Abstract
Background
Deucravacitinib is a first-in-class tyrosine kinase 2 (TYK2) inhibitor recently approved for the treatment of adults with moderate-to-severe plaque psoriasis.
Objective
To discuss the mechanism of action, efficacy, safety, and real-world applications of deucravacitinib for the treatment of psoriasis.
Methods
Literature on the mechanism of action of deucravacitinib is reviewed. The pivotal clinical studies and long-term extension studies for deucravacitinib are also examined.
Results
Deucravacitinib is a novel oral TYK2 inhibitor that binds to the regulatory domain of TYK2, a Janus kinase. By inhibiting TYK2, deucravacitinib interferes with signaling of IL-23, IL-12, and type I interferons, cytokines believed to play important roles in psoriasis pathogenesis. Nearly 60% of patients achieve PASI 75 at 16 weeks of treatment; efficacy improves over 24 weeks and is maintained through 2 years of continuous treatment. In a head-to-head comparison, deucravacitinib efficacy was superior to apremilast, an older yet commonly used oral PDE4 inhibitor approved for the treatment of psoriasis. Of note, patients with moderate-to-severe plaque psoriasis with concomitant involvement of the scalp, nails, and/or palms/soles demonstrated good improvement in these high impact areas. Deucravacitinib has an acceptable safety profile and is generally well-tolerated. Small increases in reactivation of herpesvirus infections, including herpes simplex outbreaks, have been reported. Tuberculosis evaluation, but no other blood tests, is recommended prior to initiation of deucravacitinib. Monitoring of triglyceride levels should be conducted for high-risk patients according to local guidelines.
Conclusion
Deucravacitinib is an effective, safe, and well-tolerated novel oral medication for adults with moderate-to-severe plaque psoriasis.
Keywords: deucravacitinib, tyrosine kinase, TYK2, mechanism of action, psoriasis, review, efficacy, safety
Introduction
Psoriasis is a chronic immune-mediated inflammatory disease that affects up to 8 million adults in the United States.1,2 Patients with moderate-to-severe disease often require treatment with systemic medications, including oral and biologic therapies.3,4 Innovations in oral therapies have been scant. In 1972, methotrexate, a dihydrofolate reductase inhibitor, was approved for the treatment of severe plaque psoriasis in adults. 5 Cyclosporine, a calcineurin inhibitor, and acitretin, an oral retinoid, were approved for adults with severe psoriasis in 1997.6,7 More recently, apremilast, a phosphodiesterase-4 inhibitor, was approved in 2014 for adults with psoriasis, 8 and represented a safer treatment option for patients when compared to the previous oral drugs. Apremilast, however, has limited efficacy, so the search for novel, safe, and more effective oral drugs for psoriasis has been pursued. In September 2022, deucravacitinib was approved by the U.S. Food and Drug Administration for adults with moderate-to-severe plaque psoriasis. 9 Here, the mechanism of action, efficacy, safety, and real-world applications of deucravacitinib for psoriasis are reviewed in detail.
Tyrosine Kinase 2 Background and Mechanism of Action of Deucravacitinib
