Abstract
Background
Patients with atrial fibrillation (AF) have a five-fold increase in stroke events, and ∼90% of the thrombi develop in the left atrial appendage (LAA). Left atrial appendage occlusion (LAAO) has emerged as a safe and feasible alternative to oral anticoagulation (OAC) for stroke prevention in selected patients with non-valvular AF and contraindications to OAC. Atrial fibrillation is closely associated with mitral disease, and there is a growing interest in combined procedures. More than half of patients undergoing a mitral transcatheter edge-to-edge repair (M-TEER) suffer of AF and many have high or unacceptable bleeding risk.
Case summary
We present a case of an 80-year-old woman suffering from paroxysmal AF, right carotid siphon aneurysm, and primary mitral regurgitation, with a high bleeding risk, who underwent a combined intervention of M-TEER and LAAO.
Discussion
The combination of these two procedures is a logical step once the access to the left atrium is obtained with a transseptal puncture (TSP) and a transesophageal echocardiography (TEE) is in place to guide both procedures. The turning point in LAAO procedure is a correct TSP allowing coaxial alignment of the sheath with the LAA neck. Steerable delivery sheaths are promising dedicated tools, particularly in challenging anatomy or during combined procedures requiring different TSP positions.
Keywords: Left atrial appendage occlusion, Mitral transcatheter edge-to-edge repair, Transseptal puncture, Deflectable sheath, Case report
Learning points.
The turning point in left atrial appendage occlusion is a correct transseptal puncture, in the majority of anatomies in inferoposterior position, allowing coaxial alignment of the sheath with the appendage neck.
Unfavourable puncture site or challenging appendage anatomy could be overcome with a steerable device.
Introduction
Patients with atrial fibrillation (AF) have a five-fold increase in stroke events with increased mortality, morbidity, and frequent recurrence.1 The introduction of non–vitamin K antagonist oral anticoagulants (NOAC) modestly improved adherence to oral anticoagulation (OAC) compared to warfarin (47.5% vs. 40.2%). Nevertheless, a large registry2 demonstrated that a significant percentage of patients does not adhere to OAC treatment, resulting in an increased risk of stroke among high-risk patients. The left atrial appendage (LAA) is a left atrium extension with a variable number of lobes and pectinate muscles. Approximately 90% of the thrombi that occur in patients with AF develop within this complex network of muscular ridges.3 Left atrial appendage occlusion (LAAO) has emerged as a safe and feasible alternative to OAC for stroke prevention in selected patients with non-valvular AF.4,5 We present a case of challenging LAAO performed in combination with a mitral transcatheter edge-to-edge repair (M-TEER).
Summary figure
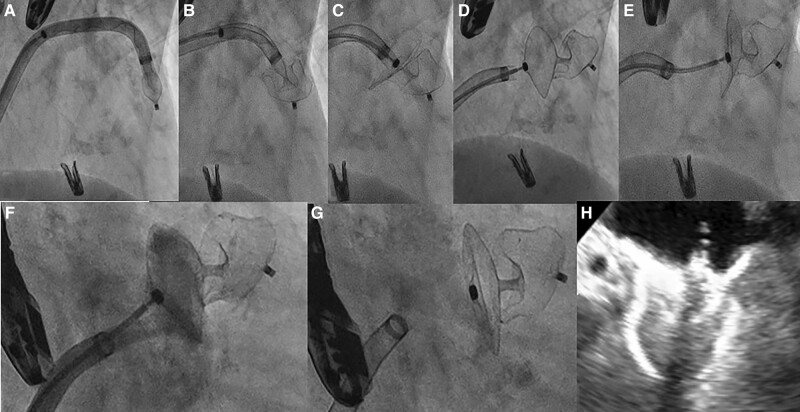
Step-by-step procedure of left atrial appendage occlusion with Amplatzer steerable delivery sheath. Amplatzer steerable delivery sheath was advanced up to LAA neck with the device in ball configuration (A). Then we deployed the device lobe (B) and disc (C). We assessed the device stability before release (D), with a tug test (E) and performed a contract injection to check the correct coverage of the appendage neck (F). Finally, we release the device (G). Last TEE evaluation confirmed the correct device position with concave shape of the disc, sealing the LAA (H).
