ABSTRACT
Matrix metalloproteinase 7 (MMP-7) is a secreted endopeptidase involved in the degradation of extracellular matrix components and the activation of cytokines and growth factors. The regulation of MMP-7 can be transcriptionally regulated by AP-1 or Wnt/β-catenin or post-translationally by proteolytic activation. MMP-7 expression is low or absent in the healthy kidney, but is significantly upregulated in kidney injury, including AKI and CKD. The function of MMP-7 in kidney disease may differ for CKD and AKI; it may have a profibrotic role in CKD and an anti-apoptotic and regenerative function in AKI. Additionally, the potential of MMP-7 as a biomarker has been studied in different kidney diseases, and the results are promising. Recently, combined unbiased kidney proteomics and transcriptomics approaches identified kidney MMP-7 as the protein having the strongest association with both fibrosis and eGFR and confirmed the predictive role of plasma MMP-7 levels for kidney function decline in over 11 000 individuals. Additionally, urinary MMP-7, combined with urinary cystatin C (CysC) and retinol binding protein (RBP) was reported to provide information on tubular injury in focal segmental glomerulosclerosis and minimal change disease. We now present an overview of research on MMP-7 expression and function in kidney diseases and discuss its potential as a biomarker of kidney diseases.
Keywords: chronic kidney disease, focal and segmental glomerulosclerosis, minimal change disease, MMP-7, urine biomarker
Chronic kidney disease (CKD) is one of the fastest growing global causes of death and is associated with increased risk of acute kidney injury (AKI) and cardiovascular and all-cause death [1]. The fast growth of CKD burden implies that current risk stratification and treatment of kidney disease is suboptimal. In the current issue of CKJ, Yin et al. assess the biomarker potential of urinary matrix metalloproteinase 7 (MMP-7) in focal and segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) [2]. The topic is relevant, as MCD usually responds to therapy, while FSGS is one of the most common glomerular causes of kidney failure [3].
Matrix metalloproteinase 7 (MMP-7): a modulator of extracellular matrix, cytokines, and growth factors
Matrix metalloproteinase 7 (MMP-7), also known as matrilysin-1, is a 30 kDa secreted zinc- and calcium-dependent endopeptidase that, along with the other MMP family members, is involved in the degradation of extracellular matrix components and the activation of several cytokines and growth factors [4, 5].
Structurally, MMP-7 is smaller than other MMPs, consisting only of a 19 kDa catalytic domain with an active Zinc binding site. It is secreted as an inactive zymogen with an additional 9 kDa cysteine-rich pro-domain near its N-terminal domain. These cysteine residues help keep the protein in an inactive state by binding with the catalytic zinc, and its disruption by proteolytic cleavage is required for MMP-7 activation [5, 6]. MMP-7 is activated by multiple proteases, including trypsin, plasmin or even other MMPs [5].
The regulation of MMP-7 is both transcriptional (synthesis of new mRNA) and post-translational (proteolytic activation). The promoter of the human MMP-7 gene contains an activator protein-1 (AP-1) site required for activation by growth factors, and a T-cell factor (TCF)-binding element (TBE) that responds to the Wnt/β-catenin pathway [7]. TGF-β1, which plays a key role in kidney fibrosis, promotes MMP-7 expression [8, 9]. Additionally, endogenous tissue inhibitors (TIMPs) can reversibly inhibit MMP-7. TIMPs are often capable of inhibiting various MMPs [5]. However, there are differences between TIMPs. As an example, while TIMP2 and TIMP3 inhibit MMPs, the impact on pro-MMP-2 activation and unilateral ureteral obstruction-induced kidney injury differs: TIMP3 inhibits activation of pro-MMP-2 and protects from kidney injury, whereas TIMP2 promotes pro-MMP-2 activation and kidney injury through MMP-2 activation [10]. However, the TIMP specificity for MMP-7 is poorly characterized [5]. TIMP-1 is a more potent inhibitor of MMP-7 than TIMP-2 or TIMP-3, but the Ki is still one order of magnitude higher than for MMP-1, MMP-2, or MMP-9, except for the TIMP-1 T2L/V4S variant that is in the same order of magnitude as for the other MMPs [11].
