Abstract
Control of breast to brain metastasis remains an urgent unmet clinical need. While chemotherapies are essential in reducing systemic tumor burden, they have shown to promote non-brain metastatic invasiveness and drug-driven neurocognitive deficits through formation of neurofibrillary tangles (NFT), independently. Now, in this study we investigated the effect of chemotherapy on brain metastatic progression and promoting tumor-mediated NFT. Results show chemotherapies increase brain-barrier permeability and facilitate enhanced tumor infiltration, particularly through the blood-cerebrospinal fluid-barrier (BCSFB). This is attributed to increased expression of matrix metalloproteinase 9 (MMP9) which, in turn, mediates loss of Claudin-6 within the choroid plexus cells of the BCSFB. Importantly, increased MMP9 activity in the choroid epithelium following chemotherapy results in cleavage and release of Tau from breast cancer cells. This cleaved Tau forms tumor-derived NFT that further destabilize the BCSFB. Our results underline for the first time the importance of the BCSFB as a vulnerable point of entry for brain-seeking tumor cells post-chemotherapy and indicate that tumor cells themselves contribute to Alzheimer’s-like tauopathy.
Keywords: brain metastasis, breast cancer, CSF, chemotherapy, blood-cerebral spinal fluid barrier, Tau
Graphical Abstract

This study explores the relationship between cancer and neuroscience, focusing on how the nervous system impacts breast to brain metastasis (BBM). Chemotherapy’s impact on tumor spread and cognitive function is explored, revealing that it increases brain-barrier permeability, aiding tumor infiltration through Matrix metalloproteinase 9 (MMP9) expression and Claudin-6 loss (A). Likewise, chemotherapy-induced MMP9 activity also contributes to tumor-derived neurofibrillary tangles leading to potential neurodegeneration (B). The study elucidates the vulnerability of the brain barrier and proposes a link between tumor cells and Alzheimer’s-like tauopathy.
Introduction:
The management of breast cancer through adjuvant/neo-adjuvant chemotherapy has been associated with negative side effects, including a persistent decline in memory and cognitive function due to neurodegeneration, commonly referred to as “chemo- brain” syndrome (Brezden, Phillips, Abdolell, Bunston, & Tannock, 2000; Wefel & Schagen, 2012). Up to 78% of breast cancer patients experience this cognitive impairment shortly after starting chemotherapy treatment (Ahles, Root, & Ryan, 2012). Furthermore, these chemotherapy-related cognitive dysfunction can last long term, even 5–10 years after treatment completion for cancer survivors (Vardy, Wefel, Ahles, Tannock, & Schagen, 2008).
The neurodegenerative processes observed in chemo-brain share similarities with Alzheimer’s disease, involving the accumulation of misfolded proteins like Tau, which leads to the formation of neurofibrillary tangles (NFTs) (Chiang, Huo, Kavelaars, & Heijnen, 2019). Tau, encoded by the MAPT gene, is well-known for its role in promoting the assembly and stability of microtubules (Iqbal, Liu, & Gong, 2016). The alternative splicing of MAPT pre-mRNA generates six different molecular isoforms, including three (3R) or four (4R) repeats of the microtubule-binding domain. In a healthy brain, there is a balanced 1:1 ratio of 3R:4R Tau. However, an imbalance in this ratio, in either direction, leads to abnormal phosphorylation of Tau protein, resulting in the formation of paired helical filaments (PHFs) and subsequent NFTs (Buee & Delacourte, 1999; Iqbal et al., 2016). An increase in the level of Tau in the cerebrospinal fluid (CSF) serves as an indicator of Tau-related pathological changes and is associated with neurodegeneration (Iqbal et al., 2016). With higher concentrations of Tau being correlated with more severe cognitive impairment and clinical disease severity (Morris, Maeda, Vossel, & Mucke, 2011).
Recent reports show chemotherapy can induce metastasis by promoting the dissemination of breast cancer cells (Karagiannis et al., 2017) and increasing the presence of circulating tumor cells (CTCs) (Gianni et al., 2009; Rastogi et al., 2008). Additionally, the incidence of breast-to-brain metastasis (BBM) has significantly increased in recent years, despite improvements in early detection and control of extracranial disease (Leone, Lee, & Brufsky, 2015). Although cognitive impairments related to chemotherapy are common, the underlying mediators of this condition in brain metastasis are not well understood. Furthermore, chemo-brain has been attributed to the disruptive effects of chemotherapy on the blood-brain barrier (BBB) and central nervous system (CNS) cells, including oxidative stress, cytokines, mitochondrial dysfunction, and apoptosis, rather than the cancer cells themselves (Ren et al., 2019).
In the present study, we aim to elucidate the mechanisms by which chemotherapy facilitates BBM through the blood-brain and blood-cerebrospinal fluid barriers, as well as the contributions of tumors and chemotherapy to the neurodegeneration frequently observed in patients with brain metastasis.
Methods and Materials
In Vitro Cell Cultures
For all in vitro experiments, human-derived CP cells (ScienceCell), primary breast cancer lines MDA-MB-231 (TN) and BT-474 (Her2+), and low-passage patient-derived breast to brain metastases (BBM) obtained from surgical resection at USC [IRB# 6CNS-07-1], USC-BBM3.1 (TN). Cells were grown in DMEM/F12 media (Gibco Life Technologies) with 10% fetal Bovine Serum (Omega Scientific), 2 mM Glutamax (ThermoFisher), 1X Antibiotic-Antimycotic (ThermoFisher) at 37°C with 5% CO2. CP cells were maintained at low passages in poly-L-lysine (PLL)-coated culture surface at 2 μg/cm2.For co-culture experiments, 2×102 choroid plexus cells were plated on PLL-coated 6-well plates (Olympus Plastics). One day post CP plating, 1×105 tumor cells were plated on top of 0.4 μm thincert cell culture insert for 6-well plates (Greiner Bio-One). Treatment of tumor cells was performed one day post plating. Cells were trypsinized and harvested 48h post treatment.
For condition media (CM) experiments, collected CM were briefly spun down at 18,000 g (Eppendorf 5427R) for 5 min. to remove dead cells and debris, and then concentrated using Amicon Ultra-4 Centrifugal Filter Unit with Ultracel-10 membrane (Millipore Sigma) and by centrifuging at 5,000g for 20 min. The concentrated media were aliquoted and stored at −80°C until use.
In Vivo Drug Administration and Permeability Assay
Animal experiments were approved by University of Southern California Institutional Animal Care and Use Committee protocol number 21017. A stock of paclitaxel (Sigma-Aldrich) was prepared at 40mg/ml in polysorbate 80: ethanol at 4:1 ratio. Adult female NSG™ mice (Jackson Laboratory) were used for in vivo experiments. Each mouse in the experimental group received an intraperitoneal (i.p) dose of 20 mg/kg paclitaxel (100μl total volume) every two days, for a total of 5 doses. The control group received an i.p injection of 100μl 4:1 polysorbate 80: ethanol. 5-Fluorouracil (5-FU) (Sigma-Aldrich) was reconstituted at a concentration of 400 mg/ml in 1:50 ethanol: PBS. Each mouse in the experimental group received an intraperitoneal (i.p) dose of 40 mg/kg 5-FU (100μl total volume) every two days, for a total of 3 doses. One day and 21 days post-treatment mice in each group were injected intraperitoneally with 200 μl of 10% fluorescent tracer sodium fluorescein (Sigma-Aldrich) in PBS. One hour after injection, mice were anesthetized with isoflurane (Vetone), and perfused with 30 ml PBS and fixed with 4% formaldehyde (EMD Millipore) through the left ventricle. Brain and liver (as control) were harvested. Tissues were weighed and subsequently homogenized in 0.5ml cold methanol (Fisher Scientific). Samples were centrifuged at 18,000 g at 4°C for 15 min. Fluorescence in prepared tissues were read with excitation at 460nm and emission at 515nm on Varioskan LUX multimode microplate reader (Thermofisher). Permeability was determined as the ratio of brain/ liver fluorescence per tissue weight.
