Abstract
Expression of cloned genes from isopropyl-β-d-thiogalactopyranoside (IPTG)-regulated promoters is lowered when the Escherichia coli CmlA/Cmr/MdfA efflux pump is overexpressed, probably due to IPTG exclusion from the cytoplasm. The previously reported cmlA1 mutation confers a similar phenotype. cmlA1 contains an IS30 insertion upstream of cmr/mdfA, which creates a putative promoter. CmlA overproduction also causes spectinomycin hypersensitivity.
The emergence of pathogenic bacteria with multiple resistance to antibiotics is a challenge for health treatments. The multidrug resistance (MDR) phenotype is often associated with increased activity of efflux pumps, which are responsible for extrusion from the cell of a broad array of sometimes unrelated toxic compounds (see references 6, 19, and 21 for reviews). It was first shown that tumor cells with MDR phenotypes overexpress P glycoproteins (P-gp), which are responsible for expulsion of antitumor agents, thus preventing their accumulation to an effective level. P-gp belong to the ABC (ATP-binding cassette) transporter family, and the extrusion energy is provided by ATP hydrolysis. To date, the LmrA protein from Lactococcus lactis is the only known bacterial P-gp homologue (29, 30). Most bacterial transporters utilize proton motive force as the driving force for drug export. They are classified into three groups: (i) the major facilitator superfamily (MFS) with 12 or 14 transmembrane segments (TMS), exemplified by EmrB in Escherichia coli; (ii) the resistance nodulation division family with 12 TMS, mainly found in gram-negative bacteria, such as AcrB in E. coli; (iii) the small multidrug resistance family with only four TMS (e.g., EmrE in E. coli). The gram negative MFS and TMS multidrug efflux transporters are often associated with periplasmic linker proteins and outer membrane channels.
We report the selection and characterization of plasmids encoding an E. coli multidrug efflux pump from the MFS group, recently characterized under the names Cmr (22) and MdfA (14); the gene was originally designated cmlA (25, 26).
Strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Genotype or phenotype | Origin or constructiona |
|---|---|---|
| Strains | ||
| C600 | supE44 thi-1 thr-1 leuB6 lacY1 tonA21 hsdR | Laboratory collection |
| UM21 | HfrC metB1 relA1 spoT1 tolZ21 sfhC | 24 |
| PhB782 | As UM21 hflB+zha-b::Tn10/pSC101lacIq | Laboratory collection |
| YYC188 | secA214 Δ(aroP-aceF)73 fhuA54? lacZ608(Am) λcI857 zbj-927::Tn10 poxB11 pflB1 pps-4 rpsL104 thi-1 | Mary Berlyn, CGSC6776 (7) |
| RE103 | proA23 lac-28 trp-30 his-51 rpsL101 cmlA1 | Mary Berlyn, CGSC4698 (25, 26) |
| RC712 | proA23 lac-28 trp-30 his-51 rpsL101 | Mary Berlyn, CGSC4413 |
| PhB1001 | As RE103 (cmlA1) zbj-927::Tn10 | RE103 + P1/YYC118 |
| PhB631 | From FB8 (prototroph) | Laboratory collection |
| PhB1010 | As PhB631 zbj-927::Tn10 | PhB631 + P1/PhB1001 |
| PhB1011 | As PhB631 zbj-927::Tn10 cmlA1 | PhB631 + P1/PhB1001 |
| Plasmids | ||
| pGB2 | From pSC101; aadA | 8 |
| pSC101lacIq | From pSC101; aadA lacIq | 28 |
| pCTCIII | From pACYC184; Ptac-λcIII | 23 |
| pIL1991 | From pUHE21; lactococcal phage bIL66 M operon | 3 |
| pKSCMLA | From pKS; cmlA | This work |
| pBRCMLA | From pBR322; cmlA | This work |
Isogenic pairs of strains were constructed by P1 vir-mediated transductions (20).
Plasmid pCTCIII-induced lethality.
The cIII protein of phage λ is responsible for E. coli growth inhibition. Plasmid pCTCIII (23) carries the λ cIII gene downstream of the Ptac promoter such that expression of the λ cIII gene can be conditionally induced by addition of the gratuitous inducer isopropyl-β-d-thiogalactopyranoside (IPTG) to the medium. The survival frequency of strain PhB782 carrying pCTCIII at 37°C on rich medium containing 10−4 M IPTG is less than 10−6.
