Abstract
This project tested the hypothesis that burn survivors can perform mild/moderate-intensity exercise in temperate and hot environments without excessive elevations in core body temperature. Burn survivors with low (23 ± 5%TBSA; N = 11), moderate (40 ± 5%TBSA; N = 9), and high (60 ± 8%TBSA; N = 9) burn injuries performed 60 minutes of cycle ergometry exercise (72 ± 15 watts) in a 25°C and 23% relative humidity environment (ie, temperate) and in a 40°C and 21% relative humidity environment (ie, hot). Absolute gastrointestinal temperatures (TGI) and changes in TGI (ΔTGI) were obtained. Participants with an absolute TGI of >38.5°C and/or a ΔTGI of >1.5°C were categorized as being at risk for hyperthermia. For the temperate environment, exercise increased ΔTGI in all groups (low: 0.72 ± 0.21°C, moderate: 0.42 ± 0.22°C, and high: 0.77 ± 0.25°C; all P < .01 from pre-exercise baselines), resulting in similar absolute end-exercise TGI values (P = .19). Importantly, no participant was categorized as being at risk for hyperthermia, based upon the aforementioned criteria. For the hot environment, ΔTGI at the end of the exercise bout was greater for the high group when compared to the low group (P = .049). Notably, 33% of the moderate cohort and 56% of the high cohort reached or exceeded a core temperature of 38.5°C, while none in the low cohort exceeded this threshold. These data suggest that individuals with a substantial %TBSA burned can perform mild/moderate intensity exercise for 60 minutes in temperate environmental conditions without risk of excessive elevations in TGI. Conversely, the risk of excessive elevations in TGI during mild/moderate intensity exercise in a hot environment increases with the %TBSA burned.
Keywords: rehabilitation, exercise, thermoregulation, air temperature
INTRODUCTION
Every year, ~150,000 patients with burn injuries are treated in emergency rooms in the United States, with 40,000 to 70,000 of these individuals being hospitalized.1–3 The American Burn Association reports that ~16% of patients hospitalized with burns have injuries covering 20% or more of their body surface area (BSA),4 that is, between 6400 and 11,200 individuals per year. Military conflicts are also a significant source of burn-related injuries, given that 5%-20% of all battlefield injuries are burn-related.5–7 Decades ago burns covering half of a person’s BSA were likely fatal, while today patients with 90% total body surface area burned (%TBSA) are surviving.8 Moreover, children with 70% TBSA burned “routinely survive” these injuries.3,9 Thus, more individuals are living with larger percentages of %TBSA burned than ever before.
After a severe burn injury, fatigue can be a significant obstacle for burn survivors when returning to work and/or carrying out their daily activities. Such fatigue is a commonly reported challenge that affects burn survivors 3-10 years following their injury.10,11 It was found that ~75% of well-healed burned survivors have an aerobic capacity in the lowest 20th percentile relative to age- and sex-matched normative values.12 In non-burned individuals, such a response is associated with a 3 to 5-fold greater mortality risk relative to the highest 20th percentile.13 Relative to matched non-burned cohorts, well-healed burn survivors experience higher rates of all-cause mortality, greater hospitalization days for “circulatory diseases” and have higher incidences of ischemic heart disease, heart failure, diabetes, and cerebrovascular disease (including stroke).14–20 Despite unclear mechanisms responsible for these adverse observations in burn survivors, sedentary non-burned individuals display similar negative responses.13,21–26 Given that burn survivors can achieve cardiovascular and metabolic benefits of physical activity,12,27,28 an important unanswered question is: What are the barriers that result in burn survivors (as a population) avoiding the physical activity necessary to maintain optimal health and to avoid the adverse consequences of a sedentary lifestyle?
It is proposed that impaired body temperature regulation and/or heightened perception of heat stress contribute to a sedentary lifestyle in the burn survivor community.29–33 This is because burn injuries and the subsequent skin grafting impair eccrine sweat gland function and skin vasodilation in response to environmental heat stress,29 which are critical for heat loss during exercise. Often burn survivors are counselled to avoid physical activity in hot environmental conditions, potentially counteracting efforts to increase physical activity in this generally sedentary population.13 In this quest to become more physically active, burn survivors and their clinical care givers need to understand the limits to which burn survivors can perform physical activity (including activities associated with rehabilitation) without excessive elevations in core body temperature. However, little is known regarding the risk of hyperthermia in adult burn survivors performing mild/moderate-intensity exercise under temperate environmental conditions; that is, conditions similar to the rehabilitation clinic and/or the gym. To that end, the first objective of this work is to test the hypothesis that burn survivors can safely perform physical activity in temperate conditions regardless of the size of their burn injury. A secondary objective is to test the hypothesis that burn survivors with small to moderate BSA burn injuries can safely perform physical activity in hot environmental conditions, while it may be unsafe for burn survivors with large BSA injuries to perform physical activity in these ambient conditions. Such information will be extremely valuable to burn survivors and their clinicians in identifying burn survivors’ potential to obtain the cardiovascular and metabolic benefits of being physically active, along with increases in functional independence.13,26,34–37
METHODS
Ethics approval
The Institutional Review Board approved this study protocol and the associated consent. All participants were informed of the risks of participation and study procedures before signing a written informed consent. All procedures conformed to the standards set forth in the Declaration of Helsinki.
