Abstract
Background:
Household air pollution exposure is a risk factor for severe pneumonia. The effect of replacing biomass cooking with liquefied petroleum gas (LPG) cookstoves on severe infant pneumonia is uncertain.
Methods:
We conducted a randomized controlled trial among 3,200 pregnant women aged 18-34 years and 9 to <20 weeks gestation in India, Guatemala, Peru, and Rwanda May 2018–September 2021. Pregnant women were randomized to unvented LPG stoves and fuel (intervention) or continued biomass fuel cooking (control). We monitored intervention adherence and measured 24-hour personal exposure to fine particulate matter (PM2.5) in pregnant women and their offspring. The trial had 4 primary outcomes; the primary outcome described in the present report was severe pneumonia in the first year of life, identified by facility surveillance or verbal autopsy of deaths.
Results:
We randomized 3,195 pregnant women who gave birth to 3,061 infants. High intervention uptake led to reduced PM2.5 personal exposures among children (intervention median 24.2 μg/m3 (interquartile range (IQR) 17.8, 36.4); control median 66.0 μg/m3 (IQR 35.2, 132.0). There were 175 severe pneumonia episodes identified during the first year of life, with an incidence rate of 5.67 (95% confidence interval (CI) 4.55, 7.07) and 6.06 (4.81, 7.62) cases per 100-child years in intervention and controls (incidence rate ratio 0.96 [98.75% CI, 0.64, 1.44; p=0.81]. No severe adverse events associated with the intervention were reported.
Conclusions:
There was no significant difference in severe pneumonia incidence among infants of women randomized to LPG compared to biomass-burning cookstoves.
Introduction
Pneumonia is a leading cause of child mortality worldwide, with most deaths in infants1. About 83% of the 808,000 annual child pneumonia deaths occur in sub-Saharan Africa, South Asia, and Latin America1. Observational studies suggest fine particulate air pollution (PM2.5) exposure from incomplete solid fuel combustion is a risk factor for pneumonia1. Nearly ~30% of global pediatric pneumonia deaths are attributed to household air pollution1. About 2.4 billion people – predominantly in low- and middle-income countries (LMICs) – use biomass (wood, charcoal, animal dung, coal) daily to cook or heat their households2.
To date, randomized controlled trials (RCTs) of cleaner cooking interventions have not found an effect on primary child pneumonia outcomes3–6. However, it is unclear if lack of benefit stemmed from insufficiently lowered pollutant levels due to inadequate cookstove intervention uptake or performance, lack of specificity in pneumonia case definitions, or low statistical power. The Household Air Pollution Intervention Network (HAPIN) trial was designed to address these limitations in assessing whether cooking with an unvented liquefied petroleum gas (LPG) stove and fuel during pregnancy and the offspring’s first year of life, compared to biomass, reduced severe infant pneumonia incidence and other health outcomes7. We previously reported no evidence of an intervention effect on birthweight8. Here, we report severe pneumonia incidence during the first year of life, one of four primary trial outcomes.
Methods
Design
HAPIN was a randomized controlled trial of unvented LPG cookstoves with free, uninterrupted fuel supply, compared to usual cooking practices (primarily or exclusively with biomass fuels), conducted in Tamil Nadu, India; Jalapa, Guatemala; Puno, Peru; and Kayonza, Rwanda from May 2018–September 20217. Sites were selected to cover a range of geographical settings in four continents where biomass is used for cooking.
Participants
Pregnant women aged 18–34 years with an ultrasound and pregnancy-test confirmed, viable, singleton fetus at 9 to <20 weeks of gestation, biomass stove use at least 4 days a week, and study area residency, were eligible. Pregnant women who smoked tobacco, planned to migrate from the study area during the study, or used or planned to switch to LPG stoves were excluded. One pregnant woman per household could be enrolled.
Randomization
We randomized participants to intervention and control groups on a 1:1 basis. India and Peru used stratified randomization to ensure balance between two and six distinct geographical study areas, respectively. While the intervention assignment could not be blinded to participants and field staff, all investigators were masked to study group at the time of data cleaning, image interpretation, or data analysis.
Intervention
Unvented LPG cookstoves all had ≥2 burners and met local safety standards. Behavioral reinforcement messaging was provided to foster exclusive, safe LPG stove use9. Staff monitored both groups for adherence to group allocation through stove temperature sensor monitoring10. Controls were provided non-monetary compensation to counterbalance the intervention incentive of free fuel provision and mitigate attrition11. As cooking fuel delivery was considered an essential service, the intervention was generally uninterrupted by COVID-19 restrictions with similar delivery times before and during COVID12.
Exposure assessment
Twenty-four-hour personal exposure to PM2.5, carbon monoxide, and black carbon were measured directly using wearable devices for pregnant women at baseline (<20 weeks) and 24-28 and 32-36 weeks’ gestation10. We estimated infants’ exposure to PM2.5, carbon monoxide, and black carbon at 1-3, 6 and 12 months of age using an indirect method13 (Supplementary Appendix).
