The SPECTRO GLIO study is the first randomized trial exploring use of a quantitative imaging modality to guide high-dose (HD) radiation therapy (RT) (72 Gy in 30 fractions) for newly diagnosed glioblastoma (GBM).1 Dr. Laprie et al. deserve congratulations on successfully completing this trial. Their ability to harmonize magnetic resonance spectroscopic imaging (MRSI) among 8 institutions for this trial should be applauded and can serve as a future blueprint for how to perform trials that utilize advanced imaging as an integral component.
While RT dose escalation for GBMs has been extensively evaluated including most recently with the NRG-BN001 trial,2 it has been largely ineffective to date. Because previous dose escalation studies only used standard MRIs to define the HD target, all regions at high risk for tumor recurrence that could potentially benefit from dose intensification may not have been identified. The premise for the SPECTRO GLIO trial is that sites with elevated Cho/NAA values (specifically, Cho/NAA > 2) on MRSI may identify these additional at-risk regions resulting in better targeting of HD RT. However, despite “improved” targeting, we are disappointed that this current dose escalation approach again did not show significant clinical benefit with a median overall survival (OS) of 22.2 months in the HD RT cohort versus 22.6 months in the control cohort.
Additional details regarding the SPECTRO GLIO trial may have affected the results and are worth further discussion. First, MRSI scans in this trial were obtained on 1.5T MR scanners and assessed only limited regions near the cavity/residual tumor seen on standard MRIs. In fact, more than a third of patients (36%) did not have Cho/NAA elevations beyond the tumor cavity using a threshold of Cho/NAA>2. In contrast, a 3D spectroscopic MRI (sMRI) technique performed on 3T MR scanners that have significantly better signal-to-noise ratio and the ability to assess the whole brain was used to guide HD RT for GBM on a 3 institution trial.3 Using sMRI, regions with significant infiltrative tumor that was at high risk for recurrence were defined by Cho/NAA elevations that were at least 2× greater than Cho/NAA values obtained from the contralateral normal-appearing white matter (“normal” Cho/NAA ratio is approximately 0.45).4,5 This criteria for determining high-risk disease identified a significantly larger volume compared with use of an absolute Cho/NAA value >2 on the SPECTRO GLIO trial (corresponding to a threshold Cho/NAA value that was approximately 4.4× that of “normal” white matter). Consistent with use of the more stringent criteria for determining the HD RT boost target volume on the SPECTRO GLIO trial, only about 1/3 of patients had high-risk regions found beyond the tumor cavity, whereas Ramesh et al. reported on the sMRI-guided dose escalation trial that Cho/NAA>2× normal white matter identified high-risk regions beyond the residual contrast-enhancing tumor in every case.1,3 This difference is illustrated in Figure 1 where the two approaches (Cho/NAA > 2× normal white matter versus Cho/NAA > 2) yielded significantly different high-risk target volumes. Careful mapping of recurrences in the brain relative to the boost target would have likely given us more information as to whether the MRSI technique used by the SPECTRO GLIO investigators identified all significant regions of high-risk disease as well as aid in our understanding of the overall effectiveness of escalated RT doses. However, the SPECTRO GLIO trial only reported crude patterns of recurrence which did not show a significant difference between the 2 arms. Finally, the control arm of the SPECTRO GLIO trial did much better than expected with median OS of 22.6 months versus approximately 16 months seen in most modern trials.6–8 This results in an extremely high bar for the HD RT arm to beat. While it is not exactly clear why the control arm did so well, the selection process whereby nearly 32% (84 of the 264) were not included for randomization due to a variety of reasons likely played a role.
Figure 1.
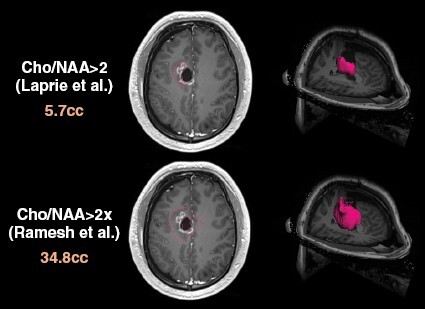
An example subject demonstrates a greater than 6-fold difference in the high-risk target volumes (outlined in red, volumes in cc labeled on left) based on absolute Cho/NAA>2 per the SPECTRO GLIO trial (Laprie et al.) versus Cho/NAA>2× normal brain per the sMRI-guided dose escalation trial (Ramesh et al.). Left side images show the respective high-risk contours overlaid on an axial contrast enhanced T1w slice while right side images show 3D volume renderings of these high-risk targets. Of note, in this example, the contour defined by Cho/NAA>2 criteria is essentially the residual enhancing tumor.
