio and triglyceride glucose index in both groups before surgery. At 6 months, SG caused 22% weight loss in both groups. Despite a reduction in HbA1c by 3.0 ± 1.3% in the diabetes group (p < 0.01), 1‐h PG, and MAGE remained elevated, and the oral disposition index, which represents pancreatic β‐cell function, remained reduced in the diabetes group when compared to the non‐diabetes group.
Conclusions
Elevation of GV markers and reduced disposition index following SG‐induced weight loss in the diabetes group underscores persistent β‐cell dysfunction and the potential residual risk of diabetes complications.
Keywords: continuous glucose monitoring, diabetes, glycemic variability, obesity, sleeve gastrectomy
This was a prospective cohort study that investigated whether glycemic variability makers: 1‐h plasma glucose levels during an oral glucose tolerance test and continuous glucose monitoring derived measurements of glucose variability reflected dysglycemia after weight loss from metabolic and bariatric surgery. Individuals with and without type 2 diabetes who underwent weight loss by sleeve gastrectomy (SG) were recruited. At 6 months, SG caused 22% weight loss in both groups. Despite the marked reduction in HbA1c in the diabetes‐group, glycemic variability markers remained elevated when compared with the non‐diabetes group. The oral disposition index, which represents pancreatic β‐cell function, remained reduced in the dīiabetes‐group after SG. Our results suggest that glycemic variability markers and disposition index reflect glycemic status better than HbA1c after metabolic and bariatric surgery.
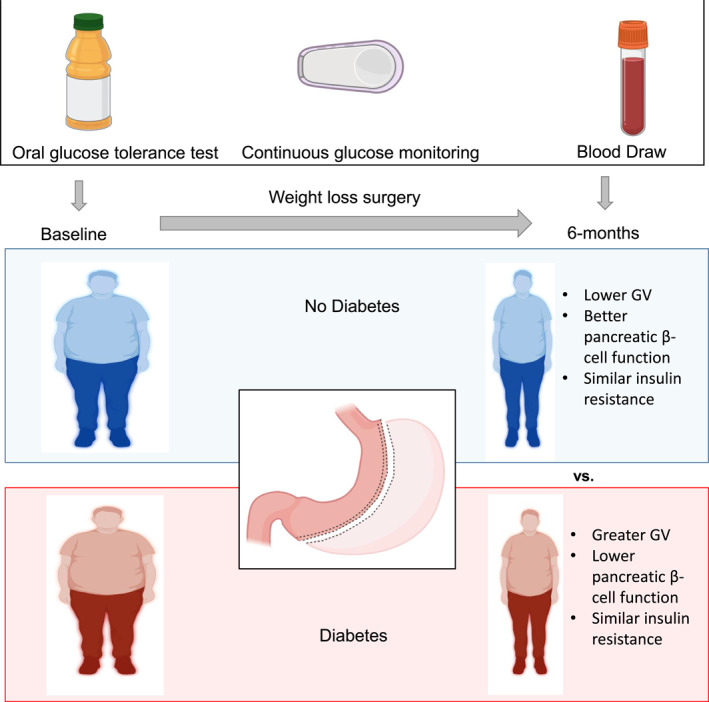
1. INTRODUCTION
Obesity leads to increased risk of diabetes and its complications such as cardiovascular disease (CVD). HbA1c is commonly used to screen for prediabetes (defined as 5.7%–6.4% [39–46 mmol/mol]) and type 2 diabetes (defined as ≥6.5% [48 mmol/mol]). 1 The measurement of HbA1c does not require fasting, capture hyperglycemia over several weeks, and has pre‐analytical stability. However, HbA1c has poor sensitivity for identifying early dysglycemia. 2 It does not provide a clear picture of day‐to‐day glucose patterns during real life settings, especially postprandial glycemia, and is insensitive for the identification of early insulin resistance and β‐cell dysfunction. 3 Variables such as age, race, anemia, chronic kidney disease, and hemoglobin variants can falsely elevate or lower HbA1c. 3 Thus, additional markers are needed to identify dysglycemia, especially after metabolic and bariatric surgery. As 30%–50% of patients who initially have diabetes remission have diabetes recurrence 3–15 years after metabolic and bariatric surgery, 4 identification of dysglycemia would allow for earlier intervention.
An emerging marker for glycemic status in lieu of HbA1c is glycemic variability (GV), which represents daily fluctuations in glucose. GV is associated with inflammation as well as mortality in diabetes. 5 Continuous glucose monitoring (CGM) was recently deployed to evaluate type 2 diabetes glycemic concentrations following sleeve gastrectomy (SG), but few studies have evaluated such changes in normoglycemic obesity. 6 Whether GV can be an alternative to HbA1c for assessing glycemic status, after metabolic and bariatric surgery, is unclear. Identifying dysglycemia after metabolic and bariatric surgery allows for earlier prescription of lifestyle modification or medications to lower glucose concentrations and to modify CVD risk factors. 7 , 8 , 9
A 1‐h plasma glucose (1‐h PG) ≥155 mg/dL (8.6 mmol/L) during an oral glucose tolerance test (OGTT) is more predictive than HbA1c or 2‐h PG for future development of diabetes, CVD, and mortality. 8 , 10 , 11 , 12 Another alternative approach for detecting dysglycemia is the implementation of CGM to identify increased GV. Elevated GV indices such as mean amplitude of glycemic excursions (MAGE), calculated from CGM‐derived interstitial glucose concentrations, are potential surrogate markers for early pancreatic β‐cell dysfunction. 13 , 14 In a previous study, both 1‐h PG during the OGTT and CGM‐derived GV indices identified individuals with dysglycemia despite having normal HbA1c. 15 In addition, both 1‐h PG and CGM during an OGTT can detect elevated GV not captured by HbA1c.
This prospective longitudinal cohort study aimed to examine GV in individuals with and without diabetes, and assess the effects of laparoscopic SG on glycemic status during acute weight loss at 6 weeks (W) and chronic weight loss at 6‐month (M) post‐SG by measuring: (1) 1‐h PG during an OGTT, and (2) CGM‐derived GV index: MAGE. Also, this study determined whether 1h‐PG correlated with insulin resistance markers–Homeostatic Model Assessment of Insulin Resistance (HOMA‐IR), triglyceride/high‐density lipoprotein (HDL) ratio, and triglyceride glucose index before and after surgery. Finally, the hypothesis that weight loss from SG would be less impactful in reducing GV than with HbA1c in individuals with diabetes was examined.
2. METHODS
This study is a prospective longitudinal cohort study that enrolled participants with obesity between 2017 and 2020, who were eligible for SG, at NYU Langone Health and Bellevue Hospital. Informed consent was obtained from each participant before the start of the study. The inclusion criteria included age 18–75 years old and candidates for SG. Participants with HbA1c <5.7% (39 mmol/mol) with no history of diabetes or not on any glucose lowering medication were assigned to the non‐diabetes group. Participants with noninsulin ‐ dependent diabetes and HbA1c ≥ 6.5% were in the diabetes group. Exclusion criteria included a history of CVD and stroke. Participants who were taking insulin were also excluded. Other exclusion criteria are listed in Table S1.