In Vivo Xenograft
For intracardiac injection, we transduced human breast cancer cell line MDA-MB-231 with a lentiviral construct carrying a bifusion reporter of green fluorescent protein (GFP) and firefly luciferase-2. A stable cell line (MDA-MB-231-FF-GFP) was established using fluorescence activated cell sorting (FACS).
For “Chemotherapy-induced brain metastasis model”, 3 experimental groups were used: 1) Control 2) Chemotherapy-treated with post-acute tumor xenograft, 3) Chemotherapy treated with post-delayed tumor xenograft (Figure 1). Specifically for group 1 control, MDA-MB-231-FF-GFP cells were trypsinized and 5×105 cells were intracardiac injected to each NSG mouse (100μl total volume) using an ultrasound-guided injection. For group 2 “Chemotherapy-treated with post-acute tumor xenograft”, NSG mice were first treated with 5 doses of 20 mg/kg paclitaxel (Huehnchen, Boehmerle, Springer, Freyer, & Endres, 2017)live imaged for tumor cells spreading and metastasis using bioluminescent imaging (BLI) on a Xenogen IVIS Spectrum In Vivo Imaging System. For in vivo imaging, animals were given D-luciferin (200 mg/kg) by tail vein injection (i.v) for 90 sec. Animals were placed onto a warmed stage inside the camera box and received continuous exposure to 2% isoflurane to remain sedated during imaging. Images were quantified as total photon counts using Living® Image Software.
Fig 1. Chemotherapy-induced brain metastasis model.
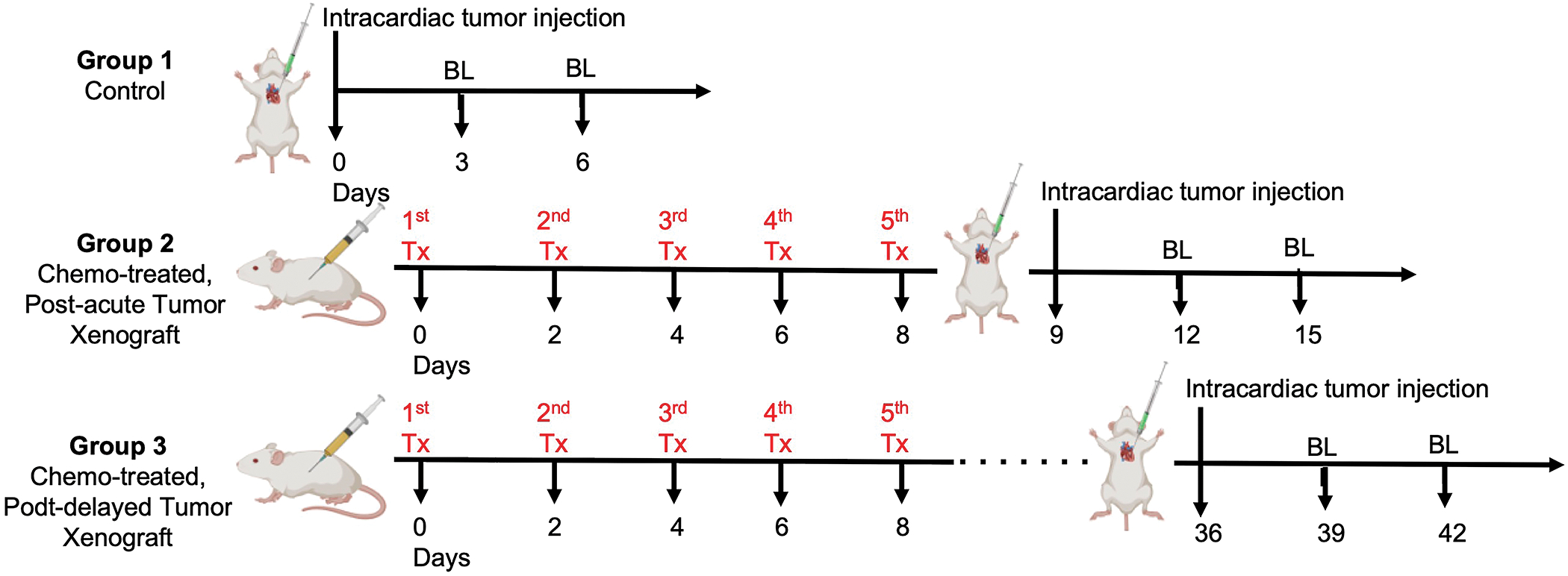
Study design to determine the effect of brain permeability for control (Group 1), acute (Group 2) and delayed (Group 3) adjuvant chemotherapy on initial brain colonization from systemic tumor cells.
For MAPT xenograft studies: MDA-MB-231-FF-GFP cells were transduced with lentiviral construct expressing a short hairpin RNA (shRNA) targeting human MAPT and fused to mCherry reporter (GeneCopoeia). Stable cell lines (MDA-MB-231-FF-GFP KDMAPT) were established using FACS and through selection of cells showing bright fluorescence for both GFP and mCherry. NSG mice (n=8) received either 2.5×105 MDA-MB-231-FF-GFP cells or MDA-MB-231-FF-GFP KDMAPT through ultrasound-guided intracardiac injection. Animals were imaged, as described above, 3, 6, 10- and 17-day post injection.
Tumor Transmigration Assay
BBB and BCSFB transwell cultures were set up as previously described (Herrera et al., 2021).
Patient Whole Brain Autopsy
Patients were enrolled in the Medical College of Wisconsin (MCW) brain bank protocol (PRO26467, PRO17446). At the time of death, the brains were removed from the body and fixed in formalin prior to sectioning[46, 47]. Patients chosen for this study presented with tumor metastasis in the brain. Primary disease included breast carcinoma, colonic adenocarcinoma or lung adenocarcinoma. Using the patient’s last MRI to death as a guide for identifying disease, samples were taken from the following areas; tumor, tumor edge, normal tissue ipsilateral to the tumor, normal tissue contralateral to the tumor, and a sample from each side of the lateral ventricle including choroid plexus. After samples were obtained, the samples were embedded in paraffin, sliced at 5 microns to create slides, and slides were stained with hematoxylin and eosin (H&E) as previously described (Bukowy et al., 2020). The stained slides were scanned using a digital microscope at 40X magnification (Huron Tissue Scope). Blocks from each sample were sent to USC for additional analysis of the BCSFB.
MRI Assessment for Periarterial Metastases
After IRB approval (Pro00081642), the preoperative contrasted magnetic resonance imaging (MRI) of 282 consecutive patients with an intracranial metastasis were screened for the presence of posterior choroidal blood supply and/or peri-atrial location (n=21, 7.5%; Table S2). All patient data was obtained from a prospectively maintained database of adult patients who underwent surgery at a single, tertiary institution between 2014–2019 for one or more intracranial metastatic lesions. The pathologies of these 21 peri-atrial lesions included metastases from the lung (n=10, 48%), (n=3, 14%), breast (n=2, 10%), prostate (n=1, 5%), kidney (n=1, 5%), bladder (n=1, 5%), esophagus (n=1, 5%), cervix (n=1, 5%), and colon (n=1, 5%). Of 21 patients, 12 identified as male and 9 as female. A total of 11 patients were dead at the time of review, with a median time to death from initial diagnosis of intracranial metastasis of 13.6 (interquartile range, IQR: 8.1–18.3) months. The remaining 10 patients remained alive at the time of chart review, with a median follow-up from initial diagnosis of intracranial metastasis of 15.4 (IQR: 10.1–24.0) months.
