Abstract
Tumor-related epilepsy (TRE) is a frequent and major consequence of brain tumors. Management of TRE is required throughout the course of disease and a deep understanding of diagnosis and treatment is key to improving quality of life. Gross total resection is favored from both an oncologic and epilepsy perspective. Shared mechanisms of tumor growth and epilepsy exist, and emerging data will provide better targeted therapy options. Initial treatment with antiseizure medications (ASM) in conjunction with surgery and/or chemoradiotherapy is typical. The first choice of ASM is critical to optimize seizure control and tolerability considering the effects of the tumor itself. These agents carry a potential for drug–drug interactions and therefore knowledge of mechanisms of action and interactions is needed. A review of adverse effects is necessary to guide ASM adjustments and decision-making. This review highlights the essential aspects of diagnosis and treatment of TRE with ASMs, surgery, chemotherapy, and radiotherapy while indicating areas of uncertainty. Future studies should consider the use of a standardized method of seizure tracking and incorporating seizure outcomes as a primary endpoint of tumor treatment trials.
Keywords: antiseizure medication, brain tumor, epilepsy, glioma, seizure
Key Points.
Seizures are a common manifestation of brain tumors and cause of morbidity to patients.
Consideration of ASM profiles is necessary to balance seizure control and adverse effects.
Seizure control may be affected by tumor progression and tumor-targeted treatments.
Tumor-related epilepsy (TRE) is a well-known consequence of primary or metastatic brain tumors. Often a seizure is the presenting symptom of a brain neoplasm. Seizure control often aligns with tumor growth and conversely can improve with tumor-directed treatment (Figure 1). Antiseizure medications (ASMs) are first-line treatment for TRE and are often followed by tumor resection. Depending on tumor type and grade, patients may receive chemotherapy, radiotherapy, and more recently immunotherapy and targeted therapy. Nearly all patients who present with a seizure or develop seizures later in the course of their disease will need ASMs as part of their treatment. Over the last 30 years, ASM availability has increased with the development of over 20 additional agents. Some of these agents have been studied in TRE and many have shown benefit in case series or systematic reviews.
Figure 1.
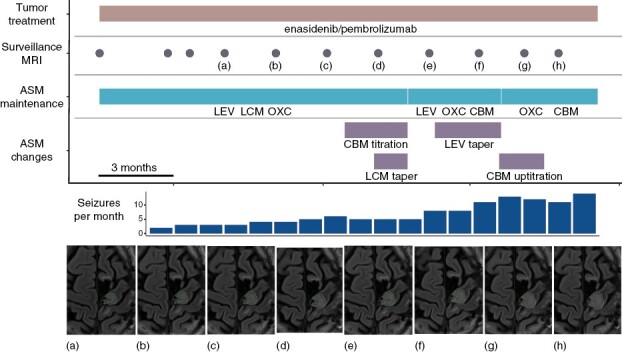
Representative example of increasing seizure frequency aligning with tumor progression in a patient with recurrent oligodendroglioma despite ASM changes. CBM, cenobamate; LCM, lacosamide; LEV, levetiracetam; OXC, oxcarbazepine.
Patients with brain tumors often experience cognitive disturbance, fatigue, and an array of side effects from tumor-directed treatment. The addition of ASM can often worsen some of these side effects or introduce new adverse effects.1 Therefore, skillful management of ASMs in addition to other tumor-directed treatments is essential for the treating physician. For this review, we will use the term tumor-related epilepsy as the defining name for this type of epilepsy. In addition, we will continue the current use of antiseizure medication as adopted by the International League Against Epilepsy (ILAE).
Tumor-Related Epilepsy Epidemiology and Incidence
TRE incidence varies by tumor type with histologic low-grade gliomas carrying a higher incidence than high-grade gliomas.2 TRE incidence viewed using WHO 2016 classification highlights several characteristics including histology and molecular profile to consider:
Lower tumor grades carry a higher incidence of TRE.
Astrocytic gliomas are less epileptogenic than tumors with oligodendroglial components.
Mutant IDH astrocytic gliomas (including grade 4 tumors), are more likely to induce TRE.3
The application of the WHO 2021 classification with TRE incidence would likely yield similar results but would need to be formally studied.
Brain and spinal cord tumors are the second most common cancer types in children secondary to leukemias.4 The overall annual incidence rate for brain tumors is 7.08/100,000 population, and stratified by age5:
| Age | Annual incidence |
|---|---|
| Birth to 14 years | 3.87/100,000 |
| 15–19 years | 2.60/100,000 |
| 20–39 years | 3.39/100,000 |
| 40–64 years | 7.96/100,000 |
| >65 years | 21.26/100,000 |
The most recent data from The Central Brain Tumor Registry of the United States (CBTRUS) shows an average annual age-adjusted incidence rate (AAAIR) for all malignant and nonmalignant tumors of 24.71/100,000 between 2015 and 2019.6 The AAAIR for malignant tumors was 7.02/100,000 and the AAAIR for nonmalignant tumors was 17.69/100,000.
In patients with primary brain tumors, seizures are more common with low-grade tumors compared to high-grade tumors.7 In a series of 1028 patients with primary brain tumors, epilepsy rates were reported at 85, 69, and 49% in patients with low-grade glioma, anaplastic glioma, and glioblastoma, respectively.2 At least one seizure occurs in up to 80% of patients with high-grade glioma at some point during the course of disease.8 Glioneuronal tumors such as ganglioglioma and dysembryoplastic neuroepithelial tumors also have a high incidence of seizures, >80%.9
Seizures are the presenting manifestation in 13–24% of children with brain tumors.10 In one study of 367 children with brain tumors, seizures occurred in 57 (16%) children, and were the initial symptom in 48 (13%) and the only clinical manifestation in 24 (7%).11 In another series, 56/184 (30%) children had seizures, of which 50/56 (89%) were supratentorial and 6/56 (10%) were infratentorial; 32% were in the temporal lobe, 21% frontal lobe, 16% parietal lobe, and 2% occipital lobe.12 Low-grade astrocytoma was the most common pathology (27%), followed by high-grade glioma (14%) and ganglioglioma (14%).
Diagnosing Tumor-Related Epilepsy
According to the ILAE, tumor-related epilepsies are etiology-specific epilepsy syndromes with associated treatment and prognostic implications.13 TRE are focal epilepsies with seizure types including focal awareness (motor and nonmotor), focal impaired awareness, and focal to bilateral tonic–clonic seizures. Adults with an unprovoked first seizure and significant abnormality on neuroimaging such as a brain tumor have their greatest risk of recurrence over the subsequent 2 years,14 and meet diagnostic criteria for epilepsy.15 Early postoperative acute symptomatic seizures carry a lesser risk of epilepsy.16,17 Long-term epilepsy-associated tumors (LEAT) present with drug-resistant epilepsy and commonly arise in the temporal lobe where focal neurological deficits are uncommon.18 Patients with TRE may develop other reasons for episodic or paroxysmal impaired awareness that can be confused with epilepsy such as increased intracranial pressure,19 or rarely psychogenic nonepileptic seizures.
TRE is a clinical diagnosis that is supported by electroencephalography (EEG). While many features of routine EEG signals are nonspecific, continuous focal delta slowing strongly correlates with an underlying structural lesion involving white matter tracts.20 Spikes and sharp waves imply a cortical phenomenon and provide support for epileptogenicity associated with focal seizures ipsilateral to the tumor. Subcortical or posterior fossa tumors can have a normal EEG. In a study of the diagnostic value of the preoperative EEG in 114 children with brain tumors, the EEG was normal in 54 (47%) and abnormal in 62 (53%).21 The EEG was abnormal in 15 children with infratentorial lesions revealing limitations of preoperative EEG in pediatric brain tumors.
Ambulatory EEG (AEEG) is an outpatient study lasting 1–3 days.22 In patients with TRE, AEEG with video is often effective for evaluating the differential diagnosis, classification of seizure type, quantification of seizure frequency, and ASM decision-making. However, the gold standard for EEG recording is inpatient long-term video-EEG monitoring (LTVEM) with or without discontinuation of ASM. Periodic patterns such as lateralized periodic discharges (LPDs) or electrographic seizures (>10 s) in patients with brain tumors lie along an interictal-ictal EEG continuum. LPDs are strongly associated with seizures and status epilepticus and should prompt continuous EEG monitoring.23 Nonconvulsive seizures and nonconvulsive status epilepticus (NCSE) are common sources of morbidity in patients with cancer.24 Clinical signs may be subtle (ie, focal aware seizures manifesting with aphasia) or absent despite the presence of NCSE on EEG. This is especially important to consider when altered baseline mental status is present in patients with brain tumors.
Neuroimaging is critical for the identification of brain tumors and defining the epileptogenic lesion. However, frequent seizures can result in neuroimaging changes, such as cortical enhancement on brain MRI and successful treatment of seizures can lead to resolution of these changes.25 Patients with focal seizures or focal status epilepticus may develop reversible CT or MRI signal changes that may mimic tumors; these resolve on follow-up imaging after seizure control in adults and children.26,27
Functional neuroimaging includes positron emission tomography (PET), single-photon emission computed tomography (SPECT), and functional MRI (fMRI). FDG (18F-2-fluoro-2-deoxy-D-glucose) PET is used to evaluate both neuro-oncology and epilepsy patients.28 Various tracers are available to evaluate both diseases, but amino acid tracers are most useful in identifying areas of epileptogenesis in patients with nontumoral epilepsies.28,29 A report of patients with grades 2–4 glioma revealed increased uptake on O-(2-18F-fluoroethyl)-L-tyrosine PET during periods of frequent seizures and status epilepticus arising from peritumoral cortex. Seizures and status epilepticus increase cerebral amino acid transport with a strict gyral uptake pattern on 18F-flouroethyl-L-tyrosine PET.30 A recent joint guideline from the Response Assessment in Neuro-Oncology working group and the European Association for Neuro-Oncology and European Association of Nuclear Medicine provides evidence-based recommendations for use, utility, and limitations of amino acid PET in the diagnosis of glioma.29
Shared Mechanisms of Epileptogenesis and Tumor Growth
Mechanisms underlying TRE are known in diffuse gliomas, although variability in epilepsy incidence across tumor pathologies indicates multiple mechanisms are contributing. Electrophysiologic epileptiform or hyperexcitable neuronal activity is used as a biomarker of epileptogenesis, which is detected in discrete sites at the brain-tumor interface and infiltrating margins.31
Glutamate is involved in promoting both glioma growth and epileptogenesis (Figure 2).32–34 Increased peritumoral extracellular glutamate may arise from increased glutamate release from glioma cells due to overexpression of the cystine-glutamate transporter (xCT), a critical component to redox homeostasis, and impaired reuptake by excitatory amino acid transporters.35,36 Excessive glutamate facilitates glioma cellular invasion and excitotoxicity via NMDA receptor and Ca(2+)-permeable AMPA receptor activation.37,38 Pathophysiologic glutamatergic signaling contributes to pro-excitable states in the glioma microenvironment,39 with downregulation of KCC2 and upregulation of NKCC1 leading to altered chloride homeostasis, impaired inhibition, and paradoxical depolarizing effects of GABAergic signaling.40,41
Figure 2.