2.1. Study protocol
2.1.1. Visits
Participants underwent a baseline metabolic evaluation before surgery, including clinical evaluation, OGTT, and CGM placement. Recruitment and retention details are presented in Figure 1. Subsequent to SG, the same metabolic evaluations occurred at 6W during active weight loss, as well as at 6M during chronic weight loss.
FIGURE 1.
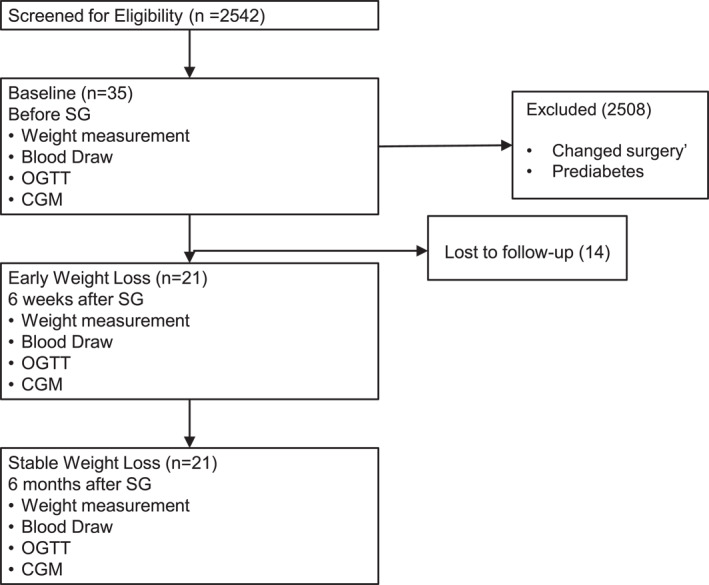
Clinical research recruitment flowchart.
2.1.2. Anthropomorphic measurements
Body weight was measured using a digital bariatric scale (Scale‐Tronix®, Welch Allyn. 5202‐X) after shoes and heavy outer clothing were removed. Height was measured to the nearest 0.25 inch using a standard height bar. Body mass index (BMI) was calculated as weight (kg)/height (m2).
2.1.3. Glycemic analysis
Prediabetes was defined by HbA1c 5.7%–6.4% (3–46 mmol/mol), impaired fasting glucose (100–125 mg/dL [5.6–6.9 mmol/L]) and/or impaired glucose tolerance (2‐h PG 140–199 mg/dL [7.8–11.0 mmol/L]). In addition to HbA1c, GV and response to a standard glycemic load were assessed. A 2‐h, 75‐g OGTT was performed at baseline, 6W, and 6M. After an overnight fast for 8–12 h, PG and insulin concentrations were drawn fasting for 1 and 2 h. The glucose area under the curve (AUC) was calculated using the trapezoid method. 16
Abbott Freestyle Libre Pro CGM (Abbott Park) was placed on the back of the arm at each visit. Participants were instructed to wear the CGM for a 14‐day period and continue their usual activities, including dietary and exercise regimens. CGM interstitial glucose concentrations were analyzed. GV index, MAGE, and other GV indices were calculated from CGM interstitial glucose using EasyGV© software (University of Oxford, www.easygv.co.uk) and calculations were previously described. 17
2.1.4. Insulin resistance and sensitivity analysis
Homeostatic Model Assessment of Insulin Resistance was analyzed to approximate insulin resistance, Matsuda index to represent whole body insulin sensitivity, and oral disposition index that measures β‐cell compensation for insulin resistance. 18 These values were calculated from PG and insulin values obtained during a 75‐g 2‐h OGTT, during times 0, 30, 60, 90, and 120 min. HOMA‐IR, Matsuda index, and oral disposition index values were obtained from an online calculator at: http://mmatsuda.diabetes‐smc.jp/MIndex.html.
2.2. Statistics
Baseline and post‐SG 6W and 6M data were compared using repeated measures analyses of variance to measure changes in data over time. For repeated measures analyses, Sphericity Assumed measurements were used. If the assumption of sphericity was violated with a p value <0.05, Greenhouse‐Geisser correction was used. Bonferroni adjustment was applied for post‐hoc pairwise comparisons and to account for multiple comparisons. Chi‐square tests were used to compare categorical variables between groups. Spearman correlation was used to measure associations between the two variables. Groups were compared using independent two‐tailed t‐tests. Data are reported as mean ± SD unless otherwise stated. Statistical analyses were conducted using SPSS version 28.0 (IBM SPSS Statistics), with alpha concentration set at p < 0.05 and GraphPad (9.4.1 for Windows, GraphPad Software) for graphs.
The NYU Langone Health Institutional Board Review approved the bariatric cohort protocol and related sub‐studies under number s16‐01995.
3. RESULTS
3.1. Weight reduction was equivalent between the non‐diabetes and diabetes group following SG
The baseline characteristics of the 21 participants are shown in Table 1. There were 11 participants in the non‐diabetes group and 10 participants in the diabetes group. The proportion of participants who were male and taking antihypertensive medications did not differ between the non‐diabetes and diabetes groups. The number of participants and ethnic breakdown was similar between the two groups.
TABLE 1.
Baseline characteristics.
| Non‐diabetes group (n = 11) | Diabetes group (n = 10) | |
|---|---|---|
| Age, years | 40 ± 12 | 49 ± 8 |
| Ethnicity, n (%) | ||
| Hispanic | 5 (45.5) | 5 (50) |
| White | 3 (27.3) | 3 (30) |
| Black | 3 (27.3) | 1 (10) |
| Sex, n (%) | ||
| Male, n (%) | 3 (27.3) | 5 (50) |
| Antihypertensive medication, n (%) | 4 (33) | 6 (60) |
| Lipid lowering medication, n (%) | 0 (0) | 4 (40)* |
| Aspirin, n (%) | 2 (18) a | 0 (0) |
Note: Age was compared using an independent t‐test, and there were no statistical group differences in age. The between proportions of all other variable were compared using Chi‐squared tests.
Aspirin was held 3 days before phlebotomy.
*p < 0.05; comparing non‐diabetes group with diabetes group proportions (Chi‐squared test).
The magnitude of weight loss from SG in each group was compared. After 6M, in most series, the average weight loss is 20%–30%. 19 There were no significant differences between BMI at baseline and weight loss percentage between both groups at 6W and 6M (Figure 2A and Table 2). In the non‐diabetes group, mean weight decreased by 22 ± 9.4% and BMI by 9 ± 2.0 kg/m2 (Figure 2A) at 6M from baseline. The weight in the diabetes group at 6M decreased by 22 ± 5% and BMI by 8.9 ± 2.0 kg/m2 from baseline. In summary, 22% weight loss from baseline to 6M was equivalent in both groups.