Participants
We recruited 29 burn survivors to participate in this study who had burn injuries covering 23 ± 5%TBSA (N = 11; categorized as the 20%TBSA group), 40 ± 5%TBSA (N = 9; categorized as the 40%TBSA group), and 60 ± 8%TBSA (N = 9; categorized as the 60%TBSA group). Participants’ burn injuries were well-healed, and participants were a minimum of 2 years after the burn injury. Participant characteristics are presented in Table 1.
Table 1.
Participant characteristics.
| 20% BSA burned | 40% BSA burned | 60% BSA burned | |
|---|---|---|---|
| N | 11 | 9 | 9 |
| Male/Female | 10/ 1 | 5/ 4 | 6/ 3 |
| Age (y) | 39 ± 12 | 40 ± 12 | 37 ± 7 |
| Body mass (kg) | 79.6 ± 13.8 | 74.9 ± 14.7 | 83.0 ± 10.9 |
| Height (m) | 1.73 ± 0.11 | 1.69 ± 0.09 | 1.74 ± 0.05 |
| BMI (kg/m2) | 26.8 ± 4.0 | 26.1 ± 3.8 | 27.4 ± 3.7 |
| BSA (m2) | 1.92 ± 0.20 | 1.83 ± 0.22 | 1.97 ± 0.12 |
| Burn injury BSA (%) | 23.1 ± 5.4 | 40.4 ± 4.9a,b | 59.6 ± 7.6a,c |
| VO2peak (ml/kg/min) | 33.3 ± 7.7 | 31.7 ± 5.4 | 31.5 ± 7.2 |
| Medications (n) | Medical marijuana (1) Diabetes (1) Weight loss (1) Allergy (1) Birth control (1) |
Medical marijuana (1) Asthma (1) Corticosteroid (1) Anxiety (2) Allergy (1) Depression (1) ADHD (1) Sleep (1) Thyroid (1) Migraines (1) Birth control (1) |
Medical marijuana (2) Acid reflux (1) Hypertension (1) Sleep (1) Anxiety (1) ADHD (1) Pain (1) |
aDifferent from 20% (P < .01).
bDifferent from 60% (P < .01).
cDifferent from 40% (P < .01)
Experimental protocol
Participants were asked to refrain from using allergy medicines, anti-inflammatory drugs, and aspirin for 36 hours, exercise and alcohol for 24 hours, and caffeine for 12 hours before coming into the laboratory. Urine specific gravity was assessed upon arrival to the laboratory, with a value of ≤1.025 confirming a euhydrated state.38
Each participant completed 3 study visits, consisting of a maximal oxygen consumption test day and 2 exercise trial days. On the maximal oxygen consumption day, participants completed a graded exercise test on a cycle ergometer in a temperate environment to determine peak oxygen consumption (V̇O2peak). In brief, this test was initiated by the participants cycling at 1 W·kg−1 body mass, with this workload increasing at a rate of 20 or 25 W·minute−1 until volitional exhaustion. Participant’s expired gases were analyzed using an indirect calorimetry system (TrueOne 2400, Parvo Medics, Sandy, UT). During this visit, participants’ body surface area was calculated from body mass and height,39 and the percentage of their body surface area covered by burn injuries was verified.
Prior to each exercise trial, participants ingested a telemetric pill for the measurement of gastrointestinal temperature (TGI; HQ Inc., Palmetto, FL). After obtaining a nude body mass (Mettler Toledo PBD655-BC120, Toledo, OH), participants were instrumented with electrodes for the recording of an electrocardiogram-based heart rate (GE Medical Systems, Madison, WI). Heart rate and TGI signals were digitized to a data acquisition system (Biopac MP150, Santa Barbara, CA) with signals sampled at 200 Hz and 0.1 Hz, respectively. Participants entered the environmental chamber set to either temperate (24.8 ± 0.2°C, 23.1 ± 3.6 % relative humidity) or hot (39.8 ± 0.28°C, 20.7 ± 3.0 % relative humidity) conditions, with the order of exposure randomized and counterbalanced. They rested (seated) in the respective environmental conditions for 30 minutes. The participants then exercised on a cycle ergometer at a metabolic heat production of ~4.5 W kg−1 body mass for 60 minutes, verified by collecting expired gases during minutes 0-10, 25-35, and 50-60, and accounting for external work on the cycle ergometer.40 Participants were permitted to drink water ad libitum during exercise, with this water temperature maintained at the participant’s internal body temperature via a circulating water bath (E100, Lauda, Germany).