Patient DCE-MRI Analysis
After IRB approval (2000859), dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was collected retrospectively from Keck Hospital of the University of Southern California. 99 DCE-MRI scans collected from a cohort of 44 patients were downloaded onto Biometrics DCE software. VIF curves were generated for each scan by manually selecting a cerebral venous sinus at three points. Regions of interest (ROI) were manually drawn on every slice with visible contrast-enhancing choroid plexus (CP) and each ROI was categorized and combined into a summed ROI labelled as fourth ventricle, left glomus, or right glomus. The perfusion parameter of Ve was analyzed within each ROI and the maximum and mean values for each ROI were collected as a single data point. Scans were excluded if there was no visible CP or if there was a technical problem with the software registering the scan. 69 patient scans from peripheral tumors with brain metastases were collected (breast n=19, liver n=1, lung n=33, melanoma n=6, kidney n=10). Of the primary peripheral tumors, 2 ROIs were from patients with no evidence of intracranial metastasis, 35 from patients with intracranial metastases without chemotherapy exposure prior to diagnosis, and 32 from patients with chemotherapy exposure prior to intracranial metastasis development.
Cytotoxicity Assays
Cytotoxicity of each chemotherapeutic agent (paclitaxel and lapatinib) for each line used in this study was determined using two different methods: Cell Titer 96® Non-Radioactive Cell Proliferation Assay (MTT) (Promega) and LIVE/DEAD Viability/Cytotoxicity Assay (ThermoFisher). For Cell Titer 96® Non-Radioactive Cell Proliferation Assay (MTT), 5×102 cells per well were plated in 50μl of culture media in a 96-well plate. After 24h, the cells were treated with different concentrations of the chemotherapeutic agent by preparing the concentration of interest in 50μl of culture media and adding it to a triplicate set of wells. 15μl of MTT solution was added to each well and the plate was incubated for 4 hours at 37°C on the day of measurement. Next, 100μl of solubilization solution was added to each well to dissolve formazan crystals and stop the reaction. The plate was incubated for at least 1 hour before reading. The absorbance was recorded at 570nm on Varioskan LUX multimode microplate reader (Thermofisher). For the LIVE/DEAD Viability/Cytotoxicity Assay, 5×103 cells were plated in 100μl of culture media in a black 96-well plate. Next day, the cells were treated with different concentrations of the chemotherapeutic agent by preparing the concentration of interest in 50μl of culture media and adding it to a triplicate set of wells. After 24 or 48h, depending on treatment time, the LIVE/DEAD reagent was freshly made. For one 96-well plate, 20μl of Ethidium homodimer-1 and 5μl of Calcein AM were added to 10 ml of PBS. After vortexing, the reagent was stored in dark at room temperature (RT). The culture media was removed from all the wells, and 100μl of DMEM media without FBS (to avoid background reading) was added to each well. Next, 100μl of LIVE/DEAD reagent was added to each well and mixed well. The plate was incubated in dark at RT for 45min. After incubation, the absorbance was recorded at 485nm excitation and 530nm emission on Varioskan LUX multimode microplate reader (Thermofisher).
In Vitro Drug Treatments
Paclitaxel (Sigma-Aldrich) was reconstituted in DMSO at 4mM, aliquoted and stored at −80°C. Cells were then treated at following concentrations of paclitaxel at their IC25 for 48h: MDA-MB-231 at 250nM, BT-474 at 2.5μM, and CP at 3nM. Lapatinib (Cayman Chemicals) was reconstituted in DMSO at a concentration of 40mM, aliquoted and stored at −20°C. Cells were then treated at following concentrations of lapatinib for 48h: MDA-MB-231 at 17uM, BT-474 at 8μM, and CP at 8μM.
In Vitro Fluorescein Assay
Choroid plexus cells were plated on top of 0.4μm PolyL-Lysine-coated (PLL) thincert cell culture insert for 24 well plates (Greiner Bio-One) at 2.1×104 cells per insert to form an 80% confluent monolayer at the time of treatment (3 days post plating), and a 100% confluent monolayer at the time of reading (one day post treatment). On the day of assay, the media from lower and upper compartments of transwell were removed and Hepes buffer (10 mM Hepes, pH 7.2, 0.1% BSA, 4.5% glucose) was immediately added. Plates were incubated at 37°C for 5 min. Hepes buffer was removed from both lower and upper compartments of transwell and 1.5ml and 490μl of buffer were added to lower and upper compartments, respectively. Next, 10μl of 5 mg/ml fluorescein sodium (Sigma-Aldrich) was added to the upper compartment. The plate was incubated at RT. After 30 min and 60min, 100μl of lower compartment was transferred to 96-well plate and fluorescence was measured at 485nm excitation and 530nm emission on Varioskan LUX multimode microplate reader (Thermofisher).
RNA Isolation and qPCR
For RNA isolation, cells were grown on 6-well cell culture plates (Olympus Plastics). Cells were plated at 1.5×106 cells per well and treated two days post plating when they were about 70% confluent. Total RNA was isolated using RNeasy Mini kit (Qiagen) according to the manufacturer’s instructions. In the final step, RNA was resuspended in 30μl of DNase, RNase free water. RNA yield was quantified using a NanoDrop spectrophotometer (ThermoFisher). For RT-qPCR, 1μg of total RNA was transcribed into cDNA using Maxima First Strand cDNA Synthesis kit (ThermoFisher) in a 20μl volume. The PowerUp SYBR Green Master Mix (ThermoFisher) was used for qPCR reaction with 10ng of cDNA per 20μl of total reaction. The list pf primers used to assess the genes in this study are listed in Table S1. Human cell junction pathway finder array plate (Qiagen) was used for screening the expression of cell junction markers. Expression levels were normalized to RPLP0. The qPCR reactions were performed on an Applied Biosystems (ABI) QuantStudio 6 Flex Real-Time PCR System.
Immunocytochemistry
For immunocytochemistry, cells were plated at 3×104 cells per well on 15mm coverslips in 24-well cell culture plates (Olympus Plastics). PLL-coated culture surfaces were used for CP cells. Cells were fixed in 4% formaldehyde at 4°C for 15min and then permeabilized in 0.3% Triton X-100 for 30 min at 37°C. After rinsing with 1X PBS, cells were blocked with 50% SEA BLOCK (ThermoFisher) in PBS at RT for 1h. Slides were incubated overnight in primary antibodies including anti-tau (Abcam ab80579 and Abcam ab76128), anti-p-Tau (Abcam ab109401), anti-CLDN6 (Abcam ab199670 and Invitrogen MA5–24076), anti-MMP9 (GeneTex GTX60482) at 4°C. The following day, the slides were rinsed in 1X PBS three times and then incubated in a cocktail of appropriate IgG secondary antibodies conjugated with Alexa Flour 488 or Cy3 (Jackson ImmunoResearch Laboratories), and Phalloidin Alexa 647 (Invitrogen A22287), protected from light, for 1hr at RT. After rinsing the slides with 1X PBS for three times, the slides were mounted with ProLong™ Gold Antifade Mountant DAPI (ThermoFisher).