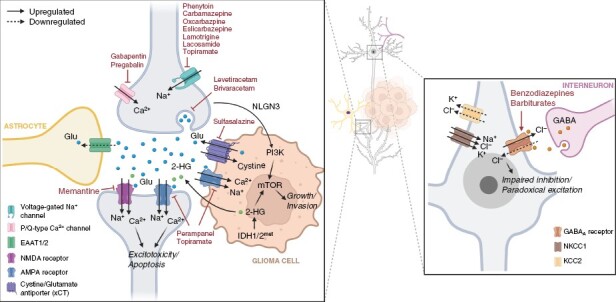
Schematic representation of established mechanisms underlying glioma-related epilepsy. Glutamatergic signaling is a key pathway affected and contributes to the development of functional neuron-glioma synapses. ASMs with selective mechanisms of action are shown. Created with BioRender.com.
Recent advances in technology with microelectrode recordings, in vivo optogenetic models, and tumor sequencing indicate a complex bidirectional relationship between glioma cells and hyperexcitability of adjacent neurons. Peritumoral neurons can form synapses directly onto glioma cells, with glial cell synaptogenesis driven by the activity-dependent release of the neuronal protein neuroligin-3 (NLGN-3).42–44 The resulting neuron-glioma synapses are capable of neurotransmission, with excitatory glutamatergic signaling AMPA receptors capable of promoting tumor cell proliferation and invasion (Figure 2).43,44 Electrochemical communication between neurons and glial cells may underlie a feed-forward mechanism where glutamate produced by glioma cells can lead to neuronal hyperexcitability and seizures, while peritumoral neuronal hyperexcitability may itself promote tumor growth and further glutamate release.43
Tumor molecular profiles may also have a significant effect on the tumor microenvironment, neuronal hyperexcitability, and tumor progression. Effects are largely determined by glioma genetic alterations, the best studied of which is the isocitrate dehydrogenase 1 (IDH1) gene mutation. IDH1 is frequently mutated in low-grade gliomas, associated with improved tumor prognosis and an independent risk factor for increased seizure risk.45,46IDH1 mutations lead to conversion of isocitrate to D-2-hydroexyglutarate (D-2HG) rather than to alpha-ketoglutarate. D-2HG may lead to hyperexcitability and seizures either by acting as a glutamate receptor agonist at NMDA receptors, due to its structural similarity to glutamate,45 or by upregulation of the mTOR signaling pathway, which is implicated in tuberous sclerosis.47
Other genetic variants, many of which converge on the PI3K-mTOR pathway, impact the tumor microenvironment and promote epileptogenesis. Specific gain-of-function missense variants in PIK3CA, coding for the PI3K catalytic subunit, induce epileptogenicity through differential cell-autonomous and cell-nonautonomous mechanisms in a glioblastoma model,48 and have been associated with poor seizure control in patients with diffuse gliomas.49 The BRAF V600E mutation observed in glioneuronal tumors is associated with the development of epileptiform activity mediated by altered regulation of ion transport and synaptic activity and is predictive of postoperative seizures.50,51
There are limited data characterizing mechanisms of epileptogenesis in meningiomas, metastatic lesions, and other nonglial brain tumors. Peritumoral edema is one of the strongest predictors of epilepsy in patients with meningiomas.52 In a patient cohort of meningiomas with genomic sequencing, somatic NF2 mutations were associated with preoperative seizures, mediated by increased edema and atypical histology.53 Local inflammatory and metabolic changes are often considered to mediate disruption to the excitatory-inhibitory balance in patients with supratentorial metastatic tumors.54,55
Anti-Seizure Medications
Few randomized clinical trials have examined the efficacy of ASMs in TRE. Most have evaluated ASMs as add-on therapy rather than monotherapy. Sample sizes are often small and include variable tumor pathologies. Recognizing limitations, a summary of the literature is provided in Table 1.
Table 1.
Antiseizure medications formulations, mechanisms of action with additional considerations and reported TRE seizure response rates.4,55–57 Reported response rates are for patients with tumor-related epilepsy and may include tumors other than gliomas including meningiomas and brain metastases. Mo, month; SE, side effect; SJS, Stevens Johnson Syndrome
| Antiseizure medication | Formulation/approved for children | Primary mechanism of action | Most commonly reported side effects | Serious side effects | Reported efficacy for use as monotherapy in TRE | Additional considerations |
|---|---|---|---|---|---|---|
| Brivaracetam | Tablet, liquid, IV/ Yes |
Binds to SV2A (a synaptic vesicle glycoprotein) |
Fatigue, dizziness, anxiety, agitation, depression | Hypersensitivity reactions | Not reported | — |
| Carbamazepine | Tablet, liquid/ Yes |
Na+ channel blocking |
Transaminitis, hyponatremia, blood dyscrasias, rash/SJS, nausea, vomiting |
Aplastic anemia, hepatotoxicity, hyponatremia, rash/ TEN/SJS, hypersensitivity reactions, decreased bone density (use > 10 years) | 6 mo seizure freedom: 28%1 12 mo seizure freedom: 30–55%1 |
CYP3A4, CYP2B6, CYP2C8, CYP2C9, CYP1A2, UGT1A4 inducer May cause bone marrow suppression |
| Cenobamate | Tablet/ No |
Na + channel blocking Enhancing GABA |
Fatigue, dizziness, hyperkalemia | QT shortening, drug reaction with eosinophilia | Not reported | CYP2C19 inhibitor, CYP3A4/5, CYP2B6 inducer |
| Clobazam | Tablet, liquid/ Yes |
GABAA-receptor agonist | Fatigue, dizziness, dry mouth, nausea |
Somnolence, sedation | Not reported | N-desmethylclobazam, the metabolite can be monitored for toxicity |
| Eslicarbazepine | Tablet, liquid/ Yes |
Na+ channel blocking |
Hyponatremia, rash/SJS, somnolence, nausea, fatigue |
Rash/TEN/SJS, hyponatremia, hepatotoxicity, blood dyscrasias, decreased T3/T4 |
Not reported | CYP2C9 inhibitor |
| Gabapentin | Tablet, liquid/ Yes |
Binding α2δ Ca channel subunit | Dizziness, fatigue, peripheral edema, weight gain, ataxia | None | Not reported | Substrate for a protein transporter likely reducing brain availability2 |
| Lacosamide | Tablet, liquid, IV/ Yes |
Na+ channel blocking |
Dizziness, gait instability, headache, fatigue, prolonged PR interval |
PR prolongation, syncope, rash/TEN/SJS, dizziness, ataxia | 6 mo seizure freedom: 55%-67%3 | Monitor for arrhythmias |
| Lamotrigine | Tablet, oral dissolving tablet/ Yes |
Na+ channel blocking |
Rash/SJS insomnia (if taken at night as bid dosing), blood dyscrasias |
Rash/TEN/SJS, blood dyscrasias, angioedema, bronchospasm | Not reported | Need for slow titration May be beneficial for mood Relatively safe in pregnancy |
| Levetiracetam | Tablet, liquid, IV/ Yes |
Binds to SV2A (a synaptic vesicle glycoprotein) |
Irritability, depression, anxiety, aggression, fatigue, lightheadedness | Suicidal ideation, depression | 6 mo seizure freedom: 39-96%1 6 mo seizure reduction≥50%: 71–100%1 |
Higher risk of psychiatric side effects in frontal lobe tumors4 Relatively safe in pregnancy |
| Oxcarbazepine | Tablet, liquid/ Yes |
Na+ channel blocking |
Hyponatremia, fatigue, lightheadedness, weight gain, alopecia, nausea | Hyponatremia, rash/TEN/SJS | 12 mo seizure freedom: 40% 12 mo seizure reduction≥50%: 88%1 |
CYP2C19 inhibitor and weakly induces CYP3A4 |
| Perampanel | Tablet, liquid/ Yes |
AMPA antagonism | Dizziness, vertigo, fatigue, aggressiveness, agitation, irritability, anxiety, nausea | Psychiatric side effects, homicidal/suicidal ideation | No studies | |
| Phenobarbital | Tablet, liquid, IV/ Yes |
Enhancing GABA | Drowsiness, fatigue, vertigo, habit forming, blood dyscrasias, cognitive slowing, rash/SJS |
Withdrawal seizures, hepatotoxicity, CNS depression, rash/TEN/SJS, blood dyscrasias | No studies |
CYP1A, CYP2A6, CYP2B, CYP2C, CYP3 A, UGT inducer Increases steroid clearance2 |
| Phenytoin | Tablet, liquid, IV/ Yes |
Na+ channel blocking |
Transaminitis, rash/SJS ataxia, dysarthria | Rash/TEN/SJS, hepatotoxicity, blood dyscrasias, gingival hyperplasia, lymphadenopathy, arrhythmias | 12 mo seizure freedom: 49–64%1 | Enzyme-inducing agent Increases steroid clearance2 Risk of birth defects |
| Pregabalin | Tablet, liquid/ Yes |
Binding α2δ Ca channel subunit | Drowsiness, sedation, weight gain, blood dyscrasias |
Peripheral edema, angioedema, hypersensitivity reactions | 12 mo seizure freedom: 75%1 | – |
| Primidone | Tablet/ Yes |
Enhancing GABA | N/A | Hypersensitivity reaction, thrombocytopenia, megaloblastic anemia | N/A | Not recommended due to similar SEs as phenobarbital and more difficult to monitor |
| Tiagabine | Tablet/ Yes, >12 years old |
Enhancing GABA | Lightheadedness, fatigue, anxiety, tremor, diarrhea, depression | CNS depression, rash | No studies | Side effects are commonly reported |
| Topiramate | Tablet, sprinkles/ Yes |
Na+ channel blocking, enhancing GABA, AMPA antagonism | Weight loss, word-finding difficulty, psychomotor slowing, metabolic acidosis, parasthesias, glaucoma | Acute angle closure glaucoma, nephrolithiasis, rash/TEN/SJS, oligohydrosis with heat stroke | 6 mo seizure freedom: 59% 12 mo seizure freedom: 57–71%1 |
CYP3A4 inducer |
| Valproic acid | Tablet, liquid, IV/ Yes > 10 years old |
Na+ channel blocking, enhancing GABA, blocking T-type Ca channels |
Weight gain, hair loss, fatigue, hyperammonemia, transaminitis, tremor, thrombocytopenia, rash/SJS | Hyperammonemia, hepatotoxicity, rash/TEN/SJS thrombocytopenia | 6 mo seizure freedom: 65% 6 mo seizure reduction≥50%: 77% 12 mo seizure freedom: 30–57% 12 mo seizure reduction ≥ 50%: 75–86%1 |
CYP2C9, UGT1A4 inhibitor (and weak inhibitor of CYP2C19 and CYP3A4) Significant risk of birth defects |
| Zonisamide | Tablet/ Yes > 16 years old |
Na+ channel blocking, blocking T-type Ca channels |
Somnolence, weight loss, lightheadedness, word-finding difficulty, renal calculi, oligohydrosis, rash | Rash/TEN/SJS, glaucoma, nephrolithiasis | No studies | Avoid in patients with history of nephrolithiasis Avoid in patients with sulfa allergies |
First Generation ASMs
The first-generation ASMs carbamazepine, phenytoin, phenobarbital, and primidone are often avoided in patients with TRE as they are potent enzyme inducers and have significant drug-drug interactions. In terms of therapeutic efficacy, carbamazepine as monotherapy has a reported six-month seizure-free rate of 28%,58 and 12-month rate of 30–55%.59 Studies report the efficacy of phenytoin monotherapy in preventing glioma-related epilepsy with six-month seizure-free rates of 67–87% and 12-month rates of 35–77%.59 None have evaluated the efficacy of phenobarbital or primidone (which is metabolized to phenobarbital as an active metabolite) as monotherapy for patients with TRE.