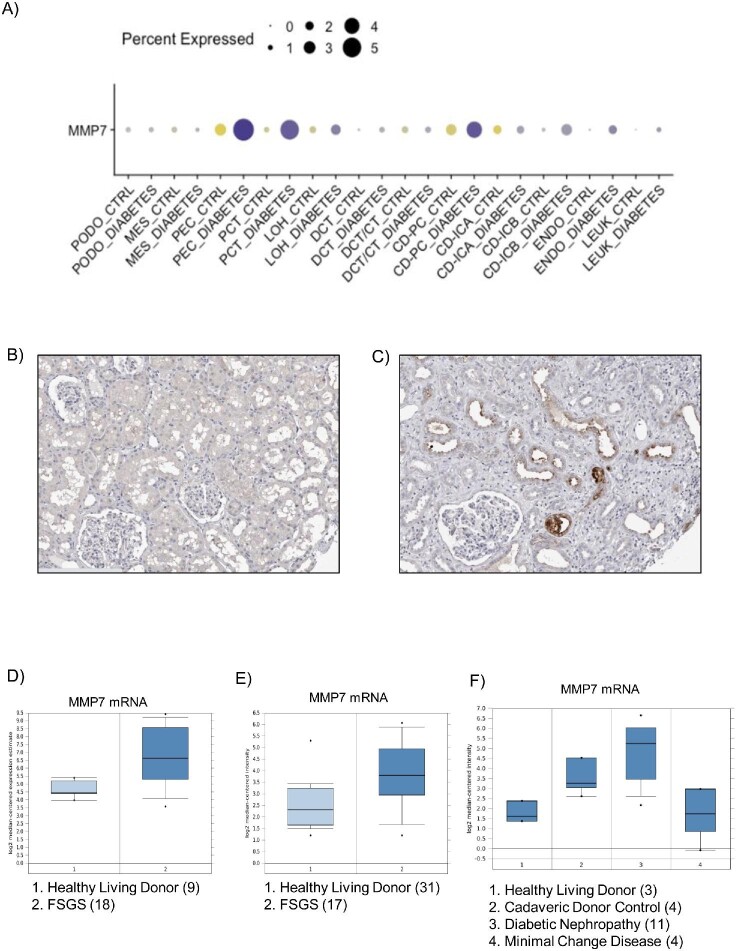
MMP-7 expression is low or absent in the healthy kidney, but it undergoes significant upregulation during kidney injury, including AKI and different forms of CKD such as diabetic, obstructive, or chronic kidney allograft nephropathy [5, 12] (Fig. 1A). The upregulation is mainly observed in the apical region of the dilated (injured) tubular epithelium, although it can also be expressed by other cell types such as podocytes, interstitial cells, or infiltrated inflammatory cells, depending on the specific disorder (Fig. 1A–C) [5, 13–16]. Increased MMP-7 expression has been observed in proximal and distal tubules.
Figure 1:
MMP-7 expression in human kidney diseases. (A) Human kidney single cell transcriptomics in control and diabetic kidney samples. Multiple cell types appear to express higher levels of MMP-7 mRNA in diabetic kidneys: PEC (parietal epithelial cells), PCT (proximal convoluted tubule), CD-PC (collecting duct principal cell), CD-ICB (collecting duct-intercalated cell type B) and endo (endothelium) [15]. https://humphreyslab.com/SingleCell/. Reproduced with permission from Prof. Ben Humphreys. (B, C) Human kidney immunohistochemistry in ProteinAtlas. Although both kidneys were classified as normal, panel A displayed back-to-back tubules, characteristic of healthy kidneys (male, age 16), while panel B shown widened interstitial spaces (female, age 59), characteristic of chronic kidney disease. Intense cytoplasmic staining is observed in distal tubules in panel B. https://www.proteinatlas.org/ENSG00000137673-MMP7/tissue/kidney#img. Image credit: Human Protein Atlas [16]. (D–F) MMP7 gene expression of micro-dissected tubulointerstitial samples from: (D) 18 focal segmental glomerulosclerosis (FSGS) patients and nine healthy living donors. Fold Change: 5.274; P‑value: 7.91E-5. ERCB Nephrotic Syndrome TubInt Dataset. http://v5.nephroseq.org/; (E) 17 FSGS patients and 31 healthy living donors. Fold change: 1.93; P‑value: 0.006. Ju CKD Tubint dataset [41]. http://v5.nephroseq.org/; (F) 3 living donors (controls), 4 minimal change disease (MCD), 4 cadaveric donors, and 11 diabetic nephropathies. Fold change Controls vs MCD: 1.09. Schmid Diabetes TubInt Dataset [42].
MMP-7 regulates multiple cellular processes due to its ability to target a broad range of substrates. These processes include extracellular matrix remodelling and epithelial-to-mesenchymal transition (EMT) by cleaving E-cadherin, laminin, fibronectin, fibrinogen or entactin, and apoptosis through cleavage of Fas ligand (FasL) and TNFα release [13, 17–22]. E-cadherin, a key target of MMP-7, plays a crucial role in maintaining intercellular adhesions and preserving the structural integrity of epithelium. E-cadherin binds to β-catenin. Upon E-cadherin degradation by MMP-7, β-catenin is released and activates downstream signalling pathways independent of Wnt, leading to expression of genes related to fibrosis, including MMP-7 [8, 9, 23].
