Abstract
Background
Glioblastoma (GBM) systematically recurs after a standard 60 Gy radio-chemotherapy regimen. Since magnetic resonance spectroscopic imaging (MRSI) has been shown to predict the site of relapse, we analyzed the effect of MRSI-guided dose escalation on overall survival (OS) of patients with newly diagnosed GBM.
Methods
In this multicentric prospective phase III trial, patients who had undergone biopsy or surgery for a GBM were randomly assigned to a standard dose (SD) of 60 Gy or a high dose (HD) of 60 Gy with an additional simultaneous integrated boost totaling 72 Gy to MRSI metabolic abnormalities, the tumor bed and residual contrast enhancements. Temozolomide was administered concomitantly and maintained for 6 months thereafter.
Results
One hundred and eighty patients were included in the study between March 2011 and March 2018. After a median follow-up of 43.9 months (95% CI [42.5; 45.5]), median OS was 22.6 months (95% CI [18.9; 25.4]) versus 22.2 months (95% CI [18.3; 27.8]) for HD, and median progression-free survival was 8.6 (95% CI [6.8; 10.8]) versus 7.8 months (95% CI [6.3; 8.6]), in SD versus HD, respectively. No increase in toxicity rate was observed in the study arm. The pseudoprogression rate was similar across the SD (14.4%) and HD (16.7%) groups. For O(6)-methylguanine-DNA methyltransferase (MGMT) methylated patients, the median OS was 38 months (95% CI [23.2; NR]) for HD patients versus 28.5 months (95% CI [21.1; 35.7]) for SD patients.
Conclusion
The additional MRSI-guided irradiation dose totaling 72 Gy was well tolerated but did not improve OS in newly diagnosed GBM.
Trial registration
NCT01507506; registration date: December 20, 2011. https://clinicaltrials.gov/ct2/show/NCT01507506?cond=NCT01507506&rank=1
Keywords: clinical trial, glioblastoma, 3D magnetic resonance spectroscopic imaging, phase III, radiotherapy
Key Points.
Magnetic resonance spectroscopic imaging (MRSI) has predictive value for the site of relapse in GBM.
This is the first multicentric randomized phase III trial of an RT boost to abnormal MRSI regions.
High-dose RT was well tolerated but did not improve OS or PFS.
Importance of the Study.
Glioblastoma is the most frequently diagnosed primitive brain tumor in adults and has a dismal prognosis, with a limited response to radiochemotherapy and early, mainly local relapses. Magnetic resonance spectroscopic imaging (MRSI) has been shown to be of predictive value for the site of relapse after radiotherapy. We thus asked whether increasing the irradiation dose to target MRSI abnormalities could increase OS in newly diagnosed GBM. This is the first prospective multicentric randomized phase III trial evaluating efficacy and safety of an irradiation boost to abnormal MRSI regions in newly diagnosed GBM. A prospective quality control was performed. In 66% of patients included in the high-dose arm, volumes were modified by MR spectroscopy. We showed that increasing the dose of radiotherapy guided by metabolic imaging with concomitant temozolomide treatment was well tolerated but failed to improve overall survival or progression-free survival in patients with glioblastoma.
Glioblastoma (GBM) is the most aggressive and common malignant primary brain tumor in adults and systematically relapses despite a standard radio-chemotherapy protocol (Stupp) combining 60 Gy radiotherapy (RT) with temozolomide (TMZ) after surgical resection or biopsy.1 Most patients exhibit local relapses within irradiation fields. Prognosis is poor, with a median overall survival (OS) of 14 months.1 Even though OS has been increased by adding tumor-treating fields after radio-chemotherapy,2 the prognosis still remains poor. There is a clear need to develop additional innovative treatment strategies to tackle radioresistance and improve local control by irradiating the target volume heterogeneously, with focal increases in dose directed at radioresistant clusters defined by different types of metabolic imaging.3,4 This dose-painting approach targets metabolic abnormalities that are not only prognostic indicators of aggressive disease but also predictors of local post-treatment relapse.
In this context, in vivo 1H magnetic resonance spectroscopic imaging (MRSI) has shown considerable promise.5–7 MRSI measures the concentration and spatial distribution of tissue metabolites such as the membrane marker choline (Cho) and the neuronal marker N-acetyl-aspartate (NAA). An elevated Cho/NAA ratio (CNR) indicates increased cellular proliferation and reduced neuron density and is assumed to highlight metabolically active parts of the tumor in high-grade gliomas.7,8 This metabolic marker is a useful predictor of survival9 and relapse location in patients with GBM.6,7
Using data from a prospective trial, our team confirmed10 the predictive value of the site of relapse after RT for the presence of metabolic abnormalities in regions with a CNR >2 (CNR2) on MRSI. Increasing the radiotherapy dose to this area may thus increase local control and survival of patients with newly diagnosed GBM.
Several dose-escalation studies using sequential stereotactic boosts have reported good tolerance and survival benefits in selected populations.11–13 These findings provided the rationale for the choice of dose in the current trial. The studies did not include concomitant TMZ, metabolic imaging to guide boost treatment, nor a concomitant daily boost during the initial RT. We therefore assessed the impact of concomitant radiochemotherapy with a simultaneous integrated boost (SIB) targeting metabolic abnormalities predictive of relapse on increasing the OS of GBM patients. Before starting the clinical trial, we assessed whether escalating the dose would increase the dose to organs at risk.14 We administered an integrated boost treatment totaling 72 Gy (2.4 Gy/day) which was equivalent to the dose delivered as sequential boosts in studies that reported improved survival. We then designed a randomized phase III trial comparing standard TMZ + 60 Gy (2 Gy/day) RT to the tumor site with the same treatment plus an additional boost of 12 Gy (0.4 Gy/day), to achieve a total SIB dose of 72 Gy (2.4 Gy/day) aimed at increasing OS in patients with newly diagnosed GBM.