Given these factors discussed above, we do not believe that MRSI should be outright ruled out as a means of improving RT targeting for GBM dose escalation especially given the promising results reported by Ramesh et al. using the more advanced whole-brain 3D sMRI sequence to guide RT dose escalation (75 Gy in 30 fractions) which yielded a median OS of 23 months (N = 30, enrolled at 3 institutions).3 Furthermore, additional advanced imaging approaches including diffusion/perfusion MRI by Kim et al. and 18F-DOPA PET by Laack et al. to guide RT dose escalation (75 Gy and 76 Gy in 30 fractions, respectively) for GBMs have been evaluated clinically at the single institutional levels with similarly promising results.9,10 Interestingly, MGMT methylated patients on the SPECTRO GLIO trial had OS curves that appeared to separate after 24 months favoring HD RT.1 This is reminiscent of the report by Laack et al. where MGMT methylated patients appear to derive greater survival benefit when treated with 18F-DOPA-guided HD RT.10 These intriguing results suggest that the presence of MGMT methylation may define a GBM subgroup that gains more benefit from advanced imaging-guided RT dose escalation. However, these results are not definitive and can only be considered hypothesis-generating at this juncture with need for more rigorous assessment in future trials.
In closing, we need to be cautious not to discount all advanced quantitative imaging techniques as a means of guiding HD RT for GBM just because the initial randomized trial assessing one such approach is negative. However, many lessons can be learned from this effort including the successes with harmonizing acquisition and use of a quantitative imaging modality across institutions as well as potential issues with determining the best threshold for identifying high-risk diseases. We hope that further efforts to utilize other advanced imaging modalities, or even more sophisticated versions of MRSI such as whole brain 3D sMRI, will still be pursued so that more definitive answers regarding their utility for guiding RT targeting can be determined.
Contributor Information
Hui-Kuo G Shu, Department of Radiation Oncology, Emory University School of Medicine, Atlanta, Georgia, USA; Winship Cancer Institute, Emory University School of Medicine, Atlanta, Georgia, USA.
Hyunsuk Shim, Department of Radiation Oncology, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Radiology and Imaging Sciences, Emory University School of Medicine, Atlanta, Georgia, USA; Department of Biomedical Engineering, Emory University School of Medicine, Atlanta, Georgia, USA; Winship Cancer Institute, Emory University School of Medicine, Atlanta, Georgia, USA.
Funding
This work was supported by R01 CA214557 (HGS, HS) and U01 CA264039 (HS) from the National Cancer Institute.
Conflicts of interest statement
None.
References
- 1. Laprie A, Noel G, Chaltiel L, et al. Randomized phase III trial of metabolic imaging-guided dose escalation of radio-chemotherapy in patients with newly diagnosed glioblastoma (SPECTRO GLIO trial). Neuro Oncol. 2024;26(2):153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gondi V, Pugh S, Tsien C, et al. Radiotherapy (RT) Dose-intensification (DI) Using Intensity-modulated RT (IMRT) versus Standard-dose (SD) RT with Temozolomide (TMZ) in Newly Diagnosed Glioblastoma (GBM): Preliminary Results of NRG Oncology BN001. Int J Radiat Oncol. 2020;108(3):S22–S23. [Google Scholar]
- 3. Ramesh K, Mellon EA, Gurbani SS, et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neurooncol Adv. 2022;4(1):vdac006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cordova JS, Kandula S, Gurbani S, et al. Simulating the effect of spectroscopic MRI as a metric for radiation therapy planning in patients with glioblastoma. Tomography. 2016;2(4):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cordova JS, Shu HK, Liang Z, et al. Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro Oncol. 2016;18(8):1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 7. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim MM, Sun Y, Aryal MP, et al. A Phase 2 study of dose-intensified chemoradiation using biologically based target volume definition in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2021;110(3):792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laack NN, Pafundi D, Anderson SK, et al. Initial results of a phase 2 trial of (18)F-DOPA PET-guided dose-escalated radiation therapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2021;110(5):1383–1395. [DOI] [PubMed] [Google Scholar]