Valproic acid is a potent enzyme inhibitor; it was initially favored due to its potential antineoplastic effect as a histone deacetylase inhibitor. Survival benefits have not been replicated and its use has declined, although it is often used as a second- or third-line agent. Valproic acid has a 6-month seizure-free rate of 65%60 and 12-month rates of 30–62%.59
Second Generation ASMs
The most commonly used ASM for TRE is levetiracetam. In a systematic review of 66 studies on ASMs in glioma-related epilepsy, 25 reported levetiracetam to be efficacious as monotherapy.59 In 9 studies, there was a 6-month seizure-free rate of 39–96% and in 4 studies, a 12-month seizure-free rate of 68–96%. The seizure reduction rate of ≥50% in 2 studies was 71–100%.59 One study followed patients over 36 months, and uncontrolled seizures were a reason for transitioning to another agent, deemed treatment failure in 18% of patients.61
Amongst the second-generation ASMs, lamotrigine is often used; however, the data evaluating its efficacy as monotherapy is limited. The advantages are that it is well-tolerated and risk of fetal birth defects is similar to that of healthy women without epilepsy.62 However, due to prolonged titration, bridging with another ASM is often necessary.
Topiramate as monotherapy was reported in one study with promising results,63 however, at seizure prevention doses, cognitive slowing and weight loss were commonly reported. Zonisamide is not an enzyme-inducer or inhibitor and the majority of patients tolerate it, although weight loss is commonly reported and it should be avoided in patients with a history of nephrolithiasis.64
Oxcarbazepine is a derivative of carbamazepine, although has fewer side effects and fewer drug interactions. As monotherapy in TRE with variable pathologies, 63% of patients were seizure-free over a mean follow-up of 16 months.65 In an open-label study, among the intent-to-treat population, 40% were seizure-free at 12 months.66
Limited data exist to support gabapentin as adjunctive therapy in TRE.67 Pregabalin is similar to gabapentin; however, it is more potent. Again, limited data in one small, randomized control trial revealed a 75% seizure-free rate at 12 months.56
Third Generation ASMs
Lacosamide is often chosen as a first- or second-line ASM with good tolerability and absence of enzyme induction properties. Small studies report it as effective as add-on therapy,68 and in monotherapy associated with a seizure-free rate of 55% at 6 months.69
Eslicarbazepine is related to carbamazepine and oxcarbazepine with once-daily dosing, favorable side effects, and was studied as add-on therapy in a small TRE series with 25% of patients being seizure-free.70
Brivaracetam is an analog of levetiracetam and an option for patients who are unable to tolerate levetiracetam due to mood effects. No studies have evaluated brivaracetam as monotherapy for patients with TRE, although retrospective studies examined add-on therapy in patients with variable tumor types, with 60% seizure-free after a mean follow-up of 10 months.71
Clobazam is well tolerated and with slow titration patients report baseline energy after 1–2 weeks. Low doses (ie, 10–15 mg daily) are often sufficient for seizure-free and may allow weaning of other ASMs. As add-on therapy, 30% of patients were seizure-free at 6 months.72
Perampanel has a unique mechanism as a noncompetitive AMPA receptor blocker. In a systematic review, 8 small studies reported add-on perampanel with variable seizure-free rates of 0–94%.57 Further studies are necessary to evaluate the role of perampanel in TRE.
Finally, cenobamate has shown promise in patients with nononcologic medically refractory epilepsy, but has not been explored in patients with TRE.73 It is a CYP2C19 inhibitor and induces both CYP3A4 and CYP2B6, an important consideration for concurrent tumor-directed treatment.
Comorbidities Influencing Drug Choice
When considering ASMs for TRE, it is essential to consider comorbid conditions and potential side effects that have a profound impact on patients. Levetiracetam is often chosen as a first line in TRE given its minimal drug-drug interactions, rapid therapeutic titration, and general tolerability; however, irritability and mood changes are reported frequently. Psychiatric side effects are more commonly seen in patients with frontal lobe tumors and in patients with histories of depression and anxiety.74 If an ASM with mood-stabilizing properties is being considered, valproic acid, lamotrigine, oxcarbazepine, eslicarbazepine, or clobazam may be considered.
Patients with comorbid migraine headache may benefit from topiramate or zonisamide,75 although the doses required for prevention of seizures are higher. Valproic acid can also be considered for brain tumor patients with migraine; a Cochrane review found a meaningful reduction in headache frequency.76
Topiramate and zonisamide can result in weight loss but should be avoided in patients with a history of nephrolithiasis. Zonisamide should be avoided in patients with sulfa allergies due to cross-reactivity.
Pregnant patients with brain tumors can be started on levetiracetam immediately at a therapeutic dose. However, if a woman is planning pregnancy, both levetiracetam and lamotrigine are reasonable options due to the low risk of birth defects.77 All women considering pregnancy should be started on at least 0.4mg of folic acid daily to reduce fetal birth defects, autistic traits, and language delay.78 If on an enzyme-inducing ASM, a high-dose contraceptive containing at least 50 mcg of ethinyl estradiol should be considered as enzyme induction accelerates metabolism of both estrogen and progesterone.78 Additionally, a secondary method of birth control should be considered. Lamotrigine has a significant interaction with estrogen-containing oral contraceptives and higher doses may be required. Considering these interactions, monitoring ASM levels in pregnancy is recommended.
Drug–Drug interactions
Pharmacokinetic interactions between ASMs and antineoplastic therapies are an important consideration in the selection of therapy (Figure 3). A majority of drugs undergo hepatic metabolism through cytochrome P450 enzymes, particularly CYP3A4; ASMs that induce these microsomal enzymes can markedly alter the clearance of cytotoxic chemotherapy, targeted agents, and corticosteroids. Phenytoin, phenobarbital, and carbamazepine are the most potent inducers. Newer agents generally have lesser to no enzyme-inducing activity, although oxcarbazepine, eslicarbazepine, and cenobamate have clinically relevant activity.79–81
Figure 3.
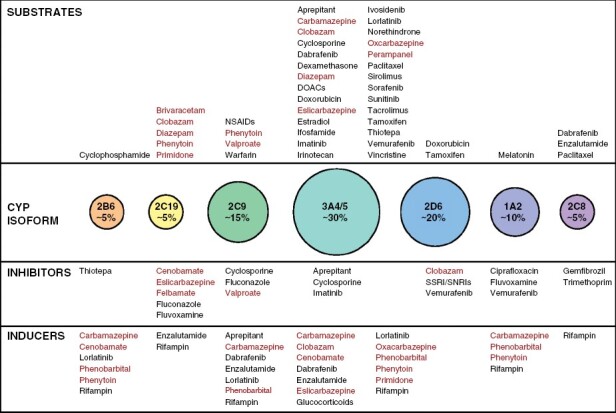
Summary of hepatic CYP isoform abundance and selected oncology and epilepsy-related substrates, inhibitors, and inducers.82 Only major CYP substrates are included (Lexicomp). ASMs are shown in red. Adapted from Fink & Deo, Handbook of Brain Tumor Chemotherapy, Molecular Therapeutics, and Immunotherapy.83
The impact of enzyme-inducing ASMs (EIASM) on chemotherapy exposure for drugs metabolized through CYP3A4 is considerable. Potent effects have been demonstrated for taxanes, irinotecan, and cyclophosphamide. The effects of strong EIASMs are seen with cyclophosphamide, camptothecin derivatives, taxanes, and topoisomerase II inhibitors, showing about a 2-fold to 3-fold higher clearance and a doubling of the maximum tolerated dose.84 Temozolomide is not metabolized through hepatic microsomal enzymes and has no pharmacokinetic interactions with ASMs.
Similar results pertain to EIASMs with tyrosine kinase, mTOR, proteasome, and IDH1 inhibitors, with a clearance rate often doubled and a corresponding decrease in AUC.85 Crizotinib, dasatinib, imatinib, lapatinib, and ivosidenib particularly show substantially faster metabolism with concurrent EIASMs. For imatinib and lapatinib, organ exposure is about 4 times lower without dose adjustment.84 Consequently, for the last 25 years neuro-oncologists and epileptologists have preferentially utilized nonEIASMs in patients with brain tumors.
EIASMs may increase the metabolism of various steroids. This is best studied in phenytoin, which increases plasma clearance of dexamethasone threefold.86
Prior to the availability of effective ASMs not metabolized through the hepatic microsomal system, early-phase glioma trials sometimes excluded patients on enzyme-inducing drugs or ran separate arms analyzing pharmacokinetics of the novel antineoplastic agent for patients on enzyme inducers. At present, the use of EIASMs is generally an exclusion criterion in clinical trials of chemotherapeutic agents metabolized through the hepatic microsomal system and should similarly be avoided in standard practice outside of clinical trials.
Adverse Effects of Anti-Seizure Medications
Optimizing the ASM regimen in TRE requires the treating physician to review side effects regularly. Distinguishing side effects from ASMs, oncologic treatments, and the underlying disease, may be challenging. Familiarity with the most common and serious side effects (Table 1) is necessary to optimize the ASM regimen.
Fatigue is common with ASMs, particularly during the first 2 weeks, and may improve gradually. However, if persistent, changing to an extended-release formulation or less sedating ASM should be considered.
Many side effects of ASMs are seen immediately after initiating the medication and checking serum levels approximately 2–4 weeks after reaching the target dose is recommended. Surveillance of hematologic, renal, and hepatic function tests is recommended. EIASMs carry the highest risk of blood dyscrasias (eg, aplastic anemia). In gliomas, hematotoxicity is more common with concurrent temozolomide or PCV chemotherapy.87 Hyponatremia is a common side effect of oxcarbazepine, carbamazepine, and eslicarbazepine, with an increased risk in older patients.88 Hepatotoxicity is common with carbamazepine, phenytoin, and valproic acid. The combination of topiramate and valproic acid increases the risk of hyperammonemia.
Stevens Johnson Syndrome (SJS), a major complication of certain aromatic ASMs, can occur with lamotrigine, phenytoin, carbamazepine, oxcarbazepine, and phenobarbital. If SJS occurs, avoidance of other aromatic ASMs is recommended. All ASMs portend a risk of cutaneous adverse drug reactions; for patients with sulfa allergies, zonisamide should be avoided due to cross-reactivity.
Cardiac side effects of ASMs are rare, although can be a potential side effect of all sodium channel blockers. Typically, an EKG is recommended prior to initiating lacosamide and repeated 1–2 weeks after the goal dose is reached to monitor for PR prolongation. Caution and potential avoidance of sodium channel blockers in patients with a known underlying arrhythmia is recommended.
Patients with epilepsy frequently have mood disorders and some ASMs may affect the risk of depression and suicidal ideation. In patients with TRE, mood inventories should be assessed regularly, and transition to mood-stabilizing or mood-neutral ASMs considered as needed.