Along with JAK1, JAK2, and JAK3, TYK2 is a member of the Janus kinase (JAK) family, enzymes involved in transducing inflammatory signals within cells. 10 The first step in this process is binding of a cytokine to the extracellular portion of its receptor. 11 These receptors span across cell membranes, with both extracellular and intracellular regions. 11 Upon cytokine binding to the extracellular region of the cytokine receptor, the intracellular region undergoes a conformational change. This shape change leads to phosphorylation, or activation, of the JAK enzymes that are attached to the intracellular portion of the cytokine receptor.11,12 Activation of JAKs then lead to phosphorylation (activation) of signal transducer and activator of transcription (STAT) proteins, which are also attached the cytokine receptor/JAK complex.11,12 Phosphorylated STAT proteins then dislodge from the complex and migrate to the nucleus, where they bind to DNA regulatory regions. 12 Genes are then transcribed, leading to the further production of inflammatory cytokines and other relevant proteins.12,13
JAKs work in pairs, either homodimerizing, e.g., JAK1/JAK1, or to another JAK, e.g., TYK2/JAK1. 11 Importantly, detailed work has shown that certain groups of cytokines signal through different types of JAKs. For example, the cytokines IL-23, IL-12, and type I interferons rely on TYK2 to mediate their pro-inflammatory effects.14–17 By contrast, another group of cytokines, IL-4, IL-13, and IL-31, signal through JAK1.18,19 This concept is important, since JAK inhibitors are now available that have different specificities for each JAK enzyme, with some being more selective than others, such as the TYK2 inhibitor deucravacitinib (approved for psoriasis) 9 and the JAK1 inhibitor abrocitinib (approved for atopic dermatitis). 20 By blocking TYK2, deucravacitinib blocks signaling of IL-23 and IL-12, similar to ustekinumab, a commonly used biologic used to treat psoriasis. 21
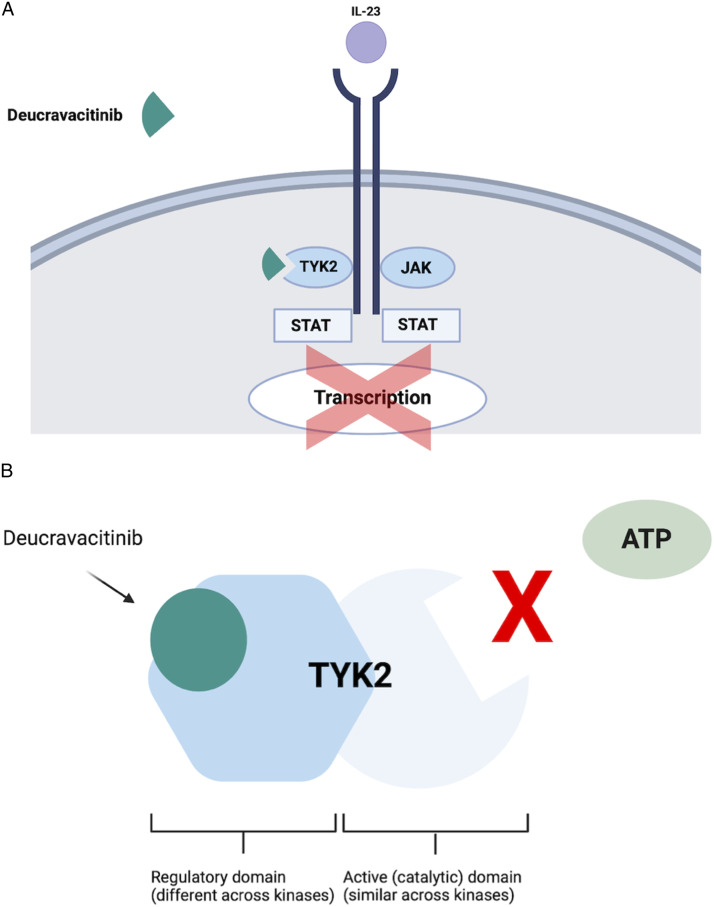
Another important feature of deucravacitinib is its selectivity for binding and inactivating TYK2 (Figure 1), while demonstrating negligible ability to block the other three members of the JAK family at relevant pharmacokinetic drug levels. 22 This selectively is imparted due to its binding in the unique regulatory region of TYK2 as opposed to the enzymatic region of the enzyme, which is a relatively conserved region of all four JAKs (Figure 1).15,23–25 Therefore, drug binding to the enzymatic region of a JAK often leads to at least some cross-inactivation of all four JAKs, i.e., less specificity. Selectively for TYK2 also is the reason deucravacitinib has not been categorized with other types of JAK inhibitors, which all have boxed warnings related to safety (see below); 20 deucravacitinib currently has no boxed warnings. Indeed, it has been designated a first-in-class drug as a selective TYK2 inhibitor. 9
Figure 1.
(A-B): Mechanism of action of deucravacitinib.
Efficacy of Deucravacitinib
Phase 3 Trials
Three phase 3 clinical trials have examined the efficacy of deucravacitinib in adults with moderate-to-severe plaque psoriasis: two pivotal trials (POETYK PSO-1 and POETYK PSO-2)26,27 and one long-term extension trial (POETYK PSO-LTE). 28 POETYK PSO-1 and POETYK PSO-2 enrolled individuals ≥18 years old with moderate-to-severe psoriasis for 6 months prior to screening.29,30 Patients were randomized to deucravacitinib 6 mg daily, placebo, or apremilast 30 mg twice a day on day 1.
The coprimary end points were achievement of ≥75% reduction from baseline in PASI (PASI 75) and sPGA 0/1 (clear/almost clear) with a ≥2-point improvement from baseline for deucravacitinib versus placebo at Week 16. Key secondary end points included achievement of ≥90% and 100% reductions from baseline in PASI (PASI 90 and PASI 100) at Weeks 16 and 24. In POETYK PSO-1 and POETYK PSO-2, clinical responses with deucravacitinib were also compared to apremilast for primary and secondary endpoints. A head-to-head comparison with an established therapy like apremilast was critical to understanding how the efficacy of deucravacitinib compared to an existing oral treatment commonly used for psoriasis.