FIGURE 2.
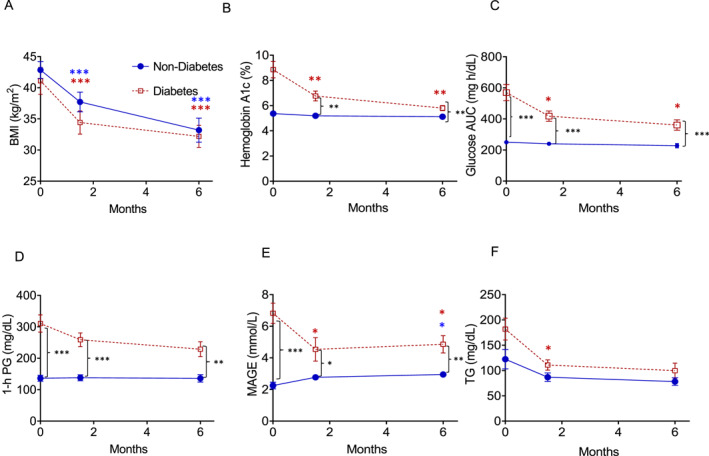
Weight reduction is equivalent between both non‐diabetes and diabetes groups after SG, but glycemic variability remains elevated in the diabetes group. Trends for (A) BMI, (B) hemoglobin A1c changes at 6W and 6M, in both the non‐diabetes and diabetes groups, (C) glucose AUC, and (D) 1‐h plasma glucose during an OGTT, and (E) mean MAGE levels. Blue circles: non‐diabetic, red squares: diabetes. In (A–D), n = 10 diabetes participants and n = 11 in the non‐diabetic participants. In (E), n = 9 diabetes participants, and n = 9 non‐diabetes participants (missing data). Error bars: Mean ± SEM. Significant differences compared to baseline *p ≤ 0.05, **p < 0.01, ***p < 0.001. Asterisks: blue—non‐diabetic group, post‐hoc Bonferroni within group comparisons, red—diabetes group; post‐hoc Bonferroni within group comparisons, black—inter‐intergroup comparisons, independent t‐test. 6M, 6M after SG; AUC, area under the curve; B, baseline; BMI, body mass index; MAGE, mean amplitude of glycemic excursions; OGTT, oral glucose tolerance test; SG, sleeve gastrectomy.
TABLE 2.
Circulating glycemic concentrations before and after sleeve gastrectomy.
| Non‐diabetes group n = 11 | Within group effect of time | Diabetes group n = 10 | Within group effect of time | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6W | 6M | Baseline | 6W | 6M | |||
| Weight (lbs.) | 262.1 ± 34.2 | 229.4 ± 33.2*** | 204.3 ± 41.2*** | F = 62.4; p < 0.001 | 254.2 ± 40.6 | 217.3 ± 34.1*** | 198.4 ± 34.9*** | F = 80.6, p < 0.001 |
| BMI (kg/m2) | 42.8 ± 4.5 | 37.7 ± 5.2*** | 33.2 ± 6.4*** | F = 62.4, p < 0.001 | 41.4 ± 6.2 | 34.9 ± 5.5*** | 32.6 ± 5.2*** | F = 80.6, p < 0.001 |
| HbA1c (%) | 5.4 ± 0.3 | 5.2 ± 0.3* | 5.1 ± 0.4* | F = 4.9, p = 0.02 | 8.9 ± 2.0††† | 6.8 ± 1.2***,††† | 5.8 ± 0.6**,††† | F = 15, p = 0.01 |
| Fasting glucose (mg/dl) | 85 ± 6.5 | 82 ± 7.4 | 80 ± 6.9 | F = 3.1, p = 0.07 | 187 ± 74††† | 108 ± 27.5**,†† | 94 ± 12.9**,†† | F = 11.3, p = 0.01 |
| 30‐min glucose (mg/dL) | 132 ± 20.0 | 148 ± 17* | 147 ± 31 | F = 4.1, p = 0.03 | 265 ± 71††† | 229 ± 49††† | 216 ± 39††† | NS |
| 1‐h glucose (mg/dL) | 136 ± 33.7 | 138 ± 30.5 | 136 ± 39.6 | NS | 310 ± 87.0††† | 259 ± 68.9††† | 229 ± 74†† | F = 3.7, p = 0.078 |
| 2‐h glucose (mg/dL) | 121 ± 21.1 | 85 ± 20* | 65 ± 24** | F = 21.6, p < 0.001 | 325 ± 85.7††† | 164 ± 61.6**,††† | 133 ± 65.9**,†† | F = 21.1, p < 0.001 |
| Glucose AUC (mg h/dL) | 250 ± 34.6 | 240 ± 31.4 | 228 ± 45.9 | NS | 569 ± 163.9††† | 418 ± 105.6*,††† | 361 ± 106**,†† | F = 7.1, p = 0.003 |
| Impaired fasting glucose n (%) | 0 (0) | ‐ | 0 (0) | 10 (100) | ‐ | 4 (40) | ||
| Impaired glucose tolerance n (%) | 2 (18.2%) | ‐ | 0 (0) | 10 (100) | ‐ | 4 (40) | ||
| Diabetes remission | ‐ | ‐ | ‐ | ‐ | ‐ | 5/10 | ||
Abbreviation: NS, not significant.
Post‐hoc Bonferroni comparisons: *p < 0.05, compared to baseline data, within group comparison; **p < 0.01, compared to baseline, within group comparison; ***p < 0.001, compared to baseline, within group comparison. Group Comparisons: † p < 0.05, group comparison at the same time point; †† p < 0.01, group comparison at the same time point; ††† p < 0.001, group comparison at the same time point.
3.2. One hour plasma glucose and MAGE remained elevated in the diabetes group after SG
The aim was to examine the differences in glycemic values after SG between both groups. A previous study found reductions in fasting and 2‐h glucose concentrations after Roux‐en‐Y gastric bypass (RYGB) in individuals with normal glucose tolerance during an OGTT. 20 However, few studies compare 1‐h PG concentrations and GV indices after weight loss from metabolic and bariatric surgery in individuals with HbA1c <5.7% (39 mmol/mol). In this study, mean HbA1c remained greater in the diabetes group when compared to the non‐diabetes group at 6W (p < 0.001) and 6M (p < 0.01). Both groups displayed reductions in HbA1c (Figure 2B), and this decrease was greater in the diabetes group. SG effectively reduced HbA1c from 5.4 ± 0.3% to 5.1 ± 0.4% at 6M (p < 0.05) in the non‐diabetes group. HbA1c was reduced to a greater degree in the diabetes group from 8.9 ± 2.0% to 5.8 ± 0.6% at 6M (p < 0.01).
Glucose AUC (Figure 2C), which was lower in the non‐diabetes group compared with the diabetes group at baseline, was examined. Glucose AUC within the non‐diabetes group did not decrease following SG. However, glucose AUC in the non‐diabetes group was lower at each time point compared with the diabetes group. Although SG effectively induced weight loss and reduced HbA1c in both groups, glucose AUC remained elevated in the diabetes group when compared with the non‐diabetes group.
