ABSTRACT
Background
Kynurenine is a protein-bound uremic toxin. Its circulating levels are increased in chronic kidney disease (CKD). Experimental studies showed that it exerted deleterious cardiovascular effects. We sought to evaluate an association between serum kynurenine levels and adverse fatal or nonfatal cardiovascular events and all-cause mortality in CKD patients.
Methods
The CKD-REIN study is a prospective cohort of people with CKD having an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m². Baseline frozen samples of total and free fractions of kynurenine and tryptophan were measured using a validated liquid chromatography tandem mass spectrometry technique. Cause-specific Cox models were used to estimate hazard ratios (HRs) for each outcome.
Results
Of the 2406 included patients (median age: 68 years; median eGFR: 25 ml/min/1.73 m2), 52% had a history of cardiovascular disease. A doubling of serum-free kynurenine levels was associated with an 18% increased hazard of cardiovascular events [466 events, HR (95%CI):1.18(1.02,1.33)], independently of eGFR, serum-free tryptophan level or other uremic toxins, cardioprotective drugs, and traditional cardiovascular risk factors. Serum-free kynurenine was significantly associated with non-atheromatous cardiovascular events [HR(95%CI):1.26(1.03,1.50)], but not with atheromatous cardiovascular events [HR(95%CI):1.15(0.89,1.50)]. The association of serum-free kynurenine with cardiovascular mortality was also independently significant [87 events; adjusted HR(95%CI):1.64(1.10,2.40)]. However, the association of serum-free kynurenine with all-cause mortality was no more significant after adjustment on serum-free tryptophan [311 events, HR(95%CI):1.12(0.90, 1.40)].
Conclusions
Our findings imply that serum-free kynurenine, independently of other cardiovascular risk factors (including eGFR), is associated with fatal or nonfatal cardiovascular outcomes, particularly non-atheromatous cardiovascular events; in patients with CKD. Strategies to reduce serum kynurenine levels should be evaluated in further studies.
Keywords: all-cause mortality, cardiovascular disease, chronic kidney disease, kynurenine
KEY LEARNING POINTS.
What was known:
Kynurenine is a protein-bound uremic toxin derived from tryptophan metabolism. Experimental studies showed that kynurenine caused endothelial cell dysfunction, abnormal angiogenesis and vascular thrombosis.
Very few clinical studies have shown the association between total kynurenine levels and cardiovascular disease in pre-dialysis CKD patients, none of them measured free kynurenine levels.
This study adds:
Serum-free kynurenine levels are associated with fatal or nonfatal cardiovascular events in patients with CKD, independently of eGFR, serum-free tryptophan level or other uremic toxins, cardioprotective drugs and traditional cardiovascular risk factors.
Kynurenine is a promising candidate for understanding the complex interplay between CKD and cardiovascular disease.
Potential impact:
Kynurenine could be considered as a predictive biomarker for cardiovascular risk in CKD patients after confirmation by predictive analysis methodologies. This could influence clinical guidelines for managing CKD patients.
Therapeutic strategies to reduce serum kynurenine levels should be evaluated in further studies, by inhibiting, for example, the IDO enzyme.
INTRODUCTION
Chronic kidney disease (CKD) is common and emerges as one of the leading non-communicable causes of death worldwide [1, 2]. Patients with CKD are at high cardiovascular risk, with cardiovascular death being the leading cause of death [3]. Traditional cardiovascular risk factors such as ageing, hypertension, and diabetes are highly prevalent in patients with CKD, but fail to explain the increase in cardiovascular risk. Non-traditional cardiovascular risk factors such as oxidative stress, and toxic compounds accumulation have been identified in patients with CKD [3,4]. Indeed, as kidney functions decline, an array of molecules called uremic toxins (UTs) will pathologically accumulate in the organism [5–7].
The majority of UTs are derived from amino acid metabolism in the gut [8]. Previous epidemiological studies have shown an association between many of them (such as para-cresyl sulfate, p-cresyl glucuronide, indoxyl sulfate, and indole-3-acetic acid) and cardiovascular outcomes [3, 9]. For example, the UTs indoxyl sulfate and kynurenine are derived from tryptophan metabolism [10]. Tryptophan is an essential aromatic amino acid, and the only protein-bound one (around 85% bound to albumin). Initially, kynurenine is derived from tryptophan metabolism in intestinal epithelial cells by the activation of the enzyme indolamine-2,3-dioxygenase-1 [11]. After absorption, the free fraction of tryptophan (i.e. the active fraction), continues to be metabolized to several bioactive compounds, particularly kynurenine, by different cell types including endothelial cells [12]. The enzymatic conversion of tryptophan is enhanced in inflammatory states, including CKD [4]. Serum kynurenine levels are increased in CKD, not only as a consequence of decreased renal clearance but also of alterations in tryptophan metabolism [4].
Previous studies on the effects of UTs mainly focused on the toxicity of indoxyl sulfate [3]. Only few examined the effects of kynurenine [3] despite being the first metabolite derived from the kynurenine pathway [4], which is the most occurring one from tryptophan extensive metabolism (Fig. S1, see online supplementary material) [13]. Kynurenine is a protein-bound molecule, whose free fraction is the toxic one [7]. In general, several analytical methods allow the quantification of both total and free fractions of protein-bound uremic toxins [14]. However, measuring the free fraction is generally complex, explaining the availability of only limited data on free kynurenine.
Experimental studies showed that kynurenine caused endothelial cell dysfunction, abnormal angiogenesis, and vascular thrombosis [4, 15, 16].
An association between total serum kynurenine concentrations and incident cardiovascular disease and all-cause mortality has previously been reported in the elderly and in patients with stable angina [17, 18]. However, to our knowledge, very few clinical studies have shown the association between total kynurenine levels and cardiovascular disease in pre-dialysis CKD patients [15, 19–28], none of them measured free kynurenine levels and none examined an association with all-cause mortality in patients with moderate or advanced stages of CKD [29], in a large representative sample. Moreover, data on kynurenine mainly focused on their association with atheromatous cardiovascular events. Given the burden of non-atheromatous cardiovascular disease in CKD, it is important to separately assess the link between kynurenine and these distinct pathologies.
The aim of the present study was to examine an association between both serum-free and total kynurenine levels with the occurrence of incident fatal or nonfatal cardiovascular events, both globally and by subtypes of cardiovascular pathologies (atheromatous cardiovascular events and non-atheromatous cardiovascular events), in a large French national sample of patients with pre-dialysis CKD, independently of traditional cardiovascular risk factors, estimated glomerular filtration rate (eGFR), and serum tryptophan levels. We further evaluated an independent association with all-cause mortality before kidney replacement therapy (KRT), both globally and by subtypes of cardiovascular death.
MATERIALS AND METHODS
Study design and participants
The Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) is a large prospective cohort enrolling 3033 adult patients between July 2013 and April 2016 from 40 representative nephrology outpatient clinics in France. The eligibility criteria were age 18 or over, a confirmed diagnosis of moderate or advanced CKD (eGFR <60 ml/min/1.73 m2), and no prior maintenance dialysis or kidney transplantation. All patients provided written informed consent before inclusion for five years of annual follow-up. Details of the study protocol have been published elsewhere [30].