Anti-Tumor Effects of Anti-Seizure Medications
In addition to their effects on controlling seizures, there has been longstanding interest in the potential antitumor effects of ASM.89 Initial focus centered on the potential antitumor effects of valproic acid (VPA). In a retrospective posthoc analysis of the European Organization for Research and Treatment of Cancer (EORTC) 26981–22981/National Cancer Institute of Canada (NCIC) trial that demonstrated the benefit of temozolomide in patients with newly diagnosed glioblastomas,90 patients who received VPA appeared to have increased survival from temozolomide and radiotherapy than patients receiving an EIAED only or patients not receiving any ASM.87 The mechanism was unclear but potentially related to the inhibitory effects of VPA on histone deacetylation or an effect of VPA in increasing temozolomide levels. This led to the suggestion that VPA may be preferred over an EIAED in patients with glioblastoma who require an ASM during temozolomide-based chemoradiotherapy. Another small retrospective study,91 and a single-arm phase II trial with high dose VPA,92 also suggested a possible survival benefit of VPA in patients with glioblastomas. However, a subsequent pooled analysis of 1869 patients from four randomized clinical trials in newly diagnosed glioblastoma found that VPA use at the start of chemoradiotherapy was not associated with improved progression-free survival (PFS) or overall survival (OS) compared with all other patients.93 Other studies also could not confirm a survival benefit with VPA.94
Levetiracetam is the most commonly used ASM for glioma patients. A number of retrospective and observational studies suggested that patients on levetiracetam may have improved outcomes compared to historic controls.95–97 In addition, a recent single-arm prospective study of 73 patients treated with levetiracetam reported improved survival compared to external controls.98 However, a meta-analysis of 5804 glioblastoma patients of which 1923 (33%) were treated with levetiracetam,99 including the study by Happold et al.93 failed to confirm that the drug increased survival. In the absence of definitive trials, it remains unclear whether levetiracetam has any antitumor effects. Levetiracetam does have antiemetic properties which may be a benefit to patients receiving temozolomide.100
Preclinical studies suggest that ASMs such as perampanel, which act via inhibition of AMPA receptors, may decrease glioma growth and have therapeutic potential.43,101 In a small study in which perampanel was used to control seizures in 12 glioma patients, there appeared to be a reduction in FLAIR signal in the majority of patients.102 Another AMPA receptor inhibitor, talampanel failed to show a therapeutic benefit in both newly diagnosed103 and recurrent glioblastoma patients.104 More definitive studies will be needed to evaluate the antitumor effect of perampanel in glioma patients.
There is currently insufficient data supporting the potential antitumor effects of any specific ASM. Further studies are warranted, especially related to ASMs that target neuronal-glioma interactions. Prospective studies dedicated to dose finding and estimating risk-benefit ratios are required because all ASMs come with relevant adverse effects when approaching the upper dosing ranges, and long-term exposure is probably required.
ASM Prophylaxis
In the absence of seizures, there is insufficient high-quality evidence to support treatment with ASM.105 Nonetheless, about 70% of tumor neurosurgeons routinely provide a short course of ASMs after craniotomy for the primary prevention of postoperative seizures.106,107 The lack of clinical equipoise in this context stems from the few under-powered randomized controlled trials in seizure-naïve patients with brain tumors comparing post operative phenytoin or phenobarbital to placebo with variable study designs.105,108 While none of these trials individually demonstrated a reduction in seizures with the use of ASM, in pooled meta-analysis, ASM treatment was associated with a decreased relative risk (RR 0.35, 95% CI 0.13–0.95) of early postoperative seizures, but not late seizures.108 The use of newer ASMs perceived to have fewer adverse effects has also contributed to this practice. Currently, the most common protocol is to treat with levetiracetam for 7 days after craniotomy and supratentorial tumor resection.
ASM Suggested Choices
Due to the high risk of seizure recurrence, it is recommended to initiate ASM treatment as soon as possible in patients with a brain tumor who experience a first seizure. Monotherapy is preferred to reduce long-term adverse effects and achieve drug adherence. Among experts in the field of neuro-oncology, levetiracetam is usually a first choice as monotherapy.109 Lacosamide and brivaracetam can be considered equivalent first choices, although there is still limited evidence to support the use of these third-generation ASMs as monotherapy in TRE. Lamotrigine could be an effective alternative option when slow titration is allowed, such as in patients with nondisabling focal seizures only or when there are no signs of tumor growth.
In patients with ongoing seizures, gradual dose escalation to the maximum tolerated dose should be pursued. Persistent seizures despite optimizing monotherapy should prompt the addition of a second ASM. To ensure maximum effectiveness of dual therapy, it is recommended to combine ASMs with different mechanisms of action, such as levetiracetam together with either valproic acid, lacosamide, lamotrigine, or perampanel. Levetiracetam combined with valproic acid has shown a better efficacy compared to other duotherapy combinations with either levetiracetam or valproic acid.110 As add-on, lacosamide and lamotrigine showed comparable efficacy and tolerability in patients with glioma.111,112 Similar results were found in smaller studies regarding effectiveness of brivaracetam and perampanel as add-on therapy.71,113 Other second-line drugs that can be considered as add-on treatment are topiramate, oxcarbazepine, and zonisamide.
Drug-resistant epilepsy is defined as “the failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom.”114 It is observed in approximately 40% of patients with low-grade glioma, compared to 10–15% of patients with glioblastoma.115 In patients with drug-resistant epilepsy, a critical review of ASM adherence is necessary. If other causes of treatment failure have been excluded, replacement of one ASM or ASM triple therapy are options depending on the level of seizure control and drug-related adverse effects. For example, the addition of a benzodiazepine such as clobazam as a third ASM could be considered (Figure 4).116
Figure 4.
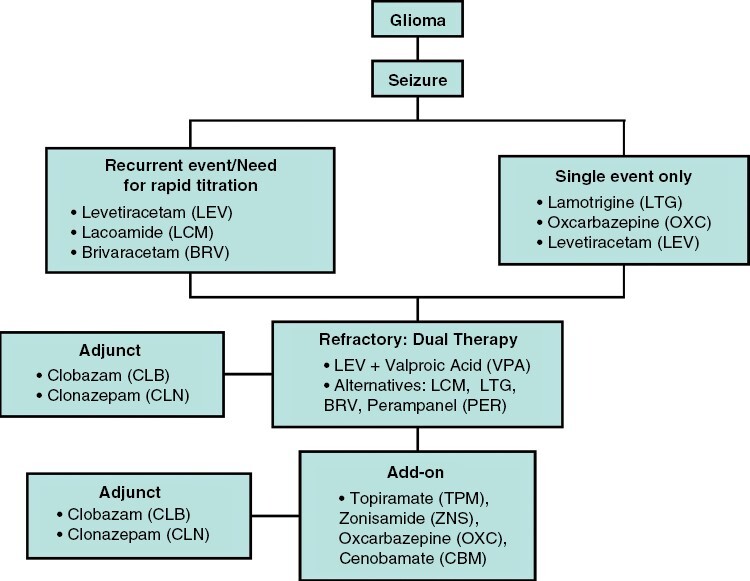
Proposed algorithm for ASM selection in glioma-related epilepsy. In polytherapy, ASMs with different mechanisms of action should be chosen to minimize adverse effects.
Dexamethasone is frequently prescribed in brain tumor patients to reduce symptom burden, preferably at the lowest effective dose to avoid long-term adverse effects. Although corticosteroids may facilitate seizure control, it is not recommended as the primary treatment for TRE. Caution is advised when tapering dexamethasone, as it may induce seizure relapse, requiring an increase in ASM dose.117 Initiation of bevacizumab in recurrent glioblastoma for radiation necrosis may prevent a dose increase or addition of ASM in patients with recurrent seizures.118 Systemic tumor-targeted treatment with temozolomide or bevacizumab in patients with stable disease should only be considered in carefully selected cases of drug-resistant epilepsy where different ASM polytherapy combinations have failed and surgery or local irradiation are not viable options.119
Due to a lack of high-quality evidence, there are no specific recommendations to guide ASM selection in subgroups of TRE such as patients with brain metastases or meningioma. Similar to patients with a glioma, nonEIASMs are generally recommended.105 In the end-of-life phase, when symptom burden increases, somnolence or dysphagia may hamper adequate ASM intake. ASMs such as intranasal midazolam, buccal clonazepam, and oral concentrated lorazepam can be easily administered by the patient’s caregiver. Subcutaneous midazolam or levetiracetam are other suitable alternatives to oral ASM treatment in an out-of-hospital setting. The administration route of choice in the end-of-life phase will eventually depend on the local availability of ASMs, the physician’s experience, and the place of care.120,121
There is limited evidence for the use of ASM in children with brain tumors and the management is generally similar to other children with epilepsy. Many reports in this population do not specify the ASMs used. A recent online survey revealed that only 18% of respondents would start an ASM after one seizure, whereas 94% would start an ASM after a second seizure; 84% chose levetiracetam as the first choice.10 We do not recommend waiting for a second seizure in children with brain tumors.
ASM Withdrawal
In patients who meet the ILAE criteria for epilepsy,15 prolonged treatment with ASM is indicated. This is particularly true for late postoperative seizures (>1 week after surgery) or recurrent seizures after completion of tumor therapy, for whom indefinite treatment is typically recommended.
A role for ASM tapering and withdrawal may be considered in select cases. Achieving at least 1–2 years of seizure freedom, similar to patients without TRE,122 is an appropriate criterion, although no evidence exists for optimal seizure-free periods in this population. Common reasons to consider ASM withdrawal include patient preference, fatigue, cognitive side effects, and polypharmacy. Several postsurgical studies support this approach in patients with preoperative TRE. Positive indicators for postoperative seizure freedom include a gross total resection, a short history of preoperative epilepsy, and completion of tumor-directed treatment with stable brain imaging.123,124 A meta-analysis involving 773 patients with low-grade gliomas and preoperative epilepsy with at least 6-month follow-up revealed an overall rate of postoperative seizure freedom in 71% of patients.123 Similarly, the 2-year postoperative seizure-free rate for patients with epilepsy-associated neuroepithelial tumors (n = 1325) in the European Epilepsy Brain Bank consortium was 77.5%, with 47% of those with long-term follow-up data having discontinued ASM at 5 years.125
If considered, ASM withdrawal requires shared decision-making between the patient and physician. If available, consultation from an epileptologist may be beneficial. Discussions should consider tumor pathology, prognosis, treatment, prior duration and associated disability of the patient’s epilepsy, current functional status, adverse effects of ASM, and driving restrictions. In the US, EEG is often used to guide ASM withdrawal. A meta-analysis across a range of epilepsy syndromes found that epileptiform EEG abnormalities during ASM withdrawal were associated with increased risk of seizure recurrence,126 however more data is needed for all epilepsy types, including TRE, regarding the timing and duration of EEG to guide ASM withdrawal.
In a prospective study of 71 patients with WHO grades 2–3 gliomas who were seizure-free for at least 1 year on ASMs, a shared decision-making process led to withdrawal of ASM in 65%, of whom 26% had seizure recurrence during follow-up compared to 8% who were continued on ASM.127 About half of patients with seizure recurrence had tumor progression. In a retrospective analysis of 169 patients with surgically treated meningiomas or low-grade gliomas, ASMs were withdrawn or never started in 66%, of whom 9.9% had seizure recurrence.128 The strongest independent predictor of seizure recurrence was the decision to continue ASM in the meningioma subset, indicating the importance of clinical judgment and consideration of patient-specific risk factors.
For other scenarios in TRE, there is minimal evidence to guide ASM withdrawal, including the prognostic indicators or optimal duration of ASM treatment. These scenarios include seizures in the early postoperative period (within 1 week after surgery) and patients with noninfiltrating or extra-axial tumors who have a single preoperative seizure and undergo gross total resection. In these cases, the authors’ practice is to extrapolate from data in acute symptomatic seizures of other etiologies that support a shorter course of ASM,129 with ranges up to 1 year determined by the treating physician’s clinical judgment and shared decision-making to review the risks, benefits, and rationale for a trial of ASM withdrawal.
Together, these data indicate that in appropriately chosen patients who are felt to be at low risk for seizure recurrence and who elect to trial withdrawal of ASM, the majority will remain seizure-free. However, in patients with residual or progressive disease and/or seizures after tumor-targeted treatment, ASM should be continued indefinitely.