An OGTT with 1‐h PG ≥155 mg/dL (8.6 mmol/L) correlates with increased progression to type 2 diabetes, CVD risk profile, and mortality. 21 , 22 In this study, mean 1‐h PG in the diabetes group decreased from a baseline concentration of 310 ± 87.0 mg/dL (p < 0.05) to 228.7 ± 74 mg/dL (Figure 2D), which is more than the 1‐h PG of 136.0 ± 39.6 mg/dL in the non‐diabetes group at 6M (p < 0.05). Mean 1‐h PG in the non‐diabetes group remained <155 mg/dL (8.6 mmol/L) and was unchanged from baseline, whereas mean 1‐h PG improved but remained elevated in the diabetes group when compared to the non‐diabetes group after SG.
Individuals with normal glucose tolerance defined by current criteria [fasting (<100 mg/dL [5.55 mmol/L]) and 2‐h PG (<140 mg/dL [7.77 mmol/L])] during an OGTT may have 1‐h PG ≥ 155 mg/dL (8.6 mmol/L). Normal glucose tolerance by standard definition does not include 1‐h PG. 23 , 24 In the diabetes group, five of 10 patients in the diabetes group achieved remission 6M after SG, defined as HbA1c <5.7%; three were not receiving diabetes medications; one remained on metformin and one on a GLP‐1 agonist. However, 9 of 10 (90%) participants had 1‐h PG ≥155 mg/dL (8.6 mmol/L) 6M after weight loss, but four of these patients had both HbA1c <5.7% (39 mmol/mol) and normal glucose tolerance during OGTT; thus, diabetes risk remains in some patients who may not be detected using HbA1c alone.
Glucose values during OGTT in the non‐diabetes group were analyzed. Although all participants had HbA1c <5.7%, 2 of 11 (18%) had prediabetes defined as 2‐h PG >140 mg/dL or impaired glucose tolerance (7.77 mmol/L) at baseline (Table 2). Additionally, 1‐h PG was elevated in a subset of patients with normal glucose tolerance. In this study, 5 of 11 (45%) participants with non‐diabetes had 1‐h PG ≥155 mg/dL (8.6 mmol/L), even though four of these participants had a normal glucose tolerance test at baseline. At 6M, three of five participants with elevated 1‐h PG at baseline had improved 1‐h PG to <155 mg/dL (8.6 mmol/L). Thus, 1‐h PG can be elevated before and after metabolic and bariatric surgery in participants with both HbA1c <5.7% and normal glucose tolerance test. A majority of participants in the diabetes group compared to the non‐diabetes group had elevated 6M 1‐h PG despite improvement in HbA1c; thus, dysglycemia persisted after weight loss from metabolic and bariatric surgery.
3.3. CGM‐derived GV Index—MAGE remained elevated after SG in individuals with diabetes
As OGTT measures static glucose concentrations after a high glucose load, changes in CGM‐derived GV indices during “free‐living” conditions were compared in both groups. Although the non‐diabetes group in this study showed a slight increase in the GV index, MAGE, at 6M, GV remained significantly lower than that in the diabetes group. MAGE decreased at 6W and 6M (Figure 2E) in the diabetes group, but remained above the reference range value (0–2.8). Other GV indices were elevated in the diabetes group when compared to the non‐diabetes group 6M after SG (Table 3).
TABLE 3.
Weight loss from SG reduces CGM‐derived glycemic variability indices in diabetes.
| Non diabetes n = 11 | Diabetes n = 10 | Reference range 17 | |||
|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | ||
| Standard deviation (SD) | 0.92 ± 0.3 | 1.10 ± 0.2 | 2.8 ± 0.8††† | 1.9 ± 0.6* | 0.0–3.0 |
| Continuous overall net glycemic action (CONGA) | 4.5 ± 0.5 | 4.3 ± 0.5 | 10.3 ± 4.7†† | 5.0 ± 0.8**,† | 3.6–5.5 |
| Lability index (LI) | 1.3 ± 0.8 | 2.1 ± 0.8 | 11.7 ± 5.4††† | 7.4 ± 4.0*,††† | 0.0–4.7 |
| J‐index | 12.54 ± 4.0 | 13.1 ± 2.7 | 84.2 ± 60.14†† | 24.1 ± 10**,†† | 4.7–23.6 |
| Low blood glucose index (LBGI) | 3.2 ± 1.6 | 3.5 ± 2.4 | 0.9 ± 1.2†† | 2.4 ± 1.4†† | 0.0–6.9 |
| High blood glucose index (HGBI) | 0.7 ± 0.5 | 1.1 ± 0.5 | 19.7 ± 17.6†† | 3.7 ± 2.1**,†† | 0.0–7.7 |
| Glycemic risk assessment in diabetes equation (GRADE) | 0.7 ± 0.66 | 0.7 ± 0.5 | 14.8 ± 10.3††† | 1.9 ± 2.2**,††† | 0.0–4.6 |
| Mean amplitude of glycemic excursion (MAGE) | 2.25 ± 0.6 | 3.0 ± 0.5* | 6.8 ± 2.0††† | 4.9 ± 1.5**,†† | 0.0–2.8 |
Abbreviations: CGM, continuous glucose monitoring; SG, sleeve gastrectomy.
*p < 0.05; 6 months compared to baseline, within group comparisons. †† p < 0.01, group comparison at the same time point. ††† p < 0.001, group comparison at the same time point.
Additionally, baseline 1‐h PG correlated with baseline MAGE (ρ = 0.787, p < 0.001) and HbA1c (ρ = 0.83, p = <0.001) and 6M 1‐h PG correlated with 6M MAGE (ρ = 0.87, p < 0.001) and HbA1c (ρ = 0.45, p = 0.04) after SG in both groups combined. In summary, SG reduced glucose AUC, but 1‐h PG and MAGE did not normalize in the diabetes group, suggesting that residual diabetes risk remains after SG in individuals with diabetes.
3.4. Weight loss improved insulin sensitivity in the diabetes group
Plasma insulin collected during OGTT was analyzed to assess changes in insulin resistance after SG in both groups (Table 4). Previous studies suggested that insulin sensitivity improves early after weight loss from metabolic and bariatric surgery in patients with normal glucose tolerance and T2D. 20 , 25 , 26 In this study, fasting insulin concentrations at baseline visits were statistically similar between both groups. After an oral glucose load at baseline visit, 1‐h insulin concentrations increased in both groups (p < 0.001), but were 52% greater in the non‐diabetes group than in the diabetes group. The non‐diabetes group demonstrated insulin concentrations that were 54% greater than the diabetes group, 2‐h after the glucose load during baseline visits (p < 0.05). Thus, the non‐diabetes group secreted more insulin than the diabetes group after a glucose load at baseline. After 6M weight loss, although insulin concentrations improved in the diabetes group and were similar to those in the non‐diabetes group (Table 4), this did not sufficiently reduce glucose concentrations to match the concentrations observed in the non‐diabetes group. As the circulating glucose concentration was elevated in the diabetes group, more pancreatic insulin secretion to compensate for the glucose concentrations was expected but not observed.