Data were collected by trained clinical research associates from medical records or patient interviews at baseline and then annually. Upon enrolment of the patients, urine and serum samples were collected. For more details concerning data collection please see the online supplementary material. The CKD-REIN study was approved by the French National Institute of Health and Medical Research (INSERM, Paris, France) institutional review board (IRB00003888) and the Ethics Committee (CCTIRS12.360/CPP).
The present study included 2406 patients who provided available biosamples within a 3-month window of inclusion (Fig. 1).
Figure 1:
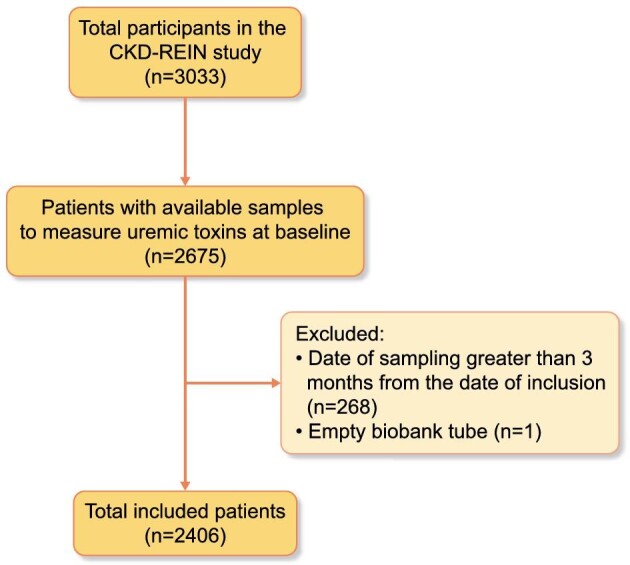
Flowchart of the study.
Quantification of uremic toxins and tryptophan
All CKD-REIN samples were stored deep frozen (−80°C) at the Biobanque de Picardie (BRIF number: BB-0033–00 017) and shipped frozen to Paris for analysis. Kynurenine, indoxyl sulfate, p-cresyl sulfate, indole-3-acetic acid and tryptophan fractions were assayed in serum using a validated liquid chromatography tandem mass spectrometry technique, for a description, see the online supplementary material [14].
Evaluation of outcomes
Fatal or nonfatal cardiovascular events before kidney replacement therapy
The primary endpoint was the occurrence of a first fatal or nonfatal cardiovascular event during the follow-up period. Nonfatal events were traced from hospital and medical records, and/or annual patient interviews. They were assessed by a physician using criteria from the Cardiovascular and Stroke Endpoint Definition for Clinical Trials [31]. All cardiovascular deaths were further adjudicated by two blinded cardiologists (MH, NM). Fatal or nonfatal cardiovascular events were studied as composite outcomes of sudden cardiac deaths, aortic aneurysm, atheromatous and non-atheromatous cardiovascular events for which distinction between them was also adjudicated by a blinded committee. Atheromatous cardiovascular events included coronary artery disease, ischemic cerebrovascular disease, and peripheral artery disease (i.e. lower limb artery disease and renal artery stenosis), and non-atheromatous cardiovascular events included heart failure, hemorrhagic stroke, atrial fibrillation and other cardiac rhythm/conduction disorders. Because of uncertainty about the underlying mechanisms for some sudden cardiac death events and about the specificity of classifying aortic aneurysm, these events were not classified in either category.
Coronary artery disease included myocardial infarction, unstable angina, and coronary interventions: angioplasty-stenting or coronary artery bypass grafting or coronary thrombolysis. Ischemic cerebrovascular disease included ischemic stroke, transient ischemic attack, and carotid interventions: thrombolysis, thrombectomy, endarterectomy, angioplasty-stenting, or bypass surgery. Lower limb artery disease included vascular claudication, and interventions such as angioplasty-stenting, endarterectomy, bypass surgery or amputation for atheromatous ischemic lesions. Heart failure was defined as new onset, or worsening heart failure. Arrhythmias and conduction disorders were documented by electrocardiography.
All the considered events occurred before any KRT. KRT was defined as kidney failure requiring initiation of maintenance dialysis or preemptive kidney transplantation [as identified from medical records or by linkage with the French National Renal Epidemiology Information Network registry (REIN)].
All-cause death events before kidney replacement therapy
All-cause mortality included any registered death, either cardiovascular or non-cardiovascular mortality. Death events were declared by family members or by clinical research associates during the 5-year follow-up, ascertained from death certificates and hospital records, or through linkage with the national death registry.
As previously mentioned, cardiovascular mortality was adjudicated by cardiologists. Any other deaths were categorized as non-cardiovascular mortality.
Statistical analysis
Participants’ baseline characteristics are described for the overall data sample (n = 2406) and by tertiles of serum free kynurenine levels. Description by tertiles of serum total kynurenine are depicted in the Table S1 (see online supplementary material). Continuous variables are presented as the median [Q1–Q3] and compared between the three groups with the Kruskal–Wallis test. Categorical variables are presented as percentages and compared between groups with the chi-square test. The variations according to CKD stages of serum free, total, and free/total kynurenine concentrations, free and total tryptophan, kynurenine/tryptophan ratio, respectively, are compared between groups with the Kruskal–Wallis test, and followed by a post-hoc analysis using Dunn's test (Figs S2–S5, see online supplementary material).
Given that the serum kynurenine values were not normally distributed they were log-transformed. For each of the six study outcomes, we used cause-specific Cox models to estimate crude and adjusted hazard ratios (HRs) (95% CI) associated with each of the free and total kynurenine levels (associations with the latter are included in the Tables S2–S5, S7, and S9, see online supplementary material): HRs of (i) fatal or nonfatal cardiovascular events; (ii) atheromatous cardiovascular events; (iii) non-atheromatous cardiovascular events; (iv) all-cause mortality; (v) cardiovascular mortality; and (vi) non-cardiovascular mortality. Fatal or nonfatal cardiovascular events (including atheromatous or non-atheromatous cardiovascular events) were censored on the date of last follow-up (fifth annual follow-up visit or study withdrawal), the date of KRT, or the date of death from a non-cardiovascular cause. Each death occurring during the 5-year follow-up period was used in the all-cause mortality model, and the data were censored at the date of last follow-up or the date of KRT. Cardiovascular death and non-cardiovascular death were censored on the date of non-cardiovascular death and the date of cardiovascular death respectively, in addition to the date of KRT and the date of last follow-up.
The Cox models were adjusted for a set of confounders, which were selected from a directed acyclic graph (DAG), avoiding adjustment for unnecessary factors, mediators, and colliders [32]. The approach has been further detailed in the online supplementary material. Associations between serum kynurenine and overall cardiovascular risks, all-cause mortality, cardiovascular and non-cardiovascular mortality risks were adjusted for age at baseline, sex, high-sensitivity C-reactive protein (CRP), urea, diabetes, dyslipidemia, systolic blood pressure, body mass index (BMI), smoking status, baseline eGFR, urine albumin or protein-to-creatinine ratio (ACR), serum free tryptophan, serum free indoxyl sulfate, indole-3-acetic acid and p-cresyl sulfate, prescriptions of diuretics, antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs. We excluded the prescription of diuretics in the adjustment models evaluating the association with atheromatous cardiovascular risk, as well as dyslipidemia and lipid-lowering drugs in the models evaluating the association with non-atheromatous cardiovascular risk. We added serum albumin to the models when evaluating serum-free kynurenine.