Neurostimulation
Neuromodulation therapies are used for the adjunctive treatment of drug-resistant epilepsy and involve the chronic implantation of a neurostimulator device.130 Currently approved modalities include vagus nerve stimulation (VNS), open-loop deep brain stimulation (DBS), and on-demand/closed-loop responsive neurostimulation (RNS). While many patients experience a reduction in seizure frequency with these approaches, it typically requires multiple years to achieve maximal efficacy, and complete seizure control is uncommon. Registry and prospective long-term follow-up data suggest improved outcomes the longer VNS, RNS, and DBS are in place.131–133 The latest models have MRI compatibility up to 3T for VNS/DBS and 1.5T for RNS, however require specialized programming at the time of each scan, and in the case of RNS and DBS, the device limits imaging interpretation adjacent to the battery and strip or depth electrodes. For these reasons, RNS is generally not considered a treatment option for adults with WHO grade 2, 3, or 4 infiltrating tumors. Given the intracranial targeting for RNS and DBS, there is also a hypothetical risk of tumor cell seeding along the electrode tracts.134
Accordingly, little data exists in the brain tumor population, and neuromodulation is generally not appropriate for patients who require ongoing surveillance imaging or in whom future tumor surgery may be indicated. Data derived from the VNS Therapy Patient Outcome Registry showed improved seizure control in patients with brain tumors at 3 months, with further improvement at 24 months, comparable to those without brain tumors.135 Other reports indicate a better response to VNS in patients with stable tumors compared to those with tumor progression.136 Evidence supporting RNS and DBS specifically in the brain tumor population is lacking, although in a small case series with tuberous sclerosis complex RNS was safe and effective with a 58% median reduction of disabling seizures at 12 months and 88% at the last follow-up.137 At this time, we reserve consideration for neuromodulation to patients with remote tumors who achieved gross total resection and are no longer undergoing regular tumor surveillance, or have demonstrated prolonged tumor stability, yet who have frequent disabling seizures despite multiple trials of ASMs.
Surgery
Surgical excision of the tumor is the most effective treatment strategy for managing most patients with TRE and is associated with improved overall survival.7 When compared with subtotal resection, gross-total resection (GTR) is most predictive of postoperative seizure freedom across a range of pathologies including gliomas, metastases, and meningiomas.138 However, ~20% of LEAT and ~40% of low-grade glioma patients have persistent seizures following maximal resection.125
Intraoperative electrocorticography (iECoG) is an electrophysiologic means to delineate epileptogenicity in patients with TRE. It is designed to aid surgical resection and improve seizure outcomes.139 Although the use of iECoG has conflicting results in the general surgical epilepsy population,140,141 iECoG significantly improves seizure-free rates in glioma patients.142 One meta-analysis of 1115 patients with drug-resistant TRE involving low-grade neoplasms, found iECoG-guided resection led to greater postoperative seizure freedom compared with lesionectomy alone.143 iECoG recording technique, electrode type, and placement varies and may impact the location, morphology, and distribution of epileptiform abnormalities.144,145 Therefore, individualizing iECoG to ensure adequate recording time, appropriate electrode usage, and biomarkers by covering both tumor and peritumoral tissue favor improved postoperative outcomes.141
When patients with TRE should undergo surgical implantation with subdural or stereotactic depth (SEEG) electrodes to better determine the seizure onset zone remains unclear and varies considerably between centers. The increasing use of SEEG, which is less invasive and safer than subdural electrodes, is generating considerable interest in the use of “epilepsy surgery” to improve seizure outcomes in TRE.
Radiotherapy
The frequency of seizures in patients with gliomas may be reduced by both radiotherapy and chemotherapy.146,147 Few studies have analyzed the impact of radiotherapy on TRE. Stereotactic interstitial irradiation was reported to improve seizure control in 40–100% of unresectable low-grade gliomas,58,148 and attributed to increased benzodiazepine receptor density. Gamma-knife radiosurgery for mesiotemporal TRE, aiming to irradiate the presumed peritumoral epileptic foci, has been effective.149 Conformal external radiotherapy achieved seizure control in 75–100% of patients with medically intractable epilepsy and low-grade gliomas.147,150 Both after interstitial and conformal external radiotherapy of low-grade gliomas, seizure reduction may begin early during treatment, even with stable disease on MRI. For example, among patients who had a significant reduction in seizures after radiotherapy, 25-68% had stable disease.147,150 Thus, in addition to a reduction of tumor burden, the effects of ionizing radiation on seizure control suggest damage to epileptogenic neurons in the peritumoral tissue or metabolic changes in the microenvironment.150 An indirect suggestion of the efficacy of conformal radiotherapy on seizures comes from the EORTC 22845 phase III trial, which compared adjuvant postoperative radiotherapy versus observation in low-grade gliomas; at 1 year, 25% of irradiated patients had seizures compared to 41% of those untreated.151
The impact of radiotherapy on seizure control in brain metastases152,153 and in meningiomas154 has not yet been investigated. Conversely, seizures may represent an adverse event following stereotactic radiosurgery for brain metastases. A recent large retrospective study has reported an 8–12% risk of seizures within 3 months of treatment, with total irradiated volume and pretreatment neurological symptoms as the major risk factors.155
Chemotherapy
Chemotherapy with alkylating agents in low-grade gliomas has been associated with improved seizure control.146,156,157 Seizure reduction has been reported in 13–60% of patients treated with PCV and 13–50% treated with temozolomide. The reduction of seizures may be evident early within the first months of treatment and is attributable to decreased infiltration of tumor cells at the periphery.158 Similar to radiotherapy, seizure reduction after chemotherapy may occur also in association with a stable disease on MRI. The rate of seizure freedom at 12 months seems higher than that observed after radiotherapy. Temozolomide in association with hypofractionated radiotherapy has a modest efficacy in seizure control compared to radiotherapy alone in elderly patients with glioblastoma.159 Thus far, there are no data on the effects of chemotherapeutics on seizures from brain metastases.
Targeted Therapy
Common pathways of tumor growth and epileptogenesis exist and may be successfully targeted with specific inhibitors. The best example is everolimus which targets the hyperactive mTOR pathway in subependymal giant cells astrocytomas of tuberous sclerosis, leading to control of both tumor growth and seizures.160,161
Another potential approach being investigated is the targeting of IDH1/2 mutations with specific inhibitors (ivosidenib, vorasidenib). Despite increasing knowledge of basic mechanisms underlying the relationship between IDH mutations and seizures, clinical data supporting the use of IDH inhibitors for seizure control are scarce and limited to a case report.162 The phase 3 INDIGO trial compared the use of an IDH1/2 inhibitor (vorasidenib) versus placebo in grade 2 gliomas with a residual or recurrent tumor after surgery. In this trial, seizure control has been included as a secondary endpoint, which may add new insights concerning the potential impact of IDH inhibitors on seizures.
Future Directions
The incorporation of seizure outcome metrics into neuro-oncology trials is necessary to advance management strategies and clinical outcomes in TRE. As biomarker, seizure frequency during and after tumor therapy should be tracked,163 however requires accurate seizure reporting. The development of novel seizure detection technologies may aid in the identification of seizures. For example, noninvasive wearables to detect peri-ictal physiologic parameters and chronically implanted EEG electrodes have shown promise in select epilepsy cohorts and may complement patient/caregiver seizure logs.164,165
Many studies of TRE to date focus on seizures around the time of tumor diagnosis, and electrophysiologic data to complement the epilepsy phenotype are lacking in neuro-oncology cohorts. Therefore, the definitions of tumor-related hyperexcitability are widely variable, and it is likely that multiple mechanisms and risk factors contribute to seizures at different times over the course of treatment and progression. The development and standardization of noninvasive hyperexcitability measures will offer greater prognostic value, as suggested by selected reports of EEG and magnetoencephalographic hyperexcitability associated with survival outcomes in patients with glioma,166–168 although further validation is warranted.169
NonEIASMs are central to the management of TRE, however minimal high-quality evidence exists to support the use of one drug over another. Randomized controlled trials with comprehensive epilepsy phenotyping are needed to optimize the standard of care (NCT01432171, NCT03048084). Additionally, controlled trials to determine the optimal dosage of ASMs, approach to monotherapy vs. polytherapy, duration of treatment, and protocols for ASM withdrawal are necessary. With mechanistic and pharmacologic advances, targeting common pathways of tumor growth and epileptogenesis will hopefully improve quality of life and neurocognitive functions in addition to survival outcomes.153,170
Conclusion
Seizures occur frequently in patients with brain tumors; however, the underlying mechanisms remain incompletely understood. Recent evidence indicates a complex interplay within the tumor microenvironment with converging molecular and physiologic pathways for tumor growth and epileptogenesis. These relationships highlight the importance of tracking seizure outcomes concurrently with tumor surveillance in clinical practice and in controlled trials. Seizure management relies primarily on the use of nonenzyme-inducing ASMs with careful consideration of tumor stability and tumor-directed therapy. As such, a multidisciplinary team is recommended for the optimal management of seizures from the diagnostic approach to the personalization of therapeutic options. Achieving a balance between maximal seizure control and minimal adverse effects of ASM is necessary to optimize quality of life. Further advances in identifying seizure-related biomarkers and mechanisms across tumor pathologies will facilitate targeted treatment strategies impacting both oncologic and epilepsy outcomes.
Contributor Information
Edward K Avila, Department of Neurology, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Steven Tobochnik, Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Department of Neurology, VA Boston Healthcare System, Boston, Massachusetts, USA.
Sara K Inati, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA.
Johan A F Koekkoek, Department of Neurology, Leiden University Medical Center, Leiden, The Netherlands; Department of Neurology, Haaglanden Medical Center, The Hague, The Netherlands.
Guy M McKhann, Department of Neurosurgery, Vagelos College of Physicians and Surgeons, Columbia University Irving Medical Center, New York, New York, USA.
James J Riviello, Division of Neurology and Developmental Neuroscience, Department of Pediatrics, Baylor College of Medicine, Texas Children’s Hospital, Houston, Texas, USA.
Roberta Rudà, Division of Neuro-Oncology, Department of Neuroscience “Rita Levi Montalcini,” University of Turin, Italy.
David Schiff, Department of Neurology, Division of Neuro-Oncology, University of Virginia School of Medicine, Charlottesville, Virginia, USA.
William O Tatum, Department of Neurology, Mayo Clinic, Jacksonville, Florida, USA.
Jessica W Templer, Department of Neurology, Northwestern University, Chicago, Illinois, USA.
Michael Weller, Department of Neurology, Clinical Neuroscience Centre, University Hospital Zurich and University of Zurich, Zurich, Switzerland.
Patrick Y Wen, Center for Neuro-Oncology, Dana-Farber Cancer Center, and Division of Neuro-Oncology, Department of Neurology, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Funding
This work was supported by a grant from the National Institutes of Health P30-A008748.
Conflict of interest statement
Dr. Avila—honoraria from UpToDate. Dr. Tobochnik—research support from Eisai. Dr. Inati—no conflicts to report. Dr. Koekkoek—no conflicts to report. Dr. McKhann—receives funding from Citizens United for Research in Epilepsy (CURE). Dr. Riviello—no conflicts to report. Dr. Ruda—no conflicts to report. Dr. Schiff—no conflicts to report. Dr. Tatum—receives research support from Mayo Clinic, Esai Inc, Liva Nova, Martin Family Foundation, McElvey Foundation. Patents held or pending: #62527896; #62770362 (intraoperative monitoring sensor devices. Dr. Templer—no conflicts to report. Dr. Weller—has received research grants from Quercis and Versameb, and honoraria for lectures or advisory board participation or consulting from Bayer, Curevac, Medac, Novartis, Novocure, Orbus, Philogen, Roche, and Sandoz. Dr. Wen—Research Support—Astra Zeneca, Black Diamond, Bristol Meyers Squibb, Celgene, Chimerix, Eli Lily, Erasca, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Servier, Vascular Biogenics, VBI Vaccines. Advisory Board/Consultant -Astra Zeneca, Black Diamond, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, VBI Vaccines.