Outcome Surveillance
We conducted active surveillance of severe pneumonia cases at pre-selected community hospitals and health centers. These facilities were identified during formative work as centers where severe cases receive care14. Passive facility and household surveillance were also conducted to identify missed facility visits, missed hospitalizations, ventilatory support, and deaths. Study staff were trained to evaluate children for severe pneumonia using a standard approach15; in brief, they passed certification examinations and received annual re-trainings. If medical care was needed, mothers could notify HAPIN staff by phone to facilitate appropriate care. In India, Peru, and Rwanda, study staff were available weekdays in-person at sentinel facilities and by phone anytime; in Guatemala, staff were available in-person continuously at the sentinel hospital. We reviewed medical charts of infant deaths and conducted a verbal autopsy to determine whether the death was related to severe pneumonia. Beginning in November 2019, sites in Rwanda increased study staff presence at outpatient clinics as surveillance identified some cases who were not hospitalized. In March 2020, COVID-19-related public health measures commenced at all sites, which limited active in person surveillance and care-seeking during lockdown periods. HAPIN staff also telephoned facility contacts to surveil for possible cases; telephone surveillance was uninterrupted during the study.
Outcomes
The primary outcome was severe pneumonia incidence in the first year of life among participant offspring. The primary case definition was adapted from World Health Organization (WHO) guidelines based on external expert input.16. In July 2019, when follow-up time of infants was <1%, we implemented additional expert recommendations to amend the case definition and improve specificity, objectivity, and to be responsive to formative data we collected (Supplementary Appendix)17,18 The primary definition of severe pneumonia was defined as (1) cough and/or difficult breathing, ≥1 general danger sign (unable to drink or breastfeed, vomiting everything, convulsions, stridor at rest, lethargy or unconscious) or ≥1 neonatal danger sign (unable to feed well, not moving at all or movement only when stimulated, grunting, severe chest indrawing), and pneumonia on imaging, (2) cough and/or difficult breathing with hypoxemia, or (3) a verbal autopsy-confirmed pneumonia death.15 Subsequent symptoms in the same child were considered separate episodes if >14 days after hospital discharge or >30 days from outpatient diagnosis. To be eligible as a case required examination by study staff except for children on ventilatory support or who died.
Chest imaging was by ultrasound (Sonosite Edge, Bothell, WA, USA)15,19 or radiography if ultrasound was unavailable. The reported sensitivity and specificity of lung ultrasound for diagnosing pneumonia in children are 95.5% and 95.3%, respectively and for chest radiography are 86.8% and 98.2%, respectively. 20. All images were interpreted by adjudication panels blinded to intervention and clinical status15,19,21. Two panelists followed pre-specified interpretation procedures and were required to agree on the presence or absence of pneumonia for the image to be classified as pneumonia. Pneumonia on imaging was a consolidation alone (meeting pre-specified size dimensions), or a pleural effusion near an infiltrate, or pleural abnormalities (ultrasound-specific)15,19,21.
Hypoxemia was defined as a peripheral arterial oxyhemoglobin saturation (SpO2) ≤92% at <2,500 meters altitude (Guatemala, India, Rwanda) or ≤86% at ≥2,500 meters altitude (Peru)15, or receipt of invasive or non-invasive ventilation or high-flow oxygen. To measure SpO2, facility study staff applied a Masimo Rad-G® pulse oximeter (Masimo, Irvine, CA, USA) and pediatric probe to the big toe of infants breathing room air. Staff collected three measurements over two-minutes, and these were averaged. SpO2 measurements were extracted from medical charts when available.
Trained, local medical staff performed verbal autopsies with caregivers of deceased infants using a validated protocol22. A physician verbal autopsy panel assigned primary and secondary causes of death using WHO 2016 ICD-10 codes. Two non-study LMIC physicians masked to randomization and other death classifications independently reviewed the autopsy open narrative and closed questions. When the assigned primary cause of death was discordant between the two physicians, a pediatrician panelist arbitrated to achieve consensus. Cases without consensus were undetermined. The final verbal autopsy classification was pneumonia if it was the primary or secondary cause of death.
Secondary outcomes were pneumonia per WHO Integrated Management of Childhood Illness (IMCI) guidelines and WHO Pocketbook guidelines23,24, hypoxemia and/or imaging-confirmed pneumonia, and any hospitalized respiratory illness (see Table S1 for secondary outcome definitions).
Statistical analysis
Based on available evidence5,6,25–28, we estimated a sample of 3,200 pregnant women would provide 80% power to detect a 36% reduction in severe pneumonia incidence between study arms assuming a baseline rate of 9/100 infant-years using an α of 0.0125 (multiple hypothesis testing for four trial outcomes)7. The primary analysis was according to intention-to-treat and was conducted independently by two teams. We used Poisson regressions with generalized estimating equations (GEE) to model the incidence of all episodes of severe pneumonia using infant days at risk as the denominator to derive incidence rate ratios (IRRs). The intervention arm was the main covariate and models were adjusted for 10 randomization strata (one in Guatemala and Rwanda, two in India, six in Peru). The threshold for statistical significance for the primary outcome was set a priori at 0.0125 to account for the four primary trial outcomes. When data were incomplete for an outcome classification, we assumed the event did not occur.
Secondary analyses estimated the intervention effect on the time to first pneumonia incidence via Cox proportional hazards models. Subgroup analyses assessed the influence of outpatient surveillance changes in Rwanda (after November 2019) and the COVID-19 pandemic (after March 2020) using GEE Poisson regression models with indicator variables for relevant time periods, as well as interaction terms between treatment arms and time periods to assess whether the intervention effect changed over time. Given the clustering of deaths very early in life, and that diagnostic accuracy may be lower in neonates, we also conducted sensitivity analyses of the primary analysis that excluded cases <7 and <30 days old.