Materials and Methods
Study Design
The trial was a multicenter, randomized, 2-arm phase III study (Figure 1). Patients who met the inclusion criteria were randomly assigned to either the standard dose (SD) or the high-dose (HD) arm, at a 1:1 ratio, based on computer-generated random numbers. Diagnoses were based on the fourth WHO 2016 brain tumor classifications.15,16 Although the current WHO classifications were updated in 2021, prior to 2021, and therefore during the inclusion period of the current trial, the WHO classification included isocitrate dehydrogenase (IDH)-mutated grade IV glioblastoma. Stratification was performed based on surgical resection versus biopsy and on DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) methylation status, age at inclusion (≤50 vs > 50 years) and center.
Figure 1.
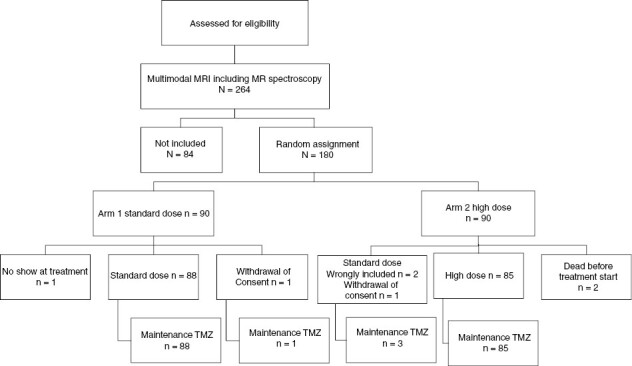
CONSORT trial flow diagram. * Two patients, that were included and randomized before checking the quality of the MRI/MRS, had inadequate acquisitions. The MRI/MRS was not repeated in these 2 cases. The 2 patients, included in the HD arm, were treated in the SD arm but analyzed as part of the intention-to-treat in the HD arm.
IDH mutation status for all patients was determined by immunohistochemistry with the anti-R132H antibody or sequencing in case of immune-negative tumors from patients younger than 55 years of age.
Both Arms Included Concomitant TMZ and RT, with TMZ Maintained for 6 months Thereafter
Standard arm: standard dose (SD).
3D conformal radiotherapy RT or intensity-modulated IMRT (IMRT) delivering 60 Gy in 30 fractions of 2 Gy to planning target volume 1 (PTV1): contrast-enhancing lesions or tumor bed+ 2 cm extended to fluid attenuated inversion recovery (FLAIR) abnormalities, with a 3 mm margin.
Experimental arm: high dose (HD).
IMRT delivered as 60 Gy in 30 fractions of 2 Gy to PTV1 and as 72 Gy in 30 fractions of 2.4 Gy to PTV2 as a simultaneous integrated boost SIB guided by MRSI to the CNR2 regions (ie, gross tumor volume 2, GTV2) + 10 mm and tumor bed or residual contrast-enhancement, with an added 3 mm for PTV. The voxel size for MRSI was 6.25 × 6.25 × 10 mm and all other imaging details are given in the supplemental text.
Study Objectives
The primary objective was to evaluate the effect of radiation dose escalation guided by MRSI on the OS of patients with newly diagnosed GBM. The secondary objectives assessed: (i) the effect of radiation dose escalation on progression-free survival (PFS); (ii) the safety of radiation dose intensification; (iii) the rate of pseudoprogression (PsP); (iv) late effects such as radiation necrosis and increases in epileptic seizures; (v) individual, imaging, and biological markers associated with OS or PFS; and (vi) the sites of relapse.
Patient Selection
Inclusion criteria were as follows: >18 years of age, performance status ≤2, histologically proven GBM (according to the WHO classification, 4th edition), available methylation status of the MGMT gene, patients’ written informed consent, surgery, or biopsy performed no more than 42 days before administration of the first RT fraction.
The exclusion criteria were poor quality MRSI data, multifocal GBM, leptomeningeal metastasis, epileptic seizure despite anticonvulsant treatment, tumor diameter >5 cm, distance from the chiasma to the tumor bed, and MRSI abnormalities <2 cm. Other standard exclusion criteria are described in our trial design paper.17
Treatment
Imaging and radiotherapy requirements, MRSI integration, radiotherapy planning, including dose constraints, chemotherapy, and quality control, have been described previously14,17 and are given as Supplementary Materials.
Follow-up
Clinical follow-up.
Patients underwent a medical examination every week during RT, every 21 days during TMZ maintenance, and every 2 months thereafter until progression.
Imaging follow-up.
The first examination was performed 3 months after the end of RT to assess the maximal effect of RT and avoid the commonly detected early appearance of pseudoprogression,18 and every 2 months thereafter. If a patient showed suspicious clinical signs within 3 months of the end of RT, MRI, and MRSI were performed earlier than 3 months. If imaging findings were suggestive of PsP, a new complete MRI scan was performed 1 month later to confirm or refute progression. Patients with stable disease or regression, were considered to be non-relapsing.
Outcome measure definition.
The primary endpoint was OS and was defined as the time interval from randomization to death from any cause or to the last known follow-up (censored). Secondary endpoints were progression free survival (time interval from randomization to progression determined according to the RANO criteria19 or death) as well as the number and type of adverse effects based on NCI-CTCAE V3.0. Treatments for relapse or progression were defined by the multidisciplinary tumor board of the corresponding treating center. None of the patients received tumor-treating fields.
Statistical Analysis
We estimated that 186 deaths would detect a hazard ration (HR) for OS of 0.66 (increase in the 2-year OS rate from 25% to 40%) with 80% power, a 2-sided significance level of 0.05, and 1:1 randomization ratio and that 220 patients would need to be enrolled to observe the required number of events. An interim analysis of efficacy was performed after 93 events using the Lan-DeMets O’Brien-Fleming boundaries. Primary and secondary endpoints (OS and PFS) were analyzed in the intention-to-treat population. Median follow-up was calculated using the reverse Kaplan–Meier method. Survival rates were estimated using the Kaplan-Meier method, and comparisons between arms were performed with the log-rank test. The supportive multivariate analysis was performed with the Cox proportional hazards model and adjusted for randomization stratification factors. Exploratory subgroup analyses allowed to evaluate treatment effects in different subpopulations. Given the exploratory nature of the subgroup analysis, no adjustments were made for multiple comparisons.
