Abstract
Ketamine may have antidepressant properties, but its acute psychoactive effects complicate successful masking in placebo-controlled trials. We present a single-center, parallel-arm, triple-masked, randomized, placebo-controlled trial assessing the antidepressant efficacy of intravenous ketamine masked by surgical anesthesia (ClinicalTrials.gov, NCT03861988). Forty adult patients with major depressive disorder who were scheduled for routine surgery were randomized to a single infusion of ketamine (0.5 mg/kg) or placebo (saline) during usual anesthesia. All participants, investigators, and direct patient care staff were masked to treatment allocation. The primary outcome was depression severity measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) at 1, 2, and 3 days post-infusion. After all follow-up visits, participants were asked to guess which intervention they received. A mixed-effects model showed no evidence of effect of treatment assignment on the primary outcome (−5.82, 95% CI −13.3 to 1.64, p=0.13). 36.8% of participants guessed their treatment assignment correctly; both groups allocated their guesses in similar proportions. In conclusion, a single dose of intravenous ketamine delivered during surgical anesthesia had no greater effect than placebo in acutely reducing the severity of depressive symptoms in adults with major depressive disorder. This trial successfully masked treatment allocation in moderate-to-severely depressed patients using surgical anesthesia. Although this masking strategy is impractical for most placebo-controlled trials, future studies of novel antidepressants with acute psychoactive effects should make efforts to fully mask treatment assignment in order to minimize subject-expectancy bias.
INTRODUCTION
Ketamine, a dissociative anesthetic with multiple molecular targets1,2, is associated with rapid-acting antidepressant effects in patients with major depressive disorder (MDD), including those with treatment-resistant depression (TRD)3–5. Across studies, an intravenous infusion of 0.5 mg/kg ketamine produces a clinical response in 41% and remission in 19% of patients with TRD at 24 hours6. Therapeutic effects appear within 2 hours of a single ketamine infusion5.
In most randomized controlled trials (RCTs) of ketamine for depression, participant masking has been nearly impossible given the drug’s obvious acute psychological effects. Inadequate masking presents a major confound to interpreting studies of ketamine, as well as other rapid-acting psychoactive therapeutics such as psilocybin and methylenedioxymethamphetamine (MDMA)7–10, to the extent that most investigations do not report on the success of participant masking10. Incomplete masking may lead to subject-expectancy bias, which occurs when a research subject has an expectation for a given result that influences the reported outcome. Subject-expectancy bias may contribute to overestimation of treatment effect sizes in antidepressant trials involving ketamine11.
We utilized ketamine’s established safety in surgical settings by conducting a randomized placebo-controlled trial in which the administration of ketamine was masked by other surgical anesthetics. The primary aim of this study was to determine whether ketamine, given at a dose of 0.5 mg/kg over 40 minutes during surgical anesthesia, produces a greater antidepressant effect than placebo. We recruited patients with depression severity comparable to previous studies and analyzed similar follow-up time points. We hypothesized that ketamine is superior to an inert placebo (0.9% sodium chloride, i.e., normal saline) in reducing depression symptoms within the first 3 days post-infusion in a population of adults with moderate-to-severe levels of MDD. A supportive aim was to test whether a conscious dissociative reaction to ketamine is needed for an antidepressant response.
Results
PARTICIPANTS
Participant recruitment occurred between February 2020 and August 2022. The first patient was enrolled in the randomized trial on February 19, 2020, and the final patient was enrolled on August 18, 2022. The last day of follow-up was September 9, 2022. For participant flow through the clinical trial, see the CONSORT diagram (Figure 1). The screening visit occurred between 27 days and 16 hours prior to surgery (mean (SD): 5.1 (4.6) days). The mean age of trial participants was 51 years; they were mostly female (70%), white (65%), non-Hispanic (87.5%), employed (62.5%), and never smoked (65%). At screening, both groups had moderate levels of depression as rated by the Montgomery-Åsberg Depression Rating Scale (MADRS; mean score for ketamine: 27.7, placebo: 30.6) and moderate levels of treatment resistance as measured by the Maudsley Staging Method (MSM; mean score for ketamine: 8.3, placebo: 7.5). The presence of symptomatic depression was also supported by the Hospital Anxiety and Depression Scale (HADS; mean score for ketamine: 24.6, placebo: 24.7). Although current MDD episode durations were longer in ketamine group, the difference did not reach significance (ketamine: median = 38 months, placebo: 17). Both groups also scored similarly on the Brief Pain Inventory Short Form (BPI-SF) at screening, except for two questions in which participants in the ketamine group reported having more pain interference with sleep (mean 7.7 versus 5.5, p=0.02) and enjoyment of life (mean 7.6 versus 5.7, p=0.04). Other characteristics were similar between groups (Table 1).
Figure 1. CONSORT Flow Diagram.
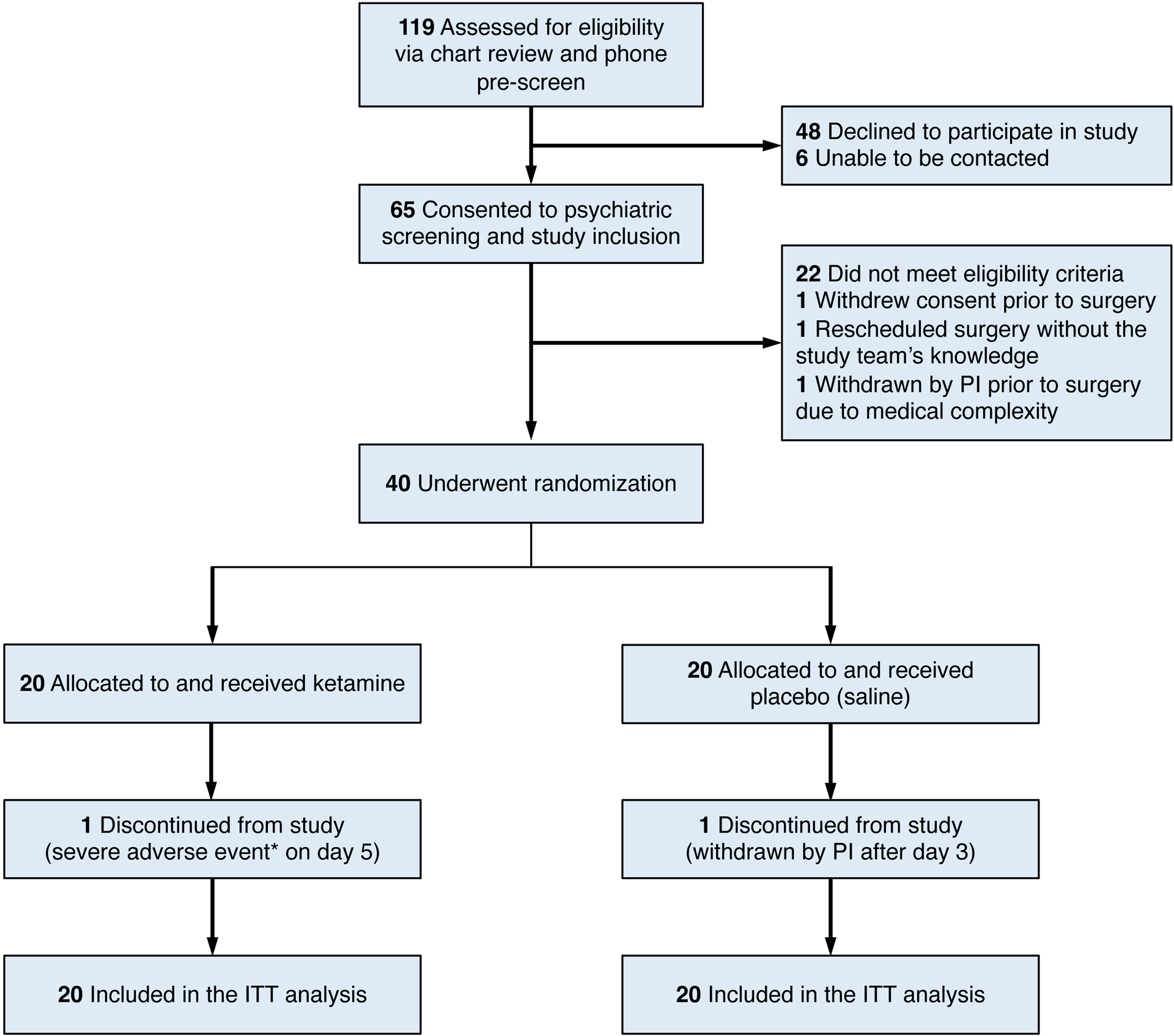
CONSORT, Consolidated Standards of Reporting Trials. PI, principal investigator. ITT, intention-to-treat. Participants were randomized to a single intravenous dose of either ketamine or saline, given during surgical anesthesia. *The severe adverse event refers to an unexpected death that occurred 2 days after the patient was discharged home without complications on postoperative day 3; this patient experienced a witnessed cardiac arrest, attributed to the participant’s medical factors and not resulting from study procedures. †One participant was withdrawn by the PI due to an unanticipated surgical revision of an implanted device, which took place on postoperative day 3 after study assessments were completed.
Table 1.