Case presentation
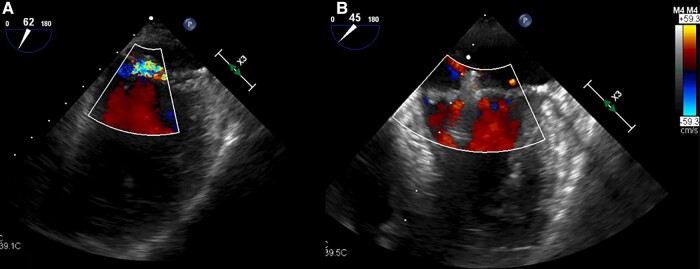
An 80-year-old women, Jehovah’s witness, affected by hepatitis C virus related liver cirrhosis, paroxysmal AF on NOAC, partially occluded right carotid siphon aneurysm, and primary mitral regurgitation (MR) was admitted to our department because of worsening dyspnoea. At presentation, physical examination revealed systemic congestion, with a respiratory rate of 23/min, normal blood pressure values and sinus tachycardia at electrocardiogram examination. The transthoracic and transoesophageal (TEE) echocardiographic evaluation showed a normal left ventricle with preserved ejection fraction (EF 58%), mild left atrium enlargement (left atrial volume index of 36 mL/m2), normal right ventricle, and mild tricuspid regurgitation with mild pulmonary hypertension. Mitral valve presented leaflets thickening, diffuse annular calcification, and posterior leaflet prolapse, with loss of coaptation due to chordal rupture, determining a severe eccentric jet (effective regurgitant orifice area of 50 mm2, vena contracta width of 0.7 cm, E-wave peak of 1.4 m/s). After multidisciplinary discussion and considering the patient’s high cardioembolic and bleeding risk (HAS-BLED score 3 and CHA2DS2VASc score 5), multiple comorbidity (Society of Thoracic Surgeon score 2,9%), and blood transfusion refusal, we decided to perform a combined percutaneous intervention of M-TEER and LAAO, after an Angio-CT scan to better study LAA morphology. The procedure was performed under general anaesthesia, under fluoroscopic and TEE guidance, via the right femoral vein (25 Fr introducer). Through a BRK™ transseptal needle (Abbott, IL, USA), the supero-posterior positioning in the fossa ovalis was evaluated in TEE short-axis, bicaval, and four-chamber view, and the site of crossing was marked in the angiogram using the EchoNavigator technology (EN, Philips Healthcare, Amsterdam, Netherlands). After transseptal puncture (TSP), we achieved a correct positioning of the MitraClip G4 XTW (Abbott, IL, USA), and we grasped the leaflets, assessed a major reduction of MR, and released the clip (Figure 2). Then we performed the LAAO procedure by advancing a pigtail marker catheter into the LAA for selective injection in two projections (Figure 1). We measured the LAA landing zone to confirm intraoperatively the device size. In order to overcome the suboptimal position of the puncture, selected to perform the MitraClip procedure, we planned to use the Amplatzer steerable delivery sheath (Abbott, IL, USA). Thanks to its steerability, we reached a distal deflection of ∼45° towards the LAA neck. Then we deployed the 22 Amplatzer Amulet (Abbott, IL, USA) (Figure 1) achieving an optimal positioning with a single attempt. The optimal device position was checked in multiple TEE views. Colour Doppler and 3D TEE confirmed the absence of peri-device leak and a good coverage of the LAA ostium. After the tug tests, the device was released and the last angiogram demonstrated successful LAAO. The patient was discharged home 3 days after, with recommendation to dual antiplatelet therapy (aspirin and a P2Y12 ADP receptor antagonist) for three months and then single antiplatelet therapy with aspirin sine die.
Figure 2.
Transoesophageal guided transcatheter mitral valve edge-to-edge repair. (A) TEE baseline assessment of mitral regurgitation. (B) Post-procedural TEE evaluation of TEER optimal result.