Qualitative variables are summarized as category frequencies and percentages, and continuous variables as medians with ranges (minimum–maximum). The number of missing data points is given for each variable, but not accounted for in percentages. Differences between randomization arms were assessed using the Chi-square or Fisher’s exact test for qualitative variables and the Kruskal–Wallis test for continuous variables.
Exploratory analyses were performed to investigate the association between PsP and OS. To minimize survivor selection bias, a landmark analysis at 6 months evaluated the association between OS and PsP, and a comparison between groups (PsP vs early true progression [ETP]) was performed using the log-rank test.
Statistical significance was set at P < .05, and all tests were 2-sided. All statistical analyses were performed using the Stata version 13 software (StataCorp LLC).
Results
Patient Characteristics
Eight centers enrolled 180 patients between March 2011 and March 2018.
A total of 264 patients were screened, 84 of these patients could not be included in the trial after having undergone MRI-MRSI. The reasons for non-inclusion were that 32 patients presented with a tumor bed diameter superior to 5 cm, 16 patients presented a distance from tumor bed to chiasm of <2 cm, in 10 patients the MGMT could not be analyzed, 17 patients presented with a second lesion at MRI and 9 patients had uninterpretable MR spectroscopic imaging results. MRSI results from these nine patients could not be interpreted due to the presence of hemorrhagic areas in one patient, a metallic artifact in two patients and inadequate acquisition in six patients. In all of these cases, we did not rescan the patients and they were not included in the trial.
The trial was prematurely closed because of slow accrual.
Patients were randomly assigned to the standard-dose (n = 90) or high-dose study (n = 90) group (Figure 1). Baseline characteristics were well balanced between treatment arms (Table 1).
Table 1.
Patient Characteristics
| Characteristics | Total | Standard Dose | High Dose | |
|---|---|---|---|---|
| Sex | P = .5461 | |||
| Female | 76 (42.2%) | 36 (40.0%) | 40 (44.4%) | |
| Male | 104 (57.8%) | 54 (60.0%) | 50 (55.6%) | |
| Age at inclusion (n = 180) | ||||
| ≤50 years | 33 (18.3%) | 17 (18.9%) | 16 (17.8%) | |
| >50 years | 147 (81.7%) | 73 (81.1%) | 74 (82.2%) | |
| Median (range) | 61.0 (26.0–84.0) | 61.5 (33.0–83.0) | 60.5 (26.0–84.0) | P = .8472 |
| Performance status (n = 165) | P = .1015 | |||
| 0 | 92 (55.8%) | 48 (59.3%) | 44 (52.4%) | |
| 1 | 62 (37.6%) | 31 (38.3%) | 31 (36.9%) | |
| 2 | 11 (6.7%) | 2 (2.5%) | 9 (10.7%) | |
| Missing data | 15 | 9 | 6 | |
| Tumor size at diagnosis (mm) (n = 115) | P = .0703 | |||
| Median | 33.0 | 35.0 | 30.0 | |
| (Range) | (3.0–95.0) | (3.0–95.0) | (6.0–62.0) | |
| Missing data | 65 (36.11%) | 31 (34.4%) | 34 (37.7%) | |
| Type of surgery (n = 180) | P = .8347 | |||
| Biopsy | 27 (15.0%) | 14 (15.6%) | 13 (14.4%) | |
| Surgical resection | 153 (85.0%) | 76 (84.4%) | 77 (85.6%) | |
| MGMT promoter methylation (N = 180) | P = .8815 | |||
| Unmethylated | 91 (50.6%) | 45 (50.0%) | 46 (51.1%) | |
| Methylated | 89 (49.4%) | 45 (50.0%) | 44 (48.9%) | |
| IDH status (n = 113) | P = .7060 | |||
| Wild type | 108 (95.5%) | 56 (94.9%) | 52 (96%) | |
| Mutated | 5 (4.4%) | 3 (5%) | 2 (3.7%) | |
| Not performed | 67 (37.2%) | 31 (34.4%) | 36 (40%) |
Since IDH-mutation status was not routinely assessed at the start of the trial, we analyzed IDH in 113 patients (62.8% of included patients) and found that 95% of patients encoded wild type IDH. IDH was not analyzed in 67 patients (37.2% of included patients), whose median age at inclusion was 63 years (range: 40–84). The GBMs were classified according to the fourth edition of the WHO classification, effective at the time of patient inclusion, application of the 5th 2021 edition WHO classification would have modified one of our subgroup classifications, and would have affected 5% of included patients.
Overall Survival
With a median follow-up of 43.9 months (95% CI [42.5, 45.5]) and 128 deaths in the intention-to-treat population (SD: n = 68, HD: n = 60), median OS was 22.6 months [18.9, 25.4] in the SD arm and 22.2 months [18.3, 27.8] in the HD arm (Figure 2). No statistical difference was found between randomization arms (unadjusted HR = 0.90 [0.64, 1.27], (logrank) = 0.5526). The multivariate analysis adjusted for stratification factors confirmed the lack of difference between treatment arms (adjusted HR = 0.91 95% CI [0.64, 1.29], P = .594). Median OS for methylated patients in the HD arm was 38 months (23.2, not reached) versus 28.5 months (21.1, 35.7) for methylated patients in the SD arm (Figure 3).
Figure 2.
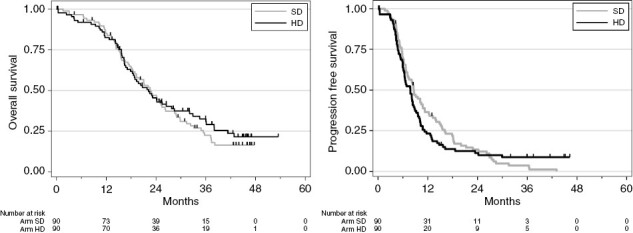
Overall survival and progression-free survival by arm. HD, high dose; OS, overall survival; SD, standard dose.