Statistical analyses
Mean changes in TGI and heart rate, referenced from the beginning of exercise, and from the 2 minutes preceding each time point (0, 15, 30, 45, and 60 minutes) were analyzed. One 20%TBSA participant was unable to complete the full 60 minutes of exercise in both 25°C and 40°C conditions. In the 25°C trial, one 20%TBSA participant’s TGI data, and another 20%TBSA participant’s heart rate data, were excluded due to equipment error. One 60%TBSA participant was unable to complete 60 minutes of exercise in the 40°C condition due to reaching the IRB-specified TGI limit of 39.5°C.
Data are presented as mean ± SD. All evaluated responses were normally distributed (Shapiro-Wilk test), and thus parametric statistical tests were performed using Prism 9.0.2 (GraphPad, La Jolla, CA). Participant anthropometrics between groups were analyzed using a one-way ANOVA. Metabolic heat production, urine specific gravity, and whole body sweat rate were compared using an ANOVA (Group × Ambient Temperature). Based upon threshold safety guidelines for workers,41,42 a participant with an absolute TGI of ≥38.5°C and/or a ΔTGI of ≥1.5°C at any time point during the trial was categorized as being at risk for hyperthermia. Heart rate and TGI responses across time for each environmental condition were analyzed using a mixed-effects analysis (Group × Time). TGI responses at the end of exercise were compared between ambient conditions and TBSA burned using a mixed-effects analysis (Group × Ambient temperature). Tukey corrected multiple comparisons were used for any variable with a significant interactive effect. Significance was set a priori at P < .05.
RESULTS
By design, TBSA differed across groups (P < .01). Urine specific gravity was similar across burn groups and trials (main effect of ambient temperature, P = .81; main effect of TBSA, P = .89). Across TBSA groups and ambient temperatures, we found no differences in metabolic heat production (main effect of ambient temperature, P = .43; main effect of TBSA, P = .21). We only observed a main effect of ambient temperature on whole body sweat rate, which was higher in the 40°C conditions across TBSA groups (P < .01).
Figure 1 presents the absolute TGI responses during exercise in both 40°C and 25°C environmental conditions, while Figure 2 presents the same data as a change in TGI across the indicated time points. As depicted in Figure 3, in the 25°C environment no participant’s TGI surpassed 38.5°C, nor did any participant’s change in TGI exceed 1.5°C. In 40°C environment, three 40%TBSA and five 60%TBSA participants’ TGI reached or exceeded 38.5°C. However, only two 60%TBSA participant’s TGI increased by more than 1.5°C during exercise. Notably, there was an interactive effect of time and TBSA on the absolute TGI and on the ΔTGI during exercise in both 40°C and 25°C environments (P < .01; see Figures 1 and 2). Consistent with the findings reported in Figure 2, we observed an interactive effect of TBSA and ambient temperature on absolute TGI and the change in TGI at the end of exercise (Figure 3, P = .02). As illustrated in Figure 3, both 40%TBSA and 60%TBSA groups exhibited greater increases in TGI, as well as greater absolute TGI, at the end of the 40°C trial relative to the same workload at the end of the 25°C trial. In contrast, there were no differences in these responses between the 40°C and 25°C trials for the 20%TBSA group.
Figure 1.
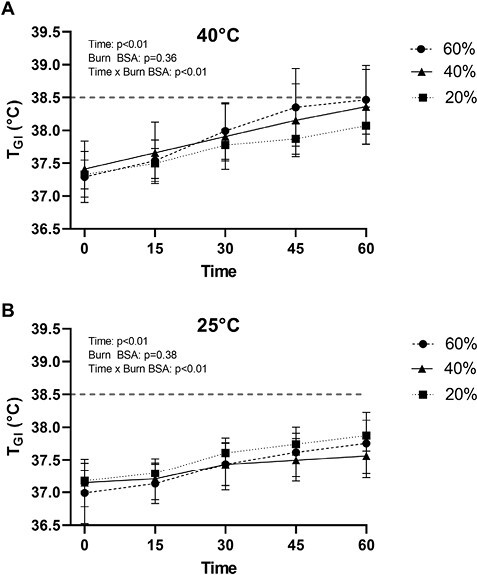
Absolute internal body temperature (TGI) responses of burn survivors to 60 minutes of exercise in a hot (A) and temperate (B) environment. The dashed line depicts the predetermined threshold to categorize excessive hyperthermia for these trials. Data represent means ± SDs.
Figure 2.