Suppression of pCTCIII-induced lethality.
Strain PhB782 carrying pCTCIII was transformed by an E. coli genomic DNA library (prepared from E. coli C600), and clones resistant to λ cIII-induced lethality were selected. On medium containing 10−4 M IPTG, the survival frequency was 6 × 10−5 per transformant, and no clones were obtained on 5 × 10−3 M IPTG. Most plasmids conferring resistance carried a fragment from the 19-min region of the E. coli chromosome. Restriction analysis suggested that the cmr/mdfA gene (see below) was responsible for the survival phenotype. We amplified solely the cmr/mdfA gene from position 882438 to 884205 of the E. coli DNA map (5) by PCR using the primers TACCCGGGTTATCACCAGTTGCCGTTGTG and CTGAAGCTTATCGAACACCAGATTGACGA. The PCR product digested by SmaI and HindIII and ligated to SmaI- and HindIII-treated pKS (Stratagene, Cambridge, United Kingdom) gave rise to pKSCMLA. When pKSCMLA was established in PhB782 carrying pCTCIII, the strain became resistant to 10−4 M IPTG. The E. coli Cmr/MdfA (14, 22) multidrug efflux pump is responsible for resistance to chloramphenicol and to many structurally unrelated toxic compounds such as lipophiles (e.g., ethidium bromide, puromycin, and tetracycline), macrolides (e.g., erythromycin), aminoglycosides (e.g., neomycin), and quinolones (e.g., norfloxacin) (14, 22). Its activity is proton motive force dependent (14, 22).
The cmlA1 mutation is an allele of cmr/mdfA.
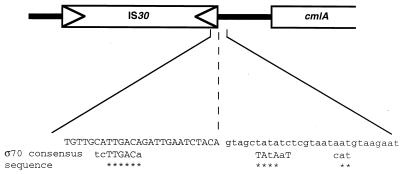
The cmlA locus was characterized in 1966 by an allele, cmlA1, conferring resistance to chloramphenicol (25, 26); multicopy plasmids carrying the cmr/mdfA gene also confer chloramphenicol resistance. Since cmlA, like cmr/mdfA, is adjacent to deoR (27), we hypothesized that cmlA is identical to cmr/mdfA. We found that the cmlA1 mutation (strain RE103) is due to an IS30 sequence upstream of cmr/mdfA (Fig. 1) which was not present in RC712, an isogenic strain. The IS30 right inverted repeat is known to carry a potential −35 transcriptional promoter which, after transposition, may induce gene activation (9–11). The IS30 insertion upstream of cmr/mdfA in the cmlA1 strain creates a putative ς70 promoter, presumably increasing cmr/mdfA activity (Fig. 1). The cmlA gene, as initially referred to on the E. coli genetic map (1), is identical to the cmr/mdfA gene; we will therefore now refer to the original name, cmlA (14, 22). However, note that it differs from the In4 integron cmlA gene of Tn1696 (4).
FIG. 1.
The cmlA1 mutation is due to IS30 transposition upstream of the cmlA (cmr/mdfA) gene. The sequence generated by the insertion creates a putative ς70 promoter as indicated in the expanded region of the IS30-cmlA junction.
The cmlA1 mutation confers resistance to pCTCIII-induced lethality.
We hypothesized that the cmlA1 mutation could also suppress pCTCIII-induced lethality. We constructed isogenic cmlA+ (PhB1010) and cmlA1 (PhB1011) strains and transformed them with pSC101lacIq and pCTCIII. Upon challenge with 5 × 10−5 M IPTG, the cmlA1 mutation conferred full resistance, while the survival frequency of the isogenic cmlA+ strain was 2 × 10−5. The cmlA1 phenotype is similar to that induced by multicopy plasmids containing wild-type cmlA, suggesting that the cmlA1 mutation was responsible for increased CmlA activity.
cmlA overexpression protects against the effects of a second IPTG-induced lethal gene.
