Abstract
Background
Breast cancer is the most common cancer worldwide, with remarkable advances in early diagnosis, systemic treatments, and surgical techniques. Robotic nipple-sparing mastectomy has been trialled; however, the complication rates, surgical outcomes, and oncological safety of this approach remain obscure.
Methods
A systematic search of the literature was conducted from conception until September 2022. Studies examining complications and operative variables where robotic nipple-sparing mastectomy was compared with conventional nipple-sparing mastectomy were included. Primary study outcomes were complications (Clavien–Dindo grade III complications, skin or nipple necrosis, seroma, haematoma, infection, implant loss, and wound dehiscence) and oncological safety (recurrence and positive margins). The secondary outcomes included operative variables, length of stay, cost-effectiveness, learning curve, and aesthetic outcome.
Results
A total of seven studies of overall fair quality, involving 1674 patients, were included in the systematic review and meta-analysis. Grade 3 complications were reduced in robotic nipple-sparing mastectomy without statistical significance (OR 0.60 (95 per cent c.i. 0.35 to 1.05)). Nipple necrosis was significantly reduced in robotic nipple-sparing mastectomy (OR 0.54 (95 per cent c.i. 0.30 to 0.96); P = 0.03; I2 = 15 per cent). Operating time (mean difference +58.81 min (95 per cent c.i. +28.19 to +89.44 min); P = 0.0002) and length of stay (mean difference +1.23 days (95 per cent c.i. +0.64 to +1.81 days); P < 0.0001) were significantly increased in robotic nipple-sparing mastectomy, whereas the opposite was true for blood loss (mean difference −53.18 ml (95 per cent c.i. −71.78 to −34.58 ml); P < 0.0001).
Conclusion
Whilst still in its infancy, robotic breast surgery may become a viable option in breast surgery. Nonetheless, the oncological safety of this approach requires robust assessment.
A total of seven studies of overall fair quality, involving 1674 patients, examining postoperative complications and operative variables that compared robotic nipple-sparing mastectomy versus conventional nipple-sparing mastectomy for breast cancer were included in this systematic review and meta-analysis. Overall, complication events were notably reduced in robotic nipple-sparing mastectomy, albeit not reaching statistical significance, namely grade 3 complications, re-operation, skin necrosis, seroma, and haematoma; however, postoperative infection, implant loss, and positive margins were increased in robotic nipple-sparing mastectomy. Although associated with a higher cost, a steep learning curve and patient-reported outcome measures favour robotic nipple-sparing mastectomy.
Introduction
In 2020, 2.3 million women were diagnosed with breast cancer globally, resulting in 685 000 deaths. By the end of 2020, there were 7.8 million women alive who had been diagnosed with breast cancer in the past 5 years, making this the world’s most prevalent malignancy1. Remarkable advances in management have occurred in the past 30 years, including early detection, improved systemic treatments, and refined surgical approaches. An effort to improve the aesthetic outcome of breast surgery, through preservation of the nipple–areolar complex (NAC), has led to the development of nipple-sparing mastectomy (NSM)2,3. Initially, concerns were voiced regarding the oncological safety of NSMs due to the potential risk of local recurrence based on residual glandular tissue remaining in situ and occult NAC involvement4. Hence, the approach was initially reserved for the prophylactic treatment of women with a high risk of developing breast cancer5. NSMs have, however, been increasingly used also for women with breast cancer where the NAC is not involved6–8. Several studies have shown that disease-free survival and local recurrence rates of NSMs are equivalent to those of skin-sparing or modified radical mastectomies in selected patients9,10. Moreover, there is evidence that cosmetic outcomes and patient-reported outcome measures are better with NAC preservation2,3,11,12. In light of this evidence, there has been a steady increase in the NSM rate among women undergoing mastectomy and reconstruction due to breast cancer13.
A total of 15 incision types for conventional NSM (CNSM) are reported across the literature14, each of which has advantages and disadvantages. As an example, inframammary incisions provide limited access to the upper areas of the breast, necessitating deep retraction, use of headlights, and awkward positioning15,16. That said, other incisions, placed closer to the NAC, might mitigate some of these access challenges, but they may also disrupt the NAC vascular supply, thus significantly increasing the risk of necrosis14,17–19. Minimally invasive surgery has been introduced, along with the mainstream conventional open operations, and new surgical innovations, including endoscopic NSM (ENSM) and robotic NSM (RNSM), have emerged. Due to the limitations of endoscopic instruments and inherent technical difficulty, ENSM is not widely adopted in the surgical management of breast cancer. RNSM is also technically demanding, but it is more practical than ENSM and is therefore being more readily explored. Robotic technology employing three-dimensional imaging, high resolution, flexible instruments with greater precision, and a wider range of motion has been developed to address the limitations of endoscopic procedures. Robotic-assisted procedures have been successfully deployed in urology, gynaecology, and colorectal surgery for a variety of indications20–24. Toesca et al.25,26 introduced RNSM in 2017 by employing a small axillary incision to complete the resection and simultaneously performing an implant reconstruction. Since then, several groups have followed27–29.
In 2017, during the 15th St Gallen International Breast Cancer Conference, robotic mastectomy was recognized as an option for selected patients30. The US Food and Drug Administration has not approved robotic breast surgery, issuing a warning in February 2019 that the safety and efficacy of robotic devices for mastectomy have not been established31. Since then, several opinion papers, an international protocol, and a consensus statement have been issued29,32–38. All have highlighted the potential benefits and need for more evidence. While research has demonstrated the feasibility and safety of RNSM, there is a steep technical learning curve involved22,24. Moreover, questions remain concerning complication rates, operative variables, and, most importantly, oncological safety24.
The aim of this systematic review and meta-analysis was to assess complication rates, differences in operative variables, and outcomes for RNSM when compared with its mainstream counterpart (CNSM).
Methods
A systematic review was performed in accordance with PRISMA guidelines38 and registered with PROSPERO, the international prospective register of systematic reviews (CRD42022381495). Five databases (Embase (Ovid), Global Health, MEDLINE (Ovid), Health Management Information Consortium (HMIC), and American Physiological Association (APA) PsycArticles) were subject to an independent literature search for relevant studies up to 13 September 2022. Additional records were not sought. The references of the included studies were scrutinized for additional relevant studies. Search limitations included human participants and English-language articles. The following search term was used in Ovid: (robotic and surgery and (breast or axill* or mastectomy or breast conserving) and breast cancer).mp.