Figure 3.
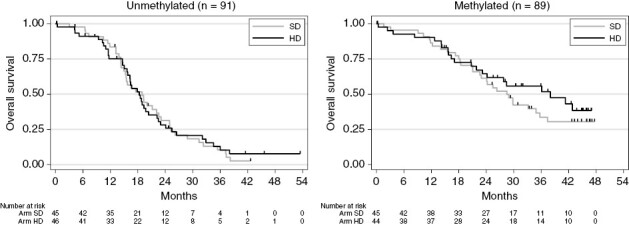
Overall survival by arm and methylation status: (A): methylated; (B): unmethylated; HD, high dose; OS, overall survival; SD, standard dose.
Progression-free Survival
With 163 relapses or deaths (SD: n = 85, HD: n = 78), median PFS was 8.6 months (95% CI [6.8; 10.8]) in the SD and 7.8 months (95% CI [6.3; 8.6]) in the HD arm (HR = 1.0 [0.80, 1.48], p-logrank = 0.6027). The adjusted HR on the stratification factor was 1.10 (95% CI [0.80, 1.50], P = .567).
Patterns of Relapse
We examined available relapse MRIs from all participating centers. There were 27 missing relapse exams.
All analyzed relapses were local, except for 3 mixed relapses (local and distant) and 2 distant relapses in the SD arm, and 2 mixed relapses and 3 distant relapses in the HD arm.
Safety Analysis
All trial patients experienced at least one adverse event (Table 2). General, neurological, and hematological adverse events were comparable across the two arms, with the exception of more frequent headaches in the SD arm (59.1% vs 44%, P = .0484) and more frequent neurological deficits in the HD arm (67.5% vs 51.1%, P = .0299). No radiation necrosis was described.
Table 2.
Maximum Grade of Adverse Events According to CTCAE by Treatment Arm
| Toxicity (n = 172) | Standard Dose | High Dose | Total | P = .9401 |
|---|---|---|---|---|
| Grade 1 | 9 (10.2%) | 8 (9.6%) | 17 (9.9%) | |
| Grade 2 | 49 (55.7%) | 45 (54.2%) | 94 (55%) | |
| Grade 3 | 22 (25%) | 20(24.1%) | 42 (24.6%) | |
| Grade 4 | 8 (9.1%) | 10 (12%) | 18 (10.5%) | |
| Missing data | 0 | 1 | 1 |
Cognitive Assessment
The Mini-Mental State Examination (MMSE) was administered to 147 patients in week 1, and the median score obtained was equivalent across the two arms (28 for SD vs 29 for HD). There was an equivalent difference between this initial score and the score 1 month after RT for each arm. MMSE scores decreased in 34.2% of patients that completed the questionnaire, were stable in 26.5%, and increased in 39.3% of patients.
Spectroscopy Data
All HD patients received a boost volume that encompassed the tumor bed. It was restricted to the tumor bed in 36% of patients. In 64% of patients, the volume was modified by the integration of MRSI abnormalities. Details are provided in the Supplementary Materials.
In HD patients, OS and PFS did not differ according to the presence or absence of MRSI abnormalities.
Pseudoprogression Versus Early True Progression
Forty-six patients were suspected to have relapsed within 6 months of chemoradiotherapy, with 28 of these patients classified as pseudoprogression (PsP) and 18 as early true progression (ETP). PsP and ETP rates were comparable between the two arms. Sixteen patients underwent biopsy to confirm or refute progression, 11 in the ETP subgroup with 7 ETP patients showing a rapid progression at the 1-month repeat MRI examination. There were no other differences in the clinical characteristics between PSP and ETP subgroups. In the landmark analysis comparing PsP with ETP patients, the median OS was longer in PsP patients (25.2 months [18.5, not reached]) than in ETP patients (20.3 months [13.2, 22.5]), P = .0092. We published recently the detailed PsP clinical and imaging findings.20
Clinical Outcomes in Subgroups with or with no Pseudoprogression at 6 Months
The 6-month landmark analysis (n = 116 patients alive and with no progression at 6 months), compared OS between PsP (n = 28) and no PsP (n = 88) in the 6-month subgroups of the two arms. For the 28 patients with PsP, median OS was longer in the HD arm (36.1 months [18.5; NR]) than in the SD arm (24.1 months [14.3; 35.7]), but this difference failed to reach statistical significance (P = .0721; Figure 4A). In the 88 patients without PsP within the first 6 months, median OS was identical across the two arms (SD: 26.3 months [22.1, 32.3]; HD: 24.1 months [16.4, 34.5]; P = .7681; Figure 4B).
Figure 4.
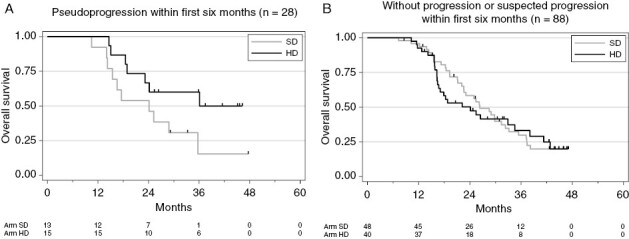
Overall survival in patients with pseudoprogression by trial arm (A). Overall survival in patients without pseudoprogression by trial arm (B).
Discussion
Ling et al.’s prophecy in their seminal paper that advances in precise dose delivery and metabolic imaging would result in physical and biological conformality in a majority of patients by 2010 has not yet come to pass.3 The difficulty in establishing the predictive value of MR and PET imaging and reliably integrating them for target volume definition explains that only a very small subset of RT trials have delivered modified doses to radioresistant clusters based on metabolic or functional imaging (ie, dose-painting).21–25 The dose-painting SPECTRO GLIO trial was based on the added value and integration of an advanced MRSI technique for predicting the site of relapse of GBM after RT (ie, CNR2).