Efficacy of Deucravacitinib: Results from POETYK PSO-1 and POETYK PSO-2
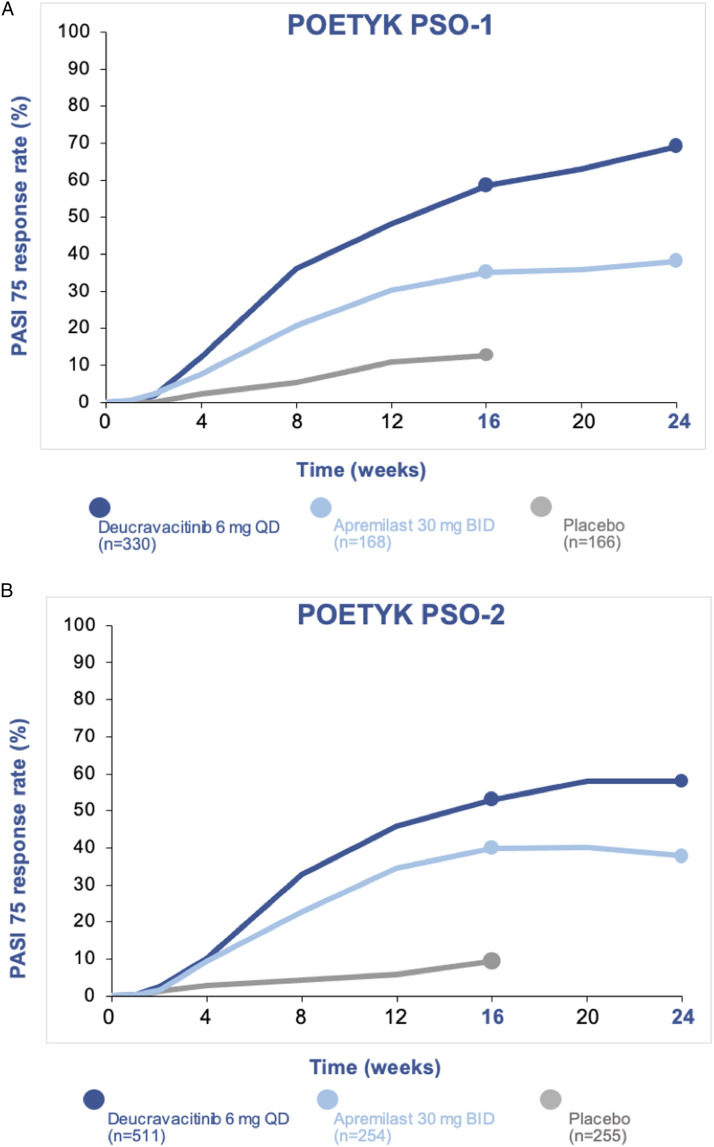
In both trials, a majority of patients had received prior systemic treatment for psoriasis (63% in POETYK PSO-1; 54% in POETYK PSO-2).29,30 Deucravacitinib demonstrated superior PASI 75 and sPGA 0/1 response rates compared to apremilast and placebo at Week 16 (Figure 2).29,30 In POETYK PSO-1, 58% of patients on deucravacitinib achieved PASI 75 at Week 16, compared to 35% of patients on apremilast and 13% of patients on placebo. 29 A total of 54% of patients on deucravacitinib achieved sPGA 0/1 at Week 16, compared to 32% of patients on apremilast and 7% of patients on placebo. 29
Figure 2.
(A-B): PASI 75 response rates in the POETYK PSO-1 and POETYK PSO-2 trials.
Deucravacitinib was also superior to apremilast in PASI 90 response rates at Weeks 16 and 24 in both trials.29,30 PASI 90 responses with deucravacitinib were 27% and 32% at Weeks 16 and 24, respectively, compared to 18% and 20% with apremilast, in POETYK PSO-2. 30 PASI 100 response rates for deucravacitinib were 14% and 10% in POETYK PSO-1 and POETYK PSO-2, respectively, compared to 1% and 1% for placebo at Week 16 (P < .001).29,30 Notably, deucravacitinib is the first oral therapy for moderate-to-severe plaque psoriasis to include PASI 100 data at Week 16 in its label.5–9
Deucravacitinib had similar efficacy among different patient sub-groups, including those with varying demographics, baseline disease activity, and prior treatment history. More specifically, on post-hoc analysis, there were no identified differences in PASI 75 responses with deucravacitinib among patient subgroups based on age, gender, race, body weight, baseline disease severity or prior systemic therapy use.31–33
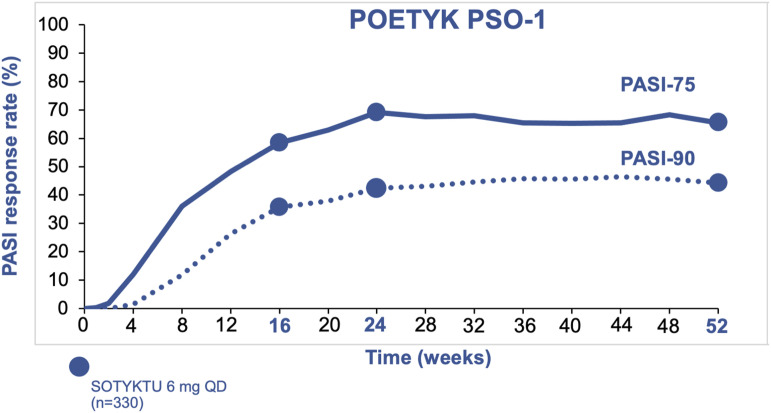
Over time, PASI response rates remained stable through 1 year for patients on deucravacitinib (Figure 3). In POETYK PSO-1, 65% of patients achieved PASI 75 at Week 52 (compared to 69% at Week 24). 34 Similarly, 44% of patients achieved PASI 90 at Week 52 (compared to 42% at Week 24). Nearly one in five patients (19%) achieved PASI 100 at Week 52. 34
Figure 3.
PASI 75 and PASI 90 response rates at 1 year of treatment with deucravacitinib.