MMP7 expression and function in kidney disease
MMP-7 is upregulated in almost all kidney diseases studied so far and it is one of the best characterized MMPs in kidney disease. Kidney MMP-7 overexpression has been detected in human kidney diseases such as ADPKD, lupus nephritis, diabetic nephropathy, IgA nephropathy, thrombotic microangiopathy and focal segmental glomerulosclerosis [13, 14, 24–27]. Additionally, high levels of MMP-7 expression have also been observed in experimental models of CKD and kidney fibrosis such as adriamycin nephropathy, adenine-induced CKD, chronic angiotensin II infusion, and unilateral ureter obstruction (UUO) [25, 27, 28], and in models of AKI induced by folic acid overdose, cisplatin, or ischemia/reperfusion [25, 29]. The Wnt/β-catenin pathway is key for MMP-7 expression in kidney diseases. In adriamycin nephropathy and in human diseased kidneys, MMP-7 expression positively correlated with β-catenin expression levels, and in both folic acid-AKI and UUO, MMP-7 colocalized with Wnt4 expression [25, 27]. In UUO kidneys, the ectopic expression of Wnt1 increased the expression of MMP-7, while inhibition of the Wnt1/β-catenin pathway repressed MMP-7 expression [27]. Moreover, tubule-specific β-catenin deficiency prevents MMP-7 overexpression induced by chronic angiotensin II infusion or Adriamycin [30]. In cultured kidney tubular cells, the ectopic expression of β-catenin promoted TCF binding to TBE domains in the MMP7 promoter and MMP7 gene expression [27]. Overall, the Wnt/β-catenin pathway is a key driver of MMP-7 expression in tubular cells during kidney disease and, as it is the case outside the kidneys, this triggers a positive feedback loop of MMP-7 expression through E-cadherin degradation and subsequent β-catenin release [27, 29].
The function of MMP-7 in kidney disease may differ in CKD and in AKI. MMP-7 global knock-out (MMP-7-KO) mice developed less severe kidney injury after UUO, characterized by higher levels of E-cadherin, and lower levels of β-catenin and fibrosis compared with wild type (WT) mice [13]. Moreover, treatment with MMP-7 inhibitor prevented β-catenin release and reduced kidney fibrosis following UUO [13]. MMP-7 degradation of E-cadherin in tubular cells drives loss of cell–cell adhesion, an initial step for tubular atrophy and kidney fibrosis, and the expression of β-catenin-dependent profibrotic genes [5, 13, 27].
MMP-7 also promotes podocyte injury. In isolated glomeruli, MMP-7 degraded the component of the podocyte slit diaphragm nephrin, resulting in increased glomerular permeability and loss of albumin [30]. Indeed, MMP-7-KO mice had better preserved nephrin and WT1 expression and milder albuminuria following chronic angiotensin II infusion than WT mice [30].
In contrast to CKD models, in experimental AKI induced by folic acid, cisplatin, or IRI, MMP-7 deficiency exacerbates early-stage kidney injury, inflammation, and cell death compared to WT mice [29]. Fas ligand (FasL) activation of the Fas receptor promotes kidney cell death [31, 32]. MMP-7 dependent-proteolytic degradation of FasL is suggested as an anti-apoptotic and protective mechanism in AKI, since FasL levels are increased in MMP-7-KO mice and MMP-7 degrades FasL in tubular cells in vitro [29]. Additionally, exogenous MMP-7 decreased the severity of kidney injury and apoptosis in MMP-7-KO mice; however, it would be necessary to treat WT mice with MMP-7 to confirm the relevance of this finding [29]. This protective effect for tubular cells contrasts with its ability to induce apoptosis in renal interstitial fibroblasts by increasing the expression and cleavage of FasL, resulting in its soluble active form [33]. In this regard, FasL-induced apoptosis of kidney fibroblasts may limit kidney fibrosis following acute injury [34]. However, knockdown of MMP-7 with a shRNA reduced the AKI-to-CKD progression in folic acid nephropathy at 14 days after injury and, thus, more studies are necessary to clear the role of MMP-7 in AKI and in AKI-to-CKD progression. More recently, MMP-7 has been also related with kidney inflammation by inducing the expression of inflammasome components NLRP6 and NLRP3 [28].
Given the kidney expression and function of MMP-7, it may play a role as a biomarker in kidney injury and, as it induces podocyte injury, it may play such role in podocytopathies, such as MCD and FSGS.
MMP7 as biomarker in kidney disease: focus on focal and segmental glomerulosclerosis (FSGS) and minimal change disease (MCD)
In this issue of CKJ, Yin et al. explore the use of uMMP-7 to evaluate tubular injury in two diseases that have not been studied before, FSGS and MCD, in young adults as well as in children, a population that is often left out of clinical studies [2]. There were no differences in serum MMP-7 between the control and kidney disease groups, but they did find differences in uMMP-7. Interestingly, the levels of all the urinary small proteins studied, MMP-7, cystatin C and retinol binding protein (RBP) were higher in FSGS than in MCD, despite similar total urinary protein, while only uMMP-7 was increased in MCD. These findings were confirmed by immunohistochemistry, where MMP-7 staining was observed in few tubular cells in MCD compared to the higher MMP-7 staining in FSGS.