References
- 1. Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–1893. [DOI] [PubMed] [Google Scholar]
- 2. Lote K, Stenwig AE, Skullerud K, Hirschberg H.. Prevalence and prognostic significance of epilepsy in patients with gliomas. Eur J Cancer. 1998;34(1):98–102. [DOI] [PubMed] [Google Scholar]
- 3. Li L, Fang S, Li G, et al. Glioma-related epilepsy in patients with diffuse high-grade glioma after the 2016 WHO update: seizure characteristics, risk factors, and clinical outcomes. J Neurosurg. 2022;136(1):67–75. [DOI] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Wagle NS, Jemal A.. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 5. Miller KD, Ostrom QT, Kruchko C, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021;71(5):381–406. [DOI] [PubMed] [Google Scholar]
- 6. Ostrom QT, Price M, Neff C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022;24(Suppl 5):v1–v95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Breemen MSM, Wilms EB, Vecht CJ.. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 8. van Breemen MSM, Rijsman RM, Taphoorn MJB, et al. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256(9):1519–1526. [DOI] [PubMed] [Google Scholar]
- 9. Villemure JG, de Tribolet N.. Epilepsy in patients with central nervous system tumors. Curr Opin Neurol. 1996;9(6):424–428. [DOI] [PubMed] [Google Scholar]
- 10. Malbari F, Zhu H, Riviello JJ, Clarke D.. Antiepileptic drug management in pediatric patients with brain tumor-related epilepsy. Epilepsy Behav. 2021;125(December):108359. [DOI] [PubMed] [Google Scholar]
- 11. Fattal-Valevski A, Nissan N, Kramer U, Constantini S.. Seizures as the clinical presenting symptom in children with brain tumors. J Child Neurol. 2013;28(3):292–296. [DOI] [PubMed] [Google Scholar]
- 12. Tsai ML, Chen CL, Hsieh KLC, et al. Seizure characteristics are related to tumor pathology in children with brain tumors. Epilepsy Res. 2018;147(November):15–21. [DOI] [PubMed] [Google Scholar]
- 13. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krumholz A, Wiebe S, Gronseth GS, et al. Evidence-based guideline: management of an unprovoked first seizure in adults. Neurology. 2015;84(16):1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482. [DOI] [PubMed] [Google Scholar]
- 16. Ersoy TF, Ridwan S, Grote A, Coras R, Simon M.. Early postoperative seizures (EPS) in patients undergoing brain tumour surgery. Sci Rep. 2020;10(1):13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horiuchi S, Kanaya K, Horiuchi T.. The occurrence and relationship of postoperative seizure and de novo epilepsy after craniotomy surgery: a retrospective single-center cohort study. Front Surg. 2022;9(April):881874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehrotra A, Singh S, Kanjilal S, et al. Long-term epilepsy-associated tumors (LEATs): a single-center, retrospective series and review of literature on factors affecting the seizure outcome. World Neurosurg. 2020;144(December):e149–e155. [DOI] [PubMed] [Google Scholar]
- 19. Lin AL, Avila EK.. Neurologic emergencies in the patients with cancer. J Intensive Care Med. 2017;32(2):99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaul N, Green L, Peyster R, Gotman J.. Structural determinants of electroencephalographic findings in acute hemispheric lesions. Ann Neurol. 1986;20(6):703–711. [DOI] [PubMed] [Google Scholar]
- 21. Preuß M, Preiss S, Syrbe S, et al. Signs and symptoms of pediatric brain tumors and diagnostic value of preoperative EEG. Childs Nerv Syst. 2015;31(11):2051–2054. [DOI] [PubMed] [Google Scholar]
- 22. Tatum WO, Halford JJ, Olejniczak P, et al. Minimum technical requirements for performing ambulatory EEG. J Clin Neurophysiol. 2022;39(6):435–440. [DOI] [PubMed] [Google Scholar]
- 23. Hirsch LJ, Fong MWK, Leitinger M, et al. American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2021 version. J Clin Neurophysiol. 2021;38(1):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spindler M, Jacks LM, Chen X, et al. Spectrum of nonconvulsive status epilepticus in patients with cancer. J Clin Neurophysiol. 2013;30(4):339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hormigo A, Liberato B, Lis E, DeAngelis LM.. Nonconvulsive status epilepticus in patients with cancer: imaging abnormalities. Arch Neurol. 2004;61(3):362–365. [DOI] [PubMed] [Google Scholar]
- 26. Kramer RE, Lüders H, Lesser RP, et al. Transient focal abnormalities of neuroimaging studies during focal status epilepticus. Epilepsia. 1987;28(5):528–532. [DOI] [PubMed] [Google Scholar]
- 27. Riela AR, Sires BP, Penry JK.. Transient magnetic resonance imaging abnormalities during partial status epilepticus. J Child Neurol. 1991;6(2):143–145. [DOI] [PubMed] [Google Scholar]
- 28. Juhász C, Mittal S.. Molecular imaging of brain tumor-associated epilepsy. Diagn. 2020;10(12):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Law I, Albert NL, Arbizu J, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hutterer M, Ebner Y, Riemenschneider MJ, et al. Epileptic activity increases cerebral amino acid transport assessed by 18F-fluoroethyl-l-tyrosine amino acid PET: a potential brain tumor mimic. J Nucl Med. 2017;58(1):129–137. [DOI] [PubMed] [Google Scholar]
- 31. Gill BJA, Wu X, Khan FA, et al. Ex vivo multi-electrode analysis reveals spatiotemporal dynamics of ictal behavior at the infiltrated margin of glioma. Neurobiol Dis. 2020;134(February):104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takano T, Lin JHC, Arcuino G, et al. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7(9):1010–1015. [DOI] [PubMed] [Google Scholar]
- 33. Buckingham SC, Campbell SL, Haas BR, et al. Glutamate release by primary brain tumors induces epileptic activity. Nat Med. 2011;17(10):1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ye ZC, Sontheimer H.. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59(17):4383–4391. [PubMed] [Google Scholar]
- 35. Ye ZC, Rothstein JD, Sontheimer H.. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19(24):10767–10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuen TI, Morokoff AP, Bjorksten A, et al. Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology. 2012;79(9):883–889. [DOI] [PubMed] [Google Scholar]
- 37. Ishiuchi S, Tsuzuki K, Yoshida Y, et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8(9):971–978. [DOI] [PubMed] [Google Scholar]
- 38. Oh MC, Kim JM, Safaee M, et al. Overexpression of calcium-permeable glutamate receptors in glioblastoma derived brain tumor initiating cells. PLoS One. 2012;7(10):e47846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee HHC, Deeb TZ, Walker JA, Davies PA, Moss SJ.. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor mediated currents. Nat Neurosci. 2011;14(6):736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pallud J, Le Van Quyen M, Bielle F, et al. Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med. 2014;6(244):244ra89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacKenzie G, O’Toole KK, Moss SJ, Maguire J.. Compromised GABAergic inhibition contributes to tumor-associated epilepsy. Epilepsy Res. 2016;126(October):185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Venkatesh HS, Tam LT, Woo PJ, et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature. 2017;549(7673):533–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532–538. [DOI] [PubMed] [Google Scholar]
- 45. Chen H, Judkins J, Thomas C, et al. Mutant IDH1 and seizures in patients with glioma. Neurology. 2017;88(19):1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang Y, Mao Q, Wang X, et al. An analysis of 170 glioma patients and systematic review to investigate the association between IDH-1 mutations and preoperative glioma-related epilepsy. J Clin Neurosci. 2016;31(September):56–62. [DOI] [PubMed] [Google Scholar]
- 47. Mortazavi A, Fayed I, Bachani M, et al. IDH-mutated gliomas promote epileptogenesis through d-2-hydroxyglutarate-dependent mTOR hyperactivation. Neuro-Oncol. 2022;24(9):1423–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu K, Lin CCJ, Hatcher A, et al. PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis. Nature. 2020;578(7793):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tobochnik S, Pisano W, Lapinskas E, Ligon KL, Lee JW.. Effect of PIK3CA variants on glioma-related epilepsy and response to treatment. Epilepsy Res. 2021;175(September):106681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koh HY, Kim SH, Jang J, et al. BRAF somatic mutation contributes to intrinsic epileptogenicity in pediatric brain tumors. Nat Med. 2018;24(11):1662–1668. [DOI] [PubMed] [Google Scholar]
- 51. Prabowo AS, Iyer AM, Veersema TJ, et al. BRAF V600E mutation is associated with mTOR signaling activation in glioneuronal tumors. Brain Pathol. 2013;24(1):52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Englot DJ, Magill ST, Han SJ, et al. Seizures in supratentorial meningioma: a systematic review and meta-analysis. J Neurosurg. 2016;124(6):1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gupte TP, Li C, Jin L, et al. Clinical and genomic factors associated with seizures in meningiomas. J Neurosurg. 2020;135(3):1–10. [DOI] [PubMed] [Google Scholar]
- 54. Schnepp PM, Lee DD, Guldner IH, et al. GAD1 upregulation programs aggressive features of cancer cell metabolism in the brain metastatic microenvironment. Cancer Res. 2017;77(11):2844–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eichler AF, Chung E, Kodack DP, et al. The biology of brain metastases—translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rossetti AO, Jeckelmann S, Novy J, et al. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A phase II randomized study. Neuro-Oncol. 2014;16(4):584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tabaee Damavandi P, Pasini F, Fanella G, et al. Perampanel in brain tumor-related epilepsy: a systematic review. Brain Sci. 2023;13(2):326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Warnke PC, Berlis A, Weyerbrock A, Ostertag CB.. Significant reduction of seizure incidence and increase of benzodiazepine receptor density after interstitial radiosurgery in low-grade gliomas. Acta Neurochir Suppl. 1997;68(12):90–92. [DOI] [PubMed] [Google Scholar]
- 59. de Bruin ME, van der Meer PB, Dirven L, Taphoorn MJB, Koekkoek JAF.. Efficacy of antiepileptic drugs in glioma patients with epilepsy: a systematic review. Neuro-Oncol Pract. 2021;8(5):501–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. You G, Sha ZY, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro-Oncol. 2012;14(2):230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van der Meer PB, Maschio M, Dirven L, Taphoorn MJB, Koekkoek JAF; Italian League Against Epilepsy Brain Tumor-Related Epilepsy Study Group. Italian League Against Epilepsy Brain Tumor-Related Epilepsy Study Group. First-line levetiracetam versus enzyme-inducing antiseizure medication in glioma patients with epilepsy. Epilepsia. 2023;64(1):162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;2017(4):CD010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maschio M, Dinapoli L, Zarabla A, et al. Outcome and tolerability of topiramate in brain tumor associated epilepsy. J Neurooncol. 2008;86(1):61–70. [DOI] [PubMed] [Google Scholar]
- 64. Maschio M, Dinapoli L, Zarabla A, et al. Zonisamide in brain tumor-related epilepsy: an observational pilot study. Clin Neuropharmacol. 2017;40(3):113–119. [DOI] [PubMed] [Google Scholar]