Demographics and Clinical Characteristics of the Participants at Baseline*
| Characteristic | Ketamine (N=20) | Placebo (N=20) | Overall (N=40) |
|---|---|---|---|
| Demographic characteristics | |||
| Age — yr | 50.5±12 | 51.9±18.9 | 51.2±15.7 |
| Female sex — no. (%) | 12 (60) | 16 (80) | 28 (70) |
| Male sex — no. (%) | 8 (40) | 4(20) | 12 (30) |
| Race — no. (%)† | |||
| White | 13 (65) | 13 (65) | 26 (65) |
| Black | 2 (10) | 2 (10) | 4 (10) |
| Asian | 0 (0) | 2 (10) | 2 (5) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 1 (5) | 1 (2.5) |
| More than one race | 2 (10) | 0 (0) | 2 (5) |
| Unknown or not reported | 3 (15) | 2 (10) | 5 (12.5) |
| Ethnicity — no. (%)† | |||
| Hispanic | 2 (10) | 2 (10) | 4 (10) |
| Non-Hispanic | 18 (90) | 17 (85) | 35 (87.5) |
| Unknown or not reported | 0 (0) | 1 (5) | 1 (2.5) |
| Married or life partnered — no. (%) | 9 (45) | 9 (45) | 18 (45) |
| Employed — no. (%) | 13 (65) | 12 (60) | 25 (62.5) |
| Never smoked — no. (%) | 14 (70) | 12 (60) | 26 (65) |
| Body mass index — kg/m2 | 29.9±5 | 29.5±4.4 | 29.7±4.7 |
| Previous ketamine exposure — no. (%) | |||
| None or did not know | 15 (75) | 19 (95) | 34 (85) |
| Received in a medical setting | 5 (25) | 1 (5) | 6 (15) |
| Used recreationally | 0 (0) | 0 (0) | 0 (0) |
| Psychiatric history | |||
| Age at first MDD onset — yr | 31.0±14.3 | 21.8±15.0 | 26.5±15.2 |
| Duration of current MDD episode — months | |||
| Mean | 80.1 | 43.7 | 61.9 |
| Median | 38 | 17 | 24 |
| Duration of current MDD episode — no. (%) | |||
| Acute (≤12 months) | 6 (30) | 10 (50) | 16 (40) |
| Subacute (13–24 months) | 1 (5) | 7 (35) | 8 (20) |
| Chronic (>24 months) | 13 (65) | 3 (15) | 16 (40) |
| Recurrent MDD — no. (%) | 11 (55) | 16 (80) | 27 (67.5) |
| Number of antidepressants trialed — no. (%) | |||
| 1–2 | 9 (45) | 7 (35) | 16 (40) |
| 3–4 | 4 (20) | 9 (45) | 13 (32.5) |
| 5–6 | 6 (30) | 2 (10) | 8 (20) |
| 7–10 | 0 (0) | 0 (0) | 0 (0) |
| >10 | 1 (5) | 0 (0) | 1 (2.5) |
| Antidepressant augmentation trialed — no. (%) | 3 (15) | 7 (35) | 10 (25) |
| Electroconvulsant therapy trialed — no. (%) | 0 (0) | 0 (0) | 0 (0) |
| Maudsley Staging Method score | 8.3±2.3 | 7.5±1.7 | 7.9±2 |
| Maudsley Staging Method resistance category — no. (%) | |||
| Mild (<7) | 4 (20) | 5 (25) | 9 (22.5) |
| Moderate (≥7 and <11) | 13 (65) | 14 (70) | 27 (67.5) |
| Severe (≥11) | 3 (15) | 1 (5) | 4 (10) |
| Depression scores | |||
| PHQ-8 total score‡ | 15.8±3.6 | 15.8±3.8 | 15.8±3.7 |
| MADRS total score§ | 27.7±7.8 | 30.6±6.3 | 29.1±7.1 |
| HADS¶ | |||
| Total score | 24.6±6.0 | 24.7±5.7 | 24.6±5.8 |
| Depression subscore | 12.0±3.2 | 10.5±3.6 | 11.2±3.5 |
| Anxiety subscore | 12.6±4.4 | 14.2±3.3 | 13.4±3.9 |
Plus–minus values represent means±SD. Participants were randomized to a single intravenous dose of either ketamine or saline, given during surgical anesthesia. Percentages may not total 100 due to rounding. MDD denotes major depressive disorder.
Race and ethnicity were reported by the participants.
Total scores on the 8-item Patient Health Questionnaire (PHQ-8) range from 0 to 27, with higher scores indicating greater severity of depression.
Total scores on the Montgomery–Åsberg Depression Rating Scale (MADRS) range from 0 to 60, with higher scores indicating greater severity of depression.
Total scores on the Hospital Anxiety and Depression Scale (HADS) range from 0 to 42, with higher scores indicating greater severity of anxiety and depression; subscales range from 0 to 21.
Missing data: Ethnicity for 1 participant in the placebo group; marital status for 2 participants in the ketamine group and 1 participant in the placebo group; age of first MDD onset for 1 participant in the placebo group; recurrent MDD type for 2 participants in the ketamine group; number of antidepressants trialed for 2 participants in the placebo group.
Table 2 summarizes participants’ surgical and anesthetic characteristics. Participants in both study arms had similar levels of disease burden, as measured by the American Society of Anesthesiologists (ASA) Physical Status Classification12, as well as the Charlson Comorbidity Index13. Patients presented to a range of surgical departments which were distributed similarly between the two groups. With regards to anesthetic type, all except one underwent general anesthesia. One participant in the ketamine group had monitored anesthesia care with a neuraxial block; however, the depth of anesthesia was within study parameters. Two participants in the ketamine arm and one participant in the placebo arm received nitrous oxide (N2O) at a concentration of ≥ 50% for ≥1 hour. Use of preoperative and intraoperative opioids and length of surgery were similar between groups.
Table 2.
Surgical and Anesthetic Characteristics of the Participants*
| Characteristic | Ketamine (N=20) | Placebo (N=20) | Overall (N=40) |
|---|---|---|---|
| ASA Physical Status Classification — no. (%)† | |||
| I | 0 (0) | 1 (5) | 1 (2.5) |
| II | 12 (60) | 15 (75) | 27 (67.5) |
| III | 8 (40) | 4 (20) | 12 (30) |
| CCI total score‡ | |||
| Mean | 2.1±2.7 | 2.7±2.6 | 2.4±2.7 |
| Median | 1 | 2.5 | 1.5 |
| Surgery department — no. (%) | |||
| General surgery | 6 (30) | 3 (15) | 9 (22.5) |
| Orthopedics, non-spine | 5 (25) | 4 (20) | 9 (22.5) |
| Otolaryngology | 2 (10) | 2 (10) | 4 (10) |
| Gynecology | 2 (10) | 3 (15) | 5 (12.5) |
| Urology | 2 (10) | 0 (0) | 2 (5) |
| Neurosurgery, non-intracranial | 1 (5) | 3 (15) | 4 (10) |
| Orthopedics, spine | 1 (5) | 2 (10) | 3 (7.5) |
| Thoracic | 1 (5) | 0 (0) | 1 (2.5) |
| Plastics | 0 (0) | 3 (15) | 3 (7.5) |
| Anesthesia type — no. (%) | |||
| General anesthesia | 19 (95) | 20 (100) | 39 (97.5) |
| Neuraxial block with sedation | 1 (5) | 0 (0) | 1 (2.5) |
| Maintenance anesthetics used — no. (%) | |||
| Nitrous oxide§ | 2 (10) | 1 (5) | 3 (7.5) |
| Propofol — no. (%) | 18 (90) | 17 (85) | 35 (87.5) |
| Sevoflurane — no. (%) | 17 (85) | 14 (70) | 31 (77.5) |
| Isoflurane — no. (%) | 1 (5) | 3 (15) | 4 (20) |
| Use of regional anesthesia — no. (%) | 1 (5) | 3 (15) | 4 (10) |
| Use of preoperative opioids — no. (%) | 9 (45) | 7 (35) | 16 (40) |
| Preoperative opioids — MME per day ¶ | |||
| Mean | 9.1±16.4 | 10.5±27.7 | 9.8±22.4 |
| Median | 0 | 0 | 0 |
| Use of intraoperative opioid infusion — no. (%) | 5 (25) | 7 (35) | 12 (30) |
| Length of surgery — min | 244±121 | 269±109 | 256±114 |
Plus–minus values represent means±SD.
American Society of Anesthesiologists (ASA) Physical Status Classification ranges from I to VI, with higher values indicating greater number and severity of pre-anesthesia medical comorbidities.
Total scores on the Charlson Comorbidity Index (CCI) range from 0 to 37, with higher scores indicating greater number and severity of pre-anesthesia medical comorbidities.
Inhaled nitrous oxide concentration of at least 50% for at least 1 continuous hour.
Milligram morphine equivalents (MME) represent potencies of opioids relative to oral morphine, with higher values indicating higher opioid doses.
OUTCOMES
For the primary outcome on post-infusion days 1, 2, and 3, the mixed-effects model (Table 3) showed no evidence of effect of group assignment on MADRS scores (95% CI −13.3 to 1.64, p=0.13, n=20 per arm). The MADRS rate of change also did not differ between groups (95% CI −1.54 to 4.93, p=0.30). An alternative model using change from pre-infusion baseline MADRS scores on the day of surgery (“day 0”) also showed no between-groups difference in change scores (95% CI −6.25 to 7.89, p=0.82). The rate of change for the MADRS change scores also did not differ between groups (95% CI −1.96 to 4.62, p=0.43). Missing MADRS scores among enrolled participants did not exceed 5% at any visit; therefore, missing data were not imputed for the primary outcome.
Table 3.