Long-Term Efficacy of Deucravacitinib: Results from POETYK PSO-LTE
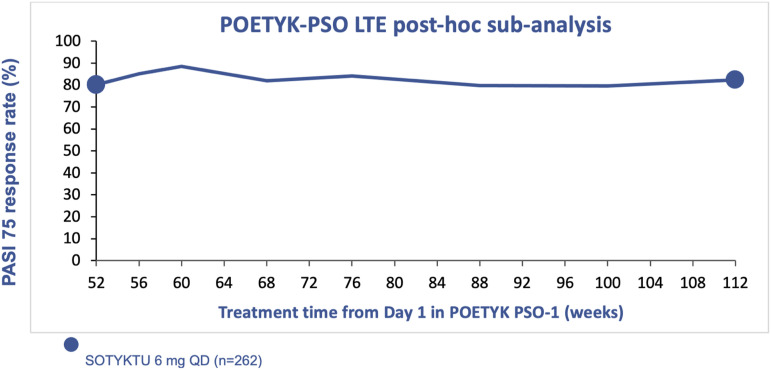
Patients who completed treatment through Week 52 in either POETYK PSO-1 or POETYK PSO-2 were eligible to enroll in the long-term extension trial, POETYK PSO-LTE. 35 All enrolled patients, regardless of treatment arm in the parent study, were blindly switched to open-label deucravacitinib at the start of POETYK PSO-LTE. Among all patients enrolled in POETYK PSO-LTE, PASI 75 and sPGA 0/1 response rates were 78% and 59%, respectively, at 2 years, compared to 67% and 52% at 1 year, using nonresponder imputation.35,36 Of note, long-term extension efficacy data may be inflated as patients who lose response or are unable to tolerate the medication may be more likely to discontinue treatment, which may raise the proportion of responders. A post-hoc sub-analysis of patients who received 2 years of continuous deucravacitinib treatment showed a PASI 75 response rate of 82% using nonresponder imputation (Figure 4). 36
Figure 4.
PASI 75 response rates through 2 years of continuous treatment with deucravacitinib.
Efficacy of Deucravacitinib in High Impact Areas
A subset of patients with moderate-to-severe psoriasis in POETYK PSO-1 and POETYK PSO-2 had concomitant disease in high impact areas, e.g., scalp, fingernails, and palms/soles.9,37 Deucravacitinib efficacy within these specific body areas was also assessed. In POETYK PSO-1, significantly more patients who had baseline moderate-to-severe scalp disease achieved clear or almost clear scalp (scalp-specific Physician’s Global Assessment [ss-PGA] 0/1) at Week 16 when treated with deucravacitinib, compared to those treated with apremilast (P < .001) and placebo (P < .001). 29 A small number of patients in both trials had concomitant moderate-to-severe nail psoriasis at baseline.29,30 In a pooled analysis of POETYK PSO-1 and POETYK PSO-2, the Physician’s Global Assessment of fingernails (PGA-F) score of 0 (clear) or 1 (almost clear) response at Week 16 was greater for deucravacitinib versus placebo (P = .0272) using nonresponder imputation. 38 Similarly, few patients had concomitant moderate-to-severe palmoplantar psoriasis at baseline.29,30 In a pooled analysis, significantly more patients receiving deucravacitinib versus placebo achieved palmoplantar Physician’s Global Assessment (pp-PGA) of 0/1 (clear/almost clear) at Week 16 (P = .0052) using nonresponder imputation. 39
Safety of Deucravacitinib
Safety of Deucravacitinib: Safety Data from POETYK PSO-1 and POETYK PSO-2
Deucravacitinib was well-tolerated; rates of headache, diarrhea, and nausea were similar or lower than in patients treated with either placebo or apremilast at Week 16 (headache: deucravacitinib 4.5%, placebo 4.5%, apremilast 10.7%; diarrhea: deucravacitinib 4.4%, placebo 6.0%, apremilast 11.8%; nausea: deucravacitinib 1.7%, placebo 1.7%, apremilast 10.0%). 40
In pooled Week 16 safety data of POETYK PSO-1 and POETYK PSO-2, patients on deucravacitinib had relatively low frequencies of upper respiratory tract infections (URI), increased blood creatine phosphokinase, herpes simplex infections, mouth ulcers, folliculitis, and acne, with slight increases in frequencies of these events compared to patients on placebo;9,40 most of these events were mild and did not require treatment discontinuation.