- 65. Maschio M, Dinapoli L, Vidiri A, et al. The role side effects play in the choice of antiepileptic therapy in brain tumor-related epilepsy: a comparative study on traditional antiepileptic drugs versus oxcarbazepine. J Exp Clin Cancer Res CR. 2009;28(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maschio M, Dinapoli L, Sperati F, et al. Oxcarbazepine monotherapy in patients with brain tumor-related epilepsy: open-label pilot study for assessing the efficacy, tolerability and impact on quality of life. J Neurooncol. 2012;106(3):651–656. [DOI] [PubMed] [Google Scholar]
- 67. Perry JR, Sawka C.. Add-on gabapentin for refractory seizures in patients with brain tumours. Can J Neurol Sci. 1996;23(2):128–131. [DOI] [PubMed] [Google Scholar]
- 68. Villanueva V, Saiz-Diaz R, Toledo M, et al. NEOPLASM study: real-life use of lacosamide in patients with brain tumor-related epilepsy. Epilepsy Behav. 2016;65(December):25–32. [DOI] [PubMed] [Google Scholar]
- 69. Mo F, Meletti S, Belcastro V, et al. Lacosamide in monotherapy in BTRE (brain tumor-related epilepsy): results from an Italian multicenter retrospective study. J Neurooncol. 2022;157(3):551–559. [DOI] [PubMed] [Google Scholar]
- 70. Zoccarato M, Basile AM, Padovan M, et al. Eslicarbazepine in patients with brain tumor-related epilepsy: a single-center experience. Int J Neurosci. 2021;131(9):879–884. [DOI] [PubMed] [Google Scholar]
- 71. Maschio M, Maialetti A, Mocellini C, et al. Effect of brivaracetam on efficacy and tolerability in patients with brain tumor-related epilepsy: a retrospective multicenter study. Front Neurol. 2020;11(August):813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brahmbhatt N, Stupp R, Bushara O, et al. Efficacy of clobazam as add-on therapy in brain tumor-related epilepsy. J Neurooncol. 2021;151(2):287–293. [DOI] [PubMed] [Google Scholar]
- 73. Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38–48. [DOI] [PubMed] [Google Scholar]
- 74. Belcastro V, Pisani LR, Bellocchi S, et al. Brain tumor location influences the onset of acute psychiatric adverse events of levetiracetam therapy: an observational study. J Neurol. 2017;264(5):921–927. [DOI] [PubMed] [Google Scholar]
- 75. Bagnato F, Good J.. The use of antiepileptics in migraine prophylaxis. Headache. 2016;56(3):603–615. [DOI] [PubMed] [Google Scholar]
- 76. Linde M, Mulleners WM, Chronicle EP, McCrory DC.. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;2013(6):CD010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Blotière PO, Raguideau F, Weill A, et al. Risks of 23 specific malformations associated with prenatal exposure to 10 antiepileptic drugs. Neurology. 2019;93(2):e167–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Meador KJ.. Epilepsy and pregnancy. Continuum. 2022;28(1):34–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Greene SA, Kwak C, Kamin M, et al. Effect of cenobamate on the single-dose pharmacokinetics of multiple cytochrome P450 probes using a cocktail approach in healthy subjects. Clin Transl Sci. 2022;15(4):899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Andreasen AH, Brøsen K, Damkier P.. A comparative pharmacokinetic study in healthy volunteers of the effect of carbamazepine and oxcarbazepine on cyp3a4. Epilepsia. 2007;48(3):490–496. [DOI] [PubMed] [Google Scholar]
- 81. Falcão A, Pinto R, Nunes T, Soares-da-Silva P.. Effect of repeated administration of eslicarbazepine acetate on the pharmacokinetics of simvastatin in healthy subjects. Epilepsy Res. 2013;106(1-2):244–249. [DOI] [PubMed] [Google Scholar]
- 82. Zanger UM, Schwab M.. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138(1):103–141. [DOI] [PubMed] [Google Scholar]
- 83. Fink KL, Deo AK.. Chapter 3 - antiepileptic drugs and chemotherapy: potential interactions and impact on treatment of patients with cancer. In: Newton HB, ed. Handbook of Brain Tumor Chemotherapy, Molecular Therapeutics, and Immunotherapy. 2nd ed. UK and USA: Academic Press; 2018:45–58. doi: 10.1016/B978-0-12-812100-9.00003-6 [DOI] [Google Scholar]
- 84. Bénit CP, Vecht CJ.. Seizures and cancer: drug interactions of anticonvulsants with chemotherapeutic agents, tyrosine kinase inhibitors and glucocorticoids. Neuro-Oncol Pract. 2016;3(4):245–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Megías-Vericat JE, Solana-Altabella A, Ballesta-López O, Martínez-Cuadrón D, Montesinos P.. Drug-drug interactions of newly approved small molecule inhibitors for acute myeloid leukemia. Ann Hematol. 2020;99(9):1989–2007. [DOI] [PubMed] [Google Scholar]
- 86. Chalk JB, Ridgeway K, Brophy T, Yelland JD, Eadie MJ.. Phenytoin impairs the bioavailability of dexamethasone in neurological and neurosurgical patients. J Neurol Neurosurg Psychiatry. 1984;47(10):1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Weller M, Gorlia T, Cairncross JG, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77(12):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Berghuis B, van der Palen J, de Haan GJ, et al. ; EpiPGX Consortium. Carbamazepine- and oxcarbazepine-induced hyponatremia in people with epilepsy. Epilepsia. 2017;58(7):1227–1233. [DOI] [PubMed] [Google Scholar]
- 89. Aronica E, Ciusani E, Coppola A, et al. Epilepsy and brain tumors: two sides of the same coin. J Neurol Sci. 2023;446(March):120584. [DOI] [PubMed] [Google Scholar]
- 90. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 91. Redjal N, Reinshagen C, Le A, et al. Valproic acid, compared to other antiepileptic drugs, is associated with improved overall and progression-free survival in glioblastoma but worse outcome in grade II/III gliomas treated with temozolomide. J Neurooncol. 2016;127(3):505–514. [DOI] [PubMed] [Google Scholar]
- 92. Krauze AV, Myrehaug SD, Chang MG, et al. A phase 2 study of concurrent radiation therapy, temozolomide, and the histone deacetylase inhibitor valproic acid for patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2015;92(5):986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Happold C, Gorlia T, Chinot O, et al. Does valproic acid or levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J Clin Oncol. 2016;34(7):731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Knudsen-Baas KM, Engeland A, Gilhus NE, Storstein AM, Owe JF.. Does the choice of antiepileptic drug affect survival in glioblastoma patients? J Neurooncol. 2016;129(3):461–469. [DOI] [PubMed] [Google Scholar]
- 95. Jabbarli R, Ahmadipour Y, Rauschenbach L, et al. How about levetiracetam in glioblastoma? An institutional experience and meta-analysis. Cancers. 2021;13(15):3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pallud J, Huberfeld G, Dezamis E, et al. Effect of levetiracetam use duration on overall survival of isocitrate dehydrogenase wild-type glioblastoma in adults: an observational study. Neurology. 2022;98(2):e125–e140. [DOI] [PubMed] [Google Scholar]
- 97. Kim YH, Kim T, Joo JD, et al. Survival benefit of levetiracetam in patients treated with concomitant chemoradiotherapy and adjuvant chemotherapy with temozolomide for glioblastoma multiforme. Cancer. 2015;121(17):2926–2932. [DOI] [PubMed] [Google Scholar]
- 98. Hwang K, Kim J, Kang SG, et al. Levetiracetam as a sensitizer of concurrent chemoradiotherapy in newly diagnosed glioblastoma: an open-label phase 2 study. Cancer Med. 2022;11(2):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen JS, Clarke R, Haddad AF, et al. The effect of levetiracetam treatment on survival in patients with glioblastoma: a systematic review and meta-analysis. J Neurooncol. 2022;156(2):257–267. [DOI] [PubMed] [Google Scholar]
- 100. Seystahl K, Oppong FB, Le Rhun E, et al. Associations of levetiracetam use with the safety and tolerability profile of chemoradiotherapy for patients with newly diagnosed glioblastoma. Neuro-Oncol Adv. 2022;4(1):vdac112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mayer J, Kirschstein T, Resch T, et al. Perampanel attenuates epileptiform phenotype in C6 glioma. Neurosci Lett. 2020;715(January):134629. [DOI] [PubMed] [Google Scholar]
- 102. Izumoto S, Miyauchi M, Tasaki T, et al. Seizures and tumor progression in glioma patients with uncontrollable epilepsy treated with perampanel. Anticancer Res. 2018;38(7):4361–4366. [DOI] [PubMed] [Google Scholar]
- 103. Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009;27(25):4155–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Iwamoto FM, Kreisl TN, Kim L, et al. Phase 2 trial of talampanel, a glutamate receptor inhibitor, for adults with recurrent malignant gliomas. Cancer. 2010;116(7):1776–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Walbert T, Harrison RA, Schiff D, et al. SNO and EANO practice guideline update: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro-Oncol. 2021;23(11):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Siomin V, Angelov L, Li L, Vogelbaum MA.. Results of a survey of neurosurgical practice patterns regarding the prophylactic use of anti-epilepsy drugs in patients with brain tumors. J Neurooncol. 2005;74(2):211–215. [DOI] [PubMed] [Google Scholar]
- 107. Dewan MC, Thompson RC, Kalkanis SN, Barker FG, Hadjipanayis CG.. Prophylactic antiepileptic drug administration following brain tumor resection: results of a recent AANS/CNS Section on Tumors survey. J Neurosurg. 2017;126(6):1772–1778. [DOI] [PubMed] [Google Scholar]
- 108. Joiner EF, Youngerman BE, Hudson TS, et al. Effectiveness of perioperative antiepileptic drug prophylaxis for early and late seizures following oncologic neurosurgery: a meta-analysis. J Neurosurg. 2018;130(4):1274–1282. [DOI] [PubMed] [Google Scholar]
- 109. van der Meer PB, Dirven L, van den Bent MJ, et al. Prescription preferences of antiepileptic drugs in brain tumor patients: an international survey among EANO members. Neuro-Oncol Pract. 2022;9(2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. van der Meer PB, Dirven L, Fiocco M, et al. Effectiveness of antiseizure medication duotherapies in patients with glioma: a multicenter observational cohort study. Neurology. 2022;99(10):e999–e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. van Opijnen MP, van der Meer PB, Dirven L, et al. The effectiveness of antiepileptic drug treatment in glioma patients: lamotrigine versus lacosamide. J Neurooncol. 2021;154(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rudà R, Houillier C, Maschio M, et al. Effectiveness and tolerability of lacosamide as add-on therapy in patients with brain tumor-related epilepsy: results from a prospective, noninterventional study in European clinical practice (VIBES). Epilepsia. 2020;61(4):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Coppola A, Zarabla A, Maialetti A, et al. Perampanel confirms to be effective and well-tolerated as an add-on treatment in patients with brain tumor-related epilepsy (PERADET Study). Front Neurol. 2020;11(June):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–1077. [DOI] [PubMed] [Google Scholar]
- 115. Jo J, Nevel K, Sutyla R, et al. Predictors of early, recurrent, and intractable seizures in low-grade glioma. Neuro-Oncol Pract. 2021;8(1):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. van der Meer PB, Dirven L, Fiocco M, et al. Effectiveness of antiseizure medication triple therapy in patients with glioma with refractory epilepsy: an observational cohort study. Neurology. 2023;100(14):e1488–e1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Toledo M, Molins A, Quintana M, et al. Outcome of cancer-related seizures in patients treated with lacosamide. Acta Neurol Scand. 2018;137(1):67–75. [DOI] [PubMed] [Google Scholar]