Linear Mixed Model Estimates for MADRS Scores
| MADRS scores from post-infusion days 1 to 3 | ||||
|---|---|---|---|---|
| Coefficient | 95% CI | t-value | p-value | |
| Intercept | 16.6 | 11.3 to 21.9 | 6.15 | <0.001 |
| Group | −5.82 | −13.3 to 1.64 | −1.53 | 0.13 |
| Time | 0.24 | −2.04 to 2.52 | 0.21 | 0.84 |
| Group×Time | 1.70 | −1.54 to 4.93 | 1.03 | 0.30 |
| Change from pre-infusion baseline MADRS scores on post-infusion days 1 to 3 | ||||
| Coefficient | 95% CI | t-value | p-value | |
| Intercept | −15.0 | −20.1 to −9.95 | −5.79 | <0.001 |
| Group | 0.82 | −6.25 to 7.89 | 0.23 | 0.82 |
| Time | 0.58 | −1.77 to 2.93 | 0.49 | 0.63 |
| Group×Time | 1.33 | −1.96 to 4.62 | 0.79 | 0.43 |
MADRS, Montgomery–Åsberg Depression Rating Scale. CI, confidence interval. The effect of group on MADRS scores from post-infusion days 1 to 3 was not significant (p=0.13 using absolute scores and p=0.82 using change from baseline scores). Significance was evaluated using likelihood ratio tests and applying the normal approximation to Wald t values from the model output. No adjustments for multiple comparisons were made for the two models presented here.
Pre-infusion baseline MADRS scores on day 0 did not differ between trial groups (ketamine: 25.1 [SD 8.3]; placebo: 29.9 [SD 7.0]). From day 0 to day 1, the average change in MADRS scores was −12.4 points (SD 9.2) in the ketamine group and −14.7 points (SD 9.0) in the placebo group, corresponding to a mean decrease of 46% and 48% on the MADRS, respectively. In both groups, MADRS scores increased slightly on day 2 relative to the nadir at day 1, but the decrease from baseline persisted through all follow-up time points up to day 14 (Figure 2A). The HADS scores also followed a trajectory similar to the MADRS scores (Extended Data Table 1).
Figure 2. Depression Severity, Masking Assessment and Other Outcomes.
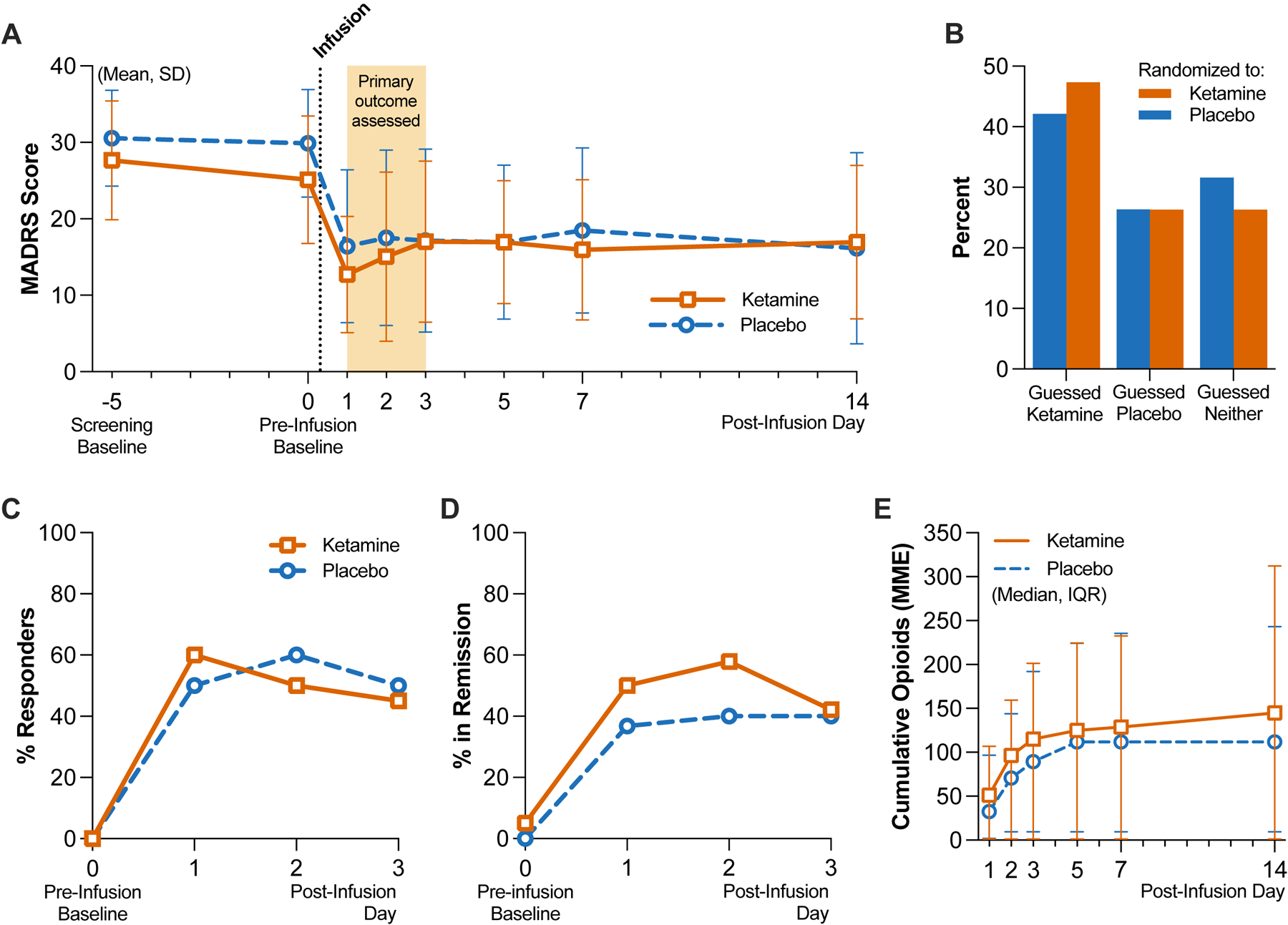
Panel A shows the mean and standard deviation (SD) scores by group on the Montgomery-Åsberg Depression Rating Scale (MADRS; scores range from 0 to 60, with higher scores indicating greater depression); the screening baseline visit occurred on average 5 days before infusion on day 0. Total N=39 independent participant responses per day, on post-infusion days 1 through 3 (see Extended Data Table 1 for group-specific counts). Panel B shows the distribution of guesses as a percentage of each group (n=19 per group) made by participants when asked to guess which treatment they received after the last follow-up visit. Panel C shows the difference in MADRS scores relative to pre-infusion baseline scores obtained on day 0 (same sample size as reflected in Panel A). Panels D shows the proportions of clinical response, respectively as a percentage of each group (n=20 per group), within the first 3 days. Panel E shows the cumulative opioid consumption in MME by group, represented as median and interquartile range (IQR); both inpatient and outpatient opioids were included in the total (n=20 per group).
At the end of the follow-up period (day 14), participants were asked to guess which intervention they had received; 36.8% of all participants guessed correctly, and the distribution of guesses between groups was comparable, with Cohen’s kappa = 0.33, indicating fair agreement between groups (Figure 2B). We performed an exploratory analysis to determine whether patients’ guesses regarding their treatment allocation were related to their final MADRS score (day 14). We regrouped MADRS data according to patient guess (“ketamine”, “placebo”, or “I don’t know”; Extended Data Figure 1). At day 14, aggregating across actual treatment allocations, mean [SD] MADRS scores were, for patients guessing “ketamine” (n=16), 10.1 [7.2]; guessing “placebo” (n=10), 19.2 [7.7]; replying “I don’t know” (n=12), 23.0 [13.7]. A simple logistic regression of day 14 MADRS score onto guessing either “ketamine” (coded ‘1’) or otherwise (coded ‘0’) suggested a significant inverse relationship between these two variables (odds ratio = 0.89 [95% CI 0.81 to 0.96]; p=0.001). MADRS change scores relative to day 0 are visualized in Figure 2C. A secondary outcome was clinical response, defined as ≥50% reduction in MADRS scores from screening baseline. On post-infusion day 1, 60% and 50% of participants in the ketamine and placebo group, respectively, met criteria for clinical response. Rates of clinical response in both trial groups remained similar to each other on post-infusion days 2 and 3 (Figure 2D, Extended Data Table 1). We also analyzed remission, which we defined in our study as MADRS score of ≤12. Remission occurred in 50% and 35% of participants in the ketamine and placebo group, respectively, on post-infusion day 1; this difference closed on post-infusion day 3, when 40% of both groups remained in remission (Extended Data Table 1).
Cumulative opioid consumption and average daily inpatient opioid use did not differ between groups (Figure 2E, Extended Data Table 2). On postoperative days 7 and 14, five participants in the ketamine group and two participants in the placebo group were still using opioids. Of note, no participants received either preoperative or postoperative methadone or buprenorphine, nor were any maintained on opioid antagonist therapy. Average pain intensity on the BPI-SF at 14 days post-infusion was not different between groups (ketamine: 4.8 [SD 1.5]; placebo: 3.7 [SD 2.0]). Pain interference on the BPI-SF at day 14 post-infusion also did not differ between groups (ketamine: 6.2 [SD 2.2]; placebo: 5.7 [SD 3.5]). Hospital length of stay was longer in the placebo group (mean 1.9 [SD 1.7] days versus 4.0 [SD 3.3] days, p=0.02).
PROTOCOL VIOLATIONS AND SAFETY
One participant in the ketamine group initially met inclusion criteria at screening but was retrospectively found not to have maintained her symptom severity on the morning of surgery (additive MADRS and HADS score of 30, below the minimum of 31). This participant was randomized and included in the intention-to-treat analysis. No protocol deviations related to the administration of the study drug occurred in this trial.