Oversight
The protocol, available at NEJM.org, was approved by all investigator-affiliated institutional review boards (see Appendix). Participants provided written informed consent. An independent data and safety monitoring board (DSMB) monitored safety and efficacy and received unblinded interim analyses. No pre-defined stopping rules were formulated due to the low intervention risk. Eric D. McCollum, William Checkley, John McCracken, Jennifer Peel, and Thomas Clasen take responsibility for the integrity and completeness of the data and fidelity of the report to the protocol.
Results
Participant characteristics
3,200 women were randomized, with 1,593 (49.8%) allocated to the LPG arm and 1,607 (50.2%) as controls (Figure 1). Baseline maternal characteristics were similar between groups (Table 1); the pregnant women and their offspring were representative of the broader population of women and infants affected by indoor air pollution from biomass cooking (Table S2). Pregnant women received LPG stoves mid-second trimester (mean 18.1 weeks (SD, 3.3). There were 1,536 livebirths overall in the intervention group and 1,525 in controls. Table 2 reports the characteristics of liveborn children by study group, including vaccination status.
Figure 1.
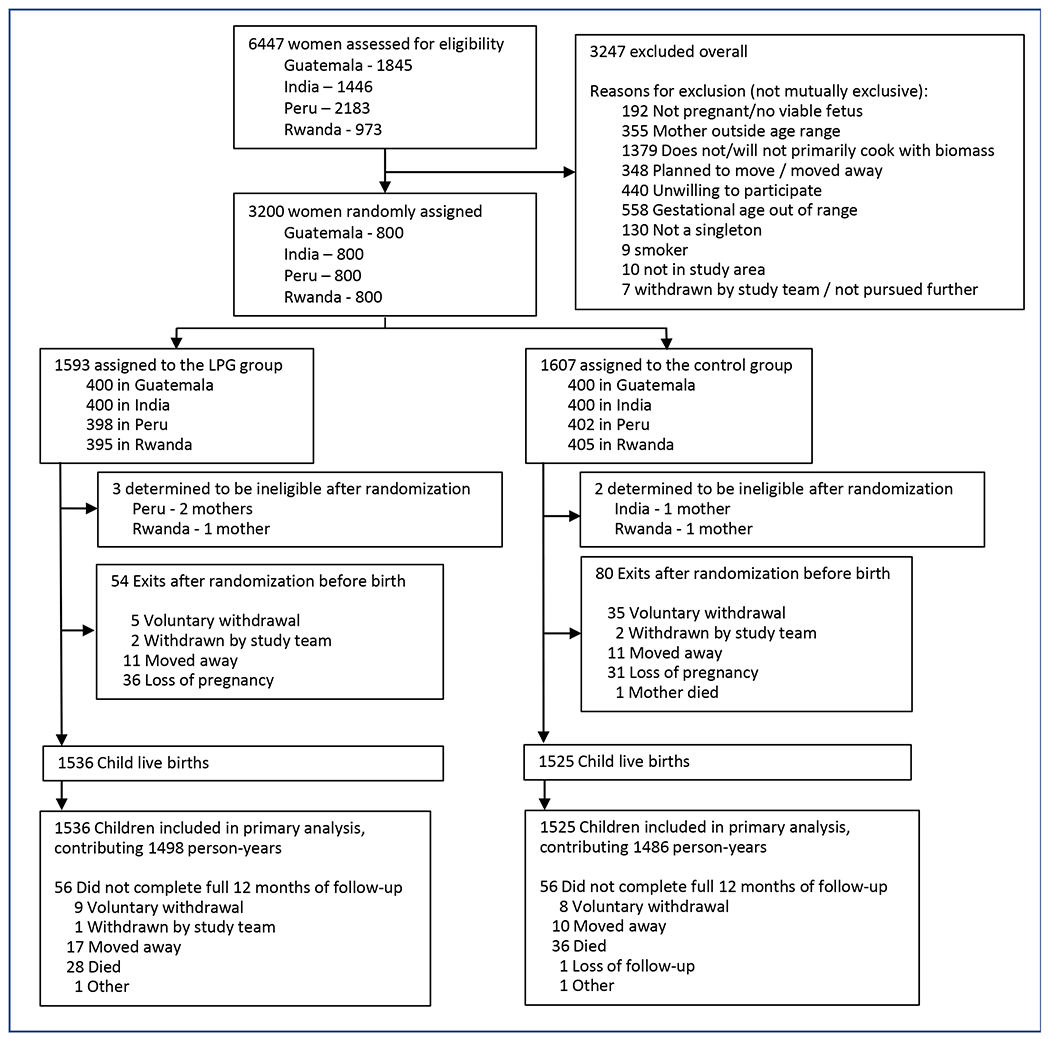
CONSORT diagram.
Table 1.