The HRs of Cox models were transformed back into the original unlogged scales in order to obtain better interpretable values.
Each model's validity (according to the proportional hazard assumption) was checked by testing the Schoenfeld residuals.
The method used to manage missing data is presented in the online supplementary material.
The threshold for statistical significance was P <0.05. All statistical analyses were performed with R software.
RESULTS
Baseline patients’ characteristics
The characteristics of the 2406 patients included in this study are presented in Table 1, compared between the tertiles of free kynurenine levels. The median (Q1–Q3) age of the participants was 68 (60–76) years, 66% were men, and the median BMI was 27.9 (24.7–31.7) kg/m2. Almost all the patients had hypertension (96%), a large proportion had dyslipidemia (74%), about half of them presented a history of cardiovascular disease (52%), and 41% had diabetes. The overall median eGFR was 24.7 (18.4–33.1) ml/min/1.73 m2, and was significantly lower in patients with the highest free kynurenine tertiles. Compared with patients in tertile 1 and tertile 2, patients in tertile 3 were older, had more comorbidities, a higher BMI, and higher serum concentrations of CRP and free tryptophan.
Table 1:
Characteristics of the study population according to the tertiles of serum-free kynurenine.
| Total | Tertiles of serum-free kynurenine (mg/L) | |||||
|---|---|---|---|---|---|---|
| T1 < 0.11 | T2 [0.11–0.16] | T3 >=0.16 | ||||
| Characteristics | N = 2406a | (n = 802)a | (n = 801)a | (n = 803)a | P-valueb | Missing data |
| Age at baseline (years) | 68 [60–76] | 67 [58–74] | 69 [60–76] | 69 [62- 77] | <0.0001 | 0% |
| Men | 66% | 68% | 67% | 63% | 0.07 | 0% |
| eGFR at baseline (ml/min/1.73 m2) | 25 [18–33] | 31 [24–40] | 25 [19–32] | 20 [15–26] | <0.0001 | 0% |
| Albumin- or protein-to-creatinine ratio (mg/g) | 104 [21–491] | 57 [14–345] | 104 [23–440] | 198 [34–721] | <0.0001 | 11.5% |
| History of acute kidney injury | 23% | 20% | 24% | 27% | 0.005 | 7.2% |
| Smoking statusNever-smokerCurrent smokerFormer smoker | 40%13%47% | 41%13%46% | 40%13%46% | 40%10%50% | 0.3 | 0.6% |
| Systolic blood pressure (mmHg) | 140 [128- 153] | 138 [127–151] | 140 [129- 152] | 140 [130–155] | 0.014 | 0.5% |
| Diabetes | 41% | 35% | 41% | 47% | <0.0001 | 0.2% |
| Dyslipidemia | 74% | 70% | 73% | 77% | 0.008 | 0.4% |
| History of cardiovascular disease | 52% | 46% | 51% | 60% | <0.0001 | 0.5% |
| Serum bicarbonate (mmol/L) | 25 [23–27] | 25.4 [23–27.4] | 25 [22.4–27] | 24.5 [22–27] | <0.0001 | 8.8% |
| Serum albumin (g/L) | 40.4 [38–43] | 41 [38.4–43.2] | 41 [38–43] | 40 [37–42] | <0.0001 | 14.5% |
| Hemoglobin (g/dL) | 13 [11.8–14.2] | 13.4[12.3–14.5] | 13 [11.9–14.2] | 12.6 [11.5–13.7] | <0.0001 | 0.9% |
| High-sensitivity C-reactive protein (mg/L) | 2.4 [1.1–5.1] | 2 [0.9–4.1] | 2.3 [1.1–4.8] | 2.9 [1.3–6.5] | <0.0001 | 5.4% |
| Body mass index (kg/m²) | 27.9 [24.7–31.7] | 27.2 [24.3–30.8] | 27.8 [24.5–31.6] | 28.8 [25.4–32.8] | <0.0001 | 1.9% |
| PPI prescription at baseline | 32% | 30% | 31% | 36% | 0.01 | 0.2% |
| Diuretics prescription at baseline | 52% | 43% | 51% | 63% | <0.0001 | 0.3% |
| Antihypertensive drugs prescription at baseline | 2243 (94%) | 722 (91%) | 753 (94%) | 768 (96%) | <0.0001 | 0.3% |
| Lipid-lowering drugs prescription at baseline | 1520 (63%) | 472 (59%) | 510 (64%) | 538 (67%) | 0.005 | 0.3% |
| Antidiabetic drugs prescription at baseline | 820 (34%) | 231 (29%) | 275 (34%) | 314 (39%) | <0.001 | 0.3% |
| Free tryptophan (mg/L) | 0.9 [0.7–1.2] | 0.7 [0.6–0.9] | 0.9 [0.8–1.1] | 1.2 [0.9–1.5] | <0.0001 | 0% |
| Total tryptophan (mg/L) | 12 [10–15] | 12 [10–15] | 12.6 [10–15] | 12 [9.9–14.6] | 0.1 | 0% |
| Free kynurenine (mg/L) | 0.1 [0.09–0.2] | 0.07 [0.06–0.08] | 0.12 [0.11–0.14] | 0.2 [0.18–0.26] | – | 0% |
| Total kynurenine (mg/L) | 1.2 [0.9–1.6] | 0.9 [0.7–1.2] | 1.2 [0.9–1.6] | 1.4 [1.2–1.8] | <0.0001 | 0% |
Median [Q1–Q3]; %
Kruskal–Wallis rank sum test; Pearson's chi-squared test eGFR: estimated glomerular filtration rate; PPI: proton pump inhibitor.
Statistically significant p-values are given in bold type.
The distribution of serum-free and total kynurenine levels, free/total kynurenine ratio, free and total tryptophan levels, and kynurenine/tryptophan ratio by CKD stage (Figs S2–S5, see online supplementary material). Patients with more advanced CKD had significantly higher free and total kynurenine concentrations with a higher free/total kynurenine ratio. Total tryptophan levels were significantly lower in more advanced CKD stages, while free levels were higher. The free kynurenine/free tryptophan ratio was significantly higher in more advanced CKD stages compared to stages 2 and 3. Similarly, the total kynurenine/free tryptophan ratio was significantly higher in stage 4 compared to stages 2 and 3.
Fatal or nonfatal cardiovascular events
Over a median [Q1–Q3] follow-up period of 5 [4.6–5.2] years, 466 out of the 2406 patients experienced a first cardiovascular event (fatal or nonfatal). The crude analysis showed that a doubling of serum free kynurenine levels was associated with a 70%-increase in the hazard of a cardiovascular event [HR (95% CI): 1.7 (1.5, 1.9) Table 2]. This association was attenuated but remained significant after multiple adjustments (Table 2): a double increase in serum free kynurenine levels was associated with an 18% increase in the hazard of a cardiovascular event [adjusted HR (95% CI): 1.18 (1.02, 1.33)], independently of kidney function (eGFR), serum free tryptophan level or other uremic toxins, cardioprotective drugs and traditional cardiovascular risk factors.