Adverse events were evaluated at every visit. Notably, one death in the ketamine group occurred 5 days post-infusion, which triggered the unmasking of treatment assignment. The patient had been discharged from the hospital on postoperative day 3, with no surgical or anesthetic complications documented prior to discharge. Subsequently, the patient experienced a witnessed cardiac arrest at home; advanced cardiac life support was initiated by paramedics and continued in the emergency room until the patient expired. One patient in the placebo group experienced a surgical complication requiring reoperation on postoperative day 3.
SENSITIVITY ANALYSES
We tested whether our results were sensitive to: 1) a possible difference in pre-infusion baseline MADRS scores, and 2) the exclusion of participants who received N2O, an anesthetic which may also have antidepressant qualities14. We adjusted for a possible difference in pre-infusion baseline MADRS scores by including it as a fixed covariate and specifying random effects only for slopes in our mixed-effects model; this showed no between-group difference in MADRS scores (95% CI −8.41 to 4.49). When we applied our original mixed-effects model for the primary outcome after excluding 3 participants who received N2O at a concentration of 50% for at least 1 hour, there was also no between-group difference in MADRS scores (95% CI −13.3 to 2.74, p=0.13).
Discussion
This randomized, triple-masked trial compared the short-term antidepressant efficacy of ketamine with placebo in adults with moderate-to-severe depression. There was no effect of treatment on our primary outcome, MADRS scores on days 1, 2, and 3 post-infusion. Baseline MADRS scores obtained at screening and on the day of surgery did not meaningfully differ between groups. In both trial groups, the observed decrease in MADRS score at day 1 was similar to, or exceeded, the decreases observed in previous ketamine trials in awake patients15–18. The variance in MADRS change scores observed in our study is also comparable to previous studies in awake patients19, supporting our a priori power calculation to detect a between-group difference. The HADS, an alternative patient-rated depression scale, yielded a similar score trajectory as the MADRS and strengthens our conclusion that ketamine and placebo did not differentially impact mood in this trial. Participant retention was excellent, with no loss to follow-up occurring within the primary outcome window. Notably, one participant death occurred in the ketamine group. However, this severe adverse event was attributed to underlying cardiovascular comorbidities rather than a direct result of trial procedures, consistent with previous analyses of cardiovascular safety outcomes after intravenous ketamine infusion19.
Both ketamine and placebo groups appeared to show a strong antidepressant response, though counter to our hypothesis, the magnitude of this response did not differ between groups. We review the available evidence for several potential interpretations of this result, while noting that the relatively small sample size, the unusual background of surgical anesthesia on which treatments were delivered, and our two-arm study design limit broadly generalizable conclusions about ketamine’s efficacy or mechanism. To the extent that the treatment effect in the ketamine group is similar to other studies, our data raise the possibility that antidepressant effects of ketamine may be achieved in the absence of a typical ketamine-induced conscious dissociative experience. However, a conscious dissociative experience may yield an even more robust response, significantly greater than that seen with placebo, in a situation where treatment-arm masking is maintained without the use of anesthesia. Furthermore, adjunctive psychotherapy may act syngergistically with the acute subjective effects of ketamine treatment (e.g. Ketamine-Assisted Psychotherapy), potentially outperforming ketamine-only treatment20, though controlled data on this form of ketamine therapy is still lacking21. Nonetheless, our successful masking of treatment allocation, and associated large apparent treatment effect across groups, may have implications for interpreting results from studies of acutely psychoactive treatments where trials are not designed for adequate treatment masking. In this study, patients’ guess regarding group allocation was strongly related with their MADRS score at the end of the study (14 days post infusion), potentially reflecting a prior belief about the efficacy of ketamine treatment for depression. Together, these data point to a major role for extra-pharmacological effects in the response to ketamine among depressed patients.
The surprisingly robust clinical response and remission rate observed in both arms of this trial raises the question of whether anesthetics besides ketamine may have antidepressant effects. N2O has been shown to improve depression symptoms in patients with TRD14,22. However, only 3/40 participants in our study were exposed to N2O at a concentration of 50% for ≥ 1 hour, making it unlikely to impact depression scores at the group level, as confirmed in our sensitivity analyses. Propofol infusions and inhaled isoflurane have also shown antidepressant properties when given at doses that suppress EEG activity (“burst suppression”) for 15 minutes, over multiple administrations23,24, though these findings are not consistent25 and differ substantially in depth and timing from standard surgical anesthesia used in our study (the recommended PSI range of 25–50 avoids burst suppression). We also considered the possibility that surgery and general anesthesia without ketamine has an antidepressant effect. However, our review of prior studies measuring symptoms of depression in the perioperative period strongly suggests otherwise. In the control arms (surgery and anesthesia alone) from studies in both depressed26–30 and non-depressed31,32 patient populations, primary mood outcomes in the first days after surgery reflect either no significant change26,29,31,33,34 or possibly worsened symptoms27,32. The anesthetic regimens used across these studies broadly resemble our own (most commonly propofol, sevoflurane and opioid-based), though the anesthetic depth was rarely noted. Taken together, these data suggest that non-pharmacological factors can strongly influence reported depression outcomes.
The lack of separation between placebo and ketamine groups in our trial may indicate that anesthetic agents blocked the antidepressant effects of ketamine. This possibility is somewhat difficult to reconcile with our observed effect size (comparing pre- to post-treatment), which is within the range of most previously observed ketamine effects sizes6. However, anesthetic agents may have interfered with neural mechanisms required for the antidepressant effect of ketamine, leaving in place, for example, large expectancy-driven antidepressant effects. Among several potential molecular and neural circuit-based mechanisms for the antidepressant effects of ketamine35–37, a popular model is that ketamine, via antagonism of cortical N-methyl-D-aspartate receptors (NMDARs), enhances cortical glutamate release and triggers neuroplastic changes through activity dependent release of neurotrophic factors. In this model, anesthetic agents could blunt ketamine effects by reducing excitability of cortical neurons. GABAergic anesthetic agents do indeed reduce cortical activity38, however emerging human data suggest that ketamine’s mechanisms may be substantially more complex than once thought. Based on a glutamate modulation theory of ketamine antidepressant action, multiple compounds have been tested as antidepressants in clinical trials and have not separated from placebo39–44, suggesting that other molecular targets may be involved. Further, interactions between ketamine and anesthetic agents like propofol may have both antagonistic and additive effects in cortical and subcortical networks45,46, possibly linked to biologically and clinically significant actions of ketamine at, among other targets1, hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (HCN1)45–49, as well as opioid receptors2,50–53. Opioids are also routinely used during surgical anesthesia, and recent evidence shows that blocking opioid receptors attenuates the antidepressant effect of ketamine50,54. No patients were on opioid antagonist or partial agonist therapy, and the average daily MME use in both groups prior to surgery was relatively low. Our review of available data suggests that surgical anesthesia does not have substantial intrinsic antidepressant efficacy, and while we cannot exclude the possibility that anesthetic agents interfered with the antidepressant effect of ketamine, the antidepressant mechanism of ketamine in humans is not well enough characterized to make such a determination at this time. We hypothesize that a minimal dose of anesthetic agent may allow for adequate treatment masking and minimal interference with putative ketamine antidepressant mechanisms.
Baseline heterogeneity in psychiatric characteristics could potentially explain the smaller-than-anticipated difference in post-treatment depression scores. Although clinical and sociodemographic characteristics were largely similar between trial groups, there was a notable difference in current MDD episode length—with the ketamine group having a longer median episode duration (38 months) compared to the placebo group (17 months). A longer current MDD episode may predict more treatment resistance to traditional antidepressants55. However, studies comparing characteristics of responders and non-responders to ketamine therapy have been mixed, with some studies showing that current MDD episode length impacts treatment response56 while other studies do not57,58. We also cannot rule out the effect of surgical heterogeneity between groups, which we did not control for in our recruitment design; however, between-group differences in case counts did not exceed 3 for any surgical department, and intraoperative factors, including length of surgery and types of anesthetic used, did not meaningfully differ between groups.
Contrary to our primary findings and other secondary findings, a between-groups difference was observed in the hospital length of stay. Participants who received ketamine were discharged 2 days earlier on average than participants who received placebo. Although this difference reached statistical significance (p=0.02), it is important to note that such significance would not withstand corrections for multiplicity. Furthermore, previous randomized trials have failed to demonstrate reductions in postoperative length of stay with intraoperative ketamine administration59–62. Nevertheless, our findings suggest the potential for a unique response to perioperative ketamine administration among clinically depressed patients.
Other studies have also evaluated the effect of ketamine on mood ratings in surgical patients30; however, numerous methodological limitations prevent direct comparison to studies of intravenous ketamine for depression in the psychiatric literature. Frequently, these trials were not conducted in a population likely to meet criteria for moderate-to-severe MDD31,32,63–65. In the Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study—the largest study to date comparing ketamine with saline in surgical patients—depression scores were analyzed as a secondary outcome from a study designed to evaluate the efficacy of ketamine for postoperative delirium in patients >60 years old. Notably, only 9.6% of participants met the PHQ-8 cutoff for moderate depression preoperatively, and no diagnostic data or clinician-rated scales were reported27. Among perioperative studies that recruited patients with at least mild-moderate symptoms of depression26,27,29,33, comparison with psychiatric trials in conscious patients is complicated by the use of non-standard ketamine doses and methods of administration26,27,33, reliance on patient-reported scales versus clinician-administered scales outcome measures26,27, and treatment masking was either not described or not assessed. Kudoh et al. and Liu et al. enrolled surgical patients with mild to moderate depression severity. A 2021 RCT testing ketamine during surgical anesthesia required moderate-to-severe depression (MADRS ≥ 22) for eligibility; however, these participants underwent intracranial tumor resection29, a population we excluded due to the possibility of mood and personality changes associated with cortical lesions and resections of such lesions66,67.