MRSI metabolic imaging was abnormal in the majority of HD patients and led to abnormalities being added to the tumor bed to boost target volume definition. Even though high radiation doses were well tolerated, no outcome differences were observed between the two arms of the study. Gondi et al. recently published results of the prospective NRG Oncology BN 001 study, comparing chemoradiotherapy dose escalation to 75 Gy in 30 fractions delivered to the tumor bed with standard treatment in 229 patients, and found no difference in survival.26 A recent review of 22 published studies following a total of 2198 patients found a survival benefit of dose escalation with RT alone, but not of TMZ + standard dose versus TMZ + higher dose.27 Dose escalation was not guided by metabolic or functional imaging in any of these 22 studies. Dose escalation was not guided by metabolic or functional imaging in NRG Oncology BN 001 nor in any of the 22 studies included in the review. Our study shows that although the boost to the tumor bed was modified in 64% of patients, this did not improve patient outcome.
Given the high radioresistance of GBM, it may be surmised that the boost dose needs to be much higher than the one we administered. By extrapolation the definition of boost volume may also need to be multimodal and include several metabolic and functional imaging modalities such as PET (18F-DOPA-PET27,28 or amino acid-PET22) and MRI (MRSI with Cho/NAA but also lactate, which has a potential prognostic value for the site of relapse,29 diffusion MRI, and perfusion MRI30–32). Indeed, we recently reported33 that glioblastoma stem cells (GSCs) isolated from a restricted apparent diffusion coefficient (ADC) map were more aggressive and generated neurospheres more quickly than GSCs isolated from other parts of the tumor and that increased relative cerebral blood volume (rCBV) areas were enriched with GSCs known to be more radioresistant.34 Only increasing the radiotherapy dose on MRSI-defined metabolic areas may therefore be insufficient to target GSCs or radioresistant cell clusters. Our work also demonstrated that GSCs are able to adapt to a clinical dose of 2 to 3 Gy by employing several mechanisms of radiation-induced plasticity such as reprogramming35 and radiation-induced transdifferentiation into endothelial like cells.36 Increasing the radiotherapy dose delivered to radioresistant clusters is therefore not expected to be sufficient to control disease and may even drive GSCs down these radiation-induced GSC plasticity pathways.37 Targeting radioresistant GSC clusters by boost irradiation may be further potentiated by combining with a specific inhibitor of these radiation-induced mechanisms such as Regorafenib, as shown in our preclinical study.38 An alternative rationale is that subventricular zones harbor GSC niches and, since these focii are not irradiated due to their distant location, they may contribute to post-therapy relapse.39
Although we did not show that increasing RT dose increases OS, our evidence, showing a similar rate of toxicity in both arms of the study, supports that SIB allows to considerably increase the dose without increasing the dose to organs at risk.14
The OS in our trial was longer than comparable OS rates reported in the literature.1,40 This may be due to the close follow-up of our patients, who frequently underwent one or more surgeries, often received bevacizumab, and were re-irradiated upon relapse.
No patient selection was performed that could explain this difference in OS rate except the 5 cm threshold which was introduced to avoid overdosing normal tissue in patients that received a boost. Median tumor size at diagnosis was nevertheless 35 mm.
Pseudoprogression is a common issue in post-RT follow-up for GBM. Its incidence varies from 4% to 30%.41 In the current study, PsP occurred at a rate of 15% and did not increase in HD patients compared to SD patients. Interestingly, PsP patients in the HD arm had a longer OS than patients in the SD arm. Other studies have also described the positive predictive value of PsP for OS and its enhancement by a higher RT dose associated with TMZ.18,42
The strengths of our study are numerous: it is a randomized, prospective, multicentric trial. A guarantee of robustness is that the MRSI analysis was a centralized analysis, with centralized contouring, and online external prospective quality control of dosimetry in the experimental arm. As confirmed in several trials and two meta-analyses, this is crucial to obtain reliable results.43–45
The active interest in GBM dose painting with MR spectroscopy is shared on both sides of the Atlantic, with a 3-institution, phase II, pilot study of a 75 Gy boost targeting CNR2 on a total of 30 patients, using whole-brain MR spectroscopy carried out in the United States (NCT03137888). Results from this trial indicate good feasibility and safety46 with the corresponding randomized trial currently being developed.47
Our trial does, however, have several limitations. First, the assessment of late cognitive effects based on MMS status is suboptimal as it is designed for assessing dementia. Second, we opted to forgo the intermediate phase II trial. A phase II study with MRSI boost had nevertheless already been performed by the previously mentioned U.S. team,46 prompting them to develop a randomized study. Third, the survival analysis of PsP versus ETP was a post-hoc subgroup analysis. Finally, we limited participation to tumors of <5 cm diameter (to avoid any increased toxicity in the treated HD arm because of tumor diameters ≥5 cm), nevertheless this exclusion is not relevant for the interpretation of this randomized trial.
The MGMT was centrally determined using the PyroMark CpG MGMT kit (ref. 972032, Qiagen, France) on FFPE samples with a cut-off of 8%, as validated by Quillien et al.48 As expected, this resulted in a 50/50 distribution of methylated and unmethylated patients. The first published cohort of 206 patients to report on the prognostic value of MGMT methylation observed a 45/55 ratio which is similar to our finding.49
In clinical trials such as RTOG 0525 and 082550 the ratio of methylated versus non-methylated patients is closer to 30/70. The cut-off therefore remains a matter of debate. Another unresolved issue is the definition of the most relevant number of methylated cytosine–phosphate–guanine-(CpG-)sites to analyze for clinical purposes. Even if the percentage of methylated patients is the same in 2 studies, depending on the specific CpG sites analyzed, the patients considered to be methylated will not be exactly the same, especially if the cut-off is high. On the whole, there are several unresolved issues in terms of methodology, definition of cut-offs, and optimal use in the clinical setting.51
Given our results, methods to improve this dose-painting approach may include 3 axes.