Table 2:
Hazard ratio of cardiovascular events associated with a double increase in baseline serum-free kynurenine level (mg/L).
| Free kynurenine (mg/L) | |
|---|---|
| Cardiovascular events | |
| Unadjusted HR [95% CI]—P-value | 1.7 [1.5, 1.9]–<0.0001 |
| Adjusted HR a [95% CI]—P-value | 1.25 [1.09, 1.4]–0.002 |
| Adjusted HR b [95% CI]—P-value | 1.18 [1.02, 1.33]–0.04 |
| Adjusted HR c [95% CI]—P-value | 1.19 [1.03, 1.39]–0.01 |
Model adjusted for age at baseline, sex, high-sensitivity C-reactive protein, serum albumin, urea, diabetes, dyslipidemia, body mass index, smoking status, baseline estimated glomerular filtration rate, urine albumin- or protein-to-creatinine ratio, systolic blood pressure, prescriptions of diuretics, antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs.
1 + serum-free tryptophan.
1 + serum-free indoxyl sulfate, indole-3-acetic acid and p-cresyl sulfate.
The results were in agreement with those of the association with total kynurenine levels: a double increase of the latter was associated with a 20% increase in the hazard of cardiovascular events, independently of eGFR and serum free tryptophan level or tryptophan-derived UTs [adjusted HR: 1.2 (1.05, 1.4)] (Table S2, see online supplementary material).
Analysis by subgroups of atheromatous vs non-atheromatous cardiovascular events
Atheromatous cardiovascular events
During follow-up, 215 patients had fatal or nonfatal atheromatous cardiovascular events. There was no significant association between serum-free kynurenine levels and the occurrence of atheromatous cardiovascular events after multiple adjustments (Table 3). Adding free tryptophan to the adjusted model did not affect these results.
Table 3:
Hazard ratio of atheromatous cardiovascular events associated with a double increase in baseline serum-free kynurenine level (mg/L).
| Free kynurenine (mg/L) | |
|---|---|
| Atheromatous cardiovascular events | |
| Unadjusted HR [95% CI]—P-value | 1.4 [1.17, 1.69]–0.0002 |
| Adjusted HR a [95% CI]—P-value | 1.15 [0.9, 1.4]–0.2 |
| Adjusted HR b [95% CI]—P-value | 1.15 [0.89, 1.5]–0.2 |
| Adjusted HR c [95% CI]—P-value | 1.08 [0.86, 1.4]–0.5 |
Model adjusted for age at baseline, sex, high-sensitivity C-reactive protein, serum albumin, urea, diabetes, dyslipidemia, body mass index, smoking status, baseline estimated glomerular filtration rate, urine albumin- or protein-to-creatinine ratio, systolic blood pressure, prescriptions of antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs.
1 + serum-free tryptophan.
1 + serum-free indoxyl sulfate, indole-3-acetic acid and p-cresyl sulfate.
However, total kynurenine levels were significantly associated with atheromatous cardiovascular events after multiple adjustments (Table S3, see online supplementary material).
Non-atheromatous cardiovascular events
A total of 313 patients had fatal or nonfatal non-atheromatous cardiovascular events in the follow-up period. A doubling of serum-free kynurenine levels was significantly associated with a 26% increase in the hazard of non-atheromatous cardiovascular events after multiple adjustments [adjusted HR (95% CI): 1.26 (1.03, 1.50)] (Table 4).
Table 4:
Hazard ratio of non-atheromatous cardiovascular events associated with a double increase in baseline serum-free kynurenine level (mg/L).
| Free kynurenine (mg/L) | |
|---|---|
| Non-atheromatous cardiovascular events | |
| Unadjusted HR [95% CI]—P-value | 1.97 [1.7, 2.27]–<0.001 |
| Adjusted HR a [95% CI]—P-value | 1.36 [1.14, 1.61]–0.0007 |
| Adjusted HR b [95% CI]—P-value | 1.26 [1.03, 1.5]–0.02 |
| Adjusted HR c [95% CI]—P-value | 1.31 [1.09, 1.6]–0.003 |
Model adjusted for age at baseline, sex, high-sensitivity C-reactive protein, serum albumin, urea, diabetes, body mass index, smoking status, baseline estimated glomerular filtration rate, urine albumin- or protein-to-creatinine ratio, systolic blood pressure, prescriptions of diuretics, antihypertensive drugs, and antidiabetic drugs.
1 + serum-free tryptophan.
1 + serum-free indoxyl sulfate, indole-3-acetic acid and p-cresyl sulfate.
The results were similar when evaluating serum total kynurenine, exhibiting a significant association with the occurrence of non-atheromatous cardiovascular events (Table S4, see online supplementary material).
All-cause mortality before kidney replacement therapy
A total of 311 patients died before KRT over the 5-year follow-up period. A doubling of serum-free kynurenine levels was significantly associated with a 22% increased hazard of all-cause mortality [adjusted HR (95% CI): 1.22 (1.02,1.50); Table 5], independently of eGFR. However, the association of serum-free kynurenine levels with all-cause mortality proved to depend on the serum-free tryptophan level; it was no longer significant after adding free tryptophan or tryptophan-derived UTs to the adjustment model (Table 5).
Table 5:
Hazard ratio of all-cause mortality associated with a double increase in baseline serum-free kynurenine level (mg/L).
| Free kynurenine (mg/L) | |
|---|---|
| Unadjusted HR [95% CI]—P-value | 1.7 [1.5, 1.9]–<0.001 |
| Adjusted HR a [95% CI]—P-value | 1.22 [1.02, 1.5]–0.03 |
| Adjusted HR b [95% CI]—P-value | 1.12 [0.9, 1.4]–0.3 |
| Adjusted HR c [95% CI]—P-value | 1.18 [0.9, 1.4]–0.07 |
Model adjusted for age at baseline, sex, high-sensitivity C-reactive protein, serum albumin, urea, diabetes, dyslipidemia, body mass index, smoking status, baseline estimated glomerular filtration rate, urine albumin- or protein-to-creatinine ratio, systolic blood pressure, prescriptions of diuretics, antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs.
1 + serum-free tryptophan.
1 + serum-free indoxyl sulfate, indole-3-acetic acid and p-cresyl sulfate.
In the multiple analyses, there was no significant association with serum total kynurenine levels in the hazard of all-cause mortality before KRT. The adjusted HR for all-cause mortality was 1.04 (0.90, 1.30) (Table S5, see online supplementary material).
Analysis by non-cardiovascular and cardiovascular mortality subgroups before kidney replacement therapy
Over the 5-year follow-up period, we observed 215 non-cardiovascular deaths and 87 cardiovascular deaths before KRT.