A key strength of our trial was the evaluation of participant masking. At their last follow-up visit, patients in both groups allocated their guesses in similar proportions and fewer than half guessed correctly. The intervention was effectively masked—an uncommon finding among antidepressant trials with ketamine. Assessment of masking is also rare among RCTs involving ketamine and electroconvulsive therapy (ECT), an important antidepressant treatment delivered to briefly anesthetized patients. Most RCTs evaluating the effect of adjunctive ketamine on ECT outcomes have found no benefit of ketamine given at doses of 0.5 mg/kg or greater68. Of the RCTs reviewed by McGirr et al., only one reported on masking effectiveness69.
Outcome expectancy related to the stated intent of the trial may drive apparent treatment effects. Previous studies of ketamine in surgical patients generally find that when patients are recruited to test ketamine’s antidepressant effect as a primary outcome, depression scores decrease postoperatively. Conversely, patients in the PODCAST study, who were recruited to participate in a trial focused on reducing postoperative delirium, depression symptoms (a secondary outcome) worsened slightly in the postoperative period.
One limitation of our study is that we did not assess the blind of the anesthesiologists who administered the study drug. While it is possible that close inspection of the intraoperative processed EEG could reveal changes consistent with a 0.5 mg/kg subanesthetic ketamine infusion70, we specifically instructed the anesthesiologists to avoid altering the patient’s anesthetic in response to the processed EEG during drug infusion (barring large excursions in PSI that correlate with patient awareness). Nonetheless, we cannot exclude the possibility that anesthesiologists who guessed the patient’s treatment allocation may have altered their anesthetic in a way that influenced postoperative mood.
Our results suggest that when differential subject-expectancy bias is minimized with successful masking, the treatment effect size of ketamine is reduced considerably. However, a major limitation of our study is that we did not measure treatment expectancies prior to randomization. Therefore, we cannot definitively conclude that subject-expectancy bias mediates the causal relationship between effective masking and smaller treatment effect sizes. Regardless of the intervention being tested, subject expectations of a positive outcome—also known as hope—may drive large decreases in depression symptoms seen in antidepressant trials71. Our trial design cannot distinguish between a null-effect of ketamine for depression and an occlusion of ketamine’s antidepressant effect through a placebo-like mechanism maintained in the absence of unmasking.
CONCLUSION
This trial utilized surgical anesthesia to successfully mask the allocation of a single antidepressant dose of ketamine or placebo in a sample of depressed patients and found that depression scores at 1, 2, and 3 days post-infusion did not differ between trial groups. Both groups improved similarly. With the exception of a shorter hospital length of stay in individuals who received ketamine, the secondary outcomes did not demonstrate an advantage of ketamine over placebo. Our primary findings differ from those of prior antidepressant trials with ketamine conducted without adequate masking, which find robust effects of ketamine. Confounding surgical and anesthetic factors in our study prevent a determination of whether ketamine, on its own, is an effective short-term treatment of MDD. However, our robust, masked placebo response suggests that previously reported large effect sizes for ketamine may reflect a degree of expectancy bias. While it is impractical to use surgical anesthesia for most placebo-controlled trials, future studies of novel antidepressants with acute psychoactive effects should make stronger efforts to mask treatment assignment to minimize the effects of subject-expectancy bias.
Methods
TRIAL OVERSIGHT
This was an investigator-initiated sponsored by the university and the Society for Neuroscience in Anesthesiology and Critical Care. The trial protocol was approved by the institutional review board at Stanford University (Protocol #49114) and all participants gave written informed consent. The trial protocol can be viewed in the Supplementary Information for this manuscript. Participants were compensated $50–100 for completing screening procedures and another $50 after all follow-up visits were completed. None of their medical costs were covered by the study. A Data Safety Monitoring Board was not required as the primary study intervention did not deviate from standard of care and posed no known incremental risk to participants. Randomization and drug compounding were handled by Stanford Health Care Investigational Drug Service.
PARTICIPANTS
Adults undergoing elective non-cardiac, non-intracranial surgery were recruited from preoperative clinics at Stanford University Medical Center. The 8-item Patient Health Questionnaire (PHQ-8) was distributed to patients through a perioperative mental health screening service. To be eligible for the study, patients must score ≥ 12 on the PHQ-8, corresponding with at least moderate depression72. Research staff screened electronic health records (EHR) of patients scheduled for surgery who scored ≥ 12 points on the PHQ-8; those without exclusion criteria documented in the EHR were introduced to the study and consented for an additional screening visit. Surgical clinics also referred patients with symptomatic depression who expressed interest in the trial. These patients were contacted by research staff for a telephone pre-screen, and qualifying patients were consented for an additional screening visit. At this visit, research staff collected information on demographics, medical, and psychiatric history, including level of antidepressant treatment resistance assessed by the Maudsley Staging Method (MSM)73. Inclusion and exclusion criteria were assessed via a hybrid approach of corroborating EHR data with patient self-report.
Inclusion criteria included English literacy, body mass index of 17–40 kg/m2, a diagnosis of MDD (single or recurrent) and a major depressive episode of ≥ 4 weeks duration prior to screening. The diagnosis of MDD was confirmed by the Mini International Neuropsychiatric Interview Module A74. Participants must also have had a combined score of ≥ 31 from the Montgomery-Åsberg Depression Rating Scale75 (MADRS) and the Hospital Anxiety and Depression Scale76 (HADS). These scales were administered in-person, or by secure video conference or telephone, which have been validated for the PHQ77, HADS78, and MADRS79,80.
Exclusion criteria included pregnancy, breastfeeding, moderate or severe substance use disorder, history of schizophrenia or schizoaffective disorder, history of psychotic symptoms in the current or previous depressive episodes, dementia or other amnestic cognitive disorder, history of surgery involving the brain or meninges, encephalitis, meningitis, or degenerative central nervous system disorder, clinically significant thyroid dysfunction within the past 6 months, and chronic use of > 90 morphine milligram equivalents (MME) per day. Patients at high risk of suicidal behavior on the Columbia-Suicide Severity Rating Scale81 were also excluded. Concurrent psychotherapy and antidepressant therapy were allowed if therapy was stable for ≥ 4 weeks prior to screening. Participation in any clinical trial with an investigational drug or device within the past month or concurrent to study participation was not allowed.
TRIAL DESIGN AND PROCEDURES
This was a triple-masked, randomized, placebo-controlled trial. Prior to randomization, five patients were recruited for an open label study to evaluate study procedures. Data from these five patients are not included in this manuscript. For the randomized trial, twenty participants were allocated to a single dose of intravenous ketamine (0.5 mg/kg diluted into 40 ml of normal saline, infused over 40 minutes using a programmable pump). Another twenty participants were allocated to 40 ml of normal saline infused similarly over 40 minutes. Pharmacy staff randomized participants using computerized block randomization with 5 blocks of 8. The participant, investigators, and direct care providers (e.g., anesthesiologists) were masked. Unmasking occurred after all 40 participants progressed through all follow-up timepoints (i.e. end of trial).
Processed electroencephalography (EEG) from a SedLine® device (Masimo Corporation, Irvine, California, USA) was used to confirm anesthetic depth, measured by the device’s Patient State Index (PSI). To ensure participant masking, the infusion was initiated after anesthetic induction and surgical incision, during maintenance anesthesia (PSI of 25 to 50, consistent with the manufacturer recommendations for general anesthesia). The study drug was provided to the anesthesiologist in an unlabeled syringe.
Anesthesiologists, masked to patient group allocation, administered routine anesthesia tailored to the surgical procedure and patient comorbidities; they were asked to avoid altering the anesthetic in response to any perceived changes to the processed EEG during the study drug infusion (excepting excessively high PSI values indicating the patient was at risk of intraoperative awareness). Anesthesiologists were asked to minimize use of nitrous oxide (N2O), which has reported antidepressant effects14. Agents used for anesthetic maintenance included intravenous propofol and inhaled sevoflurane or isoflurane. A standard multimodal analgesic regimen was used, consisting of intravenous opioid and acetaminophen, with or without intravenous lidocaine. Due to the heterogeneity of surgical cases represented in this study, we did not mandate specific anesthetic or analgesic regimens outside of the requested constraint on depth of anesthesia.
OUTCOME MEASURES
The primary outcome was the MADRS score measured 1, 2, and 3 days post-infusion, as previous studies have found the greatest antidepressant effect occurs within the first 72 hours of a single ketamine infusion82. The same sample was assessed at 1, 2 and 3 days post-infusion, as participant dropout only occurred after outcomes were assessed on day 3. Additional assessments were made 5, 7, and 14 days post-infusion and used for exploratory analyses. The MADRS is a clinical rating scale used widely in antidepressant trials; it consists of 10 items which measure the severity of depression in individuals, with a total score ranging from 0 to 60, and higher scores indicating more severe depression75. Trained clinical research personnel administered the MADRS.