Inclusion and exclusion criteria
All included studies (retrospective, prospective, and time series) examined the immediate and long-term complications of RNSM. No geographical, age, or gender restrictions were applied. Studies not directly comparing RNSM with conventional nipple sparing mastectomy (CNSM) were excluded (full-text exclusion criterion).
Data extraction
After removing duplicates, citations were screened by title and abstract, and then full texts were appraised to determine their eligibility by one author (A.N.) (Fig. S1). A total of two authors (A.N. and S.L.K.) independently conducted the abstract and full-text screening. Disagreements were resolved by a consensus meeting. Peer-reviewed full-text papers that reported comparison of postoperative complications, aesthetic outcome, and oncological outcomes were selected (Table S1). Studies were assessed for overlapping populations. Data from each article (n (percentage) or median (range)) were extracted by two authors (A.N. and S.L.K.): number of participants; number of participants and percentage treated with RNSM; robotic system/platform used; study interval; histology; cancer stage; indication; age (median (range)); post-menopausal status (n (percentage)); current smoker (n (percentage)); BMI (median (range)); breast size (A–B, C, and greater than C (n (percentage)); specimen size in grams; follow-up in months (median (range)); procedure time in minutes (median (range)); reconstruction time in minutes (median (range)); reconstruction type; incision type; length of stay in days (median (range)); and conversion to open surgery (n (percentage)). Where mean and standard deviation values were supplied, median values were calculated for homogenization (Table S2).
Outcomes
Primary study outcomes were complications (Clavien–Dindo grade III complications, skin and nipple necrosis, wound dehiscence, infection, seroma, haematoma, and implant loss) and oncological safety (positive margins and recurrence) for RNSM compared with CNSM (n (percentage)). Secondary outcomes included operative variables, operating time, length of stay, blood loss, cost-effectiveness, patient satisfaction, aesthetic outcome, and learning curve.
Quality assessment
The quality of the included studies was assessed by two independent reviewers (A.N. and S.L.K.) using the Newcastle–Ottawa scale (NOS) for observational studies39. Bias analysis was conducted via the Cochrane-recommended tool (Review Manager (RevMan) V. 5.4)40. Studies with an NOS score greater than or equal to six were of high quality. RCTs were assessed using the Cochrane RoB 2 tool41.
Data analysis and meta-analysis
Clinical study context and design were compared and suitably homogeneous studies were included in the quantitative analysis 40,42. The meta-analysis was conducted by computing the OR or mean difference (MD) random effects from the original data using the Mantel–Haenszel method with RevMan V. 5.4 software. Statistical heterogeneity was quantified using I2 statistics and Cochrane Q tests. Asymmetry was assessed by funnel plot and publication bias was assessed formally by rank correlation test (Begg’s test); RevMan V. 5.441. Given the limited number of studies meeting inclusion criteria, all studies were analysed jointly regardless of design. Clinical characteristics were employed to quantify inherent heterogeneity. Therefore, a sensitivity analysis was conducted based on median patient age across each study population, with a cut-off at 46.7 years old, which was rounded up to 47 years old.
Results
A total of 1985 citations were initially retrieved, of which 7 studies met the inclusion criteria for full-text screening (Fig. S1a); 1 study was an RCT43, 1 study was a prospective observational study44, and 5 studies were retrospective observational studies45–49 (Table S1). The single RCT was considered of good quality, with a low risk of bias, as assessed using the Cochrane RoB 2 tool41. A total of four observational studies44–46,49 were deemed of overall fair quality using the NOS, whereas two observational studies were of poor quality47,48 (Fig. S1b,c). A total of 1674 patients, of whom 853 (50.9 per cent) underwent RNSM and 821 (49.1 per cent) underwent CNSM, were included in this systematic review and meta-analysis. All participants were female, with a median age of 46.7 (interquartile range (i.q.r.) 45.38–49.48) years and a median BMI of 22.64 (i.q.r. 21.83–32.65) kg/m2. Patient post-menopausal status was reported in two studies, with 39 patients (24.22 per cent of total population examined in the relevant studies) being post-menopausal at the time of treatment46,49. A total of 245 patients (14.6 per cent) were diagnosed with ductal carcinoma in situ, 444 patients (26.52 per cent) were diagnosed with invasive ductal carcinoma, and 534 patients (31.89 per cent) were diagnosed with invasive lobular carcinoma or mixed tumour pathology, while tumour histology was not stated for 26.99 per cent of the study population. Regarding cancer stage, 301 patients (17.98 per cent) were stage 0, 443 patients (26.46 per cent) were stage I, 429 patients (25.6 per cent) were stage II, 100 patients (5.97 per cent) were stage III, and 9 patients (0.53 per cent) were stage IV. The majority of RNSM procedures were performed via an axillary incision, whereas several different approaches were utilized for CNSM (Table S3). Considering the surgical equipment, four studies utilized the da Vinci Xi Surgical System44–47.