Immunofluorescence of Formalin-Fixed Paraffin-Embedded Tissue
Paraffin-embedded tissues were pre-heated at 65°C for 10min and dewaxed with xylene. Next, they were rehydrated with an ethanol gradient (100%, 95%, 70%). Antigen retrieval was performed by treating the slides with sodium citrate buffer (10mM, pH 6) for 15min at 85°C. Permeabilization of tissue was done in 0.3% Triton X-100 in PBS for 30 min at 37°C. After rinsing with 1X PBS, the tissues were blocked with 50% SEA BLOCK in PBS plus 0.3M Glycine for 1hr at RT. The slides were incubated overnight at 4°C with primary antibodies including anti-tau (Abcam ab76128), anti-p-Tau (Abcam ab109401), anti-MMP9 (GeneTex GTX60482 and Abcam ab38898) in 50% SEA BLOCK and 0.1% Tween 20. The next day, sections were incubated with the appropriate IgG secondary antibody conjugated with either Alexa Flour 488, Cy3 or Alexa Fluor 647 (Jackson ImmunoResearch Laboratories) in PBS for 1 hr at room temperature protected from light and then mounted with ProLong™ Gold Antifade Mountant DAPI (ThermoFisher).
Microscopy and quantification:
Confocal imaging was performed using the Leica SP8 microscope. Quantification from confocal imaging was performed as previously described (Deshpande et al., 2021). Briefly, first control tissue sections stained with only secondary antibody were imaged to determine “detector gain” and “amplifier offset” for threshold signal to reduce false positivity and tissue autofluorescence. Second, confocal Z stacks of test sections, stained with desired primary antibody plus secondary antibody, were imaged under the presets determined from matched control tissue for “amplifier offset” and “detector gain.” This ensured standardization and linearity of response over the intensity ranged measured. A minimum of three regions of interest per section or coverslip. Confocal Z stacks of test sections or coverslips, stained with desired primary antibody plus secondary antibody, were imaged at 0.5-μm intervals under the presets determined from matched control tissue for “amplifier offset” and “detector gain.” Quantification of immunofluorescence (in vivo and in vitro) was done on a minimum of 3 ROIs per coverslip.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA was performed using Tau (Total) Human ELISA Kit (Invitrogen KHB0041) and MMP9 Human ELISA Kit (Invitrogen BMS2016–2) according to manufacturers’ instruction. Samples were measured on Varioskan LUX multimode microplate reader (Thermofisher).
Tau Paired Helical Filaments (PHF) Staining
A stock solution of 0.1% thioflavin S (ThS; Sigma T1892) was prepared in ethanol: water at 1:1 ratio and stored at −20°C in dark. A working solution of 0.05% was used for staining the condition media. A 1:5 dilution of concentrated condition media was used for staining. Media was used as blank. After adding ThS, the media were incubated at room temperature in dark for 30 min. The absorbance was read at 450nm excitation and 510nm emission on Varioskan LUX multimode microplate reader (Thermofisher). Tau PHF in condition media of treated cells was normalized relative to control with no treatments.
Statistical Analysis
For all in vivo experiments, the number of animals used was determined by power analysis (Alpha=0.05, Beta=0.2, Power=0.8). All in vitro experiments were conducted with at least three technical and biological replicates. Quantification of immunofluorescence (in vivo and in vitro) was done on a minimum of 3 ROIs per coverslip. Experimental data are shown as mean ± standard error mean. Statistical analyses were performed in Graphpad Prism using one- or two-way analysis of variance (ANOVA) ± Bonferroni Multiple test.
Results:
Chemotherapy facilitates metastatic tumor cell entry into the brain through the BCSFB.
Several key zones of restrictive entry have evolved for protection of the brain: the blood-brain-barrier (BBB) and the blood-cerebral spinal fluid-barrier (BCSFB) (Abbott, Patabendige, Dolman, Yusof, & Begley, 2010). The current prevailing model is that metastatic cancer cells enter the brain by crossing the BBB. However, we recently showed breast cancer cells have a preference for crossing the BCSFB (Herrera et al., 2022). First, to determine whether systemic chemotherapy opens the entry barriers of the brain, we set up an in vivo model of acute and delayed chemotherapeutic response. Since paclitaxel and 5-FU are standard of care drugs used to treat breast cancer (Waks & Winer, 2019), tumor-naïve mice were treated with 5 doses, over 10 days, of these latter chemotherapies and then euthanized either 1-day post-treatment (acute response) or 21-days post-treatment (delayed response). Results show a significant increase in fluorescein-dye uptake within the brains of mice treated with both paclitaxel (Fig. 2A) and 5-FU (Fig. 2B) in both acute and delayed response treatment compared to vehicle control groups. This indicates that chemotherapy increases permeability of BBB and BCSFB in a manner that is maintained over time.
Fig. 2. Chemotherapy facilitates breast cancer cells’ entry into the brain through the BCSFB.

In vivo analysis of brain permeability via Fluorescein-dye uptake in the brain of mice treated with paclitaxel (A) and 5-FU (B) compared to vehicle group. (C) In vivo BLI and (D) quantification of BBM signal in control (Group I), acute chemo-treated (Group II), and delayed chemo-treated (Group III) groups 3 days and 6 days post tumor injection (DPTI). Quantification (E) and imaging (F) of metastatic tumor-GFP fluorescence intensity in the brain parenchyma (blue) and in the choroid plexus of the BCSFB of the lateral and 4th ventricles (red) in Groups 1–3. (G) In vitro migration capacity of BC cells across the BBB versus BCSFB in control and paclitaxel-treatments. (H) MRI and quantification (I) of patients with brain metastasis having peri-atrial brain metastasis (red) with posterior CP blood supply involvement; Anterior of lateral ventricle (A of LV). (J) Representative histological H&E sections from whole-brain patient tissue from rapid autopsy cases with parenchymal BMs and tumor cells in the ipsilateral BCSFB. (K) Quantification of extravascular extracellular space (Ve) in the choroid plexus from DCE-MRI of patients with and without chemotherapy exposure prior to brain metastasis diagnosis. (L-M) Representative examples of Ve color map from DCE-MRI from both groups shown in the left glomus of the CP.
To determine whether such increased barrier permeability might impact the establishment of brain metastases, we developed an intracardiac metastatic tumor model. MDA-MB-231-FF-GFP cells were introduced either acutely (1 day) following paclitaxel regimens, or in delayed fashion (28 days) after treatment (Fig. 1). The degree of brain seeding was assessed with bioluminescence imaging (BLI). Results show that compared to an untreated tumor-bearing control group, mice treated with chemotherapy develop a significant increase of systemic (Supplemental Fig. 1A) and brain metastates (Fig. 2C,D) burden, regardless of the timing of tumor injection following treatment. Thus, we sought to determine whether this increased brain colonization we observed post-chemotherapy occurred through a leaky BBB or BCSFB. To do so, in the same experimental models, we assessed the relative metastatic seeding of the brain parenchyma (protected by the BBB) versus of the choroid plexus (protected by the BCSFB). Results show that there was no significant difference in the number of metastatic tumor cells in the brain parenchyma across chemotherapy treated and untreated groups (Fig. 2E,F). However, there was indeed a significant increase in the number of infiltrating metastatic tumors found in the choroid plexus of the lateral and 4th ventricles in mice exposed to chemotherapy (Fig. 2E,F). Additionally, there was a greater degree of tumor infiltration through the BCSFB in in vivo where tumors were xenografted in delayed fashion following chemotherapy treatment. In vitro, breast cancer cell migration models further corroborated these in vivo results by showing that tumor cells exhibit significant preference for crossing the BCSFB relative to the BBB in both control and paclitaxel-treated transwell co-cultures (Fig. 2G). Moreover, while there was a significant decrease in breast cancer migration across the BCSFB post-chemotherapy, this migration was still significantly higher compared to migration across the BBB (Fig. 2G).