Secondary outcomes included clinical response, defined as ≥50% reduction in MADRS scores from screening baseline83 and remission, defined as MADRS score ≤12 in our study84. Other secondary outcomes included the HADS score, postoperative pain intensity, cumulative opioid use, average daily inpatient opioid use, and presence of opioid use at 7 and 14 days postoperatively. The HADS is a self-reported questionnaire used to assess the severity of anxiety and depression in hospital patients; it consists of 14 items (7 items measuring anxiety, 7 items measuring depression) with a total score ranging from 0 to 42, with higher scores indicating more severe symptoms76. Postoperative pain was assessed by the Brief Pain Inventory Short Form modified for postoperative use (BPI-SF)85,86. The BPI-SF measures severity of pain and its impact on daily functioning; it consists of 9 items, each using a numeric rating scale from 0–10. Inpatient postoperative opioid use, calculated as total daily MME87, was abstracted from the EHR for each day of hospitalization. For discharged patients, outpatient postoperative opioid use, via pill counts, was obtained during remote scheduled assessment days (1, 2, 3, 5, 7, and 14 days post-infusion). At 14 days post-infusion, participants were asked to guess their treatment arm. Outcomes were assessed in person during postoperative hospitalization, and by video or telephone after discharge. Participants who remained in the hospital for at least 24 hours postoperatively provided blood samples for secondary immunological analyses (in progress).
STATISTICAL METHODS
Intention-to-treat analysis was performed for the primary outcome. A mixed-effects model for repeated measures was the analysis strategy pre-registered before data unmasking to evaluate the antidepressant superiority of ketamine to placebo on postoperative days 1, 2, and 3. The following fixed effects were included in the model: group, time in days, and the interaction between group and time. We included random effects for intercepts and slopes to account for variation in MADRS scores and differential treatment effects. An alternative, non-prespecified mixed-effects model using change from pre-infusion baseline scores on the day of surgery was also used to analyze the primary outcome. An unstructured covariance matrix was used in all mixed models described in this study. We calculated Cohen’s kappa statistic, in a post-hoc analysis, to assess the level of agreement between groups regarding their guesses on treatment allocation. We also performed a simple logistic regression to investigate the relationship between final MADRS score and patients’ treatment group guess (coded as ‘1’ for guessing “ketamine” and ‘0’ otherwise). Both variables were obtained at day 14 during the final assessment. Logistic regression results are reported as odds ratios. All analyses were performed using RStudio software (version 2022.07.1 for MacOS). The lme4 package was used for mixed-effects modeling. Prism GraphPad (version 9.5.0 for MacOS) was used for figure-making.
Our sample size estimation was derived from an a priori power analysis for the primary outcome. In a randomized controlled trial of ketamine versus active placebo performed by Phillips et al., participants had a mean decrease of 10.9 points (standard deviation [SD] 8.9) in MADRS total score relative to pre-infusion scores compared with a mean decrease of 2.8 points (SD 3.6) with midazolam15. For reference, the minimum clinically important difference on the MADRS is estimated to range from 3 to 9 points88. Using these results, we computed an estimated total sample size of 38 participants at a two-sided alpha level of 0.05 and 80% power to detect this difference if using parametric testing. An additional 2 participants were added to account for potential attrition, for a total of 40 participants. Interim analyses were not performed.
Extended Data
Extended Data Figure 1.
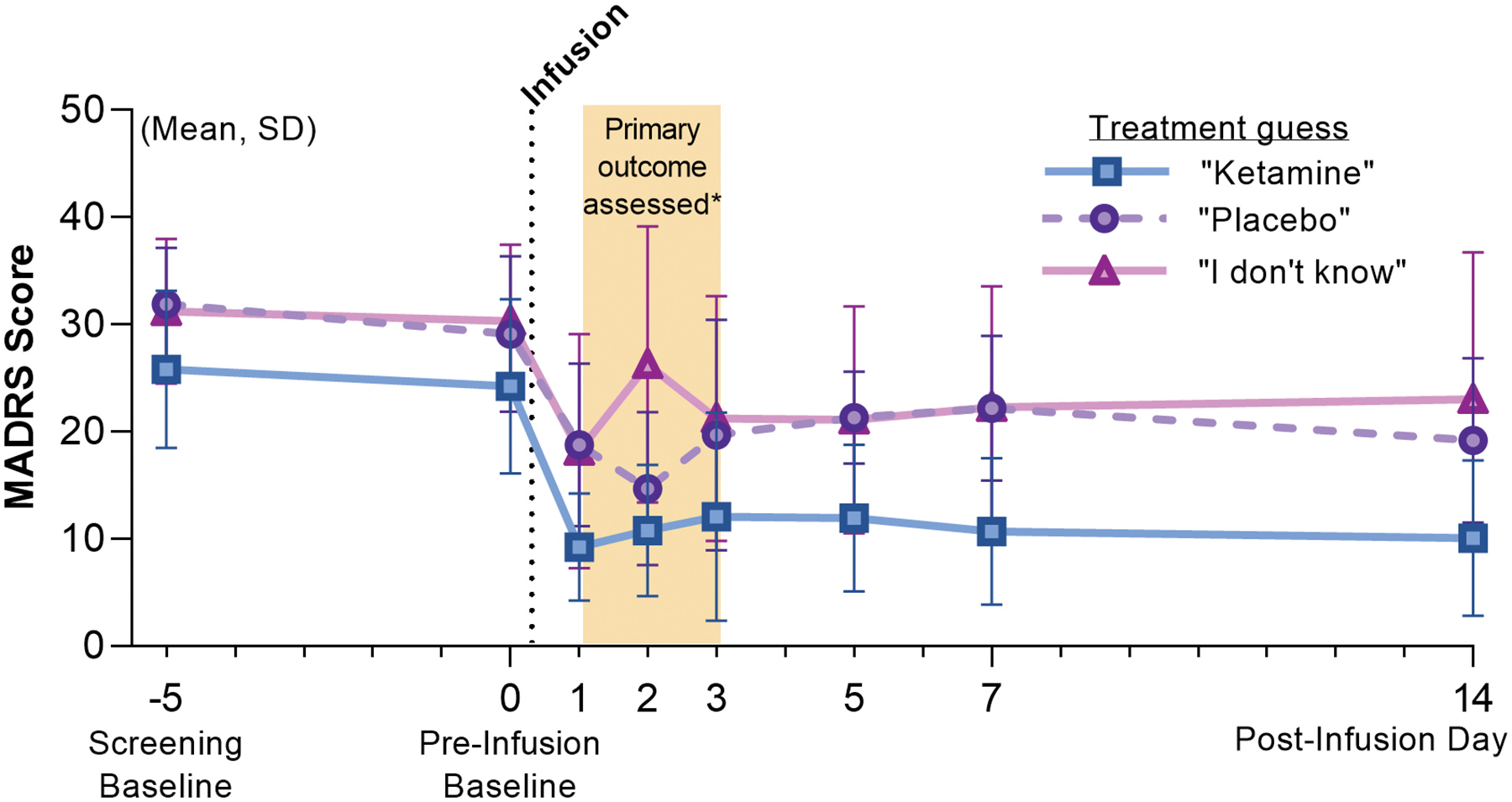
Depression Ratings Reanalyzed According to Patient Guess On day 14, the final day of patient assessments, patients were asked the following questions: “What treatment do you think you received?” MADRS scores were reanalyzed according to their guess, irrespective of their true group allocation. Mean and standard deviation (SD) MADRS scores are shown using the alternate grouping: “Ketamine”, n=17; “Placebo”, n=10; “I don’t know”, n=11.
Extended Data Table 1.
Depression Outcomes
| Measure | Ketamine | Placebo | ||||
|---|---|---|---|---|---|---|
| Mean | SD | N with data | Mean | SD | N with data | |
| MADRS total score | ||||||
| Day 0 (pre-infusion) | 25.1 | 8.3 | 20 | 29.9 | 7.0 | 19 |
| Day 1 | 12.7 | 7.6 | 20 | 16.4 | 10.0 | 19 |
| Day 2 | 15.1 | 11.1 | 19 | 17.5 | 11.5 | 20 |
| Day 3 | 17.0 | 10.5 | 19 | 17.2 | 12.0 | 20 |
| Day 5 | 16.9 | 8.0 | 19 | 16.9 | 10.1 | 18 |
| Day 7 | 15.9 | 9.2 | 18 | 18.5 | 10.8 | 19 |
| Day 14 | 16.9 | 10.0 | 19 | 16.2 | 12.5 | 19 |
| HADS total score | ||||||
| Day 0 (pre-infusion) | 22.9 | 4.2 | 20 | 24.6 | 5.6 | 19 |
| Day 1 | 17.8 | 6.6 | 20 | 19.9 | 5.5 | 19 |
| Day 2 | 18.8 | 7.5 | 18 | 21.2 | 5.9 | 20 |
| Day 3 | 19.3 | 6.3 | 19 | 20.8 | 5.4 | 20 |
| Day 5 | 18.3 | 4.8 | 19 | 18.9 | 7.3 | 19 |
| Day 7 | 18.4 | 5.3 | 16 | 19.6 | 8.1 | 19 |
| Day 14 | 16.4 | 7.2 | 19 | 17.2 | 8.5 | 19 |
| n/N in trial | % | n/N in trial | % | |||
| Clinical response on MADRS | ||||||
| Day 0 (pre-infusion) | 0/20 | 0.0 | 0/20 | 0.0 | ||
| Day 1 | 12/20 | 60.0 | 10/20 | 50.0 | ||
| Day 2 | 10/20 | 50.0 | 12/20 | 60.0 | ||
| Day 3 | 9/20 | 45.0 | 10/20 | 50.0 | ||
| Day 5 | 7/19 | 36.8 | 8/19 | 42.1 | ||
| Day 7 | 6/19 | 31.6 | 7/19 | 36.8 | ||
| Day 14 | 8/19 | 42.1 | 11/19 | 57.9 | ||
| Remission on MADRS | ||||||
| Day 0 (pre-infusion) | 1/20 | 5.0 | 0/20 | 0.0 | ||
| Day 1 | 10/20 | 50.0 | 7/20 | 35.0 | ||
| Day 2 | 11/20 | 55.0 | 8/20 | 40.0 | ||
| Day 3 | 8/20 | 40.0 | 8/20 | 40.0 | ||
| Day 5 | 7/19 | 36.8 | 6/19 | 31.6 | ||
| Day 7 | 6/19 | 31.6 | 11/19 | 57.9 | ||
| Day 14 | 8/19 | 42.1 | 9/19 | 47.4 | ||
SD, standard deviation. MADRS, Montgomery–Åsberg Depression Rating Scale. HADS, Hospital Anxiety and Depression Scale. Clinical response is defined as ≥50% reduction in MADRS scores from screening baseline. Remission is defined as MADRS score ≤12.