Non-cardiovascular mortality
There was no significant association with serum free kynurenine in the hazard of non-cardiovascular mortality before KRT after adjustment for eGFR, serum-free tryptophan, and other mortality risk factors (Table 6).
Table 6:
Hazard ratio of non-cardiovascular mortality associated with a double increase in baseline serum-free kynurenine level (mg/L).
| Free kynurenine (mg/L) | |
|---|---|
| Unadjusted HR [95% CI]—P-value | 1.5 [1.3, 1.8]–<0.001 |
| Adjusted HR a [95% CI]—P-value | 1.1 [0.9, 1.4]–0.3 |
| Adjusted HR b [95% CI]—P-value | 1.03 [0.8, 1.32]–0.8 |
| Adjusted HR c [95% CI]—P-value | 1.1 [0.9, 1.4]–0.3 |
Model adjusted for age at baseline, sex, high-sensitivity C-reactive protein, serum albumin, urea, diabetes, dyslipidemia, body mass index, smoking status, baseline estimated glomerular filtration rate, urine albumin- or protein-to-creatinine ratio, systolic blood pressure, prescriptions of diuretics, antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs.
1 + serum-free tryptophan.
1 + serum-free indoxyl sulfate indole-3-acetic acid and p-cresyl sulfate.
The results were comparable when evaluating the association with total kynurenine levels (Table S6, see online supplementary material).
Cardiovascular mortality
Serum-free kynurenine levels were significantly associated with the occurrence of cardiovascular mortality, even after adjustment for eGFR, serum-free tryptophan and other confounding factors. A doubling of serum free kynurenine levels was associated with a 64% increase in the hazard of cardiovascular mortality [adjusted HR (95% CI): 1.64 (1.10, 2.40) Table 7].
Table 7:
Hazard ratio of cardiovascular mortality associated with a double increase in baseline serum-free kynurenine level (mg/L).
| Free kynurenine (mg/L) | |
|---|---|
| Unadjusted HR [95% CI]—P-value | 2.3 [1.7, 3.03]–<0.001 |
| Adjusted HR a [95% CI]—P-value | 1.8 [1.3, 2.5]–0.001 |
| Adjusted HR b [95% CI]—P-value | 1.66 [1.1, 2.5]–0.01 |
| Adjusted HR c [95% CI]—P-value | 1.64 [1.1, 2.4]–0.007 |
Model adjusted for age at baseline, sex, high-sensitivity C-reactive protein, serum albumin, urea, diabetes, dyslipidemia, body mass index, smoking status, baseline estimated glomerular filtration rate, urine albumin- or protein-to-creatinine ratio, systolic blood pressure, prescriptions of diuretics, antihypertensive drugs, lipid-lowering drugs, and antidiabetic drugs.
1 + serum-free tryptophan.
1 + serum-free indoxyl sulfate, indole-3-acetic acid and p-cresyl sulfate.
There was no significant association between serum total kynurenine levels and cardiovascular mortality in the multiple analyses (Table S7, see online supplementary material).
DISCUSSION
This large national cohort study that included patients with CKD before any KRT showed that higher serum levels of the free fraction of kynurenine were associated with a higher incidence of adverse fatal or nonfatal cardiovascular events. There was a particularly strong association with non-atheromatous cardiovascular events, independent of kidney function, serum-free tryptophan level and traditional cardiovascular risk factors. Higher serum-free kynurenine levels were also significantly associated with a higher risk of cardiovascular mortality, but not non-cardiovascular or all-cause mortality.
As to the mechanisms involved, previous experimental studies showed that the actions of kynurenine are mainly mediated by direct activation of the aryl hydrocarbon receptor (AHR) [10, 15, 33]. AHRs interact with diverse regulatory and signaling proteins such as nuclear factor-kappa-B involved in cell proliferation, and immune responses [10]. AHR activity can be stimulated by various agents, including endogenous ligands such as the tryptophan metabolites indoxyl sulfate, indole-3-acetic acid, kynurenic acid, and kynurenine, which can induce vascular inflammation, cardiotoxicity, and a pro-coagulation status of vascular cells, as demonstrated both in mice and in humans [10, 33–35]. Kolachalama et al. [16]. showed that kynurenine promoted thrombosis after vascular injury in mice by increasing tissue factor expression in the vessel wall through AHR signaling. Arinze et al. provided evidence for an association between kynurenine and peripheral artery disease. They reported that kynurenine enhanced AHR activity in endothelial cells, resulting in the suppression of Wnt activity via the β-catenin pathway. This in turn led to impaired angiogenesis [15, 34].
Previous small studies have investigated the effect of total kynurenine on cardiovascular risk, mainly in patients receiving dialysis therapy; however, this was without considering the free, active kynurenine, and therefore probably underestimated kynurenine's full impact [15, 19, 20]. Total kynurenine concentrations have also been associated with markers of endothelial cell dysfunction, inflammation and oxidative stress. Kynurenine/tryptophan ratio was linked to abnormalities of tissue factor, contributing to a high risk of thrombotic complications and atherosclerosis, in CKD patients on conservative treatment [20–27]. Our results confirmed these previous observations and extended them by including a very large representative sample of patients with CKD, demonstrating the place of the free fraction of kynurenine, and adjusting for eGFR and serum-free tryptophan levels.
A recent report in a CKD patient cohort demonstrated a significant association between tryptophan levels and incident CVD [19]. Tryptophan is the precursor of many metabolites and UTs, including kynurenine, kynurenic acid, indoxyl sulfate, and indole-3-acetic acid. Tryptophan metabolites play a key role in incident cardiovascular disease, including myocardial infarction, atherosclerosis, major coronary events, and ischemic stroke, in the general population and in patients with heart disease [36]. This has also been shown for patients with CKD. Hence the need and importance of including serum-free tryptophan for the first time in the adjustment models of our analysis in order to isolate the true effect of serum-free kynurenine on cardiovascular diseases in predialysis CKD patients beyond the tryptophan effects.
Our study showed a significant association between kynurenine and non-atheromatous cardiovascular events. This association could be partly explained by an inhibitory effect of kynurenine on endothelial cell activity, which is critical for angiogenesis [34]. Reasons for an independent association of free kynurenine with non-atheromatous cardiovascular events but not with atheromatous disease remains to be explored. Possible explanations are lack of power due to relatively few observed atheromatous cardiovascular events and/or specific mechanisms of action.
Given that kynurenine induces inflammation and oxidative stress, one could have expecteded an impact on all-cause mortality. However, this was not the case in our cohort. One previous observational study also failed to find an association between kynurenine and mortality after kidney transplantation [29]. None examined such an association in non-transplant patients with CKD. We only found an isolated effect of free kynurenine on cardiovascular but not all-cause mortality. In any case, these findings highlight the critical role of uremic toxicity induced by kynurenine in the genesis of cardiovascular complications.