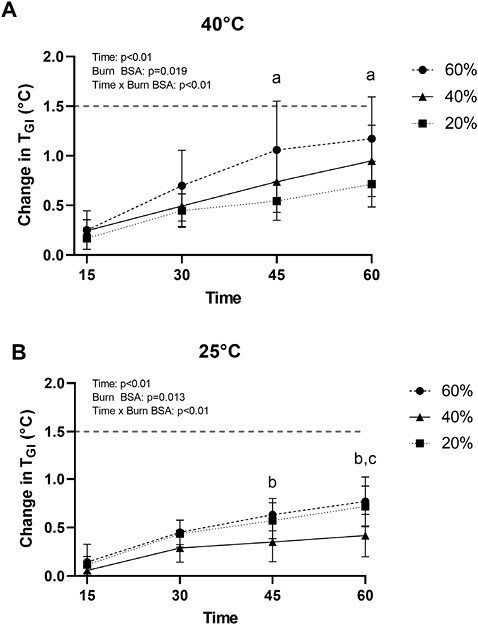
Change in internal body temperature (TGI) responses of burn survivors to 60 minutes of exercise in a hot (A) and temperate (B) environments. a60%TBSA group different from the 20%TBSA group at the indicated time points (P < .05). b40%TBSA group different from the 60%TBSA group at the indicated time points (P < .02). c20%TBSA group different from the 40%TBSA group at the indicated time points (P = .02). The dashed line depicts the predetermined threshold to categorize excessive hyperthermia for these trials. Data represent means ± SDs.
Figure 3.
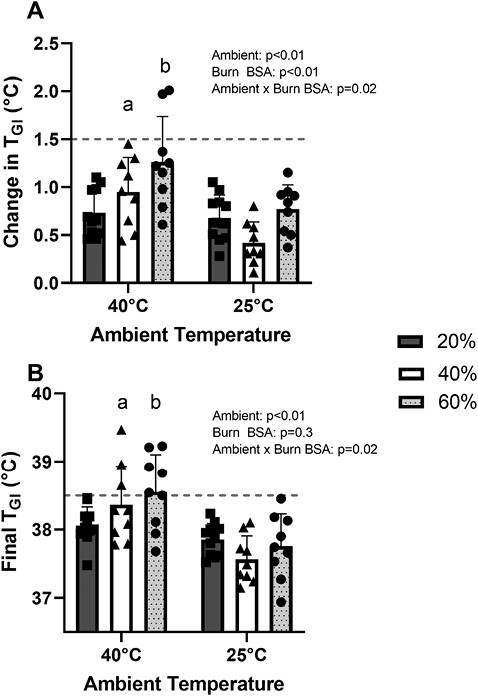
Final increases in internal body temperature (TGI, Panel A) and final absolute TGI (Panel B) in burn survivors upon cessation of exercise in hot and temperate ambient conditions. a40°C responses different from 25°C responses for the 40%TBSA group (P < .01). b40°C responses different from 25°C responses for the 60%TBSA group (P < .01). There were no differences for either variable between 40°C and 25°C bouts for the 20%TBSA group. The dashed line depicts the predetermined threshold to categorize excessive hyperthermia for these trials. Data represent means ± SDs.
As shown in Figure 4, for both environmental conditions, we observed an interaction between Time and Group (P = .02) for the increase in heart rate across the 60-minute exercise bout, though post-hoc analyses did not reveal any differences in the magnitude of the elevation in heart rate between groups for any of the assessed time points.
Figure 4.
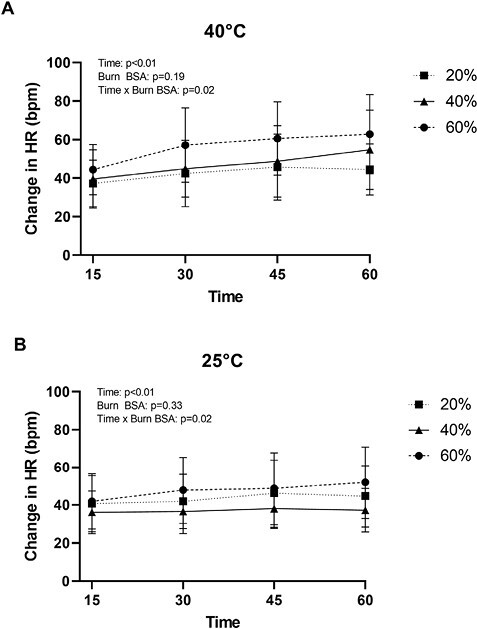
Heart rate (HR) responses of burn survivors to 60 minutes of exercise in a hot (A) and temperate (B) environment. For both climates, there were no differences in responses between groups at any of the assessed time points. Data represent means ± SDs.