Meta-analysis
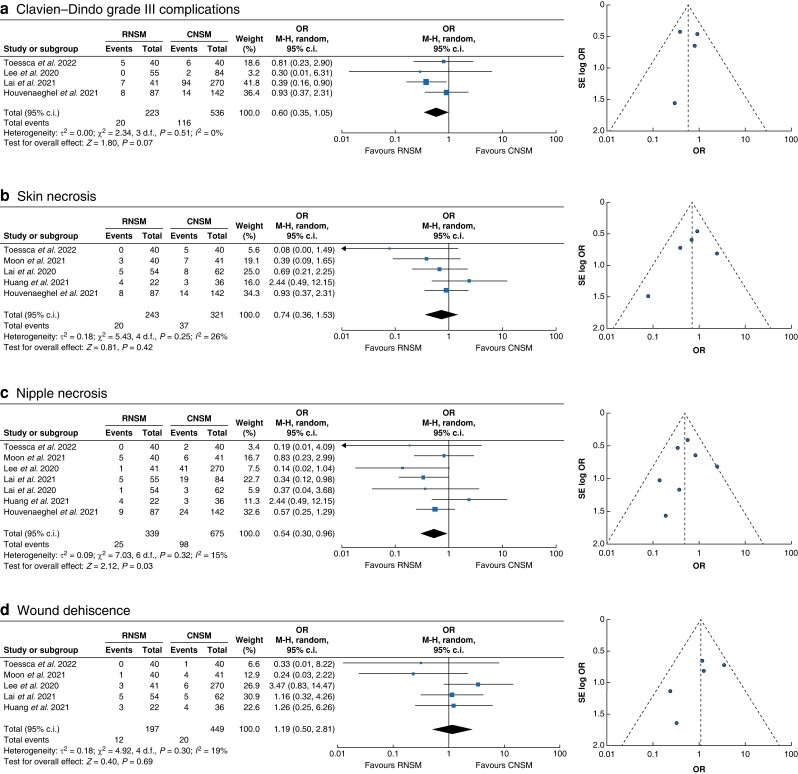
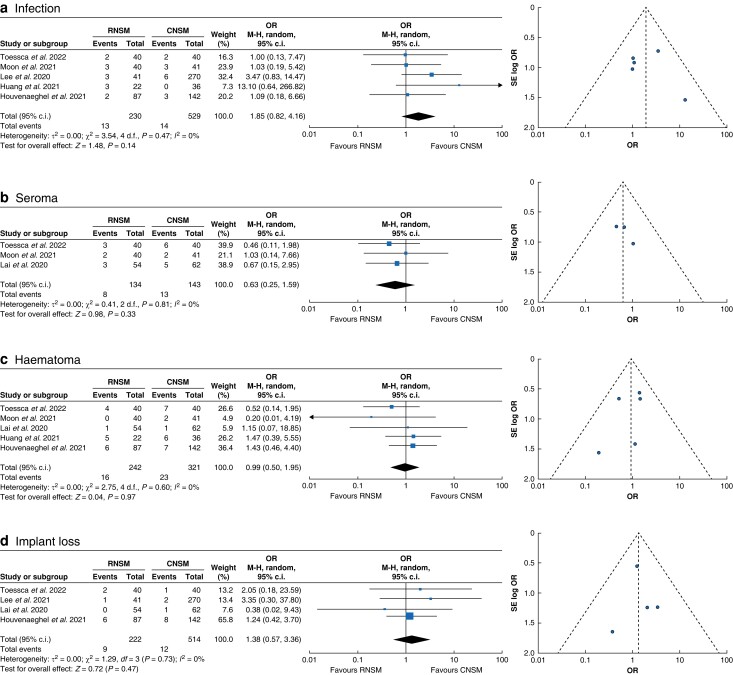
Overall, complications were lower in RNSM, albeit not reaching statistical significance. More specifically: Clavien–Dindo grade III complications (OR 0.60 (95 per cent c.i. 0.35 to 1.05); P = 0.07; I2 = 0 per cent); re-operation required (OR 0.91 (95 per cent c.i. 0.41 to 2.02); P = 0.51; I2 = 30 per cent); skin necrosis (OR 0.74 (95 per cent c.i. 0.36 to 1.53); P = 0.42; I2 = 26 per cent); seroma (OR 0.63 (95 per cent c.i. 0.25 to 1.59); P = 0.331; I2 = 0 per cent) and haematoma (OR 0.99 (95 per cent c.i. 0.50 to 1.95); P = 0.972; I² = 0 per cent) (Fig. 1). Nipple necrosis events were, however, significantly reduced in RNSM (OR 0.54 (95 per cent c.i. 0.30 to 0.96); P = 0.029; I2 = 15 per cent). There was no notable difference between RNSM and CNSM for wound dehiscence events (OR 1.19 (95 per cent c.i. 0.50 to 2.81); P = 0.691; I2 = 19 per cent). Whilst not statistically significant, postoperative infections appeared to be increased in the RNSM patient group (OR 1.85 (95 per cent c.i. 0.82 to 4.16); P = 0.138; I2 = 0 per cent), as was implant loss (OR 1.38 (95 per cent c.i. 0.57 to 3.36); P = 0.471; I2 = 0 per cent) (Fig. 2).
Fig. 1.
Mantel–Haenszel statistical method with random-effects analysis model and OR as output only for included observational studies and RCT, and funnel plots assessing respective variance
Forest plots analysing crude event numbers between robotic nipple-sparing mastectomy and conventional nipple-sparing mastectomy. a Clavien–Dindo grade III complications. b Skin necrosis. c Nipple necrosis. d Wound dehiscence. Overall heterogeneity for the respective outcomes was considered acceptable (less than 30 per cent) given the nature of the included studies. RNSM, robotic nipple-sparing mastectomy; CNSM, conventional nipple-sparing mastectomy; M-H, Mantel–Haenszel; SE, standard error.
Fig. 2.
Mantel–Haenszel statistical method with random-effects analysis model and OR as output only for included observational studies and RCT, and funnel plots assessing respective variance
Forest plots analysing crude event numbers between robotic nipple-sparring mastectomy and conventional nipple-sparing mastectomy. a Infection. b Seroma. c Haematoma. d Implant loss. Overall heterogeneity for the respective outcomes was considered acceptable (less than 30 per cent) given the nature of the included studies. RNSM, robotic nipple-sparing mastectomy; CNSM, conventional nipple-sparing mastectomy; M-H, Mantel–Haenszel; SE, standard error.