In the CKD setting, kynurenine is derived from tryptophan metabolism initially in the gut and then in different other cell types by exceptionally activating the indolamine-2,3-dioxygenase enzyme, leading to cardiovascular complications by activating the AHR pathway. Therefore, strategies targeting each of this pathway may give grounds for the development of new, more effective solutions to regulate serum levels of kynurenine and improving deleterious outcomes in a CKD setting [11, 34, 37–40]. Given that several UTs act together in the generation of deleterious effects, targeting only one UT is probably insufficient to reduce CKD-associated complications. For example, Massy and Drueke [34] suggested that AHR inhibitors are likely to be indicated as a solution via the blockade of common pathways. However, the efficacy and safety of AHR inhibitors in CKD patients need to be further investigated, especially that AHRs present diverse ligands with diversified effects. In other terms, AHRs and tryptophan-derived metabolites are dual in their activity as they can have both beneficial and/or deleterious effects [41]. Tryptophan is metabolized through several pathways, and can be a precursor of toxic compounds (UTs) and/or non-toxic molecules such as melatonin, proved to have immunoregulatory and anti-inflammatory effects in hemodialysis patients [42]. The AHR is activated by several tryptophan metabolites, among other beneficial tryptophan metabolites, which are essential for detoxification and immune and metabolic regulation [41]. Thereby, future interventions should specifically block the deleterious pathways while maintaining the beneficial ones.
Our study has important strengths. First, the present cohort study emphasizes the place of the free fraction of kynurenine in the association with cardiovascular diseases on the one hand and all-cause mortality on the other, in patients with CKD before any KRT. The stratified approach of cardiovascular diseases (according to atheromatous vs non-atheromatous mechanisms) and of cardiovascular vs non-cardiovascular mortality is a first significant step in the understanding of serum-free kynurenine's effect on CKD-related cardiovascular complications. Second, this study included a large sample size of people that are nationally representative of nephrology practices and the CKD patient population in France. This allowed for a sufficiently high statistical power and permitted extensive adjustments for confounders (including eGFR and serum free tryptophan). Third, UT assays were centralized using the validated, robust ultra-high-performance liquid chromatography tandem mass spectrometry technique. Fourth, an accurate assessment of cardiovascular events was conducted using standardized definitions [31] with two cardiologists adjudicating cardiovascular deaths. Last, the statistical analysis of the data considered competitive risk events for each outcome.
Some limitations also need to be considered. First, we did not investigate the time-varying effect of serum-free kynurenine levels on each of the studied outcomes. Second, our eligibility criteria was CKD patients with eGFR <60 ml/min/1.73 m2, excluding all patients with eGFR above that threshold. However, patients with eGFR >60 ml/min/1.73 m² may still have elevated albuminuria (>30 mg/g) or other markers of kidney damage, which also align with the CKD criteria according to KDIGO guidelines [43]. Third, the possibility of residual confounding cannot be excluded. The potential influence of dietary factors could not be included in the adjustment models as they were not available in our exhaustive database. They may have contributed to baseline serum kynurenine levels because this UT is a gut-derived one from an amino acid metabolism. However, the importance of diet on serum kynurenine concentrations is not determined and possibly has limited effects. Fourth, we did not find an independent association between free kynurenine and atheromatous CVD, despite the knowledge that kynurenine activates AHR and can contribute to thrombosis and PAD. Lastly, our study serves as an etiological evaluation, primarily aiming to enhance our understanding of the association between serum-free kynurenine and cardiovascular outcomes, rather than making predictive analyses. However, we neither explored the potential of kynurenine as a predictive biomarker for cardiovascular risk, nor compared the predictive value of other UTs known to be associated with CVD. This would require a separate investigation employing predictive analysis methodologies and external validation. It is important to note that CKD is a complex condition with various contributing factors to CVD. Kynurenine is just one piece of the puzzle.
In conclusion, our present large prospective study showed that higher serum-free kynurenine levels were associated with an elevated risk of incident fatal or nonfatal cardiovascular events in patients with CKD before any KRT particularly non-atheromatous cardiovascular events. This association was independent of kidney function and serum-free tryptophan levels. By investigating this association, we aimed to contribute to existing knowledge and shed light on the potential role of kynurenine in CVD development and progression. Future research should consider incorporating predictive analysis methodologies to further explore the potential of UTs, including kynurenine, as predictive biomarkers for cardiovascular risk. Such analyses might provide valuable insights into the predictive capabilities of UTs in relation to clinical outcomes. Moreover, experimental studies are necessary to provide a better insight into the mechanisms involved and potential therapeutic interventions targeting kynurenine in CKD-related cardiovascular complications.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the CKD-REIN study coordination staff for their efforts in setting up the CKD-REIN cohort (Marie Metzger, Elodie Speyer, Céline Lange and all the clinical research associates) as well as the CKD-REIN clinical sites and investigators:
Alsace: Prs T. Hannedouche and B. Moulin (CHU, Strasbourg), Dr A. Klein (CH Colmar). Aquitaine: Pr C. Combe (CHU, Bordeaux), Dr J.P. Bourdenx (Clinique St Augustin, Bordeaux), Dr A. Keller, Dr C. Delclaux (CH, Libourne), Dr B. Vendrely (Clinique St Martin, Pessac), Dr B. Deroure (Clinique Delay, Bayonne), Dr A. Lacraz (CH, Bayonne) Basse. Normandie: Dr T. Lobbedez (CHU, Caen), Dr I. Landru (CH, Lisieux). Ile de France: Pr Z. Massy (CHU, Boulogne—Billancourt), Pr P. Lang (CHU, Créteil), Dr X. Belenfant (CH, Montreuil), Pr E. Thervet (CHU, Paris), Dr P. Urena (Clinique du Landy, St Ouen), Dr M. Delahousse (Hôpital Foch, Suresnes). Languedoc—Roussillon: Dr C. Vela (CH, Perpignan). Limousin: Pr M. Essig, Dr D. Clément (CHU, Limoges). Lorraine: Dr H. Sekhri, Dr M. Smati (CH, Epinal) Dr M. Jamali, Dr B. Hacq (Clinique Louis Pasteur, Essey-les-Nancy), Dr V. Panescu, Dr M. Bellou (Polyclinique de Gentilly, Nancy), Pr Luc Frimat (CHU, Vandœuvre-les-Nancy). Midi-Pyrénées: Pr N Kamar (CHU, Toulouse). Nord-Pas-de-Calais: Prs C. Noël et F. Glowacki (CHU, Lille), Dr N. Maisonneuve (CH, Valenciennes), Dr R. Azar (CH, Dunkerque), Dr M. Hoffmann (Hôpital privé La Louvière, Lille). Pays-de-la Loire: Pr M. Hourmant (CHU, Nantes), Dr A. Testa (Centre de dialyse, Rezé), Dr D. Besnier (CH, St Nazaire). Picardie: Pr G. Choukroun (CHU, Amiens), Dr G. Lambrey (CH, Beauvais). Provence-Alpes—Côte d'Azur: Pr S. Burtey (CHU, Marseille), Dr G. Lebrun (CH, Aix-en-Provence), Dr E. Magnant (Polyclinique du Parc Rambot, Aix-en-Provence). Rhône-Alpes: Pr M. Laville, Pr D. Fouque (CHU, Lyon-Sud) and L. Juillard (CHU Edouard Herriot, Lyon), Dr C. Chazot (Centre de rein artificiel Tassin Charcot, Ste Foy-les-Lyon), Pr P. Zaoui (CHU, Grenoble), Dr F. Kuentz (Centre de santé rénale, Grenoble).