Extended Data Table 2.
Average Daily Inpatient Opioid Use
| Measure | Ketamine | Placebo | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Inpatient n* | Mean | SD | Inpatient n* | |
| Average Daily Inpatient Opioid Use, in MME | ||||||
| Day 1 | 81.6 | 59.6 | 14 | 69.2 | 62.2 | 16 |
| Day 2 | 83.3 | 65.2 | 8 | 77.6 | 71.3 | 13 |
| Day 3 | 87.5 | 101 | 6 | 59.7 | 84.0 | 12 |
| Day 5 | 195 | n/a | 1 | 36.7 | 41.4 | 8 |
| Day 7 | n/a | n/a | 0 | 0 | n/a | 1 |
| Day 14 | n/a | n/a | 0 | n/a | n/a | 0 |
SD, standard deviation. MME, morphine milligram equivalents,
includes only patients who remained hospitalized for the entire day.
Supplementary Material
Acknowledgements
This work was supported by a grant awarded to B.D.H. by the Society for Neuroscience in Anesthesia and Critical Care. T.R.L. received salary support through a T32 grant from the NIH National Institute on Drug Abuse (3T32DA035165-02S1). The funding bodies supporting this study had no influence on the conduct of the trial, analysis of the data, or reporting of the results.
We acknowledge Ms. Kayla Pfaff (Ohio University Heritage College of Osteopathic Medicine, Medical Student, Athens, Ohio, USA) and Dr. Rasmus Thordstein MD (Lund University, Lund, Sweden) for assistance with contacting patients, and Dr. Vishweshwara Ramachandran MBBS, MBA (Stanford University School of Medicine, Stanford, CA, USA) for implementing the PHQ-2 survey into the Anesthesia Preoperative Evaluation Clinic electronic workflow at Stanford.
Statistical support was provided by Data Studio (Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, California, USA), which is supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR003142.
Screening and outcomes data were entered into Stanford REDCap (version 13.4.10), a secure online data capture platform (http://redcap.stanford.edu) developed and operated by Stanford Medicine Research IT team. The REDCap platform services at Stanford are subsidized by a) Stanford School of Medicine Research Office, and b) the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 TR001085.
Footnotes
Code Availability
R code used for data analysis is publicly available at https://osf.io/zdkr8/ (DOI 10.17605/OSF.IO/ZDKR8).
Competing Interests Statement
B.D.H. is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant for Clairvoyant Therapeutics and Vine Ventures. Dr. Schatzberg has served as a consultant to Alto Neuroscience, ANeurotech, Compass, Magnus, NeuraWell, Parexal, Sage and Signant. He holds equity in Alto Neuroscience, Corcept, Delpor, Madrigal, Magnus, Seattle Genetics, Titan and Xhale. These interests had no role in the present trial. The other authors declare no competing interests.
Data Availability
De-identified participant data, data dictionaries, study protocol, and statistical analysis plan are available at https://osf.io/zdkr8/ (DOI 10.17605/OSF.IO/ZDKR8). All participants have consented to sharing de-identified data with outside entities for scientific research purposes.
References
- 1.Sleigh J, Harvey M, Voss L & Denny B Ketamine – More mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care 4, 76–81 (2014). [Google Scholar]
- 2.Bonaventura J et al. Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry 26, 6704–6722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berman RM et al. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 47, 351–354 (2000). [DOI] [PubMed] [Google Scholar]
- 4.McIntyre RS et al. Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. AJP 178, 383–399 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarate CA et al. A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry 63, 856–864 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Marcantoni WS et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 – January 2019. Journal of Affective Disorders 277, 831–841 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Butler M, Jelen L & Rucker J Expectancy in placebo-controlled trials of psychedelics: if so, so what? Psychopharmacology 239, 3047–3055 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall WD & Humphreys K Is good science leading the way in the therapeutic use of psychedelic drugs? Psychological Medicine 52, 2849–2851 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Aday JS et al. Great Expectations: recommendations for improving the methodological rigor of psychedelic clinical trials. Psychopharmacology 239, 1989–2010 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthukumaraswamy SD, Forsyth A & Lumley T Blinding and expectancy confounds in psychedelic randomized controlled trials. Expert Review of Clinical Pharmacology 14, 1133–1152 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Price RB et al. A Novel, Brief, Fully Automated Intervention to Extend the Antidepressant Effect of a Single Ketamine Infusion: A Randomized Clinical Trial. AJP 179, 959–968 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horvath B, Kloesel B, Todd MM, Cole DJ & Prielipp RC The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 135, 904–919 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL & MacKenzie CR A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases 40, 373–383 (1987). [DOI] [PubMed] [Google Scholar]
- 14.Nagele P et al. A phase 2 trial of inhaled nitrous oxide for treatment-resistant major depression. Sci. Transl. Med 13, eabe1376 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Phillips JL et al. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial. American Journal of Psychiatry 176, 401–409 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Murrough JW et al. Antidepressant Efficacy of Ketamine in Treatment-Resistant Major Depression: A Two-Site Randomized Controlled Trial. American Journal of Psychiatry 170, 1134–1142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sos P et al. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. 8 (2013). [PubMed] [Google Scholar]
- 18.Singh JB et al. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression. American Journal of Psychiatry 173, 816–826 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Sanacora G et al. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry 74, 399 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Dore J et al. Ketamine Assisted Psychotherapy (KAP): Patient Demographics, Clinical Data and Outcomes in Three Large Practices Administering Ketamine with Psychotherapy. J Psychoactive Drugs 51, 189–198 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Joneborg I et al. Active mechanisms of ketamine-assisted psychotherapy: A systematic review. J Affect Disord 315, 105–112 (2022). [DOI] [PubMed] [Google Scholar]
- 22.Nagele P et al. Nitrous Oxide for Treatment-Resistant Major Depression: A Proof-of-Concept Trial. Biological Psychiatry 78, 10–18 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Mickey BJ et al. Propofol for Treatment-Resistant Depression: A Pilot Study. International Journal of Neuropsychopharmacology 21, 1079–1089 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weeks HR et al. Antidepressant and Neurocognitive Effects of Isoflurane Anesthesia versus Electroconvulsive Therapy in Refractory Depression. PLoS ONE 8, e69809 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Toro M et al. Inefficacy of Burst-Suppression Anesthesia in Medication-Resistant Major Depression: A Controlled Trial: The Journal of ECT 17, 284–288 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Kudoh A, Takahira Y, Katagai H & Takazawa T Small-Dose Ketamine Improves the Postoperative State of Depressed Patients: Anesthesia & Analgesia 95, 114–118 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Mashour GA et al. Intraoperative ketamine for prevention of depressive symptoms after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. British Journal of Anaesthesia 121, 1075–1083 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu P et al. Effect of Pretreatment of S-Ketamine On Postoperative Depression for Breast Cancer Patients. Journal of Investigative Surgery 34, 883–888 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y et al. Ketamine Alleviates Depressive Symptoms in Patients Undergoing Intracranial Tumor Resection: A Randomized Controlled Trial. Anesthesia & Analgesia 133, 1588–1597 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Guo J et al. Efficacy and safety of perioperative application of ketamine on postoperative depression: A meta-analysis of randomized controlled studies. Mol Psychiatry 1–11 (2023) doi: 10.1038/s41380-023-01945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang M et al. Effect of intraoperative application of ketamine on postoperative depressed mood in patients undergoing elective orthopedic surgery. Journal of Anesthesia 30, 232–237 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Wang J et al. Effects of a single subanaesthetic dose of ketamine on pain and mood after laparoscopic bariatric surgery: A randomised double-blind placebo controlled study. Eur J Anaesthesiol 36, 16–24 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Liu P et al. Effect of Pretreatment of S-Ketamine On Postoperative Depression for Breast Cancer Patients. J Invest Surg 1–6 (2020) doi: 10.1080/08941939.2019.1710626. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z et al. Intraoperative Low-Dose S-Ketamine Reduces Depressive Symptoms in Patients with Crohn’s Disease Undergoing Bowel Resection: A Randomized Controlled Trial. J Clin Med 12, 1152 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aleksandrova LR & Phillips AG Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol Sci 42, 929–942 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Hess EM, Riggs LM, Michaelides M & Gould TD Mechanisms of ketamine and its metabolites as antidepressants. Biochem Pharmacol 197, 114892 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston JN, Henter ID & Zarate CA The antidepressant actions of ketamine and its enantiomers. Pharmacol Ther 246, 108431 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown EN, Lydic R & Schiff ND General anesthesia, sleep, and coma. N Engl J Med 363, 2638–2650 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heresco-Levy U et al. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord 93, 239–243 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Zarate CA et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry 163, 153–155 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Paterson B, Fraser H, Wang C & Marcus R A RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, SEQUENTIAL PARALLEL STUDY OF CERC-301 IN THE ADJUNCTIVE TREATMENT OF SUBJECTS WITH SEVERE DEPRESSION AND RECENT ACTIVE SUICIDAL IDEATION DESPITE ANTIDEPRESSANT TREATMENT. (2015).