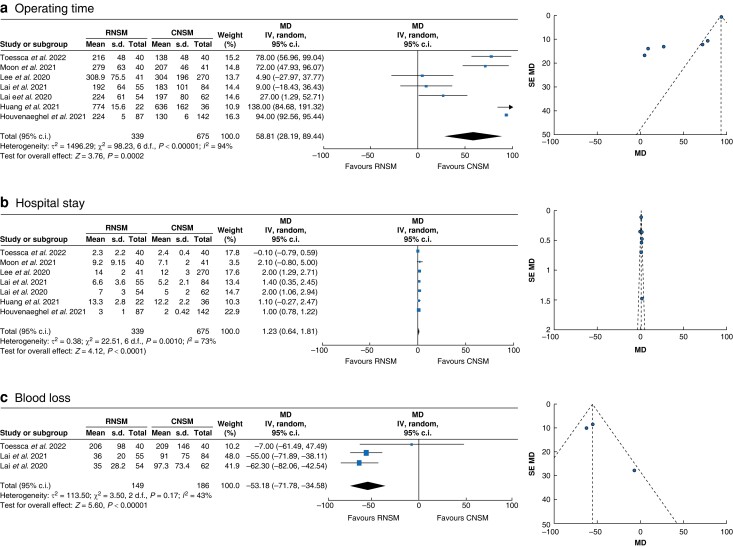
Regarding oncological safety, local recurrence events appeared to be reduced in the RNSM group (OR 0.27 (95 per cent c.i. 0.07 to 1.07); P = 0.061; I2 = 0 per cent), whereas the opposite was observed for positive margins at surgery (OR 1.66 (95 per cent c.i. 0.46 to 5.94); P = 0.439; I2 = 0 per cent) (Fig. S2). Operating time MD (in minutes) was significantly increased in RNSM (MD +58.81 min (95 per cent c.i. +28.19 to +89.44 min); P < 0.001; I2 = 94 per cent). In contrast, intraoperative blood loss was significantly reduced in the RNSM group (MD −53.18 ml (95 per cent c.i. −71.78 to −34.58 ml); P < 0.00001; I2 = 43 per cent). A statistically significant difference favouring CNSM was noted regarding length of stay (in days) (MD +1.23 days (95 per cent c.i. +0.64 to +1.81 days); P < 0.001; I2 = 73 per cent) (Fig. 3). Importantly, the subgroup analysis for younger patients (less than 47 years old) did not show changes in complication trends compared with the total population (Table S4). Equally, younger patients did not show changes in operating time compared with the total population (MD 70.87 min (7.32 to 134.42 min); P = 0.56, I2 = 91 per cent). Therefore, younger patient age does not appear to improve length of surgery in RNSM. The learning curve, however, appeared to be strongly associated with shorter operating times (Table S1). Cost-effectiveness was reported by three studies, for which RNSM was a median of 48.90 (i.q.r. 34.70–52.42) per cent more expensive than CNSM44–46. Lastly, the aesthetic outcome was assessed in five studies, by means of patient-reported outcomes (four studies) or a panel review (one study), and was uniformly found to be better in RNSM patients43–46.
Fig. 3.
Mantel–Haenszel statistical method with random-effects analysis model and mean difference for continuous variables as output only for included observational studies and RCT, and funnel plots assessing respective variance
Forest plots analysing crude event numbers between between robotic nipple-sparring mastectomy and conventional nipple-sparing mastectomy. a Operating time (in minutes). b Hospital stay (in days). c Blood loss (in millilitres). RNSM, robotic nipple-sparing mastectomy; CNSM, conventional nipple-sparing mastectomy; M-H, Mantel–Haenszel; SE, standard error; MD, mean difference.
Discussion
The present meta-analysis is a contemporary review of head-to-head comparisons of RNSM versus CNSM complications and surgical outcomes. Overall, complication rates were reduced in the RNSM group, although not reaching statistical significance for most outcomes. There was, however, a statistically significant reduction of nipple necrosis (OR 0.54 (95 per cent c.i. 0.30 to 0.96); P = 0.029; I2 = 15 per cent) in the RNSM group. Despite longer operations, intraoperative blood loss was significantly reduced in the RNSM group and the aesthetic outcome was deemed better in comparison with CNSM. In contrast to previously published evidence, suggesting that postoperative complications may be higher in patients undergoing RNSM (complication rate of 3.9 per cent for CNSM (total of 13 661 masectomies) versus complication rate of 7 per cent for RNSM (total of 225 masectomies); P = 0.070)50, the inclusion of a larger RSNM cohort (853 patients) in the present study suggests a steep upwards trend in the learning curve for RNSM. Recent observational studies also suggest an increase in RNSM surgical efficiency, although it should be noted that patient selection may significantly skew objective quantification of surgical outcomes. As an example, studies on RNSM only included patients with small to medium breast size and excluded those with breast cup sizes greater than C. Additionally, RNSM patients had a median BMI of 22.65 kg/m2 and the majority of those diagnosed with early-stage disease (70.04 per cent), denoting the uncertainty of RNSM performance in higher-risk patients.
Nipple necrosis is a major postoperative complication in NSMs. In RNSM, the incidence of nipple necrosis decreased significantly. Nevertheless, the definition of nipple necrosis varied among the included studies, with overlaps in the reporting of nipple necrosis, ischaemia, skin and flap necrosis. Consistent with the present study, Filipe et al.50 reported decreased skin and nipple necrosis rates in RNSM (Table 1). Others have reported lower nipple necrosis rates and Clavien–Dindo grade complications51. This is expected, as most complications after CNSM are associated with impaired blood flow. In RNSM, the incision is made away from the nipple in the mid-axillary line and the improved exposure enables the surgeon to dissect glandular tissue with greater precision, while preserving subcutaneous fat and vessels14,17,18. Similar rates of implant loss are recorded in the present review (4.05 per cent for RNSM and 2.33 per cent for CNSM) compared with previous reviews.
Table 1.
Comparison of crude complication rates of robotic nipple-sparing mastectomy and conventional nipple-sparing mastectomy
| Complication | RNSM | CNSM | ||
|---|---|---|---|---|
| Present study | Filipe et al.50 | Present study | Filipe et al.50 | |
| Implant loss | 4.05 | 4.1 | 2.33 | 3.2 |
| Haematoma | 6.58 | 4.3 | 7.16 | 2.0 |
| Necrosis | 8.23 (skin); 7.34 (nipple) | 4.3 | 11.52 (skin); 14.22 (nipple) | 7.4 |
| Infection | 5.56 | 8.3 | 2.64 | 4.0 |
| Seroma | 5.97 | 3.0 | 9.09 | 2.0 |
Values are %. RNSM, robotic nipple-sparing mastectomy; CNSM, conventional nipple-sparing mastectomy.
Despite previous studies reporting a high infection rate for RNSM, the present study reports a comparatively low infection rate (present study, 5.56 per cent; and Filipe et al.50, 8.3 per cent), albeit still higher than for CNSM. The opposite trend was noted for haematoma and seroma formation, favouring RNSM, although without statistical significance. The crude rates of postoperative haematoma were higher for RNSM in the present study when compared with previous studies (7.16 versus 4.3 per cent), but meta-synthesized evidence suggested no significant difference between RNSM and CNSM (OR 0.99 (95 per cent c.i. 0.50 to 1.95); P = 0.969). It is interesting to note that none of the studies reported a need of conversion to CNSM, as this likely is a rare phenomenon observed during the initial learning curve of a new technology.