Induction of the lactococcal phage bIL66 M operon in E. coli cells leads to cell death due to extensive degradation of chromosomal DNA (2, 3). Plasmid pIL1991 (3) carries the M operon cloned under the control of an early phage T7 promoter combined with two lac operators (PA1-O4/O3); this tightly repressed promoter is activated upon induction with IPTG (18). The isogenic cmlA+ (PhB1010) and cmlA1 (PhB1011) strains were transformed with pSC101lacIq and pIL1991; cells containing the cmlA1 mutation survived in the presence of 10−4 M IPTG, while growth of the isogenic cmlA+ strain was prevented by addition of only 5 × 10−5 M IPTG to the medium. Overproduction of CmlA results in nonspecific protection against λ cIII or against the lactococcal phage bIL66 M operon when their expression is induced by IPTG.
Overexpression of cmlA results in delayed IPTG-induced β-galactosidase synthesis.
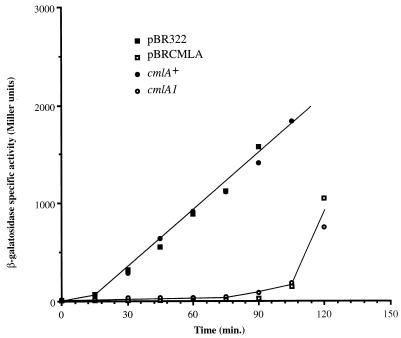
We hypothesized that the CmlA efflux pump could exclude IPTG, thus lowering its intracellular concentration and reducing expression of these toxic proteins. Endogenous β-galactosidase activity (which is under the control of the LacI repressor) was measured after IPTG induction in cmlA+ (PhB1010) and cmlA1 (PhB1011) isogenic strains and in a cmlA+ strain transformed with pBRCMLA (pBRCMLA results from the PCR product described above digested by AvaI and HindIII and ligated into AvaI- and HindIII-treated pBR322) or the control plasmid pBR322. The induction of β-galactosidase was immediate in the control strains but delayed for 90 min in the cmlA1 or cmlA+/pBRCMLA strain (Fig. 2). All strains showed an identical induced level of β-galactosidase activity when expression was induced by addition of 0.4% lactose to the medium (data not shown). We conclude that CmlA-dependent survival against IPTG-induced lethality is caused by reduced expression from IPTG promoters, probably due to IPTG exclusion.
FIG. 2.
Kinetics of endogenous β-galactosidase specific activities of strains PhB1010 (parental strain) (closed circle), PhB1011 (cmlA1) (open circle), PhB1010/pBR322 (closed square), and PhB1010/pBRCMLA (open square) after addition (at time zero) of 5 × 10−5 M IPTG to broth. β-Galactosidase was assayed according to Miller (20).
CmlA overexpression confers susceptibility to spectinomycin.
We encountered difficulties in selecting pGB2 derivatives of cmlA1 strains on plates containing spectinomycin (100 μg/ml). pGB2 (8) carries aadA (17) encoding aminoglycoside 3"-adenylyltransferase [AAD(3")(9)], a modifying enzyme which inactivates both streptomycin and spectinomycin (13). Transformants were obtained at low frequencies and grew very poorly, while they grew normally in the absence of spectinomycin. When streptomycin (50 μg/ml) was used for selection the plating efficiency was not affected by cmlA1. These observations suggested that CmlA overproduction was responsible for spectinomycin sensitivity despite the expression of aadA. The spectinomycin MIC (defined as producing <10−3 plating efficiency) in PhB1010 transformed with pGB2 and pKS (control plasmid) was >100 μg/ml. It was markedly lower, 10 μg/ml, in a strain transformed with pGB2 and pKSCMLA, which overproduces CmlA.
The spectinomycin MIC for PhB1010 transformed with pKS (control plasmid) was 25 μg/ml and decreased to 10 μg/ml for PhB1010 transformed with pKSCMLA. In addition, clones carrying pKSCMLA grew more slowly than they did with the control plasmid only in the presence of spectinomycin. These results indicate that expression of aadA is not directly involved in spectinomycin sensitivity observed in the presence of pKSCMLA. We conclude that increased CmlA activity results in spectinomycin sensitivity.
Conclusion.