Deucravacitinib had the lowest discontinuation rates due to adverse events (AE) at 16 weeks (2.4%) compared to apremilast (5.2%) and placebo (3.8%). 40 Discontinuation rates due to AEs remained lower for patients on deucravacitinib (4.4%) compared to patients on apremilast (11.6%) and placebo (9.4%) through 52 weeks of treatment. 40 Incidence (reported as exposure adjusted incident rate [EAIR] per 100 patient years [PY]) of headache, diarrhea and nausea remained significantly lower for deucravacitinib vs apremilast (headache 8.5 vs 26.0; diarrhea 7.3 vs 26.5; nausea 2.1 vs 22.9) at Week 52. 40
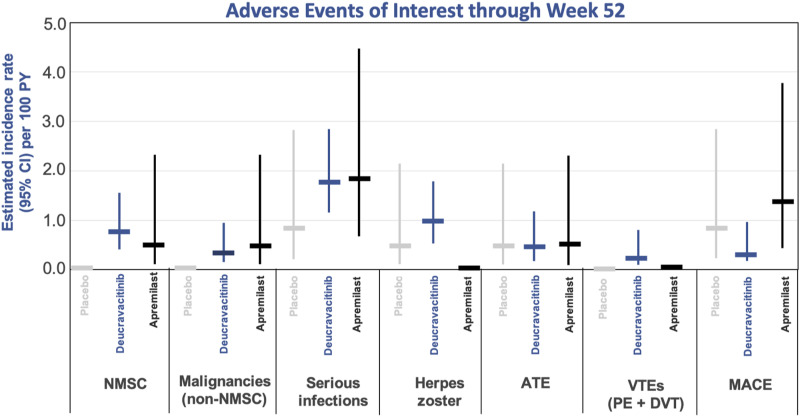
The incidence of specific AEs of interest, including nonmelanoma skin cancer (NMSC), malignancies, serious infections, arterial thromboembolic events, venous thromboembolism events (VTE), and major cardiovascular events (MACE) were low and similar among the deucravacitinib, apremilast, and placebo arms at Week 52 (Figure 5).9,36,40 Serious adverse events (SAE) occurred at low and similar rates among the deucravacitinib, placebo, and apremilast groups through 52 weeks (EAIR per 100 PY 5.7, 5.7, and 4.0, respectively). 40
Figure 5.
Adverse events of interest through week 52: pooled analysis of POETYK PSO-1 and POETYK PSO-2. NMSC, non-melanoma skin cancer; ATE, arteriothrombotic event; VTE, venous thromboembolism event; PE, pulmonary embolism; DVT, deep vein thrombosis; MACE, major adverse cardiovascular event.
Long-Term Safety of Deucravacitinib: Safety Data from POETYK PSO-LTE
Treatment with deucravacitinib was well-tolerated through 2 years and had lower rates of AEs compared to 52 weeks of treatment on pooled safety data from POETYK PSO-1, POETYK PSO-2, and POETYK PSO-LTE. 35 At 2 years of treatment with deucravacitinib, the EAIR per 100 PY of AEs leading to discontinuation was lower compared to 1 year of treatment (2.8 vs 4.4). 35 Rates of SAEs remained low and stable through 2 years (EAIR 6.2 per 100 PY) compared to 1 year (EAIR 5.7 per 100 PY). 35 The EAIR per 100 PY for AEs of interest were also reported through 2 years of treatment. The EAIRs per 100 PY for serious infections for patients receiving deucravacitinib were 1.7 at 1 year and 2.6 at 2 years. 35 The EAIRs per 100 PY were 0.9 at year 1 and 0.7 at year 2 for herpes zoster infection; 0.3 and 0.4, respectively, for MACE; 0.2 and 0.1, respectively, for VTE; and 1.0 and 0.9, respectively, for total malignancies. 35 The EAIRs per 100 PY of NMSC were 0.7 and 0.4 and 1 and 2 years, respectively. 32 The EAIRs per 100 PY of malignancies excluding NMSC were 0.3 and 0.5 and 1 year and 2 years, respectively. 35
Laboratory Parameters: Results from POETYK PSO-1 and POETYK PSO-2
In pooled laboratory data from POETYK PSO-1 and POETYK PSO-2, there were no abnormalities in hematologic parameters at Week 16. 41 The most commonly reported laboratory AE in all 3 treatment groups was elevated blood creatine phosphokinase, which was typically associated with recent exercise and resolved without intervention in most cases.29,30 While most patients had normal triglyceride levels, some patients experienced increases in triglycerides. 41 Rates of grade ≥3 triglyceride abnormalities at Week 16 were similar among the three treatment groups (grade 3: deucravacitinib 1.6%, placebo 1.5%, apremilast 2.0%; grade 4: deucravacitinib 0.2%, placebo 0%, apremilast 0.2%), and the majority of these did not lead to discontinuation from the study. 41 The mean increase in triglycerides in the deucravacitinib group was approximately 10 mg/dL higher than the placebo group, 41 but the clinical significance of this increase is uncertain. Currently, there is no universal recommendation for triglyceride monitoring in patients on deucravacitinib, but, in general, lipid evaluation with primary care providers is recommended for at-risk patients.
Real-World Applications
Which Patients are Appropriate for Deucravacitinib?
Deucravacitinib is a novel oral medication that is among the first-line treatments for adults with moderate-to-severe plaque psoriasis. It is an effective option for patients who are not interested in injectable biologic medications or have contraindications to biologics (Table 1). It would also be an appropriate treatment option for those who have demonstrated an inadequate response to or not tolerated apremilast. Deucravacitinib has similar efficacy regardless of baseline disease severity or prior systemic therapy use,31,32 making it an appealing option for both biologic-naïve and biologic-experienced patients (Table 1). Furthermore, it is an appropriate option regardless of patient weight (Table 1).31,32 Ongoing clinical trials are investigating the efficacy of deucravacitinib in treating psoriatic arthritis. 42
Table 1.