DISCUSSION
Body temperature homeostasis is maintained via a balance between heat gain (from internal and/or external sources) and heat dissipation. As such, core body temperature will increase if the rate of heat gain is greater than that of heat dissipation. Sweating and elevations in skin blood flow are the primary mechanisms by which humans dissipate heat.43–46 If these responses are insufficient relative to heat gain, core body temperature will continually rise during physical activity, culminating in a heat-related injury or death.47 Severely burned skin cannot effectively increase blood flow or sweat during a heat stress, with these limitations persisting throughout the individual’s life.29–32 Furthermore, burn survivors have a heightened perception of heat stress that accompanies these elevated core body and skin temperatures.33 Consistent with these laboratory observations, 72% of burn survivors report “problems in hot temperature” 17 ± 13 years postinjury.48 Moreover, a fear of “overheating” during physical activity by burn survivors is reinforced by rehabilitation specialists who inform burn survivors of their reduced ability to regulate their body temperature.48–50 Such recommendations could be taken too far, resulting in burn survivors greatly limiting physical activity. The result of these sequelae is a population of burn survivors that is generally sedentary and suffer from a higher incidence of the cardiovascular and metabolic consequences of insufficient physical activity.14–20 For burn survivors to fully rehabilitate following their injuries, they (as a population) need to be more physically active.
Key findings from the present work demonstrate that, when performing physical activity of a mild/moderate intensity under temperate environmental conditions, survivors with large burn injuries can maintain their core body temperatures within safe limits; findings consistent with a prior report using a simulated burn-injury model.51 These data suggest that heat-dissipating capabilities of the uninjured skin are sufficient to avoid excessive elevations in core body temperature during mild/moderate physical activity in burn survivors when the physical activity, including activities associated with rehabilitation, is performed in temperate environmental conditions (ie, typical room temperatures). Conversely, and also consistent with prior observations,33,52,53 when the identical level of physical activity is performed in a 40°C environment, individuals with ~40%TBSA and ~60%TBSA start to exhibit core body temperatures that can be categorized as excessive (eg, TGI ≥38.5°C, see Figure 3). In the present study, this was evident by three individuals (33% of the cohort) with ~40%TBSA and five individuals (56% of the cohort) with ~60%TBSA having a TGI that reached or exceeded 38.5°C while performing physical activity in the heat. That said, only two individuals with ~60%TBSA had a ΔTGI that exceeded 1.5°C, which was the other criterion employed to assess the risk of hyperthermia. When ΔTGI at the end of exercise during identical levels of physical activity was compared between temperate and hot environmental conditions (Figure 3), higher values were found for the 40% and 60%TBSA groups for the heated trial relative to the 25°C trials, but not the 20%TBSA burned group. These observations suggest that at the assessed level of physical activity and environmental conditions, individuals with ~40% and ~60%TBSA are more susceptible to hyperthermia in hot environmental conditions, whereas individuals with ~20%TBSA are not.
Only one measure of cardiovascular stress (ie, heart rate) was obtained in this trial. At these relatively mild/moderate intensity workloads, there was an interaction between Time and Group for the increase in heart rate (P = .02 for both environments, see Figure 4). However, post-hoc analyses did not reveal any differences in the magnitude of the elevation in heart rate between groups for any of the assessed time points, including at the end of exercise. This latter observation is unexpected given prior results, with burn survivors exercising at a similar exercise intensity in the heat, showing heightened heart rate responses at the end of exercise in individuals with larger burn injuries.53 There are at least 3 possible reasons for these apparent disparate results. (1) In the former trial,53 the separation of groups was different relative to the present trial, wherein that former trial burn survivors were categorized as 17%-40%TBSA and >40%TBSA burned. (2) The relative humidity in the prior study was slightly higher,53 ~30%, relative to 23% employed in the present study, which could result in reduced evaporative cooling leading to heightened cardiovascular stress in that prior trial. (3) The heart rate responses in the aforementioned trial53 were obtained after 90 minutes of exercise, whereas the present data were obtained after 60 minutes of exercise.
It is important to emphasize that the interpretation of the obtained findings should be constrained to the applied exercise intensity and environmental conditions. The level of physical activity was clamped at a metabolic heat generation of 4.5 W/kg body mass, which was equivalent to an oxygen uptake of 1.25 ± 0.21 L/minute or 4.5 ± 0.2 METs. It was recently shown that TGI responses to exercise using a simulated burn model, and across a wide range of TBSA, was greatly influenced by the exercise intensity.54 Thus, we expect that had a more intense level of physical activity been performed during the heated trial, greater separation of TGI would have been observed between the groups. However, such a trial would be challenging in burn survivors given the low level of aerobic capacity of this population.12 With respect to environmental temperatures, the hot trial was performed under extreme conditions (40°C); that is, conditions that burn survivors are unlikely to perform physical activity in many parts of the world, particularly in a rehabilitation setting. That said, less clear are the anticipated TGI outcomes should burn survivors exercise in environmental temperatures intermediate to those applied in the present conditions (ie, between 25°C and 40°C). Given the responses in Figures 2 and 3 for the higher burn groups, we anticipate intermediate environmental temperatures would result in intermediate TGI responses, relative to those depicted in these figures. Furthermore, the enrolled population had an age range from 22 to 56 years, and thus the present observations should be viewed with the understanding that they may not represent responses for younger or older individuals. Finally, there was a sex imbalance for the 20% (1 female) and 60% (3 females) BSA burned groups. However, when controlling for body morphology, as we did in this study, sex-related differences in core temperature responses are minimized during mild/moderate intensity exercise in the heat in non-burned individuals.55 Thus, we do not anticipate this sex imbalance to adversely affect the interpretation of the results.