Baseline characteristics of pregnant women (with liveborn children) by study group
| Characteristic | Intervention (N=1536) |
Control (N=1525) |
|
|---|---|---|---|
| IRC, n (%) | Guatemala | 384 (25.0%) | 386 (25.3%) |
| India | 388 (25.3%) | 387 (25.4%) | |
| Peru | 385 (25.1%) | 358 (23.5%) | |
| Rwanda | 379 (24.7%) | 394 (25.8%) | |
| Missing, n | 0 | 0 | |
| Mother’s age (years) at baseline | Mean (SD) | 25.3 (4.4) | 25.4 (4.5) |
| 18 to <25 years, n (%) | 787 (51.2%) | 758 (49.7%) | |
| 25 to <30 years, n (%) | 484 (31.5%) | 488 (32.0%) | |
| 30 to <35 years, n (%) | 265 (17.3%) | 279 (18.3%) | |
| Missing, n | 0 | 0 | |
| Mother’s highest level of education completed, n (%) | None or some primary | 461 (30.0%) | 540 (35.4%) |
| Primary or some secondary | 538 (35.0%) | 514 (33.7%) | |
| Secondary, vocational, or university/college | 537 (35.0%) | 471 (30.9%) | |
| Missing, n | 0 | 0 | |
| Gestational age (weeks) at baseline | Mean (SD) | 15.5 (3.1) | 15.3 (3.2) |
| Missing, n | 0 | 0 | |
| Gestational age (weeks) at intervention1 | Mean (SD) | 18.1 (3.3) | 17.9 (3.2) |
| 10 to <18 weeks | 767 (49.9%) | 791 (51.9%) | |
| 18 to <30 weeks | 769 (50.1%) | 734 (48.1%) | |
| Missing, n | 0 | 0 | |
| Number of siblings in the household | Mean (SD) | 1.0 (1.1) | 1.1 (1.2) |
| Missing, n | 0 | 0 | |
| Second-hand Smoking | n (%) | 146 (9.5%) | 174 (11.4%) |
| Missing, n | 1 | 2 | |
| Household food insecurity score2, n (%) | Food secure | 904 (58.9%) | 820 (53.8%) |
| Mild | 403 (26.2%) | 424 (27.8%) | |
| Moderate/severe | 208 (13.5%) | 259 (17.0%) | |
| Missing, n | 21 | 22 | |
| Socioeconomic status index3 | Mean (SD) (range) | −0.1 (1.1) (−2.2, 2.1) | 0.1 (1.0) (−2.2, 2.1) |
| Missing, n | 0 | 0 | |
| PM2.5 (μg/m3)4 | Median (IQR) | 81.6 (45.9, 150.7) | 84.2 (46.5, 143.0) |
| Missing, n | 184 | 173 | |
| Black carbon (μg/m3)4 | Median (IQR) | 10.5 (6.2, 15.4) | 10.9 (6.9, 15.5) |
| Missing, n | 313 | 314 | |
| Carbon monoxide (ppm)4 | Median (IQR) | 1.3 (0.5, 3.0) | 1.2 (0.5, 2.5) |
| Missing, n | 152 | 150 | |
IRC indicates International Research Center; SD, standard deviation; PM, particulate matter; IQR, interquartile range.
Control group calculated as gestational age at baseline plus 2.6 weeks, which is the average time between baseline and stove installation in the intervention group.
Categories (corresponding score): Food secure (0); Mild (1,2,3), Moderate (4,5,6) or Severe (7,8) food insecurity.
Principal component analysis was applied to data on number of people in the household, participant’s education level, quality of water and sanitation, access to electricity, housing materials, ownership of 24 specific household assets and food insecurity at the start of the study. Multiple imputation with chained equations was used to handle missing data. A higher index indicates worse socioeconomic status.
Missing includes invalid samples that failed to pass quantitative quality checks, including samples with unacceptable flow rates, filter damage, and measurement durations outside of 24 ± 4 hours.
Table 2.
Characteristics of liveborn children by study group
| Characteristic | Intervention (N=1536) |
Control (N=1525) |
|
|---|---|---|---|
| Child sex, n (%) | Male | 800 (52.1%) | 787 (51.6%) |
| Female | 736 (47.9%) | 738 (48.4%) | |
| Missing, n | 0 | 0 | |
| Birth weight-for-age z score | Mean (SD) | −0.8 (1.0) | −0.8 (1.0) |
| Missing, n | 24 | 3 | |
| Exclusive breastfeeding1 | n (%) | 702 (48.9%) | 747 (52.5%) |
| Missing, n | 100 | 101 | |
| Up-to-date pentavalent vaccine at study exit2 | n (%) | 1306 (95.4%) | 1311 (95.2%) |
| Missing, n | 167 | 148 | |
| Up-to-date pneumococcal conjugate vaccine at study exit2 | n (%) | 999 (97.6%) | 1000 (96.8%) |
| Missing, n | 513 | 492 | |
| Trial-period antenatal PM2.5 (μg/m3)3 | Median (IQR) | 24.8 (17.0, 40.5) | 77.0 (40.7, 132.8) |
| Missing, n | 99 | 116 | |
| Trial-period postnatal PM2.5 (μg/m3)3 | Median (IQR) | 24.2 (17.8, 36.4) | 66.0 (35.2, 132.0) |
| Missing, n | 688 | 592 | |
| Trial-period antenatal black carbon (μg/m3)3 | Median (IQR) | 2.9 (1.7, 4.8) | 10.0 (5.9, 14.1) |
| Missing, n | 123 | 149 | |
| Trial-period antenatal carbon monoxide (ppm)3 | Median (IQR) | 0.3 (0.1, 0.8) | 1.2 (0.5, 2.4) |
| Missing, n | 86 | 95 | |
| Trial-period postnatal carbon monoxide (ppm)3 | Median (IQR) | 0.3 (0.0, 0.8) | 1.3 (0.4, 3.0) |
| Missing, n | 571 | 609 | |
IRC indicates International Research Center; SD, standard deviation; PM, particulate matter; IQR, interquartile range.