Postoperative complications are of major clinical importance, but oncological safety should be the first consideration when determining whether a procedure should be performed in breast cancer. In terms of oncological safety, it appeared that there was a reduction in the number of local recurrence events in the RNSM group. Nevertheless, the follow-up duration was insufficient to draw any firm conclusions and the RNSM group is prone to selection bias. The increased risk of positive margins may reflect the inclusion of more advanced disease stages or flexibility regarding the accepted distance of the tumour to the areola. Two studies excluded disease that was greater than or equal to stage IIIb45,46 and one study excluded disease that was stage IV49 for RNSM. Toesca et al.43 included one stage IV patient in the RNSM group and Lai et al.45 included eight stage IV patients in the CNSM group. The only RCT43 reported on oncological outcomes and, with a median follow-up of 42 months, there was no significant difference in overall survival and disease-free survival between the RNSM and CNSM arms. Additionally, no nipple recurrence was noted in the RNSM arm, whereas one nipple recurrence was reported in the CNSM arm. Oncological outcomes were similar in the SORI study51. Of note, long-term oncological outcomes were not addressed in the present meta-analysis, as the oncological safety profile was not systematically reported in the included studies. For the four included studies that reported recurrence events43,45–47, the duration of follow-up was also deemed insufficient for reliable conclusions (Fig. S1b).
A learning curve is anticipated for any new technique. The increased operating time of 58 min is especially important from a health-system management point of view, as resource constrains are prevalent. Regarding the observed increased length of RNSM procedures, the time required for preparation of the operating area, docking of robotic arms, robotic breast resection, and the overall smaller operating space compared with intraperitoneal robotic surgery, and longer smoke expulsion times, may all influence the operating time. This may, however, be reduced with time. On that note, the preliminary experience and learning curve of RNSM were analysed and reported by Lai et al.52,53; the cases needed to reduce operating time for ‘docking’, ‘RNSM’, and ‘total time for RNSM and Immediate Prosthetic Breast Reconstruction (IPBR)’ were 13th, 13th, and 12th procedures respectively. Similar trends were observed in the present review, where the surgeon learning curve appeared to be strongly associated with shorter operating times (Table S1). A total of three of the included studies reported on the learning curve and demonstrated a steep learning curve45–47. The Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG)54 reported early experiences with 11 surgeons at eight institutions and demonstrated an equally rapid learning-curve stabilization, especially among second-generation surgeons, who learned from the pioneer surgeons.
Taking into account that the length of the operation remains high at this stage, the major drawback of RNSM is the high cost, shown to be an additional 3,804.80 Euros46,53,54. RNSM is significantly associated with a higher cost compared with other NSMs (P < 0.01)45. Moreover, the mean cost was reported to be higher for both RNSM-implant versus CNSM-implant (+34.7 per cent: 1749 euros) and RNSM-latissimus dorsi flap versus CNSM-latissimus dorsi flap (+30 per cent: 2357 euros) (Table S1)44. The costs of the robotic console, service contract, and disposable instrumentation are higher than the cost of a CNSM. Additional expenses were, however, minimal when approximately 300 procedures per year were performed with a single robotic system, using only two robotic instruments for dissection and a brief learning curve28. With an expanding range of clinical indications for the robotic approach, the overall cost of using the platform is likely to drop further. This will mainly result from a further improvement in the longevity of the instruments. Recently, the ‘life’ of each robotic instrument has increased from 10 uses to up to 18 uses, making the consumables significantly more cost-effective than previously. It is plausible that further development along the technical specifications of robotic systems may make robotic procedures more cost-effective in the longer term55,56. The lower postoperative complication profile of RNSM43,51, including reduction of postoperative complications, can moreover translate into improved long-term cost-effective outcomes.
In addition to postoperative outcomes and oncological safety, the aesthetic outcome is vital in terms of patient satisfaction and quality of life57. The most appealing aspect of an NSM is superior aesthetic results. The patient satisfaction or aesthetic outcome was assessed in five studies. Patient-reported outcome measures and panel-based assessment of the aesthetic outcome were reported inconsistently across studies. Toesca et al.43 documented BREAST-Q scores at 12 months after surgery and a high level of quality of life was maintained after an RNSM. Additionally, a similar trend favouring RNSM in terms of reducing psychosocial health and body-image disturbances after cancer treatment was found. It is also noteworthy that nipple sensitivity and sexual pleasure were less disturbed after the robotic approach. Houvenaeghel et al.44 examined the aesthetic outcome at 6 and 12 months after surgery using a questionnaire. Similarly, aesthetic-outcome panel questionnaires were utilized by Huang et al.47. The only study to report data on nipple sensitivity showed better preservation of nipple sensation43. Of note, a recent study has highlighted that patient and panel aesthetic-outcome assessments may significantly differ and therefore standardization of tools may be necessary to allow meaningful comparisons across studies57.
The present meta-analysis is a contemporary head-to-head comparison of RNSM versus CNSM complications and surgical outcomes, with a robust search strategy and use of Cochrane-recommended statistical methodology, in contrast to a previously published meta-synthesis58. The present study has evaluated cost-effectiveness and the learning curve, in addition to postoperative complications and outcomes. Inherent limitations of the present analysis lie in the retrospective observational design of some of the included studies, which leads to a possible risk of selection bias. The level of the evidence of the included studies is not of high quality, indicating the need of further high-quality studies. On that note, the MAstectomy with Reconstruction including Robotic Endoscopic Surgery (MARRES) study (NCT04585074) is a prospective cohort study aiming to recruit 2000 patients and evaluates surgical outcomes and complication rates between RNSM versus endoscopic mastectomy versus CNSM59. Additionally, the ROM trial (NCT05490433) aims to provide prospective results to address long-term oncological outcomes, including 5-year survival rates, as well as postoperative complication rates and cost-effectiveness of RNSM60. Contemporary and recently completed clinical trials associated with robotic breast surgery have been thoroughly summarized by Park et al.61. Multiple prospective trials have been initiated on RNSM and will provide higher level of evidence62–72.
Additionally, while the aim of the present study was to evaluate the outcomes of these operations on women with breast cancer, the included studies were conducted on very heterogeneous patient populations, including women undergoing both risk-reducing mastectomies, as well as cancer treatment. For data transparency and homogenization of cumulative outcomes, crude outcome values indication for surgery, disease stage (Table S2), and surgical parameters (Table S3) were collected and reported. Notably, the included studies utilized a variety of reconstruction techniques and it is well established that the complication profile of autologous reconstruction may differ according to the technology used. Neoadjuvant and adjuvant therapy may also influence outcomes; however, this variable was not reported uniformly in the included studies and hence was not incorporated in the meta-analysis. Of note, the proportion of invasive lobular carcinoma or mixed tumour pathology (31.89 per cent) appeared higher than expected, especially when compared with the 26.52 per cent of invasive ductal carcinoma. A likely cause could lie in lobular cancer more often being multifocal, requiring a higher rate of mastectomy, as well as geographical variations in invasive lobular carcinoma incidence.