The potential of serum, tissue, or urine MMP-7 as a biomarker for kidney disease has been studied previously (Table 1). MMP-7 (among other genes) was upregulated in kidney grafts from patients with interstitial fibrosis and tubular atrophy (IFTA). Indeed, serum MMP-7 levels were higher in transplanted patients with IFTA than in non-transplanted patients or transplanted patients without IFTA [35]. Interestingly, among over 11 000 diabetic and non-diabetic participants in the ARIC cohort, plasma MMP-7 levels correlated with both current kidney function and prospective kidney function decline [11]. However, in a smaller cohort of 141 diabetic kidney disease (DKD) patients, no association between serum MMP-7 levels and progression to kidney failure or mortality was found, while uMMP-7 correlated with risk of progression to kidney failure and mortality [36]. Similar findings were obtained when studying other kidney diseases such as lupus nephritis [37] or AKI following cardiac surgery [38]. Now, Yin et al. have observed that uMMP-7 levels increase in MCD and FSGS while serum MMP-7 levels do not change. Thus, uMMP-7 appears to be a biomarker of interest in kidney disease, but results with serum MMP-7 are conflicting and further studies are needed to determine its potential as a biomarker. Additionally, uMMP-7 may be informative in kidney transplant recipients. Among 133 kidney transplant recipients, the uMMP-7/uCreatinine ratio was higher in patients with inflammation of any kind, including glomerulonephritis, infections, IFTA, and rejection [39]. Furthermore, the combination of the uMMP-7/uCreatinine and uCXCL10/uCreatinine ratios significantly improved the sensitivity and specificity compared with either biomarker alone. uRBP (molecular weight ∼20 kDa) and uCystC (molecular weight 13 kDa) are promising biomarkers to evaluate proximal tubules injury and the resulting failure to reabsorb small-sized proteins, as they filtered by normal glomeruli and should be fully reabsorbed by proximal tubular cells [40]. Now, Yin et al. showed that uMMP-7 combined with uCystC and uRBP can contribute to evaluate renal tubular reabsorption in patients with MCD and FGSG. Circulating MMP-7, as a small protein, is also expected to be filtered by normal glomeruli and reabsorbed by proximal tubules, but MMP-7 of tubular injury may leak into urine. uCysC and uRBP were not significantly elevated in MCD compared to controls, suggesting preserved proximal tubule function. The higher uMMP-7, uRBP, and uCystC levels in FSGS than in MCD support a more severe tubular injury in FSGS.
Table 1:
Studies of MMP-7 as biomarker in kidney disease.
| Study population | n | Sample | Outcome | Comment | Ref |
|---|---|---|---|---|---|
| Hydronephrotic kidneys, diverse kidney diseases | 3 controls 9 HN kidneys |
Kidney tissue mRNA (microarray) | CKD progression | kMMP-7 belongs to a set of 9 genes upregulated in kidney disease and associated with clinical outcomes. | [26] |
| 7 controls 25 renal patients |
|||||
| Kidney transplant recipients | 7 Control tx 22 tx with IF/TA |
Microdissected kidney mRNA (microarray, RT-PCR) | IFTA diagnosis and progression | kMMP-7 increased in all kidney regions of Tx with IFTA. sMMP-7 progressively rise from control non-tx to control tx to IFTA. | [35] |
| 6 Control non-tx 6 Control tx 6 tx with IFTA |
Serum (ELISA) | ||||
| Pediatric kidney transplant recipients | 20 stable allografts 20 acute allograft rejection |
Kidney tissue mRNA (RT-PCR) | Diagnose rejection of renal allografts | kMMP-7 is increased in acute allograft rejection. | [48] |
| 10 healthy kidney donors 10 stable allografts 14 acute allograft rejection |
|||||
| Lupus nephritis | 54 lupus nephritis patients | Kidney tissue mRNA (RT-PCR) | Prediction of prognosis | kMMP-7 positive correlation with chronicity index and kidney function at time of biopsy. | [24] |
| 19 lupus nephritis patients | Kidney tissue (IHQ) | ||||
| Type 1 diabetes Type 2 diabetes with candesartan |
91 type 1 diabetes 11 type 2 diabetes |
Urine (ELISA) | Risk of DKD | uMMP-7 increased in diabetic patients with DKD compared with patients without DKD. Candesartan did not reduce MMP-7. | [53] |
| Type 2 diabetes and DKD | 141 patients with type 2 diabetes and DKD | Fasting blood and urine (ELISA) | Risk of ESRD and mortality | uMMP-7 associated with increased risk of ESRD and mortality while sMMP-7 was not. | [36] |
| Renal transplant patient | Discovery: 14 Tx patients | Urine (proteomic) | Diagnosis of subclinical and clinical inflammation/injury in Tx patients | uMMP-7 increased in inflamed/injured renal allografts compared with normal allografts. Adding urinary MMP7 to CXCL10 improved diagnosis of subclinical and clinical inflammation/injury (improved integrated discrimination). | [39] |
| Quantitative Test: 133 Tx patients | Urine (ELISA) | ||||
| Patients receiving cardiac surgery | Stage 1: 721 adults, 323 children Stage 2: 398 adults |
Urine and blood (ELISA) Before surgery and at follow-up |