Current guidelines recommend that adults perform 150 minutes of moderate-intensity exercise each week.56 which could equate to 30 minutes of exercise per day for 5 days per week. A careful review of Figures 1 and 2 shows that at 30 minutes of physical activity TGI responses were similar between groups for both environmental conditions. This observation suggests that across environmental temperatures of 25°C and 40°C, burn survivors with upwards to ~60%TBSA can safely perform physical activity for 30 minutes at an intensity of ~4.5 METS without a risk for excessive elevations in core body temperature. That said, it should be emphasized that the assessed environmental temperatures did not include an added radiant heat load that one would encounter upon exercising outside under direct exposure to the sun.
At the end of exercise in the 25°C environment, the TGI responses in the ~20%TBSA participants were slightly higher relative to TGI responses in the participants with ~40%TBSA (Figure 2B). That figure shows that subjects having ~40%TBSA exhibited mean increases in TGI of less than 0.5°C. Similarly, lower TGI values in individuals with 40% simulated burn injuries were not observed during exercising in similar environmental conditions as the present work, though the individuals exercised at higher intensities in that study.51 Given those contrasting findings, coupled with mean TGI values being higher in the ~40%TBSA group versus the ~20%TBSA group during exercise in the heat (Figure 2A), we do not have a reason/mechanism (other than selection bias) for the apparently lower elevation in TGI in the ~40%TBSA group exercising in temperate environmental conditions.
In conclusion, individuals with substantial percentages of TBSA burned can exercise at a mild/moderate intensity for 60 minutes in temperate environmental conditions without a risk of excessive elevations in core body temperature. Conversely, while exercising in hotter environmental conditions, over half of the subjects in the larger %TBSA burned groups had TGI responses that were categorized as being excessive (ie, ≥38.5°C). Given that rehabilitation of the burn survivor typically occurs in temperate environmental conditions, these data demonstrate that burn survivors can perform upwards to 60 minutes of exercise associated with rehabilitation in such conditions without a risk of hyperthermia. Importantly, such burn survivors can obtain the cardiovascular and metabolic benefits of physical activity at ~4.5 METs (eg, brisk walking, golfing, light cycling, yardwork, etc.) in a temperate environment, inclusive of a gym setting, without being concerned about overheating.
Conflict of Interest. None declared.
Funding. This study was funded by the Department of Defense (W81XWH-15-1-0647 to C.G.C.), National Institutes of Health (R01GM068865 to C.G.C), Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship to M.N.C.
Contributor Information
Luke N Belval, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas and University of Texas Southwestern Medical Center, Dallas, TX 75231, USA.
Matthew N Cramer, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas and University of Texas Southwestern Medical Center, Dallas, TX 75231, USA.
Gilbert Moralez, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas and University of Texas Southwestern Medical Center, Dallas, TX 75231, USA; Applied Clinical Research, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Mu Huang DPT, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas and University of Texas Southwestern Medical Center, Dallas, TX 75231, USA; Applied Clinical Research, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Joseph C Watso, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas and University of Texas Southwestern Medical Center, Dallas, TX 75231, USA.
Mads Fischer, Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, DK 1165, Denmark.
Craig G Crandall, Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas and University of Texas Southwestern Medical Center, Dallas, TX 75231, USA.