Of note, most studies in the present study were conducted in Taiwan and Korea, limiting the generalizability of the results to Western populations. However, in selected patients, RNSM has reduced or equivalent postoperative complications and oncological safety. Overall, the present systematic review and meta-analysis indicates a trend of reduced complication rates and improved aesthetic outcome in patients that undergo RNSM. Cost-effectiveness and learning curve-dependent longer operating times pose limitations to widespread adoption. Whilst still in its infancy, robotic breast surgery may present a viable option within the range of oncoplastic breast-surgery techniques. The oncological safety profile of this approach, however, requires more robust assessment before its widespread implementation.
Supplementary Material
Acknowledgements
The authors acknowledge NHSG Library and Knowledge Services.
Contributor Information
Ashrafun Nessa, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK; General Surgery, Aberdeen Royal Infirmary, Aberdeen, UK; Breast Surgery, Aberdeen Royal Infirmary, Aberdeen, UK.
Shafaque Shaikh, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK; General Surgery, Aberdeen Royal Infirmary, Aberdeen, UK.
Mairi Fuller, Breast Surgery, Aberdeen Royal Infirmary, Aberdeen, UK.
Yazan A Masannat, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, UK; Breast Surgery, Aberdeen Royal Infirmary, Aberdeen, UK.
Stavroula L Kastora, UCL EGA Institute for Women's Health, University College London, London, UK.
Funding
The authors have no funding to declare.
Authors contributions
Ashrafun Nessa (Conceptualization, Data curation, Formal analysis, Project administration, Writing—original draft, Writing—review & editing), Shafaque Shaikh (Conceptualization, Validation, Writing—review & editing), Mairi Fuller (Conceptualization, Validation, Writing—review & editing), Yazan A. Masannat (Conceptualization, Formal analysis, Validation, Writing—review & editing), and Stavroula L. Kastora (Conceptualization, Data curation, Formal analysis, Methodology, Writing—review & editing).
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
All crude data available upon request.
References
- 1. WHO . Breast Cancer. https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed 7 April 2023)
- 2. Didier F, Radice D, Gandini S, Bedolis R, Rotmensz N, Maldifassi A et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat 2009;118:623–633 [DOI] [PubMed] [Google Scholar]
- 3. Romanoff A, Zabor EC, Stempel M, Sacchini V, Pusic A, Morrow M. A comparison of patient-reported outcomes after nipple-sparing mastectomy and conventional mastectomy with reconstruction. Ann Surg Oncol 2018;25:2909–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boughey JC, McLaughlin SA. Breast surgery in 2015: advances in recent years. Ann Surg Oncol 2015;22:3157–3160 [DOI] [PubMed] [Google Scholar]
- 5. Newman LA, Kuerer HM, Hunt KK, Vlastos G, Ames FC, Ross MI. et al. Prophylactic mastectomy. J Am Coll Surg 2000;191:322–330 [DOI] [PubMed] [Google Scholar]
- 6. Mesdag V, Régis C, Tresch E, Chauvet MP, Boulanger L, Collinet P et al. Nipple sparing mastectomy for breast cancer is associated with high patient satisfaction and safe oncological outcomes. J Gynecol Obstet Hum Reprod 2017;46:637–642 [DOI] [PubMed] [Google Scholar]
- 7. Lago V, Maisto V, Gimenez-Climent J, Vila J, Vazquez C, Estevan R. Nipple-sparing mastectomy as treatment for patients with ductal carcinoma in situ: a 10-year follow-up study. Breast J 2018;24:298–303 [DOI] [PubMed] [Google Scholar]
- 8. Chan YH, Yau WM, Cheung PS. Oncological safety and technical feasibility of nipple-sparing mastectomy for breast cancer: the Hong Kong experience. World J Surg 2018;42:1375–1383 [DOI] [PubMed] [Google Scholar]
- 9. De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple–areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol 2015;22:3241–3249 [DOI] [PubMed] [Google Scholar]
- 10. Galimberti V, Vicini E, Corso G, Morigi C, Fontana S, Sacchini V et al. Nipple-sparing and skin-sparing mastectomy: review of aims, oncological safety and contraindications. Breast 2017; 34(Suppl 1): S82–S84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bailey CR, Ogbuagu O, Baltodano PA, Simjee UF, Manahan MA, Cooney DS et al. Quality-of-life outcomes improve with nipple-sparing mastectomy and breast reconstruction. Plast Reconstr Surg 2017;140:219–226 [DOI] [PubMed] [Google Scholar]
- 12. Wagner JL, Fearmonti R, Hunt KK, Hwang RF, Meric-Bernstam F, Kuerer HM et al. Prospective evaluation of the nipple–areola complex sparing mastectomy for risk reduction and for early-stage breast cancer. Ann Surg Oncol 2012;19:1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong SM, Chun YS, Sagara Y, Golshan M, Erdmann-Sager J. National patterns of breast reconstruction and nipple-sparing mastectomy for breast cancer, 2005–2015. Ann Surg Oncol 2019;26:3194–3203 [DOI] [PubMed] [Google Scholar]
- 14. Rocco N, Catanuto G, Nava MB. What is the evidence behind conservative mastectomies? Gland Surg 2015;4:506–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson RS, Sanders T, Park A, Buras R, Liang W, Harris C et al. Prospective study comparing surgeons’ pain and fatigue associated with nipple-sparing versus skin-sparing mastectomy. Ann Surg Oncol 2017;24:3024–3031 [DOI] [PubMed] [Google Scholar]
- 16. Kopkash K, Novak K, Kuchta K, Yashina I, Poli E, Rabbitt S et al. The “nipple whipple”?! A pilot study to assess the ergonomic effects of nipple-sparing mastectomy. Ann Surg Oncol 2019;26:3216–3223 [DOI] [PubMed] [Google Scholar]
- 17. Mota BS, Riera R, Ricci MD, Barrett J, de Castria TB, Atallah ÁN et al. Nipple- and areola-sparing mastectomy for the treatment of breast cancer. Cochrane Database Syst Rev 2016; (11)CD008932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee KT, Mun GH. Necrotic complications in nipple-sparing mastectomy followed by immediate breast reconstruction: systematic review with pooled analysis. Arch Hand Microsurg 2014;23:51–64 [Google Scholar]