To next assess tumor cell interaction with the BCSFB and choroid plexus in patients, we looked at the pre-operative contrasted magnetic resonance imaging (MRI) of patients with brain metastases. In a cohort of 282 patients with established brain metastases, 21 patients (7.5%) exhibited metastases that drew choroidal blood supply and/or were found in a peri-atrial location (Fig. 2H,I). Analysis of whole brain tissue from rapid autopsy cases showed that patients with parenchymal brain metastatic lesions have tumor cells in the ipsilateral BCSFB (Fig. 2J). Analysis of DCE-MRI perfusion scans from patients with brain metastases showed a significant increase extravascular extracellular space (Ve) in the choroid plexus of patients who had exposure to chemotherapeutic agent before they developed MRI evidence of an initial or new brain metastasis (Fig. 2K–M). All together, these results suggest that the BCSFB is a vulnerable point of entry for breast cancer cells post-chemotherapy due to enhanced permeability in the choroid endothelium.
Chemotherapy-induced upregulation of MMP9 in choroid plexus leads to downregulation of Claudin 6.
We next addressed the mechanism by which chemotherapy increases BCSFB permeability. BCSFB permeability was assessed under 3 different conditions: Choroid plexus (CP) cells were exposed in vitro to 1) paclitaxel alone; 2) breast cancer-conditioned media (CM) alone; or 3) CM from breast cancer cells treated with paclitaxel. Results shows BCSFB permeability was significantly enhanced in all three conditions compared to naïve control (Fig. 3A). While paclitaxel and CM exposure increased permeability equally when administered individually, the effect was significantly additive when choroid plexus cells were treated with CM from breast cancer cells pre-treated with paclitaxel.
Fig. 3. Chemotherapy upregulates CP-MMP9 leading to downregulation of CLDN6 tight junctions on BCSFB.

(A) In vitro BCSFB permeability assessed by fluorescein-dye on naïve CP (control), paclitaxel (Pac)-treated CP cells, or CP cells exposed to MDA-MB-231 condition media (CM) alone or MDA-MB-231CM pre-treated with paclitaxel. (B) Clustergram representing mRNA expression of junctional markers in CP cells treated with paclitaxel (PTX), MDA-MB-231 CM, MDA-MB-231 CM pre-treated with PTX, and co-cultured with MDA-MB-231 alone or MDA-MB-231 pretreated with PTX. Color scheme goes from red for no change in expression to purple for down-regulated genes. (C) Venn diagram of downregulated junctional markers in all five conditions from (B) relative to control. (D) RT-qPCR validation of four common junctional markers downregulated in all five tested conditions in CP cells. (E) Immunofluorescent (IF) imaging of Claudin-6 in choroid plexus adjacent to tumor lesion compared to CP distal to metastatic lesion in post-mortem patient tissue diagnosed with brain metastasis. Images taken at 40X. (F) IF imaging of MMP9 in CP of the BCSFB ipsilateral to tumor lesion compared to choroid plexus on the contralateral hemisphere in post-mortem patient tissue diagnosed with brain metastasis. Images taken at 40X. (G) MMP9 expression in CP cells treated with paclitaxel, tumor condition media (CM), and combination of tumor condition media and paclitaxel relative to CP cells alone in vitro. (H) Quantification of exogenous MMP9 released by CP treated alone, with primary breast cancer MDA-MB-231, or BBM3.1 CM ± paclitaxel in vitro. (I) RT-qPCR analysis of junctional markers’ expression in CP KDMMP9 cells treated with tumor CM ± paclitaxel. (J) IF imaging of Claudin-6 in CP KDMMP9 cells treated with tumor CM ± paclitaxel. Images taken at 63X (K) Flourescein dye-assay determining permeability of BCSFB (control CP and CP KDMMP9) when treated with breast cancer cells (MDA-MB-231 CM ± paclitaxel).
Next, we measured mRNA expression for 36 junctional markers in choroid plexus cells when exposed to chemotherapy ± tumor CM. Specifically, choroid plexus cells were treated with: 1) paclitaxel alone; 2) breast cancer CM alone; 3) CM from breast cancer cells treated with paclitaxel; 4) untreated breast cancer cells in co-culture; or 5) paclitaxel-treated breast cancer cells in co-culture. The mRNA expression screen revealed a wide-ranging downregulation of tight junction transcripts in choroid plexus cells (Fig 3B), with CAV1, CDH2, CLDN6, and ITB6 being downregulated under all 5 conditions (Fig. 3C). Claudin-6 (CLDN6) was the most significantly downregulated junctional marker by both mRNA (Fig. 3D) and protein in the choroid plexus (Supplemental Fig. 1B). Furthermore, analysis of post-mortem patient tissue with brain metastases showed lower expression of CLDN6 in tumor-adjacent compared to choroid plexus of the lateral ventricle, distant from the metastatic lesion (Fig 3E).
We next explored the mechanism of CLDN6 downregulation in choroid plexus. Matrix Metalloproteases (MMPs) have been linked to tumor invasion, neurodegenerative diseases, and BBB breakdown in bacterial meningitis (Brkic, Balusu, Libert, & Vandenbroucke, 2015; Leppert et al., 2000). Currently, there is no known role for choroid plexus-derived MMPs in BCSFB breakdown. Analysis of post-mortem tissue from patients diagnosed with brain metastases showed increased expression of MMP9 in tumor-adjacent choroid plexus compared to choroid plexus in the contralateral hemisphere (Fig. 3F). Thus, we hypothesized that increased choroid plexus MMP9 levels might be linked to CLDN6 downregulation and BCSFB permeability in the context of chemotherapy and tumor cell invasion.
Returning to the experimental conditions above, we found that MMP9 mRNA (Fig. 3G) and protein expression (Supplemental Fig. 1C) were both elevated in choroid plexus cells upon exposure to tumor cells treated with paclitaxel, tumor CM, or the combination of tumor CM plus paclitaxel in vitro. We next measured exogenous MMP9 from choroid plexus cells in vitro. While there was no significant exogenous MMP9 from control or paclitaxel-treated choroid plexus cells, release was significantly enhanced when choroid cells were exposed to CM from MDA-MB-231 primary breast cancer or from brain metastatic BBM3.1 cells that had or had not been treated with paclitaxel (Fig. 3H). Furthermore, there was increase of MMP9 in acute (group 1) and delayed (group 2) adjuvant chemotherapy in vivo (Supplemental Fig. 1D).
We next asked whether MMP9 knockdown in choroid plexus (CP MMP9kd) (Supplemental Fig. 1E) can rescue the expression of Claudin-6 and restore the BCSFB. Indeed, when CP MMP9kd cells were cultured in the presence of CM from tumor ± paclitaxel pre-treatment, there was an increase in both Claudin 6 mRNA (Fig. 2I) and protein (Fig. 2J) expression. Next, we tested the ability of breast cancer cells to cross the BCSFB in control or CP MMP9kd cells (Fig. 2K). While MMP9kd alone did not affect BCSFB permeability, CP MMP9kd cells showed enhanced resistance to fluorescein flow-through and significantly reduced BCSFB permeability when exposed to CM from MDA-MB-231 cells ± paclitaxel pre-treatment (Fig. 2K). Overall, this suggests that the combination of chemotherapy and tumor exposure induces choroid plexus MMP9 release, resulting in Claudin-6 downregulation, and thus increasing BCSFB permeability.