REFERENCES
- 1. CDC. Mass casualities: burn. In. Injury Fact Sheets 2007. [Google Scholar]
- 2. UDH. Work-Related Burn Surveillance in Utah, 2001. Office of Epidemiology; 2002. [Google Scholar]
- 3. Pruitt BA, Wolf SE, Mason AD.. Epidemiological, demongraphic, and outcome characteristics of burn injury. In: Herndon DN, ed. Total Burn Care. Saunders; 2007:14–32. [Google Scholar]
- 4. Association AB. National Burn Repository 2016. Version 12.0. Chicago; 2016. [Google Scholar]
- 5. Wolf SE, Kauvar DS, Wade CEet al. Comparison between civilian burns and combat burns from Operation Iraqi Freedom and Operation Enduring Freedom. Ann Surg. 2006;243(6):786–792; discussion 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chung KK, Blackbourne LH, Wolf SEet al. Evolution of burn resuscitation in operation Iraqi freedom. J Burn Care Res. 2006;27(5):606–611. [DOI] [PubMed] [Google Scholar]
- 7. Cancio LC, Horvath EE, Barillo DJet al. Burn support for Operation Iraqi Freedom and related operations, 2003 to 2004. J Burn Care Rehabil. 2005;26(2):151–161. [DOI] [PubMed] [Google Scholar]
- 8. Unknown. Burns. National Institute of General Medical Sciences. Published 2008. Accessed Februaryhttps://www.nigms.nih.gov/education/fact-sheets/Pages/burns.aspx
- 9. Baker CP, Russell WJ, MeyerW, 3rd, Blakeney P.. Physical and psychologic rehabilitation outcomes for young adults burned as children. Arch Phys Med Rehabil. 2007;88(12 Suppl 2):S57–S64. [DOI] [PubMed] [Google Scholar]
- 10. Helm P, Herndon DN, Delateur B.. Restoration of function. J Burn Care Res. 2007;28(4):611–614. [DOI] [PubMed] [Google Scholar]
- 11. Holavanahalli RK, Kowalske KJ, Helm PA.. Long-term neuro musculoskeletal outcomes in patients surviving severe burns. J Burn Care Res. 2009;30(2):S109. [Google Scholar]
- 12. Ganio MS, Pearson J, Schlader ZJet al. Aerobic fitness is disproportionately low in adult burn survivors years after injury. J Burn Care Res. 2015;36(4):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blair SN, KohlHW, 3rd, PaffenbargerRS, Jr., Clark DG, Cooper KH, Gibbons LW.. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. [DOI] [PubMed] [Google Scholar]
- 14. Duke JM, Rea S, Boyd JH, Randall SM, Wood FM.. Mortality after burn injury in children: a 33-year population-based study. Pediatrics. 2015;135(4):e903–e910. [DOI] [PubMed] [Google Scholar]
- 15. Duke JM, Boyd JH, Randall SM, Wood FM.. Long term mortality in a population-based cohort of adolescents, and young and middle-aged adults with burn injury in Western Australia: a 33-year study. Accid Anal Prev. 2015;85:118–124. [DOI] [PubMed] [Google Scholar]
- 16. Mason SA, Nathens AB, Byrne JPet al. Increased rate of long-term mortality among burn survivors: a population-based matched cohort study. Ann Surg. 2019;269(6):1192–1199. [DOI] [PubMed] [Google Scholar]
- 17. Duke JM, Boyd JH, Rea S, Randall SM, Wood FM.. Long-term mortality among older adults with burn injury: a population-based study in Australia. Bull World Health Organ. 2015;93(6):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM.. Understanding the long-term impacts of burn on the cardiovascular system. Burns. 2016;42(2):366-374. [DOI] [PubMed] [Google Scholar]
- 19. Chung SD, Chen CS, Lin HC, Kang J-H.. Increased risk for stroke in burn patients: a population-based one-year follow-up study. Burns. 2014;40(1):54-60. [DOI] [PubMed] [Google Scholar]
- 20. Hung TY, Lee YK, Huang MY, Hsu C-Y, Su Y-C.. Increased risk of ischemic stroke in patients with burn injury: a nationwide cohort study in Taiwan. Scand J Trauma Resusc Emerg Med. 2016;24:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keteyian SJ, Brawner CA, Savage PDet al. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am Heart J. 2008;156(2):292–300. [DOI] [PubMed] [Google Scholar]
- 22. Erikssen G, Liestol K, Bjornholt J, Thaulow E, Sandvik L, Erikssen J.. Changes in physical fitness and changes in mortality. Lancet. 1998;352(9130):759–762. [DOI] [PubMed] [Google Scholar]
- 23. Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT.. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330(22):1549–1554. [DOI] [PubMed] [Google Scholar]
- 24. Peters RK, CadyLD, Jr, Bischoff DP, Bernstein L, Pike M C.. Physical fitness and subsequent myocardial infarction in healthy workers. JAMA. 1983;249(22):3052-3056. [PubMed] [Google Scholar]
- 25. Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K.. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328(8):533–537. [DOI] [PubMed] [Google Scholar]
- 26. Aspenes ST, Nilsen TI, Skaug EAet al. Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc. 2011;43(8):1465–1473. [DOI] [PubMed] [Google Scholar]
- 27. Willis CE, Grisbrook TL, Elliott CM, Wood FM, Wallman KE, Reid SL.. Pulmonary function, exercise capacity and physical activity participation in adults following burn. Burns. 2011;37(8):1326–1333. [DOI] [PubMed] [Google Scholar]