During the first six months of life feeding only breast milk, not any other foods or liquids including infant formula or water.
Up-to-date vaccination at one year old when received three doses of the pentavalent vaccine for all IRCs, three doses of PCV for Rwanda, or two doses of PCV for Guatemala and Peru. PCV was not available in India.
Trial-period measurements refer to post-randomization pollutant values, which are presented as the median of the average of household-level measures. Missing includes invalid samples that failed to pass quantitative quality checks, including samples with unacceptable flow rates, filter damage, and measurement durations outside of 24 ± 4 hours. Post-natal black carbon measurement is not available.
Intervention fidelity, adherence, and effects on exposure
Intervention participants used biomass stoves on a median of 0.4% (interquartile range (IQR) 0.0, 2.3) of monitored days12,29. The averaged, post-randomization, 24-hour personal exposures to PM2.5 were overall lower in the intervention arm, compared to controls in the antenatal (median 24.8 μg/m3, interquartile range (IQR) 17.0, 40.5 vs median 77.0 μg/m3, 40.7, 132.8) as well as during postnatal periods (median 24.2 μg/m3, IQR 17.8, 36.4 vs median 66.0 μg/m3, IQR 35.2, 132.0) (Table 2, Table S3)13,30.
Primary outcome
We identified 85 severe pneumonia episodes in the intervention group and 90 in the control group (Figure 2) from 1,243 healthcare facility visits and 55 verbal autopsies (Figure S1, Tables S4–S8). Among these, there were 12 deaths attributed to pneumonia (6.9% of pneumonia outcomes), eight in the control group and 4 in the intervention group.) (Table S9). The severe pneumonia incidence rate in the first year of life was 5.67 (95% CI 4.45, 7.07) per 100 child-years in the LPG group and 6.06 (95% CI 4.81, 7.62) per 100 child-years in the control group (incidence rate ratio (98.7% CI) 0.96 (0.64, 1.44; p=0.81) (Figure 2).
Figure 2. Intervention effects on primary and secondary outcomes.
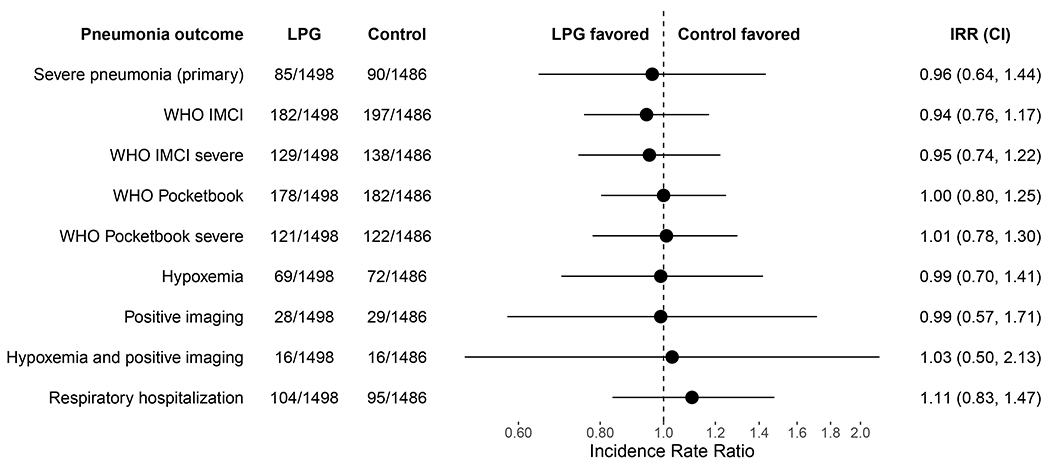
LPG indicates liquefied petroleum gas; IRR, Incidence Rate Ratio; IMCI, Integrated Management of Childhood Illnesses; WHO, World Health Organization. See Appendix Table S1 for secondary outcome case definitions. Severe pneumonia (primary) 98.75% confidence interval was adjusted for multiplicity. 95% confidence intervals of other endpoints were not adjusted for multiplicity and should not be used to infer definitive treatment effects.
Secondary outcomes, subgroup and sensitivity analyses
No evidence of an intervention effect was observed for secondary outcomes (Figure 2, Table S10) or when stratified by study site or other subgroups (Figure S2). Although the observed incidence of severe pneumonia across all study sites decreased by 77% (95% CI 61%, 86%) during the COVID-19 pandemic period (Figure 3, Figure S3, Table S11), there was no appreciable change in the IRR when our models accounted for the pandemic period and child’s age (IRR 0.96, 95% CI 0.70, 1.31). The IRRs before (0.71 IRR, 95% CI 0.12, 4.23) and after (IRR 0.80, 95% CI 0.49, 1.31) surveillance changes (November 2019) in Rwanda were also similar (Table S12).
Figure 3.