Choroid plexus derived-MMP9 promotes breast cancer-derived Tau neurofibrillary tangles
We next determined whether tumor-driven tau contributes to brain neurofibrillary tangle formation. Results show a basal level of total MAPT, MAPT 3R and MAPT 4R expression Fig. 4A), with deviation from a 1:1 ratio for MAPT 3R: MAPT 4R seen in in primary breast cancer and brain metastatic cells (Fig. 4B). MAPT knockdown (KDMAPT) in MDA-MB-231 breast cancers resulted in overall downregulation of total MAPT, MAPT 3R and MAPT 4R (Fig. 4C); Along with overall reduced metastatic potential, including loss of brain-metastatic ability, leading to significant survival advantage in KDMAPT xenografted animals in vivo (Fig. 4D).
Fig. 4. Tumors cells highjack CP-MMP9 leading to NFT and subsequent increase in BCSF permeability.

(A) mRNA analysis of MAPT 3R and MAPT 4R expression and their (B) ratio in primary breast cancer MDA-MB-231 and BT-474, and USC-BBM3.1. (C) qPCR validation of total MAPT, MAPT 3R/4R knockdown in MDA-MB-231 cells (D) BLI of MDA-MB-231 and MDA-MB-231 KDMAPT xenografts in vivo. Kaplan-Meier survival analyses of breast cancer MDA-MB-231-bearing and MDA-MB-231 KDMAPT mice. (E) Quantification of Tau NFT formation by PHF staining in BC cells treated with DMSO and Paclitaxel. (F) Expression of junctional markers in BCSFB treated with exogenous tau, and CM from MDA-MB-231 and MDA-MB-231 KDMAPT. (G) Quantification of BCSFB permeability in vitro in CP cells treated with CM from MDA-MB-231 and MDA-MB-231 KDMAPT using fluorescein assay. (H) Quantification of exogenous MMP9 released from CP alone, treated with CM from MDA-MB-231 (Control), or MDA-MB-231 KDMAPT, and from MDA-MB-231 or MDA-MB-231 KDMAPT. (I) PHF quantification in the condition media of CP cells alone, CP KDMMP9 cells, CP cells treated with CM from MDA-MB-231, CP KDMMP9 cells treated with CM from MDA-MB-231, and in MD-MB-231 CM. (J) In vitro migration assay through BCSFB quantifying the migrational capacity of MDA-MB-231 and MDA-MB-231 KDMAPT cells across the wild-type CP (Control) and CP KDMMP9 cells. (K) MAPT expression in cells found in CSF of a leptomeningeal (LMD) patient using single cell RNA-Seq analysis. (L) Tau quantification in the CSF of patients ± LMD. (M) PHF quantification in of CP cells (control, CP KDMMP9) treated with CM from MDA-MB-231 cells treated with Paclitaxel. (N) PHF quantification of CP cells treated with CM from MDA-MB-231 KDMAPT cells in two conditions (cells only, cells treated with Paclitaxel) (O) Accumulation of corpora amylacea waste vacuoles on ipsilateral brain parenchyma to tumor lesion, adjacent to lateral ventricle (LV) CSF, and CP in H&E sections from post-mortem brain metastatic patient tissue. Images taken at 40X.
Since MAPTKD tumor cells did not form observable brain metastases, we asked whether tumor-derived Tau promotes entry of chemotherapy-treated and untreated breast cancer cells through the BCSFB. For this, we first investigated the effect of chemotherapy on Tau expression in breast cancer cells. Paclitaxel-treated cancer cells showed an increase in total MAPT and MAPT 3R and 4R variants with significant deviation of MAPT 3R/4R ratios relative to DMSO-treated cells used as control (Supplemental Fig. 2A). Furthermore, there was an increase in total tau expression in paclitaxel treated cancer cells (Supplemental Fig. 2B), along with significant increase in soluble Tau (Supplemental Fig. 2C). Furthermore, there was induction of Tau tangles in paclitaxel (Fig. 4E) and lapatinib (Supplemental Fig. 2D) treated cancer cells compared to DMSO-treated controls. These chemotherapy effects were not observed in untreated MAPTKD tumor cells (Supplemental Fig. 2E–G). We thus conclude that chemotherapy leads to enhanced breast cancer Tau expression, release, and formation of NFT.
We next determined the effect of breast cancer-derived Tau on BCSFB permeability. While exposure of choroid plexus cells to exogenous Tau and MDA-MB-231 CM resulted in downregulation of 19 junctional markers of BCSFB including CLDN6, there was no change when choroid plexus cells were exposed to MDA-MB-231 MAPTKD CM (Fig. 4F). Furthermore, BCSFB permeability was significantly increased only upon exposure to MDA-MB-231 CM, but not to MDA-MB-231 MAPTKD CM (Fig. 4G).
MMP9 can target the cleavage of Tau, resulting in oligomerization and aggregation as NFT (Nubling et al., 2012). Furthermore, there is elevated MMP9 in the CSF, blood plasma, and serum of Alzheimer’s patients (Horstmann et al., 2010). Therefore, we asked whether choroid plexus MMP9 results in Tau oligomerization leading to increased BCSFB permeability and tumor migration. Results show that while choroid cells significantly increased MMP9 release upon exposure to tumor cell CM in general, the MMP9 release was significantly lower when cells were treated with MDA-MB-231 MAPTKD CM compared to control MDA-MB-231 CM (Fig. 4H). Likewise, staining of insoluble tau through paired helical filaments (PHF) (Barghorn, Davies, & Mandelkow, 2004), shows NFTs significantly increase only when choroid plexus cells are cultured with MDA-MB-231 CM. However, CP MMP9KD cells are unable to cleave tumor-derived Tau into NFTs, even when cultured with MDA-MB-231 CM (Fig. 4I).
We then asked whether knockdown of CP MMP9 and/or breast cancer-MAPT affected tumor migration through the BCSFB. Results show that MDA-MB-231 MAPTKD cells have a significant lower migration capacity compared to control tumor cells when crossing the BCSFB. Furthermore, while MMP9KD in choroid plexus significantly hindered tumor cell migration, the hindrance was greater for MDA-MB-231 MAPTKD tumor cells (Fig. 4J). This indicates that MMP9KD in choroid plexus restricts tumor entry through the BCSFB by maintaining barrier integrity, and by inhibiting the NFT formation that follows reduced cleavage of tumor-derived Tau.
Finally, we investigated whether tumor cells that crossed the BCSFB and into CSF maintained Tau expression. Single cell RNA sequencing from patients with leptomeningeal disease (LMD) showed metastatic tumor cells in these patients have higher expression of MAPT (Fig. 4K), along with a significant increase in CSF-Tau (Fig. 4L). Moreover, quantification of showed that NFT formation is significantly increased in artificial CSF from choroid plexus compared to MMP9KD cells when given with tumor cells pre-treated with paclitaxel (Fig. 4M). However, paclitaxel does not induce PHF in artificial CSF when Tau is knocked down in MDA-MB-231 (Fig. 4N). Based on these observations, we asked whether tumor-derived NFT contributes to neurodegeneration in patients with breast to brain metastasis. Whole-brain tissue from rapid autopsy cases from patients with parenchymal brain metastatic lesions was assessed for accumulation of corpora amylacea (CA) waste vacuoles, which are associated with Tau and neurodegeneration. CA was observed only in parenchyma ipsilateral and adjacent to metastatic lesions and the lateral ventricle (Fig. 4O). This suggests chemotherapy induces choroid plexus MMP9 overexpression, which then facilitates NFT formation from tumor-derived Tau, leading to BCSFB breakdown, tumor migration across the BCSFB, and CSF-derived Tau.