Real-World Applications of Deucravacitinib.
| Patients that may be appropriate for deucravacitinib | Potential candidates for treatment with deucravacitinib include biologic naïve patients, biologic experienced patients, patients with contraindications to biologic therapy, needle averse patients, patients interested in oral therapy, or patients with BMI >30 kg/m2. |
| Dosing and administration | Deucravacitinib 6 mg is a once daily oral tablet that requires no dose titration. It can be taken with or without food. There are no known drug interactions with deucravacitinib; however, patient should avoid live vaccines while on deucravacitinib and update vaccination status prior to initiating therapy, when possible. |
| Monitoring recommendations | All patients should undergo tuberculosis (TB) evaluation prior to starting deucravacitinib. Patients with latent TB should be initiated on anti-tuberculosis therapy prior to or concurrent to starting deucravacitinib. If a patient has active TB, consider consultation with infectious disease specialists. |
| Consider evaluation of baseline lipids. For patients with known or suspected liver disease, liver function enzymes and hepatitis serologies should be evaluated at baseline. Those with known liver disease should be monitored with liver function enzymes throughout treatment with deucravacitinib, according to routine patient management. |
Dosing and Administration of Deucravacitinib
Deucravacitinib is a once daily 6 mg oral tablet that does not require dose titration or dose adjustments (Table 1). 9 Patients can take deucravacitinib with or without meals, 9 which provides flexibility for patients and can increase adherence. There are no known medication interactions with deucravacitinib (Table 1). 9 However, live vaccines should be avoided while taking deucravacitinib, 9 and when possible, as with other systemic immunosuppressive medications, patients might consider updating immunizations according to current immunization guidelines prior to initiating therapy (Table 1).
Monitoring Recommendations
Prior to starting deucravacitinib, all patients should undergo tuberculosis (TB) evaluation. This recommendation stems from the fact that deucravacitinib can interfere with signaling and function of IL-12, 29 a cytokine known to play a role in fighting granulomatous infections. 43 If the patient has latent TB, the patient should be considered for anti-tuberculosis therapy prior to or concurrent to starting deucravacitinib. If the patient has active TB, deucravacitinib should not be started, and consultation with infectious disease specialists is advisable (Table 1). 9 Baseline evaluation of lipids (fasting) can be considered for patients who do not have a prior available assessment of baseline lipids. While there is no additional universal recommendation for triglyceride monitoring, patients with unmanaged hypertriglyceridemia should follow-up with their primary care providers such that they can receive periodic triglyceride evaluation according to clinical guidelines for hyperlipidemia (Table 1). 9
If a patient has known or suspected liver disease, liver enzymes and hepatitis serologies should be evaluated at baseline prior to starting deucravacitinib. Those with known liver disease should then be monitored with liver function enzymes during deucravacitinib therapy, according to routine patient management (Table 1). 9 Deucravacitinib is not recommended for patients with severe hepatic impairment or with chronic hepatitis B virus or hepatitis C virus infection. 9
Counseling Patients on Deucravacitinib
Before starting a patient on deucravacitinib, the risks, benefits and treatment alternatives should be discussed while emphasizing the overall tolerability and safety of deucravacitinib therapy. Patients should be reminded not to crush or cut deucravacitinib tablets. Patients can expect to see improvement in their skin as early as 4 weeks, which can continue to improve with continuous treatment through Week 24.29,30 At this time, there is insufficient data to support the use of deucravacitinib in pregnant or breastfeeding women. 9 Patients can be counseled on the superior efficacy of deucravacitinib as compared to apremilast, though it should be noted there is a lack of head-to-head studies comparing deucravacitinib to other oral therapies for psoriasis.
Combination Use
Deucravacitinib can be used as monotherapy to treat moderate-to-severe psoriasis but may also be used as part of combination therapy. In real-world clinical practices, as with other systemic medications, deucravacitinib is often used with adjunctive topical therapies to achieve greater clearance of plaques. In patients with recalcitrant severe plaque psoriasis, considerations may be given to using deucravacitinib in combination with another systemic agent. However, there are little data with this combination use, and further studies are necessary to understand the efficacy and safety of this approach.
Conclusion
Development and approval of deucravacitinib, a novel first-in-class TYK2 inhibitor, represents a major breakthrough for oral therapies to treat psoriasis. Overall, deucravacitinib has been shown to be an effective oral agent with an acceptable safety profile and good tolerability through 2 years of use. Efficacy has also been shown to be superior to apremilast in head-to-head studies, which positions this drug to become the gold standard oral therapy for patients with plaque psoriasis.