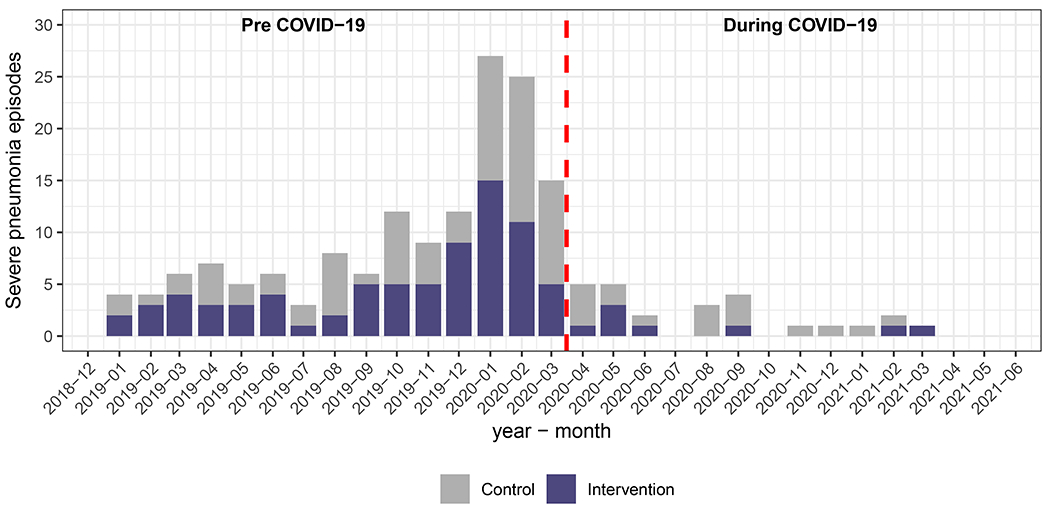
Episodes and incidence of severe pneumonia over time.
Adverse Events
Burns were reported by three infants (0.2%) in the intervention group and seven infants (0.5%) in the control arm. No burn was classified as a serious adverse event Table S13).
Discussion
Despite high LPG intervention uptake and substantial reductions in air pollutant exposure, we found no significant difference in the incidence of severe infant pneumonia between the intervention and control arms in this multi-country trial. Our findings are consistent with null findings from a cluster randomized trial in Ghana of a similar cookstove3, indicating that unvented LPG cookstoves are unlikely to reduce severe infant pneumonia. Our trial also found no difference between study arms in the other primary endpoints of birthweight8 and stunting (reported in another article in this issue of the Journal,) 31.
There are several potential explanations for our null findings for severe infant pneumonia. First, evidence suggests household air pollution is more closely linked with bacterial than viral nasopharyngeal carriage32,33. While nasopharyngeal carriage is considered a prerequisite for the development of invasive or mucosal bacterial diseases like pneumonia34, populations vaccinated against Haemophilus Influenzae type B (Hib) and Streptococcus pneumoniae (pneumococcus) are well protected from nasopharyngeal carriage progressing to disease35. Our study population had high rates of vaccination against Hib and pneumococcal pneumonia, making severe bacterial pneumonia less likely. As observed in this trial (Figure 3, Figure S3) and elsewhere, the fact that mitigation efforts during the COVID-19 pandemic dramatically reduced both respiratory virus circulation and pediatric hospitalizations provides indirect evidence on the central role of viruses in severe childhood respiratory disease36,37. However, definitively determining the etiology of severe childhood pneumonia is challenging, and we do not have information on respiratory pathogens in these infants.
Second, the PM2.5 levels we achieved were lower than levels in other trials3–6 but remained above WHO recommendations38. Although uncertain, it is possible that lower PM2.5 exposure levels than were achieved may be required to reduce the risk of severe pneumonia and greater reductions may require broader community interventions, rather than household strategies as we employed. Third, even though unvented LPG cookstoves produce nitrogen dioxide (NO2) at levels lower than biomass cookstoves, these levels are nevertheless above recommendations39. Elevated NO2 concentrations have associations with asthma in children40, and may have contributed to our null results.
Limitations of our study should be noted. Incomplete assessments at facility visits may have led to missed cases, although this is unlikely to have impacted our results because missingness among screened children was low (Figure S1). It is also possible that incomplete case ascertainment occurred due to children seeking care at clinics outside of the surveillance area or failing to seek care at all. Missed cases may have been more common during the COVID-19 pandemic period, particularly in the first months during lockdowns. We accounted for the pandemic in our analysis but did not find evidence of differential effects of the pandemic on our results (Table S11). The wide confidence intervals around our effect estimates mean that we cannot exclude clinically important reductions or increases in severe pneumonia risk with the use of unvented LPG cookstoves compared to biomass cookstoves. Also since there is no gold standard for pneumonia diagnosis, the accuracy of our primary case definition for severe pneumonia is undetermined. However, we sought and incorporated external expert recommendations intended to optimize the definition’s objectivity and specificity. The results for outcomes using alternative pneumonia definitions were also consistent with results for the primary outcome.
In conclusion, in this multicenter trial involving four LMICs, unvented LPG cookstoves did not reduce the incidence of severe infant pneumonia compared to biomass cookstoves.
Supplementary Material
ACKNOWLEDGEMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US National Institutes of Health or Department of Health and Human Services. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. A data sharing statement provided by the authors is available with the full text of this article at NEJM.org. The sponsors played an active role in study design and conduct decisions but did not participate or influence the preparation of this report. The co-first and last three authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
A multidisciplinary, independent Data and Safety Monitoring Board (DSMB) appointed by the National Heart, Lung, and Blood Institute (NHLBI) monitors the quality of the data and protects the safety of patients enrolled in the HAPIN trial. NHLBI DSMB: Catherine Karr (Chair), Nancy R. Cook, Stephen Hecht, Joseph Millum, Nalini Sathiakumar, Paul K. Whelton, Gail Weinmann and Thomas Croxton (Executive Secretaries). Program Coordination: Gail Rodgers, Bill & Melinda Gates Foundation; Claudia L. Thompson, National Institute of Environmental Health Science; Mark J. Parascandola, National Cancer Institute; Marion Koso-Thomas, Eunice Kennedy Shriver National Institute of Child Health and Human Development; Joshua P. Rosenthal, Fogarty International Center; Concepcion R. Nierras, NIH Office of Strategic Coordination Common Fund; Katherine Kavounis, Dong-Yun Kim, Antonello Punturieri, and Barry S. Schmetter, NHLBI.