TABLE 4.
Circulating insulin levels before and after sleeve gastrectomy.
| Non‐diabetes n = 8 | Within group effect of time | Diabetes n = 8 | Within group effect of time | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6W | 6M | Baseline | 6W | 6M | |||
| Fasting insulin (μU/mL) | 10.7 ± 5.3 | 6.2 ± 4* | 5.0 ± 2.5* | F = 7.5, p = 0.01 | 17.4 ± 11.2 | 9.2 ± 5.1 | 6.7 ± 3.8* | F = 5.7, p = 0.02 |
| 30‐min insulin (μU/mL) | 105.3 ± 95 | 109.1 ± 149 | 122.2 ± 92.8 | NS | 34.1 ± 20† | 64.5 ± 42.0* | 79.8 ± 44.2* | F = 5.8, p = 0.01 |
| 1‐h insulin (μU/mL) | 82.3 ± 35.1 | 115.2 ± 166.8 | 96.4 ± 107 | NS | 38.7 ± 27.2† | 83.5 ± 65.8 | 80.3 ± 29.3 | NS |
| 2‐h insulin (μU/mL) | 80.03 ± 53.4 | 90.7 ± 208 | 12 ± 7.4 | NS | 36.6 ± 33.1† | 44.1 ± 33.7 | 18.9 ± 14.2 | NS |
| Insulin AUC (uU h/mL) | 161 ± 71.4 | 133 ± 123 | 140 ± 95 | NS | 74 ± 37.7 | 129 ± 82.0 | 112 ± 39.0 | NS |
| Matsuda index | 3.8 ± 1.8 | 6.1 ± 3.0 | 6.4 ± 2.0 | NS | 2.7 ± 2.0 | 3.7 ± 2.2 | 4.6 ± 2.1** | F = 3.9, p = 0.046 |
| HOMA‐IR | 2.5 ± 1.4 | 1.4 ± 0.8* | 1.0 ± 0.5* | F = 7.5, p = 0.01 | 8.4 ± 6.5†† | 2.8 ± 2.0*,† | 1.7 ± 1.0* | F = 10.2, p = 0.01 |
| Disposition index | 4.4 ± 3.6 | 8.3 ± 4.7 | 11.7 ± 10.3* | F = 5.2, p = 0.05 | 0.9 ± 1.5† | 1.7 ± 2.2††† | 3.5 ± 2.3*,† | F = 5.2, p = 0.02 |
Abbreviation: NS, not significant.
Post‐hoc Bonferroni comparisons: *p < 0.05, compared to baseline data, within group comparison. **p < 0.01, compared to baseline, within group comparison. ***p < 0.001, compared to baseline, within group comparison.
Group Comparisons: † p < 0.05, group comparison at the same time point. †† p < 0.01, group comparison at the same time point. ††† p < 0.001, group comparison at the same time point.
Disposition index, which represents pancreatic β‐cell function, was analyzed (Table 4), with low concentrations consistent with inadequate insulin secretion to compensate for insulin resistance. 27 The disposition index in the non‐diabetes group increased from a baseline at 6M (reference range ≥1.24) (Table 4). Although the diabetes group disposition index increased (p < 0.01) at 6M from baseline, it was lower than that in the non‐diabetes group (p < 0.05). Diminished pancreatic β‐cell insulin secretion accounts for the residual dysglycemia observed in the diabetic group.
Metabolic and bariatric weight loss decreased insulin sensitivity as measured by HOMA‐IR (Table 4), a measure of hepatic insulin resistance, decreased (reference range <2.5) at 6W and 6M in both groups, and there was no difference between the two groups after weight loss. The Matsuda index, an index of whole body insulin sensitivity 28 , increased by 70% 6M after SG in the diabetes group. There were no differences in Matsuda indices between the diabetes versus the non‐diabetes groups after SG. Hence, SG improved insulin resistance and whole body insulin sensitivity in the diabetes group to concentrations similar to those in the non‐diabetes group.
Collectively, these data corroborate previous studies that demonstrated improvement in insulin sensitivity after weight loss. 28 Inadequate β‐cell compensation explains residual dysglycemia in the diabetes group after 6M of weight loss and not insulin resistance.
3.5. Triglyceride/HDL and triglyceride glucose index correlated with 1‐h PG only at baseline
Circulating lipoprotein profiles were measured at the three determined time points (Table 5). Individuals with diabetes have higher total cholesterol, low‐density‐lipiprotein (LDL) cholesterol, and triglycerides, but lower HDL cholesterol than those with non‐diabetes. 29 In this study, despite equivalent baseline weight, the only clinical lipid parameter that differed between the non‐diabetes and diabetes groups was baseline concentrations of triglycerides (Table 5). The average baseline triglyceride of 182 ± 61 mg/dL was 39% greater in the diabetes group than the concentration of 122 ± 63.6 mg/dL in the non‐diabetes group (p = 0.056). There was no difference in baseline total cholesterol, HDL cholesterol, or LDL cholesterol.
TABLE 5.
Circulating lipid levels before and after sleeve gastrectomy.
| Non‐diabetes n = 11 | Within group effects of SG | Diabetes N = 8 | Within group effects of SG | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6W | 6M | Baseline | 6W | 6M | |||
| Total cholesterol (mg/dL) | 183 ± 37 | 150 ± 29** | 173 ± 39 | F = 10.6, p < 0.001 | 182 ± 42 | 165 ± 32 | 174 ± 30 | NS |
| Triglycerides (mg/dL) | 122 ± 63.6 | 87 ± 28.1* | 78 ± 25.0* | F = 5.2, p = 0.03 | 182 ± 61 | 111 ± 32* | 100 ± 47* | F = 7.2, p = 0.01 |
| HDL (mg/dL) | 51 ± 21.3 | 44 ± 10.9 | 54 ± 12.7‡ | F = 4.2, p = 0.029 | 40 ± 8.4 | 36 ± 4.5† | 53 ± 8.0* | F = 29, p < 0.001 |
| LDL (mg/dL) | 109 ± 29 | 88 ± 22.1** | 103 ± 31.7 | F = 5.6, p = 0.01 | 105 ± 33.4 | 107 ± 31.1 | 101 ± 21.6 | NS |
Abbreviation: NS, not significant.
Post‐hoc Bonferroni comparison: *p < 0.05, compared to baseline, within group comparison. **p < 0.01, compared to baseline, within group comparison. † p < 0.05, group comparison at the same time point. ‡ p < 0.05, increase in HDL at 6M compared to 6W in the non‐diabetic group.