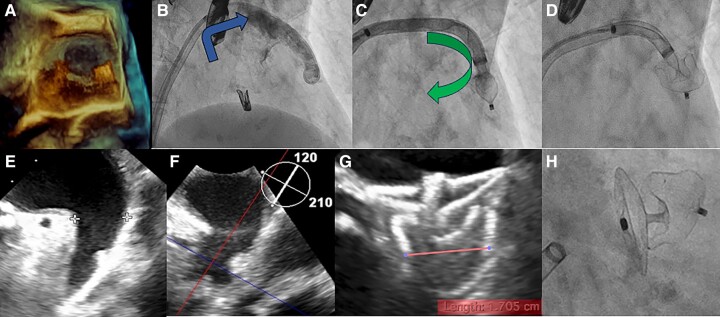
Figure 1.
Unfavourable position of the delivery sheath, due to high and posterior puncture, pointing towards the roof of the LAA overcomes with steerable delivery sheath. (A) 3D TEE evaluation of the direction of the delivery sheath that was not aligned with the main axis of the appendage. (B) Unfavourable direction of delivery sheath and anatomy of the LAA due to a sharp band directed downward. (C) LAA coaxial alignment achieved deflecting the distal curve of steerable delivery towards the main direction of the appendage. (D) Deployment of device lobe in the correct position. (E, F) TEE view of landing zone and LAA morphology. (G) After Amulet 22 release, we performed TEE with demonstration of optimal device compression and concave shape of the disc, sealing the LAA. (H) Fluoroscopic view confirmation of good procedural result.
Discussion
Left atrial appendage often presents challenging anatomies due to its different take-off and spatial location. A crucial procedural step is the TSP to achieve an optimal trajectory for coaxial alignment of delivery sheaths and devices into the LAA and a correct device deployment.5
Our patient underwent a combined procedure of M-TEER and LAAO. In the majority of anatomies, LAAO is performed with an inferoposterior puncture, while for MitraClip procedure is usually preferred a posterior puncture.6 In our case, the M-TEER requires a supero-posterior TSP to allow optimal positioning across the mitral valve. The puncture site was ∼4 cm above the mitral annulus, as recommended in degenerative MR, to guarantee enough space for optimal MitraClip manoeuvering. After M-TEER procedure, we decided to perform LAAO using a steerable sheath to overcome the unfavourable puncture site, achieving a good coaxiality between the sheath and the LAA. The Amplatzer steerable delivery sheath provides distal bidirectional steerability, due to a fixed 45° proximal curve and a distal steerable curve. The distal curve offers the possibility of controlled bidirectional deflection from 0° to 120°. In our procedure, the direction of the delivery sheath was not aligned with the main axis of the appendage, as it commonly happens with a high TSP (Figure 1). The LAA was classified as a ‘windsock’ morphology, because of the presence of a dominant lobe and a sharp band directed downward. In this challenging scenario, the availability of a deflectable sheath was instrumental in achieving success. The main drawback of the deflectable sheath, its larger diameter, was obviously irrelevant since a 25 Fr sheath was already inserted into the femoral vein and through the interatrial septum. Transseptal puncture defect did not require closure with another device. In our limited experience with steerable devices, we never found an iatrogenic atrial septal defect requiring percutaneous correction.
Atrial fibrillation is closely associated with mitral disease, and there is a growing interest in combined procedures. Important advantages are the single venous access, which reduces access site related complications, and overall patient satisfaction. There are limited data about the use of steerable sheaths during LAAO.7,8 Gonzales et al.9 described a case report supporting the utility of FEops HEARTguide combined with Amplatz steerable delivery sheath. Kleinecke et al.10 reported a 20-patients experience with LAmbre LAA occluder and the FuStar sheath that confirmed the safety and the feasibility of the procedure. Chang et al.11 also described optimal results in 20 consecutive LAAO cases and difficult LAA anatomy. To the best of our knowledge, this is the first case report about LAAO performed by Amplatzer steerable sheath during M-TEER.
More than half of patients undergoing M-TEER suffer from AF, and high or unacceptable bleeding risk is common in this population. The combination of these two procedures, as customary in cardiac surgery (ligation), is a logical step once the access to the left atrium is obtained with TSP and a TEE is in place to guide both procedures.