The investigators would like to thank the members of the advisory committee - Patrick Bryesse, Donna Spiegelman, and Joel Kaufman - for their valuable insight and guidance throughout the implementation of the trial. We would like to thank our pneumonia experts who participated in our workshops: Dr. Heather Zar of the University of Capetown (Cape Town, South Africa), Dr. Harry Campbell of the University of Edinburgh (Edinburgh, UK), Dr. Claudio Lanata of the Instituto De Investigacion Nutricional (Lima, Peru), Dr. Carina King (Karolinska Institute, Sweden) and Dr. Laura Hammitt of Johns Hopkins University (Baltimore, MD, USA). We additionally thank Dr. Carina King for independently leading the pneumonia verbal autopsy panel and analyses. We thank Dr. Marieke van der Zalm of the Desmond Tutu TB Centre, Stellenbosch University (Cape Town, South Africa) for serving as the pediatrician adjudicator of the verbal autopsy panel, and Dr. Kristen Joseph of the Johns Hopkins School of Medicine (Baltimore, United States) and Dr. Nadia Hoekstra of University of North Carolina-Chapel Hill School of Medicine (Chapel Hill, United States) for serving as the pediatrician adjudicators of the hospitalized respiratory illness panel. We extend our gratitude to Dr. Murali Arunan, Dr. Gokula Krishnan, Dr. Anupama Chandrashekharan, Dr. P. Rajeev, and Dr. P.M. Venkata Sai from Sri Ramanchandra Hospital (Tamil Nadu, India) for their work on the chest radiography reading panel. We additionally would like to thank Katerina Lescouflair and Delaney Connolly, both of Johns Hopkins University, for assistance with preparation of the protocols and development of materials. We also wish to acknowledge all the research staff and study participants for their dedication to and participation in this important trial.
Statistical analyses were conducted by Dr. Larry Moulton, Laura Grajeda, Miles Kirby, and Shakir Hossen.
(Funded by US National Institutes of Health and the Bill and Melinda Gates Foundation; NCT029446282)
Support Statement
The HAPIN trial is funded by the U.S. National Institutes of Health (cooperative agreement 1UM1HL134590) in collaboration with the Bill & Melinda Gates Foundation [OPP1131279].
Footnotes
The views expressed here are those of the authors and do not represent official statements by the National Institutes of Health or other components of the United States Government.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.GBD 2017 Lower Respiratory Infections Collaborators. Quantifying risks and interventions that have affected the burden of lower respiratory infections among children younger than 5 years: an analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2020;20:60–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The World Bank. Tracking SDG7 The Energy Progress Report 2022. Washington DC: 2022. (https://trackingsdg7.esmap.org/data/files/download-documents/sdg7-report2022-full_report.pdf) [Google Scholar]
- 3.Jack DW, Ae-Ngibise KA, Gould CF, et al. A cluster randomised trial of cookstove interventions to improve infant health in Ghana. BMJ Glob Health 2021;6:e005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tielsch JM, Katz J, Khatry S, et al. E!ect of an improved biomass stove on acute lower respiratory infections in young children in rural Nepal: a cluster-randomised, step-wedge trial. Lancet Glob Health 2016;4:S19. [Google Scholar]
- 5.Mortimer K, Ndamala CB, Naunje AW, et al. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet 2017;389:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet 2011;378:1717–26. [DOI] [PubMed] [Google Scholar]
- 7.Clasen T, Checkley W, Peel JL, et al. Design and Rationale of the HAPIN Study: A Multicountry Randomized Controlled Trial to Assess the Effect of Liquefied Petroleum Gas Stove and Continuous Fuel Distribution. Environ Health Perspect 2020;128:47008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clasen TF, Chang HH, Thompson LM, et al. Liquefied Petroleum Gas or Biomass for Cooking and Effects on Birth Weight. N Engl J Med 2022;387:1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams KN, Thompson LM, Sakas Z, et al. Designing a comprehensive behaviour change intervention to promote and monitor exclusive use of liquefied petroleum gas stoves for the Household Air Pollution Intervention Network (HAPIN) trial. BMJ Open 2020;10:e037761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson MA, Steenland K, Piedrahita R, et al. Air Pollutant Exposure and Stove Use Assessment Methods for the Household Air Pollution Intervention Network (HAPIN) Trial. Environ Health Perspect 2020;128:47009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn AK, Williams K, Thompson LM, et al. Compensating control participants when the intervention is of significant value: experience in Guatemala, India, Peru and Rwanda. BMJ Glob Health 2019;4:e001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams KN, Quinn A, North H, et al. Fidelity and adherence to a liquefied petroleum gas stove and fuel intervention: the multi-country Household Air Pollution Intervention Network (HAPIN) trial. Environ Int 2023;179:108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pillarisetti A, Ye W, Balakrishnan K, et al. Post-birth exposure contrasts for children during the Household Air Pollution Intervention Network randomized controlled trial. medRxiv 2023;2023.07.04.23292226/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simkovich SM, Underhill LJ, Kirby MA, et al. Resources and Geographic Access to Care for Severe Pediatric Pneumonia in Four Resource-limited Settings. Am J Respir Crit Care Med 2022;205:183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simkovich