Acknowledgements
AB has served as a speaker (received honoraria) for AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Regeneron, and Sanofi, served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, CTI BioPharma, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, Leo, Lipidio, Merck, Nektar, Novartis, Pfizer, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Ventyx, Vibliome, and Xencor, and has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, UCB Pharma, and Ventyx.BS has served as a consultant (honoraria) to AbbVie, Alamar, Alumis, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Immunic Therapeutics, Kangpu Pharmaceuticals, Bristol-Myers-Squibb, Capital One, Connect Biopharma, CorEvitas, Dermavant, Evelo Biosciences, Janssen, Leo, Eli Lilly, Maruho, Meiji Seika Pharma, Mindera Health, Protagonist, Nimbus, Novartis, Pfizer, UCB Pharma, Sun Pharma, Regeneron, Sanofi-Genzyme, Union Therapeutics, Ventyxbio, vTv Therapeutics; Stock Options: Connect Biopharma, Mindera Health; Speaker: AbbVie, Arcutis, Dermavant, Eli Lilly, Incyte, Janssen, Regeneron, Sanofi-Genzyme; is the Scientific Co-Director (consulting fee) for the CorEvitas Psoriasis Registry; has served as an investigator for the CorEvitas Psoriasis Registry; is the Editor-in-Chief of the Journal of Psoriasis and Psoriatic Arthritis. AWA has served as a research investigator, scientific advisor, and/or speaker to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, BI, BMS, EPI, Incyte, Leo, UCB, Janssen, Lilly, Mindera, Nimbus, Novartis, Ortho Dermatologics, Sun, Dermavant, Dermira, Sanofi, Regeneron, and Pfizer.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PK declares no conflicts of interest. AB has served as a speaker (received honoraria) for AbbVie, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer, Regeneron, and Sanofi, served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, CTI BioPharma, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, Leo, Lipidio, Merck, Nektar, Novartis, Pfizer, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Ventyx, Vibliome, and Xencor, and has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol-Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, UCB Pharma, and Ventyx. BS has served as a consultant (honoraria) to AbbVie, Alamar, Alumis, Almirall, Amgen, Arcutis, Arena, Aristea, Asana, Boehringer Ingelheim, Immunic Therapeutics, Kangpu Pharmaceuticals, Bristol-Myers-Squibb, Capital One, Connect Biopharma, CorEvitas, Dermavant, Evelo Biosciences, Janssen, Leo, Eli Lilly, Maruho, Meiji Seika Pharma, Mindera Health, Protagonist, Nimbus, Novartis, Pfizer, UCB Pharma, Sun Pharma, Regeneron, Sanofi-Genzyme, Union Therapeutics, Ventyxbio, vTv Therapeutics; Stock Options: Connect Biopharma, Mindera Health; Speaker: AbbVie, Arcutis, Dermavant, Eli Lilly, Incyte, Janssen, Regeneron, Sanofi-Genzyme; is the Scientific Co-Director (consulting fee) for the CorEvitas Psoriasis Registry; has served as an investigator for the CorEvitas Psoriasis Registry; is the Editor-in-Chief of the Journal of Psoriasis and Psoriatic Arthritis. AWA has served as a research investigator, scientific advisor, and/or speaker to AbbVie, Almirall, Arcutis, ASLAN, Beiersdorf, BI, BMS, EPI, Incyte, Leo, UCB, Janssen, Lilly, Mindera.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Consent: Participants provided written informed consent.
Ethical Statement
Ethical Approval
This research did not require IRB approval/ethical approval because data reviewed are publicly available.
ORCID iDs
Bruce Strober https://orcid.org/0000-0002-8394-2057
April W. Armstrong https://orcid.org/0000-0003-0064-8707
References
- 1.Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatology. 2021;157(8):940-946. doi: 10.1001/jamadermatol.2021.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis. JAMA. 2020;323(19):1945-1960. doi: 10.1001/jama.2020.4006 [DOI] [PubMed] [Google Scholar]
- 3.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029-1072. doi: 10.1016/j.jaad.2018.11.057 [DOI] [PubMed] [Google Scholar]
- 4.Menter A, Gelfand JM, Connor C, et al. Joint American academy of dermatology-national psoriasis foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445-1486. doi: 10.1016/j.jaad.2020.02.044 [DOI] [PubMed] [Google Scholar]
- 5.Morgantown WV. METHOTREXATE [package insert]. Morgantown, WV: Mylan Pharmaceuticals, Inc; 2022. [Google Scholar]
- 6.NEORAL [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2009. [Google Scholar]
- 7.SORIATANE [package insert]. Research Triangle Park, NC: Stiefel Laboratories, Inc; 2017. [Google Scholar]
- 8.OTEZLA [package insert]. Thousand Oaks, CA: Amgen; 2021. [Google Scholar]
- 9.SOTYKTU [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2022. [Google Scholar]
- 10.Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger JG, McInnes IB, Blauvelt A. Tyrosine kinase 2 and Janus kinase-signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J Am Acad Dermatol. 2022;86(1):148-157. doi: 10.1016/j.jaad.2021.06.869 [DOI] [PubMed] [Google Scholar]
- 12.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234-243. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Jeldres T, Tyler CJ, Boyer JD, et al. Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front Pharmacol. 2019;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77(2):175-187. [DOI] [PubMed] [Google Scholar]