- 19. Donovan CA, Harit AP, Chung A, Bao J, Giuliano AE, Amersi F. Oncological and surgical outcomes after nipple-sparing mastectomy: do incisions matter? Ann Surg Oncol 2016;23:3226–3231 [DOI] [PubMed] [Google Scholar]
- 20. Kaouk JH, Haber GP, Autorino R, Crouzet S, Ouzzane A, Flamand V et al. A novel robotic system for single-port urologic surgery: first clinical investigation. Eur Urol 2014;66:1033–1043 [DOI] [PubMed] [Google Scholar]
- 21. Badani KK, Bhandari A, Tewari A, Menon M. Comparison of two-dimensional and three-dimensional suturing: is there a difference in a robotic surgery setting? J Endourol 2005;19:1212–1215 [DOI] [PubMed] [Google Scholar]
- 22. Yu HY, Friedlander DF, Patel S, Hu JC. The current status of robotic oncologic surgery. CA Cancer J Clin 2013;63:45–56 [DOI] [PubMed] [Google Scholar]
- 23. Oehler MK. Robot-assisted surgery in gynaecology. Aust N Z J Obstet Gynaecol 2009;49:124–129 [DOI] [PubMed] [Google Scholar]
- 24. Bianchi PP, Petz W, Luca F, Biffi R, Spinoglio G, Montorsi M. Laparoscopic and robotic total mesorectal excision in the treatment of rectal cancer. Brief review and personal remarks. Front Oncol 2014;4:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toesca A, Peradze N, Galimberti V, Manconi A, Intra M, Gentilini O et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg 2017;266:e28–e30 [DOI] [PubMed] [Google Scholar]
- 26. Toesca A, Peradze N, Manconi A, Teixeira LF et al. Reply to the letter to the editor “Robotic-assisted nipple sparing mastectomy: a feasibility study on cadaveric models” by Sarfati B. et al. J Plast Reconstr Aesthet Surg 2017;70:558–560 [DOI] [PubMed] [Google Scholar]
- 27. Ahn SJ, Song SY, Park HS, Park SH, Lew DH, Roh TS et al. Early experiences with robot-assisted prosthetic breast reconstruction. Arch Plast Surg 2019;46:79–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Houvenaeghel G, Bannier M, Rua S, Barrou J, Heinemann M, Van Troy A et al. Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve. World J Surg Oncol 2019;17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Selber JC. Robotic nipple-sparing mastectomy: the next step in the evolution of minimally invasive breast surgery. Ann Surg Oncol 2019;26:10–11 [DOI] [PubMed] [Google Scholar]
- 30. Morigi C. Highlights from the 15th St Gallen International Breast Cancer Conference 15–18 March, 2017, Vienna: tailored treatments for patients with early breast cancer. Ecancermedicalscience 2017;11:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration . Caution When Using Robotically-Assisted Surgical Devices in Women's Health Including Mastectomy and Other Cancer-Related Surgeries: FDA Safety Communication. 2019
- 32. Lai HW, Toesca A, Sarfati B, Park HS, Houvenaeghel G, Selber JC et al. Consensus statement on robotic mastectomy—expert panel from International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg 2020;271:1005–1012 [DOI] [PubMed] [Google Scholar]
- 33. Margenthaler JA. Robotic mastectomy—program malfunction? JAMA Surg 2020;155:461–462 [DOI] [PubMed] [Google Scholar]
- 34. Struk S, Qassemyar Q, Leymarie N, Honart JF, Alkhashnam H, De Fremicourt K et al. The ongoing emergence of robotics in plastic and reconstructive surgery. Ann Chir Plast Esthét 2018;63:105–112 [DOI] [PubMed] [Google Scholar]
- 35. Angarita FA, Castelo M, Englesakis M, McCready DR, Cil TD. Robot-assisted nipple-sparing mastectomy: systematic review. Br J Surg 2020;107:1580–1594 [DOI] [PubMed] [Google Scholar]
- 36. Lai HW, Lin SL, Chen ST, Lin YL, Chen DR, Pai SS et al. Robotic nipple sparing mastectomy and immediate breast reconstruction with robotic latissimus dorsi flap harvest—technique and preliminary results. J Plast Reconstr Aesthet Surg 2018;71:e59–e61 [DOI] [PubMed] [Google Scholar]
- 37. Sarfati B, Struk S, Leymarie N, Honart JF, Alkhashnam H, Kolb F et al. Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: surgical technique. Plast Reconstr Surg 2018;142:624–627 [DOI] [PubMed] [Google Scholar]
- 38. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 39. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed 3 January 2023)
- 40. Sterne JA, Savovi J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 41. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101 [PubMed] [Google Scholar]
- 42. Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Including non-randomized studies on intervention effects. In: Cochrane Handbook for Systematic Reviews of Interventions. United Kingdom: Cochrane, 2019, 595–620 [Google Scholar]
- 43. Toesca A, Sangalli C, Maisonneuve P, Massari G, Girardi A, Baker JL et al. A randomized trial of robotic mastectomy versus open surgery in women with breast cancer or BRCA mutation. Ann Surg 2022;276:11–19 [DOI] [PubMed] [Google Scholar]
- 44. Houvenaeghel G, Barrou J, Jauffret C, Rua S, Sabiani L, Van Troy A et al. Robotic versus conventional nipple-sparing mastectomy with immediate breast reconstruction. Front Oncol 2021;11:637049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lai HW, Chen ST, Mok CW, Lin YJ, Wu HK, Lin SL et al. Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer-A case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg 2020;73:1514–1525 [DOI] [PubMed] [Google Scholar]
- 46. Lai HW, Chen ST, Lin YJ, Lin SL, Lin CM, Chen DR et al. Minimal access (endoscopic and robotic) breast surgery in the surgical treatment of early breast cancer—trend and clinical outcome from a single-surgeon experience over 10 years. Front Oncol 2021;11:7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang JJ, Chuang EY, Cheong DC, Kim BS, Chang FC, Kuo WL. Robotic-assisted nipple-sparing mastectomy followed by immediate microsurgical free flap reconstruction: feasibility and aesthetic results—case series. Int J Surg 2021;95:106143. [DOI] [PubMed] [Google Scholar]