Discussion:
The use of standard-of-care chemotherapies are associated with collateral damage such as chemo-brain and neuropathy (Abu Samaan, Samec, Liskova, Kubatka, & Büsselberg, 2019). In our study, we specifically examined the impact of chemotherapy on the development of brain metastasis and neurodegenerative neurofibrillary tangles
Our findings demonstrate that exposure to chemotherapy can enhance the occurrence of systemic metastases. This enhancement is likely due to multiple mechanisms, including the generation of resistant and disseminating cancer stem cells at the primary tumor site, the recruitment of immune cells that support the dissemination of the primary tumor, the mobilization and modulation of CTCs heterogeneity, and the creation of a favorable environment for metastatic growth, known as the metastatic niche (Karagiannis et al., 2017; Prasanna et al., 2021).
Moreover, we show that chemotherapy increase BCSFB permeability in the brain, which is sustained for up to one month, thus leaving the brain susceptible to tumor cell entry. To date, extravasation through the BBB was considered the primary route of tumor cell entry, with few studies reporting solitary lesions around the choroid plexus-lined ventricles of the brain (Della Puppa et al., 2010; Kitagawa et al., 2013). Our results reveal that exposure to breast cancer cells and chemotherapy results in choroid plexus MMP9-mediated loss of Claudin-6, a choroid specific tight junction protein (Kratzer et al., 2012), leading in turn to enhanced BCSFB permeability. Correspondingly, we also observe enhanced tumor infiltration across the BCSFB compared to the BBB in mice treated with chemotherapy. This discovery on the impact of chemotherapy on BCSFB further supports previous reports on the preferential route of breast cancer entry into the brain via the BCSFB (Herrera et al., 2022). Therefore, given our results, the increased CTC numbers seen after chemotherapy(Ortiz-Otero, Marshall, Lash, & King, 2020), and the positional proximity of the ventricles to CSF circulation in the brain(Lun, Monuki, & Lehtinen, 2015), it is important to consider the BCSFB as a vulnerable early point of CNS-entry for chemo-treated breast cancer cells. Furthermore, a retrospective analysis of the topography from brain metastases patients revealed breast cancer hones most frequently to the cerebellum (Neman et al., 2021). It would therefore be interesting to determine whether the location of the BCSFB-lined 4th ventricle at the base of the cerebellum, and the combined ability of chemotherapy and tumor burden to enhance BCSFB permeability contribute to the preference of BC cells to metastasize to this site.
Enhanced Tau in breast cancer cells leads to resistance to taxanes e.g. paclitaxel, because of competitive tubulin binding(Smoter et al., 2011), and is also required for reattachment of CTCs (Matrone et al., 2010). Furthermore, elevated serum tau is associated with worse survival and presence of brain metastases in breast cancer (Darlix et al., 2019). However, while abnormal neural tau has been extensively studied, the contribution of tumor-derived tau has not been studied in the progression of brain metastasis. Our results indicate that in the presence of paclitaxel, breast cancer cells upregulate Tau production and release, and that increased choroid plexus-MMP9 also contributes to cleavage of this tumor-derived Tau. This results in NFTs, which further enhance BCSFB permeability by affecting tight junction physiology in the choroid plexus. While TauKD breast cancer cells retain their ability to cross the BCSFB in vitro, they do not contribute to MMP9-mediated NFT formation, even in the presence of paclitaxel. Therefore, beyond the effects of chemotherapy, breast cancer cells may also contribute to cognitive impairments seen in patients with advanced breast cancer, through the generation of NFTs from tumor-derived Tau.
Collectively, our results show chemotherapy and breast cancer cells co-operatively enhance MMP-9 activity in the choroid plexus epithelium. This results in breakdown of the BCSFB and the formation of tumor-derived Tau tangles, which lead to both enhanced metastatic invasion and Alzheimer-like tauopathy in brain metastatic breast cancer. Our study thus fosters a bridge across oncology and neuroscience, utilizing knowledge of neurodegenerative diseases to further our understanding of the pathology of breast to brain metastasis.
Supplementary Material
Significant Statement:
The current study focuses on the emerging transdisciplinary investigation between cancer and neuroscience foundations to advance our understanding of how the nervous system contributes to breast to brain metastasis. Specifically, while chemotherapies are essential in reducing systemic tumor burden, thus far they have only been associated with tumor invasiveness outside of the central nervous system and drug-driven neurocognitive deficits through formation of neurofibrillary tangles, independently. Our results underline for the first time the significance of the BCSFB as a vulnerable point of entry for brain-seeking tumor cells post-chemotherapy and indicate tumor cells themselves contribute to Alzheimer’s-like tauopathy.
Acknowledgements:
The authors would like to thank Bavrina Bigjahan, Negin Amini, Maryam Mohammadzadzadeh, Ali Mohammadzadzadeh, and Sina Nazemi with help with data gathering. We would like to acknowledge patient advocates (Ms. Michele Rakoff, Ms. Michelle Atlan, Ms. Andrea Hutton and Ms. Sharon Schlesinger) and all breast cancer patients/survivors/family members for the invaluable role they have played in our research over the years.
Support:
National Institutes of Health/National Cancer Institute R01CA223544-01A (JN)
Department of Defense BCRP BC141728 (JN)
Susan G Komen Career Catalyst Grant CCR15332673 (JN)
METAvivor (JN)
Footnotes
Conflict of Interest Statement
Authors declare that they have no conflicting interests.