- 15.Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11(502):1-16. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L, Li Z, Rui L. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J Biol Chem. 2008;283(42):28066-28073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie J, LeBaron MJ, Nevalainen MT, et al. Role of tyrosine kinase Jak2 in prolactin-induced differentiation and growth of mammary epithelial cells. J Biol Chem. 2002;277(16):14020-14030. [DOI] [PubMed] [Google Scholar]
- 18.Kvist-Hansen A, Hansen PR, Skov L. Systemic treatment of psoriasis with JAK inhibitors: A review. Dermatology and Therapy. 2020;10(1):29-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843-862. [DOI] [PubMed] [Google Scholar]
- 20.CIBINQO [package insert]. New York, NY: Pfizer Labs; 2022. [Google Scholar]
- 21.STELARA [package insert]. Horsham, PA: Janssen Biotech, Inc; 2009. [Google Scholar]
- 22.Chimalakonda A, Burke J, Cheng L, et al. Selective inhibition of tyrosine kinase 2 with deucravacitinib (BMS-986165) compared with Janus kinase 13 inhibitors. Poster presented at: Annual Fall Clinical Dermatology Conference; October 29 to November 1, 2020; virtual meeting and at Las Vegas, NV. [Google Scholar]
- 23.Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: Focus on selective TYK2 inhibitors. Drugs. 2020;80(4):341-352. [DOI] [PubMed] [Google Scholar]
- 24.Moslin R, Zhang Y, Wrobleski ST, et al. Identification of N-methyl nicotinamide and N-methyl pyridazine-3-carboxamide pseudokinase domain ligands as highly selective allosteric inhibitors of tyrosine kinase 2 (TYK2). J Med Chem. 2019;62(20):8953-8972. [DOI] [PubMed] [Google Scholar]
- 25.Wrobleski ST, Moslin R, Lin S, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: Discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62(20):8973-8995. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov. NCT03624127. Accessed March 29, 2023, 2023. [Google Scholar]
- 27.ClinicalTrials.gov. NCT03611751. Accessed March 29, 2023, 2023. [Google Scholar]
- 28.ClinicalTrials.gov. NCT04036435. Accessed April 7, 2023, 2023. [Google Scholar]
- 29.Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29-39. doi: 10.1016/j.jaad.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51. [DOI] [PubMed] [Google Scholar]
- 31.Merola JF, Sofen H, Thaçi D, et al. Deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy analysis by baseline disease characteristics from the phase 3 POETYK PSO-1 and PSO-2 trials. Oral presentation at EADV; September 2021; Milan, Italy Poster presentation at EADV 2021. Poster 1410. [Google Scholar]
- 32.Warren RB, Armstrong A, Imafuku S, et al. Deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, versus placebo and apremilast in moderate to severe plaque psoriasis: Efficacy analysis by prior treatment in the phase 3 POETYK PSO-1 and PSO-2 trials. Oral presentation at EADV; 2021; Switzerland. Presentation FC03.06. [Google Scholar]
- 33.Data on file. BMS-REF-DEU-0010. Princeton, NJ: Bristol-Myers Squibb Company; 2022. [Google Scholar]
- 34.Data on file. BMS-REF-DEU-0020. Princeton, NJ: Bristol-Myers Squibb Company; 2022. [Google Scholar]
- 35.Warren RB, Sofen H, Imafuku S, et al. Deucravacitinib long-term efficacy and safety in plaque psoriasis: 2-year results from the phase 3 POETYK PSO program. Poster presented at: European Academy of Dermatology and Venereology Spring Symposium; May 12-14, 2022; Ljubljana, Slovenia. Poster P465. [Google Scholar]
- 36.Lebwohl M, Warren RB, Sofen H, et al. Deucravacitinib long-term efficacy with continuous treatment in plaque psoriasis: 2-year results from the phase 3 POETYK PSO study program. Oral presentation at EADV; September 2022; Milan, Italy Presentation D3T01.1F. [Google Scholar]
- 37.Data on file. BMS-REF-DEU-0075. Princeton, NJ: Bristol-Myers Squibb Company; 2023. [Google Scholar]
- 38.Blauvelt A, Rich P, Sofen H, et al. Deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, versus placebo in scalp, nail, and palmoplantar psoriasis: Subset analyses of the phase 3 POETYK PSO-1 and PSO-2 trials. SKIN J Cutan Med. 2022;6(4):s41. Oral presentation at SDDS 2022. [Google Scholar]
- 39.Blauvelt A, Rich P, Sofen H, et al. Deucravacitinib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in scalp, nail, and palmoplantar psoriasis: Subgroup analyses of the phase 3 POETYK PSO-1 and PSO-2 trials. SKIN J Cutan Med. 2022;6(6):s49. Oral presentation at FCDC 2022. [Google Scholar]
- 40.Armstrong A, Gooderham M, Warren RB, et al. Efficacy and safety of deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe plaque psoriasis: Results from the POETYK PSO-1 study. Presented at: the Annual Meeting of the American Academy of Dermatology; March 19, 2021; San Francisco, CA. Oral presentation at AAD 2021. Session S033. [Google Scholar]
- 41.Thaçi D, Gordon K, Gooderham M, et al. Deucravacitinib, an oral, selective tyrosine kinase 2 (TYK2) inhibitor, compared with placebo and apremilast in moderate to severe psoriasis: Integrated laboratory parameter results from the phase 3 POETYK PSO-1 and POETYK PSO-2 trials. SKIN J Cutan Med. 2021;5(6):s37. Poster presentation at EADV 2021; Poster P1407. [Google Scholar]
- 42.Mease PJ, Deodhar AA, van der Heijde D, et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81(6):815-822. doi: 10.1136/annrheumdis-2021-221664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larousserie F, Pflanz S, Coulomb-L’Herminé A, et al. Expression of IL-27 in human Th1-associated granulomatous diseases. J Pathol. 2004;202(2):164-171. doi: 10.1002/path.1508 [DOI] [PubMed] [Google Scholar]