- 48. Moon J, Lee J, Lee DW, Lee HS, Nam DJ, Kim MJ et al. Postoperative pain assessment of robotic nipple-sparing mastectomy with immediate prepectoral prosthesis breast reconstruction: a comparison with conventional nipple-sparing mastectomy. Int J Med Sci 2021;18:2409–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee J, Park HS, Lee H, Lee DW, Song SY, Lew DH et al. Post-operative complications and nipple necrosis rates between conventional and robotic nipple-sparing mastectomy. Front Oncol 2021;10:594388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Filipe MD, de Bock E, Postma EL, Bastian OW, Schellekens PP, Vriens MR et al. Robotic nipple-sparing mastectomy complication rate compared to traditional nipple-sparing mastectomy: a systematic review and meta-analysis. J Robot Surg 2022;16:265–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park HS, Lee J, Lai HW, Park JM, Ryu JM, Lee JE et al. Surgical and oncologic outcomes of robotic and conventional nipple-sparing mastectomy with immediate reconstruction: international multicenter pooled data analysis. Ann Surg Oncol 2022;29:6646–6657 [DOI] [PubMed] [Google Scholar]
- 52. Lai HW, Wang CC, Lai YC, Chen CJ, Lin SL, Chen ST et al. The learning curve of robotic nipple sparing mastectomy for breast cancer: an analysis of consecutive 39 procedures with cumulative sum plot. Eur J Surg Oncol 2019;45:125–133 [DOI] [PubMed] [Google Scholar]
- 53. Lai HW, Chen ST, Lin SL, Chen CJ, Lin YL, Pai SH et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol 2019;26:42–52 [DOI] [PubMed] [Google Scholar]
- 54. Ryu JM, Kim JY, Choi HJ, Ko B, Kim J, Cho J et al. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: an initial experience of the Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG). Ann Surg 2022;275:985–991 [DOI] [PubMed] [Google Scholar]
- 55. Almujalhem A, Rha KH. Surgical robotic systems: what we have now? A urological perspective. BJUI Compass 2020;1:152–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mayor N, Coppola AS, Challacombe B. Past, present and future of surgical robotics. Trends Urol Men's Health 2022;13:7–10 [Google Scholar]
- 57. Kastora SL, Holmquist A, Valachis A, Rocco N, Meattini I, Somaiah N et al. Outcomes of different quality of life assessment modalities after breast cancer therapy: a network meta-analysis. JAMA Netw Open 2023;6:e2316878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. De la Cruz-Ku G, Chambergo-Michilot D, Perez A, Valcarcel B, Pamen L, Linshaw D et al. Outcomes of robotic nipple-sparing mastectomy versus conventional nipple-sparing mastectomy in women with breast cancer: a systematic review and meta-analysis. J Robot Surg 2023;17:1493–1509 [DOI] [PubMed] [Google Scholar]
- 59. ClinicalTrials.gov . Prospective Study of MAstectomy With Reconstruction Including Robot Endoscopic Surgery (MARRES). https://clinicaltrials.gov/ct2/show/NCT04585074 (accessed 15 June 2023)
- 60. US Clinical Trials Registry . Robotic-assisted vs. Open Nipple-sparing Mastectomy With Immediate Breast Reconstruction (ROM). https://ichgcp.net/clinical-trials-registry/NCT05490433 (accessed 15 June 2023)
- 61. Park KU, Cha C, Pozzi G, Kang YJ, Gregorc V, Sapino A et al. Robot-assisted nipple sparing mastectomy: recent advancements and ongoing controversies. Curr Breast Cancer Rep 2023;15:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. ClinicalTrials.gov . Prospective Registry Trial for Single Port Robot-assisted Nipple Sparing Mastectomy (SPrNSM) (SPrNSM). https://www.clinicaltrials.gov/ct2/show/NCT04866992? cond=Robot-assisted+mastectomy&draw=2 (accessed 15 June 2023)
- 63. ClinicalTrials.gov . Prospective Pilot Study of Robot-assisted Nipple Sparing Mastectomy (RNSM). https://clinicaltrials.gov/ct2/show/NCT04537312? term=robot&cond=Breast+Cancer&draw=2 (accessed 15 June 2023)
- 64. ClinicalTrials.gov . Surgical and Patient Reported Outcomes of Robotic Nipple-Sparing Mastectomy (RNSM). https://clinicaltrials.gov/ct2/show/NCT04151368 (accessed 15 June 2023)
- 65. ClinicalTrials.gov . Robotic-Assisted da Vinci Xi Prophylactic Nipple-Sparing Mastectomy. https://clinicaltrials.gov/ct2/show/NCT03892980 (accessed 15 June 2023)
- 66. ClinicalTrials.gov . Safety and Feasibility of Robotic SP Nipple Sparing Mastectomy. https://clinicaltrials.gov/ct2/show/NCT05245812 (accessed 15 June 2023)
- 67. ClinicalTrials.gov . Surgical and Oncologic Outcomes After Robotic Nipple Sparing Mastectomy and Immediate Reconstruction (SORI). https://clinicaltrials.gov/ct2/show/NCT04108117 (accessed 15 June 2023)
- 68. ClinicalTrials.gov . Robotic Nipple-Sparing Mastectomy Vs Conventional Open Technique. https://clinicaltrials.gov/ct2/show/NCT03440398 (accessed 15 June 2023)
- 69. ClinicalTrials.gov . Robotic Versus Conventional or Endoscopic Nipple Sparing Mastectomy in the Management of Breast Cancer-Prospective Study (RCENSM-P). https://clinicaltrials.gov/ct2/show/NCT04037852 (accessed 15 June 2023)
- 70. ClinicalTrials.gov . Robotic Versus Conventional or Endoscopic Nipple Sparing Mastectomy for Breast Cancer (RCENSM-R). https://clinicaltrials.gov/ct2/show/NCT04049305 (accessed 15 June 2023)
- 71. ClinicalTrials.gov . Post Market Clinical Follow-up Study on da Vinci® Robotic-assisted Prophylactic Nipple Sparing Mastectomy With Breast Reconstruction (PREVENT). https://clinicaltrials.gov/ct2/show/NCT05251285 (accessed 15 June 2023)
- 72. ClinicalTrials.gov . Mastectomy With Retention of the Nipple-areola Complex, Robot-assisted or Not, and / or Immediate or Seconday Reconstruction by Latissimus Dorsi Flap, Robot-assisted or Not. (RMR). https://clinicaltrials.gov/ct2/show/NCT04457167 (accessed 15 June 2023)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All crude data available upon request.