The synthetic, nonmetabolizable β-galactoside IPTG is a classical gratuitous inducer of genes controlled by the Lac repressor. We show here that IPTG-induced lethality, which is obtained when expression of toxic proteins is under the control of lac operators, is markedly decreased when the wild-type cmlA (alias cmr or mdfA [14, 22]) gene is cloned on a multicopy plasmid. A similar but less marked effect was observed in the cmlA1 strain. Overproduction of CmlA by means of a multicopy plasmid (carrying cmlA) or by cmlA1 mutation also delays the time needed to induce the lac operon by IPTG. Our results suggest that an excess of CmlA lowers the IPTG concentration inside the cell. This could be due either to a membrane disturbance, which antagonizes the import of IPTG into the cell, or to direct export of IPTG by the CmlA efflux pump. Nilsen et al. pointed out the presence of an intracellular common motif for sugar transport (SDRIGRRPVMLAG) between transmembrane fragments 2 and 3 of CmlA (22). This motif is also found in YjiO (the closest E. coli CmlA homolog), in a few other MDR proteins (Bmr1 and Bmr2 in Bacillus subtilis and tetracycline resistance proteins), and in numerous sugar transporters. The presence of a sugar transport motif in CmlA leads us to ask whether it has β-galactoside export function. As the lactose operon has been extensively studied and is the paradigm for gene regulation, it is surprising that cmlA has not been previously described as a gene which could affect its expression.
It was previously observed that Pseudomonas aeruginosa nfxB and nfxC mutations, which cause overexpression of the efflux systems MexCD-OprJ and MexEF-OprN, respectively, induce β-lactam and aminoglycoside hypersensitivity (15, 16). However, these mutations could affect other targets. Here, we show a dramatic effect of direct overproduction of the efflux pump CmlA on pGB2-mediated spectinomycin resistance. Since the streptomycin resistance is not affected, AAD(3")(9) is probably fully functional. Furthermore, pKSCMLA induces spectinomycin hypersensitivity in the absence of AAD(3")(9). In contrast to that of common aminoglycosides (12), little is known concerning spectinomycin uptake; possibly, CmlA overproduction may contribute to increase the intracellular concentration of spectinomycin.
The emergence of MDR is a threat to antibacterial treatments. However, activation of MDR pumps could result in hypersensitivity to certain antibiotics. Appropriate antibiotics could be used to offset the prevalence of multidrug-resistant bacteria, as shown from our observation that spectinomycin is more efficient when CmlA is overexpressed.
Acknowledgments
We are indebted to Amos Oppenheim, Elena Bidnenko, Mary Berlin, and Hiroshi Matsuzawa for sending plasmids and strains. We thank Emmanuelle Binet, Christophe Goyon, Sandy Gruss, Sylvie Souès, and Bud Weiser for helpful discussions and warm support. We are grateful to Sandy Gruss for careful reading of the manuscript.
This work was supported in part by grant “ATIPE Microbiologie” from the Centre National pour la Recherche Scientifique and by grant “Aide à l’implantation de nouvelles équipes” from the Fondation pour la Recherche Médicale.
REFERENCES
- 1.Berlyn M K B, Low K B, Rudd K E. Linkage map of Escherichia coli K-12, edition 9. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C: American Society for Microbiology; 1996. pp. 1715–1902. [Google Scholar]
- 2.Bidnenko E, Ehrlich D, Chopin M-C. Phage operon involved in sensitivity to the Lactococcus lactis abortive infection mechanism AbiD1. J Bacteriol. 1995;177:3824–3829. doi: 10.1128/jb.177.13.3824-3829.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidnenko E, Ehrlich S D, Chopin M-C. Lactococcus lactis phage operon coding for an endonuclease homologous to RuvC. Mol Microbiol. 1998;28:823–834. doi: 10.1046/j.1365-2958.1998.00845.x. [DOI] [PubMed] [Google Scholar]