Prediction of severe AKI after cardiac surgery | uMMP-7 higher in patients who developed severe AKI within the first 6 hours compared with those with mild or no AKI. However, pMMP-7 not different in patients with or without AKI. | [38] |
| Various CKDs | 102 CKD, 20 healthy | Urine (ELISA) | Non-invasive biomarker for kidney fibrosis | uMMP-7 increased in patients with CKD and positively correlated with kidney fibrosis scores | [13] |
| 10 CKD, 2 healthy | Kidney tissue (IHQ) | ||||
| AKI patients | 28 AKI 15 healthy subjects |
Urine (time-resolved fluoroimmunoassay (IMPs-TRF) and ELISA | Novel method to quantify uMMP-7 | IMPs-TRF good correlation with ELISA | [46] |
| Adult patients with stage 1 or 2 AKI after cardiac surgery | 121 patients Same cohort that PMID 28 698 269 |
Urine (ELISA) before surgery and at follow-up | Early detection of high risk for progressive AKI post-heart surgery | uMMP-7 at time of AKI clinical diagnosis predicts AKI progression. uMMP-7 plus with clinical risk factor model identified high risk for progressive AKI post-heart surgery. | [47] |
| IgA nephropathy | Training set: 554 for 40 months Validation set: 392 for 28 months |
Urine (ELISA), at time of biopsy | IgAN progression (composite of >40% loss of eGFR, kidney failure, or death) | uMMP-7 strongest association with IgAN progression compared with other biomarkers. Adding uMMP-7 to MEST-C score and clinical data at time of biopsy improved risk prediction of IgAN progression. | [44] |
| Lupus nephritis | Stage I: Training set 88 LN, 30 extrarenal SLE, 20 healthy subjects. Bx: 10 LN Validation set: 66 |
Serum and urine (ELISA) Kidney tissue (IHQ) |
Incident renal flare in LN | High uMMP-7 in LN, while sMMP-7 are not. kMMP-7 mainly in tubular cells, correlates with uMMP-7. High uMMP-7 in LN associated with high renal disease activity. | [37] |
| MPO-AAV | Control:30 Test cohort: 90 MPO-AAV Validation cohort: 60 MPO-AAV |
Urine (ELISA) | Predicts kidney prognosis | High uMMP7 level in MPO-AAV independently associated with severe kidney injury and incident ESKD. | [45] |
| Control:10 Test cohort: 90 MPO-AAV |
Kidney tissue (IHQ) | ||||
| General non-diabetic population | Baseline: 1627 subjects, GFR and serum MMP-7 After 5.6 years, in 1324 subjects GFR |
Serum (proteomics) | Accelerated GFR decline. Incident CKD |
sMMP7 independently associated with GFR loss in persons without diabetes or pre-existing CKD. | [51] |
| DKD, and general population | 23 DKD, 10 healthy Validation set: 433 subjects (Including various CKD and healthy) |
Kidney tissue mRNA (RNA-seq) | eGFR decline and kidney fibrosis | kMMP-7 (mRNA and protein) showed the strongest association with both fibrosis and eGFR. |
[14] |
| 23 DKD, 10 healthy Validation set: 23 DKD, 10 healthy. |
Kidney tissue (proteomic) | ||||
| ARIC cohort: 1623 diabetic, 9407 non-diabetic After ± 17 years eGFR |
Plasma (proteomics) | eGFR decline by 50% or ESKD | pMMP7 levels correlated with both current kidney function and prospective kidney function decline |
Abbreviations. HN: hydronephrotic; IFTA: interstitial fibrosis and tubular atrophy; MPO-AAV: myeloperoxidase–antineutrophil cytoplasmic antibody-associated vasculitis; IHQ: immunohistochemistry; RT-PCR: real time-polymerase chain reaction ELISA: enzyme-linked immunosorbent assay; TX: transplant; RNA-seq: RNA sequencing; kMMP-7, uMMP-7, sMMP-7, pMMP-7: kidney, urine, serum, and plasma MMP-7, respectively; LN: lupus nephritis; SLE: systemic lupus erythematosus; DKD: diabetic kidney disease; IgAN: IgA nephropathy; GFR: glomerular filtration rate; ESRD: end-stage renal disease; ESKD: end-stage kidney disease.
The mechanisms underlying the observation that only uMMP-7 is elevated in both FSGS and MCD remain unclear. In this regard, data mining of the Nephroseq database (http://v5.nephroseq.org/) disclosed increased tubulointerstitial MMP7 mRNA expression in human FSGS, which may lead to increased tubular production of MMP-7 that, on top of filtered-but-not-reabsorbed MMP-7, may contribute to increased uMMP-7 (Fig. 1D–E) [41]. However, Schmid et al. reported that tubulointerstitial MMP7 mRNA was not increased in human MCD bulk transcriptomics (Fig. 1F) [42]. This leaves unexplained why uMMP-7, but not urinary levels of other small proteins, was increased in MCD. Yin et al. did observe immunostaining for MMP-7 protein in proximal tubules, suggesting that increased local protein production could contribute to increase uMMP-7. Reabsorption of filtered uMMP-7 protein may also lead to tubular MMP-7 immunostaining. Finally, the report by Schmid et al. may have lacked sensitivity to detect changes in gene expression in proximal tubular cells as they analysed bulk tubulointerstitial tissue [42].
Yin et al. did not estimate GFR by using creatinine or cystatin C-based equations. Given that they have measured urinary levels of small proteins, they could contribute to the ongoing discussion of the so-called selective glomerular hypofiltration syndrome, in which the glomerular filtration of small proteins is hypothesized to be selectively decreased [43].