1) To optimize the use of MR spectroscopic imaging for boost target definition, we are now analyzing several other metabolites, in addition to the widely used CHO/NAA, in the entire trial cohort. Another interesting approach would be to make greater use of whole-brain 3D MR spectroscopy.47 One limitation to the use of MRSI is its low resolution and, in clinical routine, the need for manual quality control of all spectra; our team recently published a technique to facilitate spectral quality control and its clinical use.52
2) Using multimodal imaging, the recent results from the phase II trial delivering 76 Gy guided by F-DOPA PET showed a high OS rate of 35 months in 24 methylated patients, compared with 23 months in a historical cohort, and an improved event-free survival of 16 months in 39 non-methylated patients.28 In our phase III trial, OS rates were similar and even slightly higher, as we found a median OS of 38 months for HD/methylated patients. Our SD/methylated arm also had a longer median OS of 28 months, which may explain why the difference between the two arms was not statistically significant. In another phase II dose-painting trial based on diffusion and perfusion imaging, in patients with a decrease in combined hypercellular/hyperperfused volume at 3 months, the median OS was 29 months versus 12 months for others.23 These studies provide support for multimodal imaging to guide RT. The SpectroGlio trial generated a large amount of multimodal imaging and dosimetry data. We have already performed imaging studies among subsets of patients included in our trial30,31 and are carrying out further studies on the whole cohort, including a radiomics-based artificial intelligence approach.53
Our objective is to describe new prognostic and predictive values for anatomical and metabolic imaging.
3) We speculate that exclusively increasing the dose is not sufficient and will probably have to be associated with inhibitors of biological pathways of radiation-induced plasticity to target the adaptation of glioblastoma stem cells to radiation as described in the discussion above.
To conclude, our dose-painting trial involving delivery of a heterogeneous dose guided by metabolic imaging showed that the dose increase was well tolerated. OS did not improve, but two subgroups of HD patients had high OS, namely, methylated patients and pseudoprogression patients. Our study yielded a large amount of longitudinal multimodal MRI and radiotherapy data, which will be useful to decipher the pathways of pseudoprogression and radioresistance in glioblastoma.
Supplementary Material
Contributor Information
Anne Laprie, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse NeuroImaging Center, Université de Toulouse, Inserm, UPS, Toulouse, France.
Georges Noel, CANS, Strasbourg, France.
Leonor Chaltiel, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France.
Gilles Truc, Centre Georges-François Leclerc, Dijon, France.
Marie-Pierre Sunyach, Centre Léon Berard, Lyon, France.
Marie Charissoux, Centre Val d’Aurelle, Montpellier, France.
Nicolas Magne, Institut de Cancérologie de la Loire, Saint-Priest en Jarez, France.
Pierre Auberdiac, Clinique Claude Bernard, Albi, France.
Julian Biau, Centre Jean-Perrin, Clermont-Ferrand, France.
Soléakhéna Ken, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, RadOpt-CRCT-INSERM, Toulouse, France.
Fatima Tensaouti, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole & ToNIC, Toulouse NeuroImaging Center, Université de Toulouse, Inserm, UPS, Toulouse, France.
Jonathan Khalifa, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France.
Ingrid Sidibe, Centre Eugène Marquis, Rennes, France.
Franck-Emmanuel Roux, Centre Hospitalier Universitaire de Toulouse, Toulouse NeuroImaging Center, Université de Toulouse, Inserm, UPS, Toulouse, France.
Laure Vieillevigne, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France.
Isabelle Catalaa, Centre Hospitalier Universitaire de Toulouse, Toulouse, France.
Sergio Boetto, Centre Hospitalier Universitaire de Toulouse, Toulouse, France.
Emmanuelle Uro-Coste, Centre Hospitalier Universitaire de Toulouse, Institut Universitaire du Cancer de Toulouse-Oncopole, RadOpt-CRCT-INSERM, Toulouse, France.
Stéphane Supiot, Institut de Cancerologie de l’Ouest, Nantes st Herblain, France.
Valérie Bernier, Institut de Cancérologie de Lorraine Centre Alexis Vautrin, Nancy, France.
Thomas Filleron, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France.
Muriel Mounier, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France.
Muriel Poublanc, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France.
Pascale Olivier, Service de Pharmacologie Médicale et Clinique, Centre Régional de Pharmacovigilance, de Pharmacoépidémiologie et d’Information sur le Médicament CHU de Toulouse, Toulouse, France.
Jean-Pierre Delord, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, Toulouse, France.
Elizabeth Cohen-Jonathan-Moyal, Institut Claudius Regaud, Institut Universitaire du Cancer de Toulouse-Oncopole, RadOpt-CRCT-INSERM, Toulouse, France.
Funding
This study was funded by the French National Institute of Cancer. Grant PHRC 2008 The funding body had no role in the design of the study; the collection, analysis, and interpretation of the data; or the writing of the manuscript.
Ethics, Informed Consent and Safety
The trial was reviewed and approved by the French National Ethics Committee on April 28, 2010 (registration no. 2009-A00594-53). This covered all the participating centers: Institut Claudius Regaud at the Institut Universitaire du Cancer de Toulouse-Oncopole- Toulouse-France; Centre Paul Strauss/ Institut de Cancérologie de Strasbourg-Europe (ICANS)- Strasbourg- France; Centre Georges-François Leclerc – Dijon- France; Centre Léon Bérard- Lyon- France; Institut de Cancérologie de la Loire- Saint-Priest en Jarez; Centre Val d’Aurelle – Montpellier- France; Clinique Claude Bernard- Albi- France. All participants signed the written informed consent form. The investigators performed the human investigations after approval by a local Human Investigations Committee and in accord with an insurance filed with and approved by the Department of Health and Human Services.