- 28. Romero SA, Moralez G, Jaffery MFet al. Progressive exercise training improves maximal aerobic capacity in individuals with well-healed burn injuries. Am J Physiol Regul Integr Comp Physiol. 2019;317(4):R563–R570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis SL, Shibasaki M, Low DAet al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res. 2007;28(3):427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis SL, Shibasaki M, Low DAet al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res. 2009;30(4):675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crandall CG, Davis SL.. Cutaneous vascular and sudomotor responses in human skin grafts. J Appl Physiol (1985). 2010;109(5):1524–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davis SL, Shibasaki M, Low DAet al. Skin grafting impairs postsynaptic cutaneous vasodilator and sweating responses. J Burn Care Res. 2007;28(3):435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ganio MS, Schlader ZJ, Pearson Jet al. Nongrafted skin area best predicts exercise core temperature responses in burned humans. Med Sci Sports Exerc. 2015;47(10):2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blair SN, KohlHW, 3rd, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA.. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 35. ACSM. American College of Sports Medicine Position Stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 1998;30(6):992–1008. [PubMed] [Google Scholar]
- 36. Spruit MA, Wouters EF, Eterman RMet al. Task-related oxygen uptake and symptoms during activities of daily life in CHF patients and healthy subjects. Eur J Appl Physiol. 2011;111(8):1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paterson DH, Govindasamy D, Vidmar M, Cunningham DA, Koval JJ.. Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc. 2004;52(10):1632–1638. [DOI] [PubMed] [Google Scholar]
- 38. Cheuvront SN, Ely BR, Kenefick RW, Sawka MN.. Biological variation and diagnostic accuracy of dehydration assessment markers. Am J Clin Nutr. 2010;92(3):565–573. [DOI] [PubMed] [Google Scholar]
- 39. Du Bois D, DuBois EF.. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII(62):863–871. [Google Scholar]
- 40. Cramer MN, Jay O.. Partitional calorimetry. J Appl Physiol. 2018;126:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. ACGIH. Heat stress and strain. In: Documentation of the Threshold Limit Values for Physical Agens Documentation. ACGIH; 2007. [Google Scholar]
- 42. Bernard TE, Kenney WL.. Rationale for a personal monitor for heat strain. Am Ind Hyg Assoc J. 1994;55(6):505–514. [DOI] [PubMed] [Google Scholar]
- 43. Johnson JM, Proppe DW.. Cardiovascular adjustments to heat stress. In: Blatteis C, Fregly M, eds. Handbook of Physiology: Adaptations to the Environment. American Physiological Society; 1996:215–243. [Google Scholar]
- 44. Rowell LB. Thermal stress. In: Human Circulation Regulation During Physical Stress. Oxford University Press; 1986:174–212. [Google Scholar]
- 45. Rowell LB, Brengelmann GL, Murray JA.. Cardiovascular responses to sustained high skin temperature in resting man. J Appl Physiol. 1969;27(5):673–680. [DOI] [PubMed] [Google Scholar]
- 46. Buono MJ, Sjoholm NT.. Effect of physical training on peripheral sweat production. J Appl Physiol. 1988;65(2):811–814. [DOI] [PubMed] [Google Scholar]
- 47. Knochel JP. Heat stroke and related heat stress disorders. Dis Mon. 1989;35(5):301–377. [PubMed] [Google Scholar]
- 48. Holavanahalli RK, Helm PA, Kowalske KJ.. Long-term outcomes in patients surviving large burns: the skin. J Burn Care Res. 2010;31(4):631–639. [DOI] [PubMed] [Google Scholar]
- 49. Kowalske K, Holavanahalli R, Carrougher GJ, et al. Exercise After Burn Injury. Model Systems Knowledge Translation Center. Published 2019. Accessed August29, 2023https://msktc.org/burn/factsheets/exercise-after-burn-injury [Google Scholar]
- 50. Carrougher GJ, Gibran NS, Caceres M, et al. Sun Protection After a Burn Injury. Model Systems Knowledge Translation Center. Published 2018. https://msktc.org/sites/default/files/SunProtectAfterBurn-508.pdf [Google Scholar]
- 51. Cramer MN, Moralez G, Huang MU, Kouda K, Poh PYS, Crandall CG.. Exercise thermoregulation with a simulated burn injury: impact of air temperature. Med Sci Sports Exerc. 2020;52(3):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ganio MS, Gagnon D, Stapleton J, Crandall CG, Kenny GP.. Effect of human skin grafts on whole-body heat loss during exercise heat stress: a case report. J Burn Care Res. 2013;34(4):e263–e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schlader ZJ, Ganio MS, Pearson Jet al. Heat acclimation improves heat exercise tolerance and heat dissipation in individuals with extensive skin grafts. J Appl Physiol (1985). 2015;119(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Belval LN, Cramer MN, Moralez Get al. Interaction of exercise intensity and simulated burn injury size on thermoregulation. Med Sci Sports Exerc. 2021;53(2):367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gagnon D, Kenny GP.. Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss. J Appl Physiol. 2012;113(5):746–757. [DOI] [PubMed] [Google Scholar]
- 56. Haskell WL, Lee IM, Pate RRet al. Physical activity and public health. Updated recommendation for Adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;39:1423–1434. [DOI] [PubMed] [Google Scholar]