Whether weight loss—known to reduce triglycerides, lower LDL cholesterol and raise HDL cholesterol 30 —had similar effects in both groups after SG was examined. Triglyceride concentrations demonstrated the greatest decrease after SG in both groups (Figure 2F). Triglyceride concentrations decreased by 29% in the non‐diabetes group (p < 0.05) and by 39% (p < 0.05) in the diabetes group at 6W. Further reductions in triglycerides occurred at 6M. Triglyceride concentrations were the same at 6M between both groups, and decreased by 44% (p < 0.05) from baseline in the non‐diabetes group and by 58% (p < 0.05) in the diabetes group. Hence, triglyceride concentrations in the diabetes group were reduced after SG almost to the same concentration as the non‐diabetes group at 6M. Also, 1‐h PG correlated with baseline triglyceride concentrations at baseline in both groups (Figure S1A,D).
HDL cholesterol decreased during active weight loss, but increased back to or greater than the baseline during weight stabilization. 31 In this study, HDL cholesterol decreased by 13.6% in the non‐diabetes group (p = 0.086), and by 11% in the diabetes group (p = 0.062) after 6W; however, these reductions did not meet statistical significance (Table 5). HDL concentrations recovered to baseline concentrations at 6M in the non‐diabetes group, and the HDL in the diabetes group improved from baseline by 25% (p < 0.05). Thus, HDL showed a biphasic response after SG.
The effects of SG on LDL cholesterol and total cholesterol concentrations were analyzed. A previous meta‐analysis revealed that total cholesterol and LDL cholesterol concentrations did not significantly decrease 1 year after SG, although these concentrations were reduced after RYBG. 30 SG reduced total cholesterol at 6W in both groups (Table 5). However, at 6M, LDL and total cholesterol returned to baseline values in both groups. Also, similar to previous findings, 30 total cholesterol and LDL concentrations did not change at 6M compared to baseline in both groups. Additionally, there were no correlations between 1‐h PG and total cholesterol, LDL‐c, or HDL‐c at 6W and 6M after SG in both groups.
Recently, log transformed triglyceride/HDL ratio and triglyceride glucose index (a product of triglyceride and glucose concentrations) were identified as markers for insulin resistance, and cardiovascular and diabetes risk. 31 , 32 In concordance with published data, 1‐h PG positively correlated with baseline insulin resistance marker—log transformed triglyceride/HDL ratio in this study 31 (Figure S1B,E). Uniquely, this study showed that baseline 1‐h PG correlated with baseline triglyceride glucose index in both groups (Figure S1C,F). These associations were not present after weight loss from SG. This study showed that 1‐h PG correlates with other markers of insulin resistance, which are triglyceride/HDL and triglyceride glucose index at baseline; but this correlation is not present after metabolic and bariatric surgery and ensuing weight loss improves insulin sensitivity.
4. DISCUSSION
Although the effects of metabolic and bariatric surgery on insulin resistance are known, 20 this study provides several insights. Foremost, although the weight reduction from SG reduces PG concentrations and HbA1c, it is less impactful in reduction of circulating glucose concentrations and MAGE in individuals with diabetes. GV is pertinent because increased concentrations are associated with the development of diabetes complications. 5 Persistent elevation of GV indices following SG‐induced weight loss suggests the presence of residual risk of diabetes complications in those with diabetes despite HbA1c reduction. 22 Moreover, even at baseline, patients with obesity and normal HbA1c had abnormal 1‐h PG and GV.
SG reduced HbA1c in both groups but was an insensitive marker in determining GV or glucose homeostasis. 2 , 33 GV indices can be analyzed from CGM interstitial glucose values and have been associated with 1‐h PG ≥155 mg/dL (8.6 mmol/L). 33 In this study, a subset of participants in the diabetes group had HbA1c <5.7% (39 mmol/mol) but a 1‐h PG ≥155 mg/dL (8.6 mmol/L) 6M after SG. Additionally, mean 1‐h PG remained elevated in the diabetes group compared to that of the non‐diabetes group at 6M. Hence, individuals with T2D have significant dysglycemia after weight loss despite improvement in HbA1c to <5.7%, and dysglycemia may be assessed by determining GV. Although MAGE increased in the non‐diabetes group after chronic weight loss from SG, MAGE remained lower than that in the diabetes group. The cause of increased GV in the no‐diabetes group after metabolic and bariatric surgery despite this overall improvement in average glucose is not clear but might be an unexpected side effect of surgery. Metabolic and bariatric surgery modifies the anatomy and physiology of the gastrointestinal system, causing rapid gastric emptying and an increase in the postprandial incretin response, which leads to greater glucose peaks followed by lower nadirs. 34 , 35
Homeostatic Model Assessment of Insulin Resistance is a commonly used marker for insulin resistance in metabolic and bariatric surgery studies. 33 HOMA‐IR represents hepatic insulin sensitivity but underestimates IR in skeletal muscle because it uses fasting parameters. In contrast, Matsuda index, incorporates postprandial glycemia, assesses plasma insulin and glucose, and represents whole body insulin sensitivity including hepatic and skeletal muscle glucose disposition. 36 In this study, SG improved insulin resistance and whole body insulin sensitivity in the diabetes group to concentrations similar to those of the non‐diabetes group. Although 1‐h PG ≥155 mg/dl (8.6 mmol/L) is associated with elevated HOMA‐IR in obesity, 37 these parameters were the same between the groups in this study after SG. Insulin resistance and sensitivity improved in the diabetes group, but 1‐h PG remained elevated. Hence, insulin resistance was not the only contributor to elevated glucose concentrations and GV index after weight loss.
Disposition index represents the ability of pancreatic β‐cell insulin secretion to compensate for insulin resistance and takes into account the first phase insulin secretion. 38 Consistent with a previous study, this study shows that metabolic and bariatric surgery improves disposition index. 18 , 39 However, the mean disposition index remained lower in the diabetes group. Thus, this group had less pancreatic β‐cell insulin response to rising glucose concentrations than the non‐diabetes group. The study results imply that pancreatic β‐cell dysfunction contributed to increased GV in individuals with T2D after metabolic and bariatric surgery.
Differences in lipoprotein concentrations after SG were also assessed. SG had a greater effect on the triglyceride concentration lowering in both groups in parallel with insulin resistance improvement. Previous studies have reported that the triglyceride/HDL ratio, a marker of insulin resistance and diabetes risk, correlates with 1‐h PG. 31 This relationship occurred before metabolic and bariatric surgery in both groups, but the association did not remain after weight loss. Another marker of insulin resistance was examined, triglyceride glucose index, and correlated it to 1‐h PG. Fasting PG indicates insulin resistance in the liver, but fasting triglycerides better indicate insulin resistance in adipocytes. 31 Triglyceride glucose index, a product of triglycerides and PG, better reflects diabetes outcomes than glucose and triglyceride values alone. 32 Baseline 1‐h PG correlated with circulating triglyceride concentrations, and baseline 1‐h PG correlated with triglyceride glucose index in both groups. Furthermore, baseline triglyceride glucose index correlates with HOMA‐IR in the diabetes group. Additionally, 1‐h PG correlates with fasting circulating triglyceride concentrations. These relationships did not remain after metabolic and bariatric surgery. After weight loss from SG, insulin resistance markers including those from lipids improve, but 1‐h PG remained elevated in the diabetes group. Thus, lipids can be an effective surrogate marker for insulin resistance but only before weight loss from surgery.