The authors would like to thank the teams of all the biological resources centers that participated in the project: the Biobanque de Picardie (BB-0033–00017), NeuroBioTec (BB-0033–00046), Centre de ressources biologiques (CRB)-Centre Hospitalier Universitaire de Nantes Hôtel Dieu (BB-0033–00040), CRB-Centre Hospitalier Universitaire Grenoble Alpes (BB-0033–00069), CRB-Centre Hospitalier Régional Universitaire de Nancy (BB-0033–00035), Service de Néphrologie, Centre Hospitalier de Perpignan, the Plateforme de Ressources Biologiques-Hôpital Henri Mondor (BB-0033–00021), the Centre d'Investigation Clinique Plurithématique CIC-1435, Plateforme de Ressources Biologiques-Hôpital européen Georges-Pompidou (BB-0033–00063), L'Etablissement Français du sang (EFS) Hauts de France—Normandie (Site de Bois-Guillaume, Site de Loos-Eurasanté), EFS Nouvelle Aquitaine (site Pellegrin), EFS Ile de France (Site Avicenne), EFS Occitanie (Site de Toulouse), EFS Grand-Est (Site de Colmar, Site de Metz), EFS PACA-Corse (Site de Marseille) (Supplementary Material).
Contributor Information
Carolla El Chamieh, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France.
Islam Amine Larabi, Department of Pharmacology and Toxicology, Raymond Poincaré Hospital, AP-HP, Garches, France; UVSQ, Université Paris-Saclay, Inserm U1018, CESP, Équipe MOODS, MasSpecLab, Montigny-le-Bretonneux, France.
Natalia Alencar De Pinho, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France.
Oriane Lambert, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France.
Christian Combe, Service de Néphrologie Transplantation Dialyse Aphérèse, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France; INSERM, U1026, University Bordeaux, Bordeaux, France.
Denis Fouque, Nephrology Dept, Centre Hospitalier Lyon Sud, Université de Lyon, Carmen, Pierre-Bénite, France; Université de Lyon, CarMeN INSERM 1060, Lyon, France.
Luc Frimat, Nephrology Department, CHRU de Nancy, Vandoeuvre-lès-Nancy, France; Lorraine University, APEMAC, Vandoeuvre-lès-Nancy, France.
Christian Jacquelinet, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France; Biomedecine Agency, Saint Denis La Plaine, France.
Maurice Laville, Université de Lyon, CarMeN INSERM 1060, Lyon, France.
Solène Laville, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, Amiens, France; MP3CV Laboratory, Jules Verne University of Picardie, Amiens, France.
Céline Lange, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France.
Jean-Claude Alvarez, Department of Pharmacology and Toxicology, Raymond Poincaré Hospital, AP-HP, Garches, France; UVSQ, Université Paris-Saclay, Inserm U1018, CESP, Équipe MOODS, MasSpecLab, Montigny-le-Bretonneux, France.
Ziad A Massy, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France; Department of Nephrology, Ambroise Paré University Hospital, APHP, Boulogne-Billancourt, France.
Sophie Liabeuf, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, Amiens, France; MP3CV Laboratory, Jules Verne University of Picardie, Amiens, France.
the Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) Study Group:
T Hannedouche, B Moulin, A Klein, C Combe, J P Bourdenx, A Keller, C Delclaux, B Vendrely, B Deroure, A Lacraz, T Lobbedez, I Landru, Z Massy, P Lang, X Belenfant, E Thervet, P Urena, M Delahousse, C Vela, M Essig, D Clément, H Sekhri, M Smati, M Jamali, B Hacq, V Panescu, M Bellou, Luc Frimat, N Kamar, C Noël, F Glowacki, N Maisonneuve, R Azar, M Hoffmann, M Hourmant, A Testa, D Besnier, G Choukroun, G Lambrey, S Burtey, G Lebrun, E Magnant, M Laville, D Fouque, L Juillard, C Chazot, P Zaoui, and F Kuentz
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
C.E.C., I.A.L., N.A.D.P., O.L., C.C., D.F., L.F., C.J., M.L., S.La., C.L., J.-C.A. and S.Li. declare no conflict of interest. Z.A.M. reports having received grants for CKD-REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp & Dohme-Chibret, Sanofi- Genzyme, Lilly, Otsuka, AstraZeneca, Vifor and the French government, as well as fees and grants to charities from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. The sponsors had no role in the design, execution, interpretation, or writing of the study.
REFERENCES
- 1. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl 2022;12:7–11. 10.1016/j.kisu.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charles C, Ferris AH.. Chronic kidney disease. Prim Care – Clin Off 2020;47:585–95. 10.1016/j.pop.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 3. El Chamieh C, Liabeuf S, Massy Z.. Uremic toxins and cardiovascular risk in chronic kidney disease: what have we learned recently beyond the past findings? Toxins 2022;14:280. 10.3390/toxins14040280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zakrocka I, Załuska W.. Kynurenine pathway in kidney diseases. Pharmacol Rep 2022;74:27–39. 10.1007/s43440-021-00329-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duranton F, Cohen G, De Smet Ret al. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 2012;23:1258–70. 10.1681/ASN.2011121175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vanholder R, Baurmeister U, Brunet Pet al. A bench to bedside view of uremic toxins. J Am Soc Nephrol 2008;19:863–70. 10.1681/ASN.2007121377 [DOI] [PubMed] [Google Scholar]
- 7. Vanholder R, Glorieux G.. Introduction: Uremic Toxicity–State of the Art 2014. Semin Nephrol 2014;34:85, –6. 10.1016/j.semnephrol.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 8. Graboski AL, Redinbo MR.. Gut-derived protein-bound uremic toxins. Toxins 2020;12:590. 10.3390/toxins12090590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glorieux G, Vanholder R, Van Biesen Wet al. Free p-cresyl sulfate shows the highest association with cardiovascular outcome in chronic kidney disease. Nephrol Dial Transplant 2021;36:998–1005. 10.1093/ndt/gfab004 [DOI] [PubMed] [Google Scholar]
- 10. Sallée M, Dou L, Cerini Cet al. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins 2014;6:934–49. 10.3390/toxins6030934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paeslack N, Mimmler M, Becker Set al. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022;54:1339–56. 10.1007/s00726-022-03161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green A, Aronson J, Curzon Get al. Metabolism of an oral tryptophan load. I: effects of dose and pretreatment with tryptophan. Br J Clin Pharmacol 1980;10:603–10. 10.1111/j.1365-2125.1980.tb00516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwiatkowska I, Hermanowicz JM, Mysliwiec Met al. Oxidative storm induced by tryptophan metabolites: missing link between atherosclerosis and chronic kidney disease. Oxid Med Cell Long 2020;2020:1. 10.1155/2020/6656033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fabresse N, Uteem I, Lamy Eet al. Quantification of free and protein bound uremic toxins in human serum by LC-MS/MS: comparison of rapid equilibrium dialysis and ultrafiltration. Clin Chim Acta 2020;507:228–35. 10.1016/j.cca.2020.04.032 [DOI] [PubMed] [Google Scholar]