Conclusion
The turning point in LAAO procedure is a correct TSP allowing coaxial alignment of the sheath with the LAA neck. Steerable delivery sheaths are promising dedicated tools, particularly in challenging anatomy or during combined procedures requiring different TSP positions. Future studies are needed to establish the role of this approach in reducing procedural times and complications in complex scenarios.
Lead author biography
 Dr Silvia Maiani received her MD from the University of Florence in 2018. She is a Cardiology resident at the University of Cagliari, with a special interest in interventional cardiology and structural heart disease. She is doing a traineeship in the Catheterization Laboratory directed by Professor Carlo Di Mario, at Careggi University Hospital of Florence.
Dr Silvia Maiani received her MD from the University of Florence in 2018. She is a Cardiology resident at the University of Cagliari, with a special interest in interventional cardiology and structural heart disease. She is doing a traineeship in the Catheterization Laboratory directed by Professor Carlo Di Mario, at Careggi University Hospital of Florence.
Consent: The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patients, according to COPE guidelines.
Funding: None declared.
Contributor Information
Silvia Maiani, Clinical Cardiology, Department of Medical Science and Public Health, University of Cagliari, SS554, 09042 Monserrato, Cagliari, Italy; Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Largo Brambilla 3, 50134, Florence.
Giulia Nardi, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Largo Brambilla 3, 50134, Florence.
Francesca Ristalli, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Largo Brambilla 3, 50134, Florence.
Carlo Di Mario, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Largo Brambilla 3, 50134, Florence.
Francesco Meucci, Structural Interventional Cardiology, Department of Clinical & Experimental Medicine, University Hospital Careggi, Largo Brambilla 3, 50134, Florence.
Data availability
The data underlying this article are available in the article and in its online supplementary material .
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2. Yao X, Abraham NS, Alexander GC, Crown W, Montori VM, Sangaralingham LR, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc 2016;5:e003074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Delgado V, Di Biase L, Leung M, Romero J, Tops LF, Casadei B, et al. Structure and function of the left atrium and left atrial appendage: AF and stroke implications. J Am Coll Cardiol 2017;70:3157–3172. [DOI] [PubMed] [Google Scholar]
- 4. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—an update. Europace 2020;22:184. [DOI] [PubMed] [Google Scholar]
- 5. Holmes DR Jr, Korsholm K, Rodés-Cabau J, Saw J, Berti S, Alkhouli MA. Left atrial appendage occlusion. EuroIntervention 2023;18:e1038–e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russo G, Taramasso M, Maisano F. Transseptal puncture: procedural guidance, challenging situations and management of complications. EuroIntervention 2021;17:720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selvais N, Leduc N, Ben Yedder M, Silberberg D, Aminian A. Use of the novel Amplatzer steerable sheath for percutaneous closure of a complex chicken-wing left atrial appendage. Acta Cardiol 2023;78:367–368. [DOI] [PubMed] [Google Scholar]
- 8. Muiños PJA, Tejero SL, Collado JR, Garibi JH, González IC. Complex left atrial appendage occlusion using the novel Amplatzer steerable delivery sheath. J Invasive Cardiol 2023;35:E158–E159. [DOI] [PubMed] [Google Scholar]
- 9. Cruz González I, Antúnez Muiños PJ, López Tejero S, Núñez García JC, Rodríguez Collado J, Martín Moreiras J, et al. Left atrial appendage occlusion using the novel Amplatzer steerable delivery sheath combine with FEops HEARTguide. JACC Cardiovasc Interv 2021;14:e301–e304. [DOI] [PubMed] [Google Scholar]
- 10. Kleinecke C, Gomez Monterrosas O, Scalone G, Lam YY, Shin ES, Bellmann B, et al. First-in-human experience of left atrial appendage occlusion with the steerable FuStar sheath. J Interv Cardiol 2018;31:532–537. [DOI] [PubMed] [Google Scholar]
- 11. Chang SN, Chiu FC, Chen JJ, Huang PS, Lin TT, Cheng HL, et al. First-in-human experience of using a universal steerable sheath in implanting left atrial appendage closure devices. JACC Asia 2022;2:780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material .