- 42.Sanacora G et al. Adjunctive Lanicemine (AZD6765) in Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Randomized, Placebo-Controlled Study. Neuropsychopharmacology 42, 844–853 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibrahim L et al. Course of Improvement in Depressive Symptoms to a Single Intravenous Infusion of Ketamine vs Add-on Riluzole: Results from a 4-Week, Double-Blind, Placebo-Controlled Study. Neuropsychopharmacol 37, 1526–1533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathew SJ, Gueorguieva R, Brandt C, Fava M & Sanacora G A Randomized, Double-Blind, Placebo-Controlled, Sequential Parallel Comparison Design Trial of Adjunctive Riluzole for Treatment-Resistant Major Depressive Disorder. Neuropsychopharmacology 42, 2567–2574 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vesuna S et al. Deep posteromedial cortical rhythm in dissociation. Nature 586, 87–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian F et al. Characterizing brain dynamics during ketamine-induced dissociation and subsequent interactions with propofol using human intracranial neurophysiology. Nat Commun 14, 1748 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Shu S & Bayliss DA HCN1 Channel Subunits Are a Molecular Substrate for Hypnotic Actions of Ketamine. J. Neurosci 29, 600–609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anand A et al. Attenuation of the Neuropsychiatric Effects of Ketamine With Lamotrigine: Support for Hyperglutamatergic Effects of N-methyl-D-aspartate Receptor Antagonists. Arch Gen Psychiatry 57, 270–276 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Cichon J et al. Ketamine triggers a switch in excitatory neuronal activity across neocortex. Nat Neurosci 26, 39–52 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams NR et al. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. American Journal of Psychiatry 175, 1205–1215 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hustveit O, Maurset A & Oye I Interaction of the chiral forms of ketamine with opioid, phencyclidine, sigma and muscarinic receptors. Pharmacol Toxicol 77, 355–359 (1995). [DOI] [PubMed] [Google Scholar]
- 52.Klein ME, Chandra J, Sheriff S & Malinow R Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc. Natl. Acad. Sci. U.S.A 117, 2656–2662 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wulf HA, Browne CA, Zarate CA & Lucki I Mediation of the behavioral effects of ketamine and (2R,6R)-hydroxynorketamine in mice by kappa opioid receptors. Psychopharmacology (2022) doi: 10.1007/s00213-022-06118-4. [DOI] [PubMed] [Google Scholar]
- 54.Marton T, Barnes DE, Wallace A & Woolley JD Concurrent Use of Buprenorphine, Methadone, or Naltrexone Does Not Inhibit Ketamine’s Antidepressant Activity. Biol Psychiatry 85, e75–e76 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Trivedi MH et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163, 28–40 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Chen M-H et al. Using classification and regression tree modelling to investigate treatment responseto a single low-dose ketamine infusion: Post hoc pooled analyses of randomized placebo-controlled and open-label trials. Journal of Affective Disorders 281, 865–871 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Pennybaker SJ, Niciu MJ, Luckenbaugh DA & Zarate CA Symptomatology and predictors of antidepressant efficacy in extended responders to a single ketamine infusion. Journal of Affective Disorders 208, 560–566 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jesus-Nunes AP et al. Clinical predictors of depressive symptom remission and response after racemic ketamine and esketamine infusion in treatment-resistant depression. Human Psychopharmacology: Clinical and Experimental 37, e2836 (2022). [DOI] [PubMed] [Google Scholar]
- 59.Ustun YB et al. Comparison of Ketamine, Dexmedetomidine and Lidocaine in Multimodal Analgesia Management Following Sleeve Gastrectomy Surgery: A Randomized Double-Blind Trial. J Perianesth Nurs 37, 820–826 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Seman MT et al. Low-Dose Ketamine Infusion for Perioperative Pain Management in Patients Undergoing Laparoscopic Gastric Bypass: A Prospective Randomized Controlled Trial. Anesthesiology Research and Practice 2021, e5520517 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moro ET et al. Ketamine does not enhance the quality of recovery following laparoscopic cholecystectomy: a randomized controlled trial. Acta Anaesthesiologica Scandinavica 61, 740–748 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Ragazzoni L et al. Intra-operative low-dose ketamine does not reduce the cost of post-operative pain management after surgery: a randomized controlled trial in a low-income country. Afr Health Sci 19, 3127–3135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mortero RF et al. The Effects of Small-Dose Ketamine on Propofol Sedation: Respiration, Postoperative Mood, Perception, Cognition, and Pain. Anesthesia & Analgesia 92, 1465 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Aubrun F et al. Effect of a low-dose ketamine regimen on pain, mood, cognitive function and memory after major gynaecological surgery: a randomized, double-blind, placebo-controlled trial. European Journal of Anaesthesiology 25, 97–105 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Lee C et al. The effects of a combination of intravenous dexamethasone and ketamine on postoperative mood in patients undergoing laparoscopically assisted-gynecologic surgery. Psychopharmacology 235, 2417–2422 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Irle E, Peper M, Wowra B & Kunze S Mood changes after surgery for tumors of the cerebral cortex. Arch Neurol 51, 164–174 (1994). [DOI] [PubMed] [Google Scholar]
- 67.Jenkins LM, Drummond KJ & Andrewes DG Emotional and personality changes following brain tumour resection. J Clin Neurosci 29, 128–132 (2016). [DOI] [PubMed] [Google Scholar]
- 68.McGirr A et al. Adjunctive ketamine in electroconvulsive therapy: Updated systematic review and meta-analysis. The British Journal of Psychiatry 210, 403–407 (2017). [DOI] [PubMed] [Google Scholar]
- 69.Anderson IM et al. Ketamine augmentation of electroconvulsive therapy to improve neuropsychological and clinical outcomes in depression (Ketamine-ECT): a multicentre, double-blind, randomised, parallel-group, superiority trial. The Lancet Psychiatry 4, 365–377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vlisides PE et al. Neurophysiologic Correlates of Ketamine Sedation and Anesthesia: A High-density Electroencephalography Study in Healthy Volunteers. Anesthesiology 127, 58–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutherford BR, Wall MM, Glass A & Stewart JW The Role of Patient Expectancy in Placebo and Nocebo Effects in Antidepressant Trials. J Clin Psychiatry 75, 1040–1046 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kroenke K et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 114, 163–173 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Fekadu A, Donocik JG & Cleare AJ Patients with Major Depressive Disorder and History of Inadequate Response to Antidepressants: A Rando. BMC Psychiatry 18, 100 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheehan DV et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20, 22–33;quiz 34–57 (1998). [PubMed] [Google Scholar]
- 75.Montgomery SA & Åsberg M A New Depression Scale Designed to be Sensitive to Change. The British Journal of Psychiatry 134, 382–389 (1979). [DOI] [PubMed] [Google Scholar]
- 76.Zigmond AS & Snaith RP The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 67, 361–370 (1983). [DOI] [PubMed] [Google Scholar]
- 77.Pinto-Meza A, Serrano-Blanco A, Peñarrubia MT, Blanco E & Haro JM Assessing Depression in Primary Care with the PHQ-9: Can It Be Carried Out over the Telephone? Journal of General Internal Medicine 20, 738–742 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hedman E et al. Telephone Versus Internet Administration of Self-Report Measures of Social Anxiety, Depressive Symptoms, and Insomnia: Psychometric Evaluation of a Method to Reduce the Impact of Missing Data. J Med Internet Res 15, e229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hermens ML et al. Administering the MADRS by telephone or face-to-face: a validity study. Ann Gen Psychiatry 5, 3 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kobak KA, Williams JBW, Jeglic E, Salvucci D & Sharp IR Face-to-face versus remote administration of the Montgomery-Asberg Depression Rating Scale using videoconference and telephone. Depress Anxiety 25, 913–919 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Posner K et al. The Columbia–Suicide Severity Rating Scale: Initial Validity and Internal Consistency Findings From Three Multisite Studies With Adolescents and Adults. Am J Psychiatry 168, 1266–1277 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kishimoto T et al. Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 46, 1459–1472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nierenberg AA & DeCecco LM Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry 62 Suppl 16, 5–9 (2001). [PubMed] [Google Scholar]
- 84.Machado M, Iskedjian M, Ruiz I & Einarson TR Remission, dropouts, and adverse drug reaction rates in major depressive disorder: a meta-analysis of head-to-head trials. Current Medical Research and Opinion 22, 1825–1837 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Keller S et al. Validity of the Brief Pain Inventory for Use in Documenting the Outcomes of Patients With Noncancer Pain. The Clinical Journal of Pain 20, 309 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Mendoza TR et al. The Utility and Validity of the Modified Brief Pain Inventory in a Multiple-Dose Postoperative Analgesic Trial. The Clinical Journal of Pain 20, 357 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors | Guidance Portal. https://www.hhs.gov/guidance/document/opioid-oral-morphine-milligram-equivalent-mme-conversion-factors-0.
- 88.Hengartner MP & Plöderl M Estimates of the minimal important difference to evaluate the clinical significance of antidepressants in the acute treatment of moderate-to-severe depression. BMJ Evidence-Based Medicine 27, 69–73 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data, data dictionaries, study protocol, and statistical analysis plan are available at https://osf.io/zdkr8/ (DOI 10.17605/OSF.IO/ZDKR8). All participants have consented to sharing de-identified data with outside entities for scientific research purposes.