- 15. Arinze NV, Yin W, Lotfollahzadeh Set al. Tryptophan metabolites suppress the Wnt pathway and promote adverse limb events in chronic kidney disease. J Clin Invest 2022;132:e142260. 10.1172/JCI142260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kolachalama VB, Shashar M, Alousi Fet al. Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. JASN 2018;29:1063–72. 10.1681/ASN.2017080929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castro-Portuguez R, Sutphin GL.. Kynurenine pathway, NAD+ synthesis, and mitochondrial function: targeting tryptophan metabolism to promote longevity and healthspan. Exp Gerontol 2020;132:110841. 10.1016/j.exger.2020.110841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solvang S-EH, Hodge A, Watne LOet al. Kynurenine pathway metabolites in the blood and cerebrospinal fluid are associated with human aging. Oxid Med Cell Long 2022;2022:5019752. 10.1155/2022/5019752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Konje VC, Rajendiran TM, Bellovich Ket al. Tryptophan levels associate with incident cardiovascular disease in chronic kidney disease. Clin Kidney J 2021;14:1097–105. 10.1093/ckj/sfaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pawlak K, Mysliwiec M, Pawlak D.. Haemostatic system, biochemical profiles, kynurenines and the prevalence of cardiovascular disease in peritoneally dialyzed patients. Thromb Res 2010;125:e40–5. 10.1016/j.thromres.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 21. Pawlak K, Mysliwiec M, Pawlak D.. Hypercoagulability is independently associated with kynurenine pathway activation in dialysed uraemic patients. Thromb Haemost 2009;102:49–55. 10.1160/TH08-10-0696 [DOI] [PubMed] [Google Scholar]
- 22. Pawlak K, Kowalewska A, Pawlak Det al. Kynurenine and its metabolites—kynurenic acid and anthranilic acid are associated with soluble endothelial adhesion molecules and oxidative status in patients with chronic kidney disease. Am J Med Sci 2009;338:293–300. 10.1097/MAJ.0b013e3181aa30e6 [DOI] [PubMed] [Google Scholar]
- 23. Pawlak K, Myśliwiec M, Pawlak D.. Kynurenine pathway–a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv Med Sci 2010;55:196–203. 10.2478/v10039-010-0015-6. [DOI] [PubMed] [Google Scholar]
- 24. Pawlak K, Brzosko S, Mysliwiec Met al. Kynurenine, quinolinic acid—The new factors linked to carotid atherosclerosis in patients with end-stage renal disease. Atherosclerosis 2009;204:561–6. 10.1016/j.atherosclerosis.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 25. Pawlak K, Domaniewski T, Mysliwiec Met al. Kynurenines and oxidative status are independently associated with thrombomodulin and von Willebrand factor levels in patients with end-stage renal disease. Thromb Res 2009;124:452–7. 10.1016/j.thromres.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 26. Pawlak K, Domaniewski T, Mysliwiec Met al. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 2009;204:309–14. 10.1016/j.atherosclerosis.2008.08.014 [DOI] [PubMed] [Google Scholar]
- 27. Pawlak K, Tankiewicz J, Mysliwiec Met al. Tissue factor/its pathway inhibitor system and kynurenines in chronic kidney disease patients on conservative treatment. Blood Coagul Fibrinolysis 2009;20:590–4. 10.1097/MBC.0b013e32832da16d [DOI] [PubMed] [Google Scholar]
- 28. Pawlak K, Buraczewska-Buczko A, Pawlak Det al. Hyperfibrinolysis, uPA/suPAR system, kynurenines, and the prevalence of cardiovascular disease in patients with chronic renal failure on conservative treatment. Am J Med Sci 2010;339:5–9. 10.1097/MAJ.0b013e3181b922a4 [DOI] [PubMed] [Google Scholar]
- 29. Mor A, Kalaska B, Pawlak D.. Kynurenine pathway in chronic kidney disease: what's old, what's new, and what's next? Int J Tryptophan Res 2020;13:117864692095488. 10.1177/1178646920954882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stengel B, Combe C, Jacquelinet Cet al. The French chronic kidney disease-renal epidemiology and information network (CKD-REIN) cohort study. Nephrol Dial Transplant 2014;29:1500–7. 10.1093/ndt/gft388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hicks KA, Mahaffey KW, Mehran Ret al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 2018;137:961–72. 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 32. Suttorp MM, Siegerink B, Jager KJet al. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant 2015;30:1418–23. 10.1093/ndt/gfu325 [DOI] [PubMed] [Google Scholar]
- 33. Addi T, Dou L, Burtey S. Tryptophan-derived uremic toxins and thrombosis in chronic kidney disease. Toxins 2018;10:412. 10.3390/toxins10100412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Massy ZA, Drueke TB.. Role of uremic toxins in vascular disease—the end of nihilism? Kidney Int 2022;101:1100–2. 10.1016/j.kint.2022.01.024 [DOI] [PubMed] [Google Scholar]
- 35. Asai H, Hirata J, Watanabe-Akanuma M.. Indoxyl glucuronide, a protein-bound uremic toxin, inhibits hypoxia-inducible factor‒dependent erythropoietin expression through activation of aryl hydrocarbon receptor. Biochem Biophys Res Commun 2018;504:538–44. 10.1016/j.bbrc.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 36. Melhem NJ, Taleb S.. Tryptophan: from diet to cardiovascular diseases. Int J Mol Sci 2021;22:9904. 10.3390/ijms22189904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barba C, Benoit B, Bres Eet al. A low aromatic amino-acid diet improves renal function and prevent kidney fibrosis in mice with chronic kidney disease. Sci Rep 2021;11:19184. 10.1038/s41598-021-98718-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koppe L, Soulage CO. The impact of dietary nutrient intake on gut microbiota in the progression and complications of chronic kidney disease. Kidney Int 2022;102:728–39. 10.1016/j.kint.2022.06.025 [DOI] [PubMed] [Google Scholar]
- 39. Koppe L, Fouque D., Microbiota and prebiotics modulation of uremic toxin generation. Panminerva Med 2016;59:173–87. 10.23736/S0031-0808.16.03282-1 [DOI] [PubMed] [Google Scholar]
- 40. Watanabe Y, Koyama S, Yamashita Aet al. Indoleamine 2, 3-dioxygenase 1 in coronary atherosclerotic plaque enhances tissue factor expression in activated macrophages. Res Pract Thromb Haemost 2018;2:726. 10.1002/rth2.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vanholder R, Nigam SK, Burtey Set al. What if not all metabolites from the uremic toxin generating pathways are toxic? A hypothesis. Toxins 2022;14:221. 10.3390/toxins14030221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marzougui H, Hammouda O, Ben Dhia Iet al. Melatonin ingestion before intradialytic exercise improves immune responses in hemodialysis patients. Int Urol Nephrol 2021;53:553–62. 10.1007/s11255-020-02643-3 [DOI] [PubMed] [Google Scholar]
- 43. Levin A, Stevens PE.. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014;85:49–61. 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