Further studies showed that uMMP-7 levels correlated with the severity of kidney injury both in AKI and in CKD of diverse causes, such as IgA nephropathy [44], lupus nephritis [37], myeloperoxidase ANCA associated vasculitis (MPO-AAV) [45], and other forms of AKI [46, 47] and of CKD [13]. Among 721 patients undergoing cardiac surgery, including both children and adults, uMMP-7 was high at 6 hours post-surgery in patients that would later develop severe AKI. In addition, higher levels of uMMP-7 had a stronger association with the probability of developing AKI in children (36-fold) than in adults (17-fold) [39]. Overall, the data are consistent with the universality of uMMP-7 as a urinary biomarker of kidney disease.
The increased uMMP-7 levels in various kidney diseases appear to be the result of an increased expression in the kidney, mostly in renal tubular cells [5]. The kidney MMP-7 mRNA expression correlated with the tubular staining by immunohistochemistry of MMP-7 protein [24]. Indeed, kidney MMP-7 staining was increased in patients with CKD of multiple causes and correlated with the degree of fibrosis [13]. Now, Yin et al. also observed that in patients with MCD a few cells express MMP-7, whereas in FSGS the tubular expression is higher. Moreover, single cell transcriptomics identified proximal tubules, connecting tubules, and principal cells as likely cellular sources of increased tissue MMP-7 expression [14]. Thus, there may be two sources of uMMP-7: one from de novo expression in tubular cells and one from glomerular filtration. MMP-7 has also been found to be a key driver of urine peptidomics patterns. MMP-7 was one of the top differentially expressed peptides in the urine peptidomes of kidney transplant recipients with acute rejection, along with peptides resulting from the proteolysis of uromodulin and various collagens [48]. As MMP-7 is an endopeptidase upregulated in kidney diseases, it may influence the urinary peptidome and analysis of the urine peptidomes in different kidney diseases could also shed light on the activity of MMP-7 and its relationship to kidney injury. In this regard, MMP-7 could explain part of the urinary peptidome characteristic of CKD patients [49]. A biomarker panel composed of 273 urinary peptides (CKD273) and variants has been shown to predict CKD progression even in persons without CKD at baseline [50–52]. Recently, combined unbiased kidney proteomics and transcriptomics identified 14 proteins with kidney tissue levels that correlated with eGFR, and 152 proteins that correlated with interstitial fibrosis in patients with DKD [14]. Of them, MMP-7 showed the strongest association with both fibrosis and eGFR.
CONCLUSION
What is the clinical practice takeaway of the findings reported by Yin et al.? We envision two potential contexts of use. First, in children with nephrotic syndrome, the combination of uMMP-7 and either urinary cystatin C or uRBP could help orient the diagnosis (and treatment) towards MCD or FSGS, potentially avoiding a kidney biopsy in the first case. In this regard, kidney biopsy is usually avoided in children, if possible, mostly because of its invasiveness and its potentially harmful, although very rare, complications, but also because of the psychological stress that this diagnostic test provokes in young patients. In a second context of use, the analysis or urinary small proteins may help suspect an underlying FSGS, even if the biopsy showed MCD, when the clinical course is atypical or eGFR progressively decreases, as the focal and segmental nature of FSGS may limit the sensitivity of the kidney biopsy to diagnose it. In any case, before widespread clinical implementation, the results should be externally validated by independent groups, ideally addressing the impact of these biomarkers in diagnosis, prognosis, treatment, and outcomes of childhood and other nephrotic syndromes in different contexts of use.
Contributor Information
Alejandro Avello, Laboratory of Experimental Nephrology, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Juan Guerrero-Mauvecin, Laboratory of Experimental Nephrology, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Ana Belen Sanz, Laboratory of Experimental Nephrology, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain; RICORS2040, Madrid, Spain.
FUNDING
Instituto de Salud Carlos III/FEDER funds (PI22/00 469, PI19/00 588, ERA-PerMed-JTC2018-PERSTIGAN AC18/00 071), Sociedad Española de Nefrología. Ramon y Cajal program to ABS (RYC2019-026916-I) and FPU program TO JG-M (FPU18/01 341).
CONFLICT OF INTEREST STATEMENT
All authors declare no competing interests.