Authorship Statement
Conception and design: Anne Laprie, Elizabeth Cohen-Jonathan-Moyal, Laure Vieillevigne, Thomas Filleron, Muriel Poublanc. Provision of study materials or patients: Elizabeth Cohen Jonathan Moyal, Anne Laprie, Georges Noel, Gilles Truc, Marie-Pierre Sunyach, Marie Charissoux, Nicolas Magne, Pierre Auberdiac, Franck-Emmanuel Roux, Sergio Boetto, Emmanuelle Uro-Coste, Stéphane Supiot, Valérie Bernier. Collection and assembly of data: Anne Laprie, Soléakhéna Ken, Fatima Tensaouti, Jonathan Khalifa, Ingrid Sidibe, Muriel Mounier, and Muriel Poublanc. Data analysis and interpretation: Anne Laprie, Elizabeth Cohen-Jonathan Moyal, Leonor Chaltiel, Thomas Filleron. Writing of the manuscript: Anne Laprie. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Conflict of interest statement. EC-J M: French coordinator of the Trident trial (Novocure). Coordinator of the STERIMGLI trial financially supported by an Astra-Zeneca grant. Received research grants from Incyte, Novocure and Bayer. Occasional interventions: member of advisory boards for Novocure. Conferences: Invitations as a speaker for Novocure.
Trial Sponsor
Institut Claudius Regaud; Institut national du Cancer INCa; Institut Universitaire du Cancer de Toulouse -Oncopole, 1 avenue Irène Joliot-Curie, F-31059 Toulouse, France. Contact: Poublanc.muriel@iuct-oncopole.fr
Data Availability
Data sharing is not applicable to this article.
References
- 1. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ling CC, Humm J, Larson S, et al. Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys. 2000;47(3):551–560. [DOI] [PubMed] [Google Scholar]
- 4. Bentzen SM, Gregoire V.. Molecular imaging-based dose painting: a novel paradigm for radiation therapy prescription. Semin Radiat Oncol. 2011;21(2):101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laprie A, Catalaa I, Cassol E, et al. Proton magnetic resonance spectroscopic imaging in newly diagnosed glioblastoma: predictive value for the site of postradiotherapy relapse in a prospective longitudinal study. Int J Radiat Oncol Biol Phys. 2008;70(3):773–781. [DOI] [PubMed] [Google Scholar]
- 6. Park I, Tamai G, Lee MC, et al. Patterns of recurrence analysis in newly diagnosed glioblastoma multiforme after three-dimensional conformal radiation therapy with respect to pre-radiation therapy magnetic resonance spectroscopic findings. Int J Radiat Oncol Biol Phys. 2007;69(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pirzkall A, Li X, Oh J, et al. 3D MRSI for resected high-grade gliomas before RT: tumor extent according to metabolic activity in relation to MRI. Int J Radiat Oncol Biol Phys. 2004;59(1):126–137. [DOI] [PubMed] [Google Scholar]
- 8. McKnight TR, von dem Bussche MH, Vigneron DB, et al. Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg. 2002;97(4):794–802. [DOI] [PubMed] [Google Scholar]
- 9. Crawford FW, Khayal IS, McGue C, et al. Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J Neurooncol. 2009;91(3):337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laprie A, Catalaa I, Cassol E, et al. Proton magnetic resonance spectroscopic imaging in newly diagnosed glioblastoma: predictive value for the site of postradiotherapy relapse in a prospective longitudinal study. International Journal of Radiation Oncology*Biology*Physics. 2008;70:773–781. [DOI] [PubMed] [Google Scholar]
- 11. Cho KH, Hall WA, Lo SS, Dusenbery KE.. Stereotactic radiosurgery versus fractionated stereotactic radiotherapy boost for patients with glioblastoma multiforme. Technol Cancer Res Treat. 2004;3(1):41–49. [DOI] [PubMed] [Google Scholar]
- 12. Nwokedi EC, DiBiase SJ, Jabbour S, et al. Gamma knife stereotactic radiosurgery for patients with glioblastoma multiforme. Neurosurgery. 2002;50(1):41–47. discussion. [DOI] [PubMed] [Google Scholar]
- 13. Sultanem K, Patrocinio H, Lambert C, et al. The use of hypofractionated intensity-modulated irradiation in the treatment of glioblastoma multiforme: preliminary results of a prospective trial. Int J Radiat Oncol Biol Phys. 2004;58(1):247–252. [DOI] [PubMed] [Google Scholar]
- 14. Ken S, Vieillevigne L, Franceries X, et al. Integration method of 3D MR spectroscopy into treatment planning system for glioblastoma IMRT dose painting with integrated simultaneous boost. Radiat Oncol. 2013;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 17. Laprie A, Ken S, Filleron T, et al. Dose-painting multicenter phase III trial in newly diagnosed glioblastoma: The SPECTRO-GLIO trial comparing arm A standard radiochemotherapy to arm B radiochemotherapy with simultaneous integrated boost guided by MR spectroscopic imaging. BMC Cancer. 2019;19(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ.. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. [DOI] [PubMed] [Google Scholar]
- 19. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 20. Sidibe I, Tensaouti F, Gilhodes J, et al. Pseudoprogression versus true progression in patients with glioblastoma: A multiapproach analysis. Radiother Oncol. 2023;181:109486. doi: 10.1016/j.radonc.2023.109486. Epub 2023 Jan 24. [DOI] [PubMed] [Google Scholar]
- 21. Nestle U, Schimek-Jasch T, Kremp S, et al. ; PET-Plan study group. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol. 2020;21(4):581–592. [DOI] [PubMed] [Google Scholar]
- 22. Oehlke O, Mix M, Graf E, et al. Amino-acid PET versus MRI guided re-irradiation in patients with recurrent glioblastoma multiforme (GLIAA) – protocol of a randomized phase II trial (NOA 10/ARO 2013-1). BMC Cancer. 2016;16(1):769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim MM, Sun Y, Aryal MP, et al. A Phase 2 study of dose-intensified chemoradiation using biologically based target volume definition in patients with newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys. 2021;110(3):792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shu H-KG, Mellon EA, Kleinberg L, et al. A multisite clinical trial of spectroscopic MRI-guided radiation dose escalation for newly-diagnosed glioblastomas. J Clin Oncol. 2021;39(15_suppl):2018–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kerkmeijer LGW, Groen VH, Pos FJ, et al. Focal boost to the intraprostatic tumor in external beam radiotherapy for patients with localized prostate cancer: results from the flame randomized phase III Trial. J Clin Oncol. 2021;39(7):787–796. [DOI] [PubMed] [Google Scholar]