Data and materials availability:
All data are available in the main text or the supplementary materials.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, & Begley DJ (2010). Structure and function of the blood-brain barrier. Neurobiol Dis, 37(1), 13–25. doi: 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- Abu Samaan TM, Samec M, Liskova A, Kubatka P, & Büsselberg D (2019). Paclitaxel’s Mechanistic and Clinical Effects on Breast Cancer. Biomolecules, 9(12). doi: 10.3390/biom9120789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Root JC, & Ryan EL (2012). Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 30(30), 3675–3686. doi: 10.1200/JCO.2012.43.0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barghorn S, Davies P, & Mandelkow E (2004). Tau paired helical filaments from Alzheimer’s disease brain and assembled in vitro are based on beta-structure in the core domain. Biochemistry, 43(6), 1694–1703. doi: 10.1021/bi0357006 [DOI] [PubMed] [Google Scholar]
- Brezden CB, Phillips KA, Abdolell M, Bunston T, & Tannock IF (2000). Cognitive function in breast cancer patients receiving adjuvant chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 18(14), 2695–2701. doi: 10.1200/JCO.2000.18.14.2695 [DOI] [PubMed] [Google Scholar]
- Brkic M, Balusu S, Libert C, & Vandenbroucke RE (2015). Friends or Foes: Matrix Metalloproteinases and Their Multifaceted Roles in Neurodegenerative Diseases. Mediators Inflamm, 2015, 620581. doi: 10.1155/2015/620581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buee L, & Delacourte A (1999). Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick’s disease. Brain Pathology, 9(4), 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowy JD, Foss H, McGarry SD, Lowman AK, Hurrell SL, Iczkowski KA, . . . LaViolette PS (2020). Accurate segmentation of prostate cancer histomorphometric features using a weakly supervised convolutional neural network. J Med Imaging (Bellingham), 7(5), 057501. doi: 10.1117/1.JMI.7.5.057501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang ACA, Huo X, Kavelaars A, & Heijnen CJ (2019). Chemotherapy accelerates age-related development of tauopathy and results in loss of synaptic integrity and cognitive impairment. Brain Behav Immun, 79, 319–325. doi: 10.1016/j.bbi.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix A, Hirtz C, Thezenas S, Maceski A, Gabelle A, Lopez-Crapez E, . . . Lehmann, S. (2019). The prognostic value of the Tau protein serum level in metastatic breast cancer patients and its correlation with brain metastases. BMC Cancer, 19(1), 110. doi: 10.1186/s12885-019-5287-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Puppa A, Dal Pos S, Zovato S, Orvieto E, Ciccarino P, Manara R, . . . Scienza R (2010). Solitary intra-ventricular brain metastasis from a breast carcinoma. J Neurooncol, 97(1), 123–126. doi: 10.1007/s11060-009-9988-z [DOI] [PubMed] [Google Scholar]
- Deshpande K, Martirosian V, Nakamura BN, Iyer M, Julian A, Eisenbarth R, . . . Neman J (2021). Neuronal exposure induces neurotransmitter signaling and synaptic mediators in tumors early in brain metastasis. Neuro-oncology. doi: 10.1093/neuonc/noab290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianni L, Baselga J, Eiermann W, Porta VG, Semiglazov V, Lluch A, . . . Bonadonna G (2009). Phase III Trial Evaluating the Addition of Paclitaxel to Doxorubicin Followed by Cyclophosphamide, Methotrexate, and Fluorouracil, As Adjuvant or Primary Systemic Therapy: European Cooperative Trial in Operable Breast Cancer. Journal of Clinical Oncology, 27(15), 2474–2481. doi: 10.1200/Jco.2008.19.2567 [DOI] [PubMed] [Google Scholar]
- Herrera RA, Deshpande K, Martirosian V, Saatian B, Julian A, Eisenbarth R, . . . Neman J (2021). Cortisol promotes breast-to-brain metastasis through the blood-cerebrospinal fluid barrier. Cancer Rep (Hoboken), e1351. doi: 10.1002/cnr2.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera RA, Deshpande K, Martirosian V, Saatian B, Julian A, Eisenbarth R, . . . Neman J (2022). Cortisol promotes breast-to-brain metastasis through the blood-cerebrospinal fluid barrier. Cancer Rep (Hoboken), 5(4), e1351. doi: 10.1002/cnr2.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann S, Budig L, Gardner H, Koziol J, Deuschle M, Schilling C, & Wagner S (2010). Matrix metalloproteinases in peripheral blood and cerebrospinal fluid in patients with Alzheimer’s disease. Int Psychogeriatr, 22(6), 966–972. doi: 10.1017/s1041610210000827 [DOI] [PubMed] [Google Scholar]
- Huehnchen P, Boehmerle W, Springer A, Freyer D, & Endres M (2017). A novel preventive therapy for paclitaxel-induced cognitive deficits: preclinical evidence from C57BL/6 mice. Transl Psychiatry, 7(8), e1185. doi: 10.1038/tp.2017.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Liu F, & Gong CX (2016). Tau and neurodegenerative disease: the story so far. Nature Reviews Neurology, 12(1). doi: 10.1038/nrneurol.2015.225 [DOI] [PubMed] [Google Scholar]
- Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, . . . Oktay MH (2017). Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med, 9(397). doi: 10.1126/scitranslmed.aan0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y, Higuchi F, Abe Y, Matsuda H, Kim P, & Ueki K (2013). Metastasis to the choroid plexus from thyroid cancer: case report. Neurol Med Chir (Tokyo), 53(11), 832–836. doi: 10.2176/nmc.cr2012-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer I, Vasiljevic A, Rey C, Fevre-Montange M, Saunders N, Strazielle N, & Ghersi-Egea JF (2012). Complexity and developmental changes in the expression pattern of claudins at the blood-CSF barrier. Histochem Cell Biol, 138(6), 861–879. doi: 10.1007/s00418-012-1001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone JP, Lee AV, & Brufsky AM (2015). Prognostic factors and survival of patients with brain metastasis from breast cancer who underwent craniotomy. Cancer Medicine, 4(7), 989–994. doi: 10.1002/cam4.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, & Hollander GA (2000). Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis, 31(1), 80–84. doi: 10.1086/313922 [DOI] [PubMed] [Google Scholar]
- Lun MP, Monuki ES, & Lehtinen MK (2015). Development and functions of the choroid plexus–cerebrospinal fluid system. Nature Reviews Neuroscience, 16(8), 445–457. doi: 10.1038/nrn3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, . . . Martin SS (2010). Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene, 29(22), 3217–3227. doi: 10.1038/onc.2010.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Maeda S, Vossel K, & Mucke L (2011). The Many Faces of Tau. Neuron, 70(3), 410–426. doi: 10.1016/j.neuron.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neman J, Franklin M, Madaj Z, Deshpande K, Triche TJ, Sadlik G, . . . Zada G (2021). Use of predictive spatial modeling to reveal that primary cancers have distinct central nervous system topography patterns of brain metastasis. J Neurosurg, 1–9. doi: 10.3171/2021.1.JNS203536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nubling G, Levin J, Bader B, Israel L, Botzel K, Lorenzl S, & Giese A (2012). Limited cleavage of tau with matrix-metalloproteinase MMP-9, but not MMP-3, enhances tau oligomer formation. Exp Neurol, 237(2), 470–476. doi: 10.1016/j.expneurol.2012.07.018 [DOI] [PubMed] [Google Scholar]
- Ortiz-Otero N, Marshall JR, Lash B, & King MR (2020). Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer, 20(1), 873. doi: 10.1186/s12885-020-07376-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna PG, Citrin DE, Hildesheim J, Ahmed MM, Venkatachalam S, Riscuta G, . . . Coleman CN (2021). Therapy-Induced Senescence: Opportunities to Improve Anticancer Therapy. J Natl Cancer Inst, 113(10), 1285–1298. doi: 10.1093/jnci/djab064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, . . . Wolmark N (2008). Preoperative chemotherapy: Updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. Journal of Clinical Oncology, 26(5), 778–785. doi: 10.1200/Jco.2007.15.0235 [DOI] [PubMed] [Google Scholar]
- Ren X, Keeney JTR, Miriyala S, Noel T, Powell DK, Chaiswing L, . . . Butterfield DA (2019). The triangle of death of neurons: Oxidative damage, mitochondrial dysfunction, and loss of choline-containing biomolecules in brains of mice treated with doxorubicin. Advanced insights into mechanisms of chemotherapy induced cognitive impairment (“chemobrain”) involving TNF-alpha. Free Radic Biol Med, 134, 1–8. doi: 10.1016/j.freeradbiomed.2018.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoter M, Bodnar L, Duchnowska R, Stec R, Grala B, & Szczylik C (2011). The role of Tau protein in resistance to paclitaxel. Cancer Chemother Pharmacol, 68(3), 553–557. doi: 10.1007/s00280-011-1696-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy J, Wefel JS, Ahles T, Tannock IF, & Schagen SB (2008). Cancer and cancer-therapy related cognitive dysfunction: an international perspective from the Venice cognitive workshop. Ann Oncol, 19(4), 623–629. doi: 10.1093/annonc/mdm500 [DOI] [PubMed] [Google Scholar]
- Waks AG, & Winer EP (2019). Breast Cancer Treatment: A Review. JAMA, 321(3), 288–300. doi: 10.1001/jama.2018.19323 [DOI] [PubMed] [Google Scholar]
- Wefel JS, & Schagen SB (2012). Chemotherapy-Related Cognitive Dysfunction. Current Neurology and Neuroscience Reports, 12(3), 267–275. doi: 10.1007/s11910-012-0264-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the main text or the supplementary materials.