REFERENCES
- 1. Ortiz A. RICORS2040: the need for collaborative research in chronic kidney disease. Clin Kidney J 2021;15:372–87. 10.1093/ckj/sfab170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yin DY, Hou GL, Yang XQet al. Urinary matrix metalloproteinase-7 is a sensitive biomarker to evaluate renal tubular injury in patients with minimal change disease and focal segmental glomerulosclerosis. Clin Kidney J, 2023. 10.1093/ckj/sfad027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. D'Agati VD, Kaskel FJ, Falk RJ.. Focal segmental glomerulosclerosis. N Engl J Med 2011;365:2398–411. 10.1056/NEJMra1106556 [DOI] [PubMed] [Google Scholar]
- 4. Gaide Chevronnay HP, Selvais C, Emonard Het al. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim Biophys. Acta (BBA)—Proteins Proteom 2012;1824:146–56. 10.1016/j.bbapap.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 5. Liu Z, Tan RJ, Liu Y.. The many faces of matrix metalloproteinase-7 in kidney diseases. Biomolecules 2020;10:960. 10.3390/biom10060960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 2005;6:931–44. 10.1038/nrn1807 [DOI] [PubMed] [Google Scholar]
- 7. Wang D, Dai C, Li Yet al. Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int 2011;80:1159–69. 10.1038/ki.2011.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He W, Dai C, Li Yet al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 2009;20:765–76. 10.1681/ASN.2008060566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ke B, Fan C, Yang Let al. Matrix metalloproteinases-7 and kidney fibrosis. Front Physiol 2017;8:21. 10.3389/fphys.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z, Famulski K, Lee Jet al. TIMP2 and TIMP3 have divergent roles in early renal tubulointerstitial injury. Kidney Int 2014;85:82–93. 10.1038/ki.2013.225 [DOI] [PubMed] [Google Scholar]
- 11. Hamze AB, Wei S, Bahudhanapati Het al. Constraining specificity in the N-domain of tissue inhibitor of metalloproteinases-1; gelatinase-selective inhibitors. Protein Sci 2007;16:1905–13. 10.1110/ps.072978507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wozniak J, Floege J, Ostendorf Tet al. Key metalloproteinase-mediated pathways in the kidney. Nat Rev Nephrol 2021;17:513–27. 10.1038/s41581-021-00415-5 [DOI] [PubMed] [Google Scholar]
- 13. Zhou D, Tian Y, Sun Let al. Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. JASN 2017;28:598–611. 10.1681/ASN.2016030354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirohama D, Abedini A, Moon Set al. Unbiased human kidney tissue proteomics identifies matrix metalloproteinase 7 as a kidney disease biomarker. JASN 2023;34:1279–91. 10.1681/ASN.0000000000000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson PC, Wu H, Kirita Yet al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci USA 2019;116:19619–25. 10.1073/pnas.1908706116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uhlén M, Fagerberg L, Hallström BMet al. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 17. Tan RJ, Liu Y.. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol 2012;302:F1351–61. 10.1152/ajprenal.00037.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGuire JK, Li Q, Parks WC.. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 2003;162:1831–43. 10.1016/S0002-9440(10)64318-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vargo-Gogola T, Crawford HC, Fingleton Bet al. Identification of novel matrix metalloproteinase-7 (matrilysin) cleavage sites in murine and human Fas ligand. Arch Biochem Biophys 2002;408:155–61. 10.1016/S0003-9861(02)00525-8 [DOI] [PubMed] [Google Scholar]
- 20. Mitsiades N, Yu WH, Poulaki Vet al. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res 2001;61:577–81. [PubMed] [Google Scholar]
- 21. Haro H, Crawford HC, Fingleton Bet al. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest 2000;105:143–50. 10.1172/JCI7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosas IO, Richards TJ, Konishi Ket al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 2008;5:e93. 10.1371/journal.pmed.0050093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 2010;21:212–22. 10.1681/ASN.2008121226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reich HN, Landolt-Marticorena C, Boutros PCet al. Molecular markers of injury in kidney biopsy specimens of patients with lupus nephritis. J Mol Diagn 2011;13:143–51. 10.1016/j.jmoldx.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Surendran K, Simon TC, Liapis Het al. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int 2004;65:2212–22. 10.1111/j.1523-1755.2004.00641.x [DOI] [PubMed] [Google Scholar]
- 26. Henger A, Kretzler M, Doran Pet al. Gene expression fingerprints in human tubulointerstitial inflammation and fibrosis as prognostic markers of disease progression. Kidney Int 2004;65:904–17. 10.1111/j.1523-1755.2004.00499.x [DOI] [PubMed] [Google Scholar]
- 27. He W, Tan RJ, Li Yet al. Matrix metalloproteinase-7 as a surrogate marker predicts renal wnt/β-catenin activity in CKD. J Am Soc Nephrol 2012;23:294–304. 10.1681/ASN.2011050490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng CM, Lu KC, Chen YJet al. Matrix metalloproteinase-7 promotes chronic kidney disease progression via the induction of inflammasomes and the suppression of autophagy. Biomed Pharmacother 2022;154:113565. 10.1016/j.biopha.2022.113565 [DOI] [PubMed] [Google Scholar]