- 26. Gondi V, Pugh S, Tsien C, et al. Radiotherapy (RT) Dose-intensification (DI) Using Intensity-modulated RT (IMRT) versus Standard-dose (SD) RT with Temozolomide (TMZ) in Newly Diagnosed Glioblastoma (GBM): Preliminary Results of NRG Oncology BN001. Int J Radiat Oncol Biol Phys. 2020;108:S22–S23. [Google Scholar]
- 27. Gondi V. Radiotherapy intensification for glioblastoma: enhancing the backbone of treatment. Chinese Clin Oncol. 2021;10:39–39. [DOI] [PubMed] [Google Scholar]
- 28. Laack NN, Pafundi D, Anderson SK, et al. Clinical investigation initial results of a phase 2 trial of 18 F-DOPA PET-guided dose-escalated radiation therapy for glioblastoma. Int J Radiat Oncol Biol Phys. 2021;110(5):1383–1395. [DOI] [PubMed] [Google Scholar]
- 29. Deviers A, Ken S, Filleron T, et al. Evaluation of the lactate-to-N-acetyl-aspartate ratio defined with magnetic resonance spectroscopic imaging before radiation therapy as a new predictive marker of the site of relapse in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2014;90(2):385–393. [DOI] [PubMed] [Google Scholar]
- 30. Khalifa J, Tensaouti F, Lotterie JA, et al. Do perfusion and diffusion MRI predict glioblastoma relapse sites following chemoradiation? J Neurooncol. 2016;130(1):181–192. [DOI] [PubMed] [Google Scholar]
- 31. Khalifa J, Tensaouti F, Chaltiel L, et al. Identification of a candidate biomarker from perfusion MRI to anticipate glioblastoma progression after chemoradiation. Eur Radiol. 2016;26(11):4194–4203. [DOI] [PubMed] [Google Scholar]
- 32. Kim MM, Aryal MP, Sun Y, et al. Response assessment during chemoradiation using a hypercellular/hyperperfused imaging phenotype predicts survival in patients with newly diagnosed glioblastoma. Neuro Oncol. 2021;23(9):1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duval T, Lotterie JA, Lemarie A, et al. Glioblastoma stem-like cell detection using perfusion and diffusion MRI. Cancers (Basel). 2022;14:2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 35. Dahan P, Martinez Gala J, Delmas C, et al. Ionizing radiations sustain glioblastoma cell dedifferentiation to a stem-like phenotype through survivin: possible involvement in radioresistance. Cell Death Dis. 2014;5(11):e1543–e1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deshors P, Toulas C, Arnauduc F, et al. Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway. Cell Death Dis. 2019;10(11):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Couturier CP, Ayyadhury S, Le PU, et al. Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat Commun. 2020;11(1):3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deshors P, Arnauduc F, Boëlle B, et al. Impact of regorafenib on endothelial transdifferentiation of glioblastoma stem-like cells. Cancers (Basel). 2022;14(6):1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hira VVV, Molenaar RJ, Breznik B, et al. Immunohistochemical detection of neural stem cells and glioblastoma stem cells in the subventricular zone of glioblastoma patients. J Histochem Cytochem. 2021;69(5):349–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson DR, O’Neill BP.. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol. 2012;107(2):359–364. [DOI] [PubMed] [Google Scholar]
- 41. Le Fèvre C, Lhermitte B, Ahle G, et al. Pseudoprogression versus true progression in glioblastoma patients: a multiapproach literature review: Part 1 - Molecular, morphological and clinical features. Crit Rev Oncol Hematol. 2021;157:103188. [DOI] [PubMed] [Google Scholar]
- 42. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 43. Carrie C, Grill J, Figarella-Branger D, et al. Online quality control, hyperfractionated radiotherapy alone and reduced boost volume for standard risk medulloblastoma: long-term results of MSFOP 98. J Clin Oncol. 2009;27(11):1879–1883. [DOI] [PubMed] [Google Scholar]
- 44. Ohri N, Shen X, Dicker AP, et al. Radiotherapy protocol deviations and clinical outcomes: A meta-analysis of cooperative group clinical trials. J Natl Cancer Inst. 2013;105(6):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weber DC, Tomsej M, Melidis C, Hurkmans CW.. QA makes a clinical trial stronger: Evidence-based medicine in radiation therapy. Radiother Oncol. 2012;105(1):4–8. [DOI] [PubMed] [Google Scholar]
- 46. Ramesh K, Mellon EA, Gurbani SS, et al. A multi-institutional pilot clinical trial of spectroscopic MRI-guided radiation dose escalation for newly diagnosed glioblastoma. Neuro-Oncology Adv. 2022;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mellon EA. Whole brain spectroscopic MRI for RT Dose Escalation in Glioblastoma. ASTRO Annu Meet. 2021. [Google Scholar]
- 48. Quillien V, Lavenu A, Ducray F, et al. Validation of the high-performance of pyrosequencing for clinical MGMT testing on a cohort of glioblastoma patients from a prospective dedicated multicentric trial. Oncotarget. 2016;7(38):61916–61929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 50. Gittleman H, Lim D, Kattan MW, et al. An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG Oncology RTOG 0525 and 0825. Neuro Oncol. 2017;19(5):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bienkowski M, Berghoff AS, Marosi C, et al. Clinical Neuropathology practice guide 5-2015: MGMT methylation pyrosequencing in glioblastoma: Unresolved issues and open questions. Clin Neuropathol. 2015;34(5):250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tensaouti F, Desmoulin F, Gilhodes J, et al. Quality control of 3D MRSI data in glioblastoma: Can we do without the experts? Magn Reson Med. 2021;14(6):1551. [DOI] [PubMed] [Google Scholar]
- 53. Tensaouti F, Bailleul J, Martin E, et al. Radiomics study from the dose-painting multicenter phase III trial on newly diagnosed glioblastoma PO-0957 Physics track: Radiobiological and predictive modelling, and radiomics. Med Phys. 2019;133(supplement 1):S518–S519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article.