SM, Underhill LJ, Kirby MA, et al. Design and conduct of facility-based surveillance for severe childhood pneumonia in the Household Air Pollution Intervention Network (HAPIN) trial. ERJ Open Res 2020;6:00308–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman D, Crocker ME, Pervaiz F, et al. Challenges in the diagnosis of paediatric pneumonia in intervention field trials: recommendations from a pneumonia field trial working group. Lancet Respir Med 2019;7:1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crocker ME, Hossen S, Goodman D, et al. Effects of high altitude on respiratory rate and oxygen saturation reference values in healthy infants and children younger than 2 years in four countries: a cross-sectional study. Lancet Glob Health 2020;8:e362–e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCollum ED, Ahmed S, Roy AD, et al. Risk and accuracy of outpatient-identified hypoxaemia for death among suspected child pneumonia cases in rural Bangladesh: a multifacility prospective cohort study. Lancet Respir Med 2023;11:769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simkovich SM, Hossen S, McCollum ED, et al. Lung Ultrasound Protocol and Quality Control of Image Interpretation Using an Adjudication Panel in the Household Air Pollution Intervention Network (HAPIN) Trial. Ultrasound Med Biol 2023;49:1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balk DS, Lee C, Schafer J, et al. Lung ultrasound compared to chest X-ray for diagnosis of pediatric pneumonia: A meta-analysis. Pediatr Pulmonol 2018;53:1130–1139. [DOI] [PubMed] [Google Scholar]
- 21.McCollum ED, Higdon MM, Fancourt NSS, et al. Training physicians in India to interpret pediatric chest radiographs according to World Health Organization research methodology. Pediatr Radiol 2021;51:1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jha P, Gajalakshmi V, Gupta PC, et al. Prospective study of one million deaths in India: rationale, design, and validation results. PLoS Med 2006;3:e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. Integrated Management of Childhood Illness: Chart Booklet. Geneva: 2014. (https://apps.who.int/iris/bitstream/handle/10665/104772/9789241506823_Chartbook_eng.pdf) [Google Scholar]
- 24.World Health Organization. Pocketbook of Hospital care for children: Guidelines for the management of common childhood illnesses, Second Edition. Geneva: 2013. (https://apps.who.int/iris/bitstream/handle/10665/81170/9789241548373_eng.pdf?s [PubMed] [Google Scholar]
- 25.Mackenzie GA, Hill PC, Sahito SM, et al. Impact of the introduction of pneumococcal conjugate vaccination on pneumonia in The Gambia: population-based surveillance and case-control studies. Lancet Infect Dis 2017;17:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta M, Kumar R, Deb AK, et al. Multi-center surveillance for pneumonia & meningitis among children (<2 yr) for Hib vaccine probe trial preparation in India. Indian J Med Res 2010;131:649–58. [PubMed] [Google Scholar]
- 27.Farooqui H, Jit M, Heymann DL, Zodpey S. Burden of Severe Pneumonia, Pneumococcal Pneumonia and Pneumonia Deaths in Indian States: Modelling Based Estimates. PLoS ONE 2015;10:e0129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broor S, Parveen S, Bharaj P, et al. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS ONE 2007;2:e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinn AK, Williams KN, Thompson LM, et al. Fidelity and Adherence to a Liquefied Petroleum Gas Stove and Fuel Intervention during Gestation: The Multi-Country Household Air Pollution Intervention Network (HAPIN) Randomized Controlled Trial. Int J Environ Res Public Health 2021;18:12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson M, Pillarisetti A, Piedrahita R, et al. Exposure Contrasts of Pregnant Women during the Household Air Pollution Intervention Network Randomized Controlled Trial. Environ Health Perspect 2022;130:97005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Checkley W, Thompson LM, Sinharoy SS, et al. Effects of cooking with liquefied petroleum gas or biomass on infant stunting. N Engl J Med. 2023. [DOI] [PubMed] [Google Scholar]
- 32.Dherani MK, Pope D, Tafatatha T, et al. Association between household air pollution and nasopharyngeal pneumococcal carriage in Malawian infants (MSCAPE): a nested, prospective, observational study. Lancet Glob Health 2022;10:e246–e256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrión D, Kaali S, Kinney PL, et al. Examining the relationship between household air pollution and infant microbial nasal carriage in a Ghanaian cohort. Environ Int 2019;133:105150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004;4:144–54. [DOI] [PubMed] [Google Scholar]
- 35.Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019;394:757–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen SJ, Azziz-Baumgartner E, Budd AP, et al. Decreased Influenza Activity During the COVID-19 Pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1305–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tempia S, Walaza S, Bhiman JN, et al. Decline of influenza and respiratory syncytial virus detection in facility-based surveillance during the COVID-19 pandemic, South Africa, January to October 2020. Euro Surveill 2021;26:2001600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Geneva: 2021. (https://apps.who.int/iris/handle/10665/345329) [PubMed] [Google Scholar]
- 39.Kephart JL, Fandiño-Del-Rio M, Williams KN, et al. Nitrogen dioxide exposures from LPG stoves in a cleaner-cooking intervention trial. Environ Int 2021;146:106196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect 2010;118:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