- 118. Hertler C, Seystahl K, Le Rhun E, et al. Improved seizure control in patients with recurrent glioblastoma treated with bevacizumab. Neuro-Oncol. 2022;24(11):2001–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ngo L, Nei M, Glass J.. Temozolomide treatment of refractory epilepsy in a patient with an oligodendroglioma. Epilepsia. 2006;47(7):1237–1238. [DOI] [PubMed] [Google Scholar]
- 120. Sutherland A, Meldon C, Harrison T, Miller M.. Subcutaneous levetiracetam for the management of seizures at the end of life: an audit and updated literature review. J Palliat Med. 2021;24(7):976–981. [DOI] [PubMed] [Google Scholar]
- 121. Koekkoek JAF, Postma TJ, Heimans JJ, Reijneveld JC, Taphoorn MJB.. Antiepileptic drug treatment in the end-of-life phase of glioma patients: a feasibility study. Support Care Cancer. 2016;24(4):1633–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ziemba KS, Wellik KE, Hoffman-Snyder C, et al. Timing of antiepileptic drug withdrawal in adult epilepsy patients after neocortical surgical resection: a critically appraised topic. Neurologist. 2011;17(3):176–178. [DOI] [PubMed] [Google Scholar]
- 123. Englot DJ, Berger MS, Barbaro NM, Chang EF.. Predictors of seizure freedom after resection of supratentorial low-grade gliomas: a review. J Neurosurg. 2011;115(2):240–244. [DOI] [PubMed] [Google Scholar]
- 124. Zhang H, Hu Y, Aihemaitiniyazi A, et al. Long-term seizure outcomes and predictors in patients with dysembryoplastic neuroepithelial tumors associated with epilepsy. Brain Sci. 2022;13(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lamberink HJ, Otte WM, Blümcke I, Braun KPJ; European Epilepsy Brain Bank writing group. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19(9):748–757. [DOI] [PubMed] [Google Scholar]
- 126. Yao J, Wang H, Xiao Z.. Correlation between EEG during AED withdrawal and epilepsy recurrence: a meta-analysis. Neurol Sci. 2019;40(8):1637–1644. [DOI] [PubMed] [Google Scholar]
- 127. Kerkhof M, Koekkoek JF, Vos MJ, et al. Withdrawal of antiepileptic drugs in patients with low grade and anaplastic glioma after long-term seizure freedom: a prospective observational study. J Neurooncol. 2019;142(3):463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Das RR, Artsy E, Hurwitz S, et al. Outcomes after discontinuation of antiepileptic drugs after surgery in patients with low grade brain tumors and meningiomas. J Neurooncol. 2012;107(3):565–570. [DOI] [PubMed] [Google Scholar]
- 129. Mauritz M, Hirsch LJ, Camfield P, et al. Acute symptomatic seizures: an educational, evidence-based review. Epileptic Disord. 2022;24(1):26–49. [DOI] [PubMed] [Google Scholar]
- 130. Ryvlin P, Rheims S, Hirsch LJ, Sokolov A, Jehi L.. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. 2021;20(12):1038–1047. [DOI] [PubMed] [Google Scholar]
- 131. Englot DJ, Rolston JD, Wright CW, Hassnain KH, Chang EF.. Rates and predictors of seizure freedom with vagus nerve stimulation for intractable epilepsy. Neurosurgery. 2016;79(3):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Nair DR, Laxer KD, Weber PB, et al. ; RNS System LTT Study. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9):e1244–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Salanova V, Witt T, Worth R, et al. ; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Haskell-Mendoza AP, Srinivasan ES, Lerner EC, et al. Risk of tract seeding following laser interstitial thermal therapy for brain tumors. Neurosurgery. 2022;93(1):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Patel KS, Labar DR, Gordon CM, Hassnain KH, Schwartz TH.. Efficacy of vagus nerve stimulation as a treatment for medically intractable epilepsy in brain tumor patients. A case-controlled study using the VNS therapy Patient Outcome Registry. Seizure J Br Epilepsy Assoc. 2013;22(8):627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Patel KS, Moussazadeh N, Doyle WK, Labar DR, Schwartz TH.. Efficacy of vagus nerve stimulation in brain tumor-associated intractable epilepsy and the importance of tumor stability. J Neurosurg. 2013;119(2):520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. McDermott DS, Mirro EA, Fetrow K, et al. Brain‐Responsive Neurostimulation for the treatment of adults with epilepsy in tuberous sclerosis complex: a case series. Epilepsia Open. 2021;6(2):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Robertson FC, Ullrich NJ, Manley PE, et al. The impact of intraoperative electrocorticography on seizure outcome after resection of pediatric brain tumors: a cohort study. Neurosurgery. 2019;85(3):375–383. [DOI] [PubMed] [Google Scholar]
- 139. Zhu Q, Liang Y, Fan Z, et al. The utility of intraoperative ECoG in tumor-related epilepsy: systematic review. Clin Neurol Neurosurg. 2022;212(January):107054. [DOI] [PubMed] [Google Scholar]
- 140. Goel K, Pek V, Shlobin NA, et al. Clinical utility of intraoperative electrocorticography for epilepsy surgery: a systematic review and meta-analysis. Epilepsia. 2023;64(2):253–265. [DOI] [PubMed] [Google Scholar]
- 141. van Klink NEC, Zweiphenning WJEM, Ferrier CH, et al. Can we use intraoperative high-frequency oscillations to guide tumor-related epilepsy surgery? Epilepsia. 2021;62(4):997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yao PS, Zheng SF, Wang F, Kang DZ, Lin YX.. Surgery guided with intraoperative electrocorticography in patients with low-grade glioma and refractory seizures. J Neurosurg. 2018;128(3):840–845. [DOI] [PubMed] [Google Scholar]
- 143. Warsi NM, Mohammad AH, Zhang F, et al. Electrocorticography-guided resection enhances postoperative seizure freedom in low-grade tumor-associated epilepsy: a systematic review and meta-analysis. Neurosurgery. 2023;92(1):18–26. [DOI] [PubMed] [Google Scholar]
- 144. Feyissa AM, Worrell GA, Tatum WO, et al. High-frequency oscillations in awake patients undergoing brain tumor-related epilepsy surgery. Neurology. 2018;90(13):e1119–e1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tatum WO, McKay JH, ReFaey K, et al. Detection of after-discharges during intraoperative functional brain mapping in awake brain tumor surgery using a novel high-density circular grid. Clin Neurophysiol. 2020;131(4):828–835. [DOI] [PubMed] [Google Scholar]
- 146. Koekkoek JAF, Kerkhof M, Dirven L, et al. Seizure outcome after radiotherapy and chemotherapy in low-grade glioma patients: a systematic review. Neuro-Oncol. 2015;17(7):924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rudà R, Magliola U, Bertero L, et al. Seizure control following radiotherapy in patients with diffuse gliomas: a retrospective study. Neuro-Oncol. 2013;15(12):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Rossi GF, Scerrati M, Roselli R.. Epileptogenic cerebral low-grade tumors: effect of interstitial stereotactic irradiation on seizures. Appl Neurophysiol. 1985;48(1-6):127–132. [DOI] [PubMed] [Google Scholar]
- 149. Schröttner O, Unger F, Eder HG, Feichtinger M, Pendl G.. Gamma-Knife radiosurgery of mesiotemporal tumour epilepsy observations and long-term results. Acta Neurochir Suppl. 2002;84:49–55. [DOI] [PubMed] [Google Scholar]
- 150. Rogers LR, Morris HH, Lupica K.. Effect of cranial irradiation on seizure frequency in adults with low-grade astrocytoma and medically intractable epilepsy. Neurology. 1993;43(8):1599–1601. [DOI] [PubMed] [Google Scholar]
- 151. van den Bent MJ, Afra D, de Witte O, et al. ; EORTC Radiotherapy and Brain Tumor Groups and the UK Medical Research Council. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 152. Wolpert F, Lareida A, Terziev R, et al. Risk factors for the development of epilepsy in patients with brain metastases. Neuro-Oncol. 2020;22(5):718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rudà R, Mo F, Pellerino A.. Epilepsy in brain metastasis: an emerging entity. Curr Treat Options Neurol. 2020;22(2):6. [DOI] [PubMed] [Google Scholar]
- 154. Baumgarten P, Sarlak M, Baumgarten G, et al. Focused review on seizures caused by meningiomas. Epilepsy Behav. 2018;88(November):146–151. [DOI] [PubMed] [Google Scholar]
- 155. Lerner EC, Srinivasan ES, Broadwater G, et al. Factors associated with new-onset seizures following stereotactic radiosurgery for newly diagnosed brain metastases. Adv Radiat Oncol. 2022;7(6):101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rudà R, Bello L, Duffau H, Soffietti R.. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro-Oncol. 2012;14(Suppl 4):iv55–iv64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rudà R, Pellerino A, Pace A, et al. Efficacy of initial temozolomide for high-risk low grade gliomas in a phase II AINO (Italian Association for Neuro-Oncology) study: a post-hoc analysis within molecular subgroups of WHO 2016. J Neurooncol. 2019;145(1):115–123. [DOI] [PubMed] [Google Scholar]
- 158. Castellano A, Donativi M, Rudà R, et al. Evaluation of low-grade glioma structural changes after chemotherapy using DTI-based histogram analysis and functional diffusion maps. Eur Radiol. 2016;26(5):1263–1273. [DOI] [PubMed] [Google Scholar]
- 159. Climans SA, Brandes AA, Cairncross JG, et al. Temozolomide and seizure outcomes in a randomized clinical trial of elderly glioblastoma patients. J Neurooncol. 2020;149(1):65–71. [DOI] [PubMed] [Google Scholar]
- 160. Krueger DA, Wilfong AA, Holland-Bouley K, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Ann Neurol. 2013;74(5):679–687. [DOI] [PubMed] [Google Scholar]
- 161. Franz DN, Agricola K, Mays M, et al. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann Neurol. 2015;78(6):929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Vo AH, Ambady P, Spencer D.. The IDH1 inhibitor ivosidenib improved seizures in a patient with drug-resistant epilepsy from IDH1 mutant oligodendroglioma. Epilepsy Behav Rep. 2022;18(January):100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Avila EK, Chamberlain M, Schiff D, et al. Seizure control as a new metric in assessing efficacy of tumor treatment in low-grade glioma trials. Neuro-Oncol. 2017;19(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Weisdorf S, Duun-Henriksen J, Kjeldsen MJ, et al. Ultra-long-term subcutaneous home monitoring of epilepsy—490 days of EEG from nine patients. Epilepsia. 2019;60(11):2204–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Naganur V, Sivathamboo S, Chen Z, et al. Automated seizure detection with noninvasive wearable devices: a systematic review and meta-analysis. Epilepsia. 2022;63(8):1930–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Tobochnik S, Lapinskas E, Vogelzang J, Ligon KL, Lee JW.. Early EEG hyperexcitability is associated with decreased survival in newly diagnosed IDH-wildtype glioma. J Neurooncol. 2022;159(1):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Derks J, Wesseling P, Carbo EWS, et al. Oscillatory brain activity associates with neuroligin-3 expression and predicts progression free survival in patients with diffuse glioma. J Neurooncol. 2018;140(2):403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Belgers V, Numan T, Kulik SD, et al. Postoperative oscillatory brain activity as an add-on prognostic marker in diffuse glioma. J Neurooncol. 2020;147(1):49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Numan T, Kulik SD, Moraal B, et al. Non-invasively measured brain activity and radiological progression in diffuse glioma. Sci Rep. 2021;11(1):18990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. van der Meer PB, Taphoorn MJB, Koekkoek JAF.. Management of epilepsy in brain tumor patients. Curr Opin Oncol. 2022;34(6):685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]