This study had limitations. The overall sample size was small, limiting generalizability. Furthermore, the majority were women in the non‐diabetes group, but the proportion of men and women was not statistically different in both groups. Finally, chronic weight loss was analyzed at 6M, but was not re‐tested at a later time point. Behavioral factors such as sleep, diet, and exercise could also contribute to differences in glycemic and lipid concentrations. Body composition measurements would provide further insight as to the anthropometric changes following SG. 40 , 41
In summary, residual dysglycemia remained after SG‐induced weight loss in individuals with diabetes. The continued abnormality in GV markers, 1‐h PG and MAGE, as well as disposition index, supports the notion that HbA1c alone is insufficient to fully reflect glycemic status after surgical weight loss. Moreover, even with HbA1c reduction in individuals with diabetes, abnormal GV and likely residual CVD risk persist. Finally, the results of this study have implications for individuals without diabetes. Since the non‐diabetes group benefited from weight loss with improvements in HbA1c and triglyceride markers, early intensive interventions for weight loss, such as metabolic and bariatric surgery, should be considered, as circulating glucose concentrations may not completely normalize once diabetes ensues. Future prospective trials are needed to delineate the impact of different weight loss surgeries, such as SG and RYGB, compared to pharmacological weight loss on GV, and determine how elevations in GV after weight loss contribute to long‐term diabetes complications such as CVD.
AUTHOR CONTRIBUTIONS
Conceptualization and methodology: José O. Alemán, Ira J. Goldberg, Brenda Dorcely. Software: Brenda Dorcely. Formal analysis: Brenda Dorcely. Investigation: Brenda Dorcely, Julie DeBermont, Akash Gujral, Migdalia Reid, Sally M. Vanegas. Resources: Ira J. Goldberg. Data Curation: Migdalia Reid, Brenda Dorcely. Writing‐Original Draft: Brenda Dorcely. Writing–review & editing: Brenda Dorcely, Julie DeBermont, Akash Gujral, Migdalia Reid, Sally M. Vanegas, Collin J. Popp, Melanie Jay, Ann Marie Schmidt, Michael Bergman, Ira J. Goldberg, José O. Alemán. Visualization: Brenda Dorcely, Julie DeBermont. Supervision: José O. Alemán, Ira J. Goldberg. Project administration: José O. Alemán, Ira J. Goldberg, Sally M. Vanegas. Funding acquisition: José O. Alemán, Ira J. Goldberg, Melanie Jay, Ann Marie Schmidt.
CONFLICT OF INTEREST
The authors have declared no conflict of interest exists.
Supporting information
Supplementary material S1
Figure S1
Table S1
ACKNOWLEDGMENTS
The research team would like to thank the NYU Langone Health Clinical &Translational Science Institute; this research is supported in part by an NYU CTSA grant: UL1 TR001445 from the National Center for Advancing Translational Sciences, National Institutes of Health. The research team would like to thank all study participants and staff.
Dorcely B, DeBermont J, Gujral A, et al. Continuous glucose monitoring captures glycemic variability in obesity after sleeve gastrectomy: a prospective cohort study. Obes Sci Pract. 2024;e729. 10.1002/osp4.729
REFERENCES
- 1. Association AD . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. J Diabetes Care. 2020;43(Suppl 1):S14‐S31. 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- 2. Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1C. Diabetes Care. 2011;34(Suppl 2):S184‐S190. 10.2337/dc11-s216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergman M, Abdul‐Ghani M, Neves JS, et al. Pitfalls of HbA1c in the diagnosis of diabetes. J Clin Endocrinol and Metabolism. 2020;105(8):2803‐2811. 10.1210/clinem/dgaa372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shah A, Laferrère B. Diabetes after bariatric surgery. Can J Diabetes. 2017;41(4):401‐406. 10.1016/j.jcjd.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Z, Sun B, Huang S, Zhu C, Bian M. Glycemic variability: adverse clinical outcomes and how to improve it? Cardiovasc Diabetol. 2020;19(1):102. 10.1186/s12933-020-01085-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sawada S, Kodama S, Tsuchiya S, et al. Continuous glucose monitoring in patients with remission of type 2 diabetes after laparoscopic sleeve gastrectomy without or with duodenojejunal bypass. Clin Obes. 2020;10(6):e12409. 10.1111/cob.12409 [DOI] [PubMed] [Google Scholar]
- 7. Li G, Zhang P, Wang J, et al. The long‐term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20‐year follow‐up study. Lancet. 2008;371(9626):1783‐1789. 10.1016/s0140-6736(08)60766-7 [DOI] [PubMed] [Google Scholar]
- 8. Pareek M, Bhatt DL, Nielsen ML, et al. Enhanced predictive capability of a 1‐hour oral glucose tolerance test: a prospective population‐based cohort study. Diabetes Care. 2018;41(1):171‐177. 10.2337/dc17-1351 [DOI] [PubMed] [Google Scholar]
- 9. Bergman M, Dankner R, Roth J, Narayan KMV. Are current diagnostic guidelines delaying early detection of dysglycemic states? Time for new approaches. Endocrine. 2013;44(1):66‐69. 10.1007/s12020-013-9873-6 [DOI] [PubMed] [Google Scholar]
- 10. Cao L, Wang P, Luan H, et al. Elevated 1‐h postload plasma glucose levels identify coronary heart disease patients with greater severity of coronary artery lesions and higher risk of 1‐year re‐admission. Diabetes Vasc Dis Res. 2020;17(1):1479164119896978. 10.1177/1479164119896978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergman M, Chetrit A, Roth J, Dankner R. One‐hour post‐load plasma glucose level during the OGTT predicts mortality: observations from the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabet Med. 2016;33(8):1060‐1066. 10.1111/dme.13116 [DOI] [PubMed] [Google Scholar]
- 12. Jagannathan R, Sevick MA, Fink D, et al. The 1‐hour post‐load glucose level is more effective than HbA1c for screening dysglycemia. Acta Diabetol. 2016;53(4):543‐550. 10.1007/s00592-015-0829-6 [DOI] [PubMed] [Google Scholar]