- 4.Bissonnette L, Champetier S, Buisson J P, Roy P H. Characterization of the nonenzymatic chloramphenicol resistance (cmlA) gene of the In4 integron of Tn1696: similarity of the product to transmembrane transport proteins. J Bacteriol. 1991;173:4493–4502. doi: 10.1128/jb.173.14.4493-4502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett G, 3rd, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Bolhuis H, van Veen H W, Poolman B, Driessen A J, Konings W N. Mechanisms of multidrug transporters. FEMS Microbiol Rev. 1997;21:55–84. doi: 10.1111/j.1574-6976.1997.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y Y, Cronan J E., Jr Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J Bacteriol. 1983;154:756–762. doi: 10.1128/jb.154.2.756-762.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 9.Dalrymple B. Novel rearrangements of IS30 carrying plasmids leading to the reactivation of gene expression. Mol Gen Genet. 1987;207:413–420. doi: 10.1007/BF00331609. [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple B, Arber W. Promotion of RNA transcription on the insertion element IS30 of E. coli K12. EMBO J. 1985;4:2687–2693. doi: 10.1002/j.1460-2075.1985.tb03988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalrymple B, Caspers P, Arber W. Nucleotide sequence of the prokaryotic mobile genetic element IS30. EMBO J. 1984;3:2145–2149. doi: 10.1002/j.1460-2075.1984.tb02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damper P D, Epstein W. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob Agents Chemother. 1981;20:803–808. doi: 10.1128/aac.20.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies J, Smith D I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- 14.Edgar R, Bibi E. MdfA, an Escherichia coli multidrug resistance protein with an extraordinarily broad spectrum of drug recognition. J Bacteriol. 1997;179:2274–2280. doi: 10.1128/jb.179.7.2274-2280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda H, Hosaka M, Hirai K, Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990;34:1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirai K, Suzue S, Irikura T, Iyobe S, Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987;31:582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollingshead S, Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid. 1985;13:17–30. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- 18.Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci USA. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 20.Miller J H. A short course in bacterial genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 21.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsen I W, Bakke I, Vader A, Olsvik O, El-Gewely M R. Isolation of cmr, a novel Escherichia coli chloramphenicol resistance gene encoding a putative efflux pump. J Bacteriol. 1996;178:3188–3193. doi: 10.1128/jb.178.11.3188-3193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obuchowski M, Giladi H, Koby S, Szalewska-Palasz A, Wegrzyn A, Oppenheim A B, Thomas M S, Wegrzyn G. Impaired lysogenisation of the Escherichia coli rpoA341 mutant by bacteriophage lambda is due to the inability of CII to act as a transcriptional activator. Mol Gen Genet. 1997;254:304–311. doi: 10.1007/s004380050420. [DOI] [PubMed] [Google Scholar]
- 24.Qu J N, Makino S I, Adachi H, Koyama Y, Akiyama Y, Ito K, Tomoyasu T, Ogura T, Matsuzawa H. The tolZ gene of Escherichia coli is identified as the ftsH gene. J Bacteriol. 1996;178:3457–3461. doi: 10.1128/jb.178.12.3457-3461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeve E C. Characteristics of some single-step mutants to chloramphenicol resistance in Escherichia coli K12 and their interactions with R-factor genes. Genet Res. 1966;7:281–286. doi: 10.1017/s0016672300009708. [DOI] [PubMed] [Google Scholar]
- 26.Reeve E C, Suttie D R. Chromosomal location of a mutation causing chloramphenicol resistance in Escherichia coli K 12. Genet Res. 1968;11:97–104. doi: 10.1017/s0016672300011228. [DOI] [PubMed] [Google Scholar]
- 27.Short S A, Singer J T. Studies on deo operon regulation in Escherichia coli: cloning and expression of the deoR structural gene. Gene. 1984;31:205–211. doi: 10.1016/0378-1119(84)90211-7. [DOI] [PubMed] [Google Scholar]
- 28.Van Melderen L, Bernard P, Couturier M. Lon-dependent proteolysis of CcdA is the key control for activation of CcdB in plasmid-free segregant bacteria. Mol Microbiol. 1994;11:1151–1157. doi: 10.1111/j.1365-2958.1994.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 29.van Veen H W, Callaghan R, Soceneantu L, Sardini A, Konings W N, Higgins C F. A bacterial antibiotic-resistance gene that complements the human multidrug-resistance P-glycoprotein gene. Nature. 1998;391:291–295. doi: 10.1038/34669. [DOI] [PubMed] [Google Scholar]
- 30.van Veen H W, Venema K, Bolhuis H, Oussenko I, Kok J, Poolman B, Driessen A J, Konings W N. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc Natl Acad Sci USA. 1996;93:10668–10672. doi: 10.1073/pnas.93.20.10668. [DOI] [PMC free article] [PubMed] [Google Scholar]