- 29. Fu H, Zhou D, Zhu Het al. Matrix metalloproteinase-7 protects against acute kidney injury by priming renal tubules for survival and regeneration. Kidney Int 2019;95:1167–80. 10.1016/j.kint.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan RJ, Li Y, Rush BMet al. Tubular injury triggers podocyte dysfunction by β-catenin-driven release of MMP-7. JCI Insight 2019;4:e122399. 10.1172/jci.insight.122399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lorz C, Ortiz A, Justo Pet al. Proapoptotic Fas ligand is expressed by normal kidney tubular epithelium and injured glomeruli. J Am Soc Nephrol 2000;11:1266–77. 10.1681/ASN.V1171266 [DOI] [PubMed] [Google Scholar]
- 32. Ortiz A, Lorz C, Egido J.. The fas ligand/fas system in renal injury. Nephrol Dial Transplant 1999;14:1831–4. 10.1093/ndt/14.8.1831 [DOI] [PubMed] [Google Scholar]
- 33. Zhou D, Tan RJ, Zhou Let al. Kidney tubular β-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep 2013;3:1878. 10.1038/srep01878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ortiz A, Lorz C, González-Cuadrado Set al. Cytokines and Fas regulate apoptosis in murine renal interstitial fibroblasts. J Am Soc Nephrol 1997;8:1845–54. 10.1681/ASN.V8121845 [DOI] [PubMed] [Google Scholar]
- 35. Rödder S, Scherer A, Raulf Fet al. Renal allografts with IF/TA display distinct expression profiles of metzincins and related genes. Am J Transplant 2009;9:517–26. 10.1111/j.1600-6143.2008.02512.x [DOI] [PubMed] [Google Scholar]
- 36. Afkarian M, Zelnick LR, Ruzinski Jet al. Urine matrix metalloproteinase-7 and risk of kidney disease progression and mortality in type 2 diabetes. J Diabetes Complications 2015;29:1024–31. 10.1016/j.jdiacomp.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang G, Wu L, Su Het al. Association of urinary matrix metalloproteinase 7 levels with incident renal flare in lupus nephritis. Arthritis Rheumatol 2021;73:265–75. 10.1002/art.41506 [DOI] [PubMed] [Google Scholar]
- 38. Yang X, Chen C, Teng Set al. Urinary matrix metalloproteinase-7 predicts severe AKI and poor outcomes after cardiac surgery. JASN 2017;28:3373–82. 10.1681/ASN.2017020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho J, Rush DN, Krokhin Oet al. Elevated urinary matrix metalloproteinase-7 detects underlying renal allograft inflammation and injury. Transplantation 2016;100:648–54. 10.1097/TP.0000000000000867 [DOI] [PubMed] [Google Scholar]
- 40. Jana S, Mitra P, Roy S.. Proficient novel biomarkers guide early detection of acute kidney injury: a review. Diseases 2022;11:8. 10.3390/diseases11010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ju W, Nair V, Smith Set al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 2015;7:316ra193. 10.1126/scitranslmed.aac7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmid H, Boucherot A, Yasuda Yet al. Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes 2006;55:2993–3003. 10.2337/db06-0477 [DOI] [PubMed] [Google Scholar]
- 43. Malmgren L, Öberg C, Bakker Eet al. The complexity of kidney disease and diagnosing it—cystatin C, selective glomerular hypofiltration syndromes and proteome regulation. J Intern Med 2023;293:293–308. 10.1111/joim.13589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang X, Ou J, Zhang Het al. Urinary matrix metalloproteinase 7 and prediction of IgA nephropathy progression. Am J Kidney Dis 2020;75:384–93. 10.1053/j.ajkd.2019.07.018 [DOI] [PubMed] [Google Scholar]
- 45. Wu L, Wang G, Yang Bet al. Urinary matrix metalloproteinase 7 activated by oxidative stress predicts kidney prognosis in myeloperoxidase-antineutrophil cytoplasmic antibody-associated vasculitis. Antioxid Redox Signaling 2022;37:246–56. 10.1089/ars.2021.0188 [DOI] [PubMed] [Google Scholar]
- 46. Liang J, Lin G, Tian Jet al. Measurement of urinary matrix metalloproteinase-7 for early diagnosis of acute kidney injury based on an ultrasensitive immunomagnetic microparticle-based time-resolved fluoroimmunoassay. Clin Chim Acta 2019;490:55–62. 10.1016/j.cca.2018.11.037 [DOI] [PubMed] [Google Scholar]
- 47. Fang F, Luo W, Yang Met al. Urinary matrix metalloproteinase-7 and prediction of AKI progression post cardiac surgery. Dis Markers 2019;2019:1. 10.1155/2019/9217571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ling XB, Sigdel TK, Lau Ket al. Integrative urinary peptidomics in renal transplantation identifies biomarkers for acute rejection. J Am Soc Nephrol 2010;21:646–53. 10.1681/ASN.2009080876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Petra E, Siwy J, Vlahou Aet al. Urine peptidome in combination with transcriptomics analysis highlights MMP7, MMP14 and PCSK5 for further investigation in chronic kidney disease. PLoS One 2022;17:e0262667. 10.1371/journal.pone.0262667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tofte N, Lindhardt M, Adamova Ket al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): a prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol 2020;8:301–12. 10.1016/S2213-8587(20)30026-7 [DOI] [PubMed] [Google Scholar]
- 51. Enoksen IT, Svistounov D, Norvik JVet al. Serum matrix metalloproteinase 7 and accelerated glomerular filtration rate decline in a general non-diabetic population. Nephrol Dial Transplant 2022;37:1657–67. 10.1093/ndt/gfab251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodríguez-Ortiz ME, Pontillo C, Rodríguez Met al. Novel urinary biomarkers for improved prediction of progressive egfr loss in early chronic kidney disease stages and In high risk individuals without chronic kidney disease. Sci Rep 2018;8:15940. 10.1038/s41598-018-34386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Afkarian M, Hirsch IB, Tuttle KRet al. Urinary excretion of RAS, BMP, and WNT pathway components in diabetic kidney disease. Physiol Rep 2014;2:e12010. 10.14814/phy2.12010 [DOI] [PMC free article] [PubMed] [Google Scholar]