- 13. Kohnert K‐D, Augstein P, Zander E, et al. Glycemic variability correlates strongly with postprandial beta‐cell dysfunction in a segment of type 2 diabetic patients using oral hypoglycemic agents. Diabetes Care. 2009;32(6):1058‐1062. 10.2337/dc08-1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohnert K‐D, Heinke P, Vogt L, Augstein, P , Salzsieder, E . Declining ß‐cell function is associated with the lack of long‐range negative correlation in glucose dynamics and increased glycemic variability: a retrospective analysis in patients with type 2 diabetes. J Clin Transl Endocrinol. 2014;1(4):192‐199. 10.1016/j.jcte.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorcely B, Sifonte E, Popp C, et al. Continuous glucose monitoring and 1‐h plasma glucose identifies glycemic variability and dysglycemia in high‐risk individuals with HbA1c < 5.7%: a pilot study. Endocrine. 2022;77(2):403‐407. 10.1007/s12020-022-03109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gagnon RC, Peterson JJ. Estimation of confidence intervals for area under the curve from destructively obtained pharmacokinetic data. J Pharmacokinet Biopharm. 1998;26(1):87‐102. 10.1023/a:1023228925137 [DOI] [PubMed] [Google Scholar]
- 17. Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Therapeut. 2011;13(9):921‐928. 10.1089/dia.2010.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2‐h glucose levels. Diabetes Care. 2009;32(2):335‐341. 10.2337/dc08-1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterli R, Wölnerhanssen BK, Peters T, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic Roux‐en‐Y gastric bypass on weight loss in patients with morbid obesity: the SM‐BOSS randomized clinical trial. JAMA. 2018;319(3):255‐265. 10.1001/jama.2017.20897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bojsen‐Møller KN, Dirksen C, Jørgensen NB, et al. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux‐en‐Y gastric bypass. Diabetes. 2014;63(5):1725‐1737. 10.2337/db13-1307 [DOI] [PubMed] [Google Scholar]
- 21. Bardini G, Dicembrini I, Cresci B, Rotella CM. Inflammation markers and metabolic characteristics of subjects with 1‐h plasma glucose levels. Diabetes Care. 2010;33(2):411‐413. 10.2337/dc09-1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bianchi C, Miccoli R, Trombetta M, et al. Elevated 1‐hour postload plasma glucose levels identify subjects with normal glucose tolerance but impaired β‐cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV study. J Clin Endocrinol Metabol. 2013;98(5):2100‐2105. 10.1210/jc.2012-3971 [DOI] [PubMed] [Google Scholar]
- 23. Jagannathan R, Sevick MA, Li H, et al. Elevated 1‐hour plasma glucose levels are associated with dysglycemia, impaired beta‐cell function, and insulin sensitivity: a pilot study from a real world health care setting. Endocrine. 2016;52(1):172‐175. 10.1007/s12020-015-0746-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Committee ADAPP . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(Suppl ment_1):S17‐S38. 10.2337/dc22-S002 [DOI] [PubMed] [Google Scholar]
- 25. Gastaldelli A, Iaconelli A, Gaggini M, et al. Short‐term effects of laparoscopic adjustable gastric banding versus Roux‐en‐Y gastric bypass. Diabetes Care. 2016;39(11):1925‐1931. 10.2337/dc15-2823 [DOI] [PubMed] [Google Scholar]
- 26. Rosen CJ, Ingelfinger JR. Bariatric surgery and restoration of insulin sensitivity ‐ it's weight loss. N Engl J Med. 2020;383(8):777‐778. 10.1056/NEJMe2024212 [DOI] [PubMed] [Google Scholar]
- 27. Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of β‐cell function. Hyperbolic Correct. 2002;51(Suppl 1):S212‐S220. 10.2337/diabetes.51.2007.S212 [DOI] [PubMed] [Google Scholar]
- 28. Douros JD, Tong J, D’Alessio DA. The effects of bariatric surgery on islet function, insulin secretion, and glucose control. Endocr Rev. 2019;40(5):1394‐1423. 10.1210/er.2018-00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. 2004;27(6):1496‐1504. 10.2337/diacare.27.6.1496 [DOI] [PubMed] [Google Scholar]
- 30. Heffron SP, Parikh A, Volodarskiy A, et al. Changes in lipid profile of obese patients following contemporary bariatric surgery: a meta‐analysis. Am J Med. 2016;129(9):952‐959. 10.1016/j.amjmed.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Correlation between serum lipids and 1‐hour postload plasma glucose levels in normoglycemic individuals. J Clin Lipidol. 2014;8(2):217‐222. 10.1016/j.jacl.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 32. Lopez‐Jaramillo P, Gomez‐Arbelaez D, Martinez‐Bello D, et al. Association of the triglyceride glucose index as a measure of insulin resistance with mortality and cardiovascular disease in populations from five continents (PURE study): a prospective cohort study. Lancet Healthy Longev. 2023;4(1):e23‐e33. 10.1016/s2666-7568(22)00247-1 [DOI] [PubMed] [Google Scholar]
- 33. Jagannathan R, Neves JS, Dorcely B, et al. The oral glucose tolerance test: 100 years later. Diabetes Metab Syndr Obes. 2020;13:3787‐3805. 10.2147/dmso.S246062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanaire H, Bertrand M, Guerci B, Anduze Y, Guillaume E, Ritz P. High glycemic variability assessed by continuous glucose monitoring after surgical treatment of obesity by gastric bypass. Diabetes Technol Therapeut. 2011;13(6):625‐630. 10.1089/dia.2010.0203 [DOI] [PubMed] [Google Scholar]
- 35. Ilesanmi I, Tharakan G, Alexiadou K, et al. Roux‐en‐Y gastric bypass increases glycemic variability and time in hypoglycemia in patients with obesity and prediabetes or type 2 diabetes: a prospective cohort study. Diabetes Care. 2020;44(2):614‐617. 10.2337/dc20-1609 [DOI] [PubMed] [Google Scholar]
- 36. Furugen M, Saitoh S, Ohnishi H, et al. Matsuda–DeFronzo insulin sensitivity index is a better predictor than HOMA‐IR of hypertension in Japanese: the Tanno–Sobetsu study. J Hum Hypertens. 2012;26(5):325‐333. 10.1038/jhh.2011.23 [DOI] [PubMed] [Google Scholar]
- 37. Haverals L, Van Dessel K, Verrijken A, et al. Cardiometabolic importance of 1‐h plasma glucose in obese subjects. Nutr Diabetes. 2019;9(1):16. 10.1038/s41387-019-0084-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lorenzo C, Wagenknecht LE, Rewers MJ, et al. Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care. 2010;33(9):2098‐2103. 10.2337/dc10-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lin E, Davis SS, Srinivasan J, et al. Dual mechanism for type‐2 diabetes resolution after Roux‐en‐Y gastric bypass. Am Surg. 2009;75(6):498‐502; discussion‐3. 10.1177/000313480907500608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB. Body composition by DXA. Bone. 2017;104:101‐105. 10.1016/j.bone.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zaffina C, Wyttenbach R, Pagnamenta A, et al. Body composition assessment: comparison of quantitative values between magnetic resonance imaging and computed tomography. Quantitative imaging Med Surg. 2022;12(2):1450‐1466. 10.21037/qims-21-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material S1
Figure S1
Table S1


